Materials Prepared via Pickering Emulsions Stabilized by Graphene Oxide: Overview and Prospects
Abstract
1. Introduction
2. Basics of Pickering Emulsions
2.1. Particles as Emulsifiers
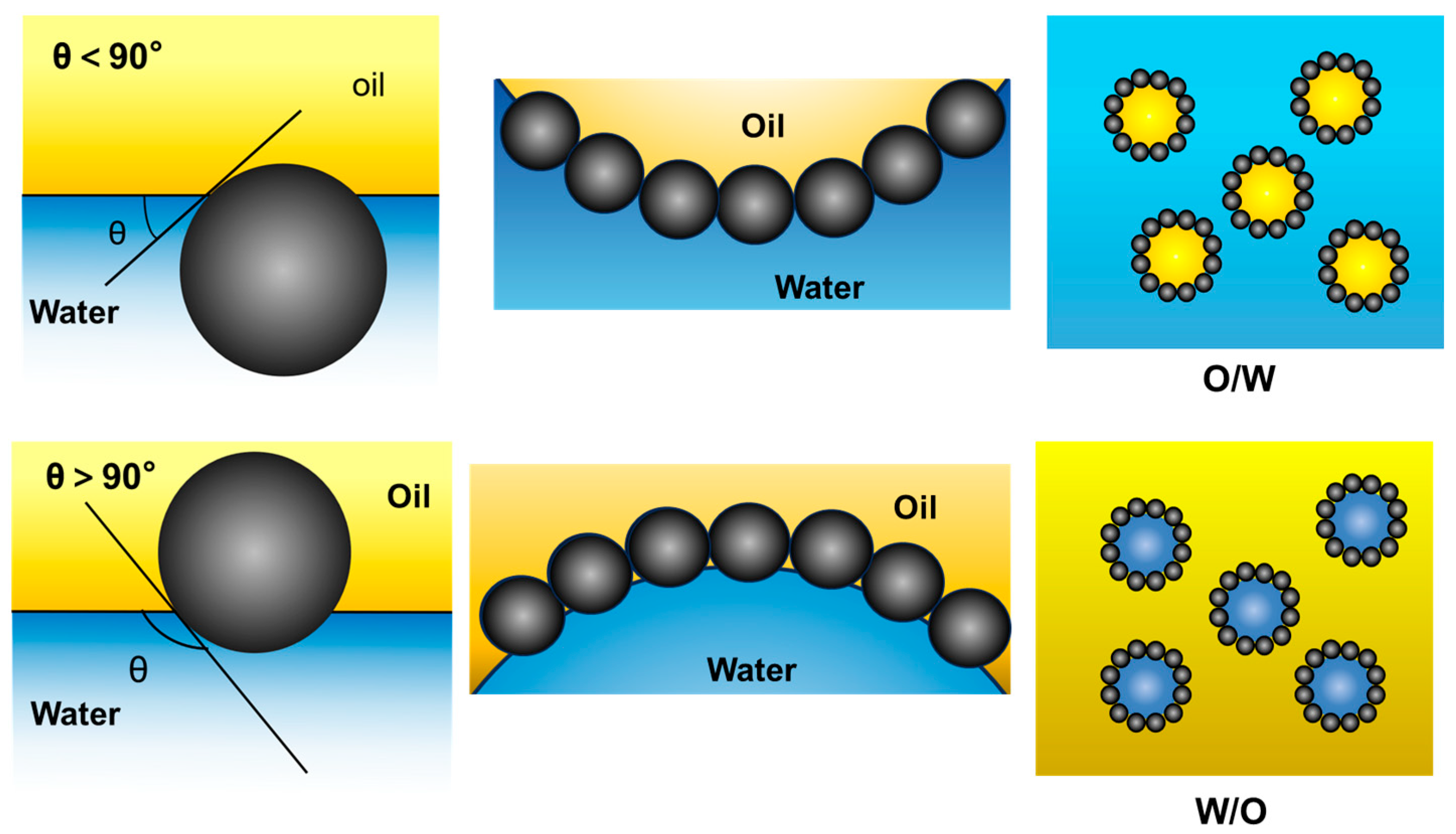
2.2. Stabilization Mechanism of Pickering Emulsion
3. Structure and Properties of GO
3.1. 2D Sheet Structure
3.2. Amphiphilicity and Interface Activity
3.3. Functional Groups in GO
3.4. Functionalized GO and Reduced GO
4. Factors Affecting the Performance of Pickering Emulsions Stabilized by GO
4.1. Inherent Characteristics of GO
4.2. Chemical Environment
4.3. Emulsion System
5. Advanced Materials Prepared via GO-Based Pickering Emulsion
5.1. Microspheres
5.2. Microcapsules
5.3. Composites Materials
5.4. Porous Materials and Sponges
6. Applications in Potential Industry
6.1. Energy Storage
6.2. Anticorrosion
6.3. Biological Medicine
6.4. Other Fields
7. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ming, L.; Wu, H.; Liu, A.; Naeem, A.; Dong, Z.; Fan, Q.; Zhang, G.; Liu, H.; Li, Z. Evolution and critical roles of particle properties in Pickering emulsion: A review. J. Mol. Liq. 2023, 388, 122775. [Google Scholar] [CrossRef]
- Ramsden, W. Separation of Solids in the Surface-layers of Solutions and ‘Suspensions’. Proc. R. Soc. 1903, 72, 156–164. [Google Scholar]
- Pickering, S.U. Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Fonseca, J.; Broto-Ribas, A.; Jiao, L.; Pei, X. Pickering emulsions stabilized by metal-organic framework nanoparticles. Adv. Colloid. Interface Sci. 2025, 342, 103532. [Google Scholar] [CrossRef]
- Gonzalez Ortiz, D.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Current Trends in Pickering Emulsions: Particle Morphology and Applications. Engineering 2020, 6, 468–482. [Google Scholar] [CrossRef]
- Wang, H.; Liu, W.; Zhou, X.; Li, H.; Qian, K. Stabilization of ASA-in-water emulsions by Laponite modified with alanine. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 294–301. [Google Scholar] [CrossRef]
- Yu, D.; Luo, Q.; Zhang, J.; Wang, Q.; Wang, H.; Song, Z.; Li, S.; Liu, W.; Zhang, F.; Ji, D. Pickering emulsions co-stabilised by cellulose nanofibres and nicotinamide mononucleotide. Cellulose 2022, 29, 8569–8585. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, D.; Zhao, R.; Hu, F.; Li, Z.; Dong, B.; Lu, P.; Song, Z.; Wang, H.; Zhang, F.; et al. Enhanced stability and biocompatibility of HIPEs stabilized by cyclodextrin-metal organic frameworks with inclusion of resveratrol and soy protein isolate for β-carotene delivery. Int. J. Biol. Macromol. 2024, 274, 133431. [Google Scholar] [CrossRef]
- Kim, J.; Cote, L.J.; Kim, F.; Yuan, W.; Shull, K.R.; Huang, J. Graphene Oxide Sheets at Interfaces. J. Am. Chem. Soc. 2010, 132, 8180–8186. [Google Scholar] [CrossRef]
- Shao, J.J.; Lv, W.; Yang, Q.H. Self-Assembly of Graphene Oxide at Interfaces. Adv. Mater. 2014, 26, 5586–5612. [Google Scholar] [CrossRef]
