The Use of Metal/ZSM-5 Nanosheet for Efficient Catalytic Cracking of Cross-Linked Polyethylene for High-Voltage Cable Insulation
Abstract
1. Introduction
2. Results and Discussion
2.1. ZSM-5 Loaded with Different Metal Ions (Ce, Mo, Ni, and Ag)
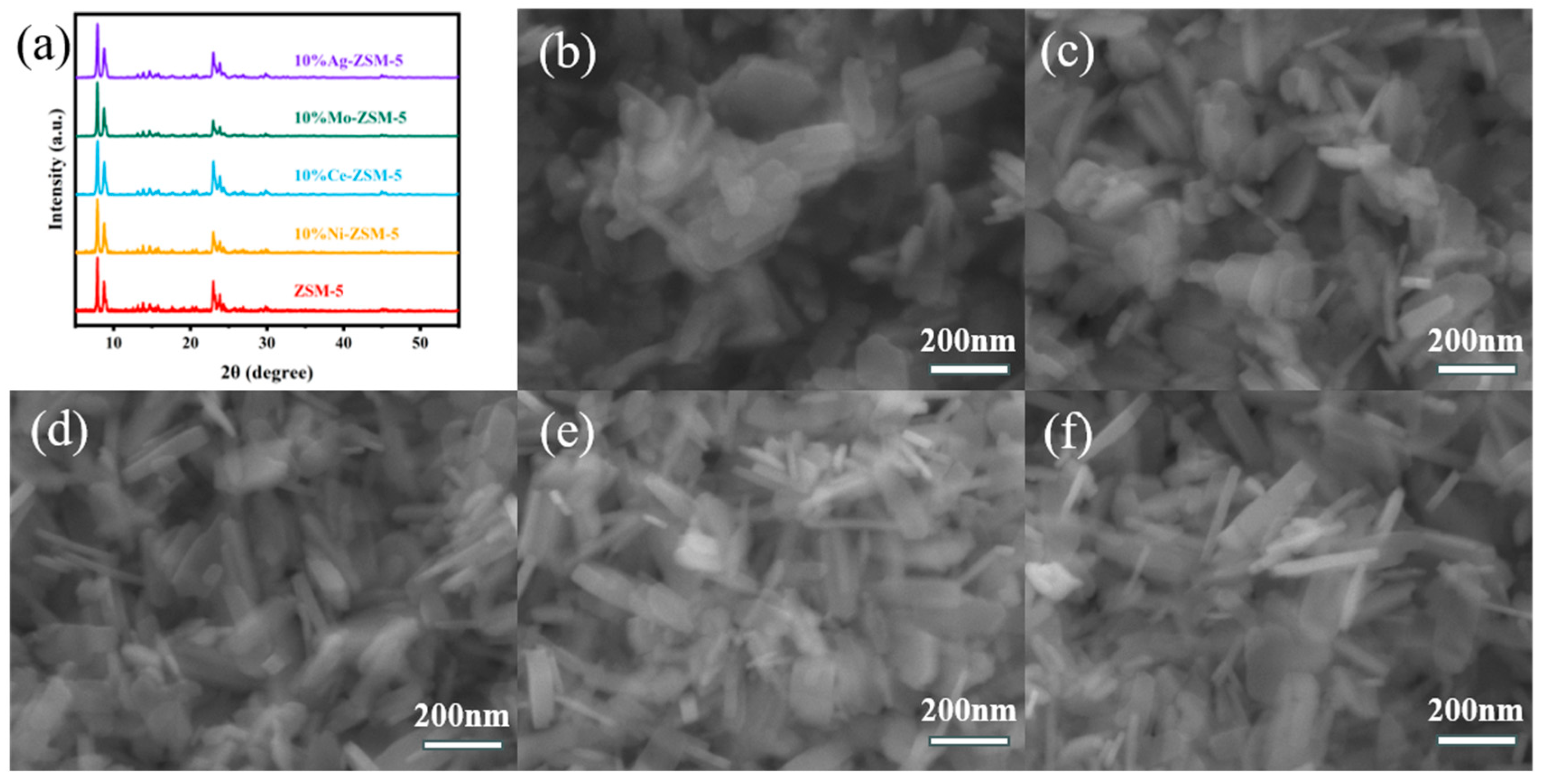
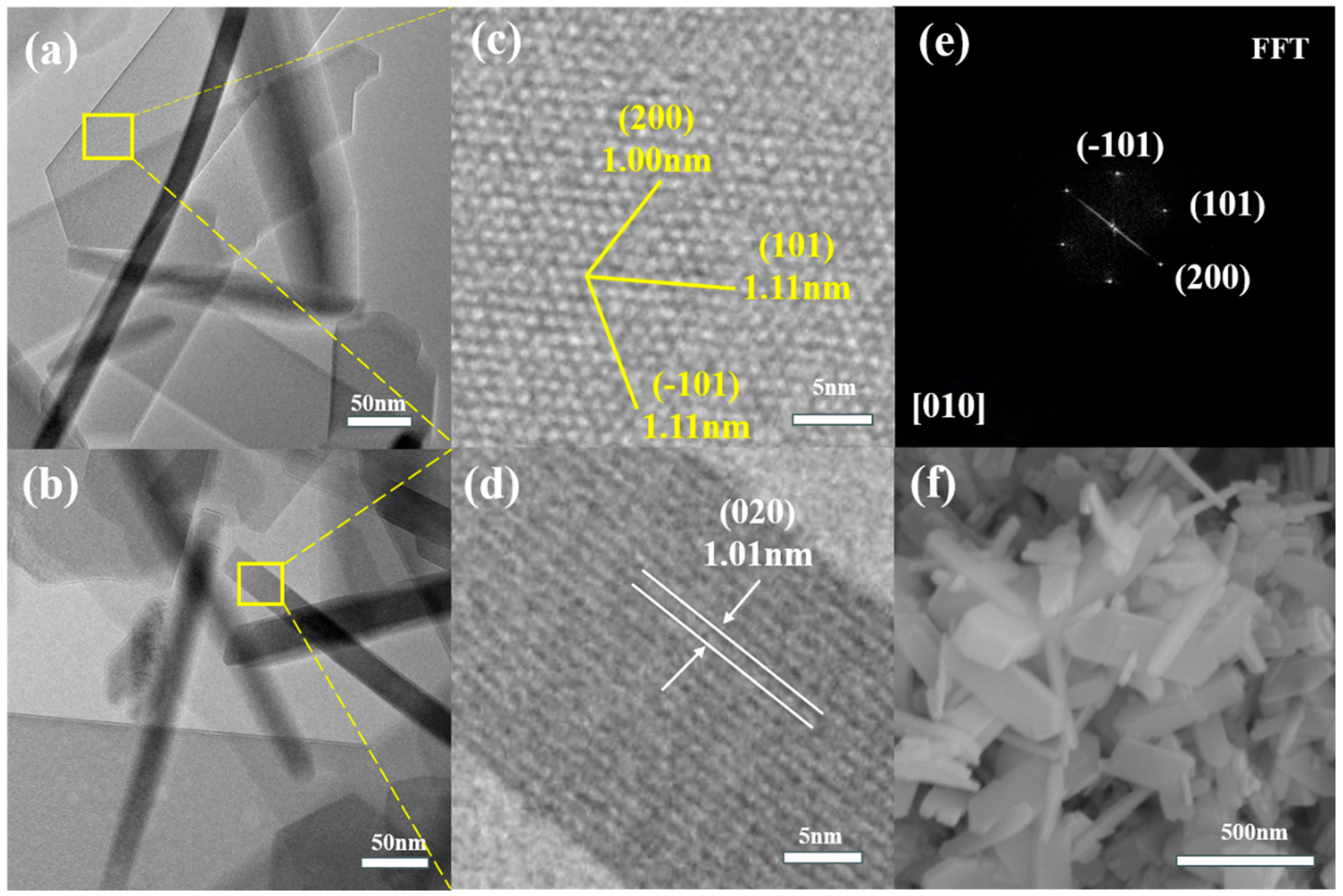
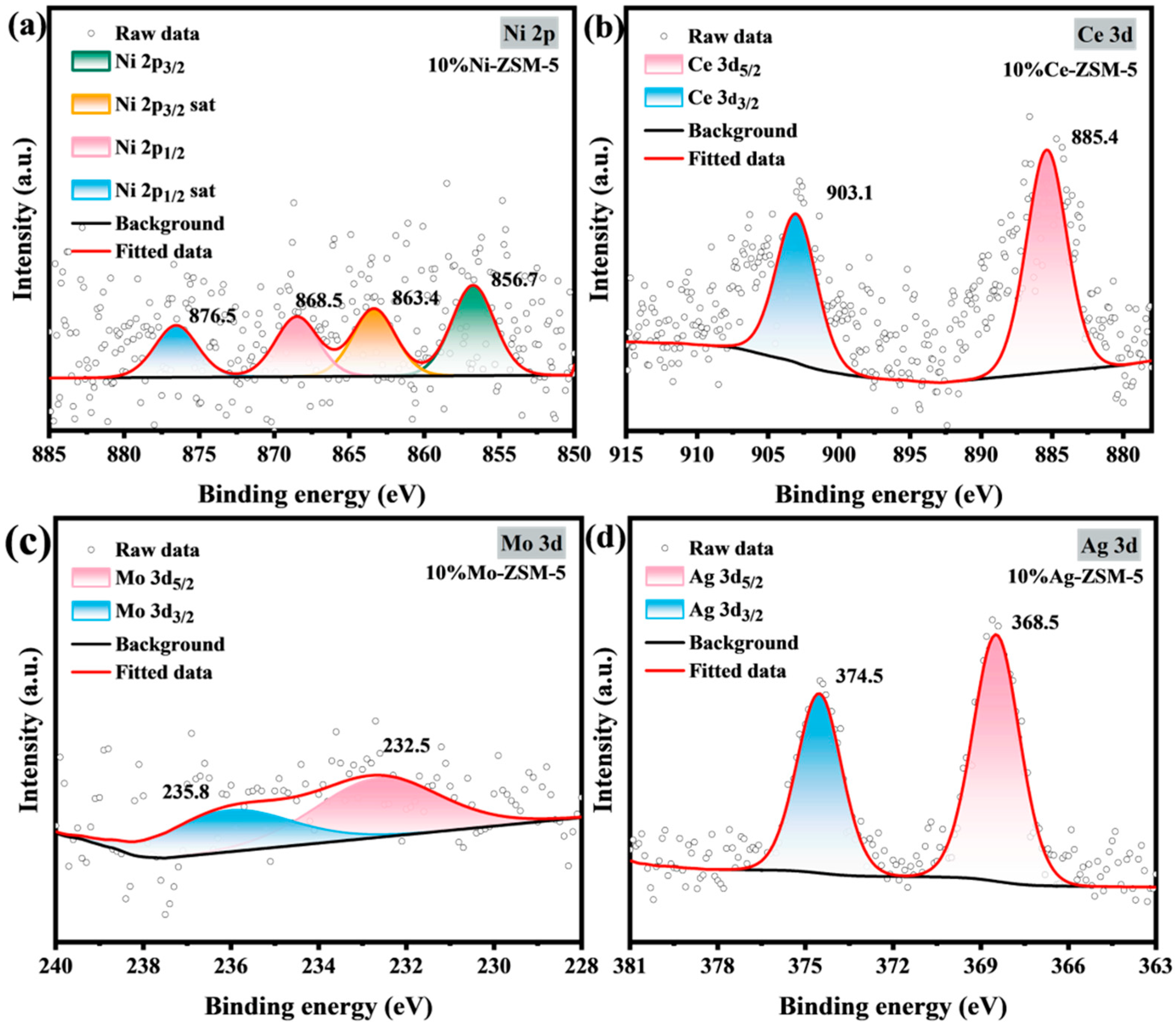
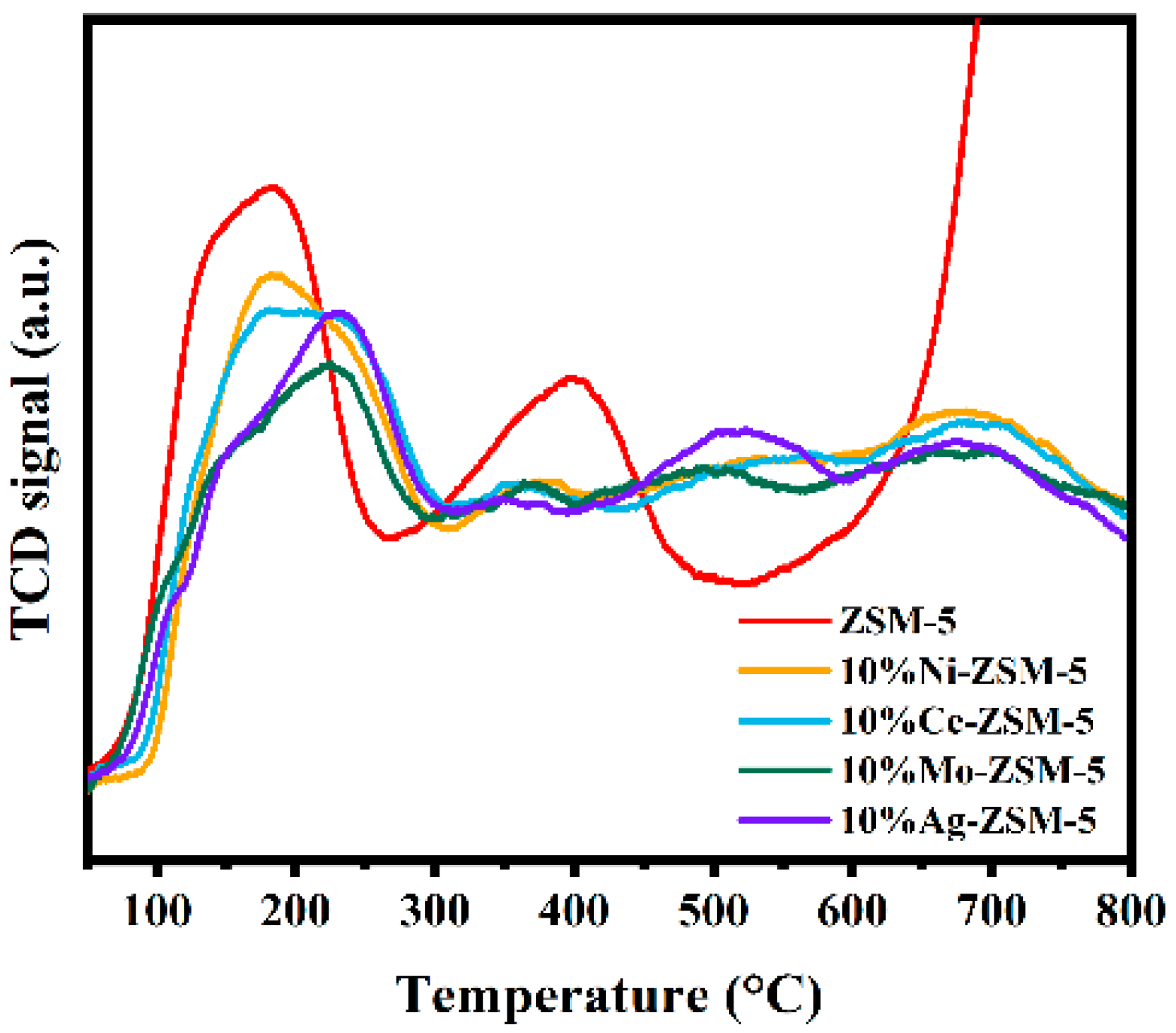
2.1.1. Catalyst Characterization
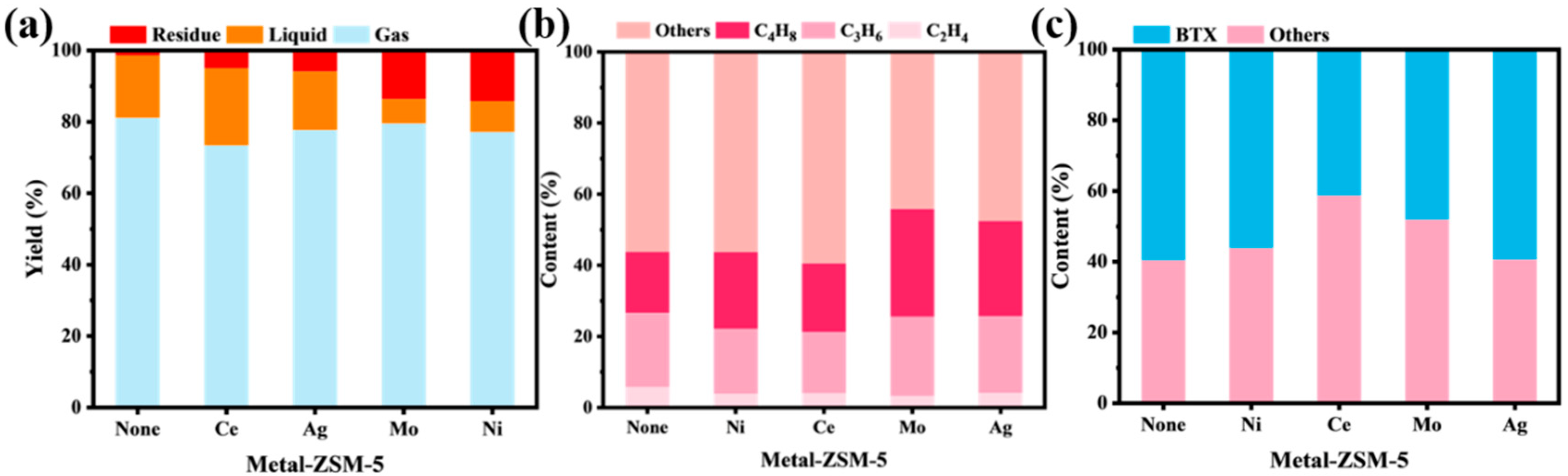
2.1.2. Catalytic Cracking Performance
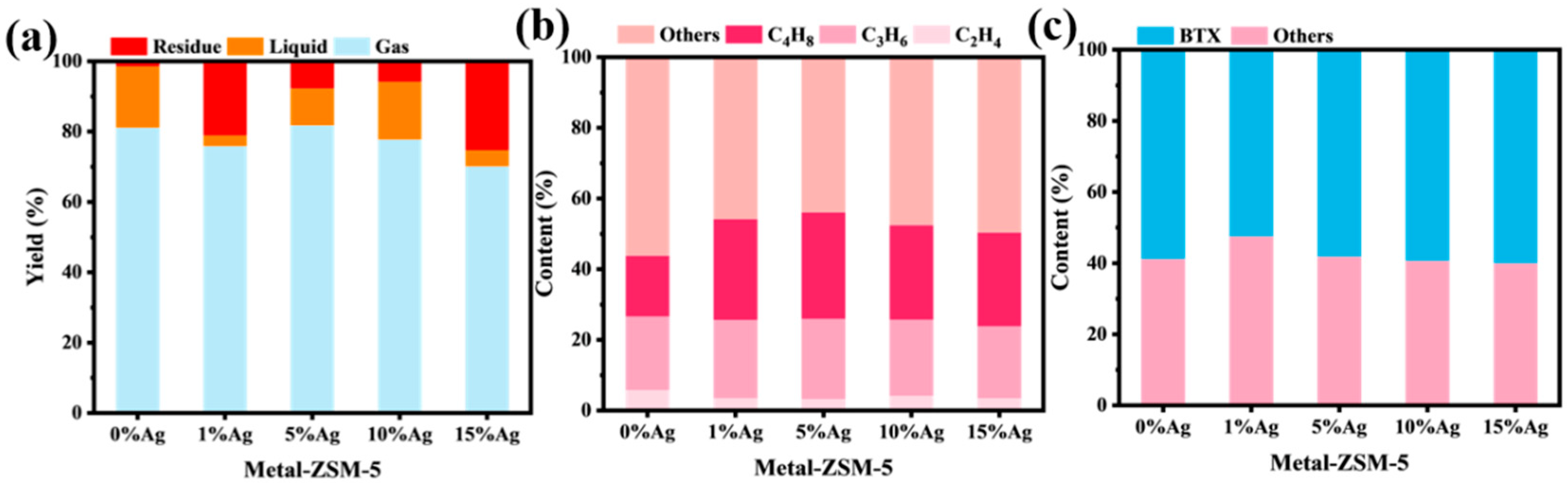
2.2. Ag-Ion Loading at Different Ratios on ZSM-5 Nanosheets
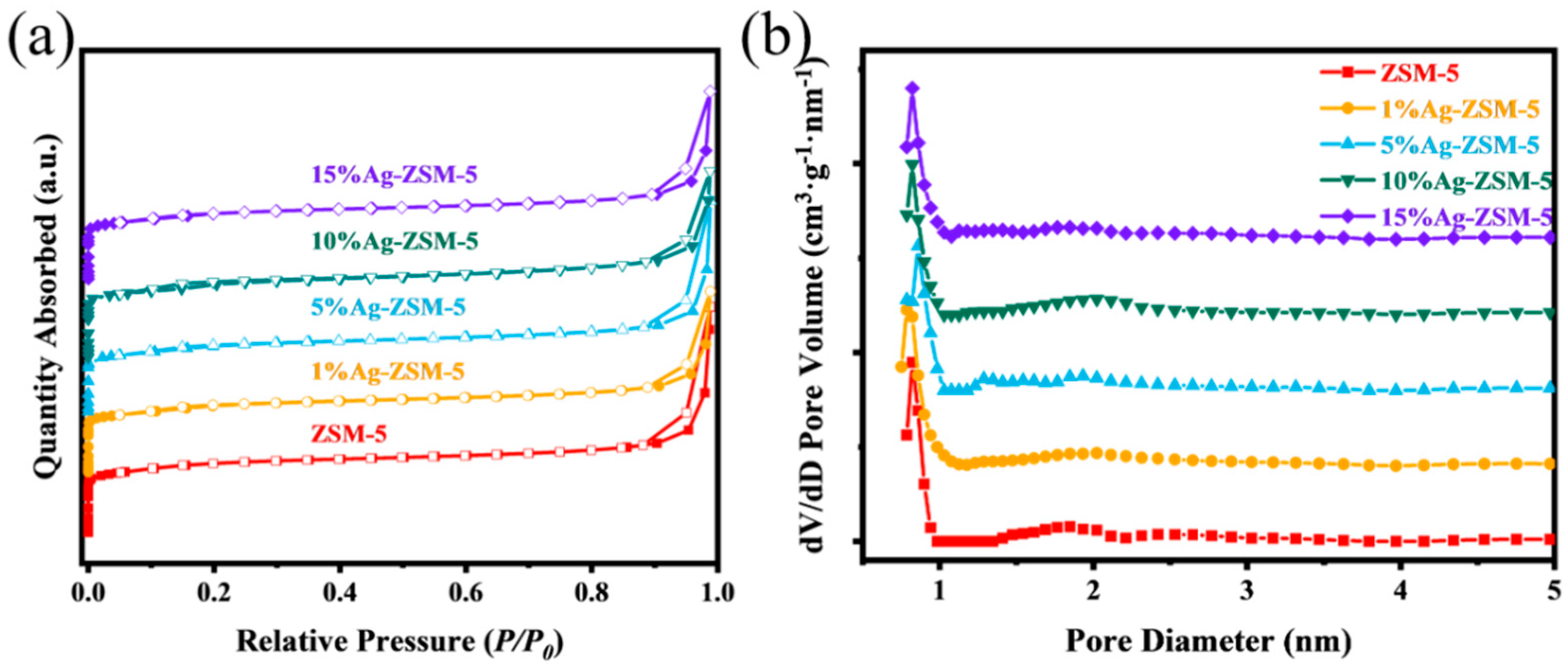
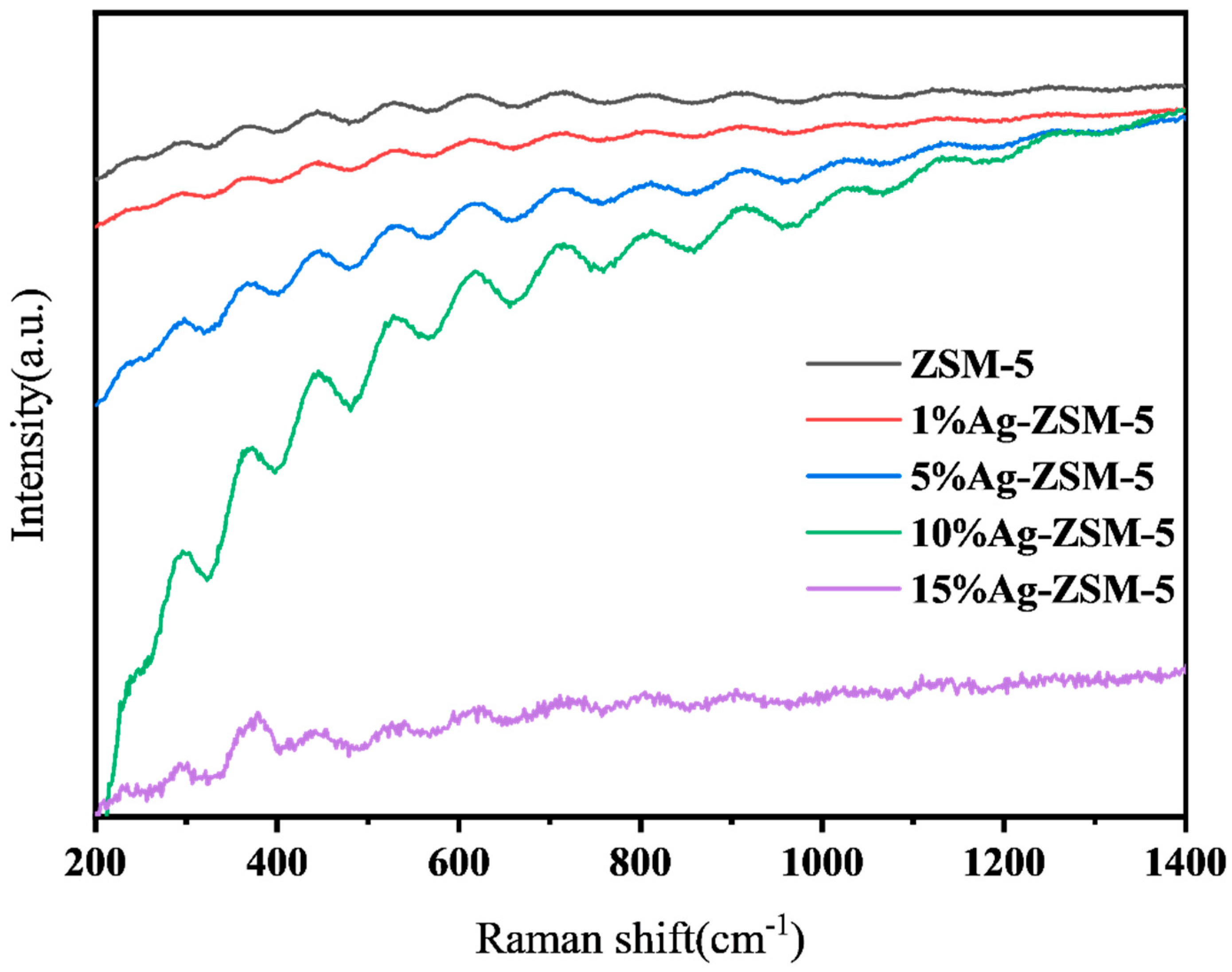
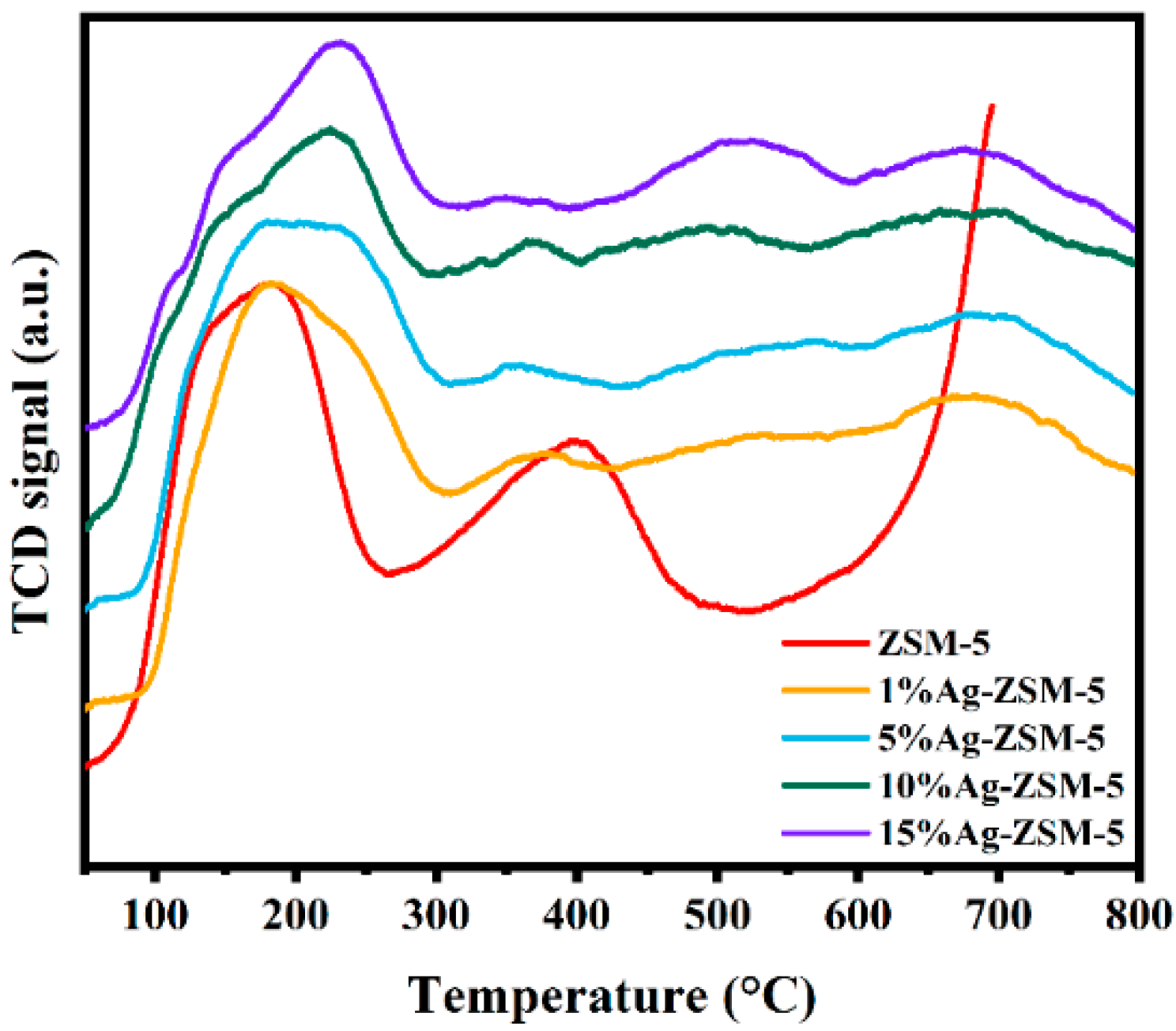
2.2.1. Catalyst Characterization
2.2.2. Catalytic Cracking Performance
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Meng, Q. XLPE Insulation Materials for High Voltage Cables. Plast. Addit. 2024, 24–26. [Google Scholar]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef]
- Peng, B.; Wu, H.; Bao, W.; Guo, S.; Chen, Y.; Huang, H.; Chen, H.; Lai, S.-Y.; Jow, J. Effects of ultrasound on the morphology and properties of propylene-based plastomer/nanosilica composites. Polym. J. 2011, 43, 91–96. [Google Scholar] [CrossRef]
- Will, T.A.; Lu, Y.; Wakabayashi, K. Effects of Polymer Properties on Solid-State Shear Pulverization: Thermoplastic Processability and Nanofiller Dispersibility. ACS Appl. Polym. Mater. 2023, 5, 1848–1858. [Google Scholar] [CrossRef]
