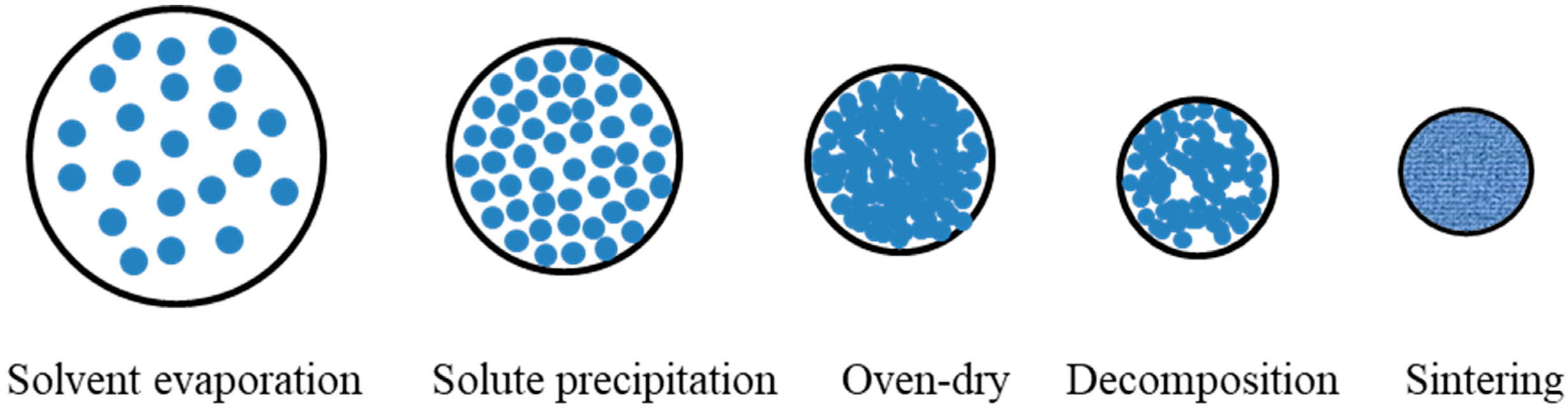
Review of Oxides Prepared by a Short Process Using Rare-Earth Chlorides
Abstract
1. Introduction
2. Research the Status of the Pyrolysis Process for Rare-Earth Chlorides
2.1. Pyrolysis of Fixed-Valence Rare-Earth Chlorides
- 1.
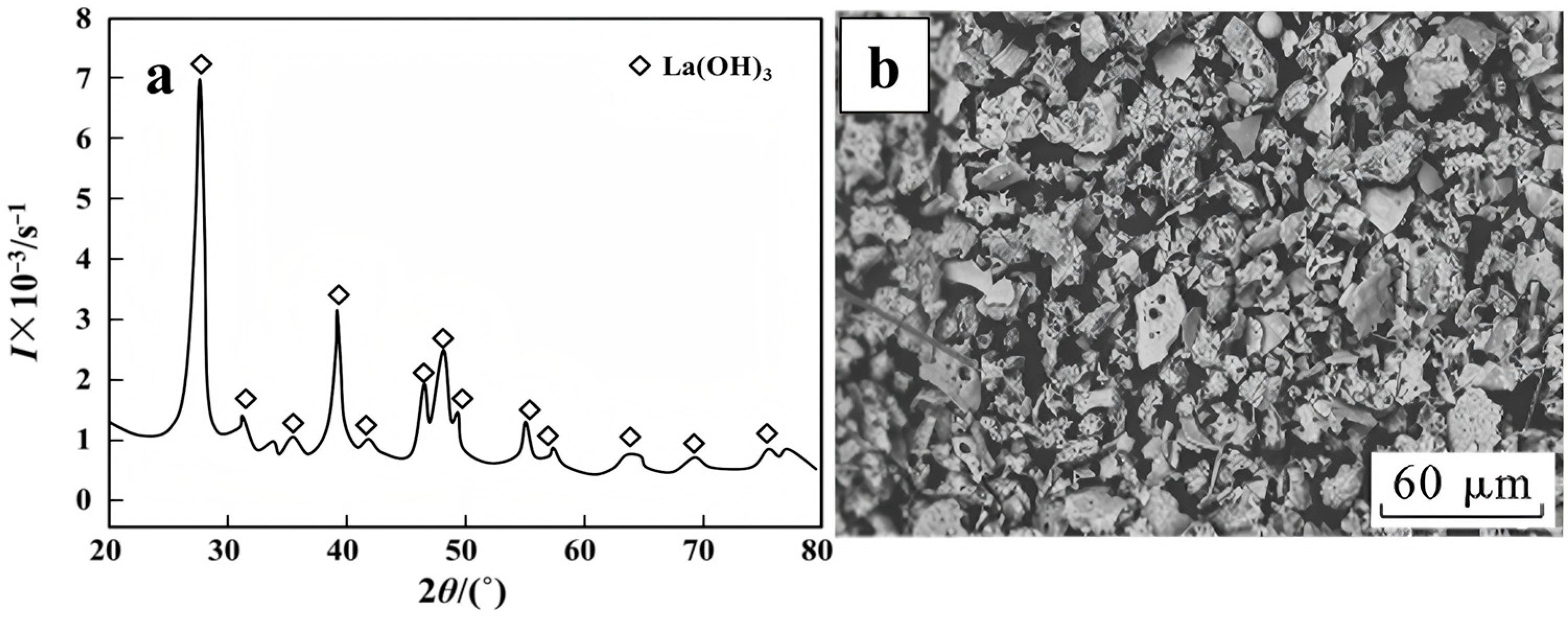
- Lanthanum (La)
- 2.
- Gadolinium (Gd)
- 3.
- Neodymium (Nd)
- 4.
- Rare-Earth Composite Oxides
2.2. Pyrolysis of Variable-Valence Rare-Earth Chlorides
- Cerium (Ce)
- 2.
- Terbium (Tb)
- 3.
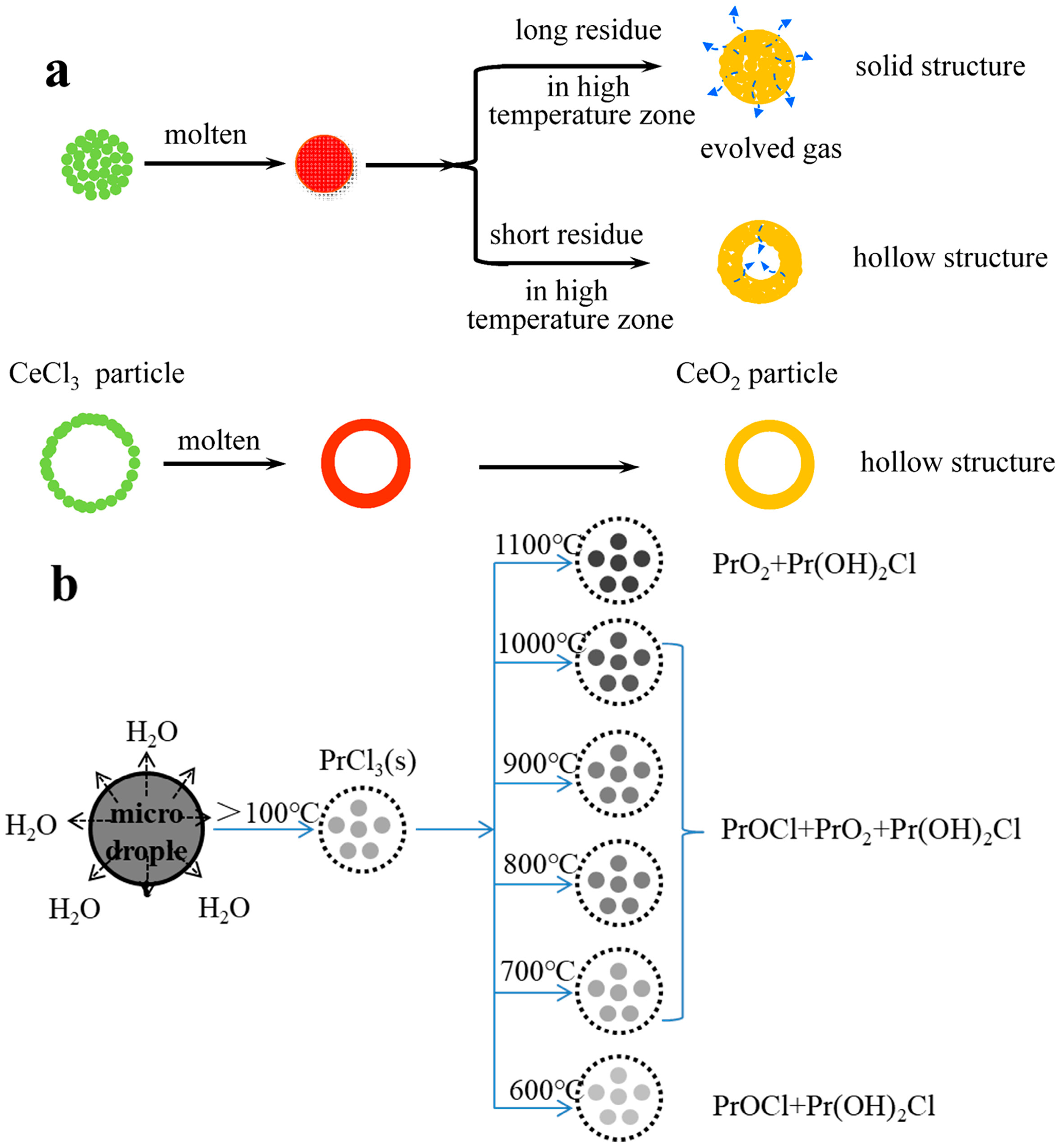
- Praseodymium (Pr)
2.3. Rare-Earth Chloride Pyrolysis Process
3. Pyrolysis Mechanism of Rare-Earth Chlorides
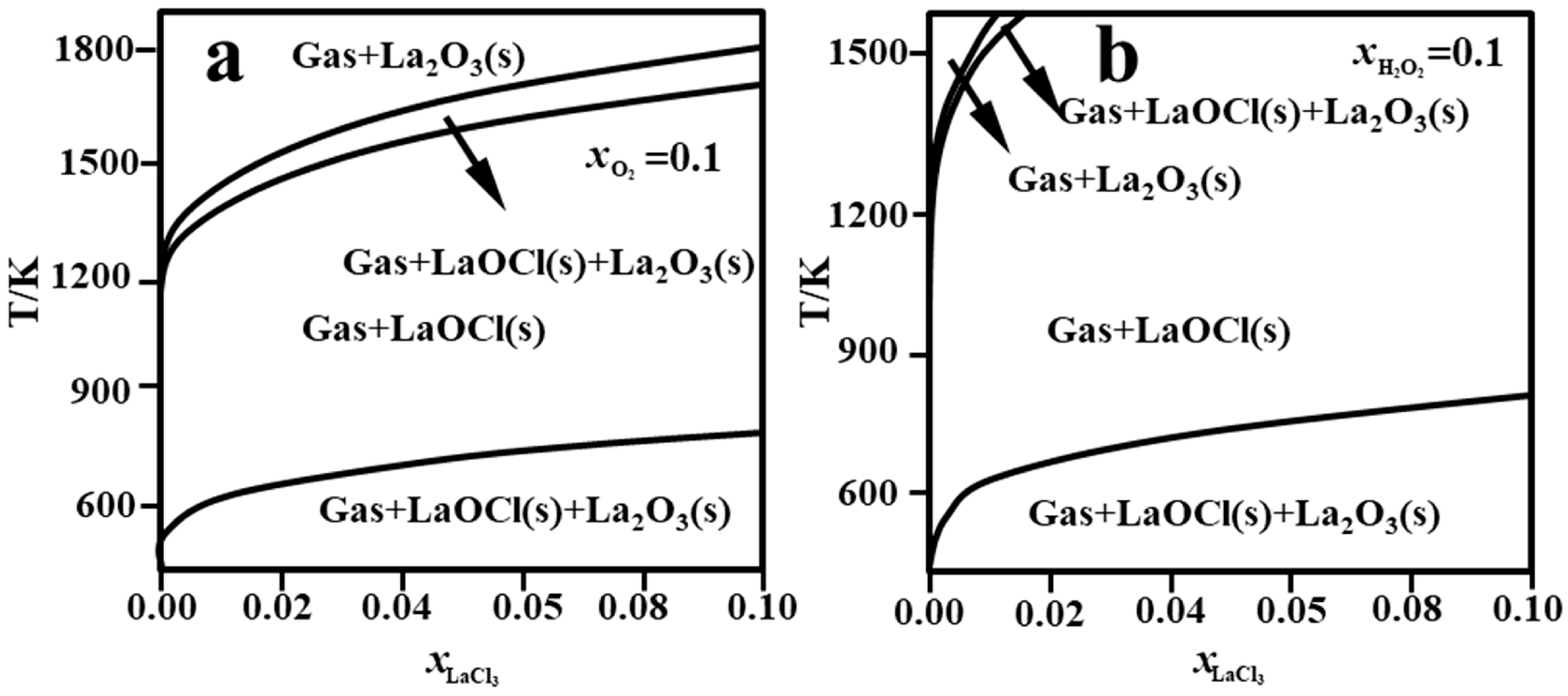
3.1. Thermodynamic Mechanism of the Pyrolysis Process
- 1.
