1. Introduction
As China accelerates its industrial development, the annual generation of large-volume industrial solid wastes—such as red mud, electrolytic manganese residues, and steel slag—continues to rise. With accumulated stockpiles surpassing 6 billion metric tons and a comprehensive utilization rate of less than 55%, these materials not only impose significant environmental pressure but also squander their precious resource value [
1]. Currently, the national level places great emphasis on the resource utilization of solid waste, the call for action emphasizes the need to accelerate the development of integrated utilization capabilities for complex and difficult-to-manage solid waste, with a focus on expanding its high-value applications in areas such as green building materials, road base materials, and soil remediation.
Electrolytic Manganese Residue (EMR) is a hazardous solid waste generated after solid–liquid separation during the filter press stage of electrolytic manganese metal production. Based on typical manganese carbonate ore grades, approximately 8–12 tons of manganese residue are generated per ton of manganese metal produced [
2]. Hazardous ions in EMR are highly susceptible to migrating into groundwater and soil under rainwater erosion, leading to a series of environmental pollution issues [
3]. Fresh EMR exhibits high environmental mobility and has a moisture content of about 30% [
4]. Its primary components include gypsum and quartz, along with soluble harmful substances such as manganese sulfate and ammonia nitrogen [
5]. Currently, open-air slag reservoirs serve as the dominant disposal method [
6], where prolonged stockpiling accelerates contaminant migration, intensifying risks to surrounding aquifers and terrestrial ecosystems [
7]. China is the world’s largest producer of electrolytic manganese metal (EMM), with an estimated production of 1.15 million metric tons, according to industry reports in 2023 [
8]. This exponential growth of residual waste now critically constrains sustainable development within the China’s electrolytic manganese sector [
9]. Consequently, the development of effective treatment strategies for EMR is imperative to mitigate its escalating environmental burden [
10].
Red mud (RM), an industrial residue from alumina extraction, derives its name from iron oxide-induced reddish coloration [
10]. It is classified as a typical bulk industrial solid waste in the aluminum production sector [
11]. Depending on the process technology and ore type, the production of 1.0 ton of alumina typically generates 1–2 tons of red mud [
12]. Compared to developed countries, China’s comprehensive utilization rate of red mud is significantly lower, standing at merely 4% [
