Synthesis and Characterisation of Metal–Glass Composite Materials Fabricated by Liquid Phase Sintering
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Metal–Glass (MG) Composite Materials
2.3. Characterisation
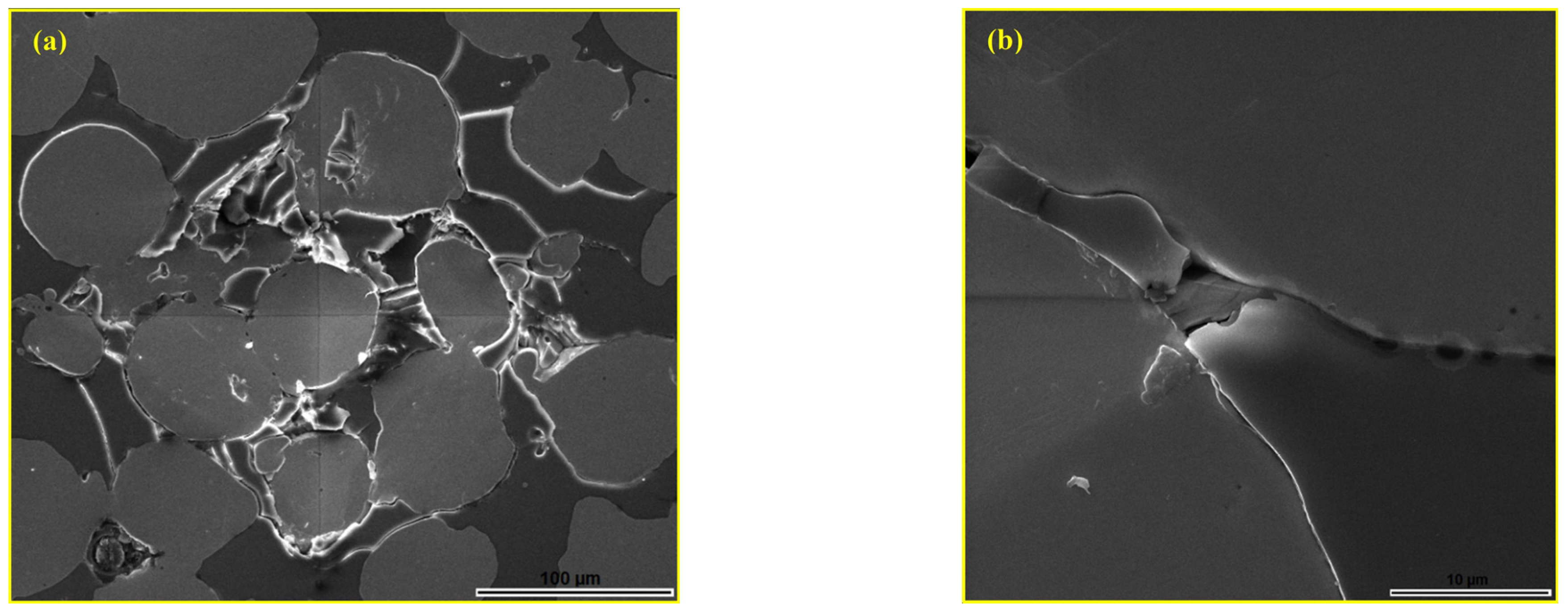
3. Results and Discussion
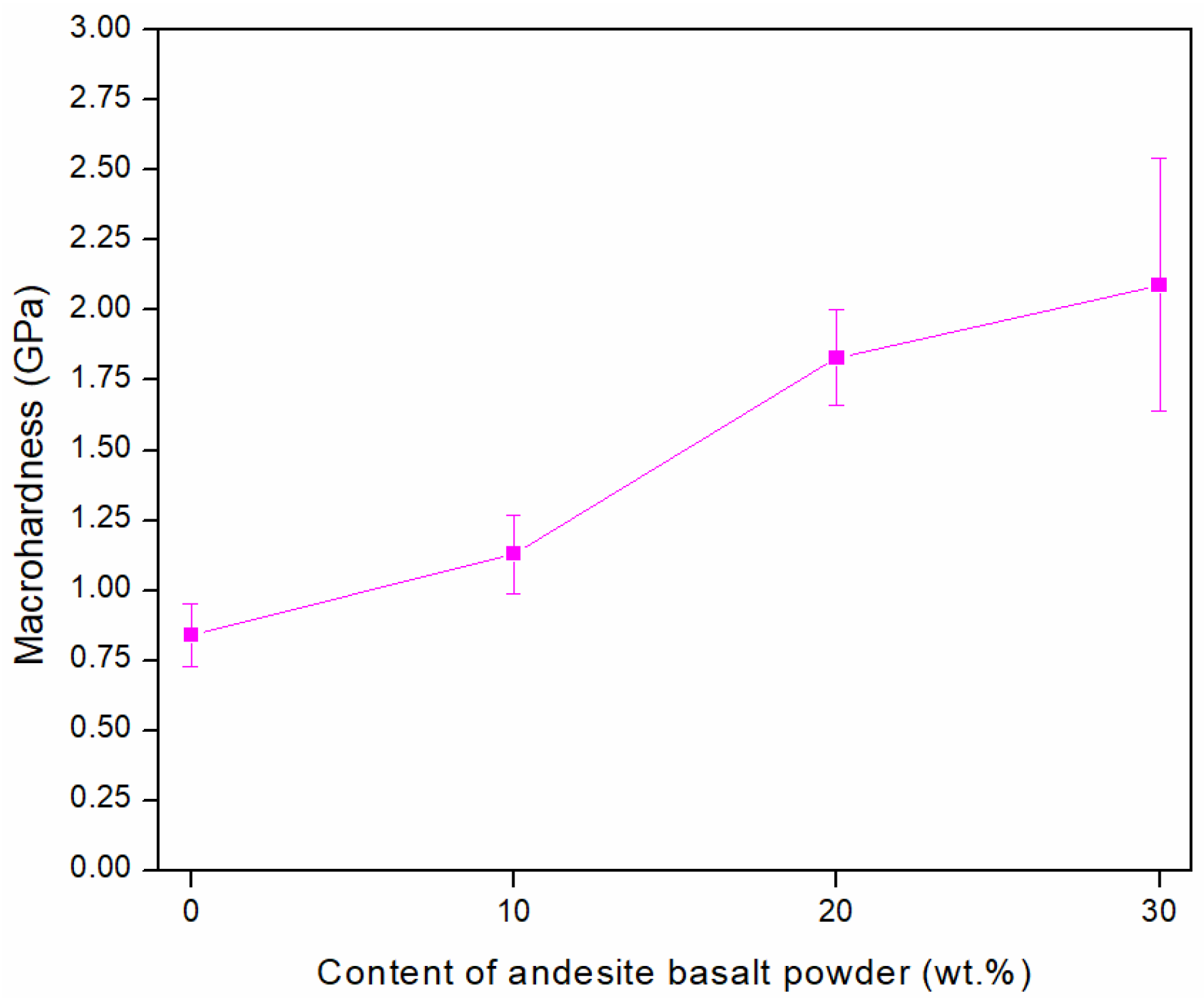
3.1. Optimisation of Andesite Basalt Content in the MG Composites
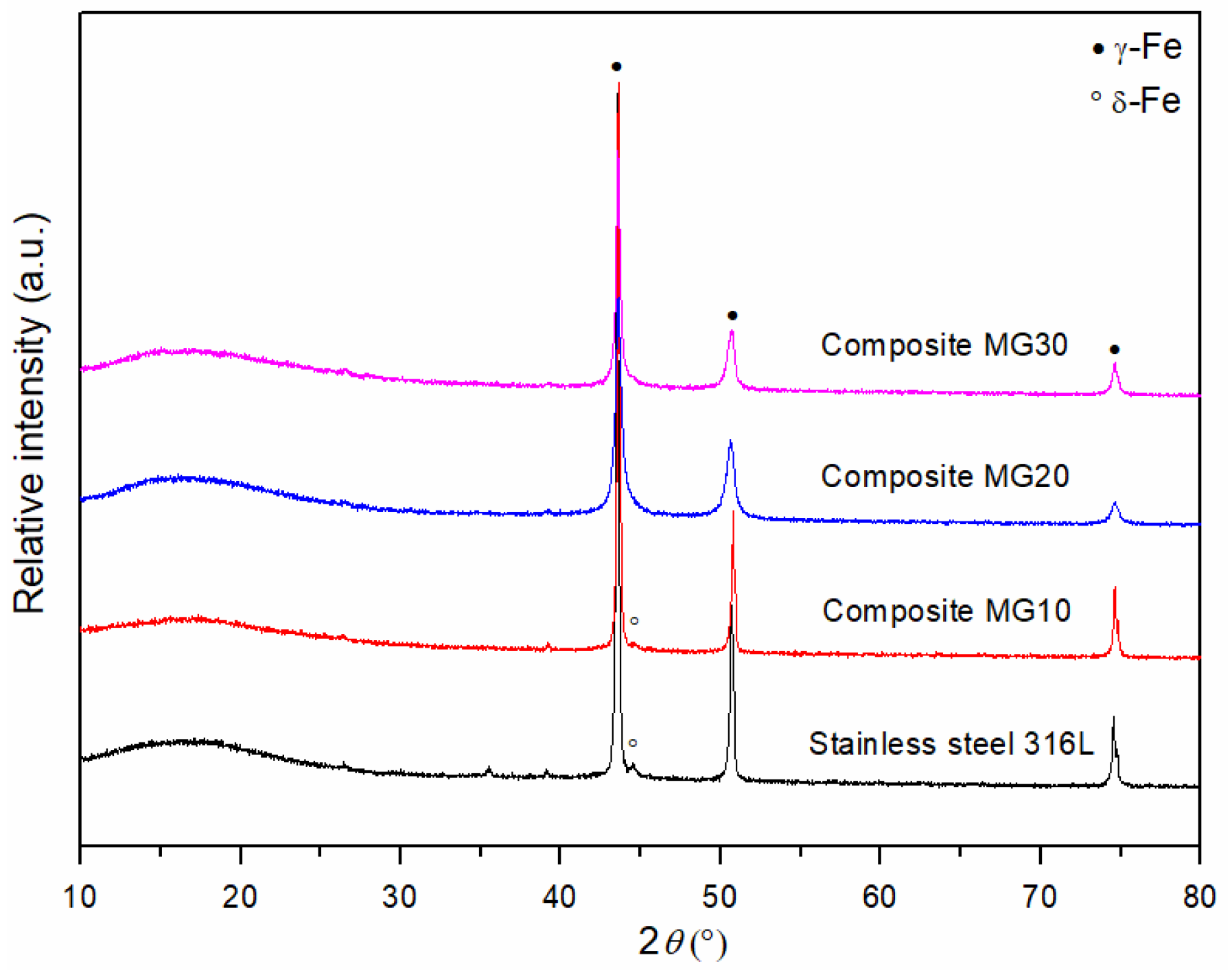
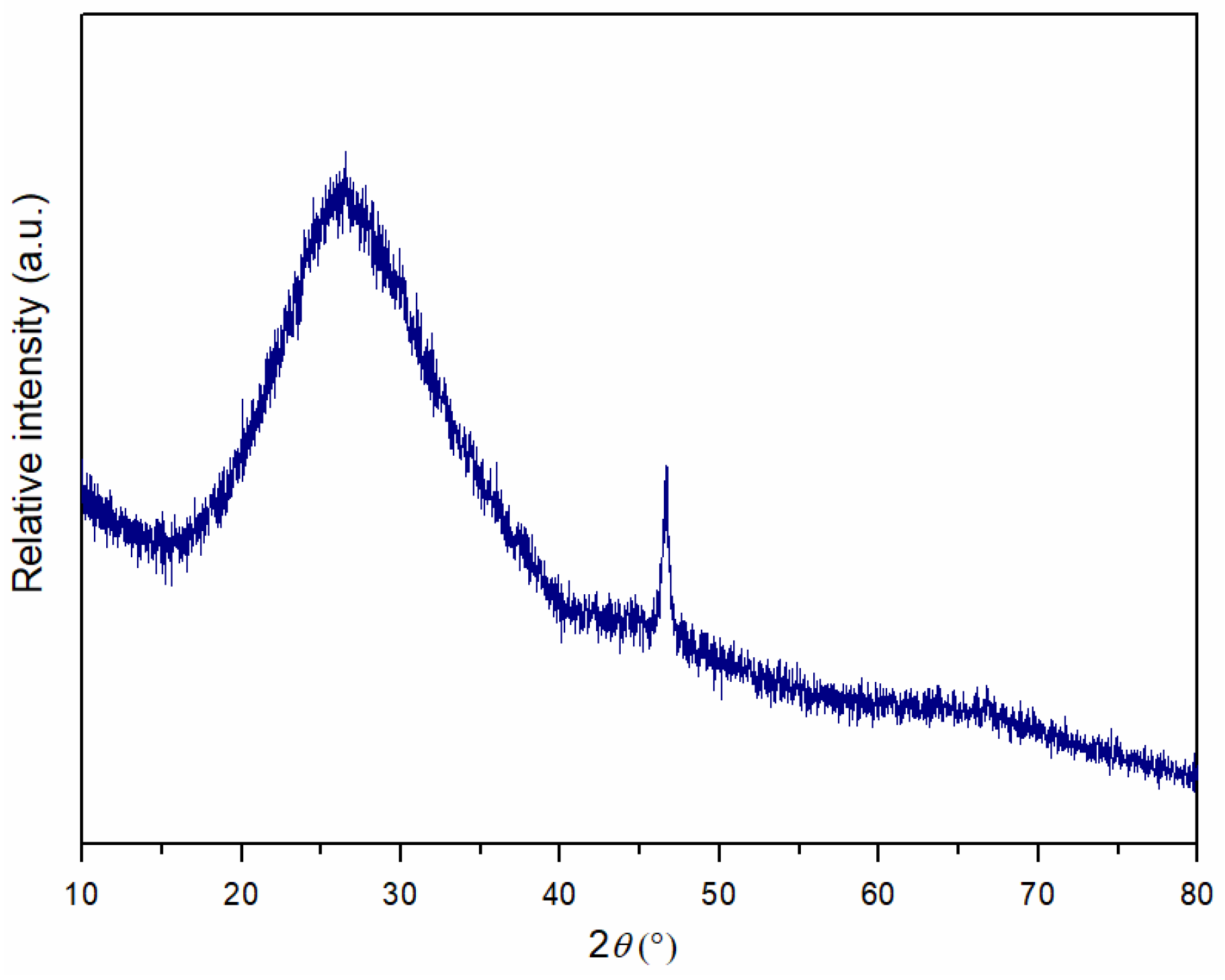
3.2. Determination of the Phase Composition of Sintered MG Composites
3.3. Hardness
4. Conclusions
- The presence of a glassy phase as a reinforcing agent in sintered 316L steel (sintered at 1250 °C for 30 min in a vacuum) reduces the porosity of the composite material by filling the pores. This effect is maintained as long as there are “bridges” between the particles, which serve as the basis for the matrix strength of the material. The elimination of porosity in the composite is attributed to the low viscosity and good wettability of the molten andesite basalt, as well as the formation of a strong bond between the matrix and the reinforcement.
