A Review of the Intrinsic Chemical Stability Challenge in Operational Perovskite Photovoltaics
Abstract
1. Introduction
2. Status of Perovskite PV Stability
3. Chemical Evolution of Perovskites
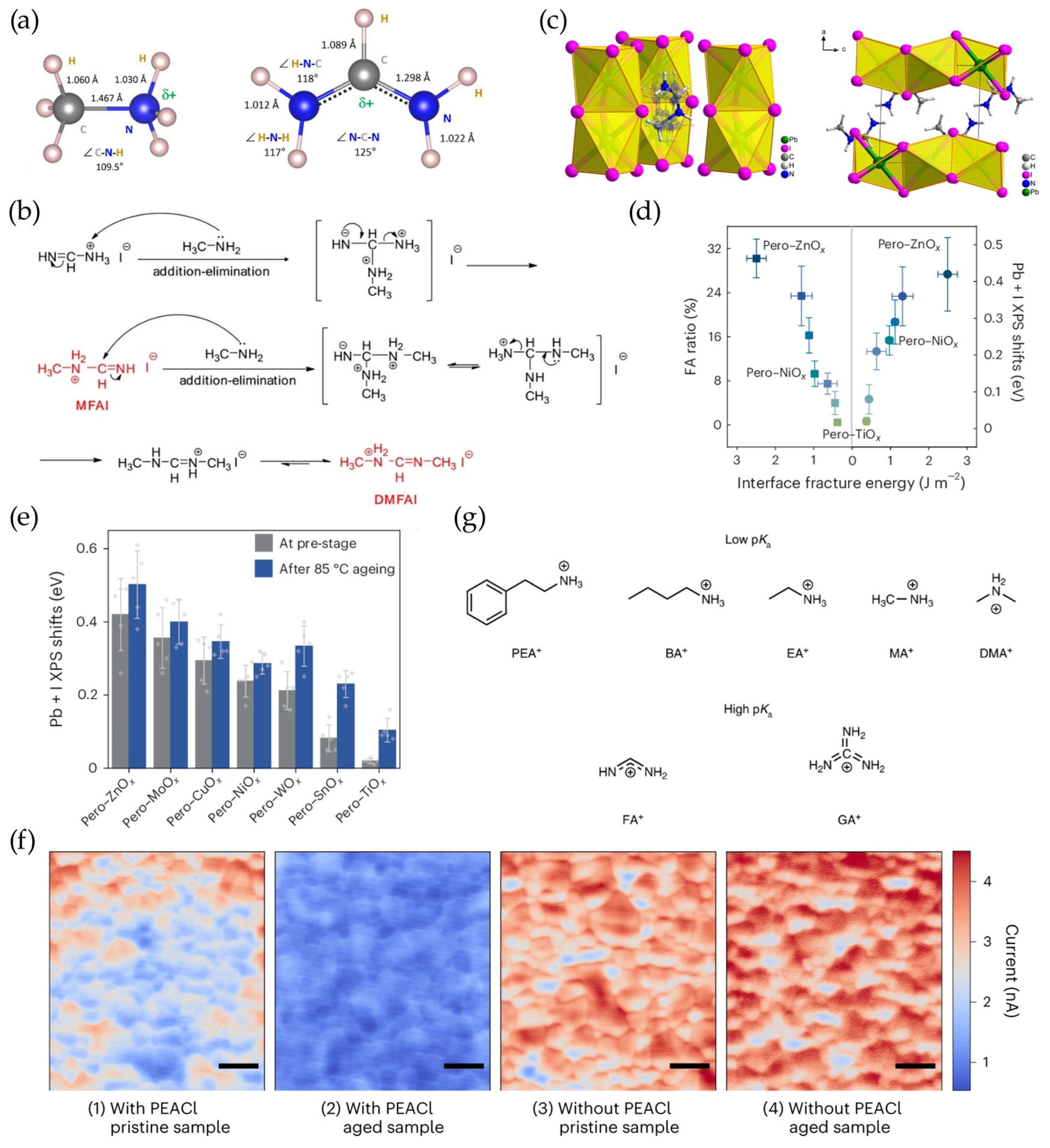
3.1. Deprotonation of A-Site Ammonium Cations
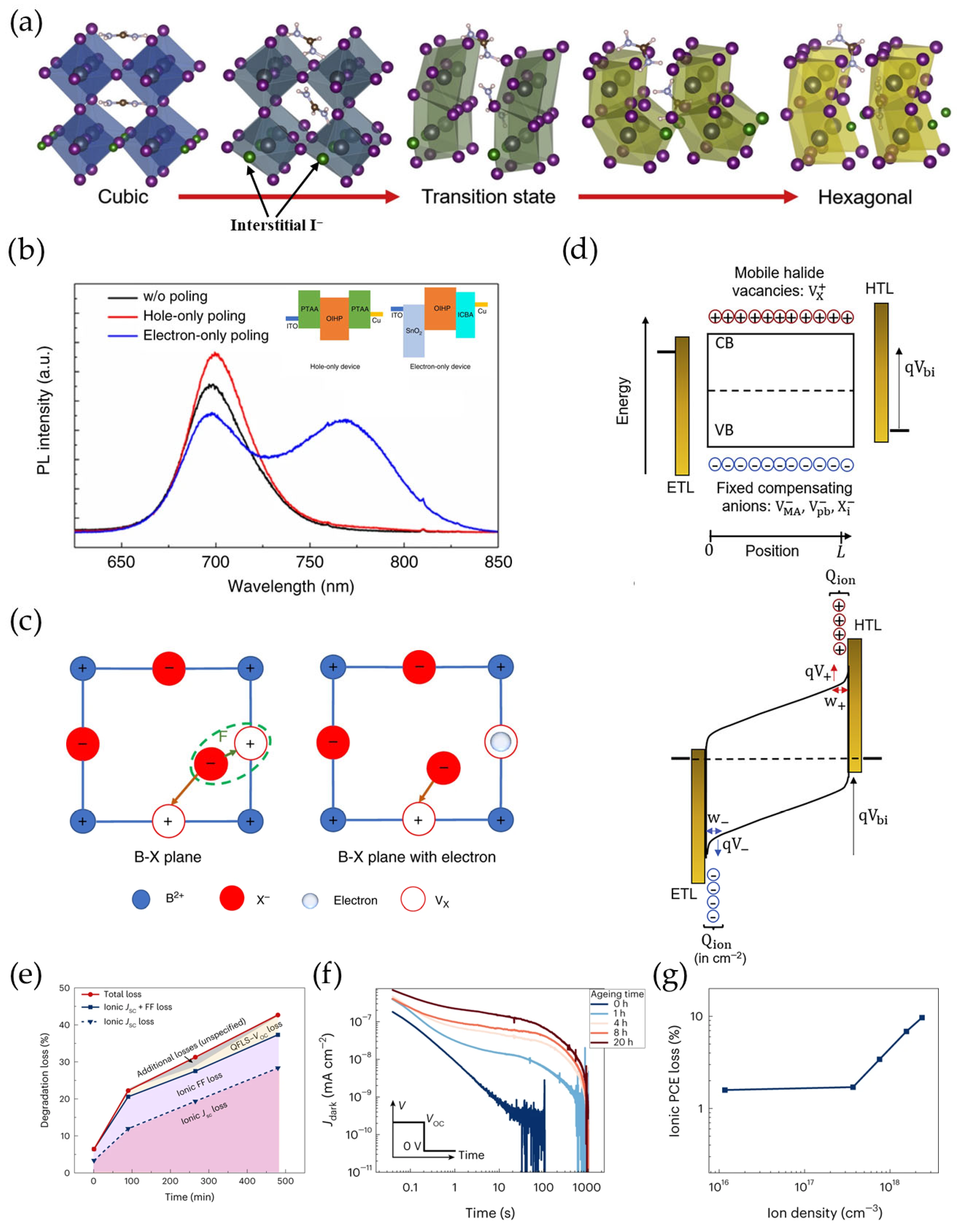
3.2. Mobile X-Site Halide Ion Migration
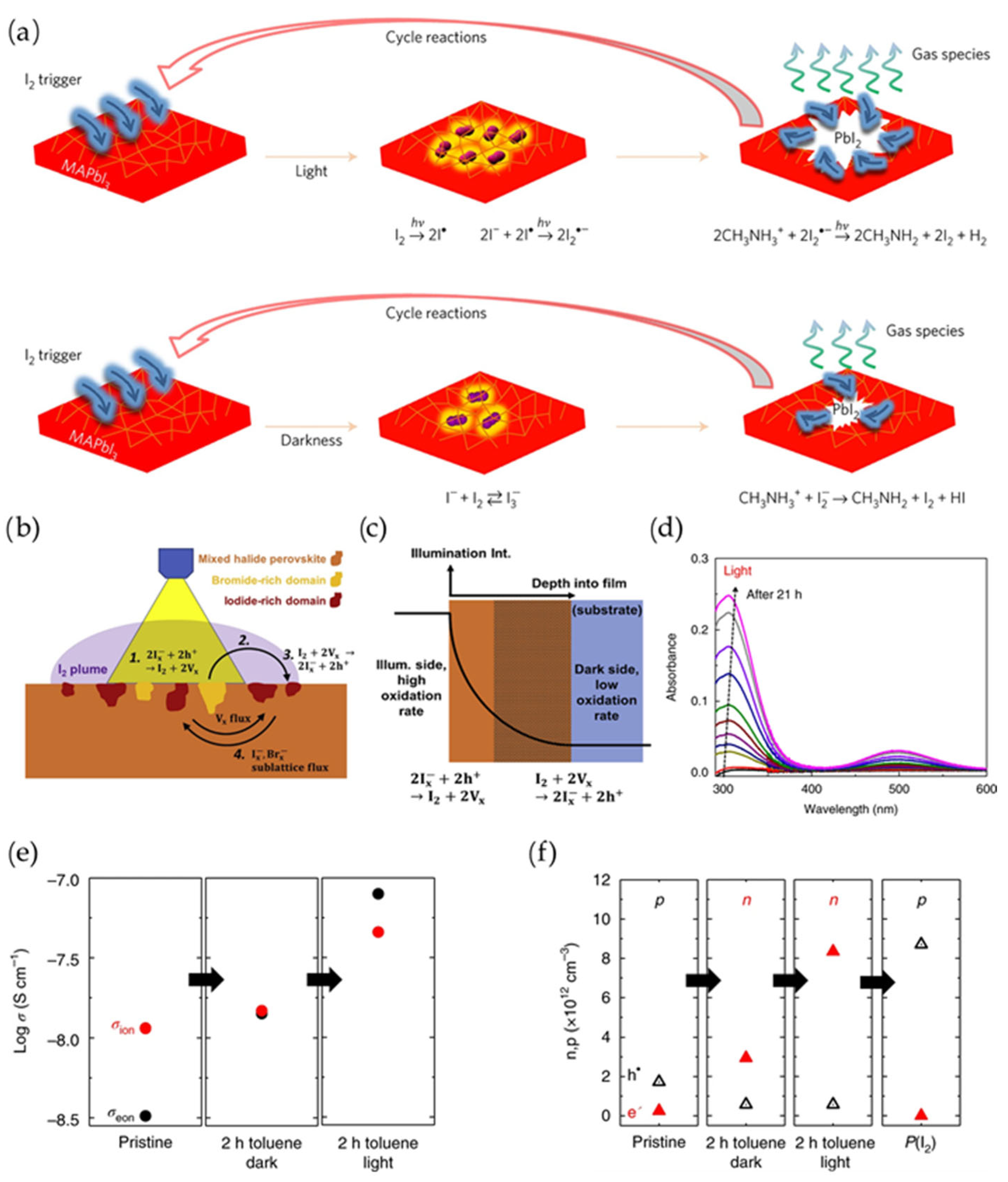
3.3. Oxidation of X-Site Halide Ions
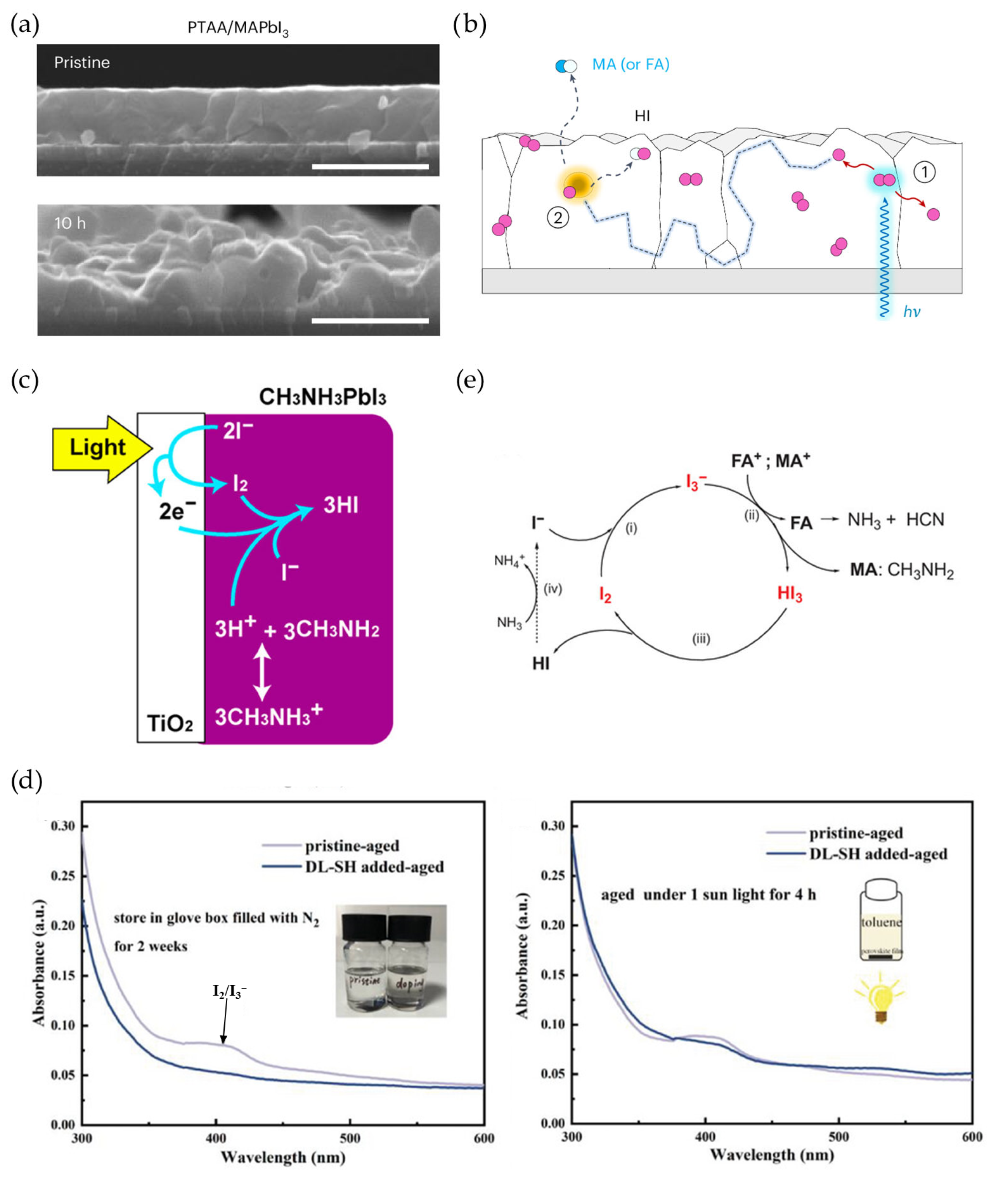
4. Strategies Against Operational PV Degradation
4.1. Stabilization of A-Site Ammonium Cation
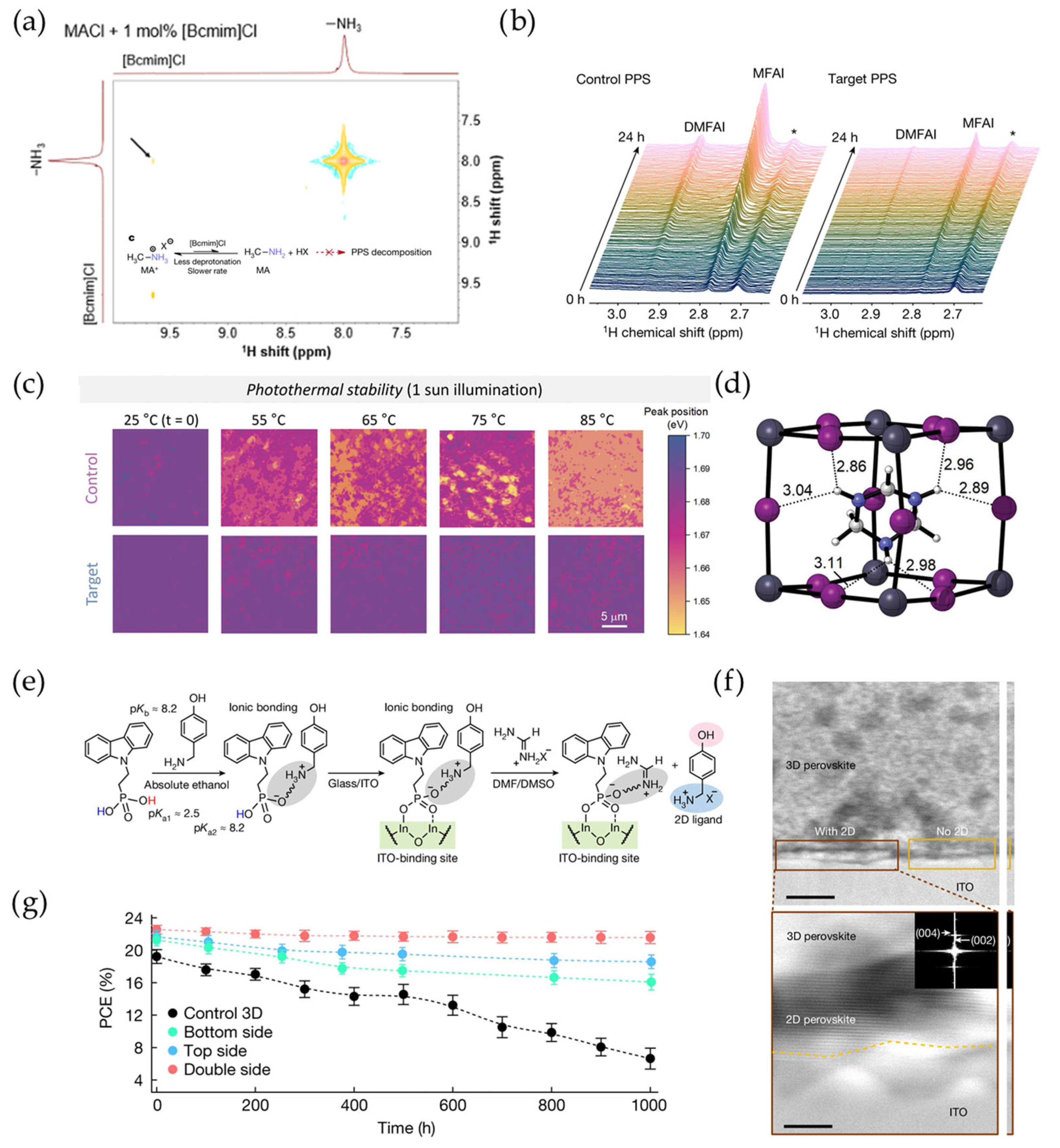
4.2. Confinement of Iodine Molecules
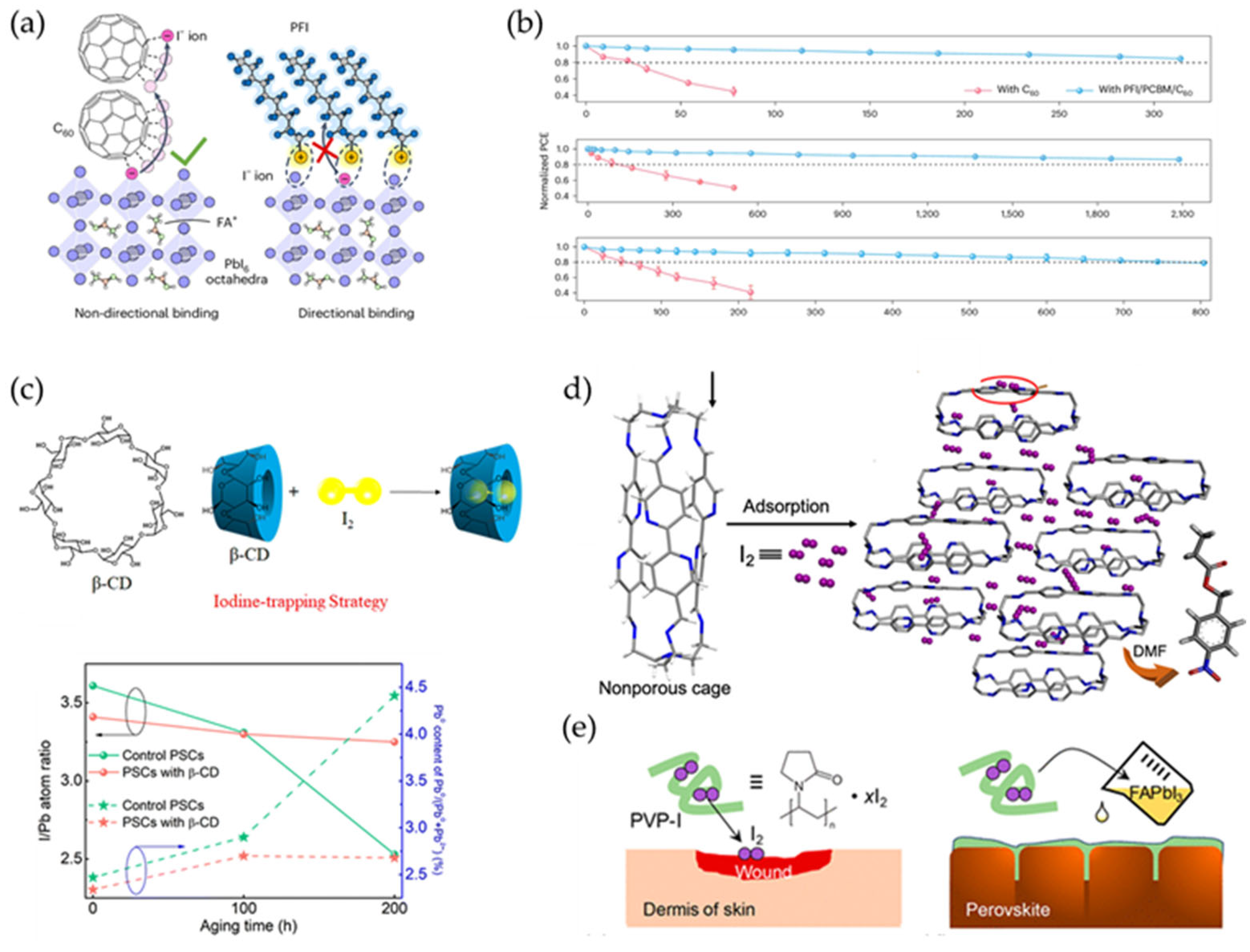
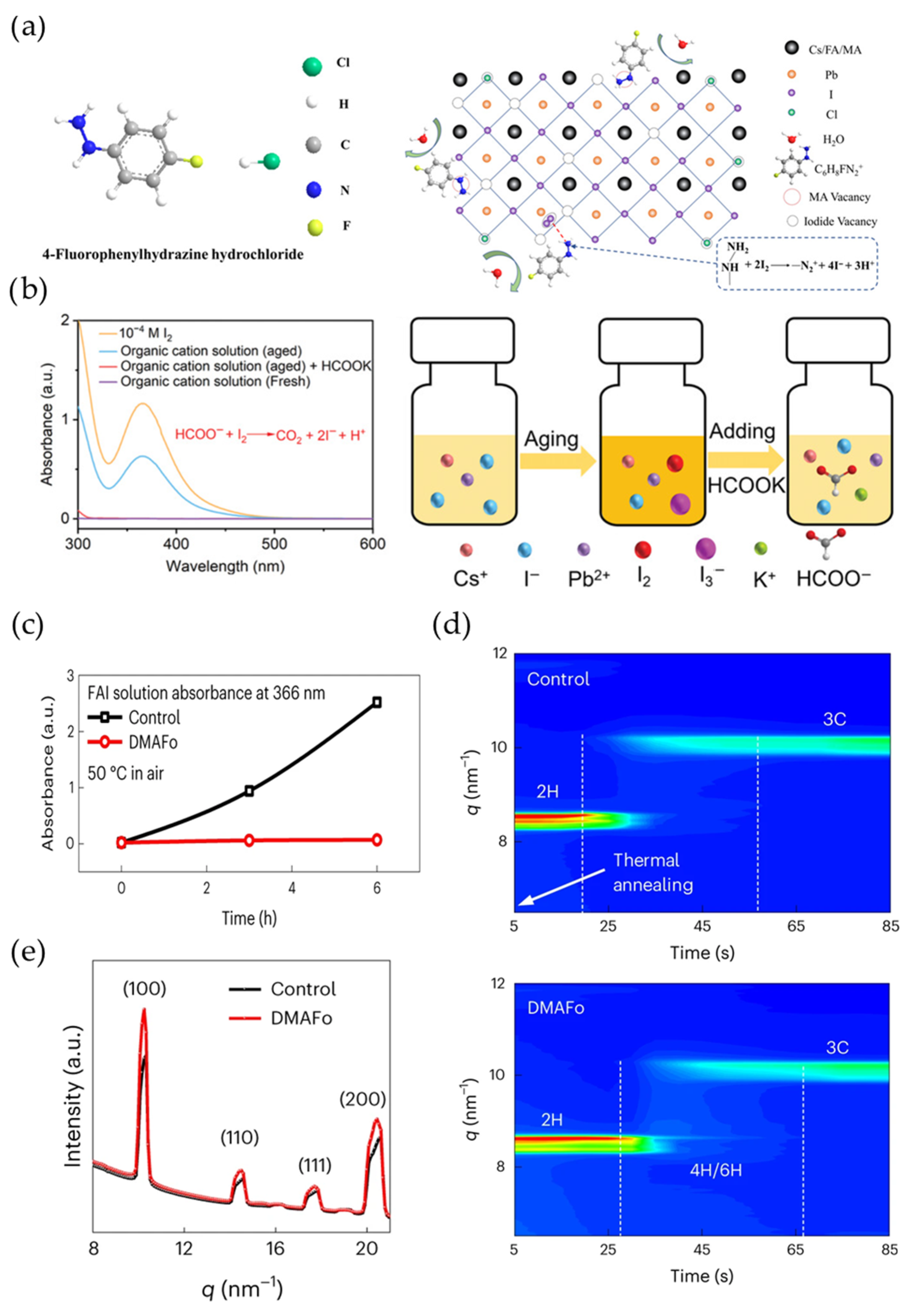
4.3. Reduction of Iodine Molecules
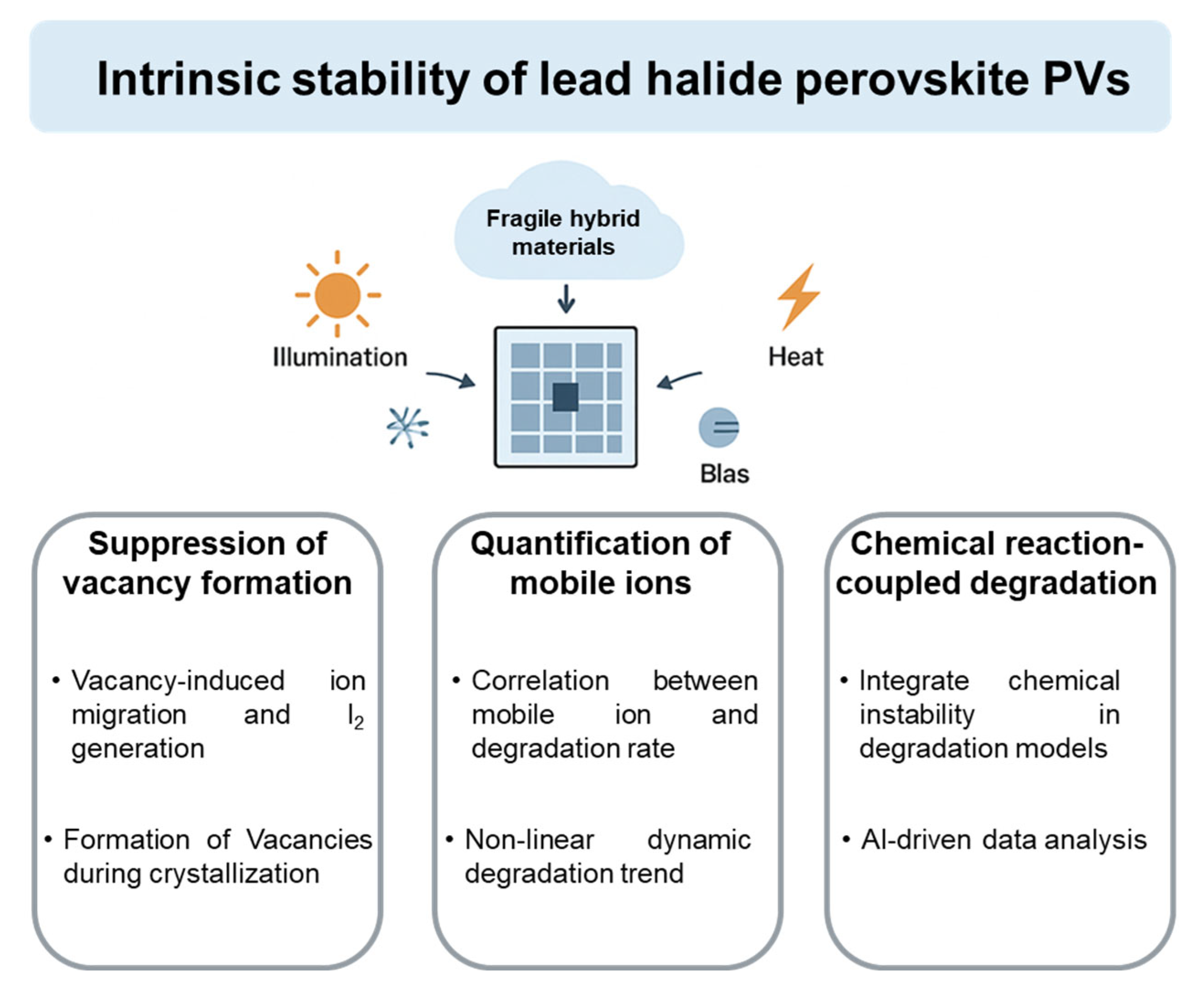
5. Conclusions and Outlook
- (1)
- Vacancy formation should be suppressed to substantially inhibit mobile ion migration and I2 generation. Ion hopping by vacancies leads to severe ion migration. The presence of mobile ions readily induces the oxidation of I− into volatile I2, which produces more halide vacancies, accelerating ion migration. Although various strategies have been developed to reduce ion migration and I2 formation, halide vacancies are the primary driver behind ion migration within perovskites [63]. Therefore, the control of vacancy formation during perovskite crystallization is essential.
- (2)
- Quantifying the relationship between mobile ion migration and perovskite PV degradation is of critical importance. Perovskite PVs show more pronounced ion movement compared to those of inorganic photoactive materials. Furthermore, these mobile ions, especially iodide ions, can trigger a series of chemical reactions, which accelerate the collapse of intrinsic perovskite lattices. Although reports claim that the suppression of ion migration leads to delayed device degradation, the number of mobile ions suppressed and their influence on device performance degradation are not clear. Therefore, it is essential to determine how the concentration of mobile ions in perovskites influences the degradation rate. We believe that device performance evolution during operation cannot follow a linear degradation trend due to the dynamic variation in mobile ion concentration. Furthermore, the increased rate of mobile ion concentration enhancement under different external stresses (e.g., illumination at different wavelengths) should be investigated systematically.
- (3)
- An accelerated aging test model for studying perovskite PV stability should be constructed. Until now, empirical insights into PV performance degradation models have largely been drawn from crystalline silicon photovoltaics [26,100]. However, the degradation of perovskite PVs associated with organic chemical reactivity differs from that of silicon PVs significantly [101], leading to more complex device degradation behavior. Therefore, the influence of organic species reactivity and halide ion migration on PV degradation pathways should be investigated, which could provide critical insights into their operational reliability under real-world conditions. In order to address the diversity of degradation mechanisms, artificial intelligence (AI), particularly machine learning and neural networks, can identify latent correlations within high-dimensional simulations and experimental datasets. Therefore, AI can predict degradation pathways and lifetimes more accurately.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, J.Y.; Lee, J.-W.; Jung, H.S.; Shin, H.; Park, N.-G. High-efficiency perovskite solar cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Hu, Y.; Mei, A.; Tan, H.; Saidaminov, M.I.; Seok, S.I.; McGehee, M.D.; Sargent, E.H.; Han, H. Challenges for commercializing perovskite solar cells. Science 2018, 361, eaat8235. