Abstract
About 120 million tons of steel slag are produced annually in China, making it one of the largest sources of industrial solid waste; however, its utilization rate remains only around 30%. The presence of f-CaO is the main factor in its widespread application. Currently, the carbonation of steel slag is mainly through indirect wet mineralization, which is difficult to implement on an industrial scale. Direct dry carbonation, on the other hand, consumes more energy due to its slow kinetics. In this study, steam coupled with CO2 was used to directly mineralize steel slag, a process fully compatible with existing iron and steel industry treatment processes. The required temperature can be achieved using the waste heat from hot steel slag, eliminating the need for additional heat supply. With 15% steam injection, the CaCO3 content increased to 12.02 g/100 g (52.8 kg CO2 t−1 slag utilization), representing a 16.7% improvement. After mineralization, the f-CaO decreased to 0.61%, with 91.73% of f-CaO in steel slag mineralized. The mineralization efficiency of f-CaO increased by 20.24%. This enhancement was attributed to steam entering the interior pores of steel slag, generating intermediate Ca(OH)2, causing steel slag particle breakage and fully exposing the previously enclosed f-CaO for complete carbonation. To further utilize flue gas, the effects of different CO2 concentrations on carbon fixation were investigated. At a concentration of 20% CO2, the carbon fixation reached 69.90% of that achieved at 100% CO2. This research not only addresses the stability issues of steel slag but also reduces CO2 emissions and effectively utilizes waste heat, making the process suitable for large-scale industrial application.
1. Introduction
Steel slag is a by-product of the steel production process and has become one of the largest sources of industrial solid waste, accounting for approximately 10% to 15% of crude steel production [1]. The global annual output of steel slag is about 190–290 million tons, and by 2025, China’s steel slag production will reach about 120 million tons [2]. In recent years, with the development of the steel industry, the output of steel slag has also been increasing rapidly. However, the effective utilization rate of steel slag remains only at about 30% [3]. A large amount of steel slag occupies land, causes soil pollution, and damages the ecological environment. Therefore, it is urgent to improve the utilization of steel slag. The composition of steel slag is similar to that of cement clinker, and it possesses certain hydraulic properties [4]. Concurrently, steel slag exhibits high mechanical strength. Therefore, steel slag can be used as an aggregate, substituting for cement and other building materials [5,6]. This is currently the most widely employed method for disposing of a large quantity of steel slag.
The existence of f-CaO in steel slag leads to poor bulk stability, which remains a significant limiting factor for its resource utilization [7]. This issue can be mitigated through the carbonation of steel slag [8]. The roll crushing−residual heat with the autoclaving process is one of the advanced technologies for steel slag treatment in China. During the process, steel slag is crushed into different particle sizes by rolling. However, this technology requires a substantial amount of water. The addition of water helps to eliminate f-CaO, thereby addressing the poor stability of steel slag [9]. It is also necessary to reduce the temperature of steel slag for safety reasons and to facilitate subsequent processing steps. Unfortunately, this leads to the loss of waste heat from the steel slag. Additionally, during the iron and steel smelting process, a large amount of low-calorific-value flue gas, rich in CO2, is generated. This gas exhibits poor combustion efficiency and high treatment costs, and its direct discharge can cause serious environmental pollution [10]. Therefore, using flue gas for the direct mineralization of hot steel slag could not only stabilize f-CaO and improve slag stability, but also effectively utilize the residual heat of the steel slag. Ultimately, this approach could achieve a win–win situation for steel slag stabilization and CO2 emission reduction.
Numerous researchers have utilized steel slag for CO2 mineral carbonation, with the methodologies mainly divided into direct and indirect approaches [11]. Currently, the carbonation of steel slag is predominantly conducted through indirect wet mineralization, both domestically and internationally. Indirect carbonation generally consists of two stages. Initially, Ca2+ and Mg2+ ions are extracted from the steel slag using a leaching solution, such as hydrochloric acid, nitric acid, citric acid, or ammonium salts, followed by carbonation in an alkaline environment [12,13,14,15]. Jo et al. [16] employed hydrochloric acid (HCl) and sodium hydroxide (NaOH) to leach Ca2+ from steel slag and subsequently synthesized high-purity nano-calcium carbonate (nCaCO3), which demonstrated that nCaCO3 with a purity of 98.5% could be successfully obtained through CO2 carbonation. Although indirect mineral carbonation offers relatively high efficiency, the process is technologically complex and energy-intensive, which significantly limits its scalability and industrial application.
Compared with indirect mineralization, direct mineralization of steel slag using CO2 is well-established, straightforward to implement, and suitable for industrial-scale production. In direct mineralization, flue gas with a low calorific value and rich in CO2 can be used for the homologous treatment of steel slag, thereby achieving a synergistic treatment of both steel slag and flue gas. However, the kinetics of the direct carbonation reaction are slow, resulting in low conversion rates. Revathy et al. [17] investigated the carbon sequestration capacity of steel slag under ambient conditions for 3 h and observed a carbonation amount of only 11.1 g CO2 kg−1 slag. Santos et al. [18] explored the carbon sequestration capacity of steel slag under high-temperature and high-pressure conditions (T = 650 °C, P (CO2) = 20 bar) and found that the content of CaCO3 generated during carbonation ranged from 9% to 26%.
The large-scale implementation of direct carbonation remains challenging due to its low mineralization efficiency. Therefore, it is essential for direct carbonation to improve its carbonation efficiency. Numerous studies have demonstrated that the introduction of steam can significantly enhance the mineralization efficiency of steel slag [19,20]. In the 1970s, Dobner et al. [21]. pioneered the investigation into the carbonation of calcined dolomite in the presence of steam and discovered that steam possessed catalytic properties when facilitating the reaction between CaO and CO2, particularly at lower temperatures (below 550 °C). Tamas et al. [22]. showed that the existence of H2O refined the particle size distribution and acted as an accelerator for the carbonate, where the output of calcite under wet conditions (0.246 kg CO2/kg) was almost three times higher than that under dry conditions (0.083 kg CO2/kg).
Symon et al. [23] and Wang et al. [24] thought that the promotion of steam on the carbonation process of steel slag was related to the formation of Ca(OH)2. Lu et al. [23] pointed out that the addition of water would force the product volume to be broken, thus affecting the particle morphology and improving the adsorption performance. While Nikulshina et al. [25] indicated that the addition of steam greatly improved the reaction kinetics, with initial reaction speeds 22 times and 9 times higher than the dry carbonization of CaO and Ca(OH)2, respectively. However, some studies showed that adding steam contributes little to CO2 absorption [18].