- Nishida, H. Development of materials and technologies for control of polymer recycling. Polym. J. 2011, 43, 435–447. [Google Scholar] [CrossRef]
- Wang, N.M.; Strong, G.; DaSilva, V.; Gao, L.; Huacuja, R.; Konstantinov, I.A.; Rosen, M.S.; Nett, A.J.; Ewart, S.; Geyer, R.; et al. Chemical Recycling of Polyethylene by Tandem Catalytic Conversion to Propylene. J. Am. Chem. Soc. 2022, 144, 18526–18531. [Google Scholar] [CrossRef]
- Zou, L.; Xu, R.; Wang, H.; Wang, Z.; Sun, Y.; Li, M. Chemical recycling of polyolefins: A closed-loop cycle of waste to olefins. Natl. Sci. Rev. 2023, 10, nwad207. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, X.; Xiang, X.; Qu, P.; Zhu, M. Recent developments in recycling of post-consumer polyethylene waste. Green Chem. 2025, 27, 4040–4093. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Ke, L.; Dai, L.; Wu, Q.; Cobb, K.; Zeng, Y.; Zou, R.; Liu, Y.; Ruan, R. A review on catalytic pyrolysis of plastic wastes to high-value products. Energy Convers. Manag. 2022, 254, 115243. [Google Scholar] [CrossRef]
- Biakhmetov, B.; Dostiyarov, A.; Ok, Y.S.; You, S. A review on catalytic pyrolysis of municipal plastic waste. WIREs Energy Environ. 2023, 12, e495. [Google Scholar] [CrossRef]
- Corma, A.; Orchillés, A.V. Current views on the mechanism of catalytic cracking. Microporous Mesoporous Mater. 2000, 35–36, 21–30. [Google Scholar] [CrossRef]
- Weitkamp, J. Catalytic Hydrocracking—Mechanisms and Versatility of the Process. ChemCatChem 2012, 4, 292–306. [Google Scholar] [CrossRef]
