Study on Decarburization Behavior in BOF Steelmaking Based on Multi-Zone Reaction Mechanism
Abstract
1. Introduction
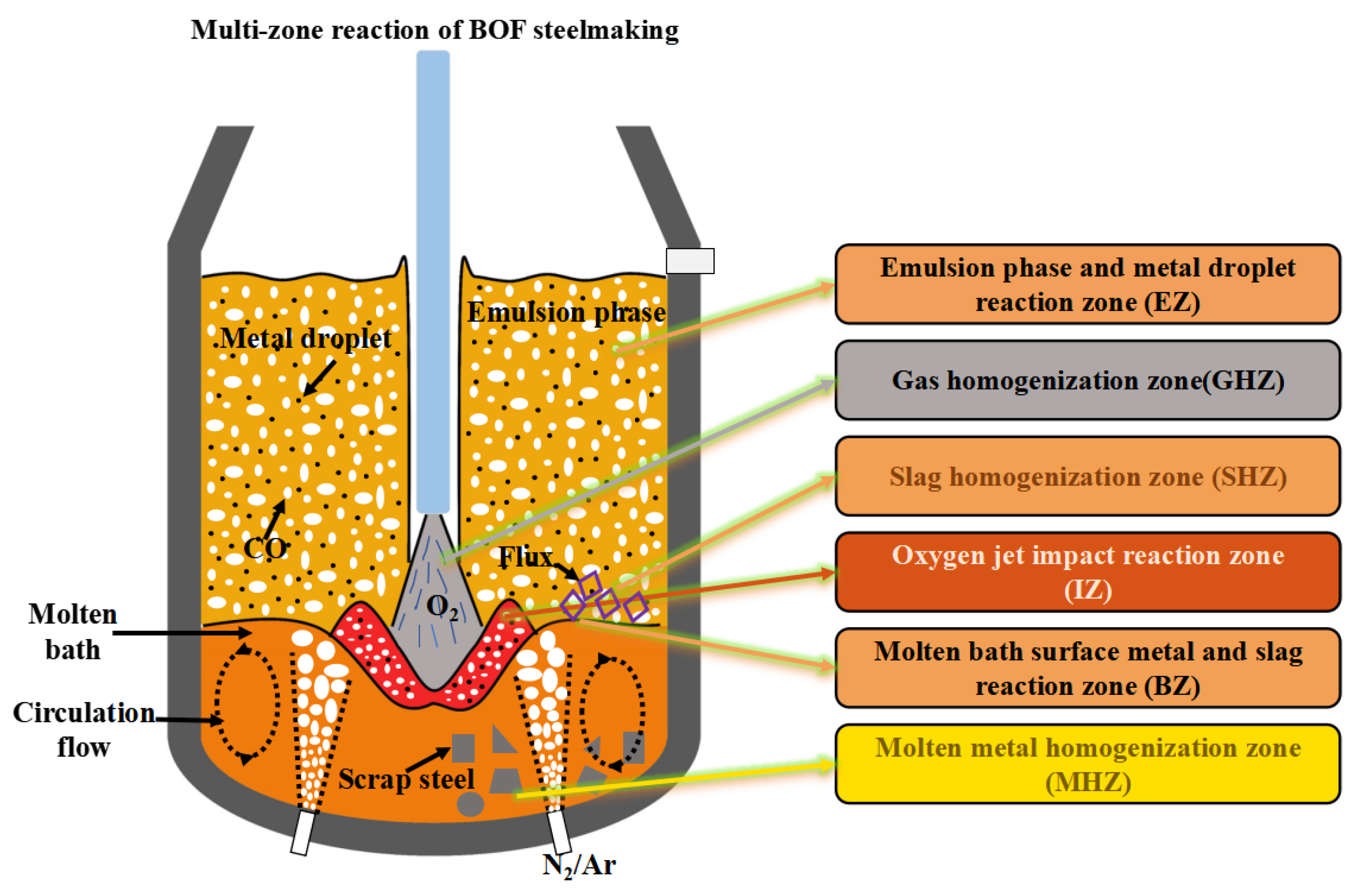
2. Method and Model
3. Results and Discussion
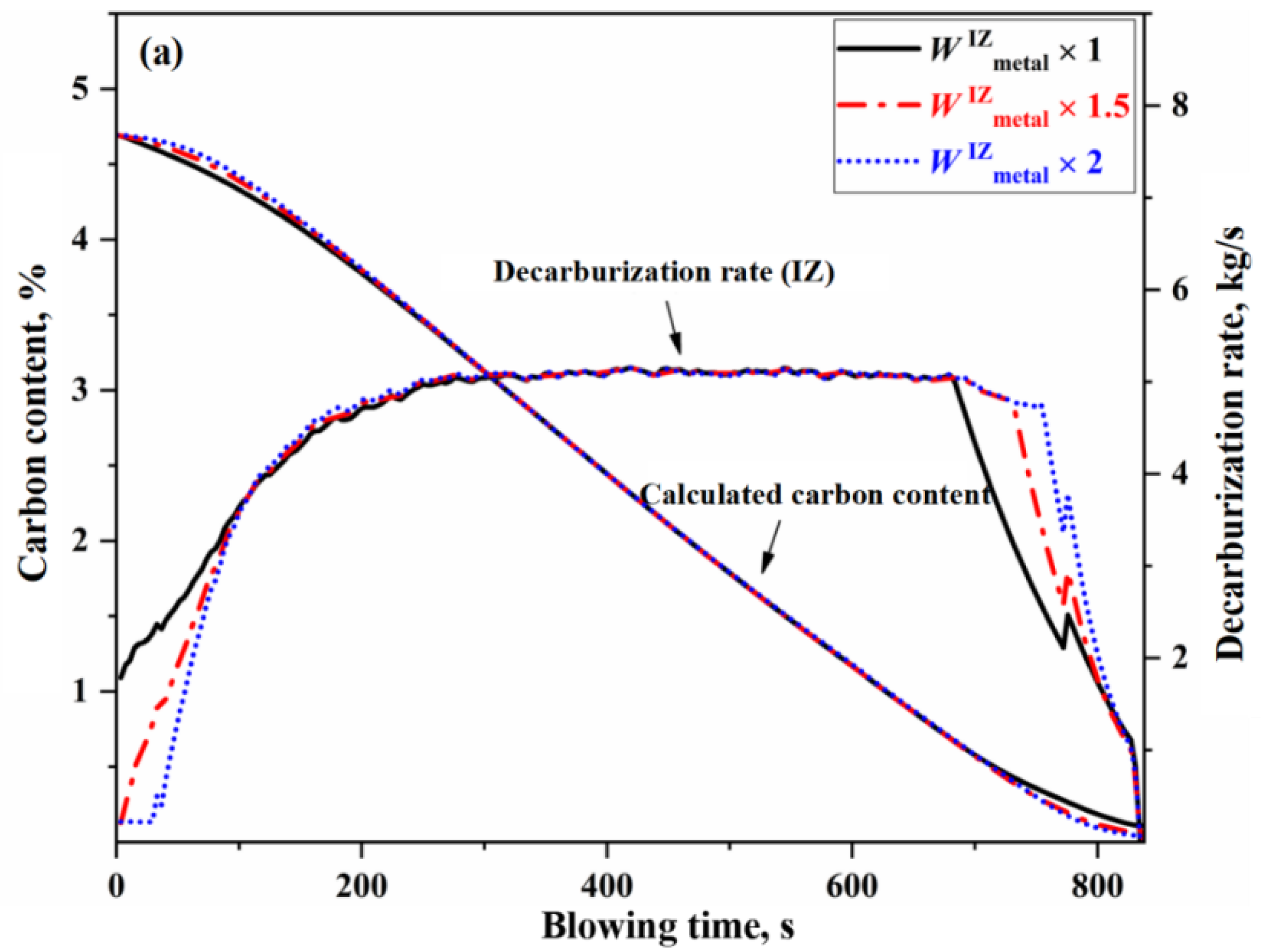