- Pyrolysis Temperature
- 2.
- O2 Partial Pressure
- 3.
- H2O and HCl Partial Pressure
3.2. Kinetic Mechanism of Pyrolysis
3.3. Phase-Transition Mechanism of Pyrolysis
4. Pyrolysis Reactors and Technology Applications
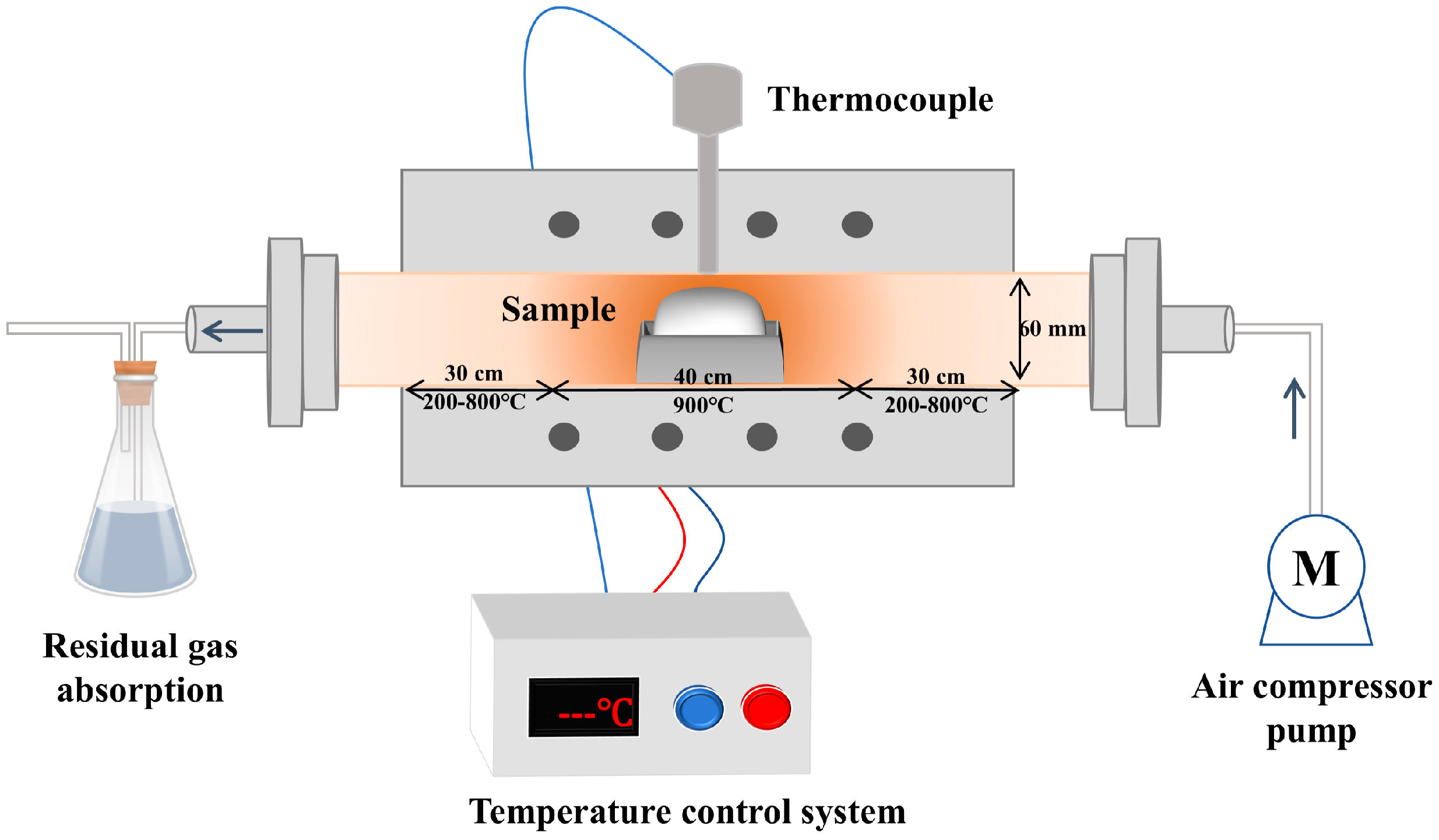
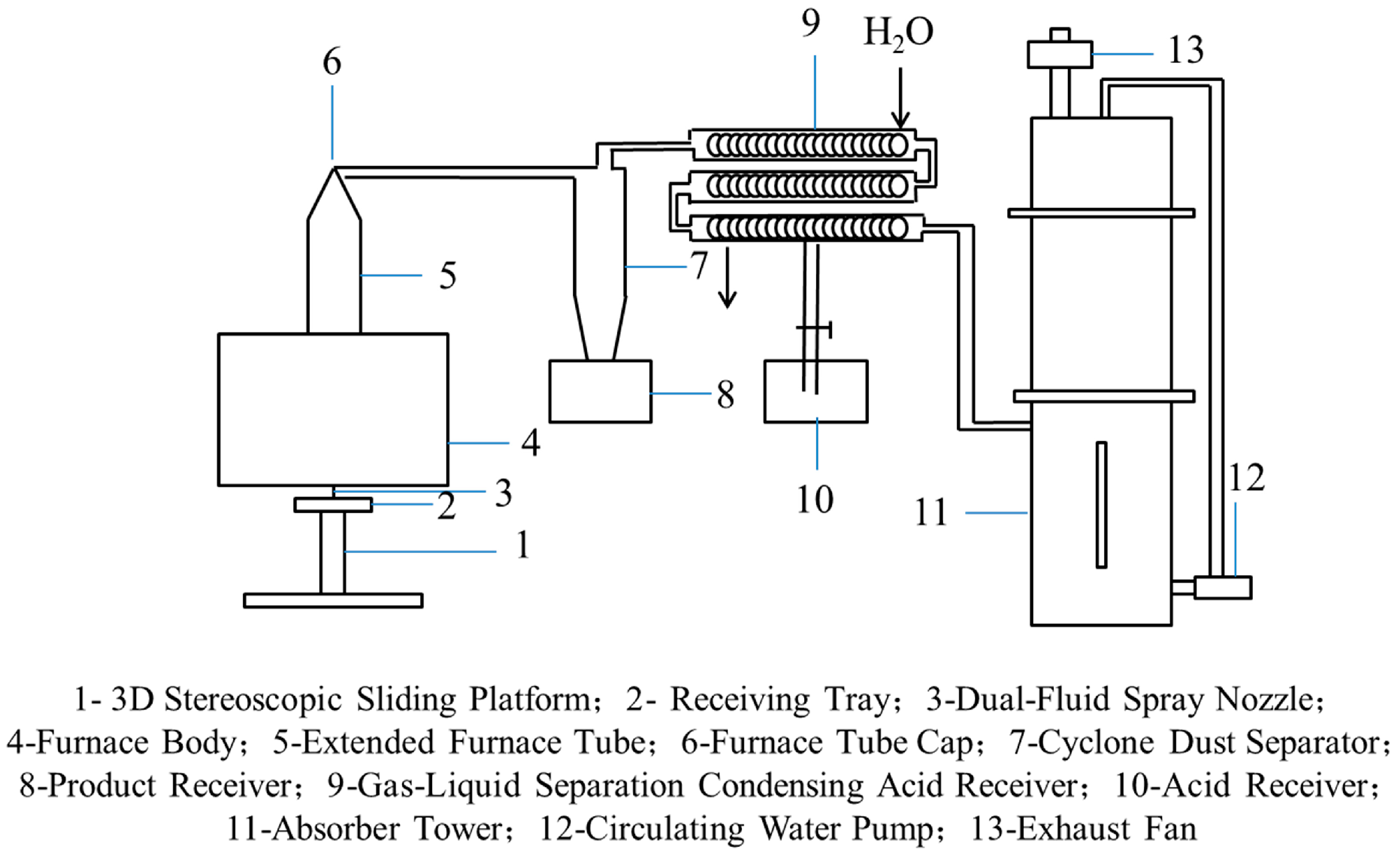
4.1. Pyrolysis Reactors
- 1.
- Horizontal Tubular Furnace Spray Pyrolysis (HTFSP)
- 2.