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Guo, X.; Lu, C.; Wei, J.; Fang, J. Constructing heterojunctions by surface sulfidation for efficient inverted perovskite solar cells. Science 2022, 375, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, A.; Prochowicz, D.; Tavakoli, M.M.; Trivedi, S.; Kumar, P.; Yadav, P. A review of aspects of additive engineering in perovskite solar cells. J. Mater. Chem. A 2020, 8, 27–54. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Y.; Chen, H.; Xu, J.; Liu, A.; Bati, A.S.; Zhu, H.; Grater, L.; Hadke, S.S.; Huang, C. Bimolecularly passivated interface enables efficient and stable inverted perovskite solar cells. Science 2023, 382, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Uddin, A. Defects and stability of perovskite solar cells: A critical analysis. Mater. Chem. Front. 2022, 6, 400–417. [Google Scholar] [CrossRef]
- Yukta; Chavan, R.D.; Mahapatra, A.; Prochowicz, D.; Yadav, P.; Iyer, P.K.; Satapathi, S. Improved Efficiency and Stability in 1, 5-Diaminonaphthalene Iodide-Passivated 2D/3D Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2023, 15, 53351–53361. [Google Scholar] [CrossRef]
- Jiang, Q.; Tirawat, R.; Kerner, R.A.; Gaulding, E.A.; Xian, Y.; Wang, X.; Newkirk, J.M.; Yan, Y.; Berry, J.J.; Zhu, K. Towards linking lab and field lifetimes of perovskite solar cells. Nature 2023, 623, 313–318. [Google Scholar] [CrossRef]
- Vidal, R.; Alberola-Borràs, J.A.; Sánchez-Pantoja, N.; Mora-Seró, I. Comparison of perovskite solar cells with other photovoltaics technologies from the point of view of life cycle assessment. Adv. Energy Sustain. Res. 2021, 2, 2000088. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Leijtens, T.; McGehee, M.D. Understanding degradation mechanisms and improving stability of perovskite photovoltaics. Chem. Rev. 2018, 119, 3418–3451. [Google Scholar] [CrossRef]
- Qin, J.; Che, Z.; Kang, Y.; Liu, C.; Wu, D.; Yang, H.; Hu, X.; Zhan, Y. Towards operation-stabilizing perovskite solar cells: Fundamental materials, device designs, and commercial applications. InfoMat 2024, 6, e12522. [Google Scholar] [CrossRef]
- Zhuang, J.; Wang, J.; Yan, F. Review on chemical stability of lead halide perovskite solar cells. Nano-Micro Lett. 2023, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, Q.; Huo, J.; Gao, F.; Gan, Z.; Zhao, Q.; Li, H. Mechanisms and suppression of photoinduced degradation in perovskite solar cells. Adv. Energy Mater. 2021, 11, 2002326. [Google Scholar] [CrossRef]
- Wang, X.; Fan, Y.; Wang, L.; Chen, C.; Li, Z.; Liu, R.; Meng, H.; Shao, Z.; Du, X.; Zhang, H. Perovskite solution aging: What happened and how to inhibit? Chem 2020, 6, 1369–1378. [Google Scholar] [CrossRef]
- Dong, B.; Wei, M.; Li, Y.; Yang, Y.; Ma, W.; Zhang, Y.; Ran, Y.; Cui, M.; Su, Z.; Fan, Q. Self-assembled bilayer for perovskite solar cells with improved tolerance against thermal stresses. Nat. Energy 2025, 10, 342–353. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Y.; Xu, J.; Wang, D.; Ding, Z.; Zhu, T.; Chen, B.; Yang, Y.; Wei, M.; Guo, R. High-efficiency and thermally stable FACsPbI3 perovskite photovoltaics. Nature 2024, 635, 82–88. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Vikram; Yang, F.; Cao, X.-L.; Dasgupta, A.; Oliver, R.D.; Ulatowski, A.M.; McCarthy, M.M.; Shen, X.; Yuan, Q. Bandgap-universal passivation enables stable perovskite solar cells with low photovoltage loss. Science 2024, 384, 767–775. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.; Xu, J.; Maxwell, A.; Zhou, W.; Yang, Y.; Zhou, Q.; Bati, A.S.; Wan, H.; Wang, Z. Improved charge extraction in inverted perovskite solar cells with dual-site-binding ligands. Science 2024, 384, 189–193. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Wang, T.; Li, J.; Chia, H.Y.; Liang, H.; Xi, S.; Liu, S.; Guo, X.; Guo, R. Determining the bonding–degradation trade-off at heterointerfaces for increased efficiency and stability of perovskite solar cells. Nat. Energy 2025, 10, 181–190. [Google Scholar] [CrossRef]
