Synthesis of Silver–Calcium Phosphate Visible Light Responsive Photocatalytic Materials and Their Antibacterial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Hue and Composition of Samples
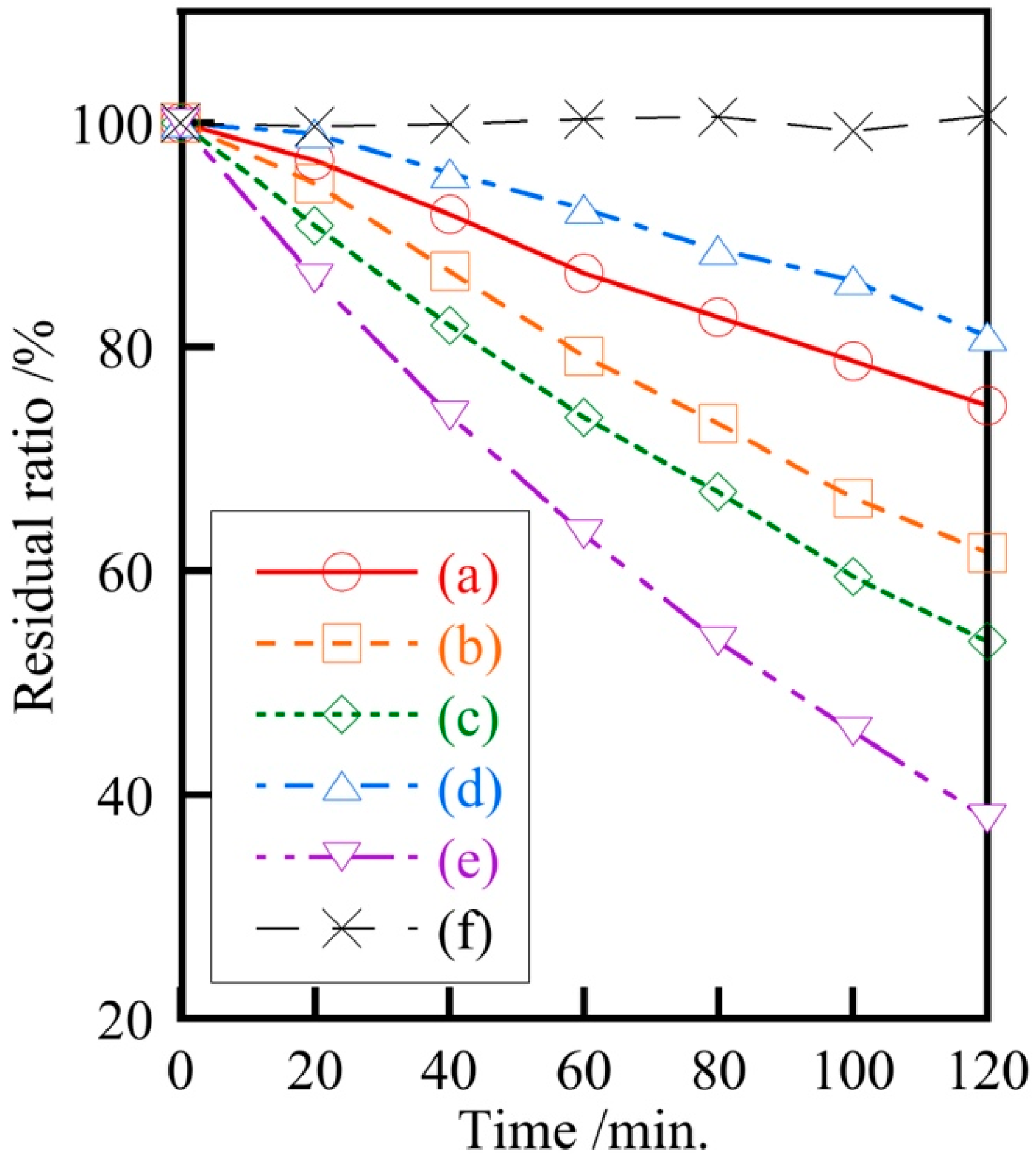
2.3. Photocatalytic and Antibacterial Properties of Samples
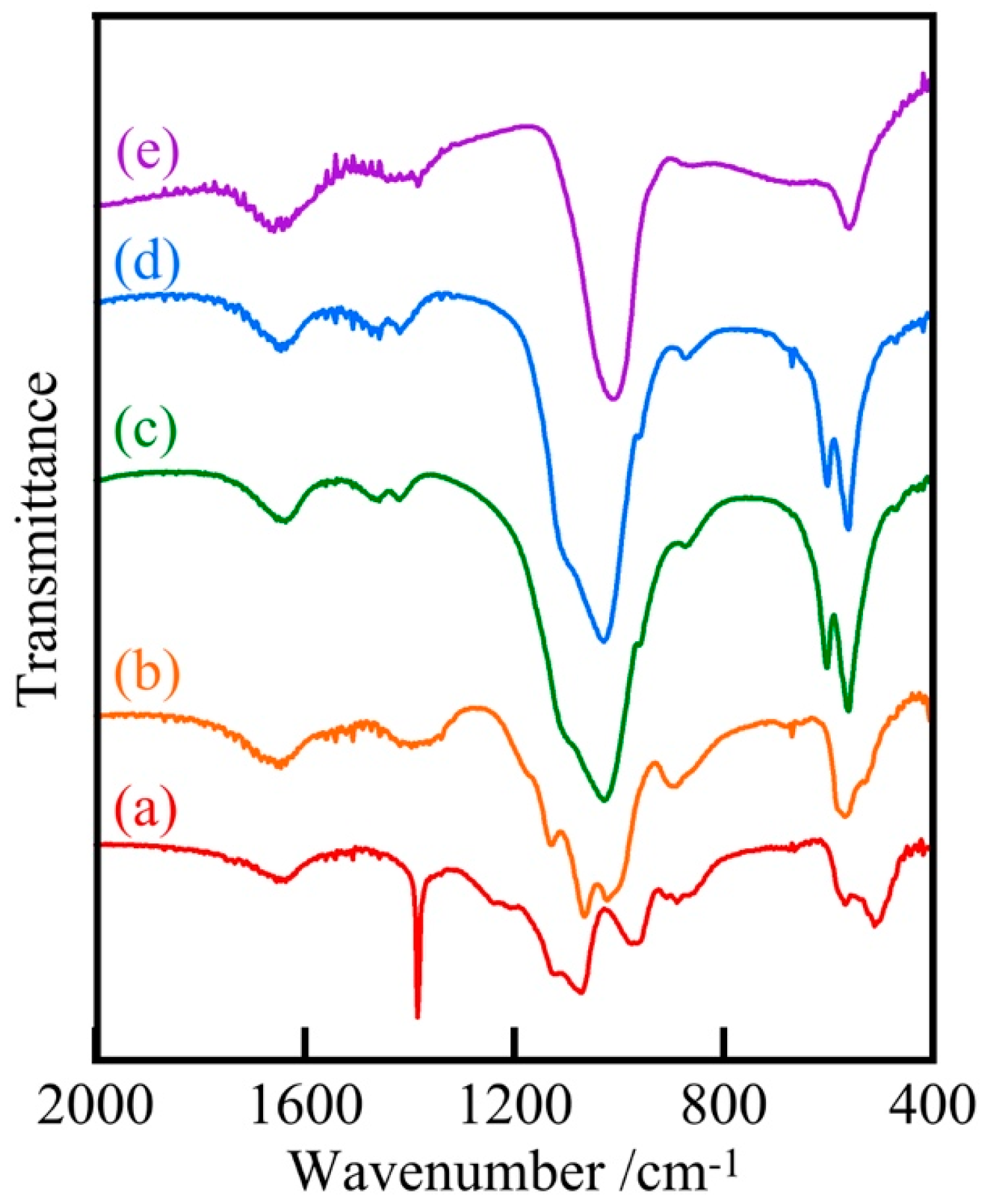
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, Y.; Li, M.; Zhu, N.; Cheng, Y.; Lam, S.S.; Chen, J.; Gao, Y.; Zhao, J. Bi-based visible light-driven nano-photocatalyst: The design, synthesis, and its application in pollutant governance and energy development. Nano Today 2022, 43, 101432. [Google Scholar] [CrossRef]
- Abu-Sari, S.M.; Daud, W.M.A.W.; Patah, M.F.A.; Ang, B.C. A review on synthesis, modification method, and challenges of light-driven H2 evolution using g-C3N4- based photocatalyst. Adv. Colloid Interface Sci. 2022, 307, 102722. [Google Scholar] [CrossRef]
- Qiu, J.; Meng, K.; Zhang, Y.; Cheng, B.; Zhang, J.; Wang, L.; Yu, J. COF/In2S3 S-Scheme Photocatalyst with Enhanced Light Absorption and H2O2-Production Activity and fs-TA Investigation. Adv. Mater. 2024, 36, 2400288. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J. Organic/inorganic composite S-scheme photocatalyst with enhanced light absorption and H2O2-production activity. J. Mater. Sci. Technol. 2026, 241, 18–20. [Google Scholar] [CrossRef]
- Saddique, Z.; Imran, M.; Javaid, A.; Latif, S.; Hussain, N.; Kowal, P.; Boczkaj, G. Band engineering of BiOBr based materials for photocatalytic wastewater treatment via advanced oxidation processes (AOPs)—A review. Water Res. Indust. 2023, 29, 100211. [Google Scholar] [CrossRef]
- Kumar, A.; Rana, S.; Dhiman, P.; Sharma, G.; Stadler, F.J. Current progress in heterojunctions based on Nb2O5 for photocatalytic water treatment and energy applications. J. Mol. Liq. 2024, 399, 124360. [Google Scholar] [CrossRef]
