Interaction of PCE and Chemically Modified Starch Admixtures with Metakaolin-Based Geopolymers—The Role of Activator Type and Concentration
Abstract
1. Introduction
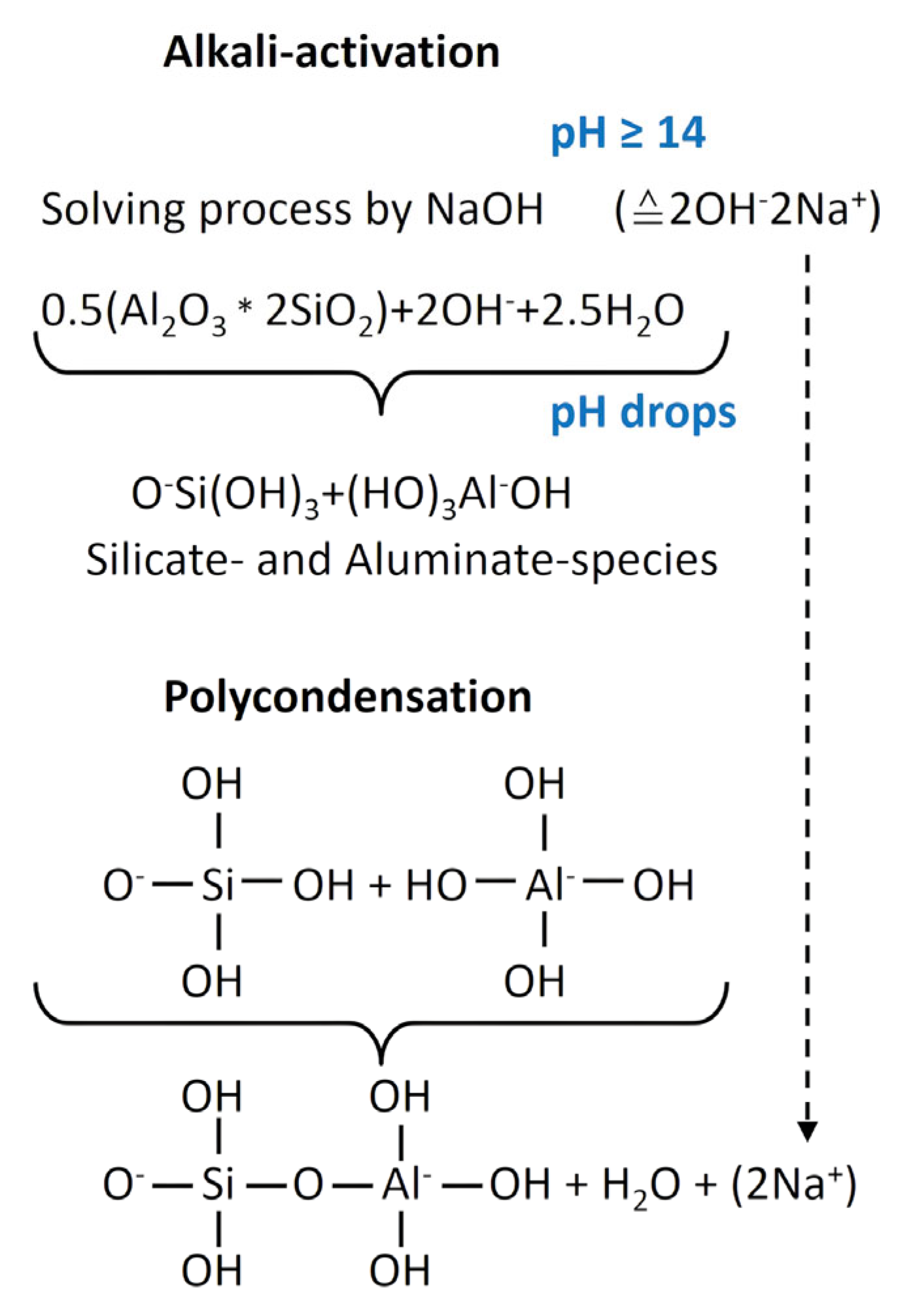
2. Materials and Methods
2.1. Materials
2.1.1. Metakaolin
2.1.2. Alkaline Activators
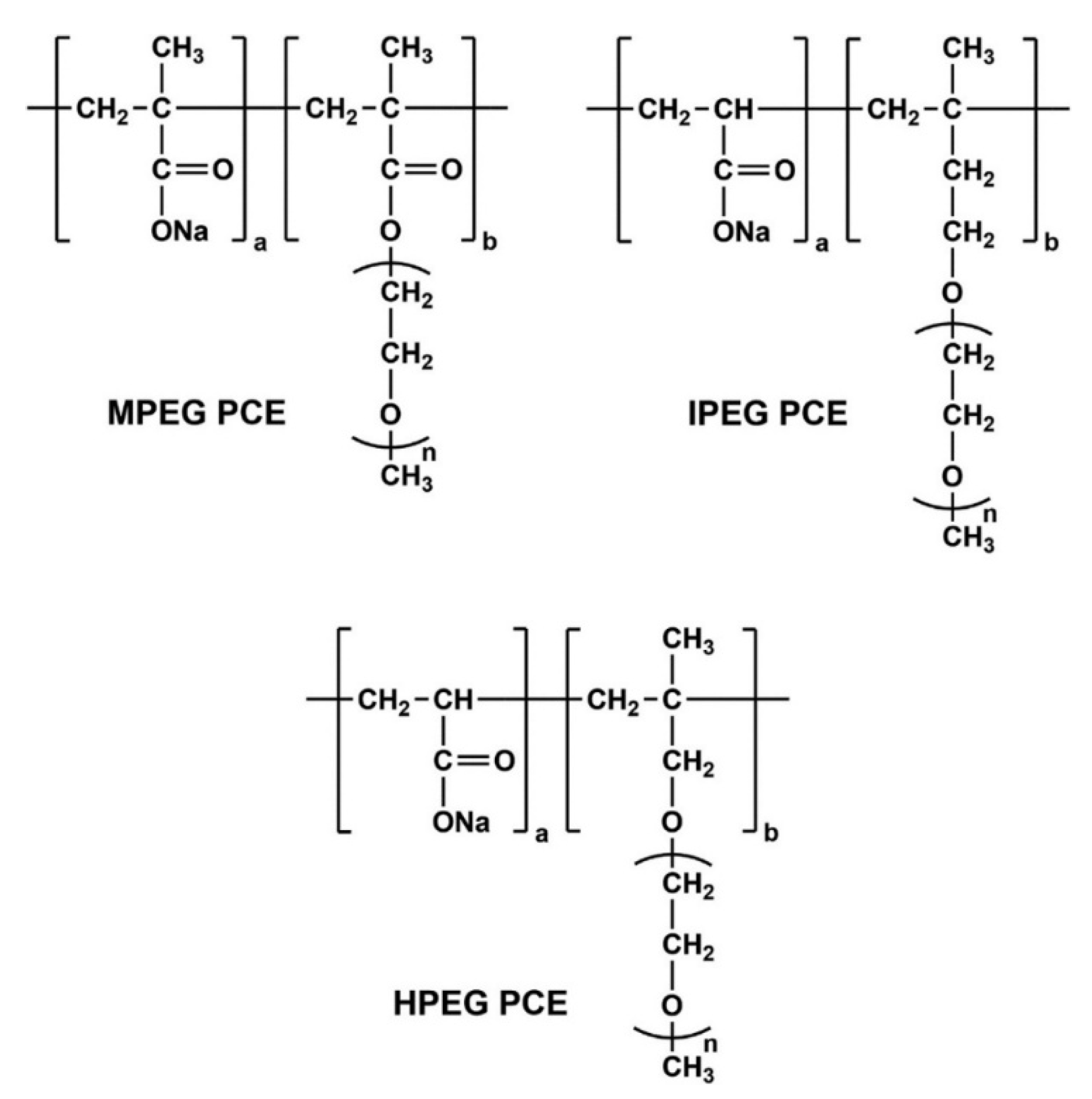
2.1.3. PCE Superplasticizers
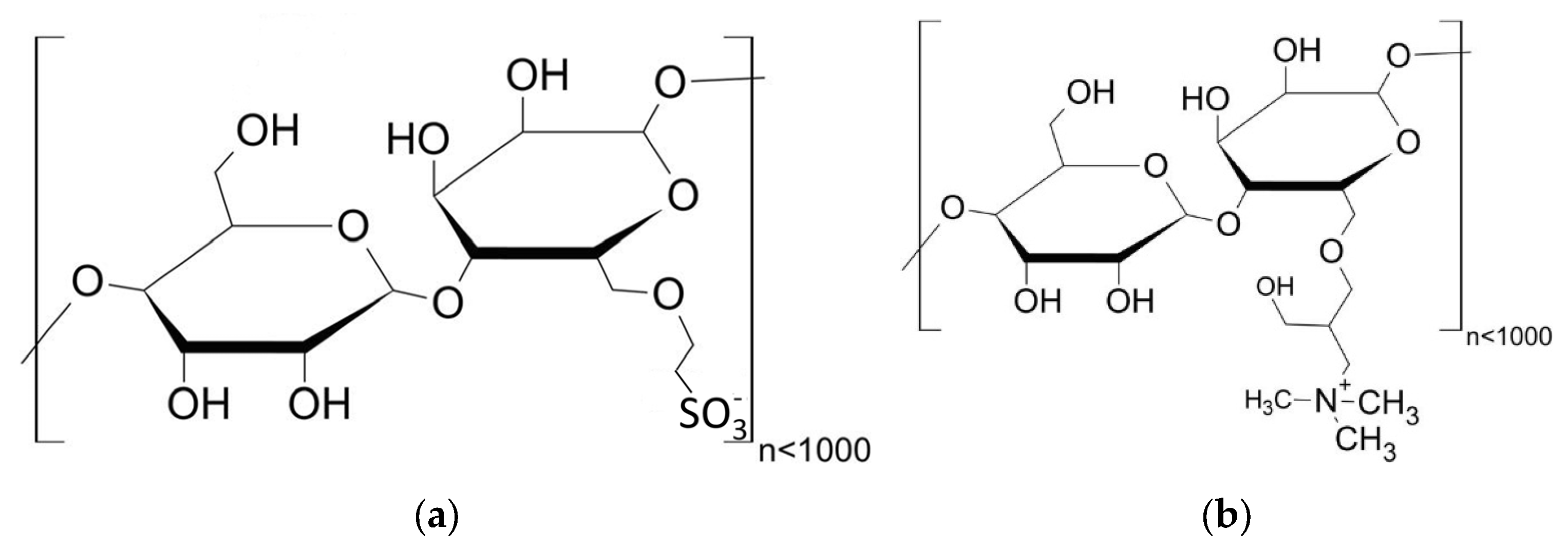