- Li, S.; Xiao, Y.; Su, R.; Xu, W.; Luo, D.; Huang, P.; Dai, L.; Chen, P.; Caprioglio, P.; Elmestekawy, K.A. Coherent growth of high-Miller-index facets enhances perovskite solar cells. Nature 2024, 635, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Shao, B.; Shen, Z.; You, S.; Yin, J.; Wehbe, N.; Wang, L.; Song, X.; Abulikemu, M.; Basaheeh, A. In situ energetics modulation enables high-efficiency and stable inverted perovskite solar cells. Nat. Photonics 2025, 19, 28–35. [Google Scholar] [CrossRef]
- Li, Q.; Liu, H.; Hou, C.-H.; Yan, H.; Li, S.; Chen, P.; Xu, H.; Yu, W.-Y.; Zhao, Y.; Sui, Y. Harmonizing the bilateral bond strength of the interfacial molecule in perovskite solar cells. Nat. Energy 2024, 9, 1506–1516. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, Z.; Almalki, M.; Hope, M.A.; Zhao, J.; Gallet, T.; Krishna, A.; Mishra, A.; Eickemeyer, F.T.; Xu, J. Stabilization of highly efficient perovskite solar cells with a tailored supramolecular interface. Nat. Commun. 2024, 15, 7139. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, S.; Zhu, X.; Li, S.; Zheng, Z.; Zhao, K.; Ji, L.; Li, R.; Liu, Y.; Liu, C. Inverted perovskite solar cells with over 2,000 h operational stability at 85 ° C using fixed charge passivation. Nat. Energy 2024, 9, 37–46. [Google Scholar] [CrossRef]
- Zhu, X.; Li, M.; Mo, K.; Yang, M.; Li, S.; Yang, Y.; Wang, H.; Li, R.; Liu, Y.; Lin, Q. A Surface-Reconstructed Bilayer Heterojunction Enables Efficient and Stable Inverted Perovskite Solar Cells. Adv. Mater. 2024, 36, 2409340. [Google Scholar] [CrossRef] [PubMed]
- Suo, J.; Yang, B.; Mosconi, E.; Bogachuk, D.; Doherty, T.A.; Frohna, K.; Kubicki, D.J.; Fu, F.; Kim, Y.; Er-Raji, O. Multifunctional sulfonium-based treatment for perovskite solar cells with less than 1% efficiency loss over 4500-h operational stability tests. Nat. Energy 2024, 9, 172–183. [Google Scholar] [CrossRef]
- Ding, Y.; Ding, B.; Shi, P.; Romano-deGea, J.; Li, Y.; Turnell-Ritson, R.C.; Syzgantseva, O.A.; Yavuz, I.; Xia, M.; Yu, R. Cation reactivity inhibits perovskite degradation in efficient and stable solar modules. Science 2024, 386, 531–538. [Google Scholar] [CrossRef]
- Azmi, R.; Utomo, D.S.; Vishal, B.; Zhumagali, S.; Dally, P.; Risqi, A.M.; Prasetio, A.; Ugur, E.; Cao, F.; Imran, I.F. Double-side 2D/3D heterojunctions for inverted perovskite solar cells. Nature 2024, 628, 93–98. [Google Scholar] [CrossRef]
- Ibaceta-Jaña, J.; Muydinov, R.; Rosado, P.; Mirhosseini, H.; Chugh, M.; Nazarenko, O.; Dirin, D.N.; Heinrich, D.; Wagner, M.R.; Kühne, T.D. Vibrational dynamics in lead halide hybrid perovskites investigated by Raman spectroscopy. Phys. Chem. Chem. Phys. 2020, 22, 5604–5614. [Google Scholar] [CrossRef]
- Chen, L.; Hu, M.; Lee, S.; Kim, J.; Zhao, Z.-Y.; Han, S.-P.; Lah, M.S.; Seok, S.I. Deciphering reaction products in formamidine-based perovskites with methylammonium chloride additive. J. Am. Chem. Soc. 2023, 145, 27900–27910. [Google Scholar] [CrossRef]
- Xu, B.; Liu, G.; Wang, P.; Li, W.; Ying, Z.; Liu, J.; Shi, Y. Interfacial Proton Precompensation: Suppressing Deprotonation-Driven Lattice Collapse for Enhanced Efficiency and Stability in Perovskite Solar Cells. Angew. Chem. 2025, 137, e202417262. [Google Scholar] [CrossRef]
- Thampy, S.; Xu, W.; Hsu, J.W. Metal oxide-induced instability and its mitigation in halide perovskite solar cells. J. Phys. Chem. Lett. 2021, 12, 8495–8506. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Ono, L.K.; Qi, Y. Thermal degradation of formamidinium based lead halide perovskites into sym-triazine and hydrogen cyanide observed by coupled thermogravimetry-mass spectrometry analysis. J. Mater. Chem. A 2019, 7, 16912–16919. [Google Scholar] [CrossRef]