13]. This has led to the accumulation of over 3.5 billion tons of red mud stored within large storage dams located across the country, with the stockpile currently increasing by approximately 100 million tons annually. The vast open-air stockpiling of red mud poses multiple threats to the surrounding ecological environment: on one hand, red mud disposal occupies substantial valuable land resources; on the other hand, its strong alkalinity and high salt content can cause severe pollution to soil, groundwater, and atmospheric environments [
14]. Furthermore, excessive stockpiling could also lead to secondary environmental disasters, such as dam failures, which pose substantial risks to ecological security, as well as human life and property. For instance, the dam collapse accident at the Ajkai Timfoldgyar alumina plant in Hungary in 2011 resulted in multiple casualties and the complete collapse of the nearby river ecosystem [
15]. Consequently, how to safely, efficiently, and scalably dispose of and utilize red mud as a resource, in order to minimize its potential threats to the ecological environment and human health, has become a critical scientific and engineering challenge urgently needing resolution.
Steel Slag (SS) is one of the primary solid wastes discharged during the steelmaking process, mainly including converter slag, electric furnace slag, and refining slag. Its output accounts for approximately 15% to 20% of crude steel production [
16]. In recent years, with the rapid development of China’s iron and steel industry, the accumulated stockpiles of steel slag have been steadily increasing. In recent years, with the rapid development of China’s iron and steel industry, the accumulated stockpiles of steel slag have been steadily accumulating. Furthermore, long-term storage of steel slag makes it susceptible to weathering, causing it to fragment into fine particles. These respirable particulate matters can disperse under wind action, resulting in atmospheric pollution that has severe impacts on the health of nearby residents and the quality of the ecological environment [
17]. However, steel slag is not “waste” in the conventional sense. The active C
2S and C
3S components within steel slag exhibit cementitious properties, which can be harnessed as they offer high strength, stability, and durability [
18].
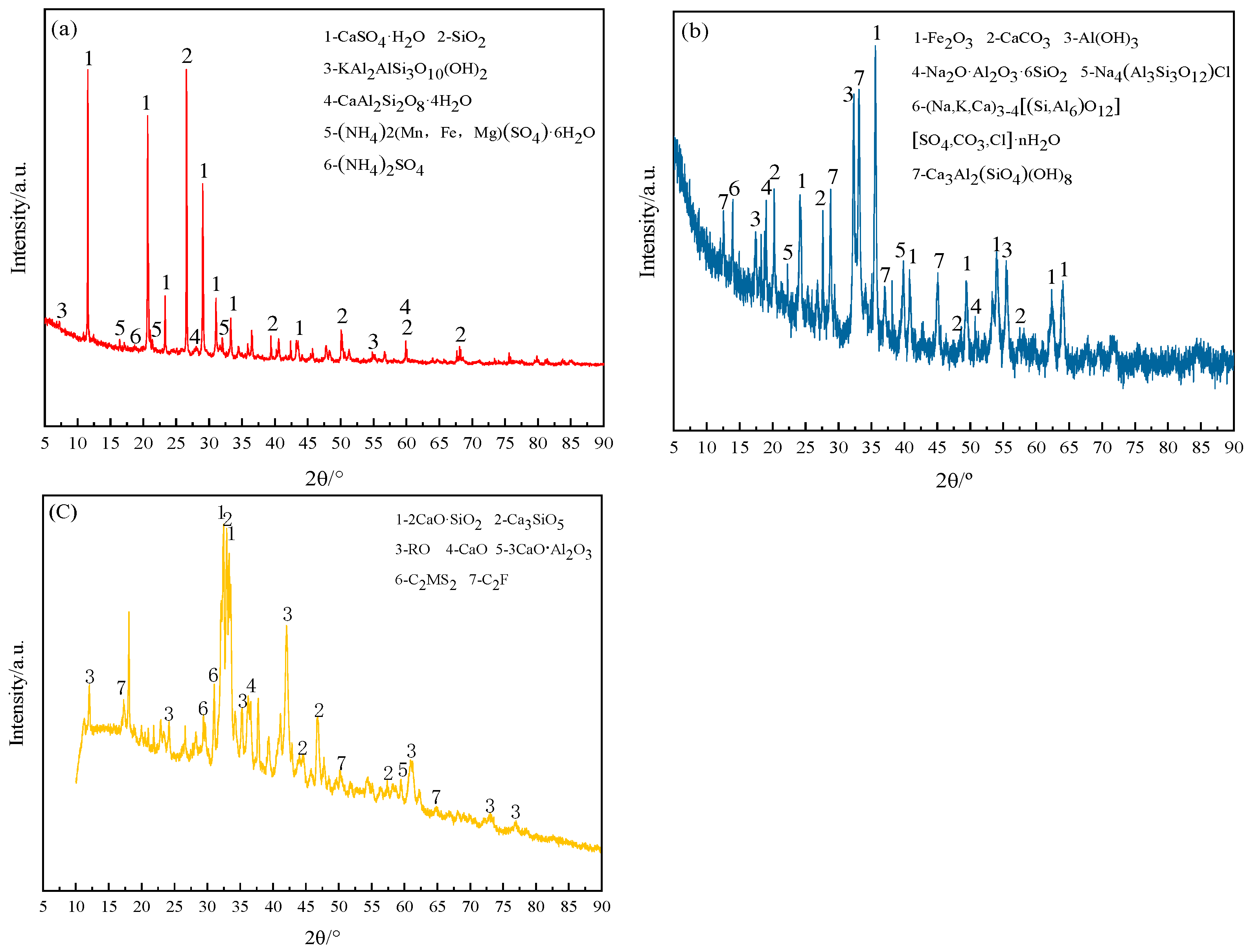
SS, EMR, and RM differ in their applications within the building materials industry. Research revealed that steel slag contained active minerals similar to cement clinker, such as C
2S, C
3S, and C