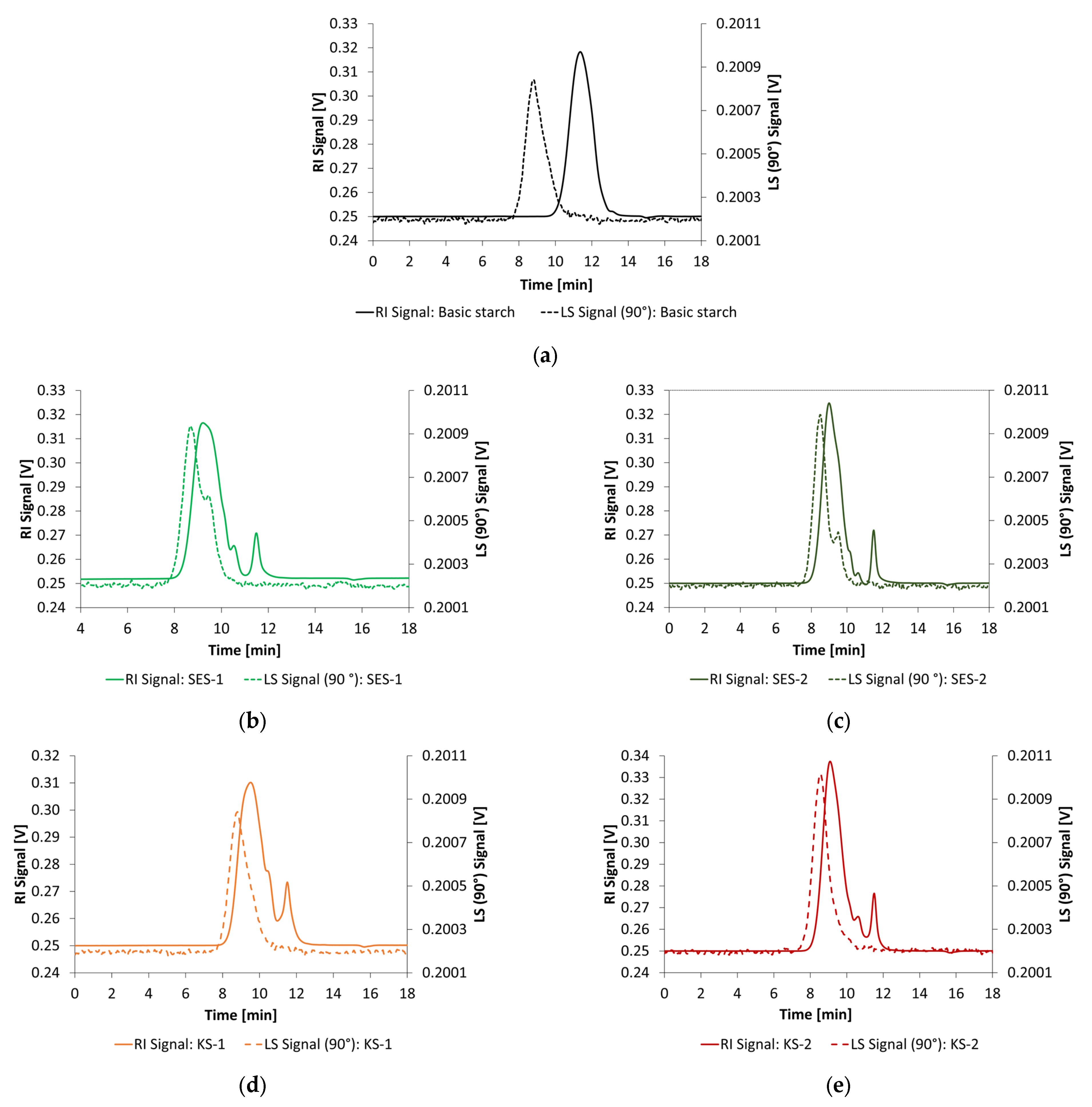
2.1.4. Preparation of Anionic and Cationic Starch Admixtures
2.1.5. Fine Aggregates
2.2. Methods
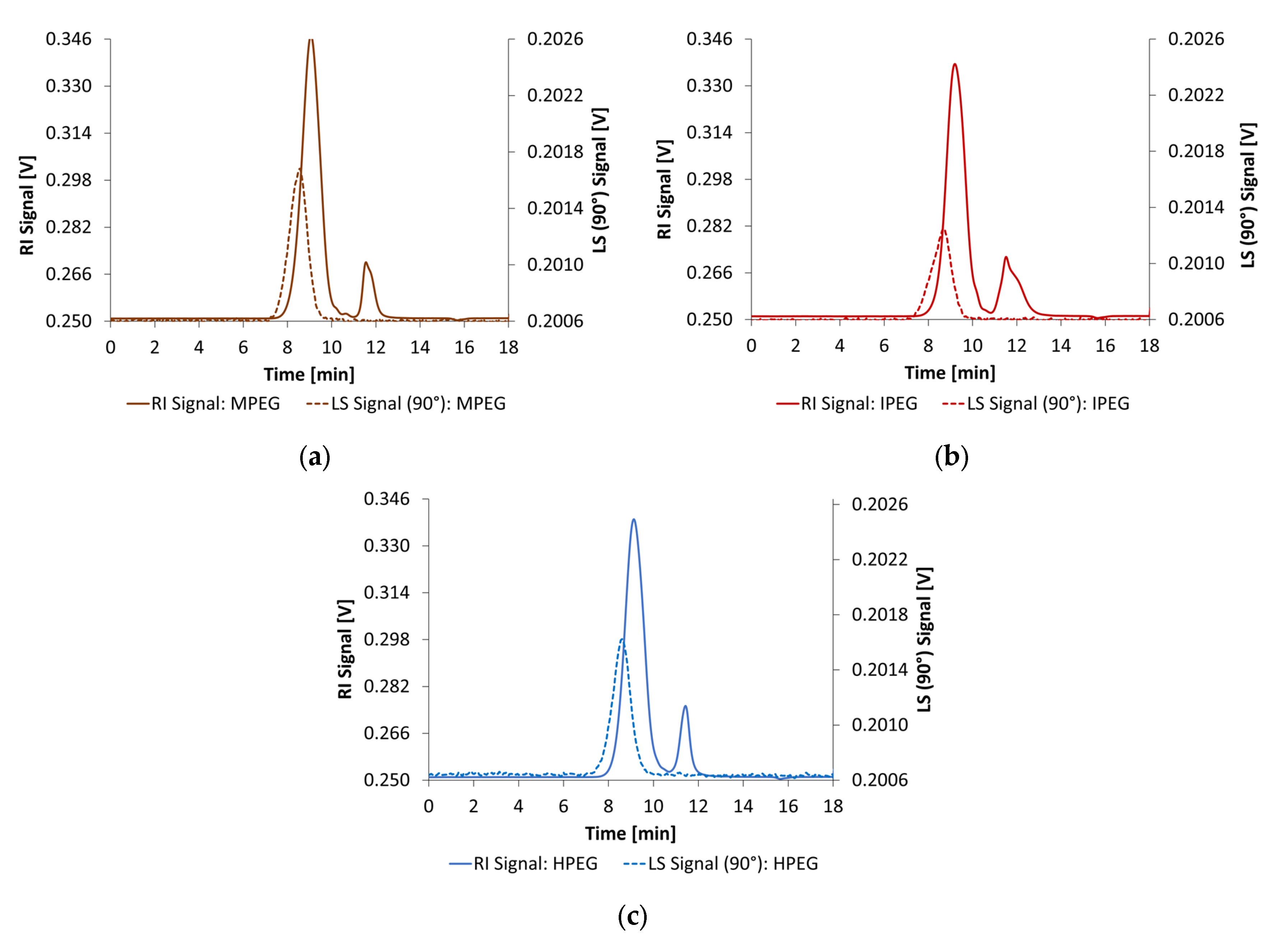
2.2.1. Dynamic Light Scattering
2.2.2. Solubility of Metakaolin in Activator Solutions
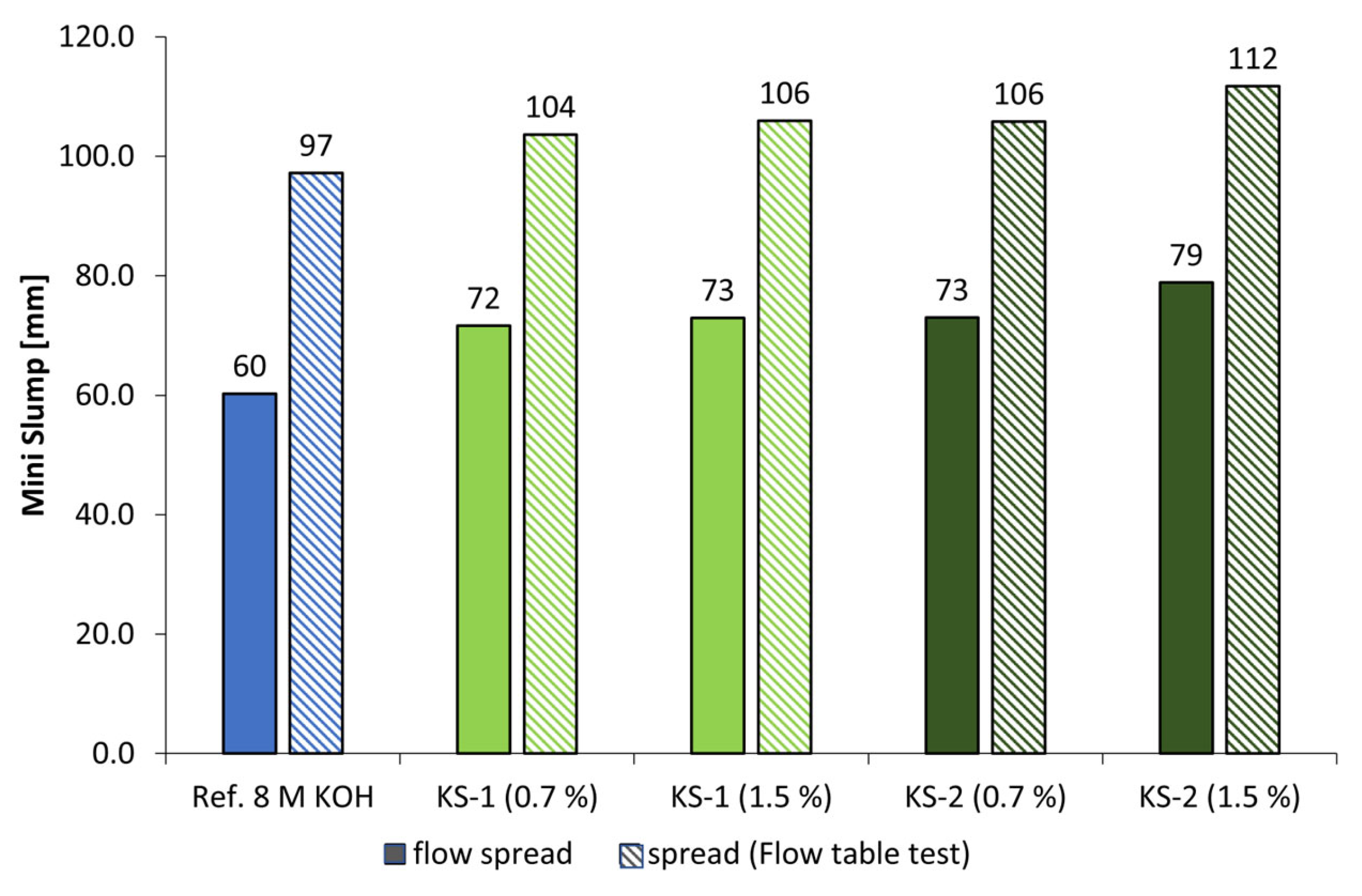
2.2.3. Mini Slump and Flow Table Test
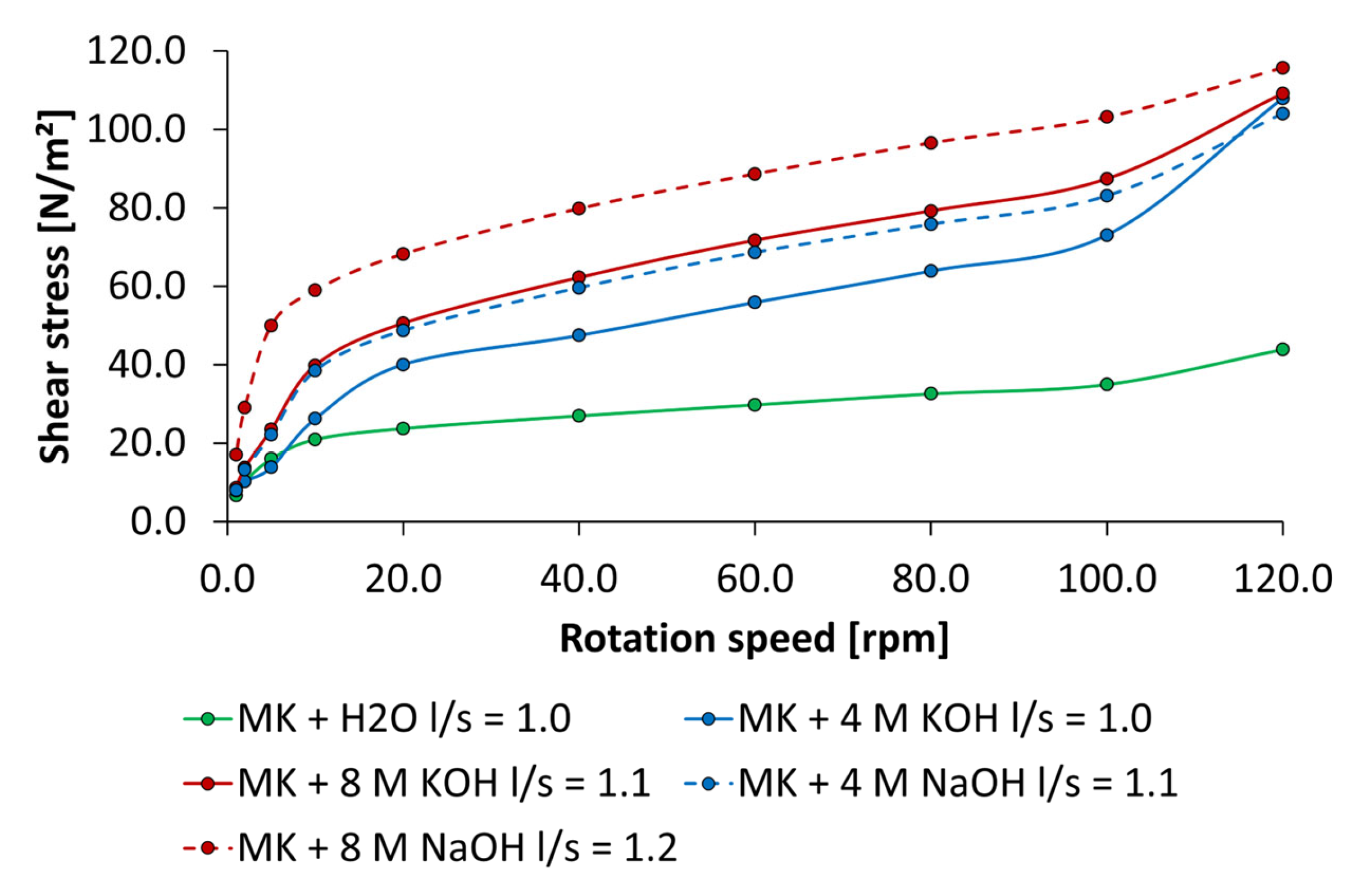
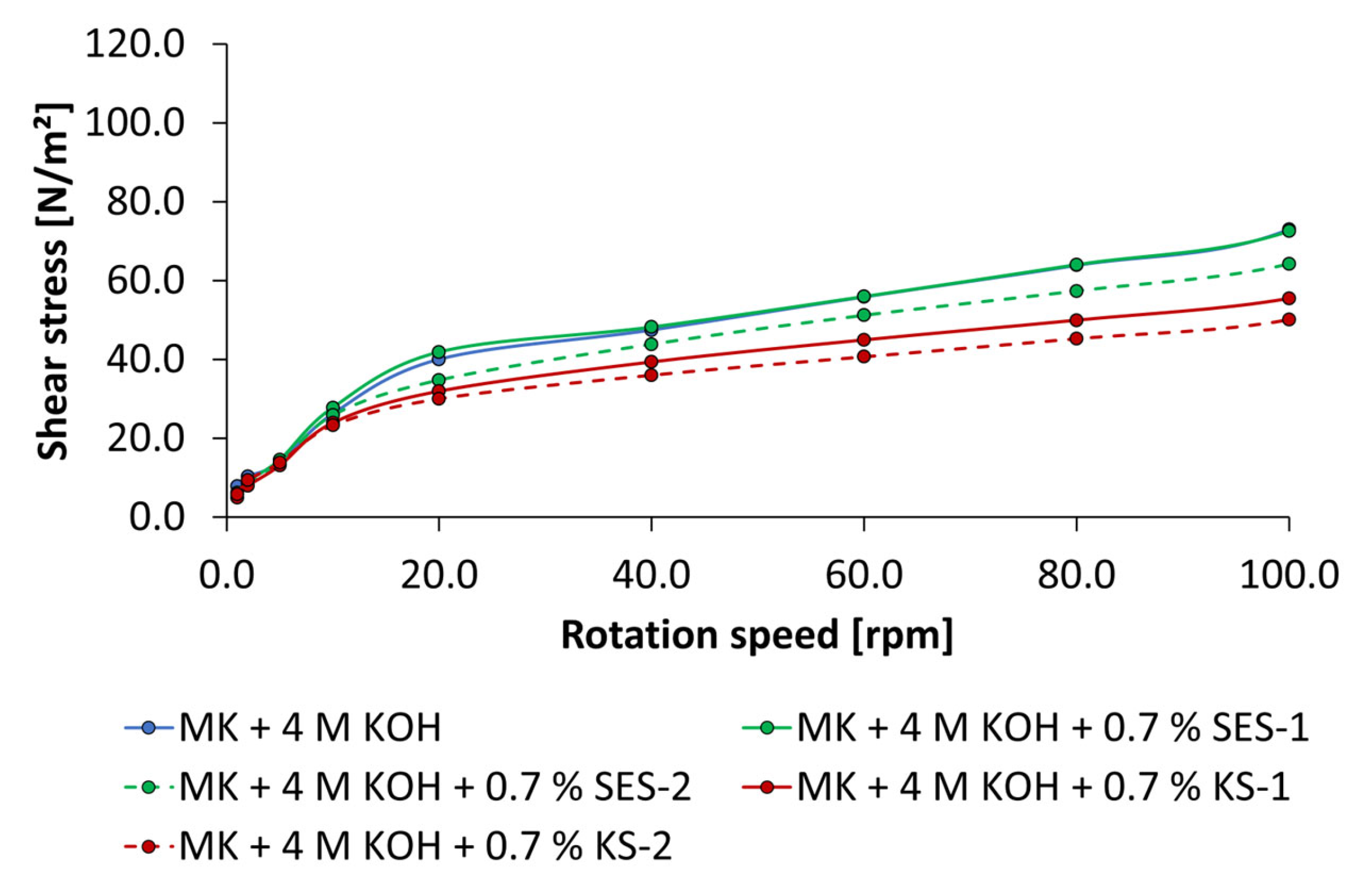
