Marginal Adaptability of Harvard MTA and Biodentine Used as Root-End Filling Material: A Comparative SEM Study
Abstract
1. Introduction
2. Materials and Methods
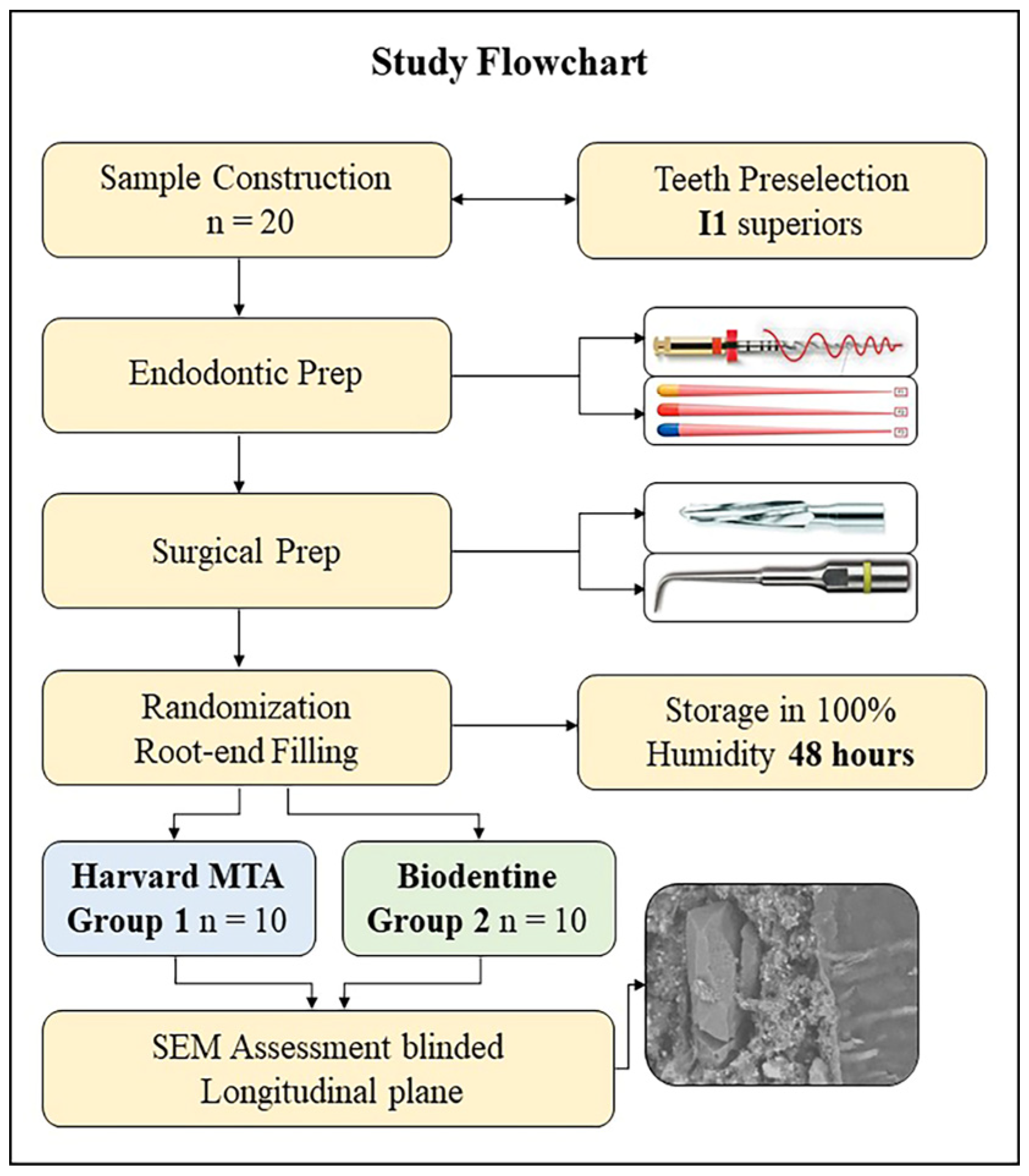
2.1. Sample Construction
2.2. Endodontic Preparation
2.3. Root-End Cavity Preparation and Root-End Filling
2.4. Marginal Adaptation Assessment (Scanning Electron Microscopy Analysis)
2.5. Statistical Analysis
3. Results
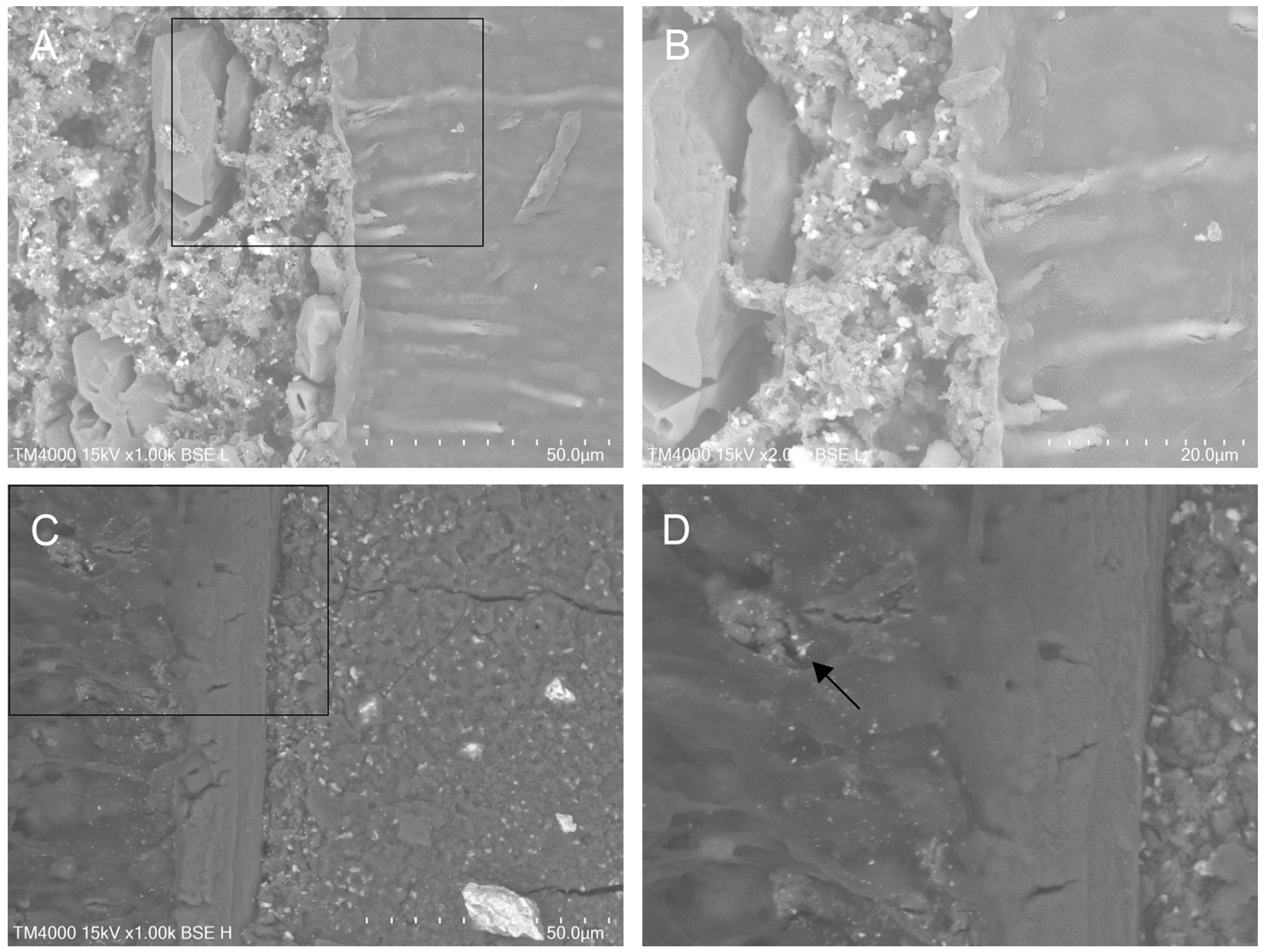
3.1. Qualitative Evaluation of Material-to-Dentine Interface
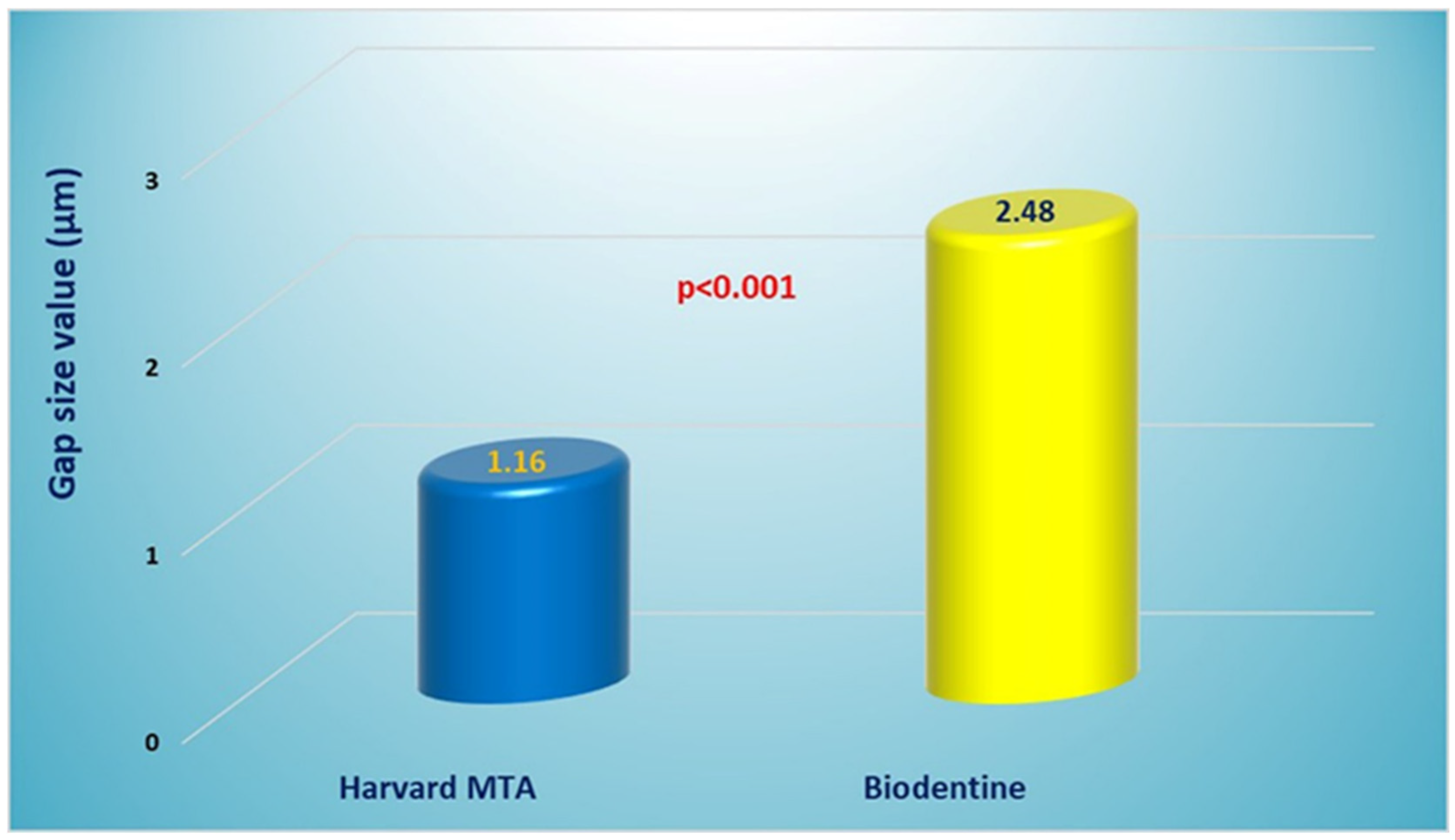
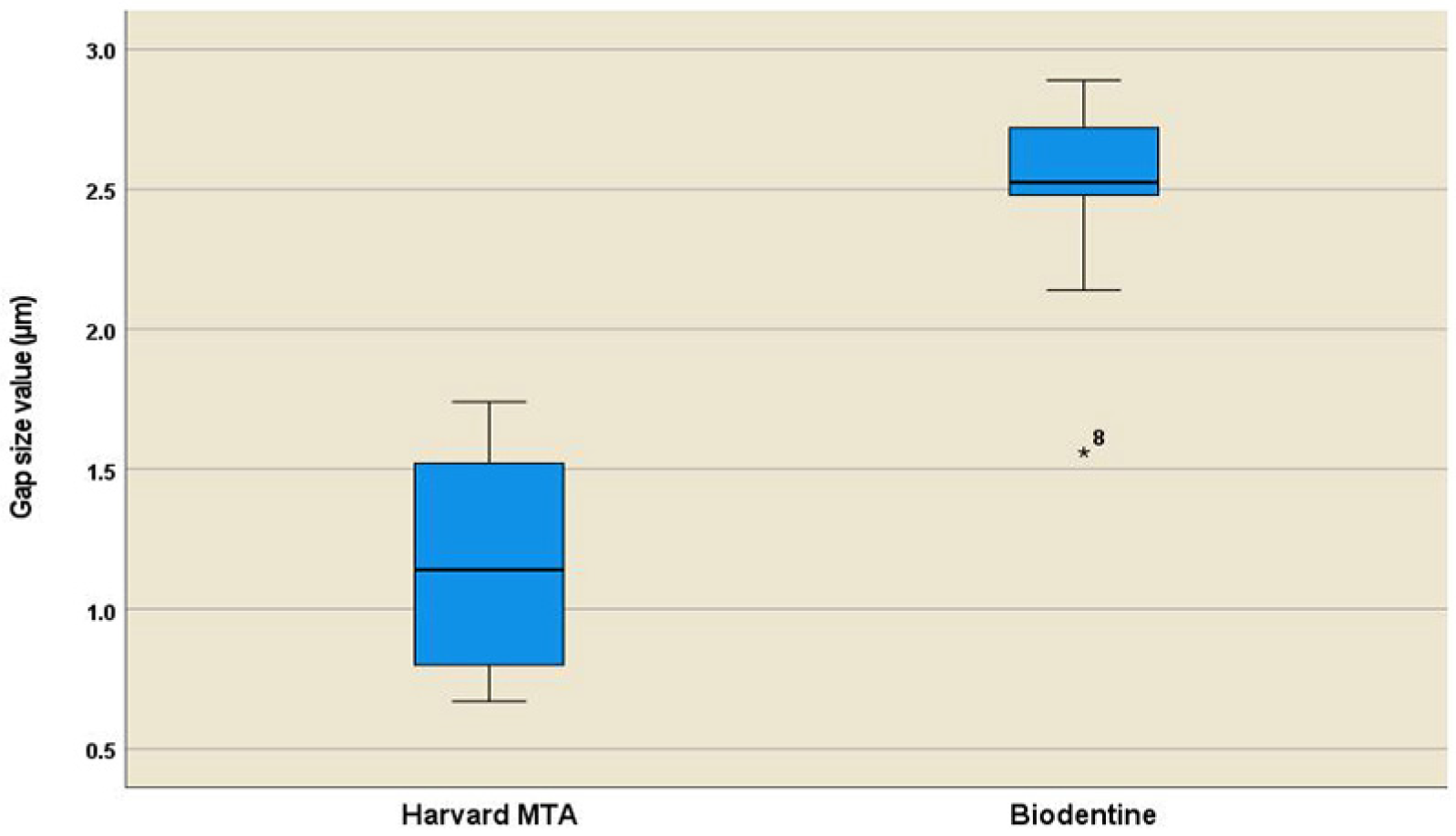
3.2. Quantitative Assessment of Interfacial Gaps
4. Discussion
5. Conclusions
Clinical Relevance
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MTA | Mineral trioxide aggregate |
| AMS | Apical microsurgery |
| CSC | Calcium silicate cement |
| SEM | Scanning electron microscope |
| PBL | Phosphate-buffered liquid |
| PC | Portland cement |
| TCS | Tricalcium silicate |
References
- Ng, Y.L.; Gulabivala, K. Factors that influence the outcomes of surgical endodontic treatment. Int. Endod. J. 2023, 56, 116–139. [Google Scholar] [CrossRef]
- Wang, H.; Xu, X.; Bian, Z.; Liang, J.; Chen, Z.; Hou, B.; Qiu, L.; Chen, W.; Wei, X.; Hu, K.; et al. Expert consensus on apical microsurgery. Int. J. Oral. Sci. 2025, 17, 2. [Google Scholar] [CrossRef]
- Yamada, M.; Kasahara, N.; Matsunaga, S.; Fujii, R.; Miyayoshi, N.; Sekiya, S.; Ding, I.; McCulloch, C.A. Critical Factors Affecting Outcomes of Endodontic Microsurgery: A Retrospective Japanese Study. J. Dent. 2024, 12, 266. [Google Scholar] [CrossRef]
- Abou ElReash, A.; Hamama, H.; Comisi, J.C.; Zaeneldin, A.; Xiaoli, X. The effect of retrograde material type and surgical techniques on the success rate of surgical endodontic retreatment: Systematic review of prospective randomized clinical trials. BMC Oral. Health 2021, 21, 375. [Google Scholar] [CrossRef]
- Chao, Y.C.; Chen, P.H.; Su, W.S.; Yeh, H.W.; Su, C.C.; Wu, Y.C.; Chiang, H.S.; Jhou, H.J.; Shieh, Y.S. Effectiveness of different root-end filling materials in modern surgical endodontic treatment: A systematic review and network meta-analysis. J. Dent. Sci. 2022, 17, 1731–1743. [Google Scholar] [CrossRef]
- Küçükkaya Eren, S.; Parashos, P. Adaptation of mineral trioxide aggregate to dentine walls compared with other root-end filling materials: A systematic review. Aust. Endod. J. 2019, 45, 111–121. [Google Scholar] [CrossRef]