- Jahandideh, H.; Nguyen, Q.A.; Tufenkji, N. Polymer-Free Emulsion-Templated Graphene-Based Sponges for Contaminant Removal. ACS Appl. Mater. Interfaces 2020, 12, 52095–52103. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liu, Y.J.; Cao, Y.Z.; Quan, G.P.; Li, W.W.; Li, D.M.; Wu, Y.H.; Xiao, L.H.; Yu, F. Fabrication of microcapsules with graphene/organic hybrid shell based on Pickering emulsions for self-healing anti-corrosive coatings. J. Appl. Polym. Sci. 2024, 141, e55653. [Google Scholar] [CrossRef]
- Du, K.; Yu, B.C.; Xiong, Y.M.; Jiang, L.; Xu, J.; Wang, Y.; Su, S.; Hu, S.; Xiang, J. Hydrodeoxygenation of Bio-Oil over an Enhanced Interfacial Catalysis of Microemulsions Stabilized by Amphiphilic Solid Particles. Catalysts 2023, 13, 573. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, D.; Zhao, R.; Li, Z.; Dong, B.; Hu, F.; Li, S.; Zhang, F.; Wang, H. A functional Pickering emulsion coating based on octadecenylsuccinic anhydride modified γ-cyclodextrin metal-organic frameworks for food preservation. Food Hydrocolloid 2024, 150, 109668. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Zhou, T.; Han, Q. High-Efficiency Alkyl Ketene Dimer Emulsions Stabilized by Polyaluminum Chloride and Chitosan Complex. Langmuir 2024, 40, 17510–17516. [Google Scholar] [CrossRef]
- Zhou, T.; Han, Q.; Yu, D.; Liu, W.; Wang, H. Encapsulation of alkenyl succinic anhydride oil droplets in emulsions Preparation characterization stability properties and application in papermaking. J. Dispers. Sci. Technol. 2024, 45, 2559–2567. [Google Scholar] [CrossRef]
- Han, Q.; Wang, H.; Zhou, T.; Wang, Y.; Shen, Z.; Yu, D.; Liu, X.; Liu, W.; Lv, W. Ultrastable Emulsion Stabilized by the Konjac Glucomannan-Xanthan Gum Complex. ACS Omega 2023, 8, 31344–31352. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, W.; Liu, X.; Yu, D.; Song, Z.; Wang, H.; Li, G.; Ge, S. Using xanthan gum and PEDOT:PSS to costabilize Ga droplets to synergistically improve the toughness and sensing performance of polyacrylamide hydrogels. Sci. China Mater. 2023, 66, 3723–3734. [Google Scholar] [CrossRef]
- Yi, D.; Jeong, G.; Seo, J.H.; Yoo, M.J.; Yang, H. Carbon Dots with Tailored Surface Wettability as Pickering Emulsifiers. ACS Appl. Nano Mater. 2022, 5, 10258–10267. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Yan, C.; Bi, J.; Han, X.; Liu, H. Tint-Adjustable Pickering Emulsion Sunscreen Based on Polydopamine-Coated Silica Nanoparticles. ACS Appl. Nano Mater. 2024, 7, 15365–15375. [Google Scholar] [CrossRef]
- Cho, H.; Sung, M.; Choi, J.; Lee, H.; Prabakaran, L.; Kim, J.W. Ultralight, Robust, Thermal Insulating Silica Nanolace Aerogels Derived from Pickering Emulsion Templates. ACS Appl. Mater. Interfaces 2024, 16, 9255–9263. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, X.; Xiao, Y.; Hu, L.; Eggersdorfer, M.; Chen, D.; Yang, Z.; Weitz, D.A. Pickering emulsions stabilized by colloidal surfactants: Role of solid particles. Particuology 2022, 64, 153–163. [Google Scholar] [CrossRef]
- Binks, B.P. Particles as surfactants—Similarities and differences. Curr. Opin. Colloid. Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Z.; Zhi, L.; Jiao, B.; Tian, Y.; Liu, H.; Hu, H.; Ma, X.; Pignitter, M.; Wang, Q.; et al. Research Progress of Food-Grade High Internal Phase Pickering Emulsions and Their Application in 3D Printing. Nanomaterials 2022, 12, 2949. [Google Scholar] [CrossRef]