Two mutually exclusive hypotheses persist regarding the role of steam in steel slag carbonation. One postulates that steam accelerates reaction kinetics, while the other contends that steam has little contribution in the carbonation process; however, neither conjecture has survived rigorous experimental verification. Based on the above research, this study conducted carbonation experiments and thermodynamic calculations to explore the role of steam in the direct mineralization of CO2 using steel slag and expounded upon the mechanism by which steam improves the carbonation efficiency. This research aimed to address the issue of free CaO(f-CaO) in steel slag by integrating water vapor with CO2 direct mineralization technology. This approach couples low-grade waste heat, steam, and CO2 in iron and steel plants, realizing a “one-step” simultaneous digestion of f-CaO, solidification of CO2, and recovery of waste heat without changing the existing slag treatment processes, which opens up a new path with low-carbon and zero additional energy consumption for the large-scale and high-value utilization of steel slag.
2. Materials and Methods
2.1. Raw Materials
The steel slag used in the experiment was obtained from Zhanjiang, China. After undergoing 2 h of grinding in a ball mill, the steel slag was sieved into samples with different particle sizes for the carbonation experiment. High-purity N2 (99.99%, Beijing Jingcheng Gas Co., Ltd., Beijing, China) was used in the experiment. High-purity CO2 (99.95%, Beijing Jingcheng Gas Co., Ltd., Beijing, China) was used in the experiment, while simulated flue gas was prepared by mixing N2 and CO2 at controlled ratios. The chemical and phase compositions of steel slag determined by X-ray fluorescence (XRF) and X-ray diffraction (XRD) are shown in Table 1 and Figure 1, respectively. According to GB/T 38216.3-2023 [26], the content of f-CaO in steel slag was determined to be 7.34%.

Table 1.
The chemical composition of the raw steel slag.
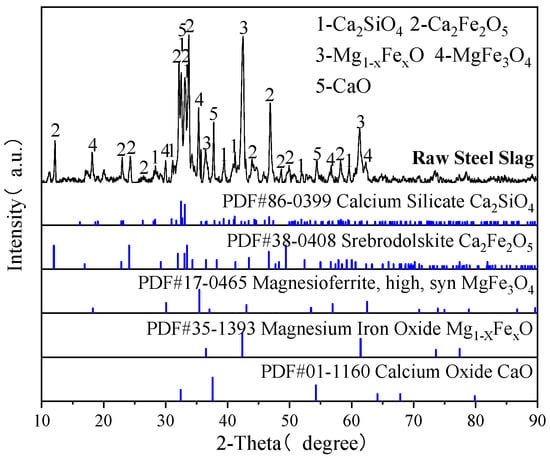
Figure 1.
XRD patterns of the raw steel slag.
2.2. Materials Characterization and Analysis
The chemical compositions of the slags after carbonation were also determined using XRF and XRD. Scanning electron microscopy (SEM) coupled with energy dispersive spectroscopy (EDS) was employed to observe the surface morphology and elemental composition of samples. The operation was conducted under a high vacuum mode with an accelerating voltage of 15 kV. Prior to testing, the samples were coated with a thin film of Au/Pt. The structures of the carbonation products were determined by Fourier transformation infrared spectroscopy (FTIR) within a wavenumber range of 4000~400 cm−1. Thermogravimetric–differential scanning calorimetry (TG-DTA) was used to analyze the mass changes in different carbonated steel slag samples, thus quantitatively describing the carbonation effect. The samples were heated from 25 °C to 1000 °C at a rate of 10 °C/min under a constant N2 flow of 100 mL/min, with continuous mass measurement throughout the process. In the experiment, the water vapor was quantitatively controlled using a steam generator. The experimental flow chart is shown in Figure 2.
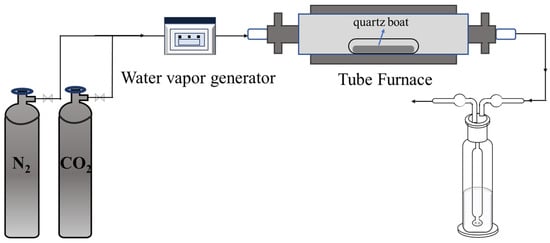
Figure 2.
Experimental flow chart.
2.3. Program for Gas–Solid Direct Carbonation of Steel Slag
The raw steel slag was crushed, ground, and sieved into five different particle size ranges: <75 μm, 75~150 μm, 150~500 μm, 500~1000 μm, and 1000~2000 μm. Approximately 1 g of each steel slag sample was placed in a quartz boat and introduced into a tube furnace for carbonation. During the experiment, N2 was used as a protective gas. The tube furnace was heated at a rate of 10 °C/min to the target carbonation temperature, which was typically 450 °C, 500 °C, 550 °C, 600 °C, or 650 °C. In the actual steelmaking process, these temperatures can be achieved during the cooling of hot slag without additional heat supplement. Once the target temperature was reached, the CO2 flow rate (30–70 mL/min), CO2 concentration (20%, 40%, 60%), and steam content (5%, 10%, 15%, 20%) were adjusted as required. The carbonation reaction was then maintained at the set temperature for 2 h.
2.4. Carbonization Efficiency Measurement
The amount of fixed CO2 and the corresponding CaCO3 formation were calculated using Equations (1) and (2),
where CO2[%] was the amount of CO2 fixed in the carbonated steel slag (%), CaCO3[%] was the CaCO3 production (%), m0 was the initial mass of the steel slag (mg), m1073k was the mass of steel slag at1073 k (mg), and m873k was the mass of steel slag at 873 k (mg). The temperature range of 873–1073 k corresponds to CaCO3 decomposition.