- Solanki, N.P.; Venkappa, K.K.; Shah, N.C. Biocompatibility and sealing ability of mineral trioxide aggregate and biodentine as root-end filling material: A systematic review. J. Conserv. Dent. 2018, 21, 10–15. [Google Scholar] [CrossRef]
- Eskandari, F.; Razavian, A.; Hamidi, R.; Yousefi, K.; Borzou, S. An Updated Review on Properties and Indications of Calcium Silicate-Based Cements in Endodontic Therapy. Int. J. Dent. 2022, 2022, 6858088. [Google Scholar] [CrossRef]
- Camilleri, J. Biodentine™: The Dentine in a Capsule or More? Septodont Learning. 2018. Available online: https://www.septodontlearning.co.uk/sites/default/files/Biodentine-The-dentine-in-a-capsule-or-more-by-Josette-Camilleri-compressed.pdf (accessed on 2 October 2025).
- Kumbhar, A.; Kamat, S.B.; Hugar, S.I.; Nanjannawar, G.S.; Kulkarni, N.R. Comparative evaluation of marginal adaptation of mineral trioxide aggregate, Biodentine, and geristore as a root end filling material: An in vitro scanning electron microscope study. J. Conserv. Dent. 2023, 26, 447–452. [Google Scholar] [CrossRef]
- Nabeel, M.; Abu-Seida, A.M.; Elgendy, A.A.; Tawfik, H.M. Biocompatibility of mineral trioxide aggregate and biodentine as root-end filling materials: An in vivo study. Sci. Rep. 2024, 14, 3568. [Google Scholar] [CrossRef]
- Paños-Crespo, A.; Sánchez-Torres, A.; Gay-Escoda, C. Retrograde filling material in periapical surgery: A systematic review. Med. Oral. Patol. Oral. Cir. Bucal 2021, 26, e422–e429. [Google Scholar] [CrossRef]
- Dong, X.; Su, Q.; Li, W.; Yang, J.; Song, D.; Yang, J.; Xu, X. The outcome of combined use of iRoot BP Plus and iRoot SP for root-end filling in endodontic microsurgery: A randomized controlled trial. Clin. Oral. Investig. 2024, 28, 188. [Google Scholar] [CrossRef]
- Dos Santos, M.M.; Só, G.B.; Siocheta, G.; Jahnke, L.T.; Krabbe, W.M.; Pinheiro, L.S.; Só, M.V.R.; da Rosa, R.A. Interfacial adaptation of NeoMTA Plus, BioRoot RCS and MTA in root-end cavities: A micro-CT study. Microsc. Res. Tech. 2024, 87, 172–178. [Google Scholar] [CrossRef]
- Knapp, J.; Kirkpatrick, T.; Ontiveros, J.C.; Jaramillo, D.E.; Kim, H.C.; Jeong, J.W. Efficacy of root-end filling techniques using premixed putty type bioceramic cements: An ex vivo study. Clin. Oral. Investig. 2024, 28, 365. [Google Scholar] [CrossRef]
- Galal, M.; Zaki, D.Y.; Rabie, M.I.; El-Shereif, S.M.; Hamdy, T.M. Solubility, pH change, and calcium ion release of low solubility endodontic mineral trioxide aggregate. Bull. Natl. Res. Cent. 2020, 44, 42. [Google Scholar] [CrossRef]