- Vertical Spray Pyrolysis (VSP)
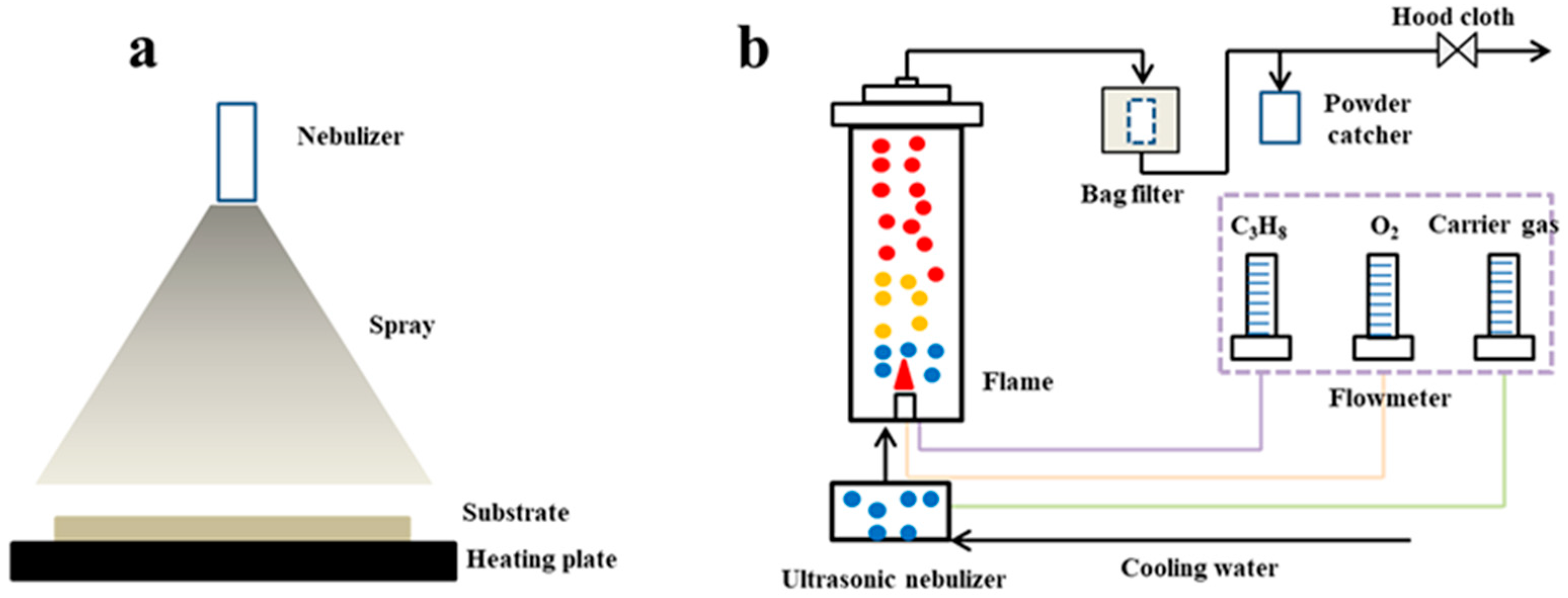
- 3.
- Jet Pyrolysis (JP)
4.2. Technology Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marion, C.; Li, R.; Waters, K.E. A review of reagents applied to rare-earth mineral flotation. Adv. Colloid Interface Sci. 2020, 279, 102142. [Google Scholar] [CrossRef]
- Xu, T.; Zheng, X.; Ji, B.; Xu, Z.; Bao, S.; Zhang, X.; Li, G.; Mei, J.; Li, Z. Green recovery of rare earth elements under sustainability and low carbon: A review of current challenges and opportunities. Sep. Purif. Technol. 2024, 330, 125501. [Google Scholar] [CrossRef]
- Das, S.; Bhaskar, R.; Narayanan, K.B. Multifunctional applications of gadolinium-doped cerium oxide (Ce1–xGdxO2–∂) ceramics: A review. J. Rare Earths 2024, 42, 1817–1834. [Google Scholar] [CrossRef]
- Qiao, J.; Li, L.; Liu, J.; Wu, N.; Liu, W.; Wu, F.; Zeng, Z. The vital application of rare earth for future high-performance electromagnetic wave absorption materials: A review. J. Mater. Sci. Technol. 2024, 176, 188–203. [Google Scholar] [CrossRef]
- Liu, S.L.; Fan, H.R.; Liu, X.; Meng, J.; Butcher, A.R.; Lahaye, Y.; Yang, K.F.; Li, X.C. Global rare earth elements projects: New developments and supply chains. Ore Geol. Rev. 2023, 157, 105428. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2023; U.S. Geological Survey: Reston, VA, USA, 2023; pp. 126–127.
- Dushyantha, N.P.; Batapola, N.M.B.; Premasiri, H.M.R.; Abeysinghe, A.M.K.B.; Rohitha, L.P.S.; Ratnayake, N.P.; Dissanayake, D.M.D.O.K.; Ilankoon, I.M.S.K.; Dharmaratne, P.G.R. A comparison of global rare earth element (REE) resources and their mineralogy with REE prospects in Sri Lanka. J. Asian Earth Sci. 2020, 200, 104475. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Geng, Y.; Ye, X.; Ma, S.; Hao, X.; Xin, W.; Chai, Y. A Review of the Main Rare Earth Ore Smelting Processes in the World: From Traditional Methods to New Technologies. J. Rare Earths, 2025; in press. [Google Scholar] [CrossRef]
- Cheng, S.; Li, W.; Han, Y.; Sun, Y.; Gao, P.; Zhang, X. Recent process developments in beneficiation and metallurgy of rare earths: A review. J. Rare Earths 2024, 42, 629–642. [Google Scholar] [CrossRef]
- Leng, J.; Wang, Z.; Wang, J.; Wu, H.-H.; Yan, G.; Li, X.; Guo, H.; Liu, Y.; Zhang, Q.; Guo, Z. Advances in nanostructures fabricated via spray pyrolysis and their applications in energy storage and conversion. Chem. Soc. Rev. 2019, 48, 3015–3072. [Google Scholar] [CrossRef]
- Raghavendra, P.V.; Bhat, J.S.; Deshpande, N.G. Enhancement of photoluminescence in Sr-doped ZnO thin films prepared by spray pyrolysis. Mater. Sci. Semicond. Process 2017, 68, 262–269. [Google Scholar] [CrossRef]
- Panatarani, C.; Faizal, F.; Florena, F.F. Tunable structure, morphology of Zn1−xMgxO fine powder prepared by spray pyrolysis method and its luminescence properties. Powder Technol. 2020, 31, 2191–2196. [Google Scholar] [CrossRef]
- Šarić, S.; Kojcinovic, J.; Tatar, D.; Djerdj, I. Overview of Recent Advances in Rare-Earth High-Entropy Oxides as Multifunctional Materials for Next-Gen Technology Applications. Molecules 2025, 30, 1082. [Google Scholar] [CrossRef]
- Siakavelas, G.I.; Charisiou, N.D.; AlKhoori, A.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Yentekakis, I.V.; Polychronopoulou, K.; Goula, M.A. Cerium oxide catalysts for oxidative coupling of methane reaction: Effect of lithium, samarium and lanthanum dopants. J. Environ. Chem. Eng. 2022, 10, 107259. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Dou, Z.; Xie, W.; Lan, C.; Li, G. Summary of the Research Progress on Advanced Engineering, Processes, and Process Parameters of Rare Earth Green Metallurgy. Materials 2024, 17, 3686. [Google Scholar] [CrossRef]
- Chivavava, J.; Petersen, J.; Lewis, A.E. Comparing the recovery of rare earth elements from ion-adsorption clay leach solutions using various precipitants. J. S. Afr. Inst. Min. Metall. 2024, 124, 731–746. [Google Scholar] [CrossRef]
- Chen, F.; Liu, F.; Wang, L.; Wang, J. Comparison of the Preparation Process of Rare Earth Oxides from the Water Leaching Solution of Waste Nd-Fe-B Magnets’ Sulfate Roasting Products. Processes 2022, 10, 2310. [Google Scholar] [CrossRef]
- Duan, L.; Yun, Q.; Jiang, G.; Teng, D.; Zhou, G.; Cao, Y. A review of chloride ions removal from high chloride industrial wastewater: Sources, hazards, and mechanisms. J. Environ. Manag. 2024, 353, 120184. [Google Scholar] [CrossRef] [PubMed]
- Tuncay, G.; Yuksekdag, A.; Kose Mutlu, B.; Koyuncu, I. A review of greener approaches for rare earth elements recovery from mineral wastes. Environ. Pollut. 2024, 357, 124379. [Google Scholar] [CrossRef]
- Messing, G.L.; Zhang, S.C.; Jayanthi, G.V. Ceramic Powder Synthesis by Spray Pyrolysis. J. Am. Ceram. Soc. 1993, 76, 2707–2726. [Google Scholar] [CrossRef]