- Tong, H.; Wang, J.; Qi, L.; Gao, Q. Starch-based Janus particle: Fabrication, characterization and interfacial properties in stabilizing Pickering emulsion. Carbohyd Polym. 2023, 313, 120867. [Google Scholar] [CrossRef] [PubMed]
- Vignati, E.; Piazza, R.; Lockhart, T.P. Pickering Emulsions: Interfacial Tension, Colloidal Layer Morphology, and Trapped-Particle Motion. Langmuir 2003, 19, 6650–6656. [Google Scholar] [CrossRef]
- Kim, I.; Worthen, A.J.; Johnston, K.P.; DiCarlo, D.A.; Huh, C. Size-dependent properties of silica nanoparticles for Pickering stabilization of emulsions and foams. J. Nanopart Res. 2016, 18, 82. [Google Scholar] [CrossRef]
- Wu, C.; Hou, D.S.; Yin, B.; Li, S.C. Synthesis and application of new core-shell structure via Pickering emulsion polymerization stabilized by graphene oxide. Compos. Part. B Eng. 2022, 247, 110285. [Google Scholar] [CrossRef]
- Zou, Y.; Guo, J.; Yin, S.; Wang, J.; Yang, X. Pickering Emulsion Gels Prepared by Hydrogen-Bonded Zein/Tannic Acid Complex Colloidal Particles. J. Agric. Food Chem. 2015, 63, 7405–7414. [Google Scholar] [CrossRef]
- Liu, L.; Ngai, T. Pickering Emulsions Stabilized by Binary Mixtures of Colloidal Particles: Synergies between Contrasting Properties. Langmuir 2022, 38, 13322–13329. [Google Scholar] [CrossRef]
- Guzman, E.; Abelenda-Nunez, I.; Maestro, A.; Ortega, F.; Santamaria, A.; Rubio, R.G. Particle-laden fluid/fluid interfaces: Physico-chemical foundations. J. Phys. Condens. Matter. 2021, 33, 333001. [Google Scholar] [CrossRef]
- Kaptay, G. On the equation of the maximum capillary pressure induced by solid particles to stabilize emulsions and foams and on the emulsion stability diagrams. Colloids Surf. A Physicochem. Eng. Asp. 2006, 282, 387–401. [Google Scholar] [CrossRef]
- Li, W.; Jiao, B.; Li, S.; Faisal, S.; Shi, A.; Fu, W.; Chen, Y.; Wang, Q. Recent Advances on Pickering Emulsions Stabilized by Diverse Edible Particles: Stability Mechanism and Applications. Front. Nutr. 2022, 9, 864943. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shen, Y.; Chen, X.; Dong, L.; Yu, H.; Bao, M.; Li, Y. Cationic surfactant-modified palygorskite particles as effective stabilizer for Pickering emulsion gel formation. Appl. Clay Sci. 2022, 219, 106439. [Google Scholar] [CrossRef]
- Hu, Y.; Yin, S.; Zhu, J.; Qi, J.; Guo, J.; Wu, L.; Tang, C.; Yang, X. Fabrication and characterization of novel Pickering emulsions and Pickering high internal emulsions stabilized by gliadin colloidal particles. Food Hydrocolloid 2016, 61, 300–310. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.; Erni, R.; Lee, Z.; Alem, N.; Gannett, W.; Zettl, A. Determination of the Local Chemical Structure of Graphene Oxide and Reduced Graphene Oxide. Adv. Mater. 2010, 22, 4467–4472. [Google Scholar] [CrossRef]
- Cote, L.J.; Kim, J.; Tung, V.C.; Luo, J.; Kim, F.; Huang, J. Graphene oxide as surfactant sheets. Pure Appl. Chem. 2010, 83, 95–110. [Google Scholar] [CrossRef]