- Dimitrova, I.; Gentscheva, G.; Spassova, I.; Kovacheva, D. Influence of Phase Composition and Morphology on the Calcium Ion Release of Several Classical and Hybrid Endodontic Cements. Materials 2024, 17, 5568. [Google Scholar] [CrossRef]
- El-Ma’aita, A.M.; Qualtrough, A.J.; Watts, D. The effect of smear layer on the push-out bond strength of root canal calcium silicate cements. Dent. Mater. 2013, 29, 797–803. [Google Scholar] [CrossRef]
- Uysal, B.A.; Sarıalioğlu Güngör, A. Effect of Trichloroacetic Acid on the Bond Strength of Calcium Silicate-Based Cements: A Modified Push-Out Test. Bezmialem Sci. 2022, 10, 194–198. [Google Scholar] [CrossRef]
- Youssef, A.R.; Elsherief, S. Evaluation of the cytotoxic effects of a new Harvard MTA compared to MTA Flow and ProRoot MTA on human gingival fibroblasts. Saudi Dent. J. 2021, 33, 679–686. [Google Scholar] [CrossRef]
- El-Sherief, S.M.; Rabie, M.I.; Negm, A.M. Marginal adaptation of a new formulation of MTA material used as root-end filling: A scanning electron microscopy (SEM) study. Egypt. Dent J. (EDJ) 2019, 65, 2813–2819. [Google Scholar]
- Jovanović, L.Z.; Bajkin, B.V. Scanning electron microscopy analysis of marginal adaptation of mineral trioxide aggregate, tricalcium silicate cement, and dental amalgam as a root end filling materials. Microsc. Res. Tech. 2021, 84, 2068–2074. [Google Scholar] [CrossRef]
- Kouzmanova, Y.; Dimitrova, I. Marginal Adaptation and Porosity of a Novel MTA Brand Applied as Root-End Filling Material: A Micro-CT Study. Appl. Sci. 2024, 14, 2758. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Sauro, S.; Sano, H.; Matinlinna, J.P.; Yamauti, M.; Hoshika, S.; Toida, Y.; Islam, R.; Tomokiyo, A. Clinical applications and classification of calcium silicate-based cements based on their history and evolution: A narrative review. Clin. Oral. Investig. 2025, 29, 187. [Google Scholar] [CrossRef]
- Dimitrova, I.; Kouzmanova, Y. Impact of photodynamic therapy on the marginal adaptation of biodentine used as root-end filling material. Biomed. Mater. Eng. 2023, 34, 277–287. [Google Scholar] [CrossRef]
- Vanti, A.; Vagarali, H.; Patil, C. Comparative evaluation of marginal adaptation of two root end filling materials in root end preparation done with diamond points and ultrasonics—An in vitro SEM study. IP Indian. J. Conserv. Endod. 2021, 6, 97–100. [Google Scholar] [CrossRef]
