Optimal Blend Between Fluorinated Esters and Fluorinated Ether for High-Performance Lithium-Ion Cells at High Voltage
Abstract
1. Introduction
2. Experimental Procedure
2.1. Electrolyte Preparation
2.2. Coin Cell Preparation
2.3. Pouch Cell Preparation
2.4. Electrochemical Testing
2.5. Safety Testing
2.6. Material Characterization Analysis
2.7. Computational Calculation
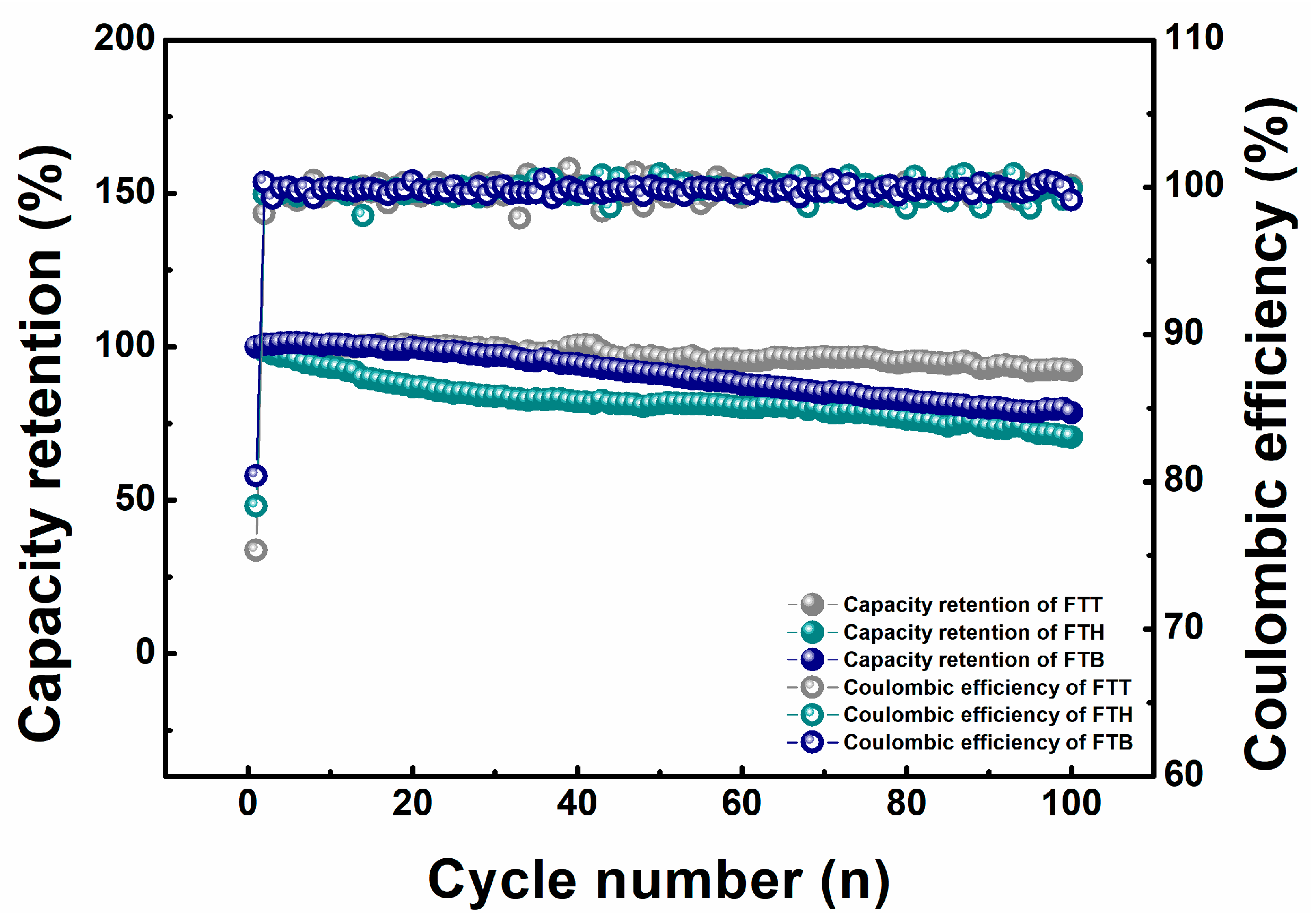
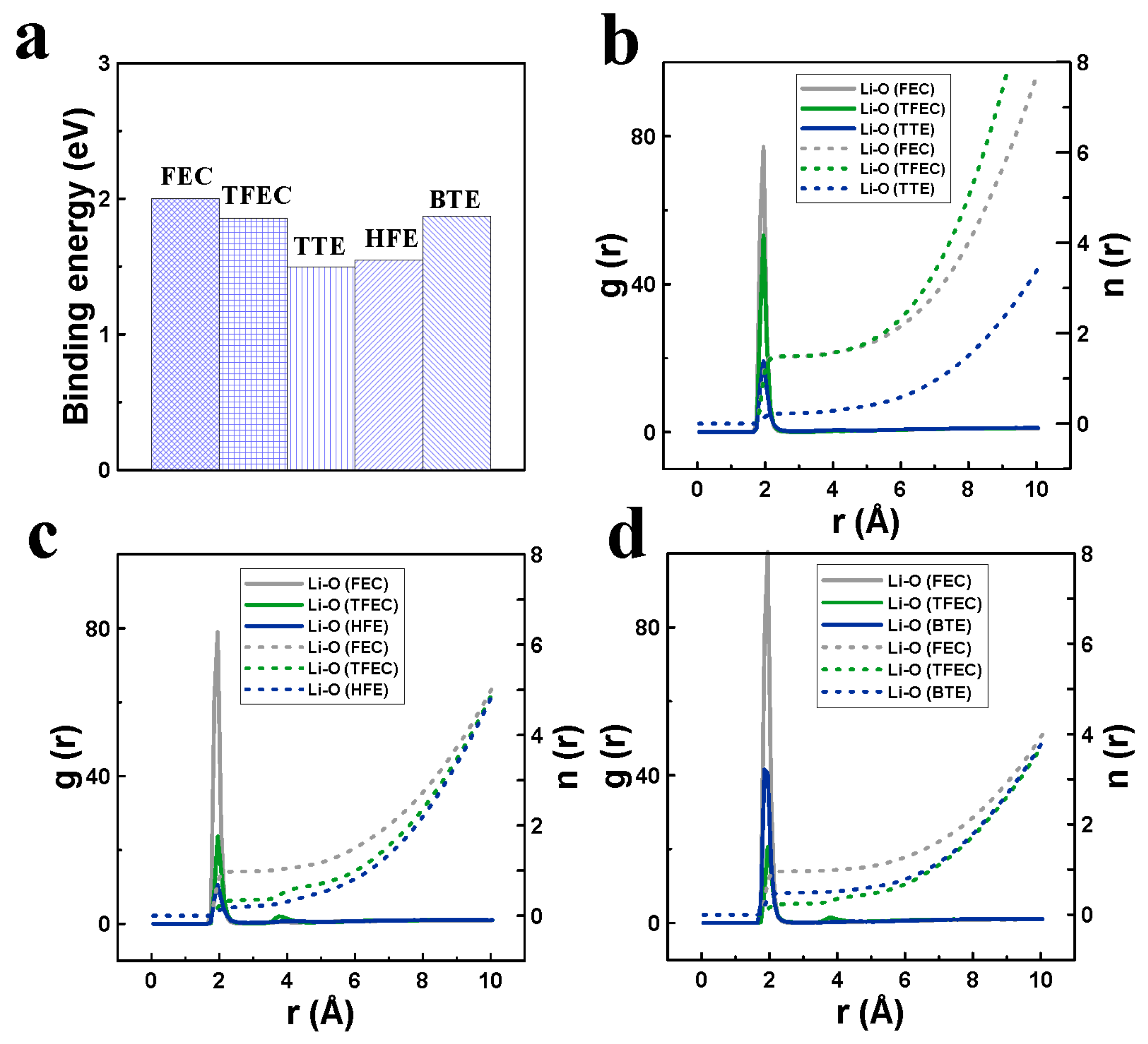
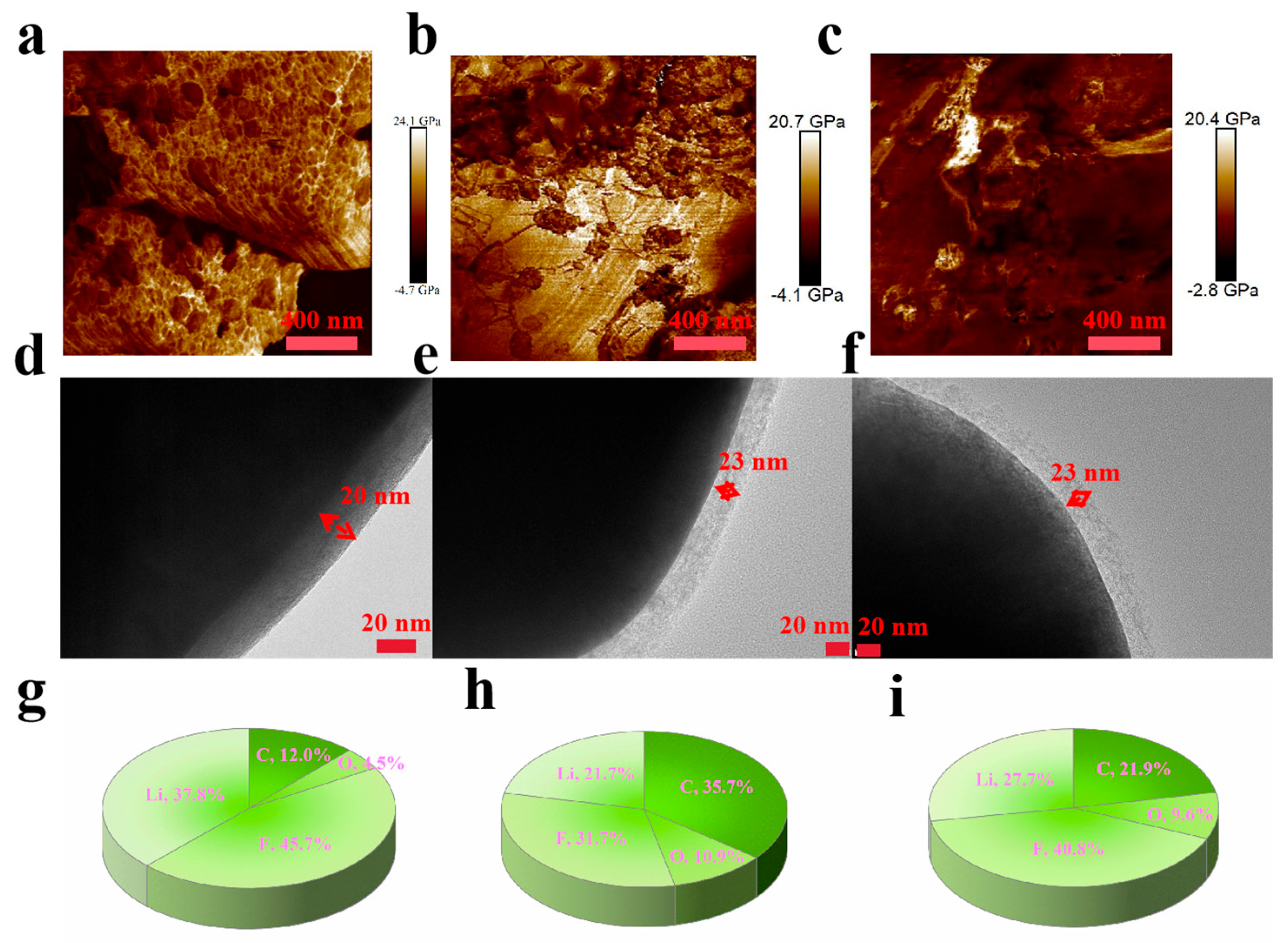
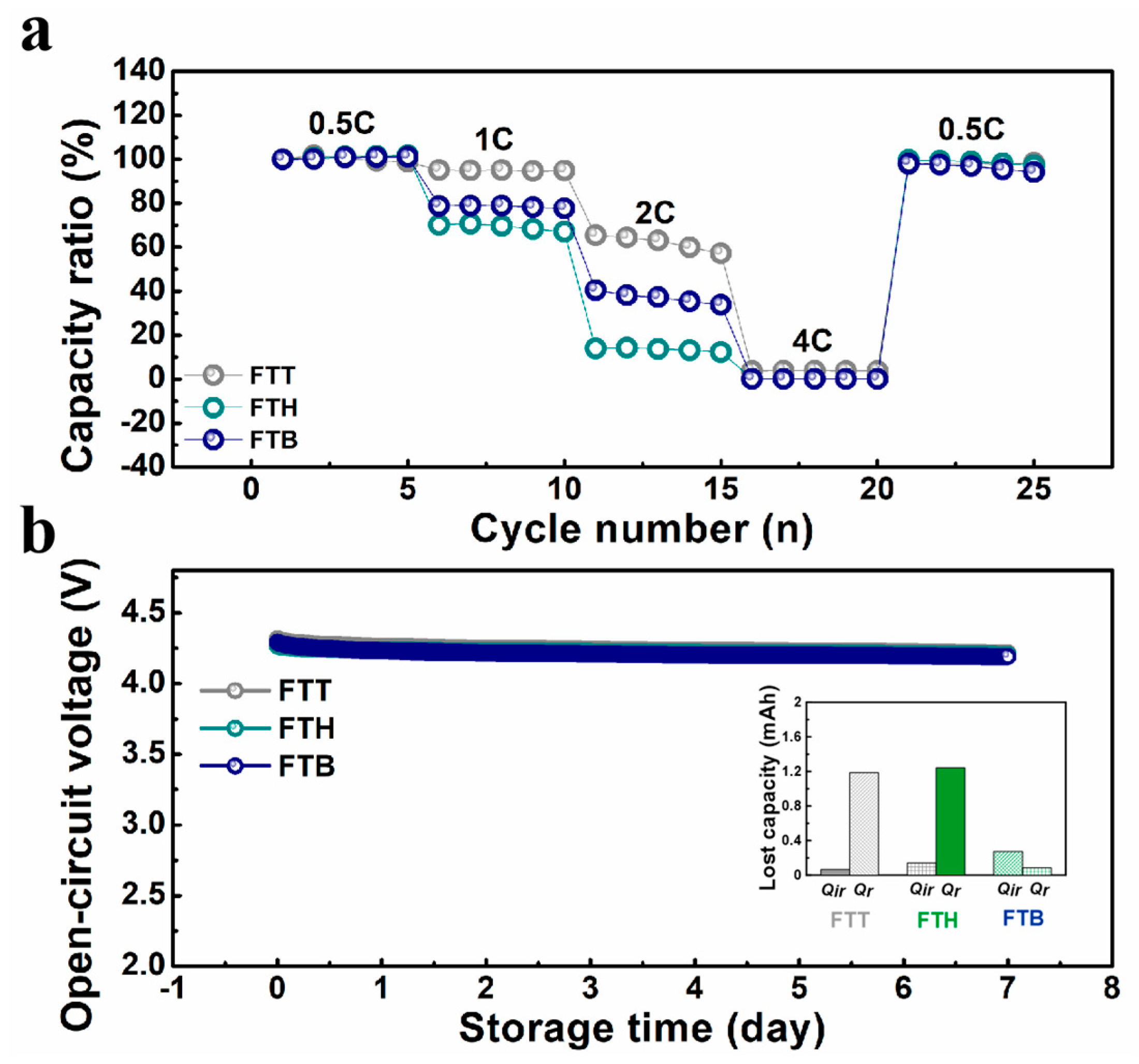
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Zhang, Y.; Dong, S.; Zhou, W.; Lee, P.; Peng, Z.; Dang, C.; Sit, P.H.; Guo, J.; Yu, D.Y.W. An all-fluorinated electrolyte toward high voltage and long cycle performance dual-ion batteries. Adv. Energy Mater. 2022, 12, 2103360. [Google Scholar] [CrossRef]
- Holoubek, J.; Yu, M.; Yu, S.; Li, M.; Wu, Z.; Xia, D. An all-fluorinated ester electrolyte for stable high-voltage Li metal batteries capable of ultra-low-temperature operation. ACS Energy Lett. 2020, 5, 1438–1447. [Google Scholar] [CrossRef]
- Su, C.-C.; He, M.; Shi, J.; Amine, R.; Yu, Z.; Cheng, L.; Guo, J.; Amine, K. Principle in developing novel fluorinated sulfone electrolyte for high voltage lithium-ion batteries. Energy Environ. Sci. 2021, 14, 3029–3034. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Meng, Y.; Wen, Z.; Han, R.; Hu, X.; Sun, B.; Kang, F.; Li, B.; Zhou, D.; et al. Fluorine Chemistry in Rechargeable Batteries: Challenges, Progress, and Perspectives. Chem. Rev. 2024, 124, 3494–3589. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, T.; Liao, X.; Song, Y.; Zhao, Y. All-fluorinated electrolyte directly tuned Li+ solvation sheath enabling high-quality passivated interfaces for robust Li metal battery under high voltage operation. Energy Storage Mater. 2023, 57, 249–259. [Google Scholar] [CrossRef]
- Su, C.-C.; He, M.; Amine, R.; Chen, Z.; Sahore, R.; Rago, N.D.; Amine, K. Cyclic carbonate for highly stable cycling of high voltage lithium metal batteries. Energy Storage Mater. 2019, 17, 284–292. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Wang, X.; Li, C.; Liu, Y.; Cheng, H.; Mao, S.; Wu, Q.; Shen, Z.; Mao, J.; et al. Nonflammable electrolyte with low exothermic design for safer lithium-based batteries. Nano Energy 2023, 114, 108639. [Google Scholar] [CrossRef]
- Xia, L.; Chen, M.; Wang, F.; Miao, H.; Yuan, J. Partially fluorinated linear carboxylate esters employed as co-solvents for high-voltage lithium-ion batteries. J. Power Sources 2022, 526, 231152. [Google Scholar] [CrossRef]
- Lai, P.; Huang, B.; Deng, X.; Li, J.; Hua, H.; Zhang, P.; Zhao, J. A localized high concentration carboxylic ester-based electrolyte for high-voltage and low temperature lithium batteries. Chem. Eng. J. 2023, 461, 141904. [Google Scholar] [CrossRef]
- He, M.; Su, C.-C.; Peebles, C.; Zhang, Z. The impact of different substituents in fluorinated cyclic carbonates in the performance of high voltage lithium-ion battery electrolyte. J. Electrochem. Soc. 2021, 168, 010505. [Google Scholar] [CrossRef]