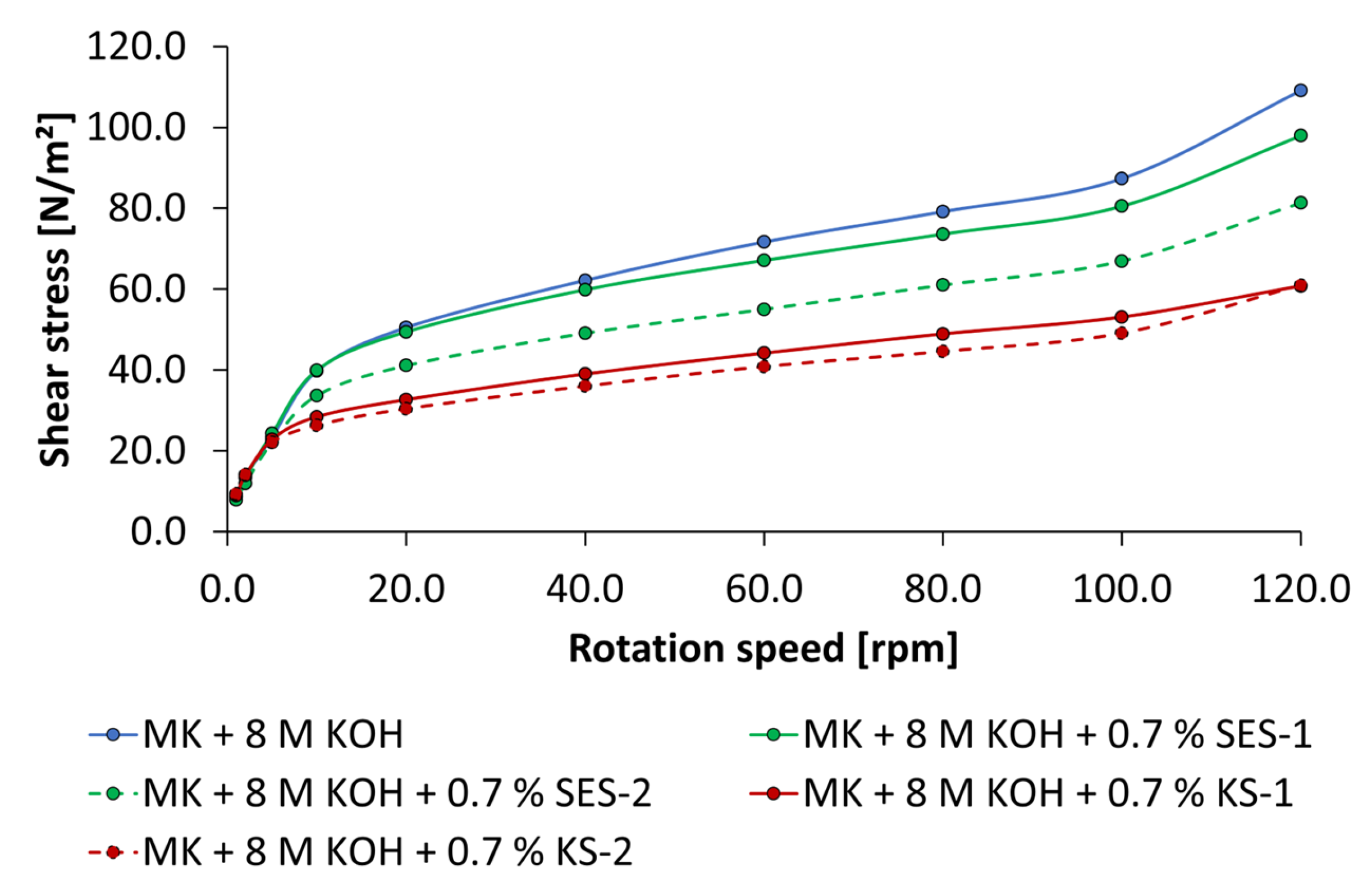
2.2.4. Rheological Tests by Rotational Viscosimeter
2.2.5. Adsorption Experiments
2.2.6. Influence of Admixture on Geopolymer Reaction
2.2.7. Mechanical Properties
3. Results and Discussion
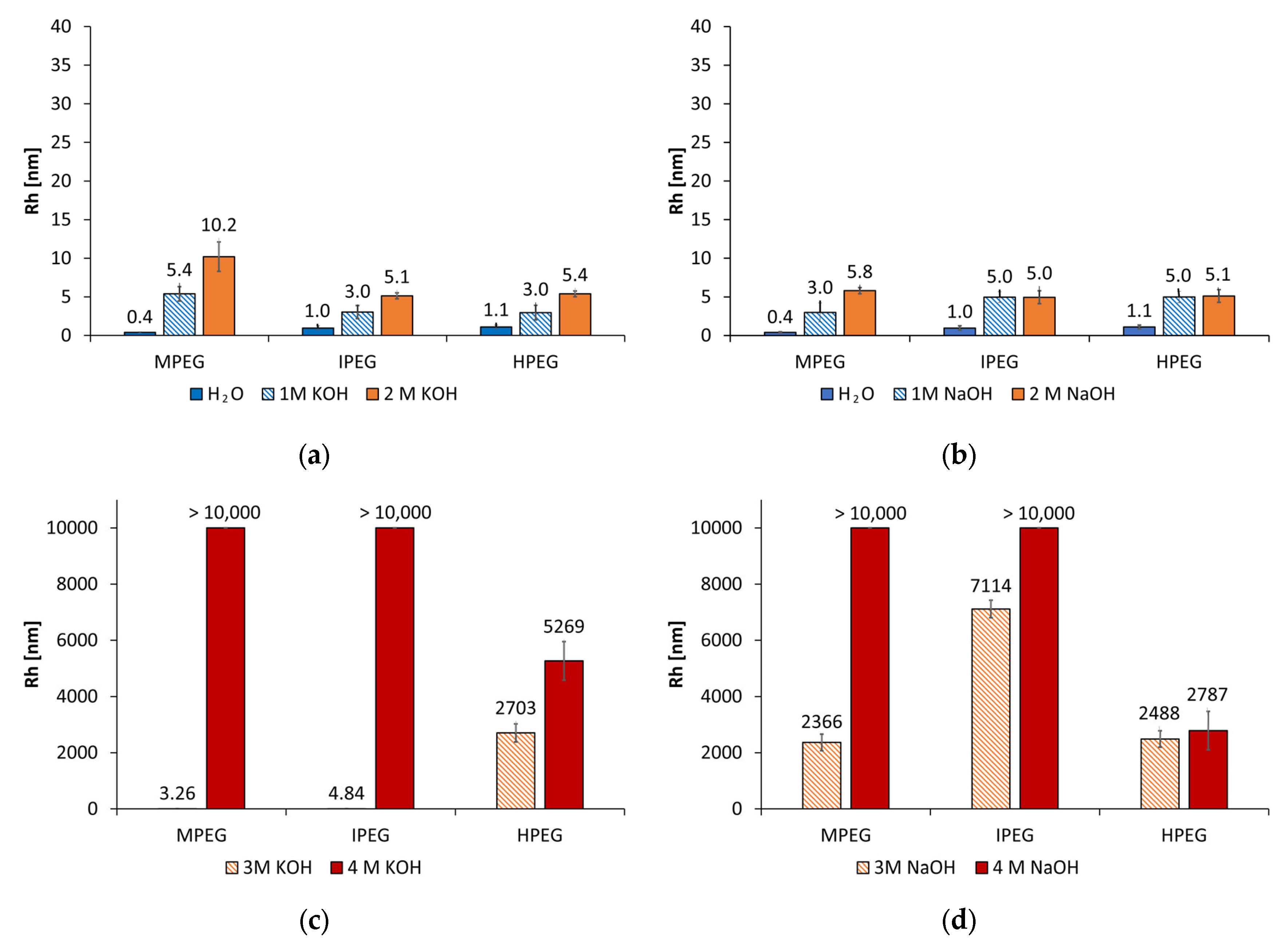
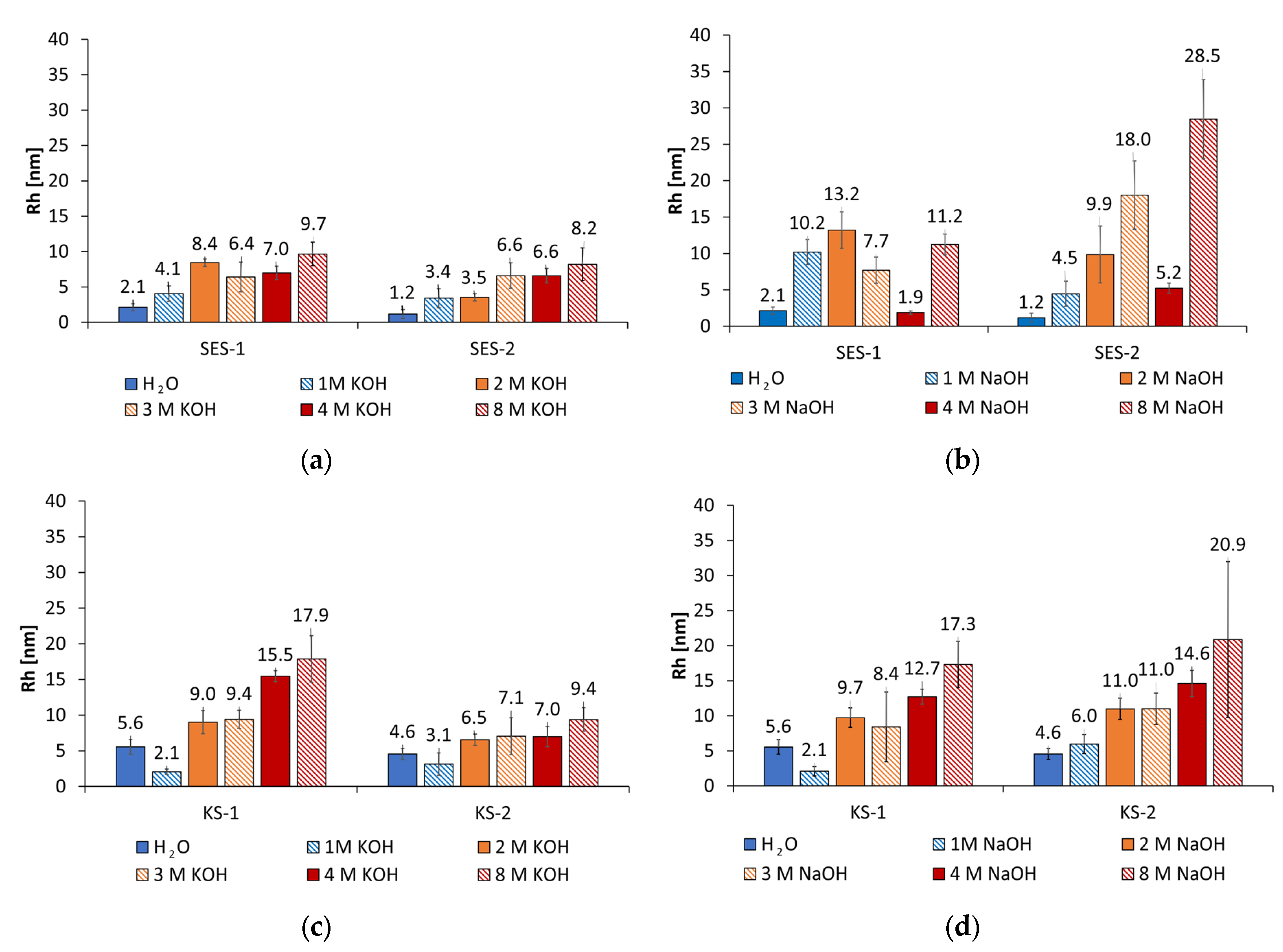
3.1. Size Analysis of the PCE and Synthesized Starch Admixtures in Alkaline Activators