- Bolbolian, M.; Seyed, M.F.; Faegh, S. Evaluation of the Marginal Adaptation of ProRoot MTA, Biodentine, and RetroMTA as Root-end Filling Materials. Dent. Hypotheses 2020, 11, 97–102. [Google Scholar] [CrossRef]
- Bolhari, B.; Ashofteh Yazdi, K.; Sharifi, F.; Pirmoazen, S. Comparative Scanning Electron Microscopic Study of the Marginal Adaptation of Four Root-End Filling Materials in Presence and Absence of Blood. J. Dent. 2015, 12, 226–234. [Google Scholar]
- Soundappan, S.; Sundaramurthy, J.L.; Raghu, S.; Natanasabapathy, V. Biodentine versus Mineral Trioxide Aggregate versus Intermediate Restorative Material for Retrograde Root End Filling: An Invitro Study. J. Dent. 2014, 11, 143–149. [Google Scholar]
- Thanatipanont, N.; Louwakul, P. Comparison of Marginal Adaptation, Surface Hardness and Bond Strength of Resected and Retrofilled Calcium Silicate-based Cements Used in Endodontic Surgery: An In Vitro Study. J. Contemp Dent Pr. 2023, 24, 638–644. [Google Scholar] [CrossRef]
- El-Aziz Ali, I.A.A.; Razek, A.A.A.; El-Gindy, A.A. Microleakage and marginal adaptation of three root-end filling materials: An in vitro study. Endod. Pract. Today 2017, 11, 191–196. [Google Scholar]
- Sunanda, Y.L.; Parvathaneni, K.P.; Raju, T.B.V.G.; Seshadri, A.; Dondapati, G.D. Effect of blood and artificial saliva contamination on marginal adaptation and sealing ability of different retrograde filling materials: A comparative analysis. J. Conserv. Dent. Endod. 2024, 27, 743–749. [Google Scholar] [CrossRef]
- Zengin, B.; Aydemir, S.; Chandler, N.P. Marginal adaptation of three root-end filling materials in cavities prepared with laser and ultrasonic tips: An in vitro comparative study. Restor. Dent. Endod. 2025, Epub ahead of print. [Google Scholar] [CrossRef]
- Bansal, R.; Bansal, M.; Matta, M.S.; Walia, S.; Kaur, B.; Sharma, N. Evaluation of marginal adaptation of MTA, Biodentine, and MTA Plus as root-end filling materials—An SEM study. Dent. J. Adv. Stud. 2019, 7, 6–11. [Google Scholar]
- Ravichandra, P.V.; Vemisetty, H.; Deepthi, K.; Reddy, S.J.; Ramkiran, D.; Krishna, M.J.N.; Malathi, G. Comparative Evaluation of Marginal Adaptation of Biodentine™ and Other Commonly Used Root End Filling Materials-An Invitro Study. J. Clin. Diagn. Res. 2014, 8, 243–245. [Google Scholar] [CrossRef]
- Küçükkaya Eren, S.; Görduysus, M.Ö.; Şahin, C. Sealing ability and adaptation of root-end filling materials in cavities prepared with different techniques. Microsc. Res. Tech. 2017, 80, 756–762. [Google Scholar] [CrossRef]