- Zhou, M.; Ou, H.; Li, S.; Qin, X.; Fang, Y.; Lee, S.-C.; Wang, X.; Ho, W. Photocatalytic Air Purification Using Functional Polymeric Carbon Nitrides. Adv. Sci. 2021, 8, 2102376. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, R.; Raizada, P.; Ahamad, T.; Alshehri, S.M.; Nguyen, V.-H.; Thakur, S.; Nguyen, C.C.; Kim, S.Y.; Le, Q.V. An overview on recent progress in photocatalytic air purification: Metal-based and metal-free photocatalysis. Environ. Res. 2022, 214, 113995. [Google Scholar] [CrossRef]
- Atacan, K.; Güy, N.; Özacar, M. Recent advances in photocatalytic coatings for antimicrobial surfaces. Curr. Opin. Chem. Eng. 2022, 36, 100777. [Google Scholar] [CrossRef]
- Ubaldi, F.; Valeriani, F.; Volpini, V.; Lofrano, G.; Spica, V.R. Antimicrobial Activity of Photocatalytic Coatings on Surfaces: A Systematic Review and Meta-Analysis. Coatings 2024, 14, 92. [Google Scholar] [CrossRef]
- Li, C.; Sun, T.; Zhang, D.; Zhang, X.; Qian, Y.; Zhang, Y.; Lin, X.; Liu, J.; Zhu, L.; Wang, X.; et al. Fabrication of ternary Ag/La-black TiO2-x photocatalyst with enhanced visible-light photocatalytic activity for tetracycline degradation. J. Alloys Compd. 2022, 891, 161960. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26. [Google Scholar] [CrossRef]
- Abdullah, F.H.; Abu Bakar, N.H.H.; Abu Bakar, M. Current advancements on the fabrication, modification, and industrial application of zinc oxide as photocatalyst in the removal of organic and inorganic contaminants in aquatic systems. J. Hazard. Mater. 2022, 424 Pt B, 127416. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.T.; Hossain, M.S.; Shariffuddin, J.H. Green synthesis and photocatalytic insights: A review of zinc oxide nanoparticles in wastewater treatment. Mater. Today Sustain. 