2F, and proper activation could develop their potential activity. Electrolytic manganese residue contained gypsum minerals accounting for over 30%, which could act as sulfate activators. The Bayer process red mud had an alkali content exceeding 10%, with soluble alkali predominantly present. Some chemically combined alkalis can be converted into soluble alkalis through calcination treatment [
19]. Upon dissolution in water, they create an alkaline environment and can serve as alkali activators. This study adopts EMR, RM, and SS as raw materials for preparing compo-site cementitious material [
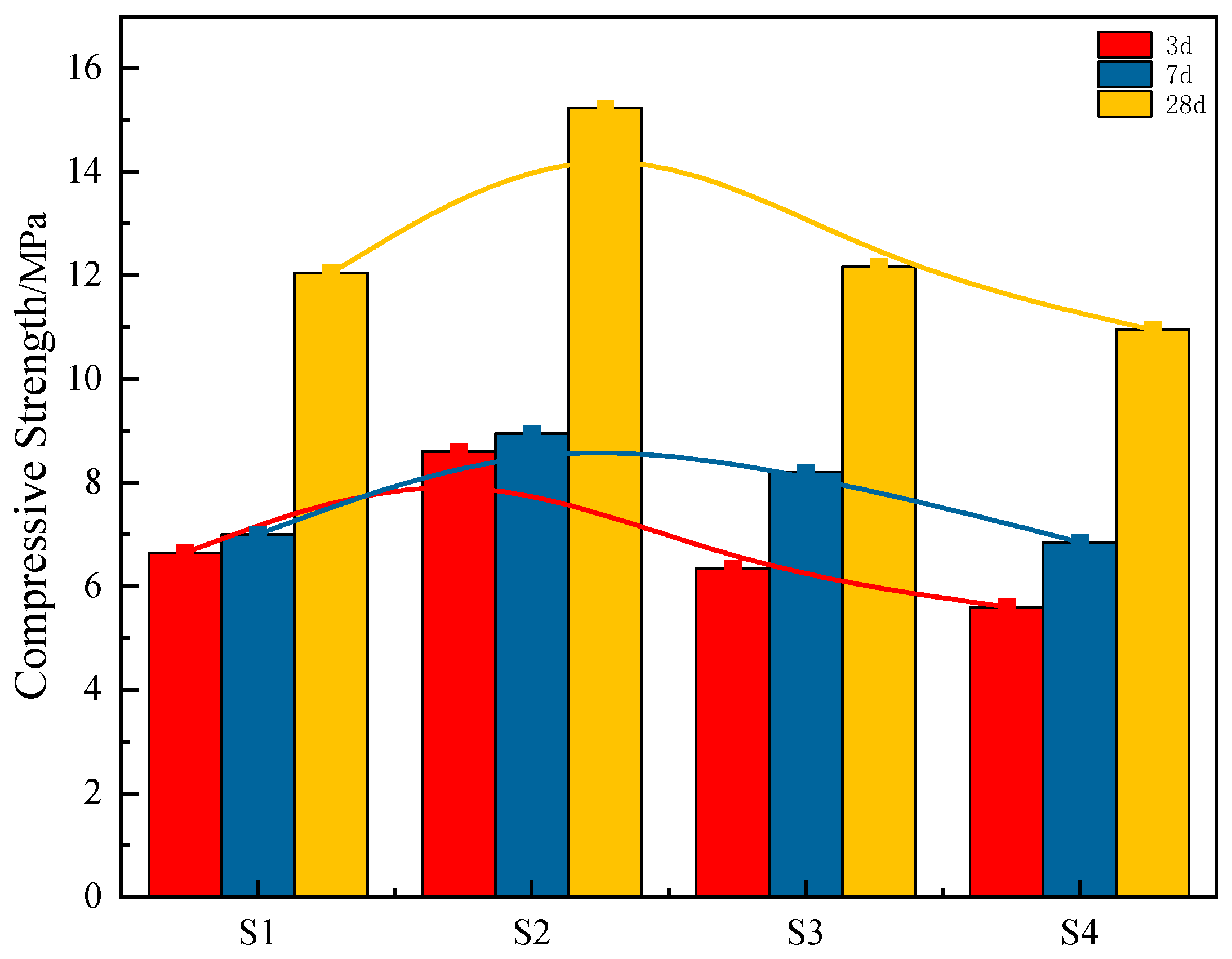
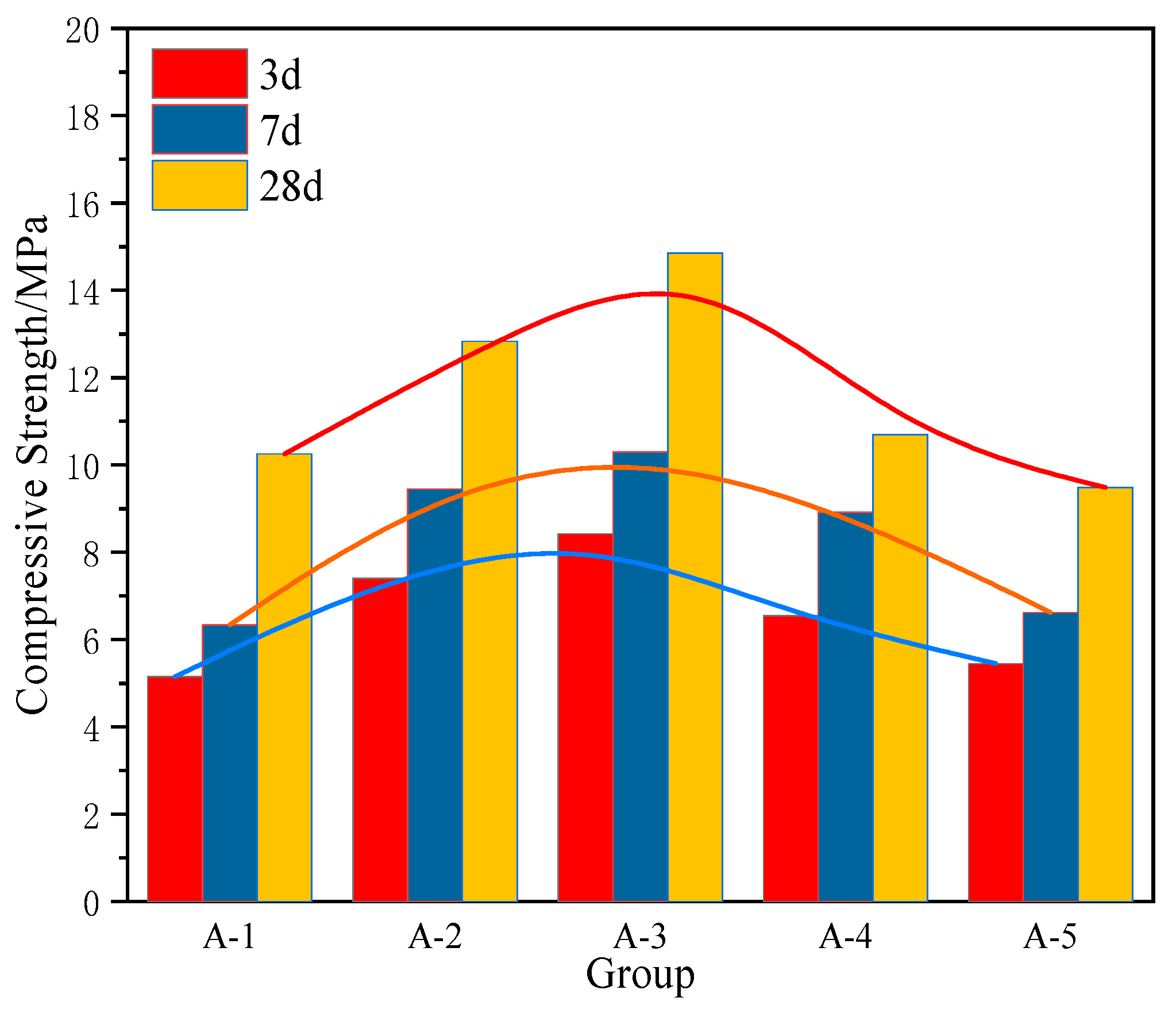
20]. Through systematic optimization of the mix ratios involving steel slag, red mud, and electrolytic manganese residue, the research delves into the hydration products and mechanisms, with a particular focus on the activation of steel slag using red mud and electrolytic manganese residue. The findings offer foundational insights for large-scale utilization of red mud and electrolytic manganese residue in China.
4. Discussion
In order to elucidate the strength development mechanism and hydration behavior of the optimized SZ-11 blend system, this study employed a suite of advanced analytical techniques to comprehensively characterize the sample’s phase composition, morphological microstructure, structural evolution, and thermal stability at various curing ages. XRD was employed for phase identification and semi-quantitative analysis of the hydration products, facilitating the tracking of crystalline mineral content. formation and transformation throughout the hydration process. FTIR was utilized to analyze the vibrational characteristics of functional groups in hydration products, uncovering changes in chemical bonding and hydration levels. SEM was utilized to monitor the microstructural changes, enabling a detailed examination of the morphology and dispersion of hydration products. This analysis is instrumental in establishing the relationship between these hydration products and the mechanical strength of the material. TGA was performed to investigate the characteristics of mass loss during hydration, thereby assessing the thermal stability of the hydration products and the variation in bound water content. Consequently, this integrated approach established the mechanistic interplay between hydration kinetics and strength evolution in the SZ-11 system across discrete curing regimes.
4.1. XRD Analysis
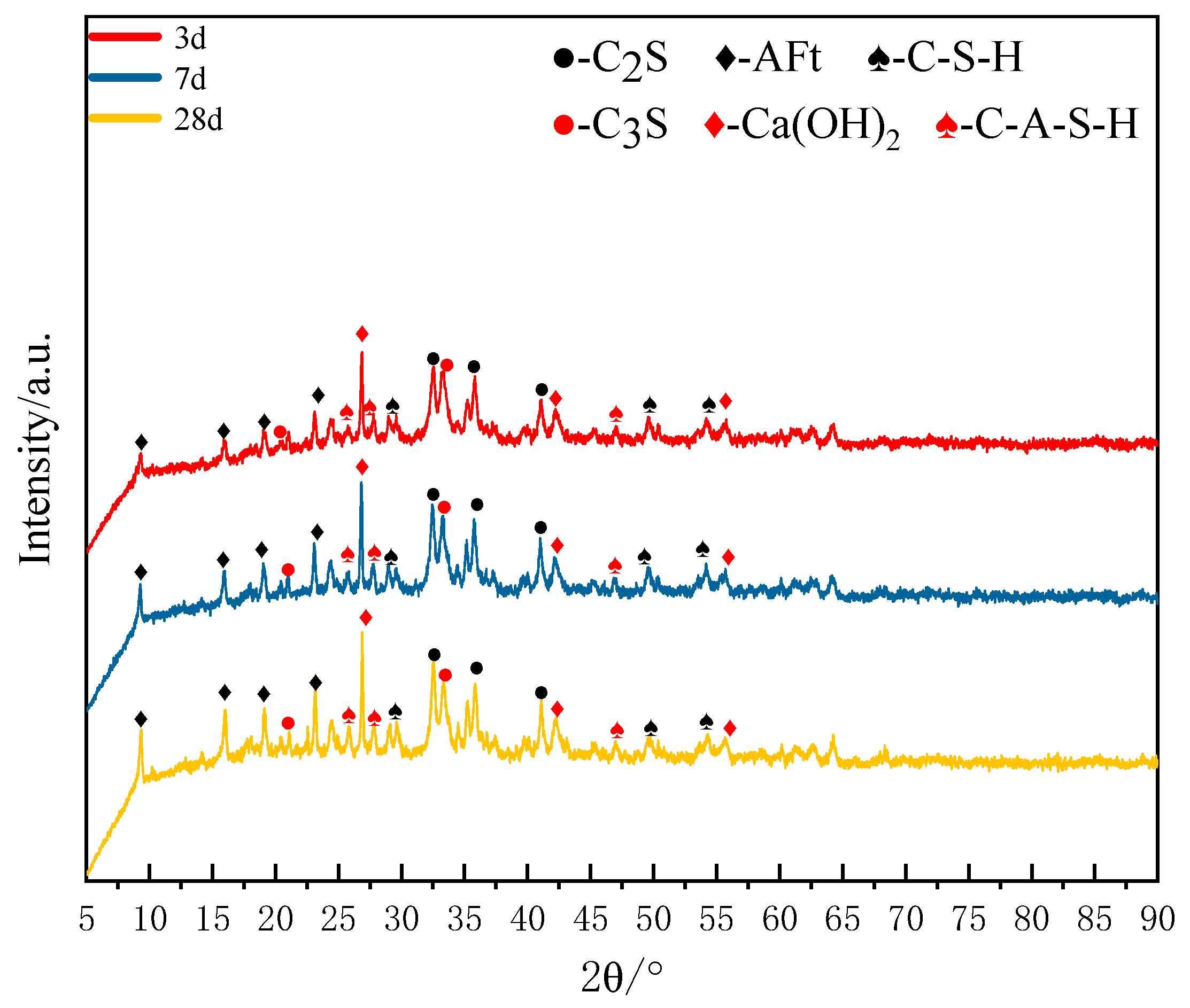
The XRD patterns of SZ-11 hydrated for 3, 7 and 28 days are presented in
Figure 6.
As shown in
Figure 6, the primary hydration products of the SZ-11 specimen comprise a series of zeolite-like crystalline phases. The inclusion of amorphous or weakly crystalline calcium silicate hydrates (C-S-H) significantly enhances the early-stage strength of cement-based systems. The layered structure of C-S-H forms a dense microscopic skeleton, which contributes to the rapid development of strength. Aft (chemical formula Ca