- Mahmoud, O.; Al-Afifi, N.A.; Salihu Farook, M.; Ibrahim, M.A.; Al Shehadat, S.; Alsaegh, M.A. Morphological and Chemical Analysis of Different Types of Calcium Silicate-Based Cements. Int. J. Dent. 2022, 2022, 6480047. [Google Scholar] [CrossRef]
- Atmeh, A.R.; Chong, E.Z.; Richard, G.; Festy, F.; Watson, T.F. Dentin-cement interfacial interaction: Calcium silicates and polyalkenoates. J. Dent. Res. 2012, 91, 454–459. [Google Scholar] [CrossRef]
- Han, L.; Okiji, T. Uptake of calcium and silicon released from calcium silicate-based endodontic materials into root canal dentine. Int. Endod. J. 2011, 44, 1081–1087. [Google Scholar] [CrossRef]
- Kim, J.R.; Nosrat, A.; Fouad, A.F. Interfacial characteristics of Biodentine and MTA with dentine in simulated body fluid. J. Dent. 2015, 43, 241–247. [Google Scholar] [CrossRef]
- Talabani, R.M.; Garib, B.T.; Masaeli, R. Bioactivity and Physicochemical Properties of Three Calcium Silicate-Based Cements: An In Vitro Study. Biomed. Res. Int. 2020, 2020, 9576930. [Google Scholar] [CrossRef]
- Teoh, Y.Y.; Liew, K.Y.; Siao, J.; Wong, S.; Chandler, N.; Bogen, G. The effects of chelation on the intratubular penetration depth of mineral trioxide aggregate. Aust. Endod. J. 2023, 49, 483–491. [Google Scholar] [CrossRef]
- Ghorbanzadeh, A.; Shokouhinejad, N.; Fathi, B.; Raoof, M.; Khoshkhounejad, M. An In Vitro Comparison of Marginal Adaptation of MTA and MTA-Like Materials in the Presence of PBS at One-Week and Two-Month Intervals. J. Dent. 2014, 11, 560–568. [Google Scholar]
- Akbulut, M.B.; Mutlu, Ş.N.; Soylu, M.A.; Şimşek, E. Interfacial characteristics of BIOfactor MTA and Biodentine with dentin. Microsc. Res. Tech. 2023, 86, 258–267. [Google Scholar] [CrossRef]
- Ozdemir, M.; Oncu, A. Penetration of Biodentine, NeoMTA 2, and NeoPUTTY into dentinal tubules in primary tooth pulpotomy: A confocal laser scanning microscopy analysis. BMC Oral. Health 2025, 25, 727. [Google Scholar] [CrossRef]
- Nica, M.L.; Boscornea-Pușcu, A.S.; Horhat, R.M.; Karsoua, M.; Cîrligeriu, L.E. Comparison of three calcium silicate cements used as retrograde filling materials. An in vitro study. Res. Clin. Med. 2020, 4, 32–36. [Google Scholar]
- Kouzmanova, Y.; Dimitrova, I. Comparative Evaluation of the Hermeticity of Sealing of Calcium-Silicate Cements Used in Furcal Perforation Repair. In Properties and Uses of Calcium Silicate, 1st ed.; Carlton, A.G., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2022; pp. 53–102. [Google Scholar]