- Ugata, Y.; Yukishita, K.; Kazahaya, N.; Takahashi, S.; Yabuuchi, N. Nonflammable fluorinated ester-based electrolytes for safe and high-energy batteries with LiCoO2. Chem. Mater. 2023, 35, 3686–3693. [Google Scholar] [CrossRef]
- Lu, H.; Zeng, F.; He, L.; Feng, R.; Yuan, Y.; Zhang, Z.; Liu, H.; Du, H.; Zheng, B. Advanced perfluorinated electrolyte with synergistic effect of fluorinated solvents for high-voltage LiNi0.5Mn1.5O4/Li cell. Electrochim. Acta 2022, 421, 140472. [Google Scholar] [CrossRef]
- Yan, G.; Li, X.; Wang, Z.; Guo, H.; Peng, W.; Hu, Q.; Wang, J. Fluorinated solvents for high-voltage electrolyte in lithium-ion battery. J. Solid State Electrochem. 2017, 21, 1589–1597. [Google Scholar] [CrossRef]
- Hotasi, B.T.; Hagos, T.M.; Huang, C.J.; Jiang, S.-K.; Jote, B.A.; Shitaw, K.N.; Bezabh, H.K.; Wang, C.-H.; Su, W.-N.; Wu, S.-H.; et al. Developing ester-based fluorinated electrolyte with LiPO2F2 as an additive for high-rate and thermally robust anode-free lithium metal battery. J. Power Sources 2022, 548, 232047. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, Z.; Liu, G.; Li, Q.; Yin, D.; Shi, X.; Cao, Z.; Tian, Z.; Kim, H.; Guo, Y.; et al. Non-flammable electrolyte enables high-voltage and wide-temperature lithium-ion batteries with fast charging. Angew. Chem. 2023, 135, e202216189. [Google Scholar] [CrossRef]
- Yoo, D.; Liu, Q.; Cohen, O.; Kim, M.; Persson, K.A.; Zhang, Z. Rational design of fluorinated electrolytes for low temperature lithium-ion batteries. Adv. Energy Mater. 2023, 13, 2204182. [Google Scholar] [CrossRef]