- With increasing content of the glassy phase in the metal–glass composite, the following effects are observed:
- The density of the composite materials increases, while their specific weight decreases;
- The macrohardness of the composite increases due to the reduction in porosity, reaching approximately 2.5 times higher than that of the sintered steel at the maximum investigated content of 30 wt.%;
- A content of 10 wt.% andesite basalt is insufficient to completely eliminate porosity within the metal matrix;
- Increasing the andesite basalt content to 20 wt.% results in the formation of the high-density composite material;
- A content of 30 wt.% andesite basalt is considered high and leads to the formation of isolated metal particles, which negatively affect the strength of the composite;
- The MG composite containing 20 wt.% andesite basalt exhibits the optimal combination of properties.
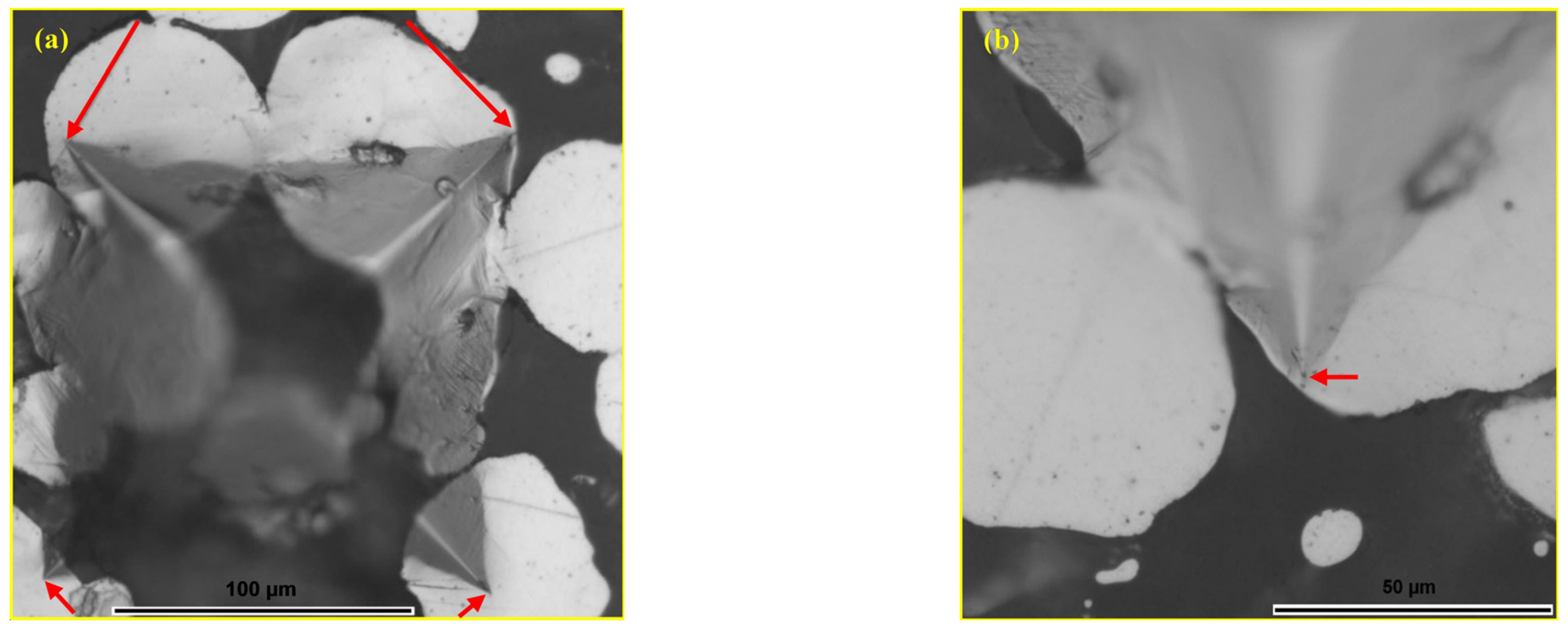
- Cracks formed within the glassy reinforcement during hardness testing propagate toward the metal matrix, where they are effectively arrested, indicating that crack propagation remains localised.
- The results of experimental investigations demonstrate that metal–glass composite materials can be successfully synthesised through powder metallurgy and under strictly defined technological parameters. Owing to their specific properties, such composites represent promising candidates for application in various industrial sectors.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- German, R.M. Particulate Composites: Fundamentals and Applications, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering: An Introduction, 10th ed.; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- McGuire, M.F. Stainless Steels for Design Engineers, 1st ed.; ASM International: Materials Park, OH, USA, 2008. [Google Scholar]
- Abosrra, L.; Ashour, A.F.; Mitchell, S.C.; Youseffi, M. Corrosion of Mild Steel and 316L Austenitic Stainless Steel with Different Surface Roughness in Sodium Chloride Saline Solutions. In WIT Transactions on Engineering Sciences, Proceedings of the Third International Conference on the Simulation of Electrochemical Processes, Bologna, Italy, 24–26 June 2009; WIT Press: Southampton, UK, 2009; pp. 161–172. [Google Scholar]
- Xu, C.; Zhang, Y.; Cheng, G.; Zhu, W. Corrosion and Electrochemical Behavior of 316L Stainless Steel in Sulfate—Reducing and Iron-Oxidizing Bacteria Solutions. Chin. J. Chem. Eng. 2006, 14, 829–834. [Google Scholar] [CrossRef]
- Xu, J.; Zhuo, C.; Tao, J.; Jiang, S.; Liu, L. Improving the Corrosion Wear Resistance of AISI 316L Stainless Steel by Particulate Reinforced Ni Matrix Composite Alloying Layer. J. Phys. D Appl. Phys. 2009, 42, 015410. [Google Scholar] [CrossRef]
- Kurgan, N.; Sun, Y.; Cicek, B.; Ahlatci, H. Production of 316L Stainless Steel Implant Materials by Powder Metallurgy and Investigation of Their Wear Properties. Chin. Sci. Bull. 2012, 57, 1873–1878. [Google Scholar] [CrossRef]
- Kurgan, N. Effect of Porosity and Density on the Mechanical and Microstructural Properties of Sintered 316L Stainless Steel Implant Materials. Mater. Des. 2014, 55, 235–241. [Google Scholar] [CrossRef]
- Malik, A.U.; Mayan Kutty, P.C.; Siddiqi, N.A.; Andijani, I.N.; Ahmed, S. The Influence of PH and Chloride Concentration on the Corrosion Behaviour of AISI 316L Steel in Aqueous Solutions. Corros. Sci. 1992, 33, 1809–1827. [Google Scholar] [CrossRef]
- Klar, E.; Samal, P.K. Powder Metallurgy Stainless Steels: Processing, Microstructure, and Properties; ASM International: Materials Park, OH, USA, 2007. [Google Scholar]
- Coovattanachai, O.; Tosangthum, N.; Morakotjinda, M.; Yotkaew, T.; Daraphan, A.; Krataitong, R.; Vetayanugul, B.; Tongsri, R. Performance Improvement of P/M 316L by Addition of Liquid Phase Forming Powder. Mater. Sci. Eng. A 2007, 445–446, 440–445. [Google Scholar] [CrossRef]
- Padmavathi, C.; Upadhyaya, A.; Agrawal, D. Corrosion Behavior of Microwave-Sintered Austenitic Stainless Steel Composites. Scr. Mater. 2007, 57, 651–654. [Google Scholar] [CrossRef]
- Morse, S.A. Basalts and Phase Diagrams: An Introduction to the Quantitative Use of Phase Diagrams in Igneous Petrology; Springer: Boston, MA, USA, 1980. [Google Scholar]
- Jamshaid, H.; Mishra, R. A Green Material from Rock: Basalt Fiber—A Review. J. Text. Inst. 2016, 107, 923–937. [Google Scholar] [CrossRef]
- Beall, G.H.; Rittler, H.L. Basalt Glass Ceramics. Am. Ceram. Soc. Bull. 1976, 55, 579–582. [Google Scholar]
- Albert, E.; Muntean, M.; Ianculescu, A.; Miculescu, F.; Albert, B. Special Ceramic Material Based On Basaltic-Andesite for Extreme Environments. Adv. Mater. Res. 2008, 59, 39–41. [Google Scholar] [CrossRef]
- Poznyak, A.I.; Levitskii, I.A.; Barantseva, S.E. Basaltic and Granitic Rocks as Components of Ceramic Mixes for Interior Wall Tiles. Glas. Ceram. 2012, 69, 262–266. [Google Scholar] [CrossRef]
- Khater, G.A.; Abu Safiah, M.O.; Hamzawy, E.M.A. Augite-Anorthite Glass-Ceramics from Residues of Basalt Quarry and Ceramic Wastes. Process. Appl. Ceram. 2015, 9, 117–123. [Google Scholar] [CrossRef]
- Matović, B.; Bošković, S.; Logar, M. Preparation of Basalt-Based Glass Ceramics. J. Serbian Chem. Soc. 2003, 68, 505–510. [Google Scholar] [CrossRef]
- Čikara, D.; Todić, A.; Todić, T. Istraživanje mogućnosti primene domaćih bazalta za proizvodnju bazaltnog stakla. IMK-14 Istraž. Razvoj 2010, 16, 1–5. (In Serbian) [Google Scholar]