3. Results and Discussion
3.1. Effect of Different Influencing Factors on Gas–Solid Direct Carbonation Ability of Steel Slag
Due to the existence of f-CaO, the stability of steel slag is poor, which greatly limits its application. Gas–solid direct carbonation of steel slag primarily proceeds through the reaction between f-CaO and CO2, during which f-CaO is converted into a stable carbonate [27]. As depicted in Figure 3, the amount of CaCO3 produced varies significantly with temperature and particle size, indicating a substantial influence of these parameters on carbonation efficiency. In contrast, the CO2 flow rate exhibits a relatively minor impact on carbonation efficiency. As illustrated in Figure 3a, with the increase in reaction temperature, the amount of CaCO3 first increases and then decreases with higher temperature. The carbonation of steel slag reaches its maximum efficiency at 550 °C, corresponding to 10.30 g CaCO3/100 g of steel slag, where the carbonation proceeds slowly due to insufficient reaction kinetics. However, when the temperature reaches a certain value, the exothermic reaction of carbonization inhibits further reaction [28,29]. Figure 3b shows the influence of steel slag particle size on CaCO3 production. Smaller particle sizes result in higher carbonation production. When the particle size is below 75 μm, the carbonation effect reaches its maximum This improvement arises because smaller particles expose more f-CaO on their surfaces, enhancing the reaction rate and overall carbonation degree. Figure 3c reveals the negligible influence of CO2 flow rate on steel slag carbonation, indicating that 30 mL/min was sufficient for the experimental slag quantity. To sum up, for a gas–solid reaction, increasing temperature and reducing particle size can accelerate the carbonation reaction. When the temperature was 550 °C, the particle size was 75 μm, and the CO2 flow rate was 30 mL/min, the maximum amount of CaCO3 was 10.3 g/100 g steel slag. After direct gas–solid carbonation, the residual f-CaO content in steel slag was 1.57%, indicating that 76.29% of f-CaO in steel slag was mineralized.
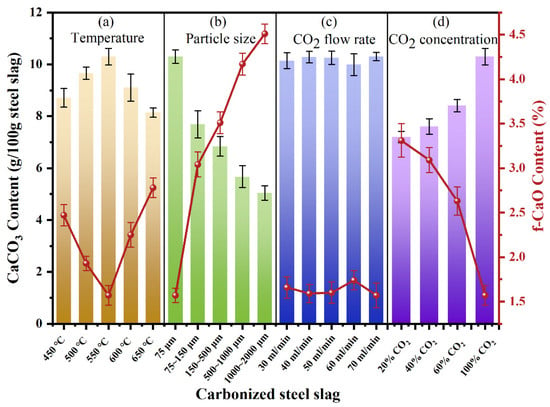
Figure 3.
CaCO3 and f-CaO content after carbonation of steel slag: (a) Temperature, (b) particle size, (c) CO2 flow rate, and (d) CO2 concentration.
To assess the feasibility of utilizing CO2-rich flue gas for direct carbonation, Figure 3d further shows the influence of different CO2 concentrations on steel slag carbonation. When the concentration of CO2 is 20%, the carbonation efficiency remains relatively high, producing 7.20 g CaCO3/100 g steel slag, equivalent to 69.90% of the efficiency under pure CO2, which confirms the feasibility of using flue gas for steel slag mineralization.
Figure 4 presents the XRD patterns of carbonated steel slag under various carbonization conditions. All carbonated steel slag samples exhibit a typical calcite diffraction peak at 2θ = 29.40°, while the intensity of f-CaO peaks decreases significantly after carbonation. Changes in carbonation temperature and particle size of steel slag cause obvious variations in phase compositions, whereas the CO2 flow rate produces negligible changes in CaCO3 and CaO peak intensities, which is consistent with CaCO3 production in Figure 3. When the CO2 concentration is 20% (Figure 4d), the characteristic peak intensity of CaCO3 in the carbonized steel slag still remains distinct. In addition, the peak intensity of dicalcium–silicate- and iron-containing minerals in carbonated steel slag samples remain essentially unchanged, indicating that only f-CaO is involved in the gas–solid carbonation reaction.
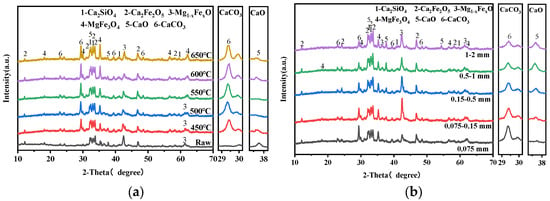
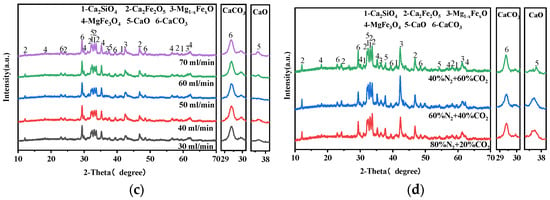
Figure 4.
XRD patterns of carbonated steel slag: (a) Temperature, (b) particle size, (c) CO2 flow rate, and (d) CO2 concentration.
Figure 5 shows the SEM and BSE results to further confirm the carbonation behavior. Steel slag appears in the form of larger particles, while CaCO3 produced by carbonation shows a smaller particle size. The elements of Ca, Fe, Mg, and O are mainly concentrated in the central area of the particles, suggesting the presence of phases such as Mg1−xFexO and Ca2Fe2O5. In addition, Ca and C also gathers loosely around the particles, indicating that small pieces of CaCO3 were formed and distributed around the surfaces of steel slag particles, forming a CaCO3 layer. It can be observed from Figure 5 that CaCO3 is mainly distributed around the steel slag particles and forms a CaCO3 layer.
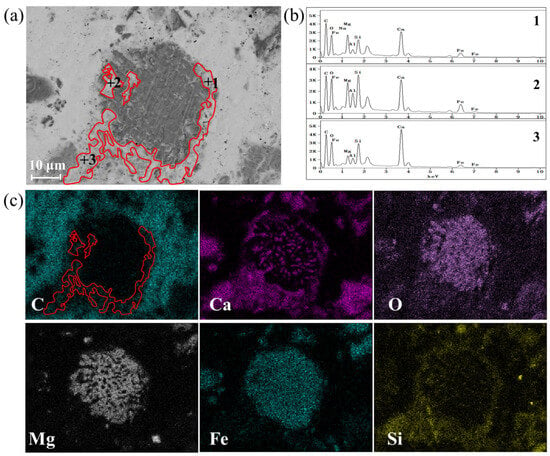
Figure 5.
BSE image and element distribution of gas–solid carbonated steel slag particles: (a) SEM of carbonated steel slag, (b) EDS result of CaCO3 formation at point 1~3, and (c) mapping of carbonated steel slag.
For gas–solid carbonation, the carbonation process occurs initially on the surface of steel slag particles. The steel slag after carbonation still presents relatively complete particles. As the reaction progresses, a CaCO3 layer forms on the surfaces of steel slag particles, creating a protective product coating, which prevents further effective contact between CO2 gas and the unreacted part of steel slag. It is difficult for CO2 to diffuse into the steel slag, which inhibits its further carbonization.