- Fiorentino, F.; Albaqami, M.D.; Poli, I.; Petrozza, A. Thermal-and light-induced evolution of the 2D/3D interface in lead-halide perovskite films. ACS Appl. Mater. Interfaces 2021, 14, 34180–34188. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, A.A.; Szostak, R.; Drigo, N.; Queloz, V.I.; Marchezi, P.; Germino, J.; Tolentino, H.C.; Nazeeruddin, M.K.; Nogueira, A.F.; Grancini, G. In situ analysis reveals the role of 2D perovskite in preventing thermal-induced degradation in 2D/3D perovskite interfaces. Nano Lett. 2020, 20, 3992–3998. [Google Scholar] [CrossRef]
- Liu, C.; Ma, L.; Zhao, P.; Yuan, L.; Li, F.; Fang, Z.; Chang, Q.; Jia, N.; Guo, P.; Guo, F. Peptide-Based Ammonium Halide with Inhibited Deprotonation Enabling Effective Interfacial Engineering for Highly Efficient and Stable FAPbI3 Perovskite Solar Cells. Adv. Funct. Mater. 2024, 34, 2405735. [Google Scholar] [CrossRef]
- Wang, M.; Shi, Z.; Fei, C.; Deng, Z.J.; Yang, G.; Dunfield, S.P.; Fenning, D.P.; Huang, J. Ammonium cations with high p K a in perovskite solar cells for improved high-temperature photostability. Nat. Energy 2023, 8, 1229–1239. [Google Scholar] [CrossRef]
- Ugur, E.; Said, A.A.; Dally, P.; Zhang, S.; Petoukhoff, C.E.; Rosas-Villalva, D.; Zhumagali, S.; Yildirim, B.K.; Razzaq, A.; Sarwade, S. Enhanced cation interaction in perovskites for efficient tandem solar cells with silicon. Science 2024, 385, 533–538. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, H.; Liu, C.; Xu, J.; Huang, C.; Malliakas, C.D.; Wan, H.; Bati, A.S.; Wang, Z.; Reynolds, R.P. Amidination of ligands for chemical and field-effect passivation stabilizes perovskite solar cells. Science 2024, 386, 898–902. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kang, D.-H.; Lee, D.-N.; Park, N.-G. Effect of functional groups in passivating materials on stability and performance of perovskite solar cells. J. Mater. Chem. A 2023, 11, 15014–15021. [Google Scholar] [CrossRef]
- Ma, C.; Kang, M.-C.; Lee, S.-H.; Zhang, Y.; Kang, D.-H.; Yang, W.; Zhao, P.; Kim, S.-W.; Kwon, S.J.; Yang, C.-W. Facet-dependent passivation for efficient perovskite solar cells. J. Am. Chem. Soc. 2023, 145, 24349–24357. [Google Scholar] [CrossRef]
- Eames, C.; Frost, J.M.; Barnes, P.R.; O’regan, B.C.; Walsh, A.; Islam, M.S. Ionic transport in hybrid lead iodide perovskite solar cells. Nat. Commun. 2015, 6, 7497. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.-J.; Shi, T.; Yan, Y. Unusual defect physics in CH3NH3PbI3 perovskite solar cell absorber. Appl. Phys. Lett. 2014, 104, 063903. [Google Scholar] [CrossRef]
- Haruyama, J.; Sodeyama, K.; Han, L.; Tateyama, Y. First-principles study of ion diffusion in perovskite solar cell sensitizers. J. Am. Chem. Soc. 2015, 137, 10048–10051. [Google Scholar] [CrossRef] [PubMed]
- Azpiroz, J.M.; Mosconi, E.; Bisquert, J.; De Angelis, F. Defect migration in methylammonium lead iodide and its role in perovskite solar cell operation. Energy Environ. Sci. 2015, 8, 2118–2127. [Google Scholar] [CrossRef]
- Nagabhushana, G.; Shivaramaiah, R.; Navrotsky, A. Direct calorimetric verification of thermodynamic instability of lead halide hybrid perovskites. Proc. Natl. Acad. Sci. USA 2016, 113, 7717–7721. [Google Scholar] [CrossRef]
- Tessler, N.; Vaynzof, Y. Insights from device modeling of perovskite solar cells. ACS Energy Lett. 2020, 5, 1260–1270. [Google Scholar] [CrossRef]
- Weber, S.A.; Hermes, I.M.; Turren-Cruz, S.-H.; Gort, C.; Bergmann, V.W.; Gilson, L.; Hagfeldt, A.; Graetzel, M.; Tress, W.; Berger, R. How the formation of interfacial charge causes hysteresis in perovskite solar cells. Energy Environ. Sci. 2018, 11, 2404–2413. [Google Scholar] [CrossRef]
- Oranskaia, A.; Yin, J.; Bakr, O.M.; Brédas, J.-L.; Mohammed, O.F. Halogen migration in hybrid perovskites: The organic cation matters. J. Phys. Chem. Lett. 2018, 9, 5474–5480. [Google Scholar] [CrossRef]
- Li, D.; Sun, F.; Liang, C.; He, Z. Effective approach for reducing the migration of ions and improving the stability of organic–inorganic perovskite solar cells. J. Alloys Compd. 2018, 741, 489–494. [Google Scholar] [CrossRef]