- Workie, A.B.; Ningsih, H.S.; Shih, S.J. A comprehensive review on the spray pyrolysis technique: Historical context, operational factors, classifications, and product applications. J. Anal. Appl. Pyrolysis 2023, 170, 105915. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, T.; Sun, W.; He, D.; Lu, C.; Fu, X. Acid recovering and iron recycling from pickling waste acid by extraction and spray pyrolysis techniques. J. Clean. Prod. 2021, 312, 127747. [Google Scholar] [CrossRef]
- Harris, L.J.F. Introduction to spray roasting process for hydrochloric acid regeneration and its application to mineral processing. In Hydrometallurgy ’94; Springer: Dordrecht, The Netherlands, 1994; pp. 923–937. [Google Scholar]
- Kladnig, W.F. New development of acid regeneration in steel pickling plants. J. Iron Steel Res. Int. 2008, 15, 1–6. [Google Scholar] [CrossRef]
- Lv, C.; Sun, M.-H.; Yin, H.-X. The Effect of Pyrolysis Conditions on the Preparation of Fe2O3 Particles Using Simulated Pickling Liquor in a Venturi Reactor. Front. Mater. 2021, 8, 733257–733263. [Google Scholar] [CrossRef]
- Beck, M.; Scherer, V.; Wirtz, S. Experimental Setup to Examine Fe2O3 Particle Formation in Spray Roasting Reactors. Chem. Eng. Technol. 2005, 28, 659–663. [Google Scholar] [CrossRef]
- Zaspalis, V.; Kolenbrander, M. Design principles for spray-roasted iron oxides for the manufacturing of ferrites. Powder Technol. 2006, 161, 169–174. [Google Scholar] [CrossRef]
- Beck, M.; Scherer, V.; Wirtz, S. Numerical Calculations of Spray Roasting Reactors of the Steel Industry with Special Emphasis on Fe2O3-Particle Formation. Chem. Eng. Technol. 2007, 30, 1347–1354. [Google Scholar] [CrossRef]
- Regel-Rosocka, M. A review on methods of regeneration of spent pickling solutions from steel processing. J. Hazard. Mater. 2010, 177, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Schiemann, M.; Wirtz, S.; Scherer, V.; Bärhold, F. Spray roasting of iron chloride FeCl2: Numerical modelling of industrial scale reactors. Powder Technol. 2013, 245, 70–79. [Google Scholar] [CrossRef]
- Schiemann, M.; de Haan, A.; Wirtz, S. Numerical investigation on particle swelling in spray roasting reactors. Int. J. Multiph. Flow 2016, 85, 38–47. [Google Scholar] [CrossRef]
- Luo, S.; Li, L.; Chang, M.; Zhou, B. Modeling and simulation of droplet-to-particle formation during spray pyrolysis. Int. J. Therm. Sci. 2024, 197, 107374. [Google Scholar] [CrossRef]
- Lv, C.; Yin, H.; Sun, M.; Zhu, H. Simulation study of citric acid effects on pyrolysis of hydrochloric acid pickling waste liquor. Chem. Ind. Chem. Eng. Q. 2023, 29, 53–59. [Google Scholar] [CrossRef]
- Hong, Y.; Qiao, L.; Liu, X. Application of Ruthner-spray hydrochloric acid regeneration technology in cold rolling. Mod. Chem. Ind. 2005, 25, 48–50. [Google Scholar] [CrossRef]
- Wang, Q.; Hao, H.; Wu, Z.; Wang, M.; Feng, Z.; Zhang, Y.; Xue, X.; Huang, X. Mechanism study on the effect of H2O2 on spray pyrolysis of MgCl2 wastewater in rare earth extraction and separation process. J. Water Process. Eng. 2024, 60, 105089. [Google Scholar] [CrossRef]
- Wang, Q.; Song, C.; Hao, H.; Wang, M.; Feng, Z.; Zhang, Y.; Xue, X.; Huang, X. Full recycling of MgCl2 wastewater generated in rare earth extraction and separation process by spray pyrolysis. Process Saf. Environ. Prot. 2024, 183, 544–554. [Google Scholar] [CrossRef]
- Jenish, S.L.; Valanarasu, S.; Raj, I.L.P.; Juliet, A.V.; Isaac, R.S.R.; Ganesh, V.; Yahia, I.S. Enhanced photo detection performance of Fe doped Co3O4 thin films prepared via the nebulizer spray pyrolysis method. Opt. Mater. 2025, 163, 117019. [Google Scholar] [CrossRef]
- Mulla, M.G.; Pittala, R.K. Fabrication and physicochemical properties of nickel oxide (NiO) thin films. Ceram. Int. 2025, 51, 22255–22265. [Google Scholar] [CrossRef]
- Wang, L. Preparation and Characterization of Alumina by the Direct Spray Pyrolysis of AlCl3 Solution. Master’s Thesis, Northeastern University, Shengyang, China, 2014. [Google Scholar]
- Yang, Y.; Wang, N.; Pang, X.; Yasinskiy, A.; Tan, Y.; Yu, J.; Wang, Z.; Shi, Z. Thermodynamics of the decomposition of aluminum chloride hexahydrate to prepare alumina. J. Mater. Res. Technol. 2021, 15, 6640–6646. [Google Scholar] [CrossRef]
- Shibayama, R.; Tsuchida, N.; Tanaka, T. Studies on the Pyrohydrolysis of Metal Chlorides I. The Pyrohydrolysis of NiCl2, CoCl2, and FeCl2. Denki Kagaku Oyobi Kogyo Butsuri Kagaku 1980, 48, 545–553. [Google Scholar] [CrossRef]
- Pereverzev, D.I.; Giniyatullin, I.M.; Vladimirova, E.V.; Zhuravlev, V.D.; Dmitriev, A.V. Influence of the synthesis method on the morphology, dispersion and porosity of nanostructured powders of nickel–cobalt spinel NiCo2O4. J. Sol-Gel Sci. Technol. 2022, 103, 935–942. [Google Scholar] [CrossRef]
- Hou, D.; Wang, G.; Gao, J.; Luo, K.H. Molecular dynamics study on evaporation of metal nitrate-containing nanodroplets in flame spray pyrolysis. Nanoscale 2023, 15, 5877–5890. [Google Scholar] [CrossRef]
- Santos-Gómez, L.D.; García, J.Z.; Vázquez, J.M.P.; Losilla, E.R.; López, D.M. Highly oriented and fully dense CGO films prepared by spray-pyrolysis and different precursor salts. J. Eur. Ceram. Soc. 2020, 40, 3080–3088. [Google Scholar] [CrossRef]