- Lin, S.; Zhao, J. Functional electrolyte of fluorinated ether and ester for stabilizing both 4.5 V LiCoO2 cathode and lithium metal anode. ACS Appl. Mater. Interfaces 2020, 12, 8316–8323. [Google Scholar] [CrossRef]
- Zheng, X.; Liao, Y.; Zhang, Z.; Zhu, J.; Ren, F.; He, H.; Xiang, Y.; Zheng, Y.; Yang, Y. Exploring high-voltage fluorinated carbonate electrolytes for LiNi0.5Mn1.5O4 cathode in Li-ion batteries. J. Energy Chem. 2020, 42, 62–70. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, W.; Li, Y.; Feng, X.; Ma, Z.; Ren, D.; Lu, L.; Xu, G.-L.; Amine, K.; Ouyang, M. Solid-state interphases design for high-safety, high-voltage and long-cyclability practical batteries via ethylene carbonate-free electrolytes. Energy Storage Mater. 2024, 65, 103165. [Google Scholar] [CrossRef]
- Hou, W.; Zhu, D.; Ma, S.; Yang, H.; Dai, Y. High-voltage nickel-rich layered cathodes in lithium metal batteries enabled by a sulfolane/fluorinated ether/fluoroethylene carbonate-based electrolyte design. J. Power Sources 2022, 517, 230683. [Google Scholar] [CrossRef]
- Hai, F.; Tian, X.; Yi, Y.; Wu, Z.; Zheng, S.; Guo, J.; Tang, W.; Hua, W.; Li, M. A sulfolane-based high-voltage electrolyte with dispersed aggregates for 5 V batteries. Energy Storage Mater. 2023, 54, 641–650. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, T.; Ashirov, T.; El Kazzi, M.; Cancellieri, C.; Jeurgens, L.P.H.; Choi, J.W.; Coskun, A. Fluorinated ether electrolyte with controlled solvation structure for high voltage lithium metal batteries. Nat. Commun. 2022, 13, 2575. [Google Scholar] [CrossRef]