- Ojovan, M.I.; Lee, W.E. Glassy Wasteforms for Nuclear Waste Immobilization. Metall. Mater. Trans. A 2011, 42, 837–851. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Lee, W.E. New Developments in Glassy Nuclear Wasteforms; Nova Science Pub Inc.: New York, NY, USA, 2007. [Google Scholar]
- Selcuk, S.; Ahmetoglu, U.; Gokce, E.C. Basalt Fiber Reinforced Polymer Composites (BFRP) Other than Rebars: A Review. Mater. Today Commun. 2023, 37, 107359. [Google Scholar] [CrossRef]
- Chen, C.; Ding, Y.; Wang, X.; Bao, L. Recent Advances to Engineer Tough Basalt Fiber Reinforced Composites: A Review. Polym. Compos. 2024, 45, 12559–12574. [Google Scholar] [CrossRef]
- Abraham, C.B.; Nathan, V.B.; Jaipaul, S.R.; Nijesh, D.; Manoj, M.; Navaneeth, S. Basalt Fibre Reinforced Aluminium Matrix Composites—A Review. Mater. Today Proc. 2020, 21, 380–383. [Google Scholar] [CrossRef]
- Vatin, N.I.; Hematibahar, M.; Gebre, T.H. Impact of Basalt Fiber Reinforced Concrete in Protected Buildings: A Review. Front. Built Environ. 2024, 10, 1407327. [Google Scholar] [CrossRef]
- Ayyanar Raja, M.; Manikandan, V.; Amuthakkannan, P.; Rajesh, S.; Balasubramanian, I. Wear Resistance of Basalt Particulate-Reinforced Stir-Cast Al7075 Metal Matrix Composites. J. Aust. Ceram. Soc. 2018, 54, 119–128. [Google Scholar] [CrossRef]
- AbuSahmin, F.; Algellai, A.; Tomić, N.; Vuksanović, M.M.; Majstorović, J.; Husović, T.V.; Simić, V.; Heinemann, R.J.; Toljić, M.; Kovačević, J. Basalt-Polyester Hybrid Composite Materials for Demanding Wear Applications. Sci. Sinter. 2020, 52, 67–76. [Google Scholar] [CrossRef]
- Abenojar, J.; Velasco, F.; Bautista, A.; Campos, M.; Bas, J.A.; Torralba, J.M. Atmosphere Influence in Sintering Process of Stainless Steels Matrix Composites Reinforced with Hard Particles. Compos. Sci. Technol. 2003, 63, 69–79. [Google Scholar] [CrossRef]
- Kurgan, N.; Varol, R. Mechanical Properties of P/M 316L Stainless Steel Materials. Powder Technol. 2010, 201, 242–247. [Google Scholar] [CrossRef]
- Rosso, M.; Grande, M.A. High Density Sintered Stainless Steels With Improved Properties. J. Achiev. Mater. Manuf. Eng. 2007, 21, 97–102. [Google Scholar]
- German, R.M. Powder Metallurgy of Iron and Steel, 1st ed.; Wiley-Interscience: New York, NY, USA, 1998. [Google Scholar]
- Boch, P.; Nièpce, J.-C. Ceramic Materials: Processes, Properties, and Applications; ISTE Ltd.: London, UK, 2007. [Google Scholar]
- Nayar, H.S.; Wasiczko, B. Nitrogen Absorption Control during Sintering of Stainless Steel Parts. Met. Powder Rep. 1990, 45, 611–614. [Google Scholar] [CrossRef]
- Rawers, J.; Croydon, F.; Krabbe, R.; Duttlinger, N. Tensile Characteristics of Nitrogen Enhanced Powder Injection Moulded 316L Stainless Steel. Powder Metall. 1996, 39, 125–129. [Google Scholar] [CrossRef]
- Ji, C.H.; Loh, N.H.; Khor, K.A.; Tor, S.B. Sintering Study of 316L Stainless Steel Metal Injection Molding Parts Using Taguchi Method: Final Density. Mater. Sci. Eng. A 2001, 311, 74–82. [Google Scholar] [CrossRef]
- German, R.M. Sintering Theory and Practice; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Nikolić, Z.S.; Ristić, M.M. Modeling and Computer Simulation of Diffusion Process during Liquid Phase Sintering. Facta Univ. 1997, 2, 223–239. [Google Scholar]
- German, R.M. Liquid Phase Sintering; Plenum Press: New York, NY, USA, 1985. [Google Scholar]
- German, R.M.; Suri, P.; Park, S.J. Review: Liquid Phase Sintering. J. Mater. Sci. 2009, 44, 1–39. [Google Scholar] [CrossRef]
- Kingery, W.D.; Norasimhan, M.D. Densification during Sintering in the Presence of a Liquid Phase. I Theory. J. Appl. Phys. 1959, 30, 307–311. [Google Scholar] [CrossRef]
- SurfitTM 316L Product Data Sheet. Available online: http://www.serfla.com.br/pdf/316L.pdf (accessed on 13 August 2025).
- Pavkov, V.; Bakić, G.; Maksimović, V.; Cvijović-Alagić, I.; Bučevac, D.; Matović, B. Novel Basalt-Stainless Steel Composite Materials with Improved Fracture Toughness. Sci. Sinter. 2023, 55, 145–158. [Google Scholar] [CrossRef]
- Pavkov, V.D. Cuнmeзa u Kapaкmepuзaцuja Koмnoзumнux Mamepujaлa нa Бaзu Memaл-Cmaклo-Kepaмuкa (Synthesis and Characterization of Metal-Glass-Ceramic Composite Materials); Faculty of Mechanical Engineering—University of Belgrade: Belgrade, Serbia, 2023. (In Serbian) [Google Scholar]
- Vaz, R.F.; Silvello, A.; Sanchez, J.; Albaladejo, V.; Garcia Cano, I. The Influence of the Powder Characteristics on 316L Stainless Steel Coatings Sprayed by Cold Gas Spray. Coatings 2021, 11, 168. [Google Scholar] [CrossRef]
- Puichaud, A.-H.; Flament, C.; Chniouel, A.; Lomello, F.; Rouesne, E.; Giroux, P.-F.; Maskrot, H.; Schuster, F.; Béchade, J.-L. Microstructure and Mechanical Properties Relationship of Additively Manufactured 316L Stainless Steel by Selective Laser Melting. EPJ Nucl. Sci. Technol. 2019, 5, 23. [Google Scholar] [CrossRef]
- Čikara, D.; Todić, A.; Čikara-Anić, D. Possibilities of Production of Wear Resistant Construction Elements by Processing of Serbian Basalt. FME Trans. 2010, 38, 203–207. [Google Scholar]
- Pavkov, V.; Bakić, G.; Maksimović, V.; Cvijović-Alagić, I.; Prekajski Ðorđević, M.; Bučevac, D.; Matović, B. High-Density Ceramics Obtained by Andesite Basalt Sintering. Process. Appl. Ceram. 2022, 16, 143–152. [Google Scholar] [CrossRef]
- MLS ParaplastTM. Available online: https://www.mls.be/en/p/paraplast (accessed on 10 August 2025).