- Lin, D.; Shi, T.; Xie, H.; Wan, F.; Ren, X.; Liu, K.; Zhao, Y.; Ke, L.; Lin, Y.; Gao, Y. Ion migration accelerated reaction between oxygen and metal halide perovskites in light and its suppression by cesium incorporation. Adv. Energy Mater. 2021, 11, 2002552. [Google Scholar] [CrossRef]
- Hidalgo, J.; An, Y.; Yehorova, D.; Li, R.; Breternitz, J.; Perini, C.A.; Hoell, A.; Boix, P.P.; Schorr, S.; Kretchmer, J.S. Solvent and A-site cation control preferred crystallographic orientation in bromine-based perovskite thin films. Chem. Mater. 2023, 35, 4181–4191. [Google Scholar] [CrossRef]
- Han, E.; Lyu, M.; Choi, E.; Zhao, Y.; Zhang, Y.; Lee, J.; Lee, S.M.; Jiao, Y.; Ahmad, S.H.A.; Seidel, J. High-Performance Indoor Perovskite Solar Cells by Self-Suppression of Intrinsic Defects via a Facile Solvent-Engineering Strategy. Small 2024, 20, 2305192. [Google Scholar] [CrossRef]
- Tan, S.; Yavuz, I.; Weber, M.H.; Huang, T.; Chen, C.-H.; Wang, R.; Wang, H.-C.; Ko, J.H.; Nuryyeva, S.; Xue, J. Shallow iodine defects accelerate the degradation of α-phase formamidinium perovskite. Joule 2020, 4, 2426–2442. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, B.; Fang, Y.; Zhao, J.; Bao, C.; Yu, Z.; Deng, Y.; Rudd, P.N.; Yan, Y.; Yuan, Y. Excess charge-carrier induced instability of hybrid perovskites. Nat. Commun. 2018, 9, 4981. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Kubicki, D.J.; Omrani, M.; Alam, F.; Abdi-Jalebi, M. The race between complicated multiple cation/anion compositions and stabilization of FAPbI 3 for halide perovskite solar cells. J. Mater. Chem. C 2023, 11, 2449–2468. [Google Scholar] [CrossRef]
- Kazemi, M.A.A.; Folastre, N.; Raval, P.; Sliwa, M.; Nsanzimana, J.M.V.; Golonu, S.; Demortiere, A.; Rousset, J.; Lafon, O.; Delevoye, L. Moisture-Induced Non-Equilibrium Phase Segregation in Triple Cation Mixed Halide Perovskite Monitored by In Situ Characterization Techniques and Solid-State NMR. Energy Environ. Mater. 2023, 6, e12335. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, J.; Wang, K.; Zhang, C.; Wang, Y.; Wang, R.; Zhao, J.; Zhong, X.; Ren, H.; Hou, G. Performance promotion strategies for wide bandgap perovskite solar cells. Sustain. Energy Fuels 2025, 9, 303–322. [Google Scholar] [CrossRef]
- Li, F.; Lo, T.W.; Deng, X.; Li, S.; Fan, Y.; Lin, F.R.; Cheng, Y.; Zhu, Z.; Lei, D.; Jen, A.K.Y. Plasmonic local heating induced strain modulation for enhanced efficiency and stability of perovskite solar cells. Adv. Energy Mater. 2022, 12, 2200186. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, C.; Zhang, H.; Li, D.; Tian, D.; Li, G.; Jing, X.; Zhang, W.; Xiao, W.; Liu, Q. Anomalously large interface charge in polarity-switchable photovoltaic devices: An indication of mobile ions in organic–inorganic halide perovskites. Energy Environ. Sci. 2015, 8, 1256–1260. [Google Scholar] [CrossRef]
- Bertoluzzi, L.; Boyd, C.C.; Rolston, N.; Xu, J.; Prasanna, R.; O’Regan, B.C.; McGehee, M.D. Mobile ion concentration measurement and open-access band diagram simulation platform for halide perovskite solar cells. Joule 2020, 4, 109–127. [Google Scholar] [CrossRef]
- Schmidt, M.C.; Ehrler, B. How Many Mobile Ions Can Electrical Measurements Detect in Perovskite Solar Cells? ACS Energy Lett. 2025, 10, 2457–2460. [Google Scholar] [CrossRef]
- Thiesbrummel, J.; Shah, S.; Gutierrez-Partida, E.; Zu, F.; Peña-Camargo, F.; Zeiske, S.; Diekmann, J.; Ye, F.; Peters, K.P.; Brinkmann, K.O. Ion-induced field screening as a dominant factor in perovskite solar cell operational stability. Nat. Energy 2024, 9, 664–676. [Google Scholar] [CrossRef]
- Baptayev, B.; Tashenov, Y.; Aliakbarova, A.; Adilov, S.; Balanay, M. Ternary NiCuS electrocatalyst for iodide/triiodide reduction in dye-sensitized solar cells. Mater. Today Proc. 2022, 71, 94–99. [Google Scholar] [CrossRef]
- Frolova, L.A.; Dremova, N.N.; Troshin, P.A. The chemical origin of the p-type and n-type doping effects in the hybrid methylammonium–lead iodide (MAPbI 3) perovskite solar cells. ChemComm 2015, 51, 14917–14920. [Google Scholar]