- Zhou, T.; Zhao, Y.; El Kazzi, M.; Choi, J.W.; Coskun, A. Integrated ring-chain design of a new fluorinated ether solvent for high-voltage lithium-metal batteries. Angew. Chem. Int. Ed. 2022, 61, e202115884. [Google Scholar] [CrossRef]
- Amanchukwu, C.V.; Yu, Z.; Kong, X.; Qin, J.; Cui, Y.; Bao, Z. A new class of ionically conducting fluorinated ether electrolytes with high electrochemical stability. J. Am. Chem. Soc. 2020, 142, 7393–7403. [Google Scholar] [CrossRef]
- Wang, R.; Li, J.; Han, B.; Wang, Q.; Ke, R.; Zhang, T.; Ao, X.; Zhang, G.; Liu, Z.; Qian, Y.; et al. Unique double-layer solid electrolyte interphase formed with fluorinated ether-based electrolytes for high-voltage lithium metal batteries. J. Energy Chem. 2024, 88, 532–542. [Google Scholar] [CrossRef]
- Lee, K.; Kwon, S.-H.; Kim, J.; Park, E.; Kim, I.; Ahn, H.C.; Coskun, A.; Choi, J.W. Fluorinated Cyclic Ether Diluent for High-Voltage Lithium Metal Batteries. ACS Energy Lett. 2024, 9, 2201–2211. [Google Scholar] [CrossRef]
- Wu, L.-Q.; Li, Z.; Fan, Z.-Y.; Li, K.; Li, J.; Huang, D.; Li, A.; Yang, Y.; Xie, W.; Zhao, Q. Unveiling the Role of Fluorination in Hexacyclic Coordinated Ether Electrolytes for High-Voltage Lithium Metal Batteries. J. Am. Chem. Soc. 2024, 146, 5964–5976. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, R.; Zhang, J.; Song, X.; Liu, J.; Xu, N.; Zhang, H.; Wan, X.; Ji, X.; Ma, Y.; et al. Long-cycling and High-voltage Solid State Lithium Metal Batteries Enabled by Fluorinated and Crosslinked Polyether Electrolytes. Angew. Chem. Int. Ed. 2024, 63, e202400303. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, T.; El Kazzi, M.; Coskun, A. Fluorinated cyclic ether co-solvents for ultra-high-voltage practical lithium-metal batteries. ACS Appl. Energy Mater. 2022, 5, 7784–7790. [Google Scholar] [CrossRef]
- Ren, Q.; Wang, Q.; Su, L.; Liu, G.; Song, Y.; Shangguan, X.; Li, F. Inorganic/organic composite fluorinated interphase layers for stabilizing ether-based electrolyte in high-voltage lithium metal batteries. J. Mater. Chem. A 2024, 12, 1072–1080. [Google Scholar] [CrossRef]
- Xia, L.; Lee, S.; Jiang, Y.; Li, S.; Liu, Z.; Yu, L.; Hu, D.; Wang, S.; Liu, Y.; Chen, G.Z. Physicochemical and electrochemical properties of 1, 1, 2, 2-Tetrafluoroethyl-2, 2, 3, 3-tetrafluoropropyl ether as a co-solvent for high-voltage lithium-ion electrolytes. ChemElectroChem 2019, 6, 3747–3755. [Google Scholar] [CrossRef]
- Ouyang, D.; Liu, B.; Wan, X.; Guan, J.; Miao, C.; Wang, Z. An all-fluorinated electrolyte with 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropylether as co-solvent for lithium-ion cells with advanced electrochemical and safety properties at high voltage. J. Power Sources 2024, 615, 235095. [Google Scholar] [CrossRef]
- Fan, X.; Chen, L.; Borodin, O.; Ji, X.; Chen, J.; Hou, S.; Deng, T.; Zheng, J.; Yang, C.; Liou, S.-C.; et al. Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries. Nat. Nanotechnol. 2018, 13, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, S.; Liu, L.; Zhao, P. Cell-to-cell variability in Li-ion battery thermal runaway: Experimental testing, statistical analysis, and kinetic modeling. J. Energy Storage 2022, 56, 106024. [Google Scholar] [CrossRef]