6Al
2(SO
4)
3(OH)
12·26H
2O) exists as slender acicular crystals, which further improve the long-term strength of the specimen by filling capillary pores and generating micro-expansion effects. Additionally, the calcium aluminate silicate hydrate (C-A-S-H) phase is detected, exhibiting structural features that integrate the cross-linked characteristics of both C-S-H and C-A-S-H. Its three-dimensional network effectively confines Al
3+ and Si
4+ ions, thereby yielding a more compact structure. The XRD pattern of the raw materials simultaneously reveals diffraction peaks of dicalcium silicate (C
2S) and tricalcium silicate (C
3S). As pivotal mineral phases in Portland cement clinker, these compounds provide abundant sources of Ca
2+ and SiO
44− ions. Under the action of the alkaline activator, they rapidly dissociate, dissolve, and participate in early-stage hydration reactions. By the 3 d, Characteristic diffraction peaks of Aft were clearly observed within the SZ-11 system. This indicates that the ion concentrations of SO
42− and AlO
2− in the SS-EMR-RM alkali-activated system had reached the supersaturation threshold, thereby promoting the rapid nucleation and growth of Aft. As the hydration age continuously extended from 3 days to 7 days and then to 28 days, the intensity of AFt’s diffraction peaks exhibited a significant increasing trend. This signifies a continuous increase in the crystal size of Aft, an improvement in its crystallinity, and a corresponding rise in the AFt content within the system. This result provides indirect confirmation that continuously supplied ions such as Ca
2+, Al(OH)
4−, and SO
42− diffuse to the surface of Aft crystals and participate in crystal growth via a dissolution-precipitation mechanism, consequently strengthening the microstructure of the matrix [
39]. SS and RM-bearing reactive CaO, SiO
2, Al
2O
3-promptly liberate significant Ca
2+/OH
− ions with trace alkali cations (Na
+, K
+) in alkaline media. This rapid ion release elevates pore solution pH > 12.5, establishing critical conditions for C-S-H gel nucleation and Ca(OH)
2 crystallization. Relevant chemical equations for this system are as follows [
40,
41,
42]:
In general, the early stage (3 d) is primarily driven by dissolution–nucleation, with the initial formation of AFt and C-S-H. The mid-term stage (7 d) shows accelerated reactions and refinement of the gel structure. In the later stage (28 d), the reaction has approached completion, characterized by the full development of AFt crystals, highly polymerized C-S-H, and microstructural densification.