- Kinoshita, T.; Murakami, D.; Wada, Y.; Okada, Y. Synthesis of nickel–cerium oxide solid-solution hollow fine particles by ultrasonic spray pyrolysis with citric acid addition. Adv. Powder Technol. 2024, 35, 104563. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Fan-Yuan, L.; Tsong-Huei, C.; Chuin-Tih, Y. Thermal Decomposition of Metal Nitrates in Air and Hydrogen Environments. J. Phys. Chem. B 2003, 107, 1044–1047. [Google Scholar] [CrossRef]
- Shih, S.J.; Zeng, W.L.T.; Kuo, W.L. Fabrication of ceria particles using glycine nitrate spray pyrolysis. Surf. Coat. Technol. 2014, 259, 302–309. [Google Scholar] [CrossRef]
- Shih, S.J.; Wu, Y.Y.; Chen, C.Y.; Yu, C.Y. Morphology and formation mechanism of ceria nanoparticles by spray pyrolysis. J. Nanopart. Res. 2012, 14, 879. [Google Scholar] [CrossRef]
- Stoia, M.; Barvinschi, P.; Barbu-Tudoran, L. Thermal decomposition of metal nitrates. J. Therm. Anal. Calorim. 2012, 113, 21–30. [Google Scholar] [CrossRef]
- Weber, S.B.; Lein, H.L.; Grande, T.; Einarsrud, M.A. Influence of the precursor solution chemistry on the deposition of thick coatings by spray pyrolysis. Surf. Coat. Technol. 2013, 221, 53–58. [Google Scholar] [CrossRef]
- Kim, E.J.; Kang, Y.C.; Park, H.D.; Ryu, S.K. UV and VUV characteristics of (YGd)2O3: Eu phosphor particles prepared by spray pyrolysis from polymeric precursors. Mater. Res. Bull. 2003, 38, 515–524. [Google Scholar] [CrossRef]
- Bian, X.; Wang, Z.F.; Wang, J.Y.; Wu, W.Y. Preparation of Lanthanum Hydroxide by Spray Pyrolysis of Lanthanum Chloride. J. Northeast. Univ. (Nat. Sci.) 2015, 36, 966–969. [Google Scholar] [CrossRef]
- Fu, L. Preparation of Gadolinium Oxide and Neodymium Praseodymium Oxide by Ultrasonic Spray Pyrolysis. Master’s Thesis, Jiangxi University of Science and Technology, Ganzhou, China, 2021. [Google Scholar]
- Balaji, R.; Mohan, P.; Vinoth, S.; Farahim, F.; Shkir, M. Fabrication and evaluation of CeO2: Er thin films by spray pyrolysis for enhanced NH3 sensing applications. Inorg. Chem. Commun. 2025, 177, 120–125. [Google Scholar] [CrossRef]
- Falcony, C.; Aguilar-Frutis, M.A.; Garcia-Hipolito, M. Spray Pyrolysis Technique; High-K Dielectric Films and Luminescent Materials: A Review. Micromachines 2018, 9, 414. [Google Scholar] [CrossRef]
- Zhu, Y.; Choi, S.H.; Fan, X.; Shin, J.; Ma, Z.; Zachariah, M.R.; Choi, J.W.; Wang, C. Recent Progress on Spray Pyrolysis for High Performance Electrode Materials in Lithium and Sodium Rechargeable Batteries. Adv. Energy Mater. 2017, 7, 1601578. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Chen, Z.; Zeng, H.; Su, W.; Zhao, Z.; Du, C.; Li, C.; Li, T. Preferential lithium extraction and simultaneous ternary cathode precursor synthesis from spent lithium-ion batteries using a spray pyrolysis-based process. Sep. Purif. Technol. 2025, 353, 12486. [Google Scholar] [CrossRef]
- Jin, R. Practical Basic Research on Application of Jet Reaction in the Preparation of Rare Earth Compounds. Master’s Thesis, Northeastern University, Shengyang, China, 2015. [Google Scholar]
- Jin., R.; Dou, Z.; Lv, G.; Niu, L.; Liu, C.; Zhang, T. Thermodynamic analysis of rare-earth chloride solution pyrolysising rare earth oxides. In Proceedings of the 17th (2013) National Conference on Metallurgical Reaction Engineering, Taiyuan, China, 5 August 2013. [Google Scholar]
- Korzun, I.V.; Zakir’yanova, I.D.; Nikolaeva, E.V. Mechanism and Caloric Effects of the Thermal Dehydration of GdCl3·6H2O Crystalline Hydrate. Russ. Metall. (Metally) 2018, 2018, 722–727. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Mishra, R.; Singh, H.; Krishnamurthy, N. Determination of thermodynamic stability of lanthanum chloride hydrates (LaCl3·xH2O) by dynamic transpiration method. J. Alloy. Compd. 2014, 588, 578–584. [Google Scholar] [CrossRef]
- Okhrimenko, L.; Dussouillez, J.; Johannes, K.; Kuznik, F. Thermodynamic equilibrium and kinetic study of lanthanum chloride heptahydrate dehydration for thermal energy storage. J. Energy Storage 2022, 48, 103562. [Google Scholar] [CrossRef]
- Zhang, T.; Dou, Z.; Liu, Y.; Zhang, Z.; Lv, G.; Zhao, Q.; Niu, L.; Jiang, X. A Method for Spray Pyrolysis of Rare Earth Chloride Solution to Prepare Rare Earth Oxides. Chinese Patent CN201410003498.7, 29 July 2015. [Google Scholar]
- Neumann, A.; Walter, D. The thermal transformation from lanthanum hydroxide to lanthanum hydroxide oxide. Thermochim. Acta 2006, 445, 200–204. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Liu, Z.; Ma, Y.; Chen, J.; Li, Y.; Xu, Z. One Uses Subcritical/Preparation of Rare Earth Oxides by Supercritical Steam Pyrolysis Method. Chinese Patent CN202010436812.6, 15 August 2023. [Google Scholar]
- Lei, X.; Wang, H.; Fu, L.; Wang, J.; Xu, S. Efficient Preparation of Rare Earth Oxides from Back Extraction Praseodymium Neodymium Chloride by Flash Pyrolysis. Chem. Sel. 2022, 7, 202200244. [Google Scholar] [CrossRef]
- Liu, C. Basic Research on Preparing Rare Earth Oxides Through Pyrolysis with Rare Earth Chloride Solution and Device Design. Master’s Thesis, Northeastern University, Shengyang, China, 2013. [Google Scholar]