- Dong, Z.; Chen, W.; Xu, K.; Liu, Y.; Wu, J.; Zhang, F. Understanding the Structure–Activity Relationships in Catalytic Conversion of Polyolefin Plastics by Zeolite-Based Catalysts: A Critical Review. ACS Catal. 2022, 12, 14882–14901. [Google Scholar] [CrossRef]
- Chen, Z.; Monzavi, M.; Latifi, M.; Samih, S.; Chaouki, J. Microwave-responsive SiC foam@zeolite core-shell structured catalyst for catalytic pyrolysis of plastics. Environ. Pollut. 2022, 307, 119573. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, N.; Cobb, K.; Chen, P.; Wang, Y.; Liu, Y.; Zou, R.; Lei, H.; Mohamed, B.A.; Cheng, Y.; et al. Insights into structure–performance relationship in the catalytic cracking of high density polyethylene. Appl. Catal. B Environ. 2022, 318, 121835. [Google Scholar] [CrossRef]
- Tarach, K.A.; Pyra, K.; Siles, S.; Melián-Cabrera, I.; Góra-Marek, K. Operando Study Reveals the Superior Cracking Activity and Stability of Hierarchical ZSM-5 Catalyst for the Cracking of Low-Density Polyethylene. ChemSusChem 2019, 12, 633–638. [Google Scholar] [CrossRef]
- Tarach, K.A.; Akouche, M.; Pyra, K.; Valtchev, V.; Jajko, G.; Gilson, J.-P.; Góra-Marek, K. Polypropylene cracking on embryonic and ZSM-5 catalysts—An operando study. Appl. Catal. B Environ. 2023, 334, 122871. [Google Scholar] [CrossRef]
- Mintova, S.; Grand, J.; Valtchev, V. Nanosized zeolites: Quo Vadis? Comptes Rendus. Chim. 2016, 19, 183–191. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, L.; Zhou, A.; Li, W.; Shang, S.; Liu, Y.; Jia, Z.; Liu, W.; Zhang, A.; Guo, X.; et al. Tailored Synthesis of ZSM-5 Nanosheets with Controllable b-Axis Thickness and Aspect Ratio: Strategy and Growth Mechanism. Chem. Mater. 2022, 34, 3217–3226. [Google Scholar] [CrossRef]