3.2. Effect of Steam on Gas–Solid Direct Carbonation Performance of Steel Slag
Introducing steam into gas–solid carbonation significantly enhances the carbon fixation of steel slag [19,20].
Figure 6 shows the amount of CaCO3 produced by carbonated steel slag with different steam contents. The content of carbonized CaCO3 initially increases and then decreases with rising steam content. The amount of CaCO3 reaches 12.02 g/100 g steel slag upon the injection of 15% steam, which is an increase of 16.70% when compared to its content under pure CO2. Correspondingly, the residual f-CaO content decreases sharply by 0.61%, indicating that 91.73% of the initial f-CaO has been mineralized. Compared with the mineralization process without steam injection, the mineralization efficiency of f-CaO has increased by 20.24%, suggesting that the injection of steam promoted the almost complete reaction of f-CaO. However, excessive steam inhibits carbonation because thick water films form on particle surfaces, which prevents CO2 entry and restricts the reaction to the outer surface layer [30]. Table 2 lists the performance comparison between the results of this study and others, in reference to the strengthening effects of steam in the direct carbonization of steel slag. In this study, the carbonation efficiency and the residual amount of f-CaO in steel slag are better than those reported in the references.
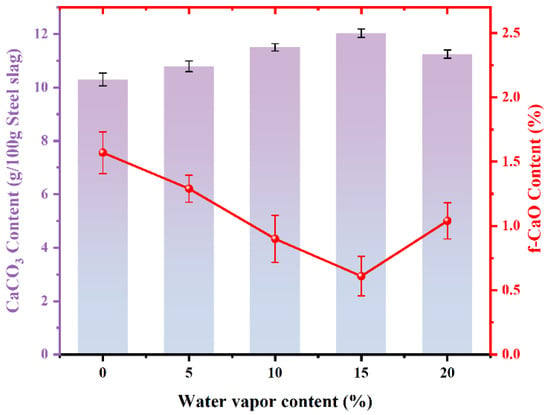
Figure 6.
Effect of steam on carbonation of steel slag CaCO3 and f-CaO production.

Table 2.
Comparison of properties of steam promoting direct carbonation of steel slag.
Figure 7a shows the XRD patterns of carbonated steel slag with various steam contents. The characteristic calcite peak intensity reaches the maximum at 15% steam, while the f-CaO peak intensity nearly disappears. Other phases remain unchanged, confirming that f-CaO is the main phase involved in the reaction. Compared with pure CO2 gas carbonation (Figure 4), the peak intensity of calcite is significantly stronger in the presence of steam.
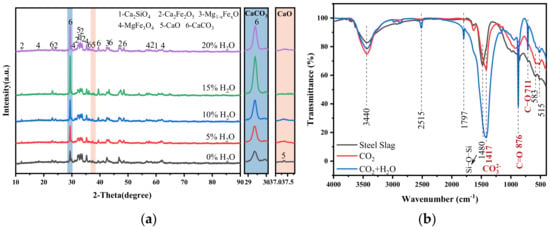
Figure 7.
Effect of steam on X-ray diffraction (XRD) and Fourier transformation infrared spectroscopy (FTIR) of carbonated steel slag. (a) XRD diagram of carbonated steel slag with different steam content; (b) FTIR analysis of steel slag before and after carbonation.
Figure 7b displays the FTIR spectra of steel slag before and after carbonization. For the original steel slag sample, silicate minerals in steel slag, such as dicalcium silicate and tricalcium silicate, have characteristic absorption peaks around 1480 cm−1, corresponding to the tensile and bending vibration of Si-O-Si [35]. The absorption band of carbonated steel slag around 711 cm−1 can be ascribed to the vibration of the C-O bond in calcite. The band at 876 cm−1 represents the out-of-plane bending of the C=O bond in calcite. The absorption peaks around 1417 cm−1, caused by carbonate, are usually related to the symmetric stretching vibration mode of the carbonate ion (CO32−) in calcium carbonate [36,37]. It is clearly observed that when steel slag was injected with steam, the signal intensities of the absorption peaks at 876 cm−1 and 1417 cm−1 increase obviously. Meanwhile, in the FTIR diagrams of carbonized steel slag injected with steam, absorption peaks of calcite near 1797 cm−1 and 2515 cm−1 appear, indicating that the presence of steam promotes the carbonization process of steel slag [38,39]. This observation is consistent with the XRD results (Figure 7a) and CaCO3 production (Figure 6) of carbonated steel slag after adding steam, suggesting that the increased strength of absorption peaks in the FTIR spectra and the changes in XRD patterns, along with the increased CaCO3 production, support the conclusion that steam enhanced the carbonization process.
Figure 8 shows the BSE images and corresponding element distribution of carbonated steel slag after adding steam. It is clearly observed that the carbon steel slag particles are obviously broken after steam treatment. Compared with pure CO2 carbonation (Figure 8), the sample with steam exhibits smaller particle size, and a more dispersed distribution of CaCO3. Under the steam/CO2 mixed gas atmosphere, the generated CaCO3 is more dispersed throughout the slag matrix as small particles (Point 1~4 in Figure 8). Overall, the addition of steam can pulverize the particles of steel slag, increase the contact area between CO2 and steel slag, and consequently promote the generation of CaCO3.
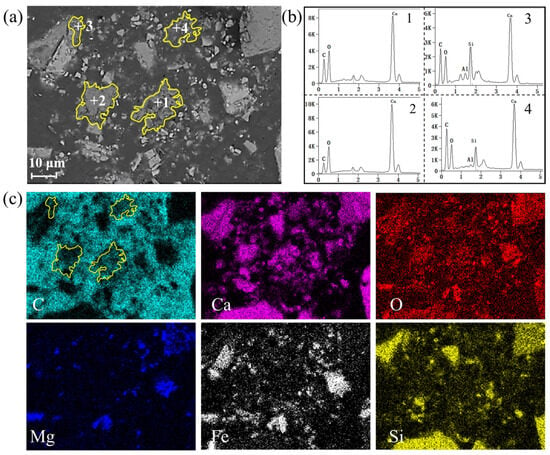
Figure 8.
Effect of steam on microstructure of carbonated steel slag: (a) SEM of carbonated steel slag, (b) EDS result of CaCO3 formation at point 1~4 (The point 1~4 respectively represent +1~+4 in Figure 8a), and (c) mapping of carbonated steel slag.