2024, 26, 100764. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Li, X.; Luo, Q.; An, J.; Yue, J. Sunlight photocatalytic activity of polypyrrole–TiO2 nanocomposites prepared by ‘in situ’ method. Catal. Commun. 2008, 9, 1162–1166. [Google Scholar] [CrossRef]
- Abdo, S.M.; El-Hout, S.I.; Rashed, M.N.; El-Dosoqy, T.I.; El-Sheikh, S.M. Modified silver phosphate nanocomposite as an effective visible-light-driven photocatalyst in the reduction of aqueous Cr(VI). Mater. Res. Bull. 2024, 169, 112511. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, B.; Yang, Y.; Liu, W.; Hou, Z.; Shang, J.; Cheng, X. Synthesis of silver phosphate/graphene oxide aerogel and its high visible light photocatalytic elimination of formaldehyde. J. Environ. Chem. Eng. 2022, 10, 107171. [Google Scholar] [CrossRef]
- Li, L.; Feng, H.; Dong, Z.; Yang, T.; Xue, S. Indium selenide/silver phosphate hollow microsphere S-scheme heterojunctions for photocatalytic hydrogen production with simultaneous degradation of tetracycline. J. Colloid Interface Sci. 2023, 649, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Lin, Y.; Zhang, S.; Nie, J.; Luo, C.; Zhang, Y.; Wu, S.; Yang, C. Performance and mechanisms of photocatalysis of silver phosphate modified by carbon nanosheets doped with both nitrogen and boron for removal of norfloxacin. J. Environ. Chem. Eng. 2023, 11, 110595. [Google Scholar] [CrossRef]
- Santos, R.K.; Martins, T.A.; Silva, G.N.; Conceicao, M.V.S.; Nogueira, I.C.; Longo, E.; Botelho, G. Ag3PO4/NiO composites with enhanced photocatalytic activity under visible light. ACS Omega 2020, 5, 21651–21661. [Google Scholar] [CrossRef] [PubMed]
- Kanemoto, R.; Onoda, H. Synthesis and visible light photocatalytic activity of silver zinc phosphates. J. Met. Mater. Miner. 2024, 34, 2055. [Google Scholar] [CrossRef]