- Borane, N.; Boddula, R.; Odedara, N.; Singh, J.; Andhe, M.; Patel, R. Comprehensive review on synthetic methods and functionalization of graphene oxide: Emerging Applications. Nano-Struct. Nano-Objects 2024, 39, 101282. [Google Scholar] [CrossRef]
- Suk, J.W.; Piner, R.D.; An, J.; Ruoff, R.S. Mechanical properties of monolayer graphene oxide. ACS Nano 2010, 4, 6557–6564. [Google Scholar] [CrossRef]
- Medhekar, N.V.; Ramasubramaniam, A.; Ruoff, R.S.; Shenoy, V.B. Hydrogen Bond Networks in Graphene Oxide Composite Paper: Structure and Mechanical Properties. ACS Nano 2010, 4, 2300–2306. [Google Scholar] [CrossRef]
- Kumar, P.V.; Bardhan, N.M.; Tongay, S.; Wu, J.; Belcher, A.M.; Grossman, J.C. Scalable enhancement of graphene oxide properties by thermally driven phase transformation. Nat. Chem. 2014, 6, 151–158. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Ibrahim, S.; Chakraborty, K.; Ghosh, S.; Pal, T. Stepwise reduction of graphene oxide and studies on defect-controlled physical properties. Sci. Rep. 2024, 14, 294. [Google Scholar] [CrossRef] [PubMed]
- Gudarzi, M.M.; Sharif, F. Self assembly of graphene oxide at the liquid-liquid interface: A new route to the fabrication of graphene based composites. Soft Matter 2011, 7, 3432–3440. [Google Scholar] [CrossRef]
- Sali, S.; Mackey, H.R.; Abdala, A.A. Effect of Graphene Oxide Synthesis Method on Properties and Performance of Polysulfone-Graphene Oxide Mixed Matrix Membranes. Nanomaterials 2019, 9, 769. [Google Scholar] [CrossRef]
- Song, X.; Yang, Y.; Liu, J.; Zhao, H. PS Colloidal Particles Stabilized by Graphene Oxide. Langmuir 2011, 27, 1186–1191. [Google Scholar] [CrossRef]
- Fei, X.; Xia, L.; Chen, M.; Wei, W.; Luo, J.; Liu, X. Preparation and Application of Water-in-Oil Emulsions Stabilized by Modified Graphene Oxide. Materials 2016, 9, 731. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, Y.; Yoo, E.; Wei, P.; Pentzer, E. Ionic Liquid-Containing Pickering Emulsions Stabilized by Graphene Oxide-Based Surfactants. Langmuir 2018, 34, 10114–10122. [Google Scholar] [CrossRef]
- Alberto Arenas-Blanco, B.; Muñoz-Rugeles, L.; Cabanzo-Hernández, R.; Mejía-Ospino, E. Molecular Dynamics study of the effect on the interfacial activity of Alkylamine-Modified graphene oxide. J. Mol. Liq. 2022, 362, 119724. [Google Scholar] [CrossRef]
- Tang, M.; Wang, X.; Wu, F.; Liu, Y.; Zhang, S.; Pang, X.; Li, X.; Qiu, H. Au nanoparticle/graphene oxide hybrids as stabilizers for Pickering emulsions and Au nanoparticle/graphene oxide@polystyrene microspheres. Carbon 2014, 71, 238–248. [Google Scholar] [CrossRef]
- He, Y.; Wu, F.; Sun, X.; Li, R.; Guo, Y.; Li, C.; Zhang, L.; Xing, F.; Wang, W.; Gao, J. Factors that affect Pickering emulsions stabilized by graphene oxide. ACS Appl. Mater. Interfaces 2013, 5, 4843–4855. [Google Scholar] [CrossRef]