3.3. Carbonation Mechanism by Steam
The above experiments demonstrate that steam has a positive effect on the carbonation process of steel slag. Based on the thermodynamic principle, the influence of steam on the carbonation of steel slag was analyzed in the standard state. Figure 9a shows the change in Gibbs free energy of reactants under the condition of a steam/CO2 atmosphere. As illustrated in Figure 9a and summarized in Table 3, Equations (3)–(5) can proceed spontaneously within the temperature range of 273 to 814 K, under standard conditions. At 550 °C (823 K), the reactivities of Equations (3) and (5) are comparable and both more favorable than that of Equation (4). Therefore, when the reactive gas contacts the steel slag, CO2 preferentially reacts with f-CaO exposed on the surface of steel slag (Equation (3)). At the same time, steam permeates the porous slag structure and reacts with f-CaO to generate Ca(OH)2 (reaction 4). The formation of Ca(OH)2 involves a volume expansion of about 98% [40], which pulverizes the steel slag and exposes the f-CaO inside the steel slag. The newly exposed f-CaO subsequently reacts with CO2, while Ca(OH)2 is further carbonated according to reaction (5). Therefore, the presence of steam enables a more complete conversion of f-CaO in steel slag.
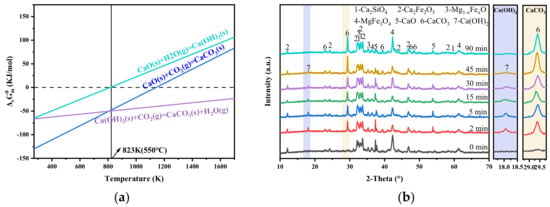
Figure 9.
Effect of adding steam on gas–solid carbonation of steel slag. (a) Effect of steam on Gibbs free energy of CaO; (b) XRD diagram of carbonated steel slag after adding steam at different time periods.

Table 3.
Equation and thermodynamic calculation of aqueous carbonation reaction.
To verify this conjecture, Figure 9b analyses the phase evolution of carbonized steel slag at different time periods in the presence of steam. During the process of steel slag carbonization, the extension of carbonization time has a significant effect on the phase composition of steel slag. At the initial stage, the characteristic peaks of CaCO3 and Ca(OH)2 appear simultaneously, while the peak intensity of f-CaO weakens, indicating simultaneous formation of CaCO3 and Ca(OH)2 upon gas–slag contact. With increasing carbonization time, the peak intensity of CaCO3 increases, whereas the peak intensities of Ca(OH)2 and f-CaO gradually decrease, suggesting progressive conversion of f-CaO and Ca(OH)2 into CaCO3. After 90 min, the characteristic peak of Ca(OH)2 disappears, indicating that all Ca(OH)2 has been converted into CaCO3. With the introduction of steam, the content of f-CaO in the mineralized steel slag decreases to 0.61% (Figure 6) compared to that of 1.57% without steam injection, demonstrating a 20.24% improvement in the mineralization efficiency.
The above experimental results strongly support the positive promotion of steam on the carbonation process of steel slag. The carbonation of steel slag is obviously accelerated by the formation of the intermediate of Ca(OH)2. To further confirm the role of steam, this dynamic process was clearly conducted by in situ FTIR. Figure 10 shows the in situ FTIR spectra of steel slag in CO2 and CO2 + H2O atmospheres at 550 °C. The doublets at 2359 and 2334 cm−1 correspond to the ν3 asymmetric stretching vibration of gaseous CO2 split by Fermi resonance. A new band at 2306 cm−1, appearing only in the CO2 + H2O atmosphere, can be attributed to linearly adsorbed CO2 species on basic sites, implying that steam enhances CO2 chemisorption, possibly via surface HCO3−. The bands at 1788, 876, and 711 cm−1 are assigned to the ν2 bending, ν4 in-plane bending, and ν2 lattice modes of CO32−, respectively, indicating the presence of calcitic CaCO3. Their integrated intensities increase monotonically over time and are significantly larger in the presence of steam, confirming that H2O accelerates CaCO3 formation. These observations support the sequential pathway CaO → Ca(OH)2 → CaCO3, which proceeds faster than direct gas–solid carbonation. The 3629 cm−1 peak, assigned to surface -OH groups and possibly including transient Ca(OH)2 stabilized on the slag matrix, intensifies within the first minute under CO2 + H2O, indicating rapid water adsorption and hydroxylation of the slag surface.
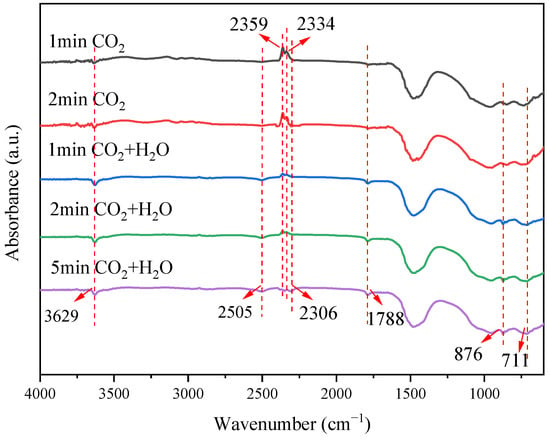
Figure 10.
In situ FTIR spectra of steel slag in CO2 and CO2/H2O atmosphere.
To sum up, in a pure CO2 atmosphere, the characteristic peak of carbonate increases slowly, because the reaction rate is limited by the direct carbonation of surface-active CaO sites. After the introduction of steam, H2O significantly enhances the carbonation rate and the adsorption capacity of CO2 through the formation of the Ca(OH)2 intermediate.
Figure 11 presents the N2 adsorption–desorption isotherms and pore structure parameters of steel slag carbonated at 550 °C for 2 h under CO2 and CO2 + H2O atmospheres. Compared to conditions under pure CO2, steam introduction increases the BET surface area from 2.23 m2·g−1 to 4.46 m2·g−1, indicating enhanced activation of Ca/Mg bearing phases and the creation of micro- and mesopores. The mean pore size decreases from 32 nm to 17 nm, while the pore volume increases by 31% to 0.0185 cm3·g−1. The transition from “fewer, larger” to “numerous, medium sized” pores facilitates CO2 diffusion.
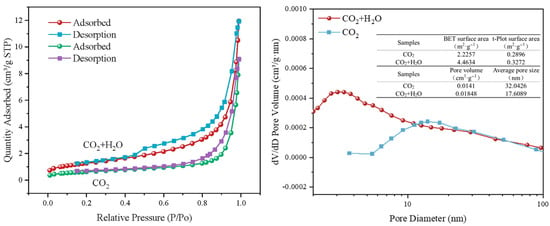
Figure 11.