- Ifijen, I.H.; Maliki, M.; Anegbe, B. Synthesis, photocatalytic degradation and antibacterial properties of selenium or silver doped zinc oxide nanoparticles: A detailed review. OpenNano 2022, 8, 100082. [Google Scholar] [CrossRef]
- Bee, S.-L.; Bustami, Y.; Ul-Hamid, A.; Lim, K.; Hamid, Z.A.A. Synthesis of silver nanoparticle-decorated hydroxyapatite nanocomposite with combined bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 2021, 32, 106. [Google Scholar] [CrossRef]
- Yang, N.; Wang, S.; Ding, P.; Sun, S.; Wei, Q.; Jafari, H.; Wang, L.; Han, Y.; Okoro, O.V.; Wang, T.; et al. Magnesium-doped biphasic calcium phosphate nanoparticles with incorporation of silver: Synthesis, cytotoxic and antibacterial properties. Mater. Lett. 2022, 322, 132478. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Ali, A.H. Fabrication and characterization of mesoporous calcium silicate and silver-incorporated mesoporous calcium silicate nanoparticles with low cytotoxicity and antibacterial properties as a dental biomaterial. Res. Mater. 2024, 22, 100555. [Google Scholar] [CrossRef]
- Cao, X.; Zhu, L.; Bai, Y.; Li, F.; Yu, X. Green one-step synthesis of silver nanoparticles and their biosafety and antibacterial properties. Green Chem. Lett. Rev. 2022, 15, 28–34. [Google Scholar] [CrossRef]
- Ren, S.; Yuan, X.; Liu, F.; Fang, F.; Iqbal, H.M.N.; Zahran, S.A.; Bilal, M. Bacteriocin from Lacticaseibacillus rhamnosus sp. A5: Isolation, Purification, Characterization, and Antibacterial Evaluation for Sustainable Food Processing. Sustainability 2022, 14, 9571. [Google Scholar] [CrossRef]