- Estrela, C.; Cintra, L.T.A.; Duarte, M.A.H.; Rossi-Fedele, G.; Gavini, G.; Sousa-Neto, M.D. Mechanism of action of Bioactive Endodontic Materials. Braz. Dent. J. 2023, 34, 1–11. [Google Scholar] [CrossRef]
- Biočanin, V.; Antonijević, Đ.; Poštić, S.; Ilić, D.; Vuković, Z.; Milić, M.; Fan, Y.; Li, Z.; Brković, B.; Đurić, M. Marginal Gaps between 2 Calcium Silicate and Glass Ionomer Cements and Apical Root Dentin. J. Endod. 2018, 44, 816–821. [Google Scholar] [CrossRef]
- Claverie, J.; Wang, Q.; Kamali-Bernard, S.; Bernard, F. Assessment of the reactivity and hydration of Portland cement clinker phases from atomistic simulation: A critical review. Cem. Concr. Res. 2022, 154, 106711. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Orangi, J.; Asatourian, A.; Gutmann, J.L.; Garcia-Godoy, F.; Lotfi, M.; Sheibani, N. Calcium silicate-based cements and functional impacts of various constituents. Dent. Mater. J. 2017, 36, 8–18. [Google Scholar] [CrossRef]
- de Siqueira, P.C.; Gonçalves de Alencar, A.H.; de Almeida Decurcio, D.; Silva, J.A.; Estrela, C. Characterization of chemical elements of calcium silicate-based cements. RSBO 2022, 19, 304–312. [Google Scholar]
- An, S.Y.; Lee, M.J.; Shim, Y.S. X-Ray Diffraction Analysis of Various Calcium Silicate-Based Materials. J. Dent. Hyg. Sci. 2022, 22, 191–198. [Google Scholar] [CrossRef]
- Grazziotin-Soares, R.; Nekoofar, M.H.; Davies, T.; Hübler, R.; Meraji, N.; Dummer, P.M.H. Crystalline phases involved in the hydration of calcium silicate-based cements: Semi-quantitative Rietveld X-ray diffraction analysis. Aust. Endod. J. 2019, 45, 26–32. [Google Scholar] [CrossRef]
- Abu Zeid, S.T.; Alamoudi, N.M.; Khafagi, M.G.; Abou Neel, E.A. Chemistry and Bioactivity of NeoMTA Plus™ versus MTA Angelus® Root Repair Materials. J. Spectrosc. 2017, 2017, 8736428. [Google Scholar] [CrossRef]
- Rodriguez Rocha, A.C.R.; Hernandez Padron, G.; Garcia Garduno, M.V.; Garcia Aranda, R.L. Physicochemical analysis of MTA Angelus® and Biodentine® conducted with X ray difraction, dispersive energy spectrometry, X ray fluorescence, scanning electron microscope and infra red spectroscopy. Rev. Odont. Mex. 2015, 19, 174–180. [Google Scholar] [CrossRef]
- Utrap, A.; Pielmeier, M.R.P.; Lange, T.; Gädt, T.; Nilges, T. An Ordered Alite Cement Clinker Phase (Ca3SiO5, aP162) from Flux Synthesis. Z. Anorg. Allg. Chem. 2021, 647, 2105–2112. [Google Scholar] [CrossRef]