BET diagram of mineralized steel slag before and after steam injection.
The steep uptake at P/P0 < 0.1 and the hysteresis loop (type H3/H4) imply a higher micropore fraction and slit shaped pores formed by the stacking of lamellar particles. Under pure CO2, the predominance of macropores and low pore volume suggest partial blockage by nascent carbonates. In the presence of steam, transient Ca(OH)2-like surface hydroxyls are formed and subsequently carbonated to CaCO3, reopening and enlarging the pore network.
Consequently, steam not only increases the number of reactive sites but also generates a more open and accessible pore structure, thereby enhancing the gas–solid carbonation efficiency of steel slag.
Figure 12 shows the BSE images of carbonated steel slag at different time periods in the presence of steam.
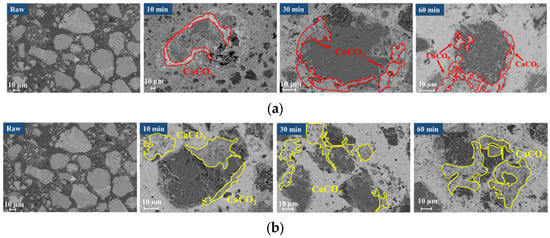
Figure 12.
Microscopic morphology of carbonated steel slag. (a) Gas–solid carbonation; (b) Gas–solid carbonation with added steam.
In the pure CO2 atmosphere (Figure 12a), steel slag particles remain at larger particle sizes and relatively intact morphologies after 60 min of reaction. With the gradual generation of CaCO3, unreacted regions became coated with a dense CaCO3 layer, confining the reaction to surface regions.
In contrast, under the steam/CO2 mixed atmosphere (Figure 12b), combined with the analysis in Figure 9, the f-CaO in the steel slag reacts with steam to generate Ca(OH)2, accompanied by a significant volume-expansion effect, leading to the effective crushing of steel slag particles into smaller sizes, as clearly observed in Figure 12b. Compared with the particles in Figure 12a, those in Figure 12b exhibit a significantly reduced size. The crushing of steel slag particles exposes the f-CaO within the steel slag, which greatly improves further contact between f-CaO and CO2, and then promotes the generation of CaCO3, resulting in a denser distribution of CaCO3 in the mixed atmosphere of steam/CO2. These microstructural changes align well with the experimental results of CaCO3 content in the presence of steam (Figure 6) and the BET results (Figure 7), providing intuitive evidence for the positive role of steam in promoting carbonation of steel slag.
Based on the above thermodynamic calculation and gas–solid carbonation experimental results, a schematic diagram of the steam-assisted carbonation mechanism of steel slag is proposed in Figure 13.
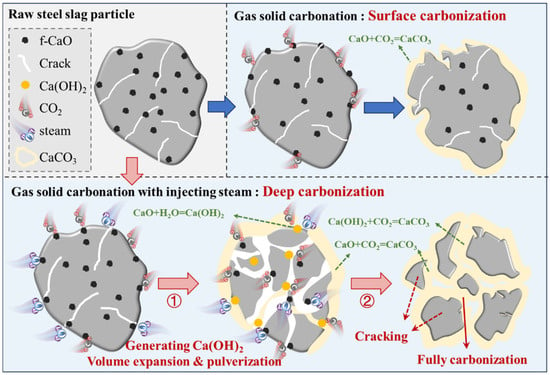
Figure 13.
Diagram of steam carbonation mechanism.
In a pure CO2 atmosphere, the formation of CaCO3 is primarily due to the direct reaction between f-CaO and CO2 on the surface of steel slag. As the reaction progresses, a CaCO3 layer eventually encapsulates the unreacted steel slag, limiting further carbonation reactions.
When steam was introduced, f-CaO on the surface of steel slag initially reacted with CO2 for carbonation. Simultaneously, steam permeates into the steel slag through its surface pores and reacted with f-CaO to generate Ca(OH)2. This process was accompanied by a significant volume expansion, leading to the pulverization of steel slag into smaller particles and a substantial increase in the specific surface area of the steel slag. This volume expansion and pulverization exposed the previously enclosed f-CaO within the steel slag to the surface, further increasing the contact area between f-CaO and CO2, thereby promoting the generation of CaCO3. The Ca(OH)2 formed by the reaction between steam and f-CaO in steel slag can be further carbonized by CO2 to produce CaCO3. The presence of steam significantly improves the process by promoting the digestion of free CaO (f-CaO) in steel slag and thereby enhancing carbonation efficiency.
To sum up, steam promotes the digestion of f-CaO and improves carbonation efficiency through the volume-expansion effect, which significantly advances the carbonation process of steel slag by increasing the surface area available for reaction and facilitating the exposure of internal f-CaO to CO2. Moreover, the process is compatible with existing technological conditions in the iron and steel industry, utilizing the residual heat of hot steel slag without additional energy input. Consequently, this process simultaneously stabilizes steel slag, reduces CO2 emissions, and enables large-scale industrial application.
4. Conclusions
In this research, steam was used to directly enhance mineralization of steel slag. The effects of CO2 flow rate, CO2 concentration, and steam addition on gas–solid carbonation of steel slag were discussed, and the strengthening mechanism of steam on steel slag carbonation was expounded.
- Under the condition of CO2 atmosphere, the carbonation effect of steel slag exhibits optimal performance at 550 °C with a particle size of 75 μm and a CO2 flow rate of 70 mL/min. The amount of CaCO3 produced reaches 10.3 g/100 g of steel slag. After direct gas–solid carbonation, the f-CaO content in steel slag decreases to 1.57%, indicating that approximately 76.29% of f-CaO in steel slag is mineralized. When the CO2 concentration is reduced to 20%, the carbon fixation remains at 7.60 g CaCO3/100 g steel slag, equivalent to 73.79% of that obtained with pure CO2.
- Under a steam/CO2 atmosphere, when the addition amount of steam is 15%, the carbonation of steel slag reaches its maximum efficiency. The amount of CaCO3 produced reaches 12.02 g/100 g steel slag, which is 16.7% higher than that achieved in the pure CO2 atmosphere. After mineralization, the f-CaO content decreases to 0.61%, corresponding to a 91.73% mineralization efficiency. Overall, the f-CaO conversion increases by 20.24% compared with carbonation without steam.