4.2. FTIR Analysis
In this study, FTIR spectra were obtained for optimally blended SZ-11 specimens at various hydration stages (3, 7, and 28 days), capturing the molecular vibrational information over the wavenumber range of 4000–500 cm
−1. These spectra, which are crucial for understanding the chemical composition and structural changes during hydration, are detailed in
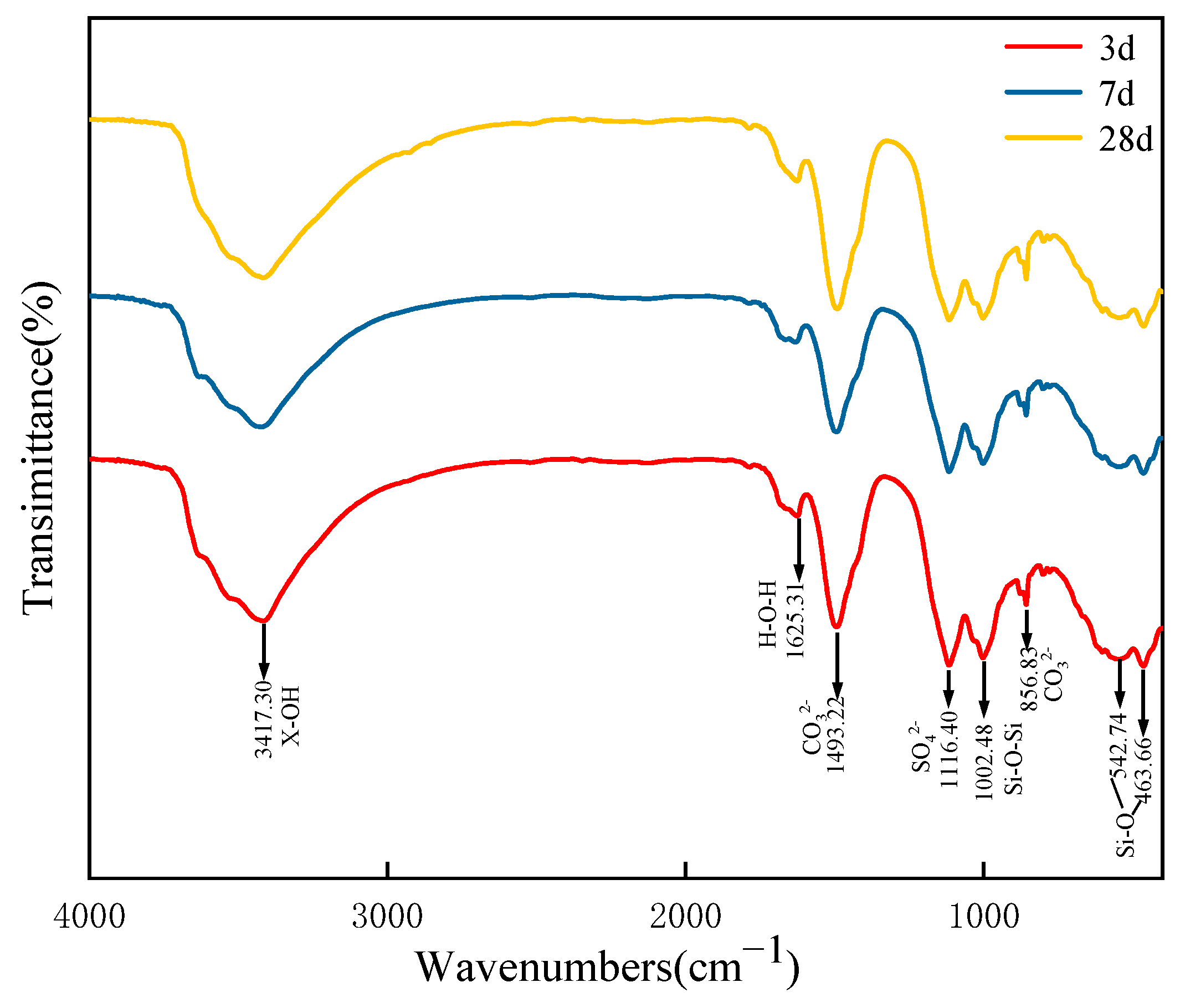
Figure 7. Characteristic absorption peaks were observed at 3417, 1625, 1493, 1116, 1002, 856, 542 and 463 cm
−1 for all samples.
As shown in
Figure 7, an absorption peak appeared at 3417 cm
−1, primarily attributed to the asymmetric stretching vibration of (X-OH) [
43]. The bending vibration of H-O-H in C-S-H gel is mainly characterized by the absorption peak at 1625 cm
−1 [
44]. These two absorption peaks collectively demonstrate that the hydration reaction process results in substantial substances rich in water of crystallization. In the infrared spectroscopy results of the 3-day sample, the SO
42− stretching vibration of AFt was observed, characterized by an absorption peak at 1116 cm
−1 [
45]. This indicates the formation of AFt in the specimen at 3 days, a result consistent with the XRD analysis. The absorption peak at 1493 cm
−1 is associated with the vibration of CO
32−, while the peak at 856 cm
−1 corresponds to the bending vibration of CO
32−. These sharp peaks arise from the reaction between atmospheric carbon dioxide and alkali components in geopolymer, combined with asymmetric stretching of CO
32− [
46]. The absorption peaks observed at 542 cm
−1 and 463 cm
−1 are attributed to vibrations of the Si-O bond.
In addition, by comparing the absorption peak at 1116 cm
−1 for specimens of different ages, it was observed that this peak sharpen progressively (or gradually) with increasing age. This result indicates that the degree of hydration reaction increases gradually with age, leading to a continuous increase in the degree of polymerization of the AFt (or resulting AFt) produced by the hydration. The absorption peak near 1002 cm
−1 corresponds to the asymmetric stretching vibrations of Si-O-Si and Si-O-Al bonds within the C-S-H gel. This peak sharpens with increasing curing age, indicating an increase in the degree of polymerization of the C-S-H gel [