- Ouyang, D.; Wang, K.; Yang, Y.; Wang, Z. Fluoroethylene carbonate as co-solvent for Li(Ni0.8Mn0.1Co0.1)O2 lithium-ion cells with enhanced high-voltage and safety performance. J. Power Sources 2022, 542, 231780. [Google Scholar] [CrossRef]
- Ouyang, D.; Wang, K.; Pang, Y.; Wang, Z. A fluorinated carbonate-based electrolyte for high-voltage Li(Ni0.8Mn0.1Co0.1)O2 lithium-ion cells. J. Power Sources 2022, 529, 231247. [Google Scholar] [CrossRef]
- Ouyang, D.; Guan, J.; Wan, X.; Liu, B.; Miao, C.; Wang, Z. Nonflammable All-Fluorinated Electrolyte Enabling High-Voltage and High-Safety Lithium-Ion Cells. ACS Appl. Mater. Interfaces 2024, 16, 42894–42904. [Google Scholar] [CrossRef]
- Gupta, A.; Manthiram, A. Designing advanced lithium-based batteries for low-temperature conditions. Adv. Energy Mater. 2020, 10, 2001972. [Google Scholar] [CrossRef]
- Xia, L.; Miao, H.; Zhang, C.; Chen, G.Z.; Yuan, J. Recent advances in non-aqueous liquid electrolytes containing fluorinated compounds for high energy density lithium-ion batteries. Energy Storage Mater. 2021, 38, 542–570. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, T.; Xiong, J.; Zhu, B.; Cheng, Y.J.; Xia, Y. Stable electrode/electrolyte interface for high-voltage NCM 523 cathode constructed by synergistic positive and passive approaches. ACS Appl. Mater. Interfaces 2021, 13, 57107–57117. [Google Scholar] [CrossRef]