- ASTM E92-17; Standard Test Methods for Vickers Hardness and Knoop Hardness of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- Speidel, M.O.; Speidel, H.J.C. Austenitic Stainless Steels of High Strength and Ductility. Int. J. Mater. Res. 2004, 95, 596–600. [Google Scholar] [CrossRef]
- Kalpakjian, S.; Schmid, S.R. Manufacturing, Engineering and Tehnology, 6th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Božić, D.; Vilotijević, M.; Ružić, J.; Jovanović, U.; Stašić, J. Microstructure and Properties of Gravity Sintered 316L Stainless Steel Powder with Nickel Boride Addition. Sci. Sinter. 2016, 48, 293–302. [Google Scholar] [CrossRef]
- Schatt, W. Pulvermetallurgie, Sinter-Und Verbundwerkstoffe; VEB Deutscher Verlag für Grundstoffindustrie: Leipzig, Germany, 1979. (In German) [Google Scholar]
- Dewidar, M. Influence of Processing Parameters and Sintering Atmosphere on the Mechanical Properties and Microstructure of Porous 316L Stainless Steel for Possible Hard-Tissue Applications. Int. J. Mech. Mech. Eng. 2012, 12, 10–24. [Google Scholar]
- Lindstedt, U.; Karlsson, B. Microstructure and Mechanical Behaviour of Single Pressed and Vacuum Sintered Gas and Water Atomised 316L Stainless Steel Powders. Powder Metall. 1998, 41, 261–268. [Google Scholar] [CrossRef]
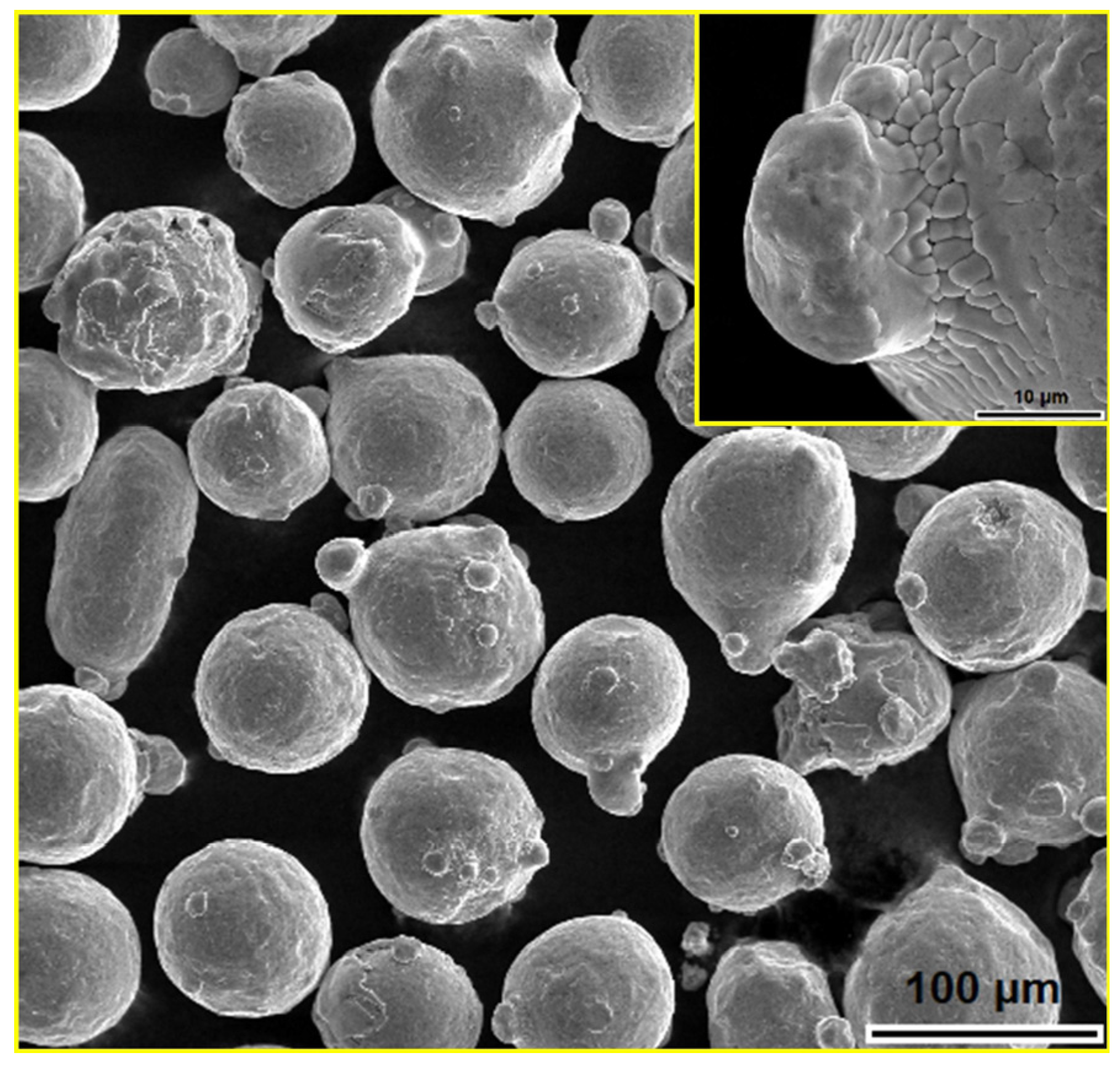
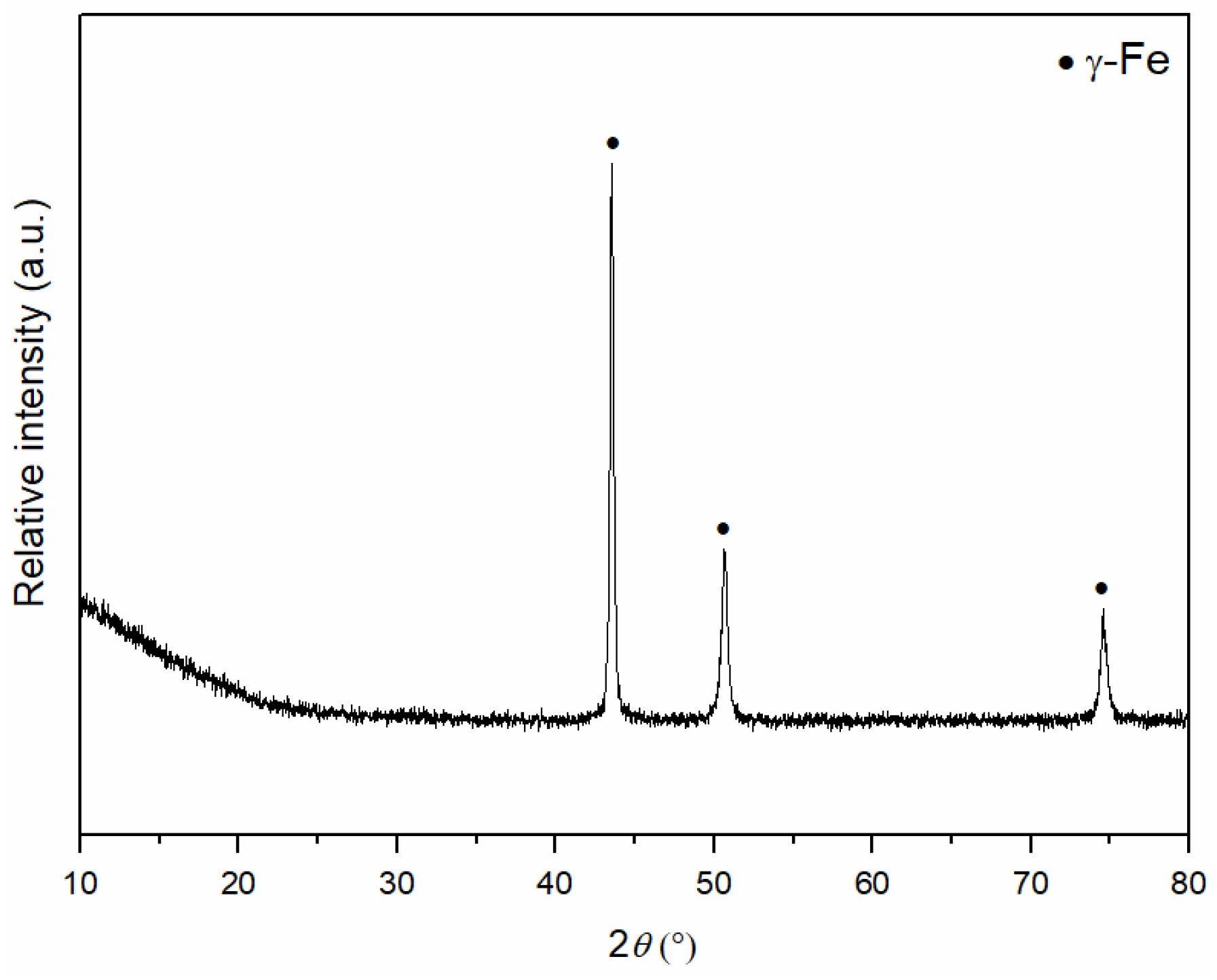
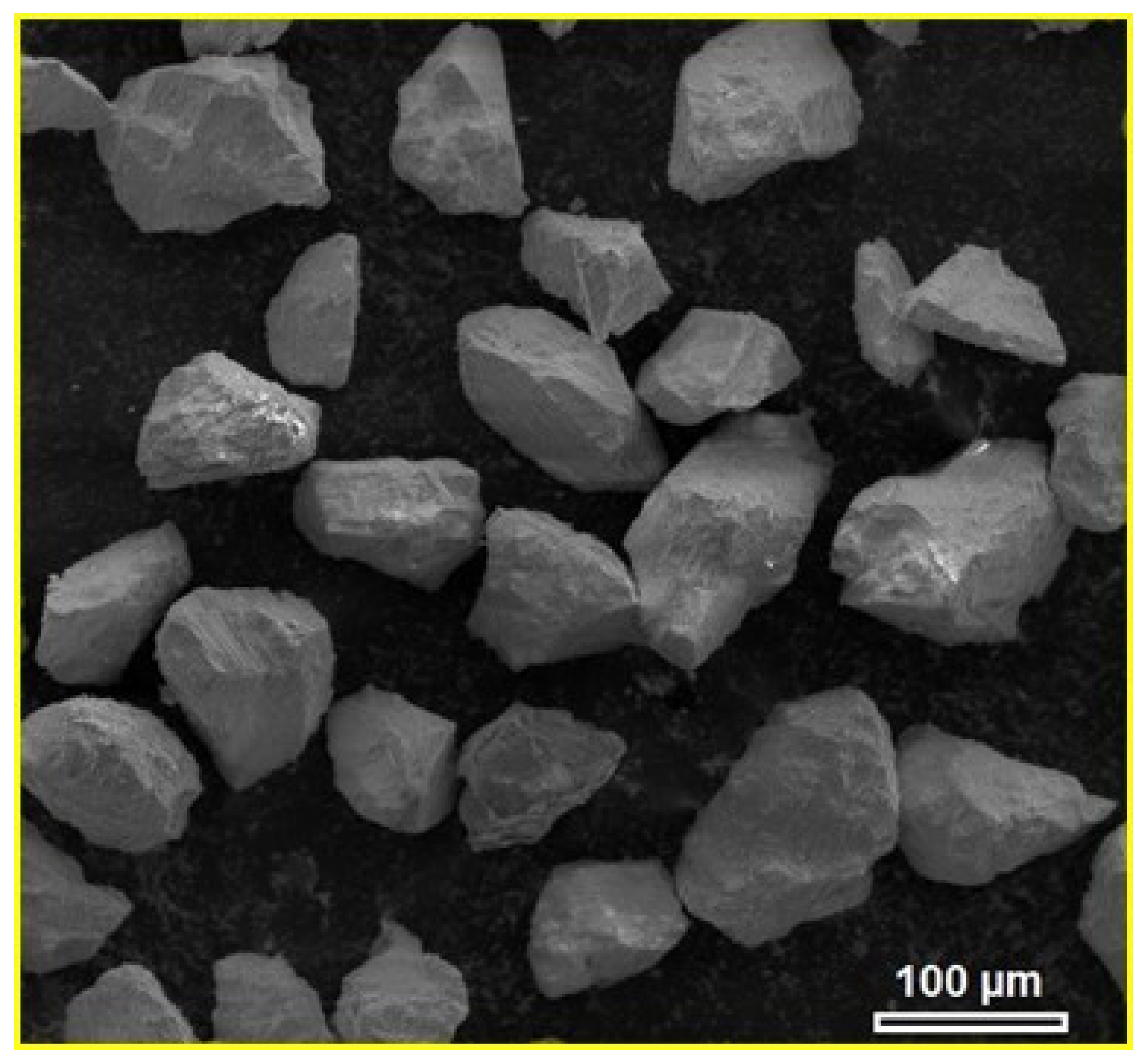

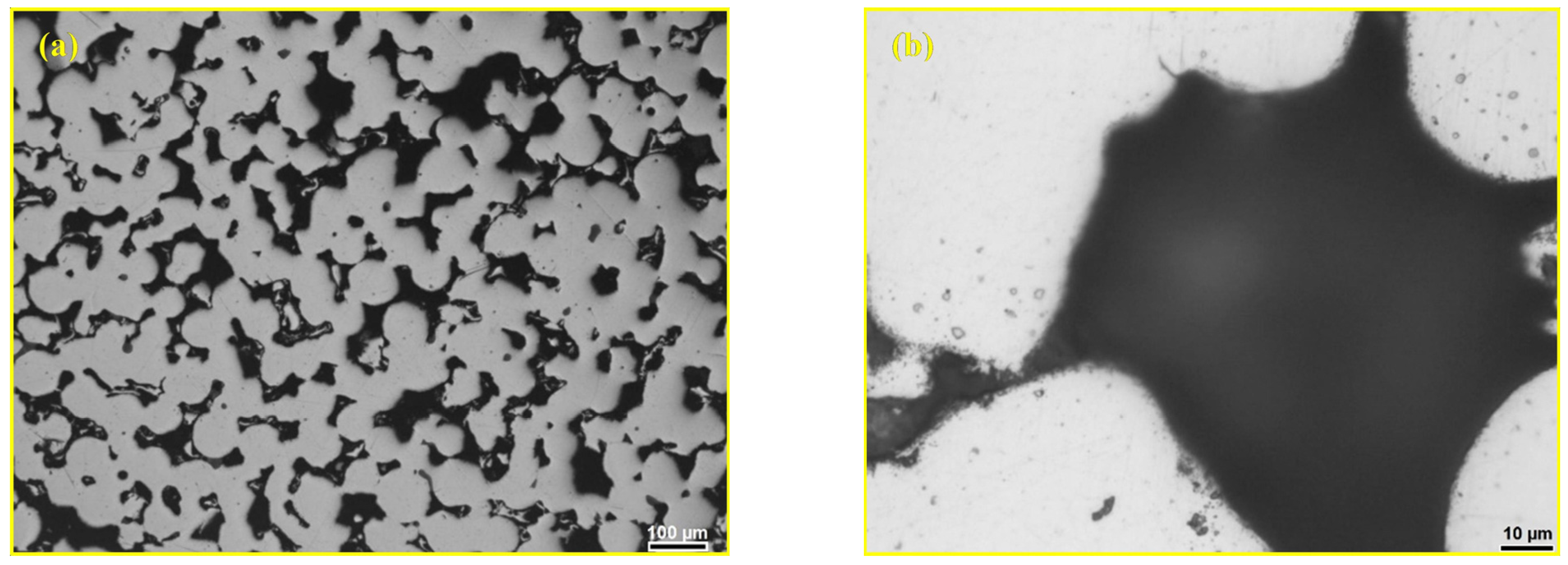