- Wu, S.; Yan, Y.; Yin, J.; Jiang, K.; Li, F.; Zeng, Z.; Tsang, S.-W.; Jen, A.K.-Y. Redox mediator-stabilized wide-bandgap perovskites for monolithic perovskite-organic tandem solar cells. Nat. Energy 2024, 9, 411–421. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, Y.; Juarez-Perez, E.J.; Ono, L.K.; Qi, Y. Accelerated degradation of methylammonium lead iodide perovskites induced by exposure to iodine vapour. Nat. Energy 2017, 2, 16195. [Google Scholar] [CrossRef]
- Liang, J.; Hu, X.; Wang, C.; Liang, C.; Chen, C.; Xiao, M.; Li, J.; Tao, C.; Xing, G.; Yu, R. Origins and influences of metallic lead in perovskite solar cells. Joule 2022, 6, 816–833. [Google Scholar] [CrossRef]
- Gao, Y.; Raza, H.; Zhang, Z.; Chen, W.; Liu, Z. Rethinking the role of excess/residual lead iodide in perovskite solar cells. Adv. Funct. Mater. 2023, 33, 2215171. [Google Scholar] [CrossRef]
- Ren, X.; Wang, J.; Lin, Y.; Wang, Y.; Xie, H.; Huang, H.; Yang, B.; Yan, Y.; Gao, Y.; He, J. Mobile iodides capture for highly photolysis-and reverse-bias-stable perovskite solar cells. Nat. Mater 2024, 23, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Kerner, R.A.; Xu, Z.; Larson, B.W.; Rand, B.P. The role of halide oxidation in perovskite halide phase separation. Joule 2021, 5, 2273–2295. [Google Scholar] [CrossRef]
- Kim, G.Y.; Senocrate, A.; Yang, T.-Y.; Gregori, G.; Grätzel, M.; Maier, J. Large tunable photoeffect on ion conduction in halide perovskites and implications for photodecomposition. Nat. Mater. 2018, 17, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yavuz, I.; Wang, M.; Weber, M.H.; Xu, M.; Lee, J.-H.; Tan, S.; Huang, T.; Meng, D.; Wang, R. Suppressing ion migration in metal halide perovskite via interstitial doping with a trace amount of multivalent cations. Nat. Mater. 2022, 21, 1396–1402. [Google Scholar] [CrossRef]
- Ito, S.; Tanaka, S.; Manabe, K.; Nishino, H. Effects of surface blocking layer of Sb2S3 on nanocrystalline TiO2 for CH3NH3PbI3 perovskite solar cells. J. Phys. Chem. C 2014, 118, 16995–17000. [Google Scholar] [CrossRef]
- Gong, X.; He, A.; Tang, P.; Hao, X.; Wu, L.; Wang, W.; Zhang, J. DL-Serine Hydrazide Hydrochloride Multiple-site Synergy Induced Effective and Stable Formamidine-Rich Perovskite Solar Cells. Small 2024, 20, 2401877. [Google Scholar] [CrossRef]
- Hu, J.; Xu, Z.; Murrey, T.L.; Pelczer, I.; Kahn, A.; Schwartz, J.; Rand, B.P. Triiodide attacks the organic cation in hybrid lead halide perovskites: Mechanism and suppression. Adv. Mater. 2023, 35, 2303373. [Google Scholar] [CrossRef]
- Hu, J.; Ahn, J.W.; Xu, Z.; Jeong, M.J.; Kim, C.; Noh, J.H.; Min, H.; Rand, B.P. Iodine modulates the MACl-assisted growth of FAPbI3 for high efficiency perovskite solar cells. Adv. Energy Mater. 2024, 14, 2400500. [Google Scholar] [CrossRef]
- Ju, Y.; Hu, X.; Wu, X.-g.; Wang, C.; Baranov, A.; Pushkarev, A.; Zhong, H. The interactions between halide perovskites and oxygen: From stages to strategies. Matter 2024, 7, 3756–3785. [Google Scholar] [CrossRef]
- Aristidou, N.; Eames, C.; Sanchez-Molina, I.; Bu, X.; Kosco, J.; Islam, M.S.; Haque, S.A. Fast oxygen diffusion and iodide defects mediate oxygen-induced degradation of perovskite solar cells. Nat. Commun. 2017, 8, 15218. [Google Scholar] [CrossRef]
- Zhou, Q.; Gao, Y.; Cai, C.; Zhang, Z.; Xu, J.; Yuan, Z.; Gao, P. Dually-passivated perovskite solar cells with reduced voltage loss and increased super oxide resistance. Angew. Chem. 2021, 60, 8303–8312. [Google Scholar] [CrossRef]
- Liao, L.; Jin, B.; Guo, Z.; Zhao, Y.; Zheng, T.; Fan, L.; Wang, C.; Peng, R. Generation and Clearance of Superoxide Radicals at Buried Interfaces of Perovskites with Different Crystal Structures. Small 2024, 20, 2404677. [Google Scholar] [CrossRef]
- Ding, B.; Ding, Y.; Peng, J.; Romano-deGea, J.; Frederiksen, L.E.; Kanda, H.; Syzgantseva, O.A.; Syzgantseva, M.A.; Audinot, J.-N.; Bour, J. Dopant-additive synergism enhances perovskite solar modules. Nature 2024, 628, 299–305. [Google Scholar] [CrossRef]