- Chen, H.; Wang, D.; Wang, X.; Ye, Z.; Han, L.; Xu, Q. Triple Phase Inversion of Emulsions Stabilized by Amphiphilic Graphene Oxide and Cationic Surfactants. ACS Omega 2020, 5, 23524–23532. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, W.; Zhang, H.; He, F.; Yan, H.; He, R.; Zhang, K.; Fan, J. Graphene oxide Pickering phase change material emulsions with high thermal conductivity and photo-thermal performance for thermal energy management. Colloids Surf. A Physicochem. Eng. Asp. 2019, 575, 42–49. [Google Scholar] [CrossRef]
- Zhao, L.H.; Shu, M.Y.; Chen, H.L.; Shi, K.L.; Li, Z.Y. Preparation of graphene oxide-stabilized Pickering emulsion adjuvant for Pgp3 recombinant vaccine and enhanced immunoprotection against Chlamydia trachomatis infection. Front. Immunol. 2023, 14, 1148253. [Google Scholar] [CrossRef] [PubMed]
- Abu Zaid, N.S.K.; Nasser, M.S.; Onaizi, S.A. Pickering Emulsions Stabilized by Metal-Organic Frameworks, Graphene-Based Materials, and Carbon Nanotubes: A Comprehensive Review. J. Mol. Liq. 2024, 393, 123617. [Google Scholar] [CrossRef]
- Thickett, S.C.; Zetterlund, P.B. Graphene oxide (GO) nanosheets as oil-in-water emulsion stabilizers: Influence of oil phase polarity. J. Colloid. Interface Sci. 2015, 442, 67–74. [Google Scholar] [CrossRef]
- Liao, W.B.; Huang, X.X.; Zhong, G.Y.; Ye, L.Y.; Zheng, S.N. Fabrication of poly (styrene-acrylate)/silver nanoparticle-graphene oxide composite antibacterial by in situ Pickering emulsion polymerization. J. Mech. Behav. Biomed. Mater. 2023, 144, 105877. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, M.V.; Suneesh, A.S.; Selvan, B.R.; Jain, A.; Madapu, K.K.; Ramanathan, N. A Polymer-Functionalized GO Composite Prepared through Pickering Emulsion Polymerization for Selective Removal of Zr from Acidic Solutions. ChemistrySelect 2024, 9, e202401504. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Y.; Chen, Y.; Li, J.; Wang, L.; Li, C. A review of multiple Pickering emulsions: Solid stabilization, preparation, particle effect, and application. Chem. Eng. Sci. 2022, 248, 117085. [Google Scholar] [CrossRef]
- Hosseinzadeh, B.; Nikfarjam, N.; Kazemi, S.H. Hollow molecularly imprinted microspheres made by w/o/w double Pickering emulsion polymerization stabilized by graphene oxide quantum dots targeted for determination of L-cysteine concentration. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125978. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, Y.; Chen, Z.; Wei, P.; Yoo, E.; Pentzer, E. Pickering Emulsion-Templated Encapsulation of Ionic Liquids for Contaminant Removal. ACS Appl. Mater. Interfaces 2019, 11, 9612–9620. [Google Scholar] [CrossRef]
- Yu, F.; Feng, H.Y.; Leng, J.P.; Xue, H.Y.; Zhong, Z.Y.; Yan, Z.; Liu, X.D.; Liu, Y.; Xiao, L.H. Self-assembled graphene oxide microcapsules in Pickering emulsions for photo-responsive self-healing epoxy coatings. J. Appl. Polym. Sci. 2022, 139, e52685. [Google Scholar] [CrossRef]
- Xu, C.Y.; Gou, W.W.; Wang, X.M.; Zhou, J.L.; Liu, J.Y.; Chen, K.L. Synthesis of paraffin@PS/reduced graphene oxide microcapsules via Pickering emulsion for multi-protective coatings. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126054. [Google Scholar] [CrossRef]