- Wang, Z. Synthesis of RE/Al Composite Oxide by Spray Pyrolysis-Roasting Using Chloride Solution as Raw Material. Ph.D. Thesis, Northeastern University, Shengyang, China, 2018. [Google Scholar]
- Wang, Z.; Wu, W.; Bian, X.; Wu, Y. Low temperature green synthesis of LaAlO3 using microcrystalline LaOCl and amorphous Al2O3 precursors derived from spray pyrolysis. Green Process 2017, 6, 49–54. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, W.; Bian, X.; Wu, Y. Synthesis and characterization of amorphous Al2O3 and γ-Al2O3 by spray pyrolysis. Green Process. Synth. 2016, 6, 305–310. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Z.; Bian, X.; Wu, Y. Study on the Preparation of Light Rare Earth Oxide from Rare Earth Chloride. In Proceedings of the 5th International Conference on Civil. Architectural and Hydraulic Engineering (ICCAHE 2016), Zhuhai, China, 30 July 2016. [Google Scholar]
- Kaya, E.E.; Kaya, O.; Stopic, S.; Gürmen, S.; Friedrich, B. NdFeB Magnets Recycling Process: An Alternative Method to Produce Mixed Rare Earth Oxide from Scrap NdFeB. Metals 2021, 11, 716. [Google Scholar] [CrossRef]
- Powell, B.R.; Bloink, R.L.; Eickel, C. Preparation of Cerium Dioxide Powders for Catalyst Supports. J. Am. Ceram. Soc. 1988, 71, 1234–1240. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Conde, F.; Nicolopoulos, S.; Ragel, C.V.; González-Calbet, J.M. Synthesis and Characterization of CeO2 Obtained by Spray Pyrolysis Method. Mater. Sci. Forum 1996, 235–238, 291–296. [Google Scholar] [CrossRef]
- Xue, S.; Wu, W.; Bian, X.; Wu, Y. Dehydration, hydrolysis and oxidation of cerium chloride heptahydrate in air atmosphere. J. Rare Earths 2017, 35, 1156–1163. [Google Scholar] [CrossRef]
- Xue, S.; Wu, W.; Bian, X.; Wang, Z. Study on the Preparation Process for CeO2 Superfine Powder. J. Northeast. Univ. (Nat. Sci.) 2015, 36, 800–804. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, S.; Wang, Z.; Dong, Y.; Liu, Z.; Liu, Y. Numerical simulation of cerium chloride droplet pyrolysis in a spray roasting reactor. China Nonferrous Metall. 2024, 53, 46–56. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, H.; Liu, Z.; Wu, W.; Bian, X. Study on Reaction Kinetics of Direct Preparation of Cerium Oxide by Single Droplet CeCl3 Solution. Chin. Rare Earths 2021, 42, 104–111. [Google Scholar] [CrossRef]
- Liu, H. Process Kinetics and Thermal Performance of Cerium Oxide Prepared by Single Drop of Cerium Chloride Research. Master’s Thesis, Inner Mongolia University of Science & Technology, Baotou, China, 2020. [Google Scholar]
- Xiang, L. Study on the Preparation of Light Rare Earth Oxide from Rare Earth Chloride. Master’s Thesis, Northeastern University, Shengyang, China, 2010. [Google Scholar]
- Ardekani, S.R.; Aghdam, A.S.R.; Nazari, M.; Bayat, A.; Yazdani, E.; Saievar-Iranizad, E. A comprehensive review on ultrasonic spray pyrolysis technique: Mechanism, main parameters and applications in condensed matter. J. Anal. Appl. Pyrolysis 2019, 141, 104631. [Google Scholar] [CrossRef]
- Wu, Y.; Li, H.; Bian, X.; Wu, W.; Wang, Z.; Liu, Y. Green and Short Preparation of CeO2 Nanoparticles with Large Specific Surface Area by Spray Pyrolysis. Mater. 2021, 14, 4963. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Bian, X.; Cui, L.; Xie, B.; Yao, Y.; Wu, W. Study on Preparation of Nano Cerium Oxide Powder by Spray Roasting of Cerium Chloride Solution. Chin. Rare Earths 2017, 38, 72–77. [Google Scholar] [CrossRef]
- Shih, S.J.; Wu, Y.; Chen, C.; Yu, C. Controlled Morphological Structure of Ceria Nanoparticles Prepared by Spray Pyrolysis. Procedia Eng. 2012, 36, 186–194. [Google Scholar] [CrossRef]
- Xue, S.; Wu, W.; Bian, X.; Cui, L.; Xie, B.; Yao, Y.; Wu, W. Mechanism of Thermal Decomposition of PrCl3 Aerosol at High Temperature in Air Atmosphere. Chin. J. Rare Met. 2018, 42, 640–649. [Google Scholar] [CrossRef]
- Xue, S.; Wu, W.; Bian, X. Thermal Decomposition Mechanism of Terbium Chloride Aerosol at High Temperature. J. Northeast. Univ. (Nat. Sci.) 2018, 39, 1423–1427. [Google Scholar] [CrossRef]
- Xue, S. Investigation on the mechanism of Rare Earth Oxide prepared by Ultrasonic Spray Pyrolysis of Variable Valence Rare Earth Chloride Solution. Ph.D. Thesis, Northeastern University, Shengyang, China, 2017. [Google Scholar]
- Fang, Z.; Dixon, D.A. Hydrolysis of ZrCl4 and HfCl4: The Initial Steps in the High-Temperature Oxidation of Metal Chlorides to Produce ZrO2 and HfO2. J. Phys. Chem. C 2013, 117, 7459–7474. [Google Scholar] [CrossRef]
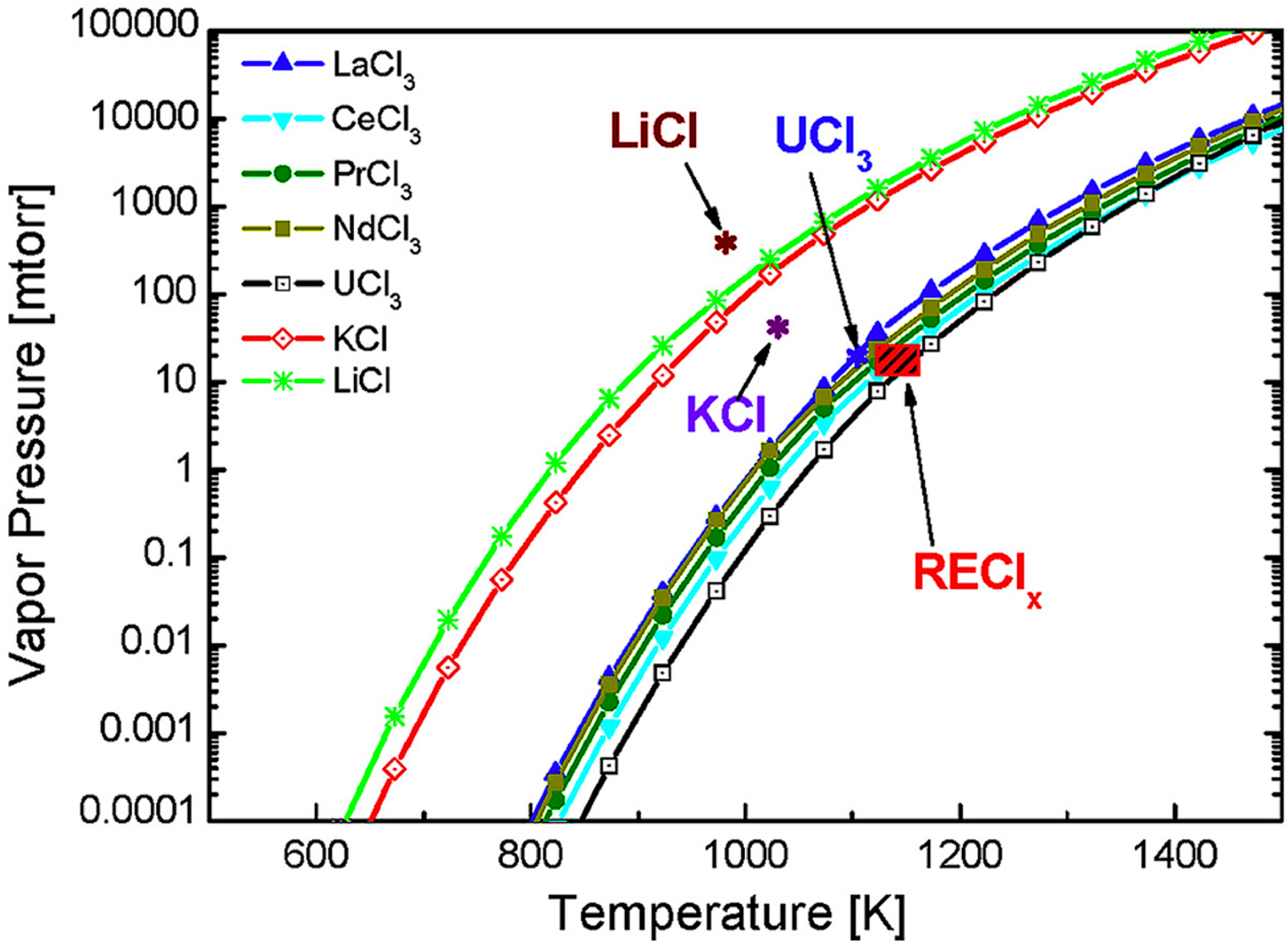
- Jang, J.; Kim, T.; Park, S.; Kim, G.Y.; Kim, S.; Lee, S. Evaporation behavior of lithium, potassium, uranium and rare earth chlorides in pyro processing. J. Nucl. Mater. 2017, 497, 30–36. [Google Scholar] [CrossRef]
- Guo, X.; Su, H.; Wu, Y.; Wu, W. Thermodynamic Analysis of Phase Diagrams Simulated by FactSage in Process of Rare Earth Oxides Production by Spray Pyrolysis Method. J. Chin. Soc. Rare Earths 2020, 38, 788–797. [Google Scholar]