- Jiang, Q.; Tong, J.; Xian, Y.; Kerner, R.A.; Dunfield, S.P.; Xiao, C.; Scheidt, R.A.; Kuciauskas, D.; Wang, X.; Hautzinger, M.P. Surface reaction for efficient and stable inverted perovskite solar cells. Nature 2022, 611, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Perez, E.J.; Ono, L.K.; Maeda, M.; Jiang, Y.; Hawash, Z.; Qi, Y. Photodecomposition and thermal decomposition in methylammonium halide lead perovskites and inferred design principles to increase photovoltaic device stability. J. Mater. Chem. A 2018, 6, 9604–9612. [Google Scholar] [CrossRef]
- Xiao, G.-B.; Wang, L.-Y.; Mu, X.-J.; Zou, X.-X.; Wu, Y.-Y.; Cao, J. Lead and iodide fixation by thiol copper (II) porphyrin for stable and environmental-friendly perovskite solar cells. CCS Chem. 2021, 3, 25–36. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.; Liu, A.; Lu, C.; Yuan, H.; Zhang, W.; Fang, J. Iodine-trapping strategy for light-heat stable inverted perovskite solar cells under ISOS protocols. Energy Environ. Sci. 2023, 16, 6071–6077. [Google Scholar] [CrossRef]
- Luo, D.; He, Y.; Tian, J.; Sessler, J.L.; Chi, X. Reversible iodine capture by nonporous adaptive crystals of a bipyridine cage. J. Am. Chem. Soc. 2021, 144, 113–117. [Google Scholar] [CrossRef]
- Ma, X.; Huang, J.; Li, X.; Yu, H.; Liu, Z.; Hu, Y.; Sun, T.; Shen, Y.; Wang, M. Enhancing the Stability of Perovskite Solar Cells Through an Iodine Confinement Strategy in Covalent Organic Frameworks. Adv. Funct. Mater. 2025, 35, 2501623. [Google Scholar] [CrossRef]
- Kang, D.-H.; Ma, C.; Park, N.-G. Antiseptic povidone–iodine heals the grain boundary of perovskite solar cells. ACS Appl. Mater. Interfaces 2022, 14, 8984–8991. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Q.; Liu, G.; Chen, Y.; Guo, Z.; Li, N.; Niu, X.; Qiu, Z.; Zhou, W.; Huang, Z. Improved fatigue behaviour of perovskite solar cells with an interfacial starch–polyiodide buffer layer. Nat. Photonics 2023, 17, 1066–1073. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, X.; Gu, H.; Huang, J. Iodine reduction for reproducible and high-performance perovskite solar cells and modules. Sci. Adv. 2021, 7, eabe8130. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, N.; Wang, L.; Li, L.; Xu, Z.; Jiao, H.; Liu, P.; Zhu, C.; Zai, H.; Sun, M. Impacts of alkaline on the defects property and crystallization kinetics in perovskite solar cells. Nat. Commun. 2019, 10, 1112. [Google Scholar] [CrossRef]
- Meng, H.; Mao, K.; Cai, F.; Zhang, K.; Yuan, S.; Li, T.; Cao, F.; Su, Z.; Zhu, Z.; Feng, X. Inhibition of halide oxidation and deprotonation of organic cations with dimethylammonium formate for air-processed p–i–n perovskite solar cells. Nat. Energy 2024, 9, 536–547. [Google Scholar] [CrossRef]
- Sun, D.; Gao, Y.; Raza, H.; Liu, S.; Ren, F.; Hu, X.; Wang, H.; Meng, X.; Wang, J.; Chen, R. Chemical reduction of iodine impurities and defects with potassium formate for efficient and stable perovskite solar cells. Adv. Funct. Mater. 2023, 33, 2303225. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Zhu, L.; Leng, S.-B.; Liang, J.; Zheng, Y.; Zhang, Z.; Zhang, Z.; Liu, X.X.; Liu, F. Favorable grain growth of thermally stable formamidinium-methylammonium perovskite solar cells by hydrazine chloride. Chem. Eng. J. 2022, 430, 132730. [Google Scholar] [CrossRef]
- Li, M.; Gao, D.; Zhang, B.; Xu, S.; Zhuang, X.; Wang, C.; Yang, L.; Ma, X.; Zheng, S.; Song, H. Multifunctional reductive molecular modulator toward efficient and stable perovskite solar cells. Solar Rrl 2021, 5, 2100320. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.; Hu, J.; Huang, B.; Sun, M.; Dong, B.; Zheng, G.; Huang, Y.; Chen, Y.; Li, L. A Eu3+-Eu2+ ion redox shuttle imparts operational durability to Pb-I perovskite solar cells. Science 2019, 363, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Sun, K.; Wang, Y.; Liu, C.; Meng, Y.; Lang, X.; Xiao, C.; Tian, R.; Song, Z.; Zhu, Z. Dynamic Reversible Oxidation-Reduction of Iodide Ions for Operationally Stable Perovskite Solar Cells under ISOS-L-3 Protocol. Adv. Mater. 2024, 36, 2400852. [Google Scholar] [CrossRef]