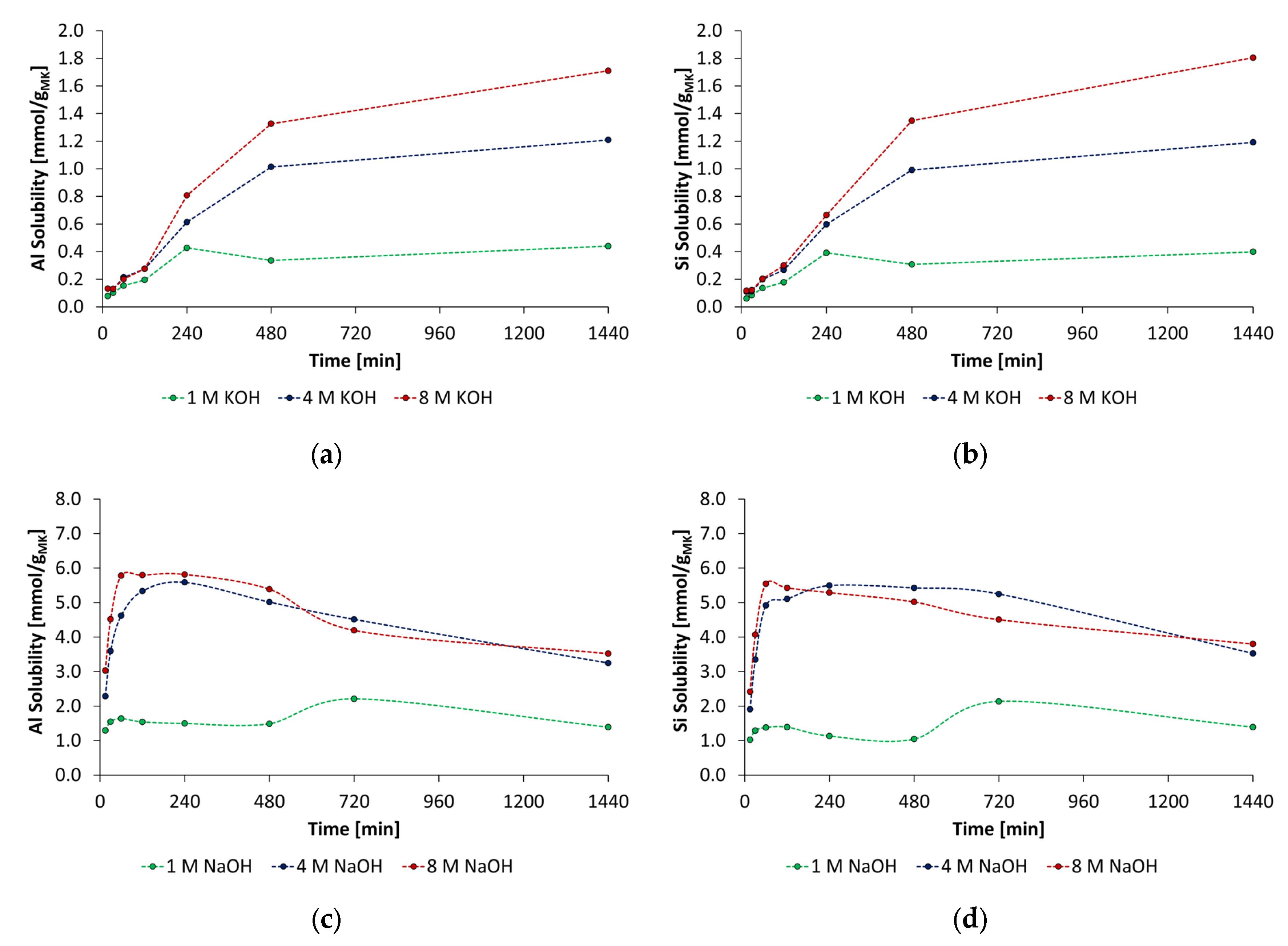
3.2. Solubility of Metakaolin in the Alkaline Activator Solutions
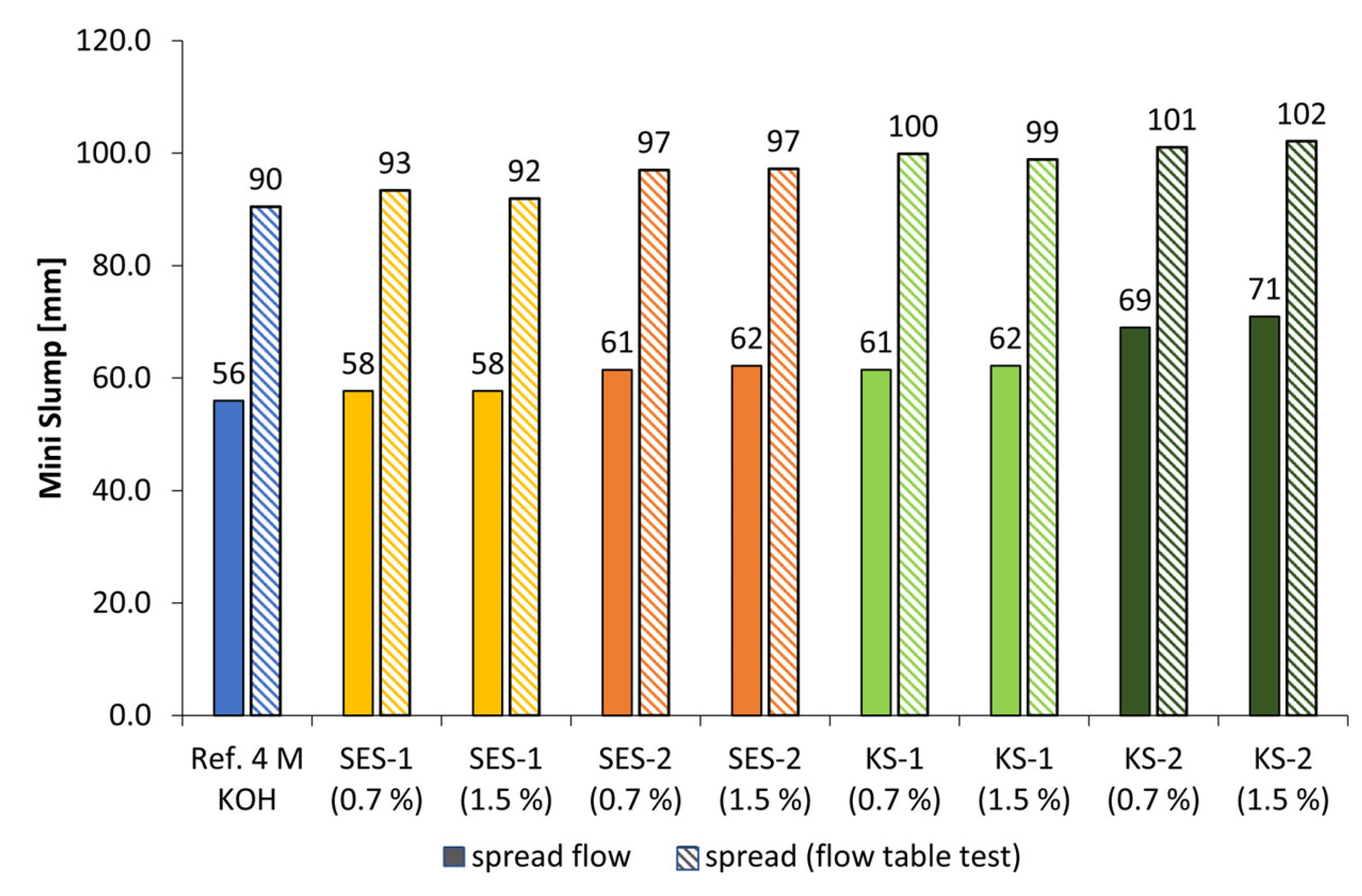
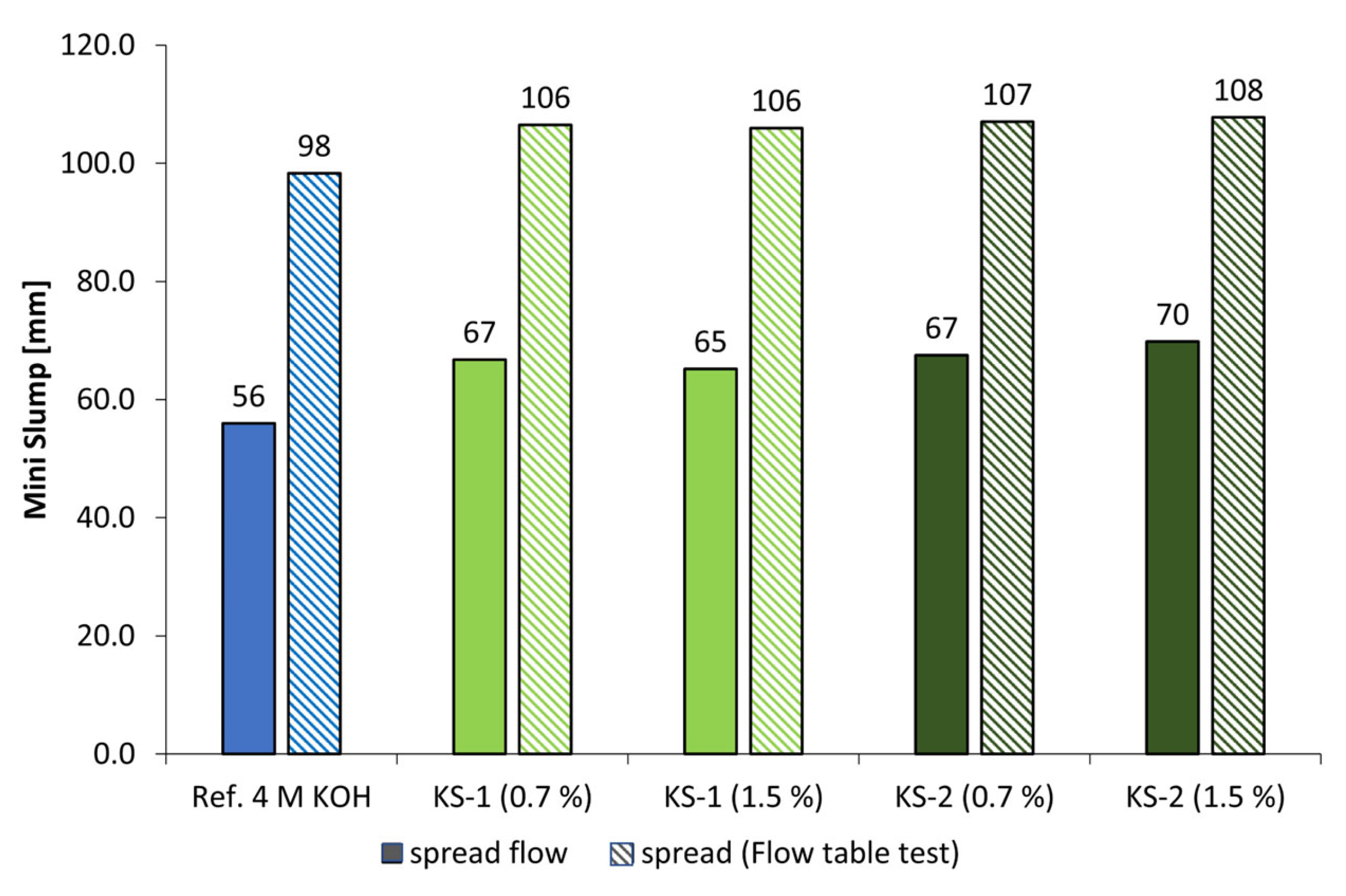
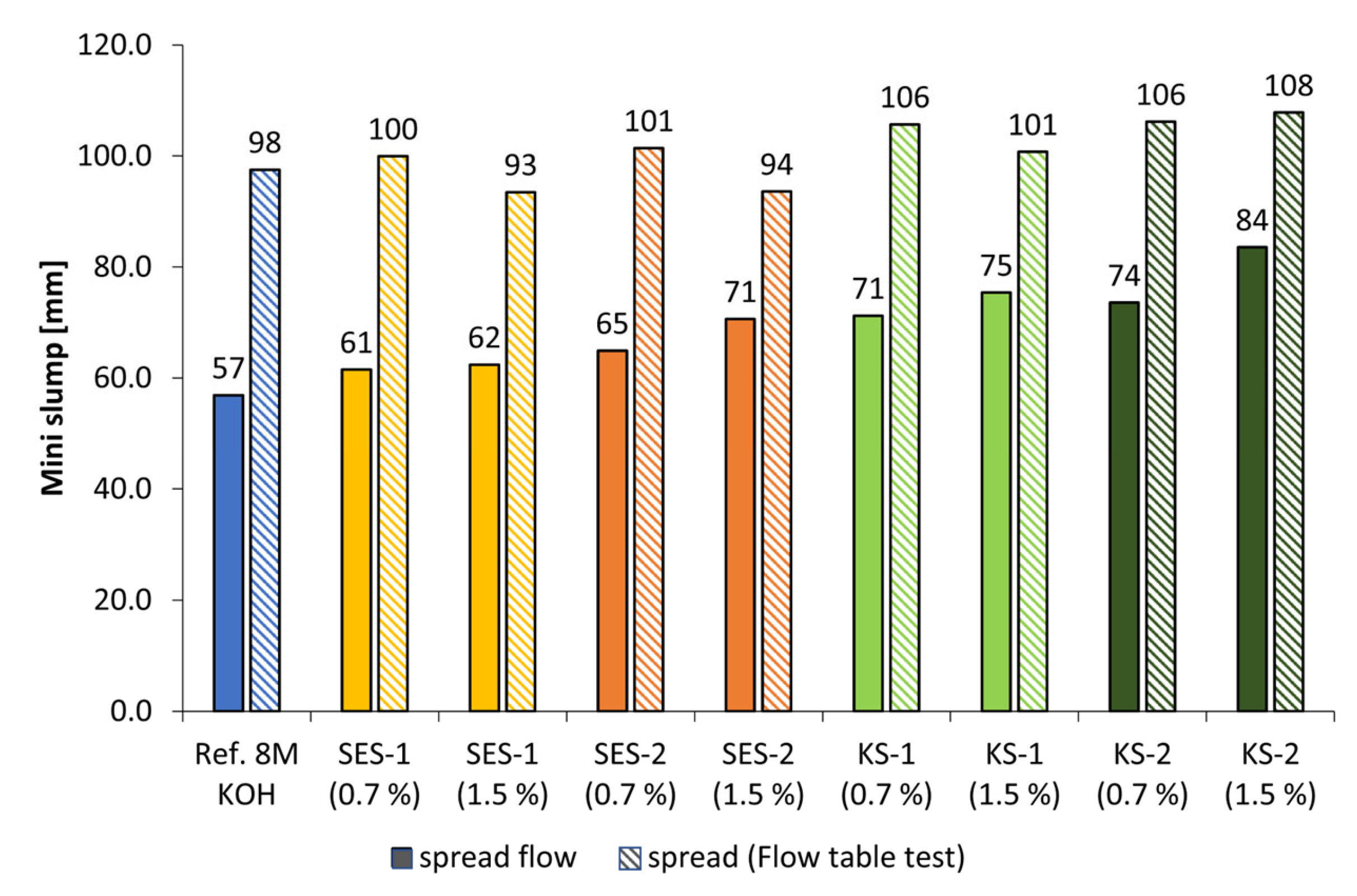
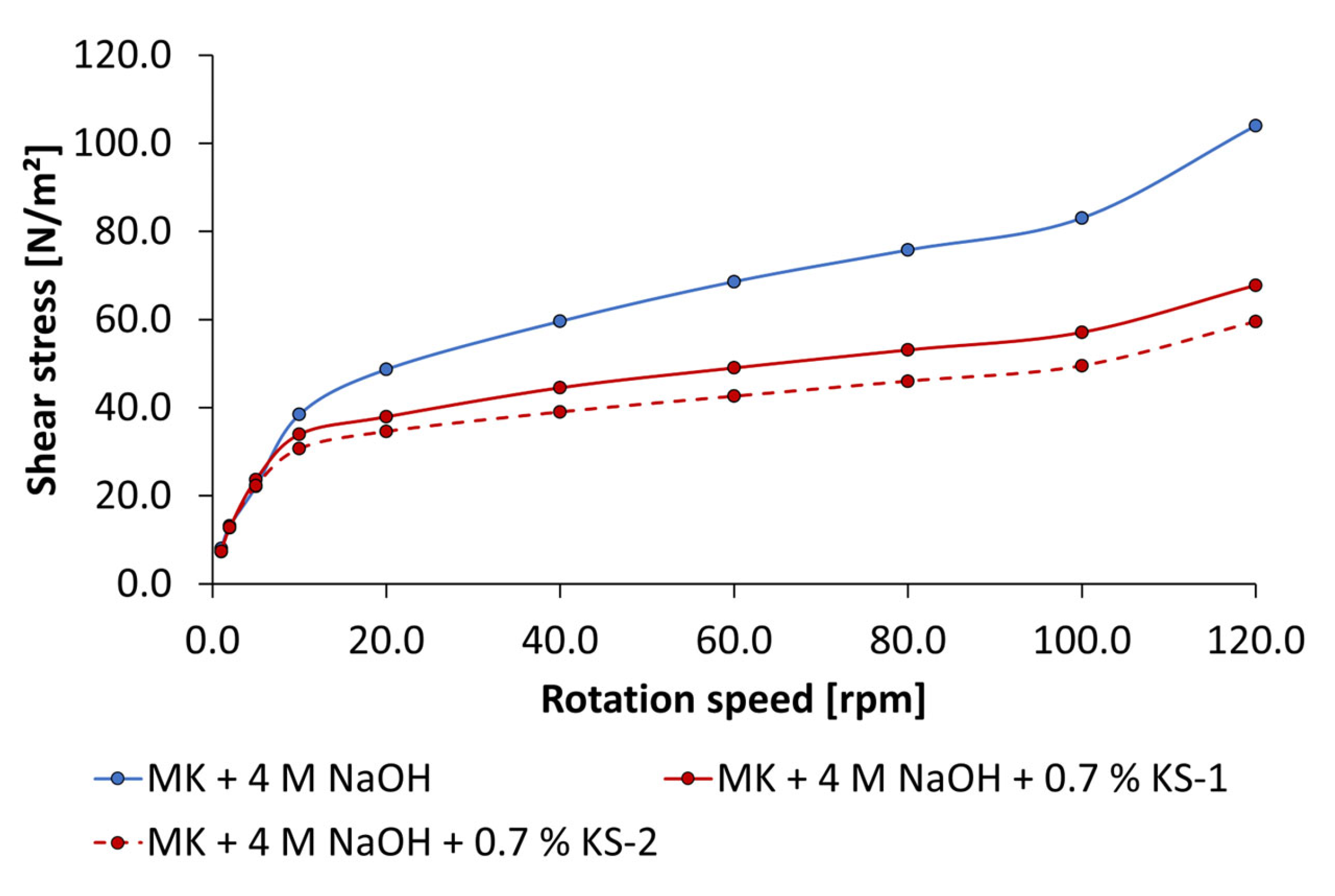
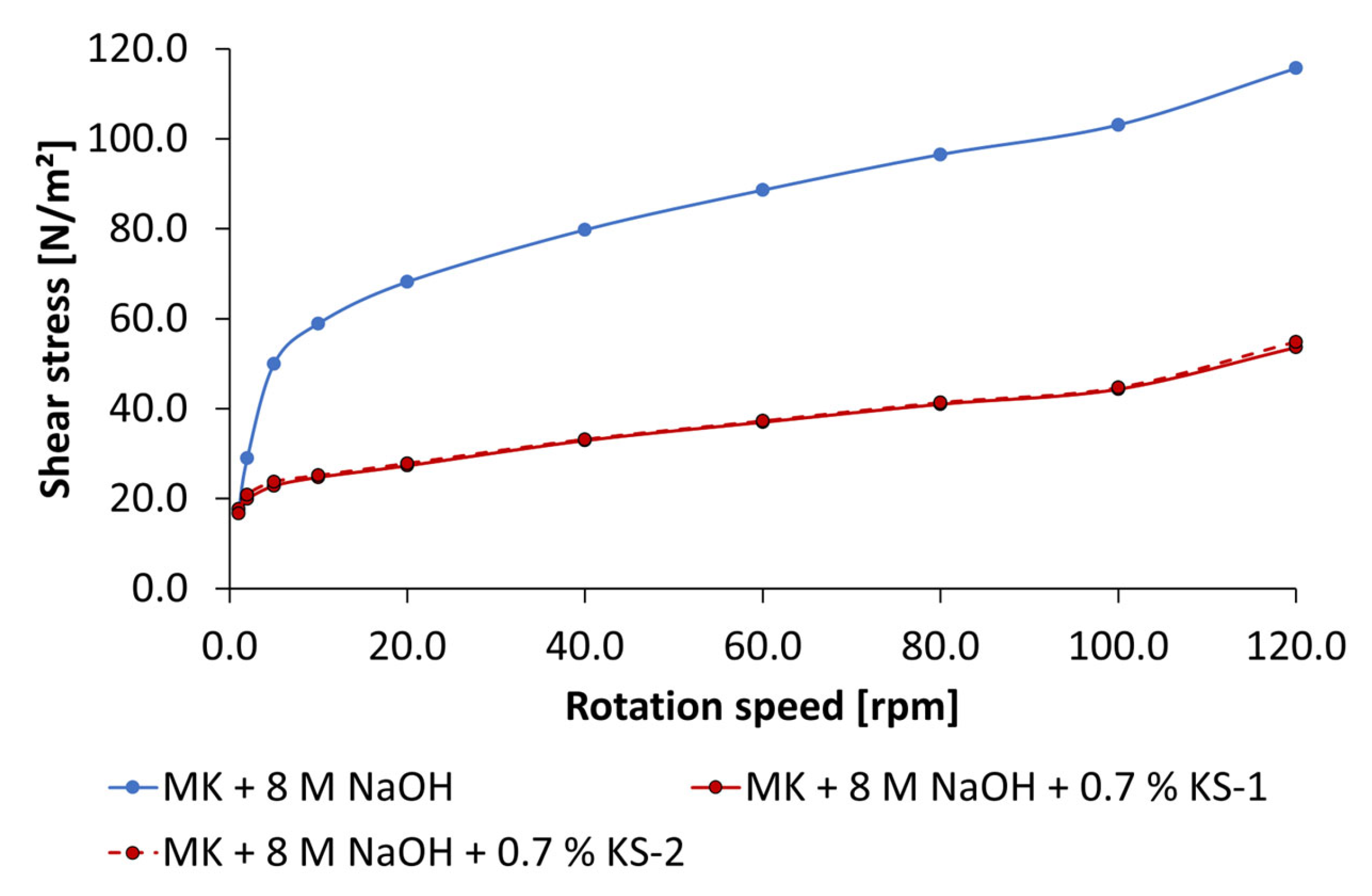
3.3. Dispersing Performance of the Synthesized Starch Admixtures
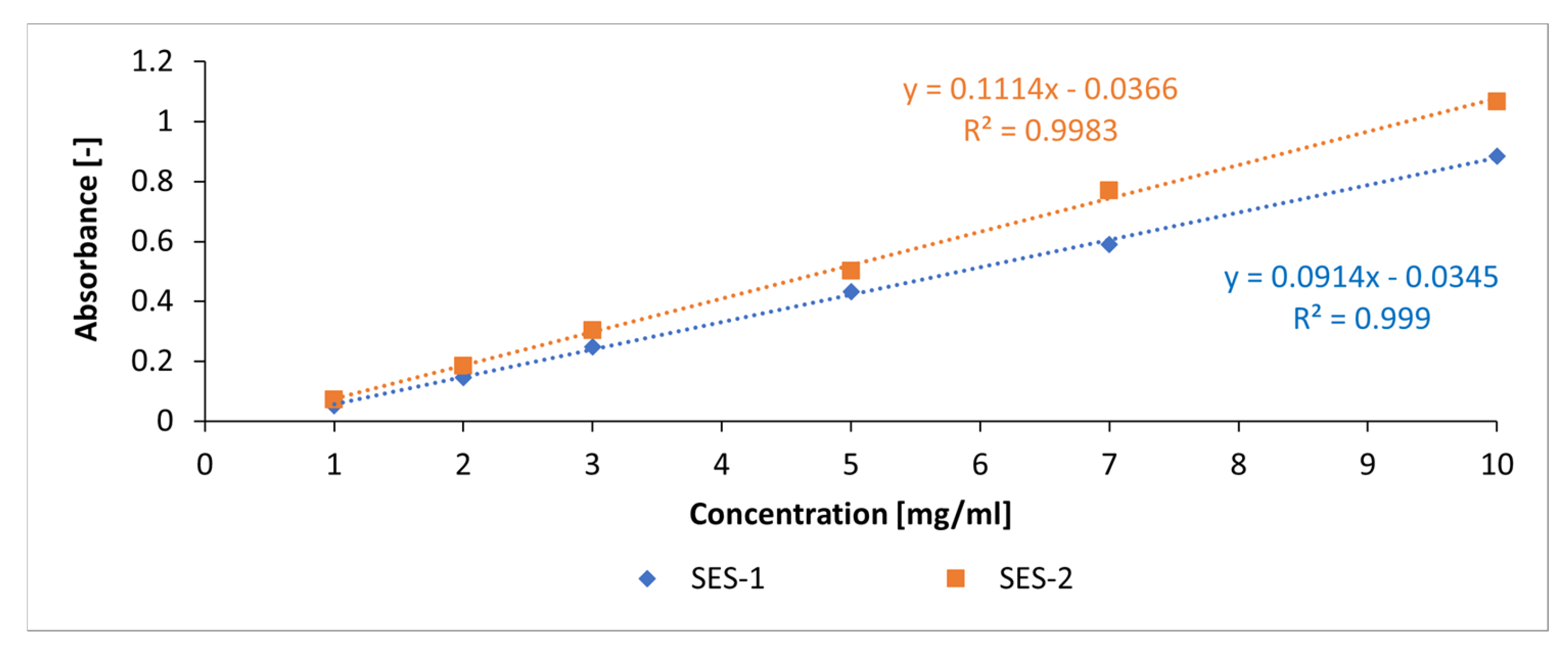