- The addition of steam significantly promotes the activation of f-CaO and leads to the formation of additional micro/mesoporous structures. The average pore size decreases from 32 nm to 17 nm, while the pore volume increases from 0.0141 cm3·g−1 to 0.0185 cm3·g−1, representing an increase of 31%, indicating a transition of the pore structure from “few in number and large in scale” to “many in number and medium in scale”. The presence of steam enhances both the carbonation rate and CO2 adsorption capacity by generating the Ca(OH)2 intermediate.
- The mechanism of direct mineralization of steel slag enhanced by steam was expounded. Steam enters the open pores of the slag and reacts with f-CaO to generate Ca(OH)2, leading to volume expansion and consequently causing physical crushing and refinement of steel slag particles. The volume expansion and pulverization caused the f-CaO to be exposed to the surface, facilitating an increased contact between the f-CaO and CO2. Meanwhile, the newly generated Ca(OH)2 further reacts with CO2 to produce CaCO3, thereby accelerating the entire carbonation reaction.
This study successfully achieved the dual goals of solving the stability problem and reducing CO2 emissions simultaneously. Moreover, the waste heat generated during the steel slag production process is effectively utilized, improving overall energy efficiency. The process maintains production stability without requiring new equipment, thereby avoiding additional costs and technical risks. Hence, the proposed process is compatible with the existing steel slag treatment process and can be directly applied on a large scale without changing the existing process flow.
However, certain limitations remain in this study. In the gas–solid carbonation system, the promoting effect of steam primarily targeted f-CaO, thereby restricting the theoretical carbon fixation capacity to the f-CaO content. In the follow-up study, high calcium/magnesium-based industrial by-products can be introduced through the multi-element solid waste synergistic mineralization strategy to expand the carbonation reaction site, thereby achieving a significant improvement in overall carbon sequestration and the co-treatment of multi-solid waste. Furthermore, although this study proposes a new strategy for low-carbon utilization of steel slag, its large-scale application will require systematic investigations in reactor amplification, working condition optimization, and life cycle assessments.
Author Contributions
Conceptualization, M.Z. and C.Y.; data curation, C.Y. and M.Z.; formal analysis, G.L.; funding acquisition, C.Y. and M.Z.; investigation, X.H.; methodology, H.L. and M.G.; supervision, G.Y. and M.Z.; validation, X.W.; writing—original draft, X.W.; writing—review and editing, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work received support from the Science and Technology Special Plan Project of China Minmetals Corporation (Chang sheng Yue: 2023ZXA03) and the China Baowu Low-Carbon Metallurgy Innovation Fund Project (Mei Zhang: No.BWLCF202310).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Authors Changsheng Yue and Guanghua Lu were employed by the companies Energy Conservation and Environment Protection Co., Ltd. and MCC Group. Authors Guilan Yi and Haokun Li were employed by the company Taiyuan Iron and Steel (Group) Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhao, L.; Wu, D.; Hu, W.; Li, J.; Zhang, Z.; Yang, F. Coupling Mineralization and Product Characteristics of Steel Slag and Carbon Dioxide. Minerals 2023, 13, 795. [Google Scholar] [CrossRef]
- Wang, Z.; Sohn, I. A Review on Reclamation and Reutilization of Ironmaking and Steelmaking Slags. J. Sustain. Metall. 2019, 5, 127–140. [Google Scholar] [CrossRef]
- Tajabadipour, M.; Esmaeili, M.; Askari, A. Experimental evaluation of cyclic performance of steel slag and grids of truck scrap tire as a novel reinforced ballast material. Constr. Build. Mater. 2024, 411, 134472. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, C.; Yu, H.; Qian, G.; Zheng, X.; Zhou, H. Research on the Properties of Steel Slag with Different Preparation Processes. Materials 2024, 17, 1555. [Google Scholar] [CrossRef] [PubMed]
- Laís, C.; Carvalho, V.; Ferreira, L.; Bruno, E.; Ricardo, A. Utilizing steel slag to prolong the durability of reinforced concrete: Corrosion resistance mechanisms. J. Build. Eng. 2024, 98, 111505. [Google Scholar] [CrossRef]
- Cai, X.; Cao, Z.; Sun, J.; Wang, H.; Wu, S. Influence of Steel Slag on Properties of Cement-Based Materials: A Review. Buildings 2024, 14, 2985. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, T.; Shi, C.; Pan, S. Characteristics of steel slags and their use in cement and concrete—A review. Resour. Conserv. Recycl. 2018, 136, 187–197. [Google Scholar] [CrossRef]
- Pan, S.; Chen, Y.; Fan, L.; Kim, H.; Cao, X.; Ling, T. CO2 mineralization and utilization by alkaline solid wastes for potential carbon reduction. Nat. Sustain. 2020, 3, 399–405. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, G.; Wang, H.; Guo, M.; Cheng, F.; Zhang, M. In-Suit Industrial Tests of the Highly Efficient Recovery of Waste Heat and Reutilization of the Hot Steel Slag. ACS Sustain. Chem. Eng. 2021, 9, 3955–3962. [Google Scholar] [CrossRef]
- He, Y.; Jia, S.; Yi, H.; Tang, X.; Yu, Q.; Gao, F. Utilization of steel slag in air pollution and greenhouse gas emission reduction-application, mechanism and challenge: A review. J. Environ. Chem. Eng. 2024, 12, 114090. [Google Scholar] [CrossRef]
- Liu, W.; Teng, L.; Rohani, S.; Qin, Z.; Zhao, B.; Xu, C. CO2 mineral carbonation using industrial solid wastes: A review of recent developments. Chem. Eng. J. 2021, 416, 129093. [Google Scholar] [CrossRef]
- Ragipani, R.; Bhattacharya, S.; Suresh, A. Kinetics of steel slag dissolution: From experiments to modelling. Proc. R. Soc. A 2019, 475, 20180830. [Google Scholar] [CrossRef]