- Hu, S.; Shan, J.; Zhang, Q.; Wang, Y.; Liu, Y.; Gong, Y.; Wu, Z.; Dou, T. Selective formation of propylene from methanol over high-silica nanosheets of MFI zeolite. Appl. Catal. A Gen. 2012, 445–446, 215–220. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Wang, R.; Sun, C.; Zeng, S.; Jiang, S.; Zhang, D.; Zhu, L.; Zhang, Z. N-Methyl-2-pyrrolidone assisted synthesis of hierarchical ZSM-5 with house-of-cards-like structure. RSC Adv. 2014, 4, 21301–21305. [Google Scholar] [CrossRef]
- Huang, H.-w.; Zhu, H.; Zhang, S.-h.; Zhang, Q.; Li, C.-y. Effect of silicon to aluminum ratio on the selectivity to propene in methanol conversion over H-ZSM-5 zeolites. J. Fuel Chem. Technol. 2019, 47, 74–82. [Google Scholar] [CrossRef]
- Hu, H.; Lyu, J.; Rui, J.; Cen, J.; Zhang, Q.; Wang, Q.; Han, W.; Li, X. The effect of Si/Al ratio on the catalytic performance of hierarchical porous ZSM-5 for catalyzing benzene alkylation with methanol. Catal. Sci. Technol. 2016, 6, 2647–2652. [Google Scholar] [CrossRef]
- Qian, K.; Tian, W.; Yin, L.; Yang, Z.; Tian, F.; Chen, D. Aromatic production from high-density polyethylene over zinc promoted HZSM-5. Appl. Catal. B Environ. 2023, 339, 123159. [Google Scholar] [CrossRef]
- Wang, W.; Yao, C.; Ge, X.; Pu, X.; Yuan, J.; Sun, W.; Chen, W.; Feng, X.; Qian, G.; Duan, X.; et al. Catalytic conversion of polyethylene into aromatics with Pt/ZSM-5: Insights into reaction pathways and rate-controlling step regulation. J. Mater. Chem. A 2023, 11, 14933–14940. [Google Scholar] [CrossRef]