- Wang, K.; Cao, Y.; Yang, Y.; Wang, Z.; Ouyang, D. Exploring fluorinated electrolyte for high-voltage and high-safety Li-ion cells with Li(Ni0.8Mn0.1Co0.1)O2 cathode. Int. J. Energy Res. 2022, 46, 24243–24253. [Google Scholar] [CrossRef]
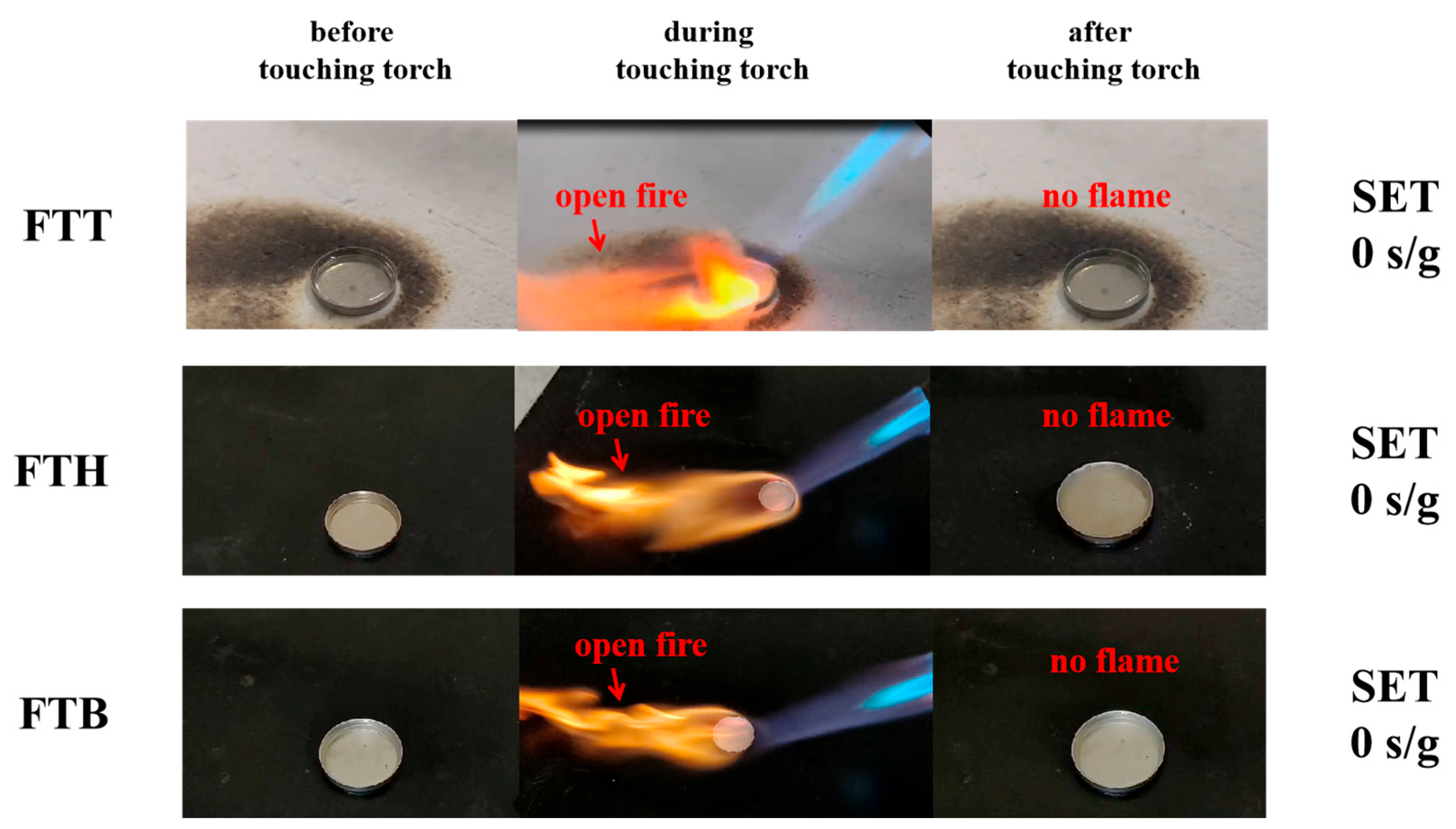
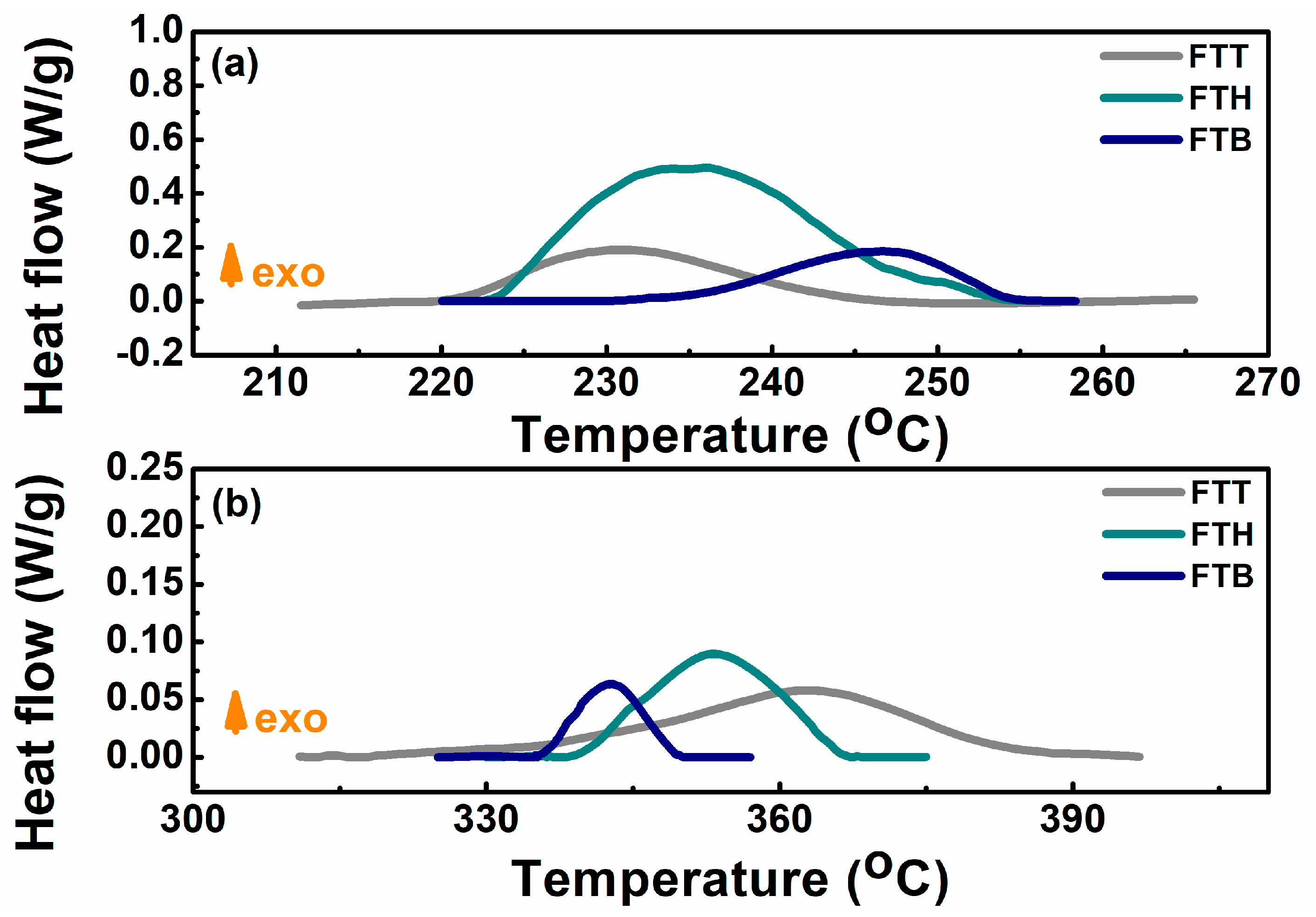


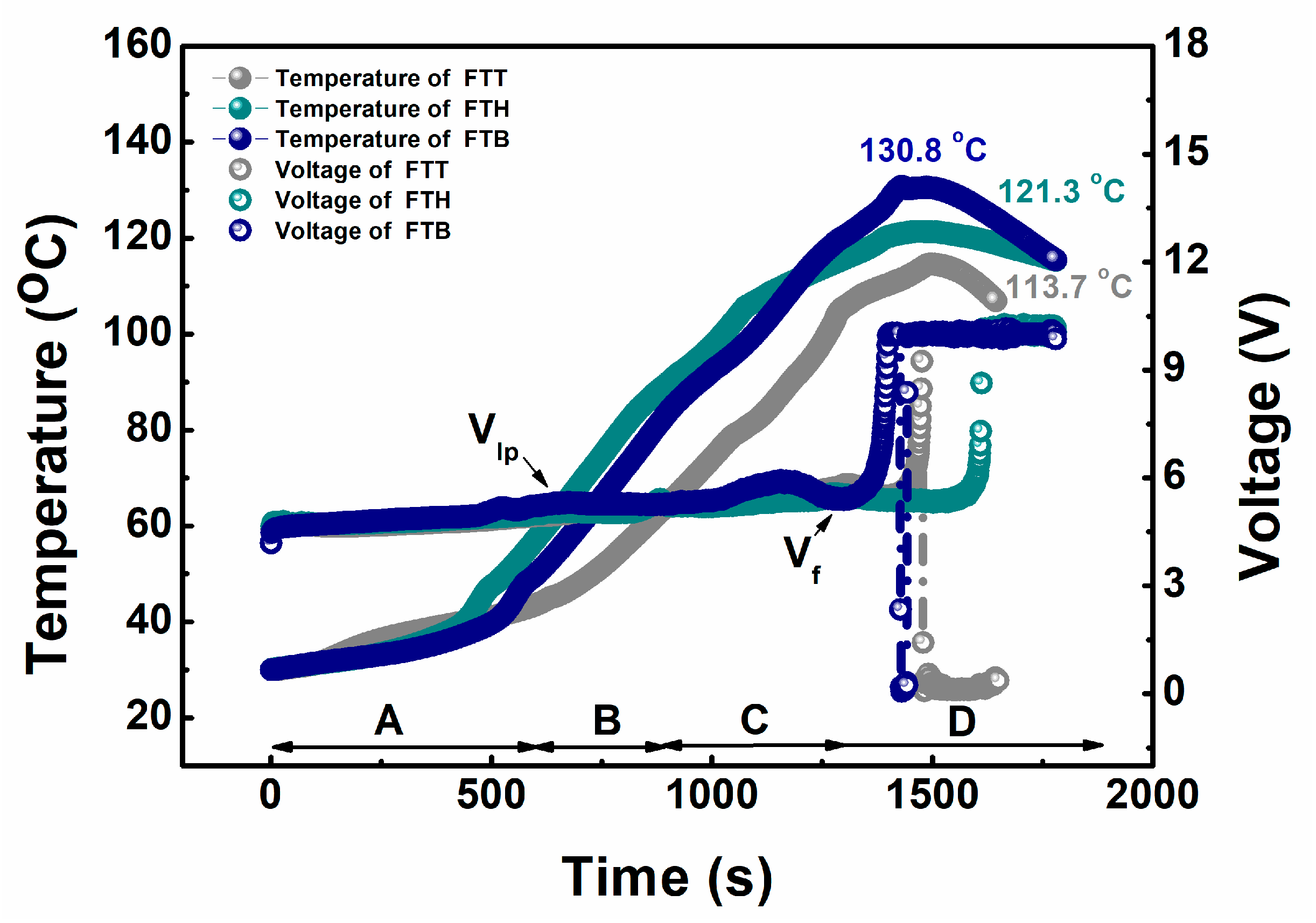

| Cell | Ttr (°C) | dT/dt (°C/min) | Tm (°C) | Total Heat Generation (J) |
|---|---|---|---|---|
| FTT | 152.6 | 8648 | 658 | 10,104 |
| FTH | 161.6 | 5553 | 621 | 9184 |
| FTB | 174.0 | 9220 | 647 | 9456 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, Y.; Liu, B.; He, J.; Zhi, M.; Ouyang, D. Optimal Blend Between Fluorinated Esters and Fluorinated Ether for High-Performance Lithium-Ion Cells at High Voltage. Materials 2025, 18, 274. https://doi.org/10.3390/ma18020274
Sheng Y, Liu B, He J, Zhi M, Ouyang D. Optimal Blend Between Fluorinated Esters and Fluorinated Ether for High-Performance Lithium-Ion Cells at High Voltage. Materials. 2025; 18(2):274. https://doi.org/10.3390/ma18020274
Chicago/Turabian StyleSheng, Yong, Bo Liu, Junjiang He, Maoyong Zhi, and Dongxu Ouyang. 2025. "Optimal Blend Between Fluorinated Esters and Fluorinated Ether for High-Performance Lithium-Ion Cells at High Voltage" Materials 18, no. 2: 274. https://doi.org/10.3390/ma18020274
APA StyleSheng, Y., Liu, B., He, J., Zhi, M., & Ouyang, D. (2025). Optimal Blend Between Fluorinated Esters and Fluorinated Ether for High-Performance Lithium-Ion Cells at High Voltage. Materials, 18(2), 274. https://doi.org/10.3390/ma18020274