- Yan, N.; Bian, C.; Li, H.Y.; Wang, J.N.; Xu, M.; Huang, H.T. Pickering emulsion-templated encapsulation of ammonium dinitramide by graphene sheets for hygroscopic inhibition. Appl. Surf. Sci. 2021, 537, 147994. [Google Scholar] [CrossRef]
- Wang, Y.F.; Quevedo, K.; Pentzer, E. Inter-capsule fusion and capsule shell destruction using dynamic covalent polymers. Polym. Chem. 2021, 12, 2695–2700. [Google Scholar] [CrossRef]
- Suresh, I.K.; Chidambaram, K.; Vinod, V.; Rajender, N.; Venkateswara, R.M.; Miroslav, Č. Synthesis, characterization and optical properties of graphene oxide-polystyrene nanocomposites. Polym. Adv. Technol. 2015, 26, 214–222. [Google Scholar] [CrossRef]
- Lu, Q.; Jin, H.J.; Choi, H.J. Pickering emulsion polymerized Fe3O4@graphene oxide-polystyrene composite particles and their electro/magnetorheological responses. J. Mol. Liq. 2022, 365, 120083. [Google Scholar] [CrossRef]
- Bian, Z.; Zhang, S.; Zhu, X.; Li, Y.; Liu, H.; Hu, J. In situ interfacial growth of zeolitic imidazolate framework (ZIF-8) nanoparticles induced by a graphene oxide Pickering emulsion. RSC Adv. 2015, 5, 31502–31505. [Google Scholar] [CrossRef]
- Wang, C.; Ge, H.; Liu, H.; Guo, S. Microstructure and properties of carbon fiber sized with pickering emulsion based on graphene oxide sheets and its composite with epoxy resin. J. Appl. Polym. Sci. 2015, 132, 42285. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Min, P.; Yue, G.Y.; Niu, B.C.; Li, L.L.; Yu, Z.Z.; Zhang, H.B. Emulsion-Based Multiscale Structural Design Realizes Lightweight and Superelastic Graphene Aerogels for Electromagnetic Interference Shielding. Small 2024, 20, 2405950. [Google Scholar] [CrossRef]
- Su, W.G.; Hu, M.Y.; Wang, L.; Kokogiannakis, G.; Chen, J.; Gao, L.Y.; Li, A.Q.; Xu, C.H. Microencapsulated phase change materials with graphene-based materials: Fabrication, characterisation and prospects. Renew. Sustain. Energy Rev. 2022, 168, 112806. [Google Scholar] [CrossRef]
- Wei, H.; Yang, W.; He, F.; Li, Y.; Lou, L.; Wang, R.; He, R.; Fan, J.; Zhang, K. Core@double-shell structured multifunctional phase change microcapsules based on modified graphene oxide Pickering emulsion. Int. J. Energy Res. 2021, 45, 3257–3268. [Google Scholar] [CrossRef]
- Maithya, O.M.; Li, X.; Feng, X.; Sui, X.; Wang, B. Microencapsulated phase change material via Pickering emulsion stabilized by graphene oxide for photothermal conversion. J. Mater. Sci. 2020, 55, 7731–7742. [Google Scholar] [CrossRef]
- Shao, F.L.; Wang, L.L.; Luo, R.R.; Yu, W.; Xie, H.Q. Shape-Stable Hybrid Emulsion Gel with Sodium Acetate Trihydrate and Paraffin Wax for Efficient Solar Energy Storage and Building Thermal Management. ACS Appl. Mater. Interfaces 2023, 15, 38474–38484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.C.; Wu, K.Y.; Chen, Y.X.; Liu, R.; Luo, J. The preparation of linseed oil loaded graphene/polyaniline microcapsule via emulsion template method for self-healing anticorrosion coatings. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129771. [Google Scholar] [CrossRef]