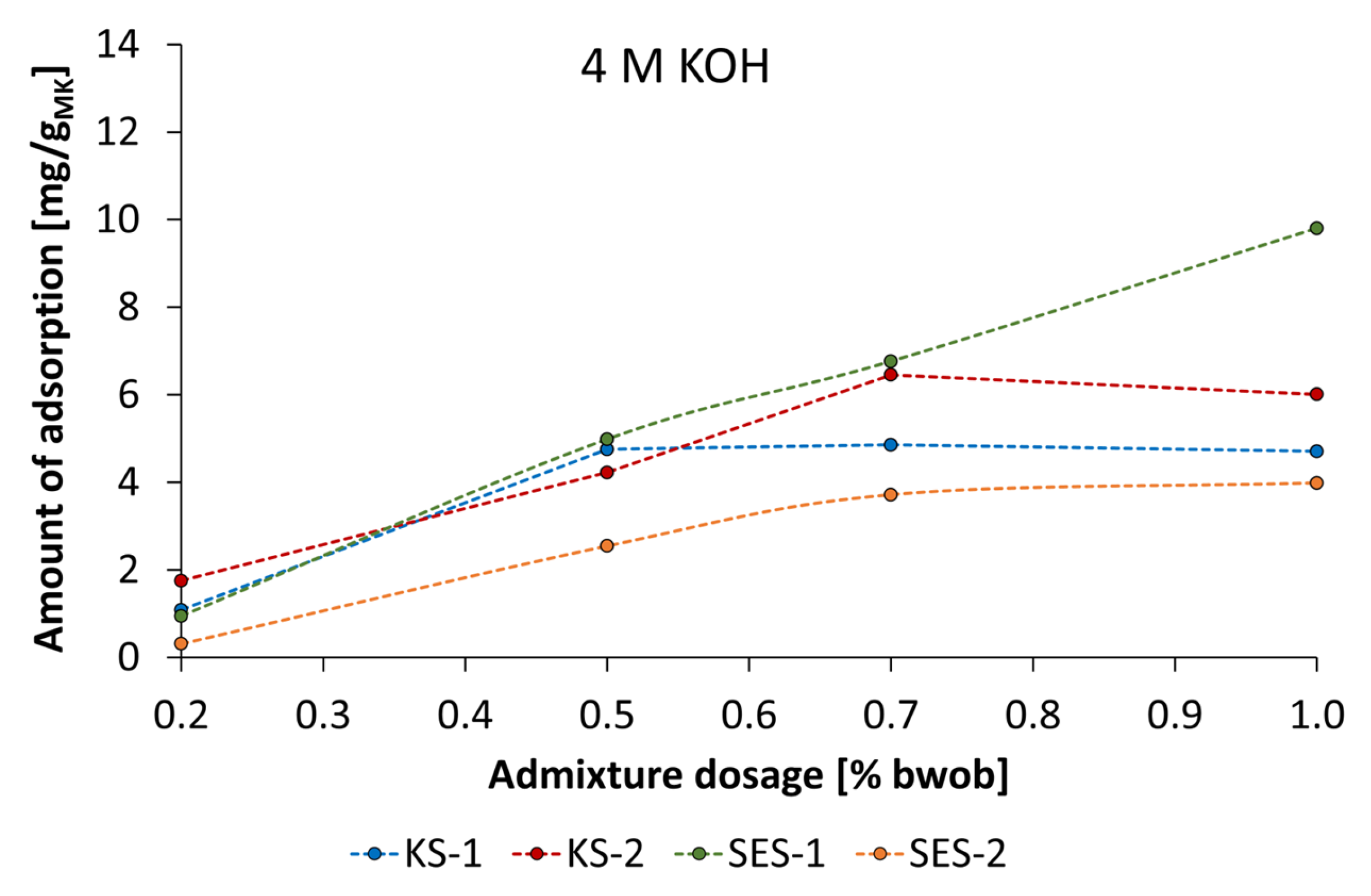
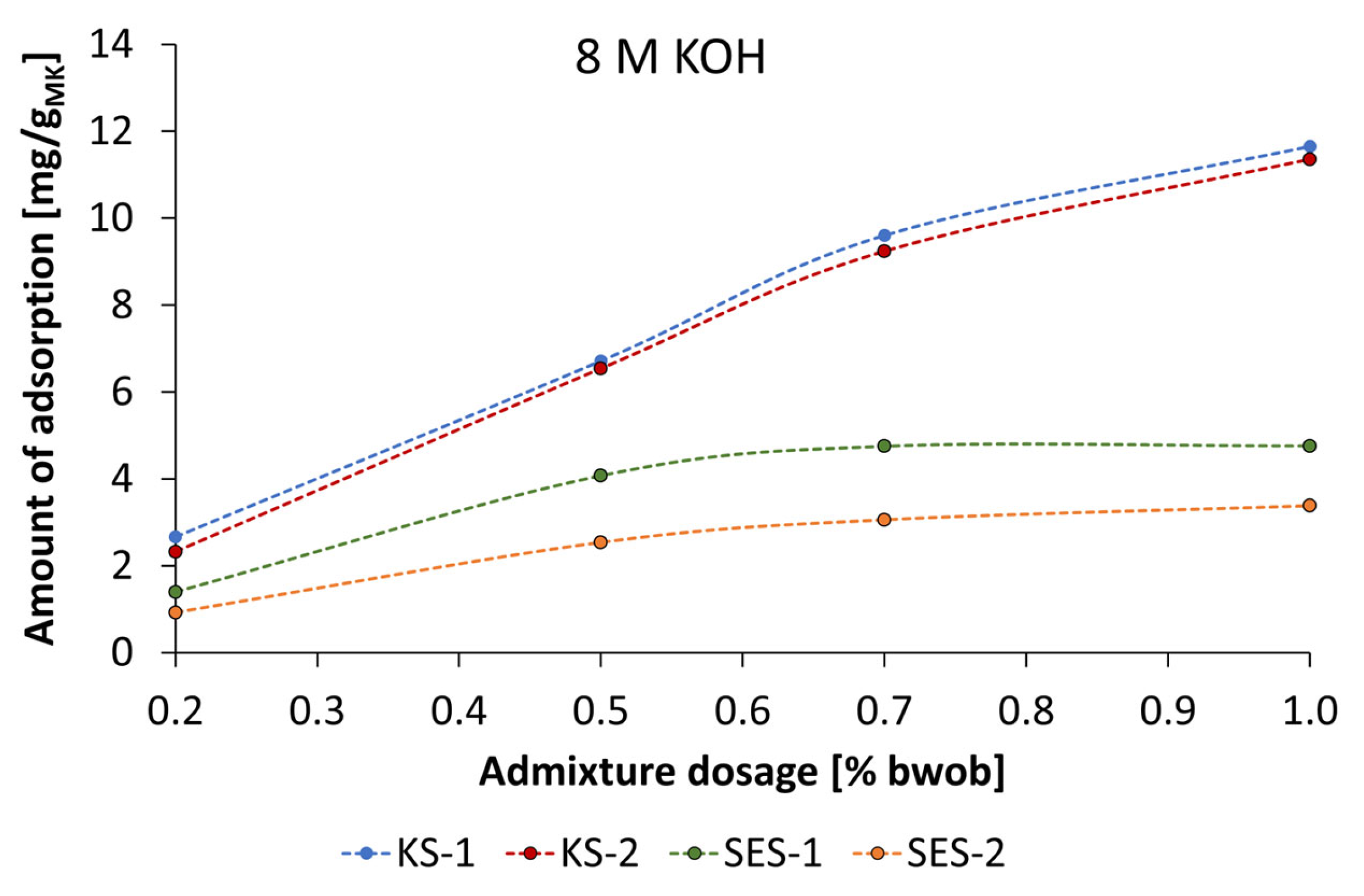
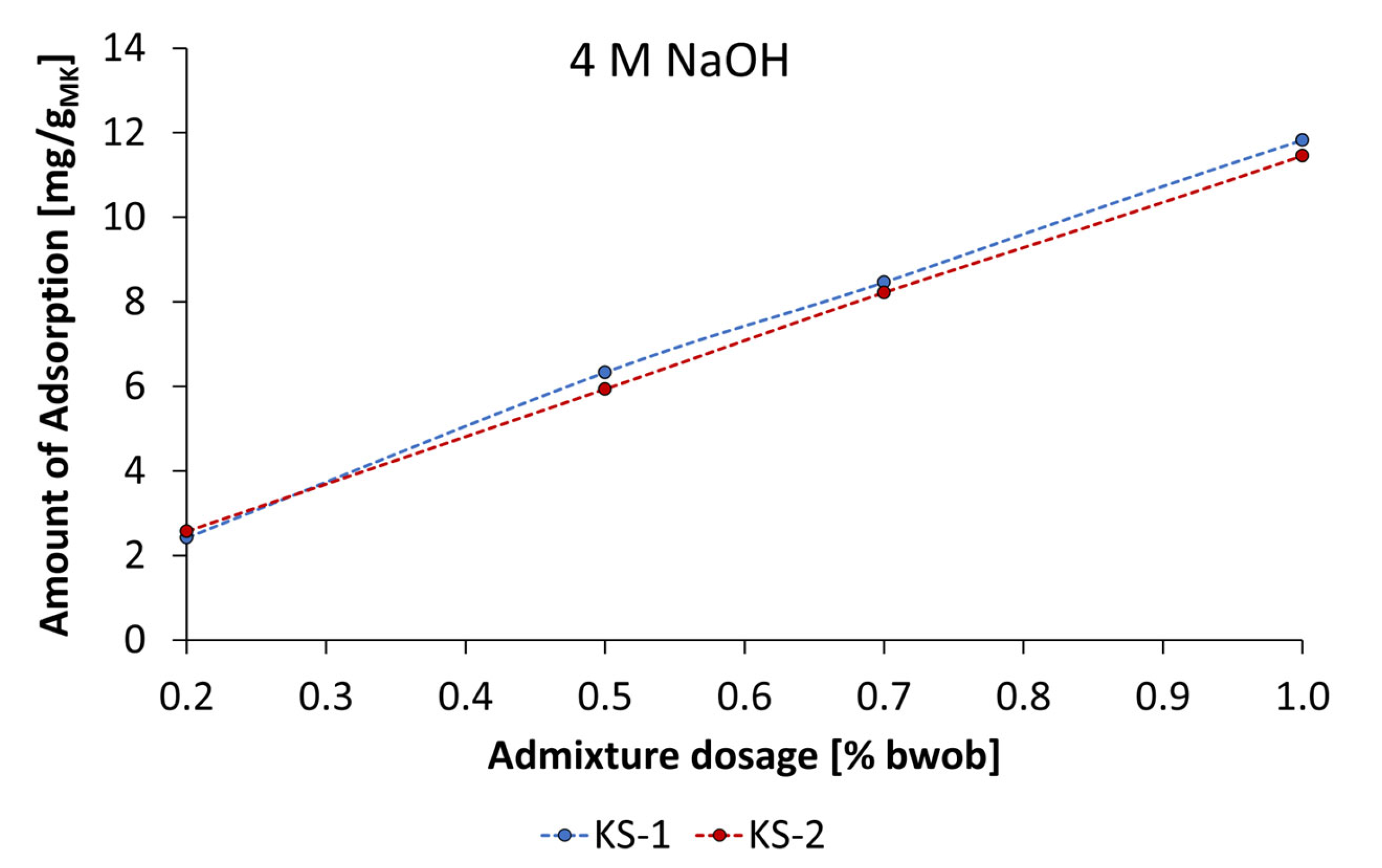
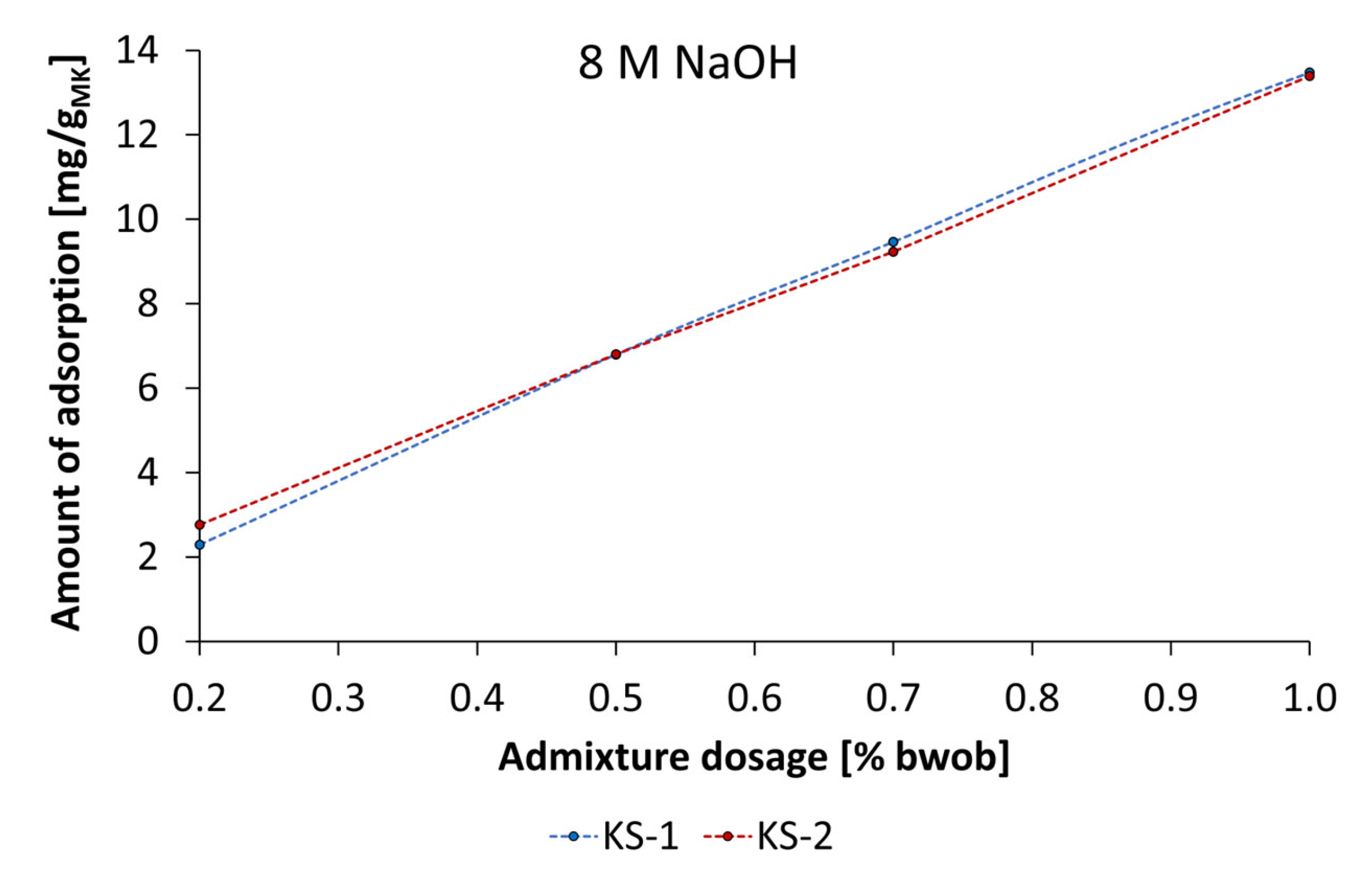
3.4. Adsorbtion Results of the Synthesized Starch Admixtures on MetakaolinParticles
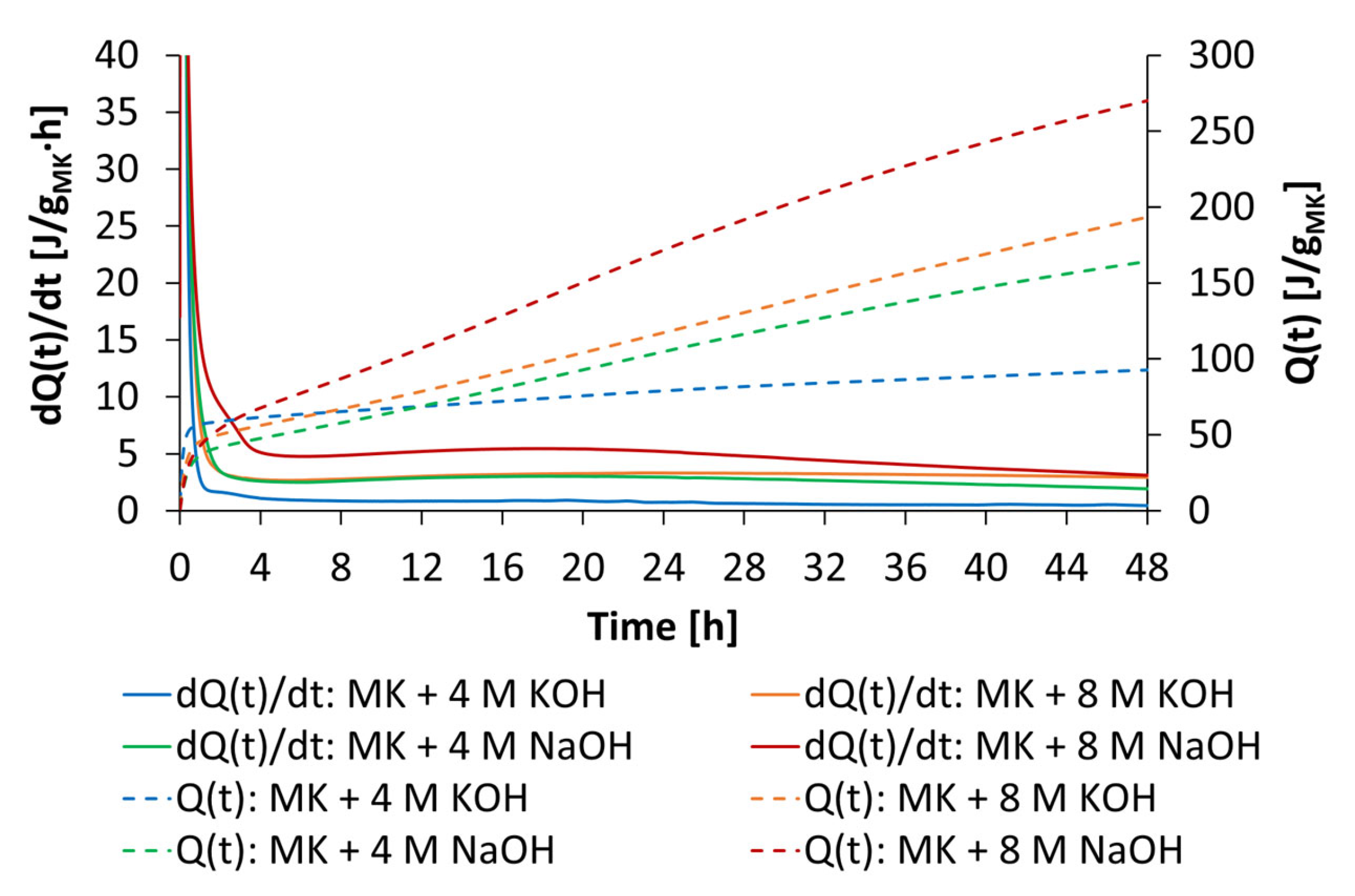
3.5. Influence of Starch Admixtures on Geopolymer Reaction
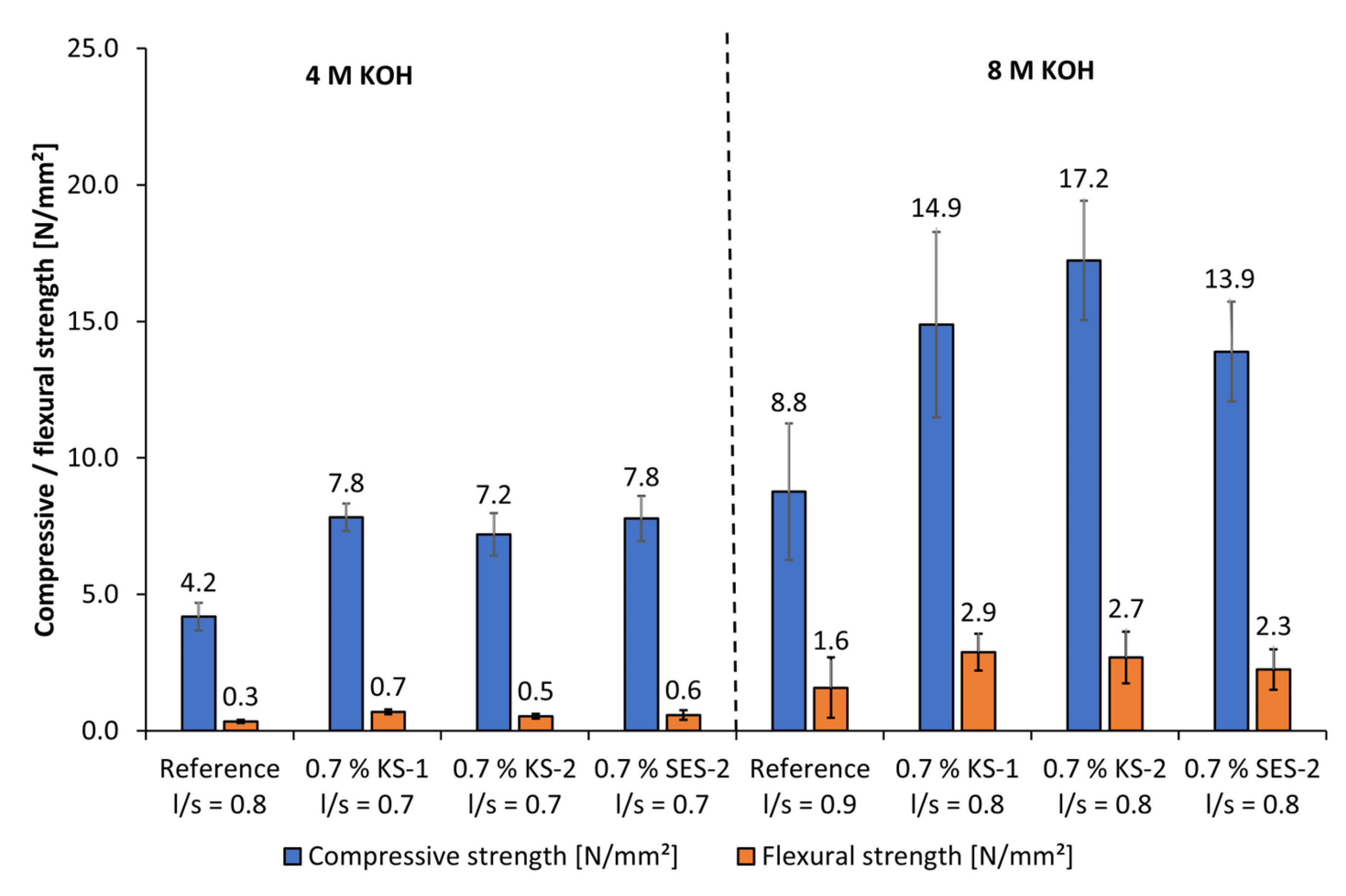
3.6. Influence of Starch Admixtures on Mechanical Properties and Bulk Densities of Geopolymer Fine Mortars
4. Conclusions
- At all types of activators and their concentrations, the metakaolin dissolved congruently, and the amount of dissolved aluminum and silicon depends strongly on the concentration of the activator. Furthermore, in the NaOH activator, the dissolution process of silicon and aluminum species occurs faster and in higher amounts.