47]. This peak sharpens with increasing curing age, indicating an increase in the degree of polymerization of the C-S-H gel. These results are consistent with the XRD and SEM findings, which show an increase in AFt formation from 3 days to 7 days.
4.3. SEM Analysis
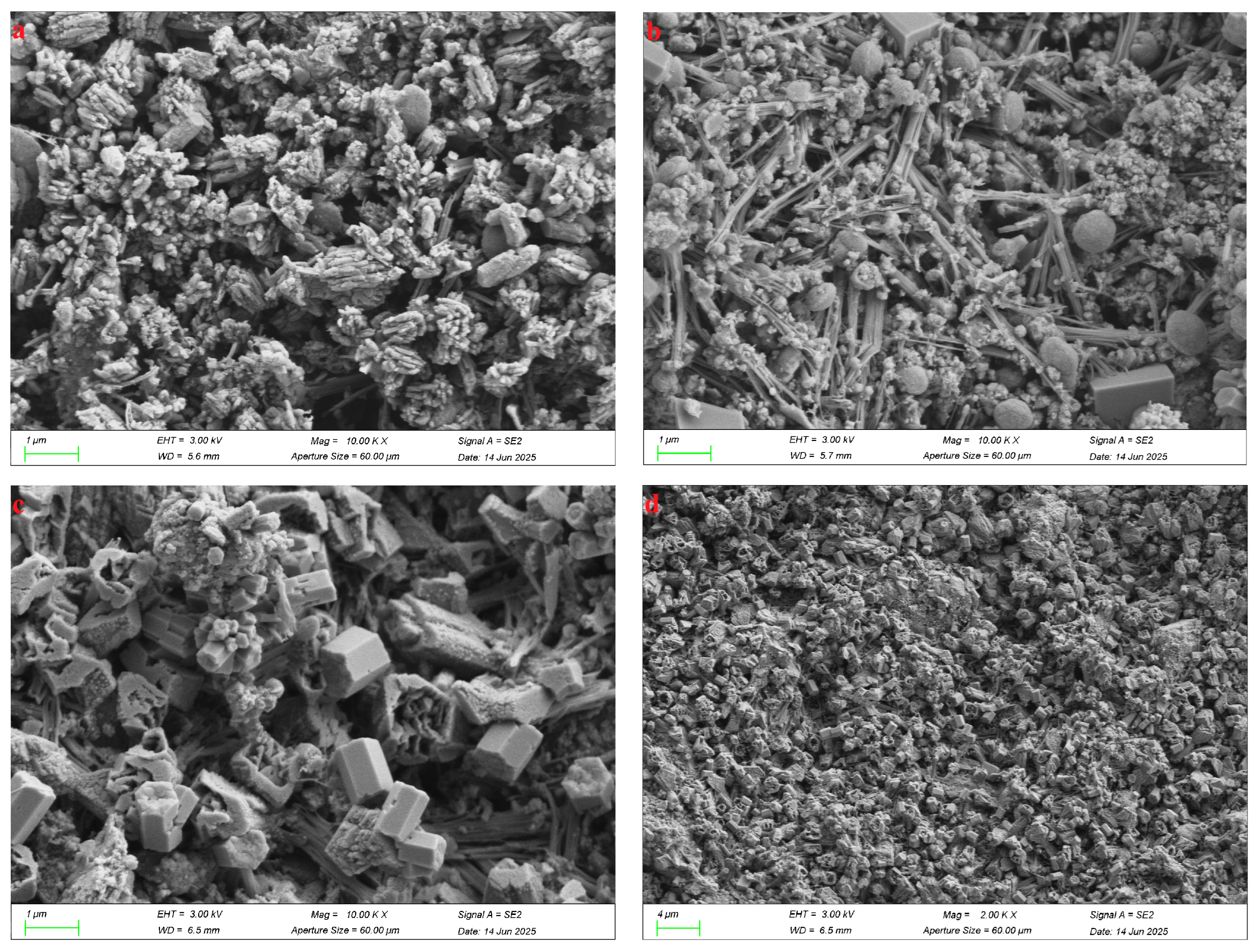
Figure 8 presents the microstructural evolution of the SZ-11 specimen at three critical curing ages: 3 d, 7 d, and 28 d. Through XRD confirmation of the crystalline phase of the AFt phase and the FTIR spectral evidence from intensity variations in the Si–O–T stretching/bending vibrational bands at 1116 cm
−1 and 463 cm
−1 (indicating the polymerization degree of C–S–H), the SEM images further reveal, in high resolution, temporal disparities in the spatial distribution and morphological characteristics of hydration products.
The AFt crystals formed after 3 days progressively develop into coarse, rod-like morphologies over 28 days of hydration (
Figure 8a). At 3 days, a high spatial density of AFt is evident within the specimen, a finding that corroborates the results obtained from X-ray diffraction, infrared spectroscopy, and thermogravimetric analysis. Owing to the limited degree of hydration, the SZ-11 sample exhibits poor compactness and pronounced interstitial porosity after 3 days of curing. With prolonged hydration up to 28 days, the microstructure becomes markedly denser (
Figure 8c,d). This densification is attributed to the continuous precipitation of calcium–silicate–hydrate (C-S-H) gel and the concurrent growth of AFt crystals, which progressively infill the internal voids of the SZ-11 matrix and overlap the surfaces of unreacted particles. Consequently, the initially discrete solid phases become bridged, yielding a more compact, cohesive, and mechanically robust microstructure.
4.4. Thermogravimetric Analysis
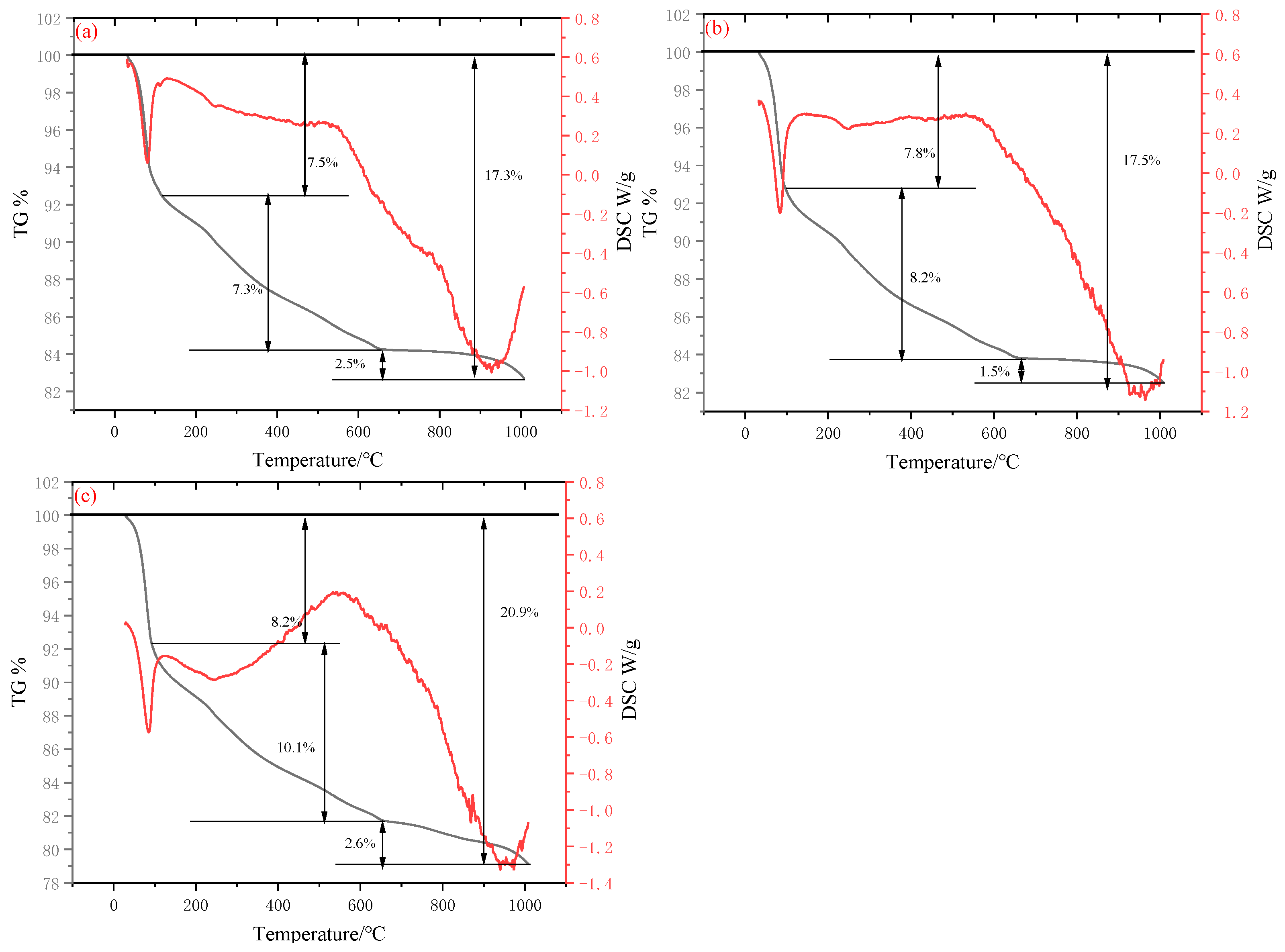
As illustrated in
Figure 9, the specimens exhibit three pronounced mass-loss regions at all investigated hydration ages, and the cumulative mass loss at 28 d consistently exceeds that at 3 d. The three regions are centred at 30–150 °C, 200–600 °C and 650–1000 °C.