- Rostamizadeh, M.; Taeb, A. Highly selective Me-ZSM-5 catalyst for methanol to propylene (MTP). J. Ind. Eng. Chem. 2015, 27, 297–306. [Google Scholar] [CrossRef]
- Nuhma, M.J.; Alias, H.; Tahir, M.; Jazie, A.A. Ce-Loaded HZSM-5 Composite for Catalytic Deoxygenation of Algal Hydrolyzed Oil into Hydrocarbons and Oxygenated Compounds. Molecules 2022, 27, 7251. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, M.; Li, B.; Liu, J.; Song, X.; Wu, M.; Yin, Y. Co-pyrolysis of waste plastics and black liquor catalyzed by Mo-Ni/HZSM-5 for preparing high-quality bio-oil. J. Anal. Appl. Pyrolysis 2024, 180, 106540. [Google Scholar] [CrossRef]
- Kubo, K.; Iida, H.; Namba, S.; Igarashi, A. Comparison of steaming stability of Cu-ZSM-5 with those of Ag-ZSM-5, P/H-ZSM-5, and H-ZSM-5 zeolites as naphtha cracking catalysts to produce light olefin at high temperatures. Appl. Catal. A Gen. 2015, 489, 272–279. [Google Scholar] [CrossRef]
- Wang, X.; Wu, X.; Zhao, M.; Zhang, R.; Wang, Z.; Li, Y.; Zhang, L.; Wang, X.; Song, S.; Zhang, H. High-efficiency Ce-modified ZSM-5 nanosheets for waste plastic upgrading. Nano Res. 2024, 17, 5645–5650. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Z.; Chen, L.; Yang, S.; Xie, X.; Gao, M.; Zhao, B.; Si, H.; Li, J.; Hua, D. Catalytic Fast Pyrolysis of Biomass into Aromatic Hydrocarbons over Mo-Modified ZSM-5 Catalysts. Catalysts 2020, 10, 1051. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, A.; Zhang, Y.S.; Xie, W.; Liu, C.; Peng, Y.; Zhang, H.; Kang, Y.; Qu, B.; Ji, G. In-situ catalytic pyrolysis of polyethylene to co-produce BTX aromatics and H2 by Ni/ZSM-5 in the rotary reactor with solid heat carriers. Fuel 2024, 371, 131950. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Lu, J.; Hu, Y.; Chen, J.; Ren, D.; Li, Z.; Zhang, Q.; Yan, H.; Chen, X.; et al. Insight into catalytic cracking pathways of n-pentane over bifunctional catalysts to produce light olefins. AIChE J. 2024, 70, e18266. [Google Scholar] [CrossRef]
- Aspromonte, S.G.; Mizrahi, M.D.; Schneeberger, F.A.; López, J.M.R.; Boix, A.V. Study of the Nature and Location of Silver in Ag-Exchanged Mordenite Catalysts. Characterization by Spectroscopic Techniques. J. Phys. Chem. C 2013, 117, 25433–25442. [Google Scholar] [CrossRef]
- Gęsikiewicz-Puchalska, A.; Zgrzebnicki, M.; Michalkiewicz, B.; Kałamaga, A.; Narkiewicz, U.; Morawski, A.W.; Wrobel, R. Changes in porous parameters of the ion exchanged X zeolite and their effect on CO2 adsorption. Molecules 2021, 26, 7520. [Google Scholar] [CrossRef]
- David, E. Evaluation of behavior of 13X zeolite modified with transition metals for catalytic applications. Bioinorg. Chem. Appl. 2022, 2022, 7352074. [Google Scholar] [CrossRef]
- Ravi Chandra Raju, N.; Jagadeesh Kumar, K.; Subrahmanyam, A. Physical properties of silver oxide thin films by pulsed laser deposition: Effect of oxygen pressure during growth. J. Phys. D Appl. Phys. 2009, 42, 135411. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Isothermal and non-isothermal kinetics of thermally stimulated reactions of solids. Int. Rev. Phys. Chem. 1998, 17, 407–433. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Goryachko, V. Potentialities of software for kinetic processing of thermoanalytical data by the isoconversion method. Thermochim. Acta 1992, 194, 221–230. [Google Scholar] [CrossRef]