- Sokol, E.V.; Kokh, S.N.; Sharygin, V.V.; Danilovsky, V.A.; Seryotkin, Y.V.; Liferovich, R.; Deviatiiarova, A.S.; Nigmatulina, E.N.; Karmanov, N.S. Mineralogical Diversity of Ca2SiO4-Bearing Combustion Metamorphic Rocks in the Hatrurim Basin: Implications for Storage and Partitioning of Elements in Oil Shale Clinkering. Minerals 2019, 9, 465. [Google Scholar] [CrossRef]
- Alecu, A.E.; Costea, C.C.; Surdu, V.A.; Voicu, G.; Jinga, S.I.; Busuioc, C. Processing of Calcium Magnesium Silicates by the Sol-Gel Route. Gels 2022, 8, 574. [Google Scholar] [CrossRef]
- Youness, R.A.; Tag El-deen, D.M.; Taha, M.A. A Review on Calcium Silicate Ceramics: Properties, Limitations, and Solutions for Their Use in Biomedical Applications. Silicon 2023, 15, 2493–2505. [Google Scholar] [CrossRef]
- Kasaniya, M.; Thomas, M.D.A.; Moffatt, T.; Hossack, A. Microstructure and microanalysis of portland cement pastes with high w/c ratios. Cem. Concr. Res. 2024, 183, 107575. [Google Scholar] [CrossRef]
- Staněk, T.; Sulovský, P.; Boháč, M. Berlinite substitution in the cement clinker. Cem. Concr. Res. 2017, 92, 21–28. [Google Scholar] [CrossRef]
- Suzuki, M.; Umesaki, N.; Okajima, T.; Tanaka, T. Effect of thermal history on high-valence chromium ion dissolution in merwinite (3CaO·MgO·2SiO2). J. Am. Ceram. Soc. 2018, 101, 2653–2665. [Google Scholar] [CrossRef]
- Yoo, K.H.; Kim, Y.I.; Yoon, S.Y. Physicochemical and Biological Properties of Mg-Doped Calcium Silicate Endodontic Cement. Materials 2021, 14, 1843. [Google Scholar] [CrossRef]
- Bernard, E.; Nguyen, H.; Kawashima, S.; Lothenbach, B.; Manzano, H.; Provis, J.; Scott, A.; Unluer, C.; Winnefeld, F.; Kinnunen, P. MgO-Based Cements—Current Status and Opportunities. RILEM Tech. Lett. 2023, 8, 65–78. [Google Scholar] [CrossRef]
- Kriskova, L.; Pontikes, Y.; Cizer, Ö.; Malfliet, A.; Dijkmans, J.; Sels, B.; Van Balen, K.; Blanpain, B. Behavior of Mechanically and Chemically Activated Synthetic Merwinite. J. Am. Ceram. Soc. 2014, 97, 3973–3981. [Google Scholar] [CrossRef]
- Song, Q.; He, Q.; Nie, J.; Song, T.; Zhou, H.; Hu, Y.; Chen, Y.; Deng, Y.; Cheng, F. The Properties of Magnesium Silicate Hydrate Prepared from the Magnesium Silicate Minerals in the Earth’s Crust. Buildings 2024, 14, 1188. [Google Scholar] [CrossRef]
- Harvard Dental International. Safety Data Sheet According to 1907/2006/EC, Article 31. Available online: https://harvard-dental-international.de/wp-content/uploads/2021/12/MSDS-Harvard-MTA-PT.pdf (accessed on 12 July 2025).