- Seo, S.; Kwon, C.; Kim, S.; Lee, C. Experiment and kinetic modeling for leaching of blast furnace slag using ligand. J. CO2 Util. 2018, 27, 188–195. [Google Scholar] [CrossRef]
- Eloneva, S.; Said, A.; Fogelholm, C.; Zevenhoven, R. Preliminary assessment of a method utilizing carbon dioxide and steelmaking slags to produce precipitated calcium carbonate. Appl. Energy 2012, 90, 329–334. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.; Jeong, S.; Youn, M.; Nguyen, D.; Chang, S. Calcium extraction from steelmaking slag and production of precipitated calcium carbonate from calcium oxide for carbon dioxide fixation. J. Ind. Eng. Chem. 2017, 53, 233–240. [Google Scholar] [CrossRef]
- Jo, H.; Lee, M.; Park, J.; Jung, K. Preparation of high-purity nano-CaCO3 from steel slag. Energy 2016, 120, 884–894. [Google Scholar] [CrossRef]
- Rushendra, T.; Palanivelu, K.; Ramachandran, A. Direct mineral carbonation of steelmaking slag for CO2 sequestration at room temperature. Environ. Sci. Pollut. Res. Int. 2016, 23, 7349–7359. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Ling, D.; Sarvaramini, A.; Guo, M.; Elsen, J.; Larachi, F. Stabilization of basic oxygen furnace slag by hot-stage carbonation treatment. Chem. Eng. J. 2012, 203, 239–250. [Google Scholar] [CrossRef]
- Biava, G.; Depero, E.; Bontempi, E. Accelerated Carbonation of Steel Slag and Their Valorisation in Cement Products: A Review. Span. J. Soil Sci. 2024, 14, 12908. [Google Scholar] [CrossRef]
- Lei, X.; Yu, H.; Feng, P.; Ling, T. Flue gas carbonation curing of steel slag blocks: Effects of residual heat and water vapor. Constr. Build. Mater. 2023, 384, 131330. [Google Scholar] [CrossRef]
- Dobner, S.; Sterns, L.; Graff, R.; Squires, A. Cyclic Calcination and Recarbonation of Calcined Dolomite. Ind. Eng. Chem. Process Des. Dev. 1997, 16, 557–565. [Google Scholar] [CrossRef]
- Tamás, K.; Gábor, M.; Sanjay, K.; Kristály, F. Carbon-dioxide sequestration by mechanical activation of Linz-Donawitz steel slag; the effect of water on CO2 capture. Fuel 2023, 352, 128951. [Google Scholar] [CrossRef]
- Symonds, R.; Lu, D.; Hughes, R.; Anthony, E.; Macchi, A. CO2 Capture from Simulated Syngas via Cyclic Carbonation/Calcination for a Naturally Occurring Limestone: Pilot-Plant Testing. Ind. Eng. Chem. Res. 2009, 48, 8431–8440. [Google Scholar] [CrossRef]
- Wang, C.; Jia, L.; Tan, Y.; Anthony, E. Carbonation of fly ash in oxy-fuel CFB combustion. Fuel 2008, 87, 1108–1114. [Google Scholar] [CrossRef]
- Nikulshina, V.; Gálvez, M.; Steinfeld, A. Kinetic analysis of the carbonation reactions for the capture of CO2 from air via the Ca(OH)2-CaCO3-CaO solar thermochemical cycle. Chem. Eng. J. 2007, 129, 75–83. [Google Scholar] [CrossRef]
- GB/T 38216.3-2023; Steel slag—Determination of f-Cao—EDTA Titration and Thermogravimetric Analysis. China Iron and Steel Industry Association: Beijing, China, 2023.
- DiGiovanni, C.; Hisseine, A.-O.; Awolayo, N.-A. Carbon dioxide sequestration through steel slag carbonation: Review of mechanisms, process parameters, and cleaner upcycling pathways. J. CO2 Util. 2024, 81, 102736. [Google Scholar] [CrossRef]
- Lackner, K.; Wendt, C.; Butt, D.; Joyce, E.-L., Jr.; Sharp, D. Carbon dioxide disposal in carbonate minerals. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Kohlmann, J.; Mukherjee, A. Direct dry mineral carbonation for CO2 emissions reduction in Finland. In Proceedings of the 27th International Technical Conference on Coal Utilization & Fuel Systems, Clearwater, FL, USA, 4–7 March 2002. [Google Scholar]
- Wei, X.; Ni, W.; Wang, X.; Li, K. Current Research of the Carbonization Technology of Steel Slag. Conserv. Util. Miner. Resour. 2019, 39, 99–104. [Google Scholar] [CrossRef]
- Fan, Y.; Liang, W.; Hu, X. The Direct Carbonation Behavior of Steel Slag with CO2-Containing Water Vapor. Metals 2025, 15, 237. [Google Scholar] [CrossRef]
- Liu, W.; Su, S.; Xu, K.; Chen, Q.; Xu, J.; Sun, Z.; Wang, Y.; Hu, S.; Wang, X.; Xue, Y.; et al. CO2 sequestration by direct gas–solid carbonation of fly ash with steam addition. J. Clean. Prod. 2018, 178, 98–107. [Google Scholar] [CrossRef]
- Diao, J. Experimental Study of Using Carbon Dioxide to Digesting Free calcium Oxide of Steel Slag. Master’s Thesis, University of Science and Technology Liaoning, Anshan, China, 2015. [Google Scholar]
- Peng, B. Modification and Its Physicochemical Property of Molten Steel Slag. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2016. [Google Scholar]
- Zhang, Q.; Feng, P.; Shen, X.; Cai, Y.; Zhen, H.; Liu, Z. Comparative analysis of carbonation strengthening mechanisms in full solid waste materials: Steel slag vs. carbide slag. Cem. Concr. Compos. 2025, 157, 105927. [Google Scholar] [CrossRef]
- Long, W.; Zhu, C.; Zhang, Y. Elucidating hardening mechanism of carbonatable binders prepared with steel slag based on distribution of microhardness. Constr. Build. Mater. 2024, 424, 135895. [Google Scholar] [CrossRef]
- Long, W.; Zhao, L.; Zhang, Y. Strengthening effect of mechanical vibration on the carbonation properties of steel slag compact. Constr. Build. Mater. 2024, 443, 137741. [Google Scholar] [CrossRef]
- Wang, S.; Wang, M.; Liu, F.; Song, Q.; Deng, Y.; Ye, W. A Review on the Carbonation of Steel Slag: Properties, Mechanism, and Application. Materials 2024, 17, 2066. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, D.; Fang, Y. Effects of mineralogical changes in BOFS during carbonation on pH and Ca and Si leaching. Constr. Build. Mater. 2018, 192, 584–592. [Google Scholar] [CrossRef]
- Mo, L.; Lin, S.; Meng, X.; Qu, L.; Chang, W.; Xiao, Y. Research on volume expansion characteristics of steel slag and simulation of bulging crack. China J. Highw. Transp. 2021, 34, 180–189. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).