- The PCE superplasticizers show coiling and formation of insoluble aggregates from concentrations of 3 mol/L in both KOH and NaOH. Therefore, PCE superplasticizers are globally incompatible superplasticizers for geopolymer binders activated by high alkaline solutions higher than 3 mol/L.
- Anionic and cationic starch admixtures were synthesized with high and low charge density, which are soluble in each type and concentration of activator and show no coiling effects or formation of insoluble aggregates.
- The anionic starch admixture shows lower dispersing performance in KOH than cationic ones and no dispersing effect in NaOH independent of concentration.
- The cationic starch admixtures are more effective in both types of activators, where especially the high-charged sample reached the highest dispersing performance.
- The mechanism of action is obviously based on adsorption of dissolved metakaolin particles. The adsorption behavior can be described by Langmuir-type isotherms for the anionic starch admixtures in pastes prepared by KOH and the Freundlich-type isotherm for cationic starch admixture in pastes activated by NaOH at the investigated concentrations. Furthermore, the amount of adsorbed cationic starch admixture is remarkably higher at all concentrations.
- The reaction degree and reaction kinetics of geopolymer pastes depend strongly on activator concentration, where 8 mol/L caused the highest heat rate and cumulative heat release. This fact is consistent with solubility investigations, where at the highest activator concentration, the highest amounts of silicon and aluminum were released and can react to an alumino-silicate network. Both types of starch admixture caused no significant effect on reaction kinetics and reaction degree at both activator types and concentrations of 4 mol/L and 8 mol/L.
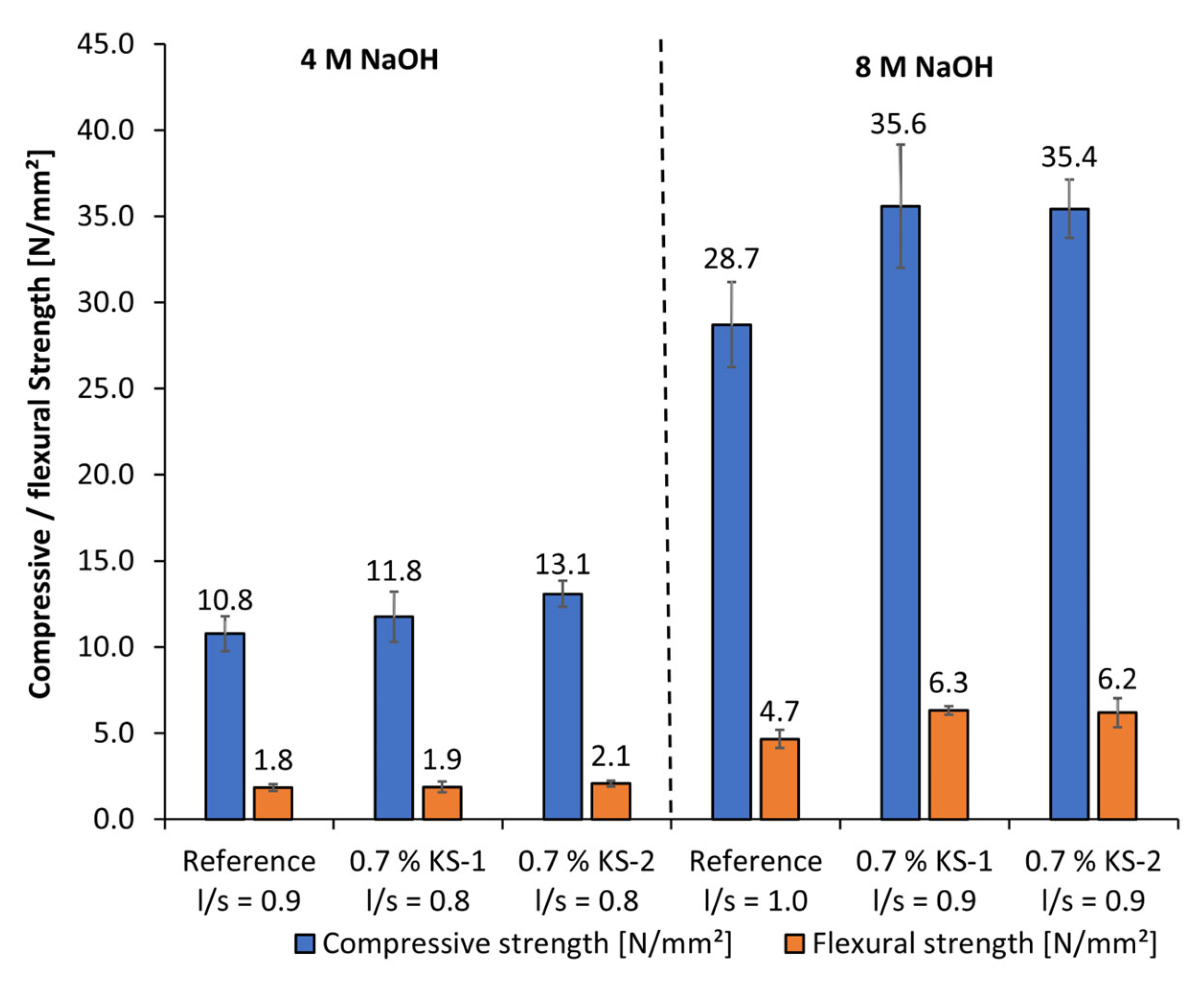
- Only at high concentrations of the examined alkaline activators were viable mechanical properties reached. Therefore, classical PCE superplasticizers are not suitable admixtures for such materials. In contrast, especially cationic starch admixtures are able to disperse the highly alkali-activated metakaolin. Therefore, the l/s ratio could be reduced, which leads to an increase in flexural and compressive strengths.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benhelal, E.; Zahedi, G.; Shamsaei, E.; Bahadori, A. Global strategies and potentials to curb CO2 emissions in cement industry. J. Clean. Prod. 2013, 51, 142–161. [Google Scholar] [CrossRef]
- Pol Segura, I.; Ranjbar, N.; Juul Damø, A.; Skaarup Jensen, L.; Canut, M.; Arendt Jensen, P. A review: Alkali-activated cement and concrete production technologies available in the industry. Heliyon 2023, 9, e15718. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 5th ed.; Institut Géopolymère: Saint-Quentin, France, 2020; ISBN 2954453117. [Google Scholar]
- Kaps, C.; Hohmann, M.; Partschefeld, S. Zur Reaktivität von Säure-/Base-aktivierten Metatonen in Spezialbindemitteln. In Proceedings of the ibausil—International Conference on Building Materials, Weimar, Germany, 12–15 September 2012; Bauhaus-Universität Weimar: Weimar, Germany, 2012. [Google Scholar]
- Agashua, L.O.; Arum, C.; Oluyemi-Ayibiowu, B.D.; Ikumapayi, C.M. A systematic review of geopolymer materials: Innovations, prevailing constraints and resolutions. Sinergi 2024, 28, 473. [Google Scholar] [CrossRef]
- Nasir, M.; Mahmood, A.H.; Bahraq, A.A. History, recent progress, and future challenges of alkali-activated binders—An overview. Constr. Build. Mater. 2024, 426, 136141. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Liew, Y.-M.; Heah, C.-Y.; Mohd Mustafa, A.B.; Kamarudin, H. Structure and properties of clay-based geopolymer cements: A review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Nodehi, M.; Taghvaee, V.M. Alkali-Activated Materials and Geopolymer: A Review of Common Precursors and Activators Addressing Circular Economy. Circ. Econ. Sust. 2022, 2, 165–196. [Google Scholar] [CrossRef]
- Farooq, F.; Jin, X.; Faisal Javed, M.; Akbar, A.; Izhar Shah, M.; Aslam, F.; Alyousef, R. Geopolymer concrete as sustainable material: A state of the art review. Constr. Build. Mater. 2021, 306, 124762. [Google Scholar] [CrossRef]
- Anudeep, P.; Reddy, M.A.K.; Khed, V.C.; Adamu, M.; Varalakshmi, M.; Ibrahim, Y.E.; Ahmed, O.S. Effect of superplasticizer in geopolymer and alkali-activated cement mortar/concrete: A review. Rev. Adv. Mater. Sci. 2024, 63, 20230173. [Google Scholar] [CrossRef]
- Plank, J.; Sakai, E.; Miao, C.W.; Yu, C.; Hong, J.X. Chemical admixtures—Chemistry, applications and their impact on concrete microstructure and durability. Cem. Concr. Res. 2015, 78, 81–99. [Google Scholar] [CrossRef]
- Lei, L.; Palacios, M.; Plank, J.; Jeknavorian, A.A. Interaction between polycarboxylate superplasticizers and non-calcined clays and calcined clays: A review. Cem. Concr. Res. 2022, 154, 106717. [Google Scholar] [CrossRef]
- Ng, S.; Plank, J. Interaction mechanisms between Na montmorillonite clay and MPEG-based polycarboxylate superplasticizers. Cem. Concr. Res. 2012, 42, 847–854. [Google Scholar] [CrossRef]
- Marchon, D.; Sulser, U.; Eberhardt, A.; Flatt, R.J. Molecular design of comb-shaped polycarboxylate dispersants for environmentally friendly concrete. Soft Matter 2013, 9, 10719. [Google Scholar] [CrossRef]
- Chen, X.; Jin, B.; Suraneni, P. Understanding the dissolution of metakaolin in sodium hydroxide solutions. Mater. Struct. 2024, 57, 102. [Google Scholar] [CrossRef]
- Weng, L.; Sagoe-Crentsil, K. Dissolution processes, hydrolysis and condensation reactions during geopolymer synthesis: Part I—Low Si/Al ratio systems. J. Mater. Sci. 2007, 42, 2997–3006. [Google Scholar] [CrossRef]
- Moghul, S.; Zunino, F.; Flatt, R.J. Flow loss in superplasticized limestone calcined clay cement. J. Am. Ceram. Soc. 2025, 108, e20344. [Google Scholar] [CrossRef]
- Sposito, R.; Beuntner, N.; Thienel, K.-C. Characteristics of components in calcined clays and their influence on the efficiency of superplasticizers. Cem. Concr. Compos. 2020, 110, 103594. [Google Scholar] [CrossRef]
- Schmid, M.; Plank, J. Dispersing performance of different kinds of polycarboxylate (PCE) superplasticizers in cement blended with a calcined clay. Constr. Build. Mater. 2020, 258, 119576. [Google Scholar] [CrossRef]
- Chen, J.; Plank, J. Which factors impact the effectiveness of PCEs in alkali-activated slag cements? Cem. Concr. Res. 2025, 190, 107807. [Google Scholar] [CrossRef]
- Tutal, A.; Partschefeld, S.; Schneider, J.; Osburg, A. Effects of Bio-Based Plasticizers, Made from Starch, on the Properties of Fresh and Hardened Metakaolin-Geopolymer Mortar: Basic Investigations. Clays Clay Miner. 2020, 68, 413–427. [Google Scholar] [CrossRef]
- Palacios, M.; Houst, Y.F.; Bowen, P.; Puertas, F. Adsorption of superplasticizer admixtures on alkali-activated slag pastes. Cem. Concr. Res. 2009, 39, 670–677. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Alrefaei, Y.; Dai, J.-G. Influence of coal fly ash on the early performance enhancement and formation mechanisms of silico-aluminophosphate geopolymer. Cem. Concr. Res. 2020, 127, 105932. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, S.; Oh, S.R. The Effects of Ester and Ether Polycarboxylate Superplasticizers on the Fluidity and Setting Behavior of Alkali-Activated Slag Paste. Materials 2024, 17, 4951. [Google Scholar] [CrossRef] [PubMed]
- Palacios, M.; Puertas, F. Stability of superplasticizer and shrinkage-reducing admixtures Stability of superplasticizer and shrinkage-reducing admixtures in high basic media. Mater. Constr. 2004, 54, 65–86. [Google Scholar] [CrossRef]
- Palacios, M.; Puertas, F. Effect of superplasticizer and shrinkage-reducing admixtures on alkali-activated slag pastes and mortars. Cem. Concr. Res. 2005, 35, 1358–1367. [Google Scholar] [CrossRef]
- Favier, A.; Hot, J.; Habert, G.; Roussel, N.; d’Espinose de Lacaillerie, J.-B. Flow properties of MK-based geopolymer pastes. A comparative study with standard Portland cement pastes. Soft Matter 2014, 10, 1134–1141. [Google Scholar] [CrossRef]
- Vieira, M.C.; Klemm, D.; Einfeldt, L.; Albrecht, G. Dispersing agents for cement based on modified polysaccharides. Cem. Concr. Res. 2005, 35, 883–890. [Google Scholar] [CrossRef]
- Crépy, L.; Petit, J.-Y.; Wirquin, E.; Martin, P.; Joly, N. Synthesis and evaluation of starch-based polymers as potential dispersants in cement pastes and self leveling compounds. Cem. Concr. Compos. 2014, 45, 29–38. [Google Scholar] [CrossRef]
- NF P18-513; Betonzusatzstoffe—Metakaolin—Festlegung und Konformitätskriterien: Addition pour béton Hydraulique—Métakaolin—Spécifications et Critères de Conformité. AFNOR: La Plaine Saint-Denis, France, 2012.