- Bolhari, B.; Noori, F.; Assadian, H.; Raee, A.; Ghabraei, S.; Shamshiri, A.R.; Heboyan, A. The effect of three additives on properties of mineral trioxide aggregate cements: A systematic review and meta-analysis of in vitro studies. BMC Oral. Health 2024, 24, 335. [Google Scholar] [CrossRef]
- Saingern, S.; Monmaturapoj, N.; Sinsareekul, C.; Kamnoedboon, P.; Osathanon, T.; Srinivasan, M.; Chivatxaranukul, P.; Singthong, T.; Makornpan, C.; Nantanapiboon, D. The comparison of physicochemical and bioactivity properties of different nanoparticles modified calcium silicate-based cement. BMC Oral. Health 2025, 25, 1335. [Google Scholar] [CrossRef]
- Staněk, T.; Sulovský, P. The Impact of Basic Minor Oxides on the Clinker Formation. Mater. Sci. Forum 2017, 908, 3–9. [Google Scholar] [CrossRef]
- Chaudhari, P.S.; Chandak, M.G.; Jaiswal, A.A.; Mankar, N.P.; Paul, P. A Breakthrough in the Era of Calcium Silicate-Based Cements: A Critical Review. Cureus 2022, 14, e28562. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, H.J. Enhancing the Physical Properties of Calcium Silicate Cement Modified with Elastin-like Polypeptides and Bioactive Glass. J. Funct. Biomater. 2025, 16, 188. [Google Scholar] [CrossRef]
- Sinsareekul, C.; Jearanaiphaisarn, T.; Hiran-Us, S. Assessment of volume and depth loss of incompletely set hydraulic calcium silicate cements used as root-end fillings in a simulated surgical model. Sci. Rep. 2025, 15, 30870. [Google Scholar] [CrossRef]
- Sinsareekul, C.; Jearanaiphaisarn, T.; Hiran-Us, S. The Effect of Set Conditions on Volumetric Changes of Different Hydraulic Cements: An In Vitro Root-End Model. Int. Dent. J. 2025, 75, 103859. [Google Scholar] [CrossRef]


| Material Type | N | Mean (µm) | Std. Error | 95% CI for Mean | SD | Min | Max |
|---|---|---|---|---|---|---|---|
| Harvard MTA | 10 | 1.16 a | 0.12 | 0.89–1.42 | 0.37 | 0.67 | 1.74 |
| Biodentine | 10 | 2.48 b | 0.12 | 2.20–2.75 | 0.38 | 1.56 | 2.89 |
| Mineral Content and Crystal Structure | Alite (Typical Composition) | Pure TCS (99.8% Purity) | Hatrurite | Merwinite | |
|---|---|---|---|---|---|
| Content (wt%) | CaO | 71.6% | 73.6% | 72.8% | 49.96% |
| SiO2 | 25.2% | 26.2% | 26.1% | 35.5% | |
| Al2O3 | 1.0% | 0.29% | 0.4% | 0.66% | |
| Fe2O3 | 0.7% | — | 0.2% | — | |
| MgO | 1.1% | 0.1% | trace | 11.62% | |
| Other oxides (Impurities) | P2O5-0.2%; Na2O-0.1%; K2O-0.1%, etc. | Free lime-0.31% | Ti, Al, P | FeO-1.22% | |
| Crystal structure | Polymorphic structure * | Triclinic | Trigonal | Monoclinic | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouzmanova, Y.; Dimitrova, I. Marginal Adaptability of Harvard MTA and Biodentine Used as Root-End Filling Material: A Comparative SEM Study. Materials 2025, 18, 4598. https://doi.org/10.3390/ma18194598
Kouzmanova Y, Dimitrova I. Marginal Adaptability of Harvard MTA and Biodentine Used as Root-End Filling Material: A Comparative SEM Study. Materials. 2025; 18(19):4598. https://doi.org/10.3390/ma18194598
Chicago/Turabian StyleKouzmanova, Yaneta, and Ivanka Dimitrova. 2025. "Marginal Adaptability of Harvard MTA and Biodentine Used as Root-End Filling Material: A Comparative SEM Study" Materials 18, no. 19: 4598. https://doi.org/10.3390/ma18194598
APA StyleKouzmanova, Y., & Dimitrova, I. (2025). Marginal Adaptability of Harvard MTA and Biodentine Used as Root-End Filling Material: A Comparative SEM Study. Materials, 18(19), 4598. https://doi.org/10.3390/ma18194598