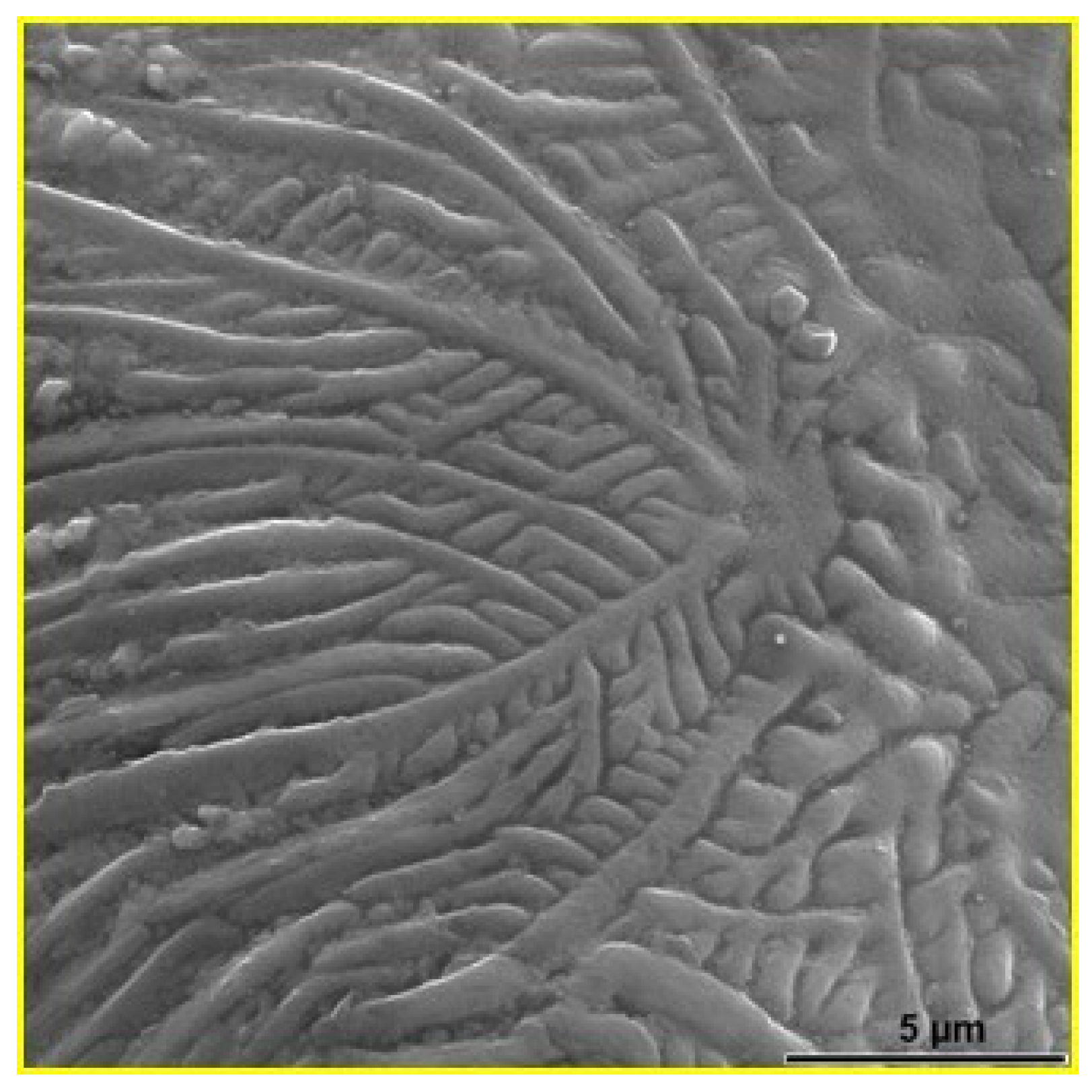


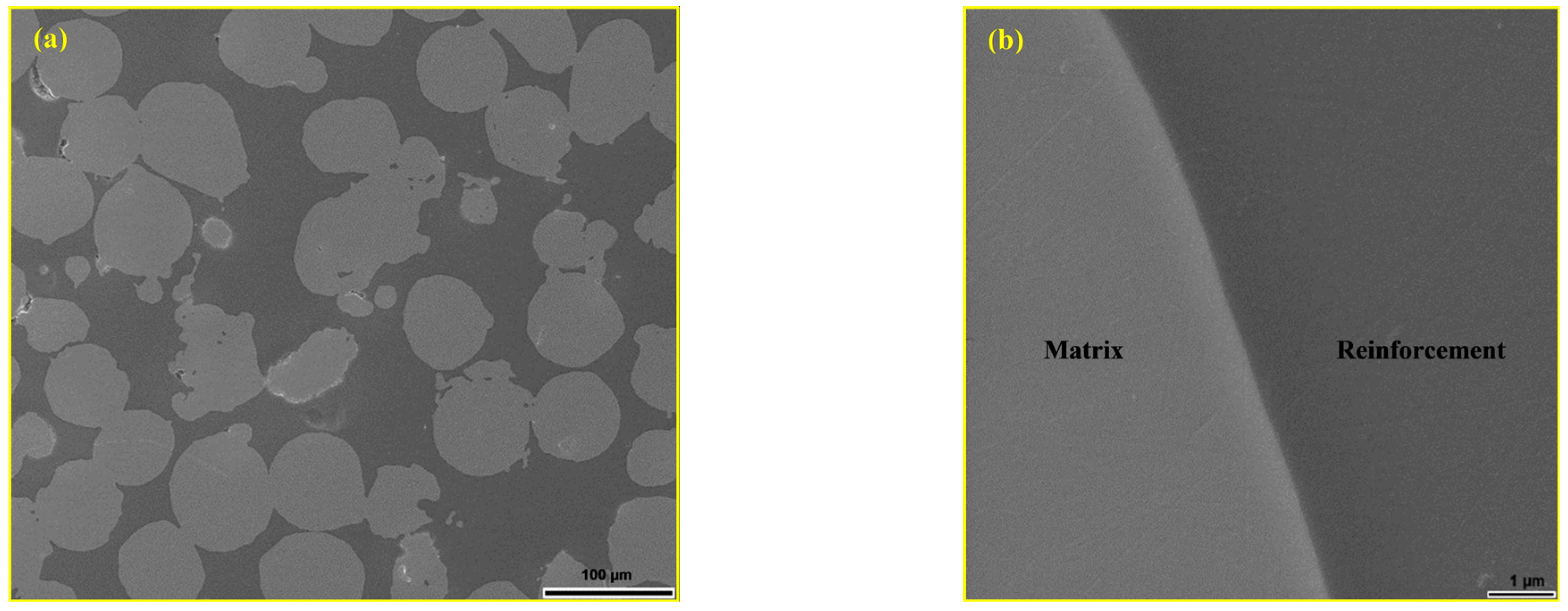
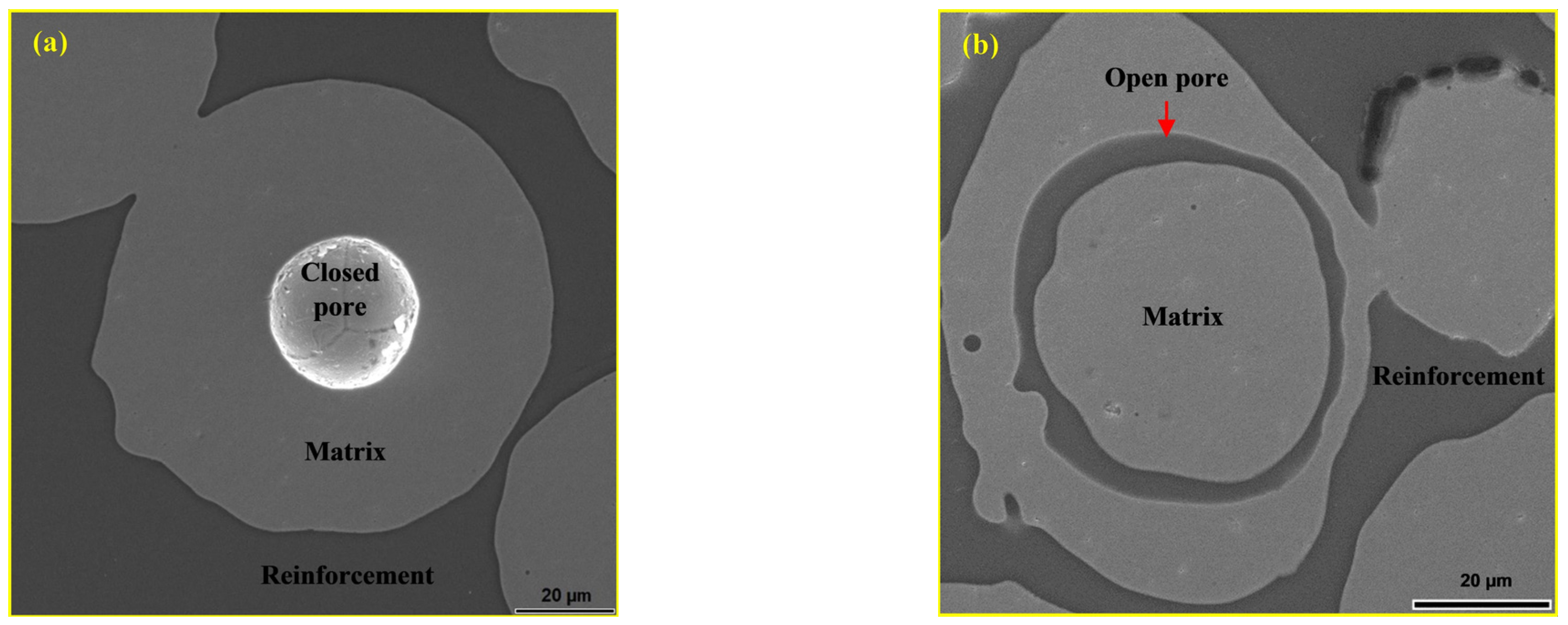
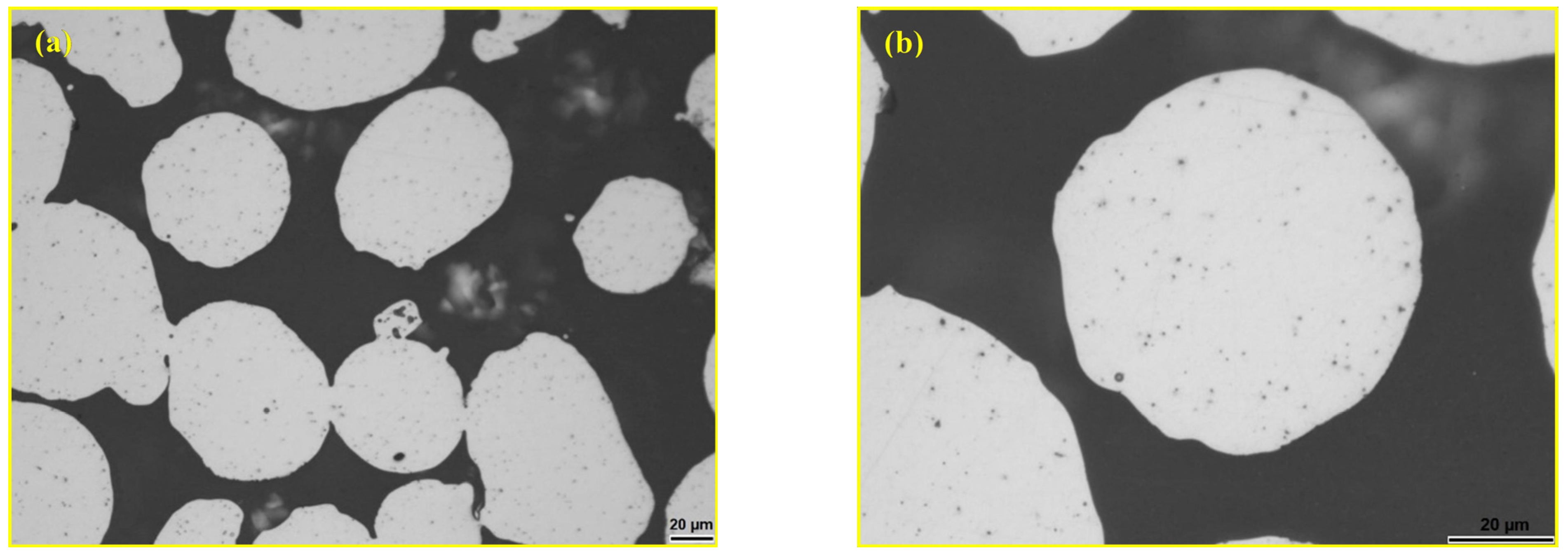
| Materials | Sintering Parameters | Hardness (GPa) | Reference |
|---|---|---|---|
| Andesite basalt aggregate | - | 6.00 ± 0.06 HV1 | [44] |
| Sintered ceramic from andesite basalt | at 1060 °C for 60 min in the air | 6.70 ± 0.05 HV3 | [48] |
| Andesite basalt glass | at 1250 °C for 30 min in a vacuum | 7.94 ± 0.15 HV0.05 | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavkov, V.; Bakić, G.; Maksimović, V.; Stopić, S. Synthesis and Characterisation of Metal–Glass Composite Materials Fabricated by Liquid Phase Sintering. Materials 2025, 18, 4622. https://doi.org/10.3390/ma18194622
Pavkov V, Bakić G, Maksimović V, Stopić S. Synthesis and Characterisation of Metal–Glass Composite Materials Fabricated by Liquid Phase Sintering. Materials. 2025; 18(19):4622. https://doi.org/10.3390/ma18194622
Chicago/Turabian StylePavkov, Vladimir, Gordana Bakić, Vesna Maksimović, and Srećko Stopić. 2025. "Synthesis and Characterisation of Metal–Glass Composite Materials Fabricated by Liquid Phase Sintering" Materials 18, no. 19: 4622. https://doi.org/10.3390/ma18194622
APA StylePavkov, V., Bakić, G., Maksimović, V., & Stopić, S. (2025). Synthesis and Characterisation of Metal–Glass Composite Materials Fabricated by Liquid Phase Sintering. Materials, 18(19), 4622. https://doi.org/10.3390/ma18194622