- Araujo, A.S.; Fernandes, V.J.; Fernandes, G.J.T. Thermogravimetric kinetics of polyethelyne degredation over silicoaluminophosphate. Thermochim. Acta 2002, 392–393, 55–61. [Google Scholar] [CrossRef]



| Sample | SBET (m2/g) | Sext a (m2/g) | Vmicro a (cm3/g) | Vmero b (cm3/g) | Vtotal c (cm3/g) | Daver d (nm) |
|---|---|---|---|---|---|---|
| ZSM-5 | 419.440 | 62.395 | 0.154 | 0.477 | 0.580 | 0.822 |
| 10%Ni-ZSM-5 | 431.431 | 71.319 | 0.154 | 0.492 | 0.600 | 0.857 |
| 10%Ce-ZSM-5 | 392.462 | 63.624 | 0.144 | 0.333 | 0.427 | 0.798 |
| 10%Mo-ZSM-5 | 390.593 | 58.251 | 0.152 | 0.379 | 0.469 | 0.803 |
| 10%Ag-ZSM-5 | 392.621 | 65.866 | 0.141 | 0.419 | 0.502 | 0.812 |
| Acid Site Concentration (µmol/g) | ||||
|---|---|---|---|---|
| Weak-Medium | Strong | Metal-Strong | Total | |
| ZSM-5 | 152.35 | 122.83 | / | 275.18 |
| 10%Ni-ZSM-5 | 159.46 | 80.98 | 193.44 | 433.88 |
| 10%Ce-ZSM-5 | 169.10 | 127.24 | 90.17 | 386.51 |
| 10%Mo-ZSM-5 | 139.16 | 133.36 | 83.41 | 355.93 |
| 10%Ag-ZSM-5 | 123.56 | 94.56 | 79.54 | 297.66 |
| Sample | SBET (m2/g) | Sext a (m2/g) | Vmicro a (cm3/g) | Vmeso b (cm3/g) | Vtotal c (cm3/g) | Daver d (nm) |
|---|---|---|---|---|---|---|
| ZSM-5 | 419.440 | 62.395 | 0.154 | 0.477 | 0.580 | 0.822 |
| 1%Ag-ZSM-5 | 398.798 | 64.675 | 0.147 | 0.369 | 0.465 | 0.785 |
| 5%Ag-ZSM-5 | 392.920 | 68.711 | 0.143 | 0.450 | 0.542 | 0.819 |
| 10%Ag-ZSM-5 | 392.621 | 65.866 | 0.141 | 0.419 | 0.502 | 0.812 |