- Seesanong, S.; Seangarun, C.; Boonchom, B.; Laohavisuti, N.; Chaiseeda, K.; Boonmee, W. Composition and Properties of Triple Superphosphate Obtained from Oyster Shells and Various Concentrations of Phosphoric Acid. ACS Omega 2021, 6, 22065–22072. [Google Scholar] [CrossRef]
- Seesanong, S.; Seangarun, C.; Boonchom, B.; Sronsri, C.; Laohavisuti, N.; Chaiseeda, K.; Boonmee, W. Recrystallization of Triple Superphosphate Produced from Oyster Shell Waste for Agronomic Performance and Environmental Issues. Minerals 2022, 12, 254. [Google Scholar] [CrossRef]
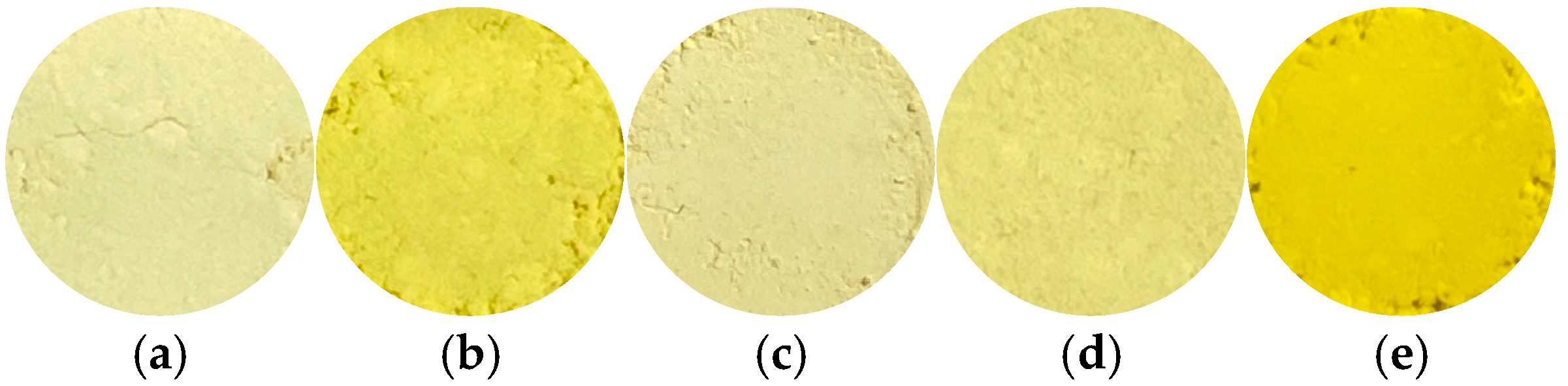





| x | pH | Yield/% | L* | a* | b* |
|---|---|---|---|---|---|
| 10 | 1 | 50.6 | 93.67 | −0.38 | 4.56 |
| 25 | 1 | 38.6 | 89.94 | 1.54 | 24.13 |
| 30 | 1 | 15.8 | 94.95 | 0.33 | 46.05 |
| 40 | 1 | 22.5 | 93.46 | 1.55 | 50.12 |
| 25 | 5 | 129 | 85.38 | −0.46 | 37.27 |
| 25 | 7 | 107 | 89.55 | 0.43 | 22.47 |
| 25 | 9 | 110 | 85.55 | −1.17 | 31.44 |
| Ag3PO4 | 9 | - | 86.83 | 0.79 | 70.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onoda, H.; Izaki, C. Synthesis of Silver–Calcium Phosphate Visible Light Responsive Photocatalytic Materials and Their Antibacterial Properties. Materials 2025, 18, 4789. https://doi.org/10.3390/ma18204789
Onoda H, Izaki C. Synthesis of Silver–Calcium Phosphate Visible Light Responsive Photocatalytic Materials and Their Antibacterial Properties. Materials. 2025; 18(20):4789. https://doi.org/10.3390/ma18204789
Chicago/Turabian StyleOnoda, Hiroaki, and Chihiro Izaki. 2025. "Synthesis of Silver–Calcium Phosphate Visible Light Responsive Photocatalytic Materials and Their Antibacterial Properties" Materials 18, no. 20: 4789. https://doi.org/10.3390/ma18204789
APA StyleOnoda, H., & Izaki, C. (2025). Synthesis of Silver–Calcium Phosphate Visible Light Responsive Photocatalytic Materials and Their Antibacterial Properties. Materials, 18(20), 4789. https://doi.org/10.3390/ma18204789