Thermogravimetric (TG) analysis revealed that the cementitious material exhibited a characteristic stepwise mass loss pattern throughout the heating process. As the temperature increased progressively from room temperature, the first distinct weight loss step occurred within the temperature range of 30 °C to 150 °C. Concurrently, a prominent endothermic valley was observed in the Differential Scanning Calorimetry (DSC) curve over this same temperature interval. This behavior is primarily attributed to the evaporation of free water and the removal of weakly bound water, such as interlayer water molecules within C-S-H gel. This phenomenon represents a typical thermoresponsive signature associated with the initial hydration products of cementitious materials [
48]. Subsequently, a second weight loss step can be observed between 200 °C and 600 °C. This stage is primarily attributed to the thermal decomposition of Ca(OH)
2 [
49]. The third weight loss step occurs in the range of 650 °C to 1000 °C. This stage primarily involves the final dehydration of both C-S-H gel and AFt [
50]. The observed endothermic range around 850 °C in the curve may correspond to the crystallization of cementitious materials, as supported by the hydration heat data and the chemical reactions of cement components. As the amount of hydration products increases, both the internal structure and mechanical properties are enhanced. Alkaline substances in RM combine with Ca
2+, forming calcium hydroxide that further reacts with active silica and alumina to generate gels such as AFt and C-S-H. Cementitious materials exhibit significant synergistic effects among RM, EMR, and SS, ultimately enhancing the hydration degree of the material.
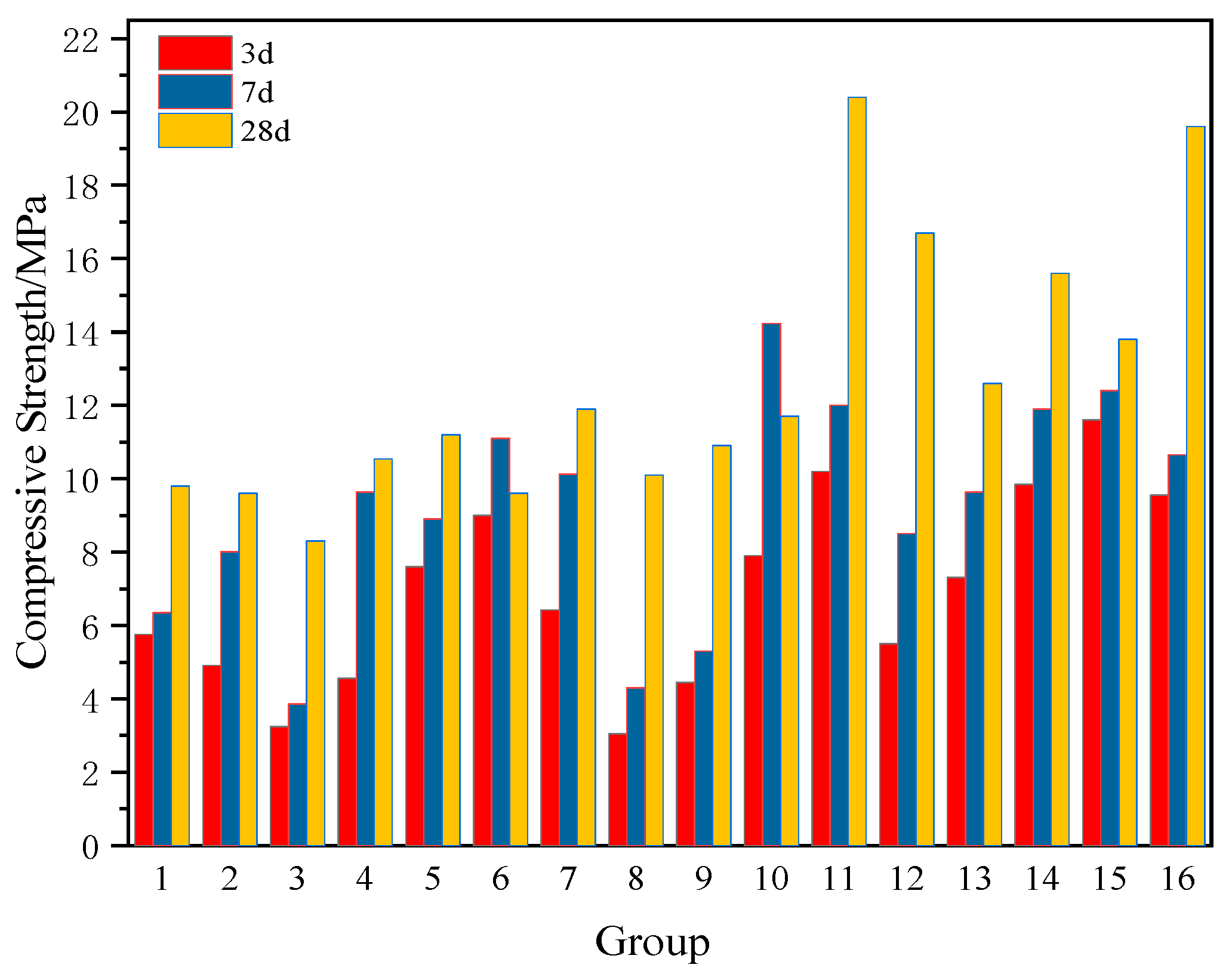
It is noteworthy that as the total hydration products accumulate over time, the cumulative weight loss corresponding to each stage of TG increased from 17.3% at 3 days (10.27 MPa) to 20.9% at 28 days (20.48 MPa). This indicates a significant increase in chemically bound water content and densification of the internal microstructure. The alkaline components in RM (primarily NaOH and KOH) rapidly release OH− during early hydration, reacting with dissolved Ca2+ to form Ca(OH)2. This further undergoes pozzolanic reactions with reactive SiO2 and Al2O3 from EMR and SS, continuously generating C-S-H and AFt gels. The synergistic effects among the three solid wastes manifest as: RM provides an alkaline environment and Al3+, EMR contributes soluble silicon and trace SO42−, while SS supplements Ca2+. This forms a multi-reaction system of “alkaline activation-pozzolanic-sulfoaluminate”, significantly enhancing both the hydration degree and mechanical properties of the cementitious material.