- Liu, X. Study on the Ceriumxie Prepare by Pyrolysis of Cerium Chloride Solution. Master’s Thesis, Northeastern University, Shengyang, China, 2011. [Google Scholar]
- Yang, G. Study on the Rare Earth Oxide and the Polishing Powder Prepared by Pyrolysis of Cerium Chloride. Master’s Thesis, Northeastern University, Shengyang, China, 2017. [Google Scholar]
- Talwar, P.; Agudelo, M.; Nanda, S. Pyrolysis Process, Reactors, Products, and Applications: A Review. Energies 2025, 18, 2979. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Vijayan, K.; Vijayachamundeeswari, S.P.; Sivaperuman, K.; Ahsan, N.; Logu, T.; Okada, Y. A review on advancements, challenges, and prospective of copper and non-copper based thin-film solar cells using facile spray pyrolysis technique. Sol. Energy 2022, 234, 81–102. [Google Scholar] [CrossRef]
- Dimitriou, C.; Psathas, P.; Solakidou, M.; Deligiannakis, Y. Advanced Flame Spray Pyrolysis (FSP) Technologies for Engineering Multifunctional Nanostructures and Nanodevices. Nanomaterials 2023, 13, 3006. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, J.K.; Hong, J.H.; Cho, J.S.; Kang, Y.C. Advances in the synthesis and design of nanostructured materials by aerosol spray processes for efficient energy storage. Nanoscale 2019, 11, 19012–19057. [Google Scholar] [CrossRef] [PubMed]
- Motl, N.; Manna, K.; Skrabalak, S.E. Aerosol-assisted synthesis and assembly of nanoscale building blocks. J. Mater. Chem. A 2013, 1, 10170–10182. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Luo, L. Spray pyrolysis technology and its application in environmental pollution control. J. Environ. Eng. Technol. 2023, 13, 1425–1433. [Google Scholar] [CrossRef]
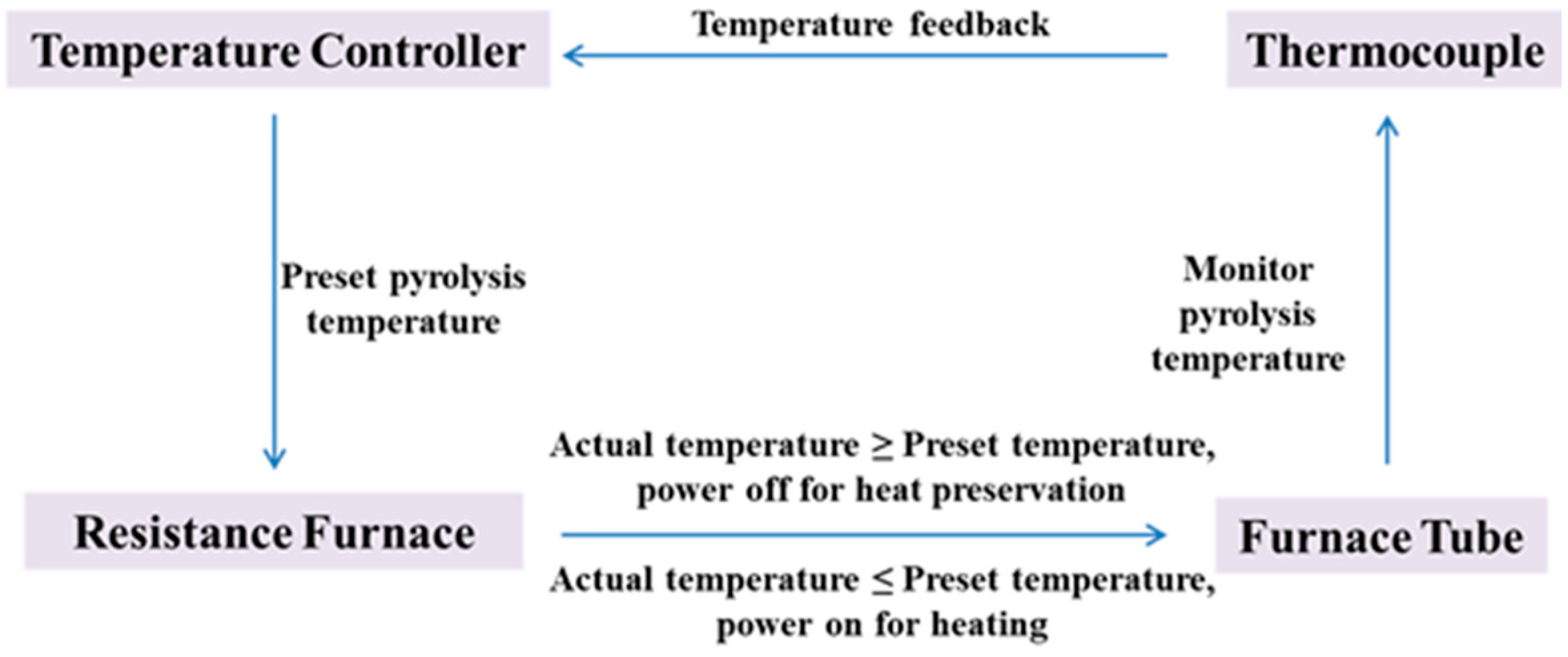
- Jia, Z. Study on the Monitoring System of Chloride Solution Spray Roasting. Master’s Thesis, Northeastern University, Shengyang, China, 2013. [Google Scholar]
- Lv, C.; Sun, M.; Chen, X.; Zhao, H.; Liu, Y.; Yin, H. Simulation and Analysis of Atomization Performance of a Venturi Jet Pyrolysis Reactor. Metall. Mater. Trans. B 2023, 54, 807–822. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Nan, X.; Lv, C.; Zhang, C.; Zhang, T. Numerical Simulation on the Device of Rare Earth Oxide Preparation by Rare Earth Chloride Spray Pyrolysis. Math. Probl. Eng. 2015, 2015, 704874. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, Z.; Zhao, Q.; Dou, Z.; Zhang, T.; Zhao, H. Numerical simulation: Preparation of La2O3 in a jet pyrolysis reactor. Rare Met. 2015, 34, 600–606. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, T.; Dou, Z.; Zhao, Q. Numerical simulation of preparation of ultrafine cerium oxides using jet-flow pyrolysis. Rare Met. 2019, 38, 1160–1168. [Google Scholar] [CrossRef]
- Lv, C. Numerical Simulation on the Recovery Process of Acid Pickling Waste Liquor by Jet-Flow Pyrolysis. JOM 2019, 71, 4944–4949. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, T.; Dou, Z.; Zhao, Q. Numerical Simulations of Irregular CeO2 Particle Size Distributions. JOM 2019, 71, 34–39. [Google Scholar] [CrossRef]
- Xu, H.; Dong, H.; Cheng, D. Exergy Analysis and Modeling of Pilot-Scale Pyrolysis for Magnesium Oxide Preparation from Salt Lake Bischofite Industrial Waste. ACS Omega 2023, 49, 47153–47162. [Google Scholar] [CrossRef]
- IMR. Spray Pyrolysis System Market Growth Analysis, Dynamics, Key Players and Innovations, Outlook and Forecast 2025–2032; Intel Market Research: USA, 2025; Available online: https://www.intelmarketresearch.com (accessed on 9 September 2025).

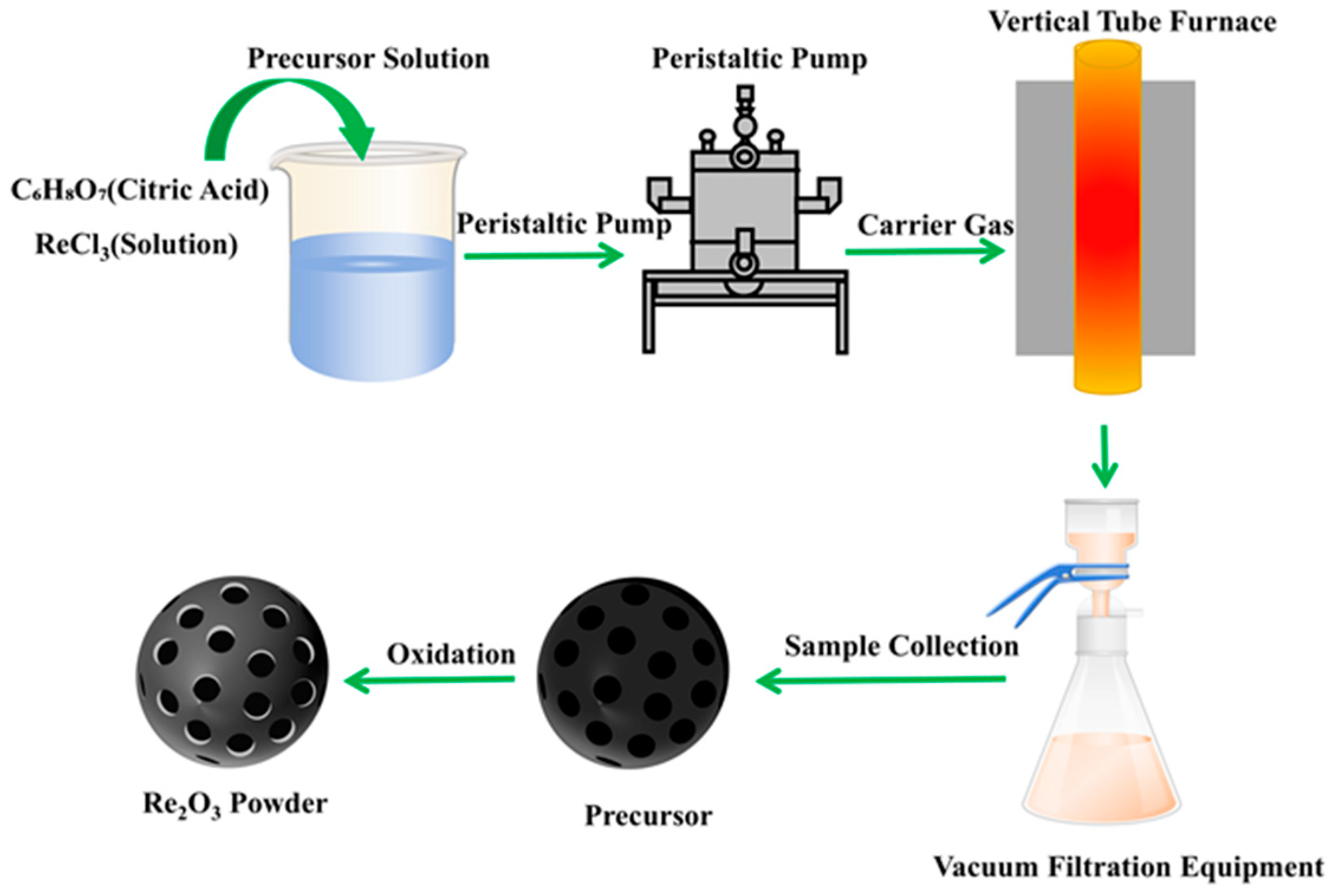


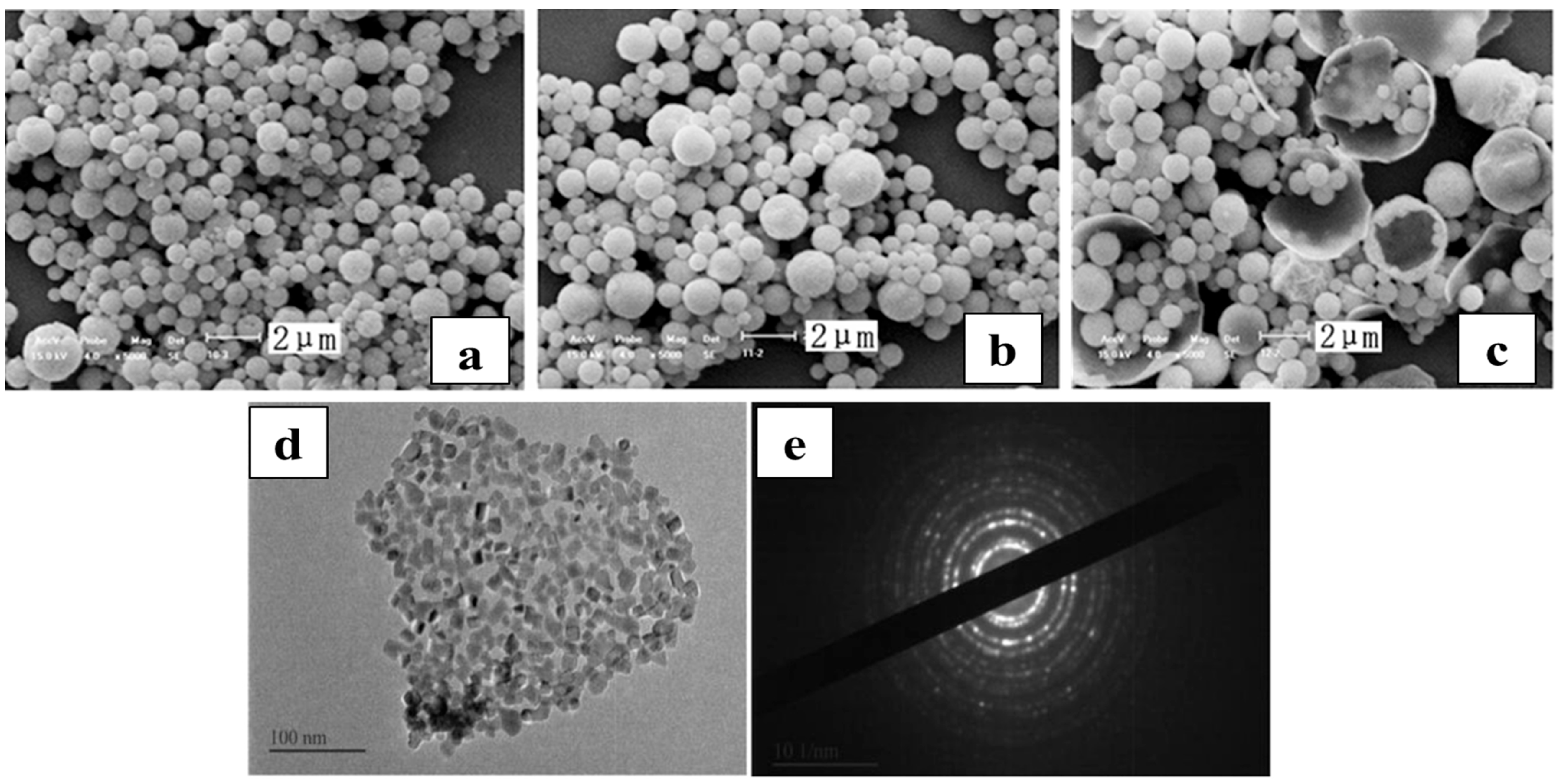
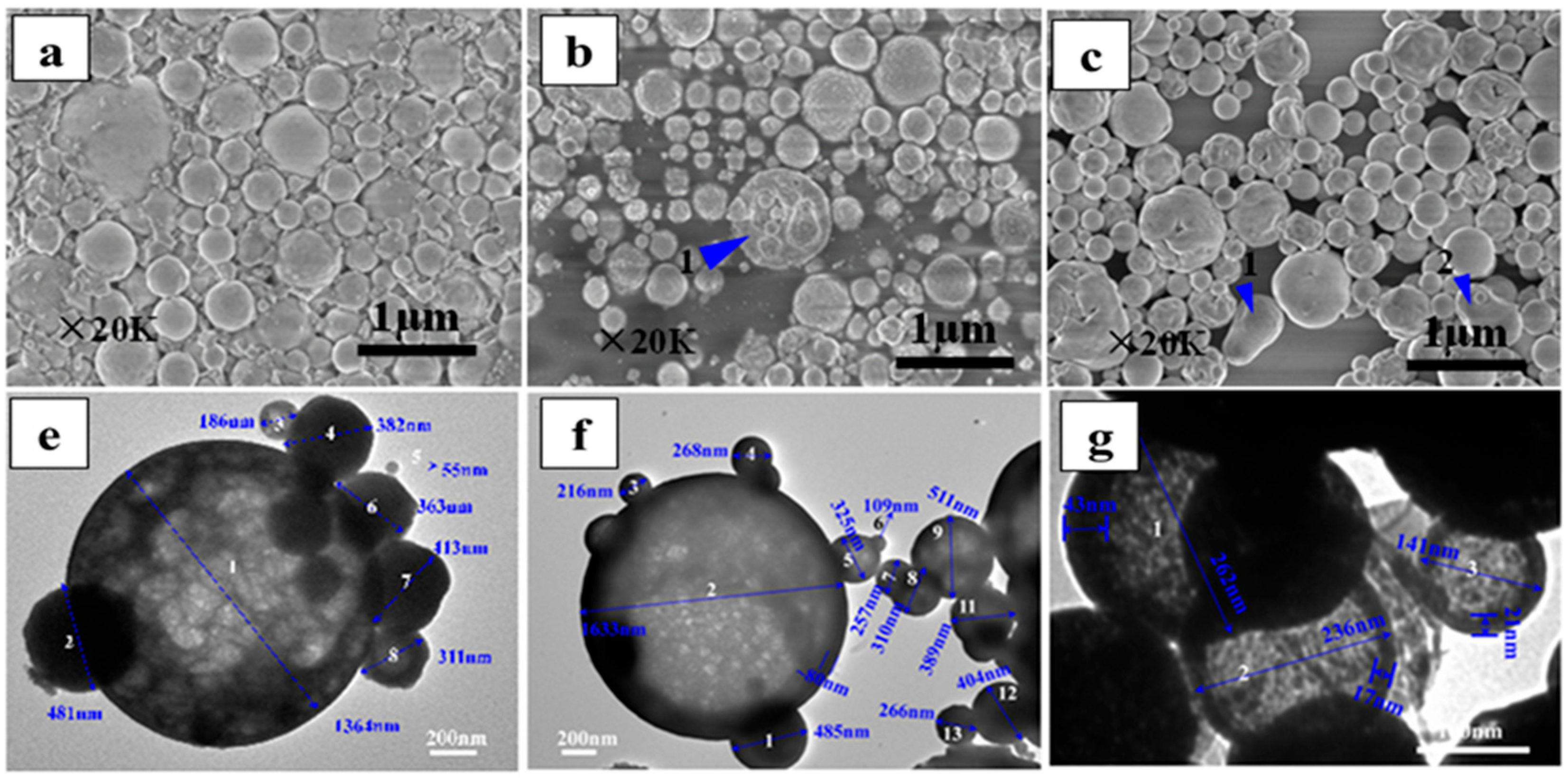



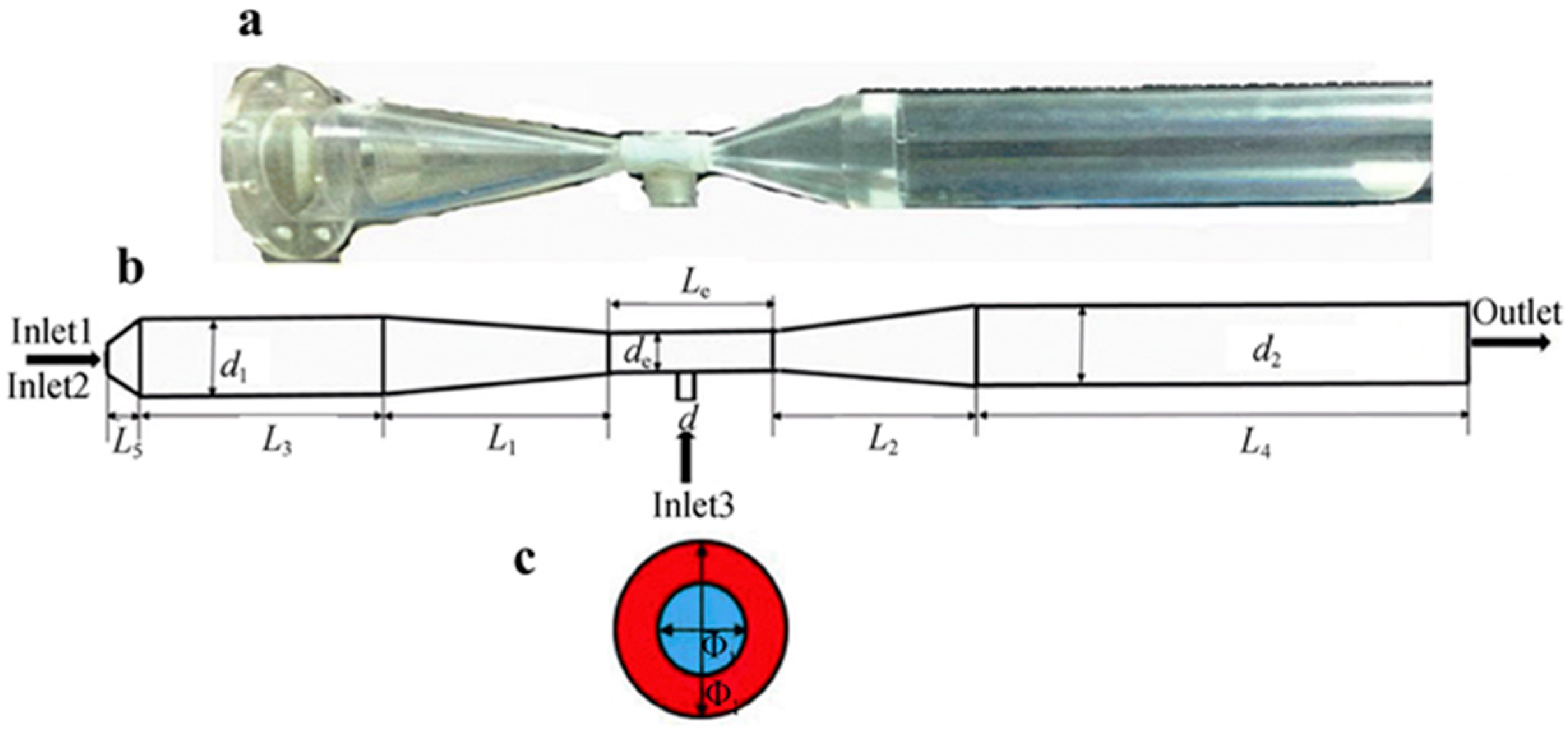
| Rare-Earth Elements | Material Compositions | Preparation Process | Product Features | References | |
|---|---|---|---|---|---|
| Constant—Valence Rare-earths | La | AlCl3·6H2O, >99.0%; LaCl3·7H2O, >99.0%; C2H5OH, >99.0% | Temperature: 800 °C; Time: 2 h; Concentration: 20 wt% | LaAlO3 purity > 99.9%; LaOCl and Al2O3 particle size: 0.5–15.0 μm; hollow spheres and plate-like particles. | [68,69,70,71] |
| LaCl3 crystals, >99%; LaCl3, 400 g/L; Additives: H2O2(AR) | Temperature: 600 °C; Carrier gas pressure: 0.4 MPa; H2O2(AR): 5% | Primary product is lanthanum hydroxide; conversion rate 99.96%; fragmented particle morphology. | [52] | ||
| LaCl3(Industrial grade), 0.4 mol/L; H(OCH2CH2)nOH (molecular weight 20,000, AR); NaOH(AR) | 608–750 °C→La(OH)3; 750 °C (2 h)→La2O3 | Specific surface area: 36.54 m2/g; equivalent diameter: 25.22 nm. | [64] | ||
| Gd | GdCl3, 347.41 g/L; Additives: C6H8O7·H2O, ≥99.5% | Temperature: 750 °C; Time: 2 h | Approximately spherical or fragmented morphology; chlorine content < 500 ppm. | [53] | |
| Nd | NdCl3, 207.75 g/L; C6H8O7·H2O, ≥99.5% | Temperature: 1050 °C; Time: 4 h | Particle size: 1.929–3.830 μm; loose, porous spherical particles with flaky surface; Cl− content well below 500 ppm. | [53] | |