- Suraneni, P. Recent developments in reactivity testing of supplementary cementitious materials. RILEM Tech. Lett. 2021, 6, 131–139. [Google Scholar] [CrossRef]
- Hamer, W.J.; Wu, Y.-C. Osmotic Coefficients and Mean Activity Coefficients of Uni-univalent Electrolytes in Water at 25 °C. J. Phys. Chem. Ref. Data 1972, 1, 1047–1100. [Google Scholar] [CrossRef]
- Hausmann, J.N.; Traynor, B.; Myers, R.J.; Driess, M.; Menezes, P.W. The pH of Aqueous NaOH/KOH Solutions: A Critical and Non-trivial Parameter for Electrocatalysis. ACS Energy Lett. 2021, 6, 3567–3571. [Google Scholar] [CrossRef]
- Matthys, S.; Proia, A. Proceedings of the DuRSAAM 2023 Symposium on Advancing Alkali-Activated Materials; UGent University: Ghent, Belgium, 2023. [Google Scholar]
- Tan, Z.; Bernal, S.A.; Provis, J.L. Reproducible mini-slump test procedure for measuring the yield stress of cementitious pastes. Mater. Struct. 2017, 50, 235. [Google Scholar] [CrossRef]
- Perche, F. Adsorption de Polycarboxylates et de Lignosulfonates sur Poudre Modèle et Ciments. Ph.D. Thesis, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 2004. [Google Scholar]
- Provis, J.L.; van Deventer, J.S.J. Alkali Activated Materials; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-007-7671-5. [Google Scholar]
- Zhang, Y.; Chan, H.-K.; Han, Z.; Lei, L. Why do conventional MAA-MPEG PCEs not work in alkali-activated slag systems? Cem. Concr. Res. 2024, 184, 107599. [Google Scholar] [CrossRef]
- Werling, N.; Kaltenbach, J.; Weidler, P.G.; Schuhmann, R.; Dehn, F.; Emmerich, K. Solubility of Calcined Kaolinite, Montmorillonite, and Illite in High Molar NaOH and Suitability as Precursors for Geopolymers. Clays Clay Miner. 2022, 70, 270–289. [Google Scholar] [CrossRef]
- Werling, N.; Dehn, F.; Krause, F.; Steudel, A.; Schuhmann, R.; Emmerich, K. Solubility of precursors and carbonation of waterglass-free geopolymers. Clays Clay Miner. 2020, 68, 524–531. [Google Scholar] [CrossRef]
- Dathe, F.; Overmann, S.; Koenig, A.; Dehn, F. The Role of Water Content and Binder to Aggregate Ratio on the Performance of Metakaolin-Based Geopolymer Mortars. Minerals 2024, 14, 823. [Google Scholar] [CrossRef]
- Lange, A.; Plank, J. Contribution of non-adsorbing polymers to cement dispersion. Cem. Concr. Res. 2016, 79, 131–136. [Google Scholar] [CrossRef]
- Wu, B.; Gu, L.; Chun, B.-W.; Kuhl, T.L. Adsorption and interaction forces of commercial Poly (naphthalene sulfonate) (PNS) and Poly (carboxylate ether) (PCE) polyelectrolytes with negatively charged surfaces in monovalent and divalent electrolytes. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127992. [Google Scholar] [CrossRef]
- Alrefaei, Y.; Dai, J.-G. Effects of delayed addition of polycarboxylate ether on one-part alkali-activated fly ash/slag pastes: Adsorption, reaction kinetics, and rheology. Constr. Build. Mater. 2022, 323, 126611. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Für Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Physical Chemistry [Medienkombination], 6th ed.; Oxford University Press: Oxford, UK; ISBN 0-19-850101-3.
- Partschefeld, S.; Osburg, A. Synthesis of dispersing agents from starch—Influence on rheological properties and early age hydration of OPC. Constr. Build. Mater. 2020, 240, 117913. [Google Scholar] [CrossRef]
| Oxides [wt.-%] | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | TiO2 | K2O | Na2O | SO3 | P2O5 | DL 1 | LOI 2 |
| 52.0 | 41.4 | 0.6 | 0.0 | 0.1 | 0.93 | 0.3 | 0.03 | 0.1 | 0.1 | 0.4 | 3.7 | |
| Phases [%] | Kaolinite | Anatase | Quartz | Calcite | Amorphous | |||||||
| 23.9 ± 1.5 | 0.9 ± 0.2 | 2.7 ± 0.3 | 0.9 ± 0.4 | 71.6 ± 1.5 | ||||||||
| Particle size [µm] | d10 0.8 | d50 8.19 | d90 20.2 | |||||||||
| BET [m2/g] | 11.5 | |||||||||||
| Puzzolanic reactivity | R3-Test (7 d, 40 °C) | Chapelle Test | ||||||||||
| Q = 959.3 J/gMK | C = 1250 mgCaO/gMK | |||||||||||
| KOH Solutions | |||
|---|---|---|---|
| Molarity [mol/L] | 1 mol/L | 4 mol/L | 8 mol/L |
| Molality [mol/kg] | 0.99 | 3.5 | 6 |
| pH value [-] | 13.89 | 14.61 | 15.28 |
| Activity coefficient [-] | 0.733 | 1.184 | 2.18 |
| Activity [mol/L] | 0.733 | 4.736 | 17.44 |
| NaOH Solutions | |||
| Molarity [mol/L] | 1 mol/L | 4 mol/L | 8 mol/L |
| Molality [mol/kg] | 0.99 | 3.5 | 6 |
| pH value [-] | 14.01 | 14.53 | 15.04 |
| Activity coefficient [-] | 0.668 | 0.847 | 1.302 |
| Activity [mol/L] | 0.668 | 3.388 | 10.416 |
| Parameter | HPEG | IPEG | MPEG |
|---|---|---|---|
| Mn [g/mol] | 8286 | 7388 | 7984 |
| Mw [g/mol] | 13,390 | 11,460 | 17,340 |
| PDI [-] | 1.62 | 1.55 | 2.17 |
| Anionic charge density in H2O 1 [µeq/g] | 6989 ± 18 2 | 9162 ± 10 2 | 9871 ± 33 2 |
| Parameter | Basic Starch | SES-1 | SES-2 | KS-1 | KS-2 |
|---|---|---|---|---|---|
| Mn [g/mol] | 9629 | 17,880 | 36,860 | 19,670 | 34,890 |
| Mw [g/mol] | 38,890 | 41,410 | 39,620 | 31,270 | 37,500 |
| PDI [-] | 4.04 | 2.32 | 1.08 | 1.59 | 1.08 |
| Anionic charge density in H2O 1 [µeq/g] | - | 946 ± 1 2 | 2422 ± 1 2 | - | - |
| Cationic charge density in H2O 1 [µeq/g] | - | - | - | 1017 ± 1 3 | 2142 ± 2 3 |
| Oxides [wt.-%] | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | TiO2 | MnO | K2O | Na2O | SO3 | P2O5 | DL 1 | LOI 2 |
| 67.3 | 14.8 | 3.4 | 2.4 | 1.4 | 0.29 | 0.18 | 4.27 | 3.29 | 0.4 | 0.51 | 0.1 | 1.6 | |
| Phases [%] | Chlorite | Muscovite | Orthoclase | Albite | Andesine | Pyrite | Quartz | ||||||
| 3.5 ± 0.9 | 11.8 ± 1.5 | 21.6 ± 1.5 | 28.4 ± 2.4 | 6.7 ± 2.3 | 0.3 ± 0.1 | 27.7 ± 1.0 | |||||||
| Particle size [µm] | d10 | d50 | d90 | ||||||||||
| 63 | 250 | 500 | |||||||||||
| BET [m2/g] | 2.5 | ||||||||||||
| Sample | dQ(t1)/dt [J/gMK∙h] | dQ(t2)/dt [J/gMK∙h] | dQ(t3)/dt [J/gMK∙h] | Q(t1) [J/gMK] | Q(t2) [J/gMK] | Q(t3) [J/gMK] |
|---|---|---|---|---|---|---|
| MK + 4 M KOH | 1.2 | 1.2 | 0.9 | 46.8 | 66.4 | 92.1 |
| 0.7% SES-1 | 1.3 | 1.3 | 1.0 | 42.3 | 63.9 | 92.0 |
| 0.7% SES-2 | 1.3 | 1.3 | 1.0 | 41.1 | 61.6 | 88.6 |
| 0.7% KS-1 | 1.3 | 1.3 | 1.0 | 49.4 | 65.4 | 92.8 |
| 0.7% KS-2 | 1.3 | 1.3 | 1.0 | 47.2 | 62.5 | 89.1 |
| MK + 8 M KOH | 3.0 | 3.3 | 2.9 | 78.7 | 117.3 | 193.4 |
| 0.7% SES-1 | 3.0 | 3.3 | 2.9 | 84.9 | 123.0 | 197.9 |
| 0.7% SES-2 | 3.1 | 3.3 | 2.9 | 89.4 | 128.5 | 204.4 |
| 0.7% KS-1 | 3.0 | 3.3 | 2.9 | 84.3 | 122.2 | 196.7 |
| 0.7% KS-2 | 3.0 | 3.3 | 2.9 | 86.4 | 124.3 | 199.4 |
| MK + 4 M NaOH | 2.9 | 3.0 | 1.9 | 68.9 | 104.8 | 164.2 |
| 0.7% KS-1 | 2.8 | 2.9 | 1,9 | 66.4 | 101.8 | 159.7 |
| 0.7% KS-2 | 2.9 | 3.0 | 1.9 | 68.0 | 103.8 | 162.5 |
| MK + 8 M NaOH | 5.2 | 5.2 | 3.1 | 107.2 | 171.7 | 270.1 |
| 0.7% KS-1 | 5.3 | 5.3 | 3.1 | 109.0 | 174.1 | 272.5 |
| 0.7% KS-2 | 5.1 | 5.1 | 3.1 | 107.0 | 170.4 | 267.9 |
| Sample | Bulk Density [g/cm3] | Sample | Bulk Density [g/cm3] |
|---|---|---|---|
| 4 M KOH | 8 M KOH | ||
| Reference, l/s = 0.8 | 1.38 ± 0.06 | Reference, l/s = 0.9 | 1.46 ± 0.08 |
| 0.7% KS-1, l/s = 0.7 | 1.53 ± 0.05 | 0.7% KS-1, l/s = 0.8 | 1.64 ± 0.10 |
| 0.7% KS-2, l/s = 0.7 | 1.50 ± 0.07 | 0.7% KS-2, l/s = 0.8 | 1.57 ± 0.04 |
| 0.7% SES-2, l/s = 0.7 | 1.50 ± 0.07 | 0.7% SES-2, l/s = 0.8 | 1.55 ± 0.12 |
| 4 M NaOH | 8 M NaOH | ||
| Reference, l/s = 0.9 | 1.42 ± 0.04 | Reference, l/s = 1.0 | 1.48 ± 0.05 |
| 0.7% KS-1, l/s = 0.8 | 1.44 ± 0.06 | 0.7% KS-1, l/s = 0.9 | 1.57 ± 0.03 |
| 0.7% KS-2, l/s = 0.8 | 1.48 ± 0.02 | 0.7% KS-2, l/s = 0.9 | 1.54 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Partschefeld, S.; Aschoff, J.; Osburg, A. Interaction of PCE and Chemically Modified Starch Admixtures with Metakaolin-Based Geopolymers—The Role of Activator Type and Concentration. Materials 2025, 18, 4154. https://doi.org/10.3390/ma18174154
Partschefeld S, Aschoff J, Osburg A. Interaction of PCE and Chemically Modified Starch Admixtures with Metakaolin-Based Geopolymers—The Role of Activator Type and Concentration. Materials. 2025; 18(17):4154. https://doi.org/10.3390/ma18174154
Chicago/Turabian StylePartschefeld, Stephan, Jasmine Aschoff, and Andrea Osburg. 2025. "Interaction of PCE and Chemically Modified Starch Admixtures with Metakaolin-Based Geopolymers—The Role of Activator Type and Concentration" Materials 18, no. 17: 4154. https://doi.org/10.3390/ma18174154
APA StylePartschefeld, S., Aschoff, J., & Osburg, A. (2025). Interaction of PCE and Chemically Modified Starch Admixtures with Metakaolin-Based Geopolymers—The Role of Activator Type and Concentration. Materials, 18(17), 4154. https://doi.org/10.3390/ma18174154