- Wu, C.; Hou, D.S.; Yin, B.; Li, S.C.; Wang, X.P. Investigation of Composite Protective Coatings Coregulated by Core-Shell Structures and Graphene Oxide Interfaces. ACS Appl. Mater. Interfaces 2022, 14, 40297–40312. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, R.N.; Du, A.; Zhang, X.R.; Yang, H.Z.; Fan, Y.Z.; Zhao, X.; Cao, X.M. Investigation of the anticorrosion properties of graphene oxide doped thin organic anticorrosion films for hot-dip galvanized steel. Appl. Surf. Sci. 2019, 480, 646–654. [Google Scholar] [CrossRef]
- Li, H.X.; Xia, Y.Z.; Wu, S.; Zhang, D.; Oliver, S.; Li, X.Y.; Chen, X.N.; Lei, L.; Shi, S.X. Micron-dimensional sulfonated graphene sheets co-stabilized emulsion polymerization to prepare acrylic latex used for reinforced anticorrosion coatings. Prog. Org. Coat. 2022, 165, 106762. [Google Scholar] [CrossRef]
- Wu, K.Y.; Chen, Y.X.; Zhang, Q.Q.; Gu, Y.; Liu, R.; Luo, J. Preparation of Graphene Oxide/Polymer Hybrid Microcapsules via Photopolymerization for Double Self-Healing Anticorrosion Coatings. ACS Appl. Mater. Interfaces 2024, 16, 38564–38575. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Feng, H.Y.; Xiao, L.H.; Liu, Y. Fabrication of graphene oxide microcapsules based on Pickering emulsions for self-healing water-borne epoxy resin coatings. Prog. Org. Coat. 2021, 155, 106221. [Google Scholar] [CrossRef]
- Wang, X.; Yu, K.; An, R.; Han, L.; Zhang, Y.; Shi, L.; Ran, R. Self-assembling GO/modified HEC hybrid stabilized pickering emulsions and template polymerization for biomedical hydrogels. Carbohyd Polym. 2019, 207, 694–703. [Google Scholar] [CrossRef]
- Wu, Q.; Yuan, Z.J.; Fang, Y.; Wu, L.B.; Bo, Z.H.; Peng, C.J.; Wu, B. Natural product of angelica essential oil developed as a stable Pickering emulsion for joint interface lubrication. Colloids Surf. B Biointerfaces 2024, 240, 113993. [Google Scholar] [CrossRef] [PubMed]
- Edgehouse, K.J.; Rosenfeld, N.; Bergbreiter, D.E.; Pentzer, E.B. Capsules of the Poly(α-olefin) PAO432 for Removal of BTEX Contaminants from Water. Ind. Eng. Chem. Res. 2021, 60, 14455–14463. [Google Scholar] [CrossRef]
- Isari, A.A.; Ghaffarkhah, A.; Hashemi, S.A.; Yousefian, H.; Rojas, O.J.; Arjmand, M. A Journey from Structured Emulsion Templates to Multifunctional Aerogels. Adv. Funct. Mater. 2024, 34, 2402365. [Google Scholar] [CrossRef]
- Narukulla, R.; Ojha, U.; Sharma, T. Facile one pot green synthesis of -NH2 surface functionalized graphene-polymer nano-composite: Subsequent utilization as stabilizer in pickering emulsions. Colloid Surf. A 2022, 641, 128594. [Google Scholar] [CrossRef]
- Tran, B.N.; Thickett, S.C.; Agarwal, V.; Zetterlund, P.B. Influence of Polymer Matrix on Polymer/Graphene Oxide Nano-composite Intrinsic Properties. Acs Appl. Polym. Mater. 2021, 3, 5145–5154. [Google Scholar] [CrossRef]
- Hu, J.; Bian, Q.; Li, M.; Zhang, J.Y.; Peng, P.; Guo, Y.; Meng, C.Y.; Zhang, H.; Jia, X. Surfactant-free essential oil emulsions enabled by non-covalent self-assembly of Janus nanosheets upgrade the sustainability and efficiency of fungicides. Chem. Eng. J. 2025, 519, 165148. [Google Scholar] [CrossRef]