| Variable—Valence Rare-earth | Ce | CeCl3·7H2O, 100 g/L | Temperature: 600 °C | Hollow spherical particles with regular morphology, clear boundaries, good dispersibility; particle size: 0.06–3.28 μm; polycrystalline nanocrystals with small grain sizes. | [76] |
| CeCl3, 20 wt% | Temperature: 650 °C; Jet velocity: 10 m/s; Ambient pressure: 1 atm | Smooth, solid spheres; narrow size distribution: 0.5–1 μm; high purity. | [77] | ||
| CeCl3·7H2O, 30 wt%; C6H8O7·H2O, 99.5% | Pressure 0.3 MPa; Temperature: 650 °C; Time: 2 h; AR:CeCl3(molar ratio): 1.5 | Minimum particle size approximately 15 nm; agglomerated nanoparticles; maximum specific surface area 59.72 m2/g; well-crystallized; uniformly distributed spherical polycrystals; high purity. | [78] | ||
| Pr | PrCl3, 65.73 g/L; C6H8O7·H2O, ≥99.5% | Temperature: 1050 °C; Time: 4 h | Particle size: 1.929–3.830 µm; loosely porous spherical particles with lamellar surface; Cl− content well below 500 ppm. | [53] | |
| Tb | TbCl3·6H2O, 0.05 mol/L | Carrier gas flow rate; 10 L/min; Temperature: 600 °C | Uniform spherical particles with regular morphology; particle size: 0.1–1.3 μm; d50 approximately 0.5 μm; high specific surface area. | [86,87] | |
| Compounds | Conditions | Ea (kJ/mol) | Rate-Limiting Mechanisms | Kinetic Equations | References |
|---|---|---|---|---|---|
| CeCl3 | Static | 25.836 | Internal diffusion control | 1 − (2/3)a − (1 − a) = 0.706exp(25.836/RT)t | [80,91] |
| Dynamic | 22.431 | Mixed-control (diffusion + reaction) | 1 − (2/3)α − (1 − α)^(2/3) = 0.646exp(−22.431/RT) t. | [92] | |
| Droplet size effect (V = 2–10 μL) | 36–62.5 | Mixed-control (diffusion + reaction) | (0.627–0.012)exp(Ea/RT) t | [78,79] | |
| DSC-TG | 33.914 | Chemical reaction | - | [80] | |
| PrCl3 | Static | 132.479 | Chemical reaction | 1 − (1 − α)^(1/3) = 664.390exp(−132.479/RT) t | [80] |
| DSC-TG | 107.04 | Chemical reaction | - | [80] | |
| NdCl3 | Static | 181.544 | Chemical reaction | 1 − (1 − α)^(1/3) = 21451exp(−181.544/RT) t | [80] |
| DSC-TG | 107.22 | Chemical reaction | - | [80] |
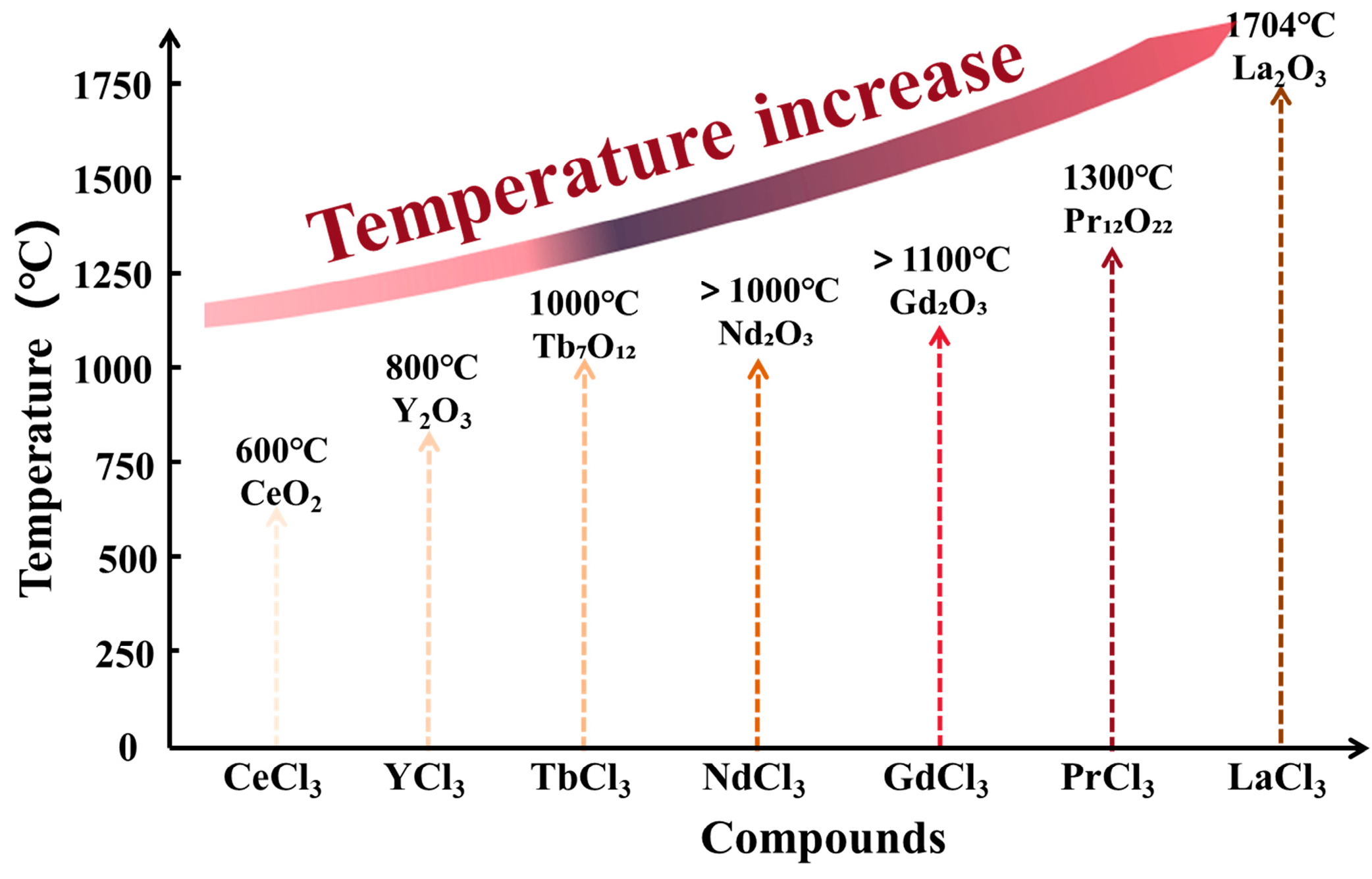
| REEs | Phase Transformation Pathway |
|---|---|
| Pr | PrCl3 → Pr(OH)2Cl → PrOCl → Pr6O11 → Pr2O3 |
| Ce | CeCl3 → CeO2 |
| Tb | TbCl3 → Tb(OH)2Cl → TbOCl → TbO2 → 2Tb7O12 |
| TbCl3 → Tb(OH)2Cl → TbOCl → 2Tb7O12 | |
| Sm, Eu | RECl3 → RE2O3 |
| Gd, Nd | RECl3 → RE (OH)2Cl → REOCl → RE2O3 |
| Reactor Types | Advantages | Disadvantages | References |
|---|---|---|---|
| Static Pyrolysis (Sealed Tube Furnace) | Simple structure; easy operation; suitable for small-scale research. | Low productivity; high energy consumption; batch operation limits industrial scalability. | [93] |
| HTFSP | Continuous operation; high efficiency; one-step synthesis; low cost; stable. | Low crystallinity and tap density; requires post-treatment (calcination, oxidation, reduction). | [10] |
| VSP | Gravity-assisted trajectory reduces wall interactions; denser particles. | Limited control over nucleation/agglomeration; high temperatures required in some configurations. | [56,95] |
| FSP | Suitable for nano-metal oxides and doped oxides; rapid synthesis. | High temperature required; difficult to synthesize non-oxides; poor control over agglomeration. | [96,97,98] |
| SPD | Enables thin-film fabrication; high control over composition and morphology. | Limited to film deposition; potential non-uniformity issues. | [95] |
| JP | Low decomposition temperature; clean production; low energy consumption; low emissions. | Scalability requires optimization; parameter sensitivity in large-scale operation. | [58,67,101] |
| Companies | Spray Methods | Main Application Areas |
|---|---|---|
| Holmarc Opto-Mechatronics Pvt. Ltd. (Kerala, India) | Ultrasonic spray | Research in thin films; Coating |
| Sono-Tek Corporation (New York, USA) | Ultrasonic atomization | Semiconductor coating |
| Siansonic Technology Co., Ltd. (Beijing, China) | Ultrasonic spray | Precision spraying; spray pyrolysis; spray drying |
| Sonaer Inc. (New York, USA.) | High-throughput spray | Semiconductor |
| Zhengzhou CY Scientific Instrument Co., Ltd. (Zhengzhou, China) | Ultrasonic spray | Spray Pyrolysis Coating System |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Bian, X.; Zhu, X.; Huang, H.; Ye, C.; Sun, S.; Zhong, L.; Tu, G. Review of Oxides Prepared by a Short Process Using Rare-Earth Chlorides. Materials 2025, 18, 4669. https://doi.org/10.3390/ma18204669
Wei J, Bian X, Zhu X, Huang H, Ye C, Sun S, Zhong L, Tu G. Review of Oxides Prepared by a Short Process Using Rare-Earth Chlorides. Materials. 2025; 18(20):4669. https://doi.org/10.3390/ma18204669
Chicago/Turabian StyleWei, Jing, Xue Bian, Xinmiao Zhu, Hao Huang, Chunlin Ye, Shuchen Sun, Liqin Zhong, and Ganfeng Tu. 2025. "Review of Oxides Prepared by a Short Process Using Rare-Earth Chlorides" Materials 18, no. 20: 4669. https://doi.org/10.3390/ma18204669
APA StyleWei, J., Bian, X., Zhu, X., Huang, H., Ye, C., Sun, S., Zhong, L., & Tu, G. (2025). Review of Oxides Prepared by a Short Process Using Rare-Earth Chlorides. Materials, 18(20), 4669. https://doi.org/10.3390/ma18204669