| 15%Ag-ZSM-5 | 393.797 | 63.145 | 0.144 | 0.387 | 0.483 | 0.796 |
| Sample | Acid Site Concentration (µmol/g) | |||
|---|---|---|---|---|
| Weak-Medium | Strong | Metal-Strong | Total | |
| ZSM-5 | 152.35 | 122.83 | / | 275.18 |
| 1%Ag-ZSM-5 | 121.54 | 118.68 | 57.40 | 297.62 |
| 5%Ag-ZSM-5 | 116.81 | 105.54 | 70.14 | 292.49 |
| 10%Ag-ZSM-5 | 123.56 | 94.56 | 79.54 | 297.66 |
| 15%Ag-ZSM-5 | 141.31 | 87.54 | 90.14 | 318.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Z.; Pan, Y.; Wang, R.; Suo, S.; Wang, Z.; Peng, X.; Fang, P. The Use of Metal/ZSM-5 Nanosheet for Efficient Catalytic Cracking of Cross-Linked Polyethylene for High-Voltage Cable Insulation. Materials 2025, 18, 4675. https://doi.org/10.3390/ma18204675
Fu Z, Pan Y, Wang R, Suo S, Wang Z, Peng X, Fang P. The Use of Metal/ZSM-5 Nanosheet for Efficient Catalytic Cracking of Cross-Linked Polyethylene for High-Voltage Cable Insulation. Materials. 2025; 18(20):4675. https://doi.org/10.3390/ma18204675
Chicago/Turabian StyleFu, Zhenfei, Yuqi Pan, Rui Wang, Shilong Suo, Zheng Wang, Xiangyang Peng, and Pengfei Fang. 2025. "The Use of Metal/ZSM-5 Nanosheet for Efficient Catalytic Cracking of Cross-Linked Polyethylene for High-Voltage Cable Insulation" Materials 18, no. 20: 4675. https://doi.org/10.3390/ma18204675
APA StyleFu, Z., Pan, Y., Wang, R., Suo, S., Wang, Z., Peng, X., & Fang, P. (2025). The Use of Metal/ZSM-5 Nanosheet for Efficient Catalytic Cracking of Cross-Linked Polyethylene for High-Voltage Cable Insulation. Materials, 18(20), 4675. https://doi.org/10.3390/ma18204675