- Wang, J.; Dai, X.-Y.; Bi, L.; Sun, J.; Liu, M.; Ji, X.; Lin, F.R.; Fu, Q.; Jen, A.K.-Y. Synergistic iodine and lead chelation with redox cycling via supramolecular engineering for stable and sustainable perovskite solar cells. Joule 2025, 9, 102105. [Google Scholar] [CrossRef]
- Luo, J.; Liu, B.; Yin, H.; Zhou, X.; Wu, M.; Shi, H.; Zhang, J.; Elia, J.; Zhang, K.; Wu, J. Polymer-acid-metal quasi-ohmic contact for stable perovskite solar cells beyond a 20,000-hour extrapolated lifetime. Nat. Commun. 2024, 15, 2002. [Google Scholar] [CrossRef]
- Liu, R.; Li, X.; Liu, Y.; Zhang, Z.; Wu, M. Accelerated Aging Method of Performance Attenuation of Crystalline Silicon Photovoltaic Modules Under Full-Spectrum Conditions. Materials 2025, 18, 1507. [Google Scholar] [CrossRef] [PubMed]
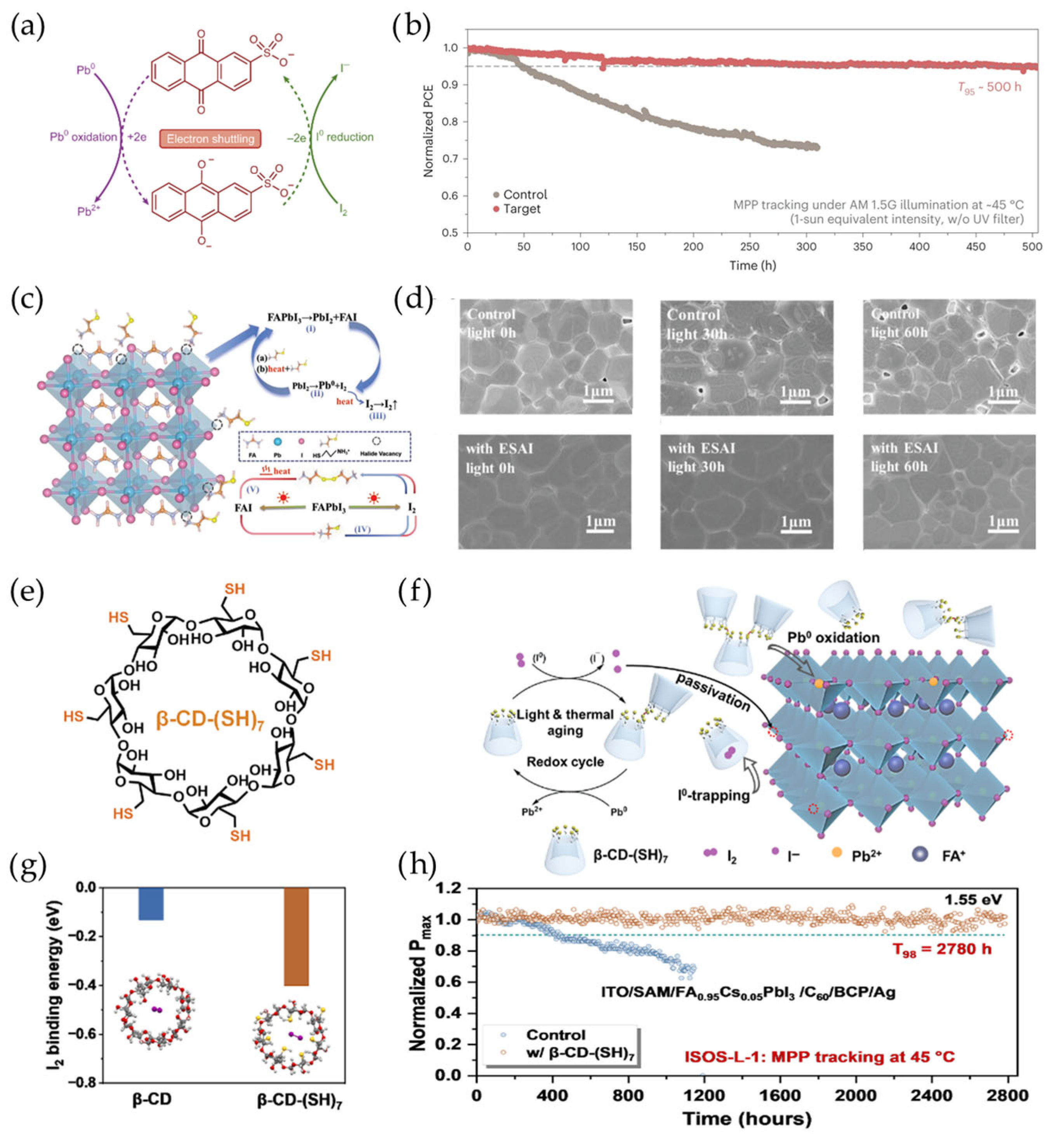
| Operational Stability Test Conditions | Initial PCE | Lifetime | Device Structure | References |
|---|---|---|---|---|
| 55 °C, 85%RH | 24.7% | T98 = 1000 h | 2PACz/FA0.84 Cs0.05MA0.11Pb(I0.987Br0.013)3 /C60/SnO2/Ag | Dong, B. et al. Nat. Energy. 10, 342–353 (2025) [15] |
| 85 °C, 60%RH | 23.0% | T95 = 2000 h | SnO2/FA0.96Cs0.04PbI3 /HTL(Unknown)/Au | Li, S. et al. Nature. 635, 82–88 (2024) [16] |
| Unknown | 20.1% | T95 = 1600 h | SAM/FA0.87Cs0.13Pb(I0.9Br0.1)3 /PCBM/BCP/Cr/Au | Lin, Y.-H. et al. Science. 384, 767–775 (2024) [17] |
| 65 °C, 50%RH | 23.2% | T95 = 1200 h | SAM/Cs0.05MA0.1FA0.85PbI3/C60/SnO2/Ag | Chen, H. et al. Science. 384, 189–193 (2024) [18] |
| 25 °C, 85%RH | 23.8% | T90 = 1000 h | NiOX/FA0.9Cs0.1PbI3/C60/BCP/Ag | Chen, J. et al. Nat. Energy. 10, 181–190 (2025) [19] |
| 40 °C, 45%RH | ~23.5% | T90 = 1142 h | SAM/FA0.85Cs0.05MA0.05Rb0.05Pb (I0.95Br0.05)3/PCBM/BCP/Cr/Au | Li, S. et al. Nature. 635, 874–881 (2024) [20] |
| 60 °C | 25.1% | T97 = 1800 h | PTAA/FA0.85Cs0.05MA0.1Pb(I0.97Br0.03)3 /C60/BCP/Cu | Zhu, H. et al. Nat. Photonics. 1–8 (2024) [21] |
| 45 °C, N2 | 25.95% | T92 = 500 h | Unknown | Li, Q. et al. Nat. Energy. 9, 1506–1516 (2024) [22] |
| 45 °C | 25.5% | T88 = 500 h | TiO2/(FAPbI3)0.97(MAPbBr3)0.03 /Spiro-OMeTAD/Au | Zhao, C. et al. Nat. Commun. 15, 7139 (2024) [23] |
| 85 °C, 50%RH | ~20.0% | T100 = 2000 h | NiOX/FA0.79Cs0.05MA0.16Pb3 /C60/BCP/Cu | Yang, Y. et al. Nat. Energy. 9, 37–46 (2024) [24] |
| 65 °C, 50%RH | ~22.0% | T94 = 1200 h | NiOX/FA0.90Cs0.04MA0.06Pb3 /C60/BCP/Cu | Zhu, X. et al. Adv. Mater. 36, 2409340 (2024) [25] |
| 25 °C, N2 | 22.7% | T99 = 4500 h | TiO2/FAPbI3/PTAA/Au | Suo, J. et al. Nat. Energy. 9, 172–183 (2024) [26] |
| 85 °C, 85%RH | 23.2% | T87 = 1900 h | TiO2/SnO2/FA0.9Cs0.05MA0.05PbI3/ Spiro-OMeTAD/MoOx/ITO/Au | Ding, Y. et al. Science. 386, 531–538 (2024) [27] |
| 40 °C, 50%RH | ~23.0% | T90 = 1000 h | 2PACz/FA0.95Cs0.05PbI3/C60/SnO2/IZO/Cu | Azmi, R. et al. Nature.628, 93–98 (2024) [28] |
| 65 °C, 50%RH | 23.5% | T96 = 2000 h | NiOX/FA0.90Cs0.05MA0.05Pb3 /C60/BCP/Ag | Liu, C. et al. Science. 382, 810–815 (2023) [5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bì, H.; Wang, Z.; Xu, Z. A Review of the Intrinsic Chemical Stability Challenge in Operational Perovskite Photovoltaics. Materials 2025, 18, 4776. https://doi.org/10.3390/ma18204776
Bì H, Wang Z, Xu Z. A Review of the Intrinsic Chemical Stability Challenge in Operational Perovskite Photovoltaics. Materials. 2025; 18(20):4776. https://doi.org/10.3390/ma18204776
Chicago/Turabian StyleBì, Huān, Zhen Wang, and Zhenhua Xu. 2025. "A Review of the Intrinsic Chemical Stability Challenge in Operational Perovskite Photovoltaics" Materials 18, no. 20: 4776. https://doi.org/10.3390/ma18204776
APA StyleBì, H., Wang, Z., & Xu, Z. (2025). A Review of the Intrinsic Chemical Stability Challenge in Operational Perovskite Photovoltaics. Materials, 18(20), 4776. https://doi.org/10.3390/ma18204776