4.5. Hydration Mechanism
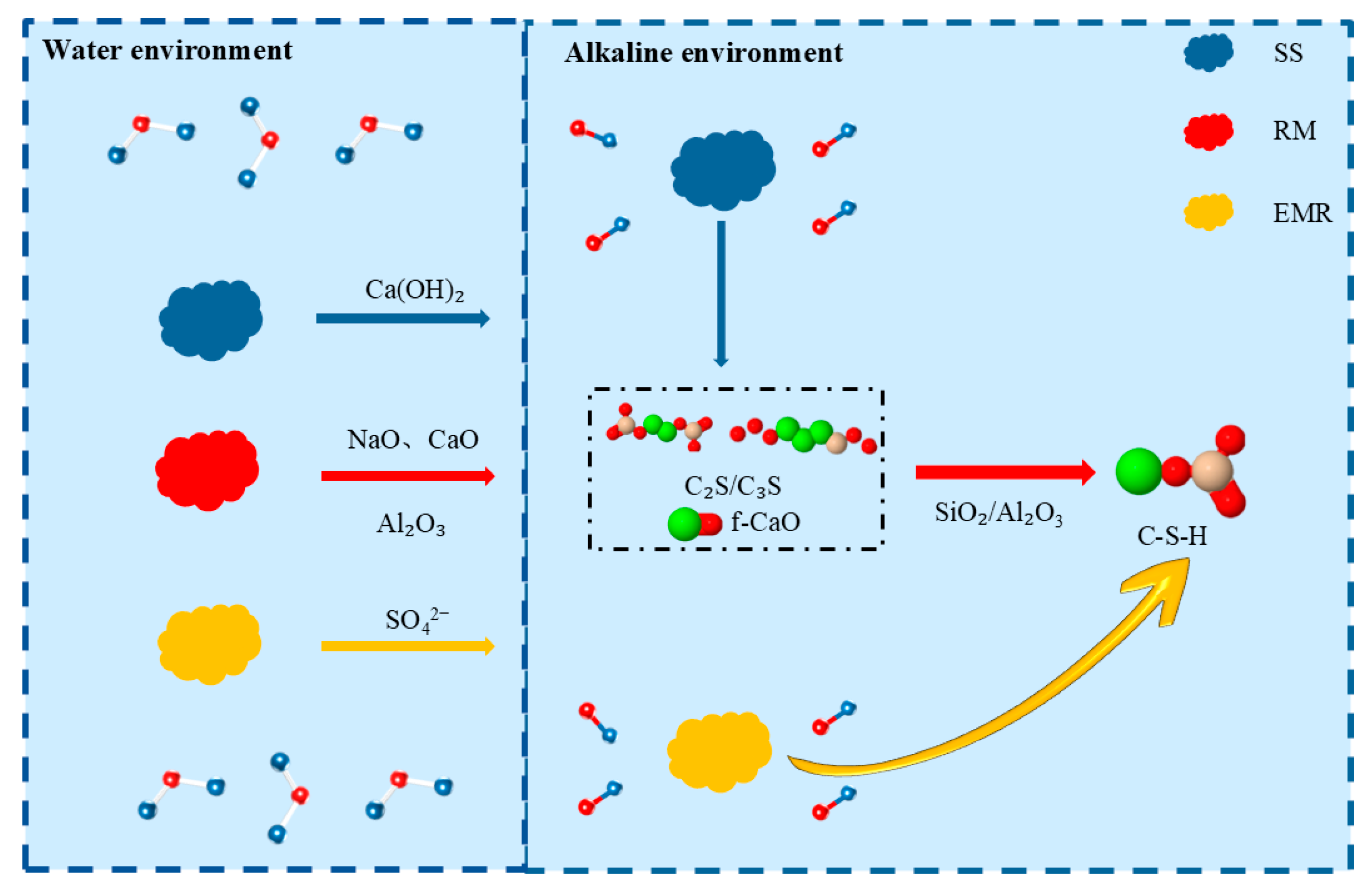
The hydration mechanism of the composite system is schematically illustrated in
Figure 10. Red mud functions as an alkaline activator by rapidly releasing Na
2O and CaO, thereby establishing a highly alkaline environment (pH > 13) that effectively activates the latent reactivity of both steel slag and electrolytic manganese residue. The Al
2O
3 present in red mud concurrently serves as an essential precursor for the subsequent formation of hydration products such as calcium aluminate hydrates. Steel slag constitutes the principal source of long-term strength development and alkalinity regulation within the system. The C
2S and C
3S phases are the dominant contributors to mechanical strength, whereas the progressive hydration of these phases continuously generates Ca(OH)
2, which sustains the alkaline conditions required for pozzolanic reactions. The free CaO (f-CaO) contained in steel slag is rapidly consumed by the reactive SiO
2 and Al
2O
3 supplied by red mud and electrolytic manganese residue under the prevailing alkaline conditions, thereby converting a potentially deleterious component into additional C-S-H gel. Electrolytic manganese residue functions as both a supplementary cementitious material and a micro-aggregate. Under an alkaline environment, the vitreous phase of manganese residue undergoes depolymerization and activation, resulting in the formation of supplementary C-S-H gel. The abundant SiO
2 present in manganese residue serves as a key reactant for f-CaO consumption, thereby further promoting C-S-H precipitation. Additionally, the fine-grained fraction of electrolytic manganese residue effectively refines the pore structure by physical packing, thereby optimizing the overall microstructural density of the composite system.
This ternary system avoids the detrimental effects of “alkali excess” often induced by high red mud content, while simultaneously harnessing the inherent cementitious properties of steel slag. An optimal balance is achieved between alkaline activation efficiency and gel-forming contribution. The high alkalinity derived from red mud activates the pozzolanic/reactivity potential of both steel slag and electrolytic manganese residue. Meanwhile, the active Si–Al components provided by electrolytic manganese residue and red mud effectively consume the free CaO in steel slag, thereby preventing harmful expansion. Through combined chemical reactions and physical densification mechanisms among the three solid wastes, a cementitious system with robust strength development and enhanced volumetric stability is formed.