- Lak, S.N.; Ahmed, S.; Shamberger, P.J.; Pentzer, E.B. Encapsulation of hygroscopic liquids via polymer precipitation in non-aqueous emulsions. J. Colloid. Interf. Sci. 2022, 628, 605–613. [Google Scholar] [CrossRef]
- Wang, X.D.; He, J.X.; Ma, L.H.; Yan, B.; Shi, L.Y.; Ran, R. Self-assembling graphene oxide/modified amphipathic hydroxyethyl cellulose hybrid stabilized Pickering emulsion polymerization for functional hydrogel. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125742. [Google Scholar] [CrossRef]
- Chen, Y.W.; Szkopek, T.; Cerruti, M. Functional porous graphene materials by pickering emulsion templating: From emulsion stabilization to structural design and fabrication. Adv. Colloid. Interface Sci. 2025, 342, 103536. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Starch based Pickering emulsions: Fabrication, properties, and applications. Trends Food Sci. Tech. 2019, 85, 129–137. [Google Scholar] [CrossRef]
- Wu, B.; Yang, C.; Xin, Q.; Kong, L.; Eggersdorfer, M.; Ruan, J.; Zhao, P.; Shan, J.; Liu, K.; Chen, D.; et al. Attractive Pickering Emulsion Gels. Adv. Mater. 2021, 33, 2102362. [Google Scholar] [CrossRef]
- Dai, H.; Wu, J.; Zhang, H.; Chen, Y.; Ma, L.; Huang, H.; Huang, Y.; Zhang, Y. Recent advances on cellulose nanocrystals for Pickering emulsions: Development and challenge. Trends Food Sci. Technol. 2020, 102, 16–29. [Google Scholar] [CrossRef]
- Zhang, Q.; Kong, B.; Liu, H.; Du, X.; Sun, F.; Xia, X. Nanoscale Pickering emulsion food preservative films/coatings: Compositions, preparations, influencing factors, and applications. Compr. Rev. Food. Sci. Food Saf. 2024, 23, e13279. [Google Scholar] [CrossRef]
- Karamoko, B.A.; Dey, S.; Mujib, S.B.; Liu, J.F.; Wang, W.S.; Li, J.; Singh, G.; Voiry, D.; Salameh, C.; Yao, B.K.; et al. Polymer-Derived Silicon Oxycarbide/Graphene Oxide Porous Ceramic Monoliths Obtained from Pickering Emulsions: Application as Active Electrodes for Lithium-Ion Batteries. Chem. Mater. 2024, 36, 3138–3149. [Google Scholar] [CrossRef]
- Ni, L.; Yu, C.; Wei, Q.; Liu, D.; Qiu, J. Pickering Emulsion Catalysis: Interfacial Chemistry, Catalyst Design, Challenges, and Perspectives. Angew. Chem. Int. Ed. 2022, 61, e202115885. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zhu, W.; Wang, H. Materials Prepared via Pickering Emulsions Stabilized by Graphene Oxide: Overview and Prospects. Materials 2025, 18, 4790. https://doi.org/10.3390/ma18204790
Liu M, Zhu W, Wang H. Materials Prepared via Pickering Emulsions Stabilized by Graphene Oxide: Overview and Prospects. Materials. 2025; 18(20):4790. https://doi.org/10.3390/ma18204790
Chicago/Turabian StyleLiu, Manman, Wenle Zhu, and Huili Wang. 2025. "Materials Prepared via Pickering Emulsions Stabilized by Graphene Oxide: Overview and Prospects" Materials 18, no. 20: 4790. https://doi.org/10.3390/ma18204790
APA StyleLiu, M., Zhu, W., & Wang, H. (2025). Materials Prepared via Pickering Emulsions Stabilized by Graphene Oxide: Overview and Prospects. Materials, 18(20), 4790. https://doi.org/10.3390/ma18204790