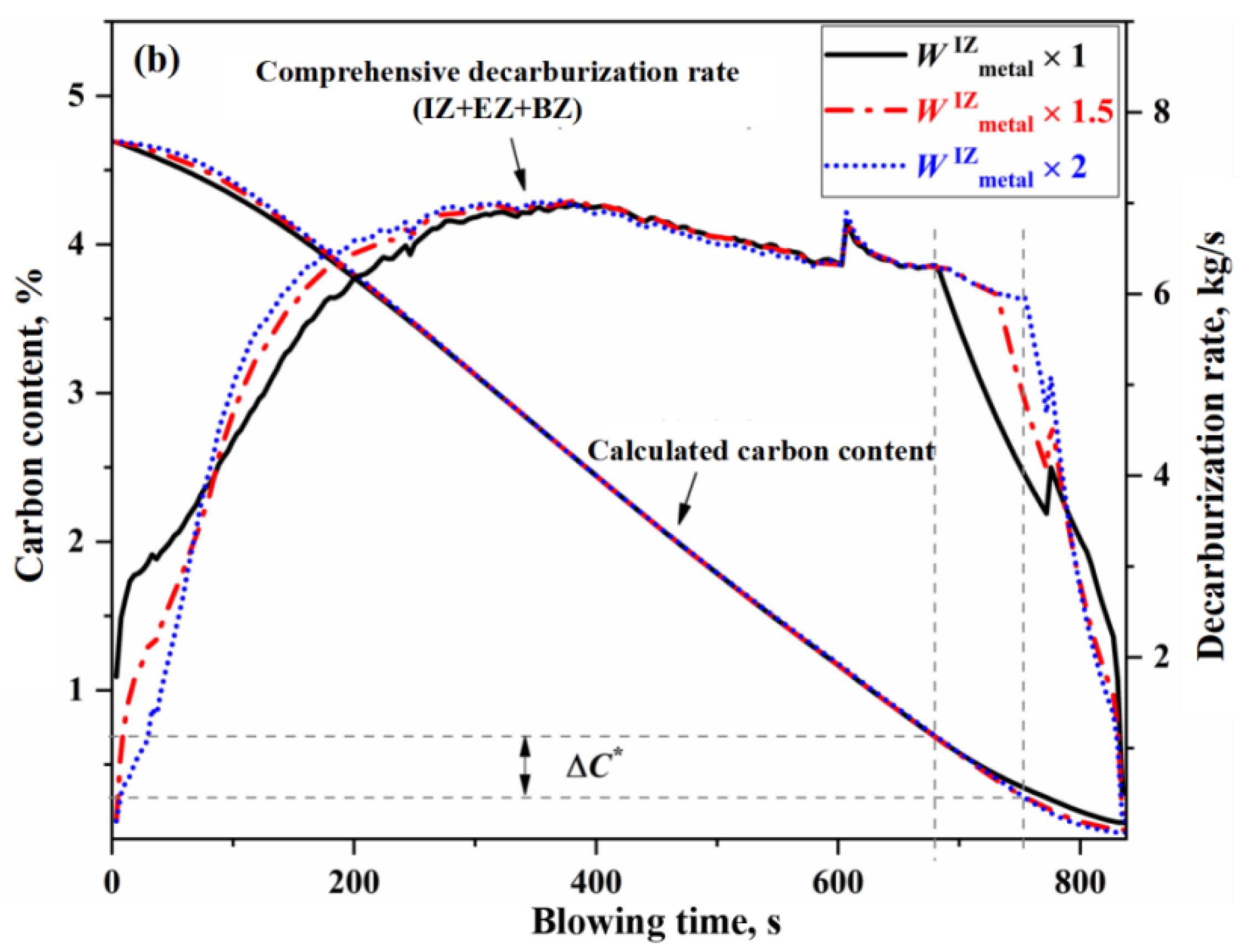
3.1. Effect of Main Reaction Zone on Decarburization Process
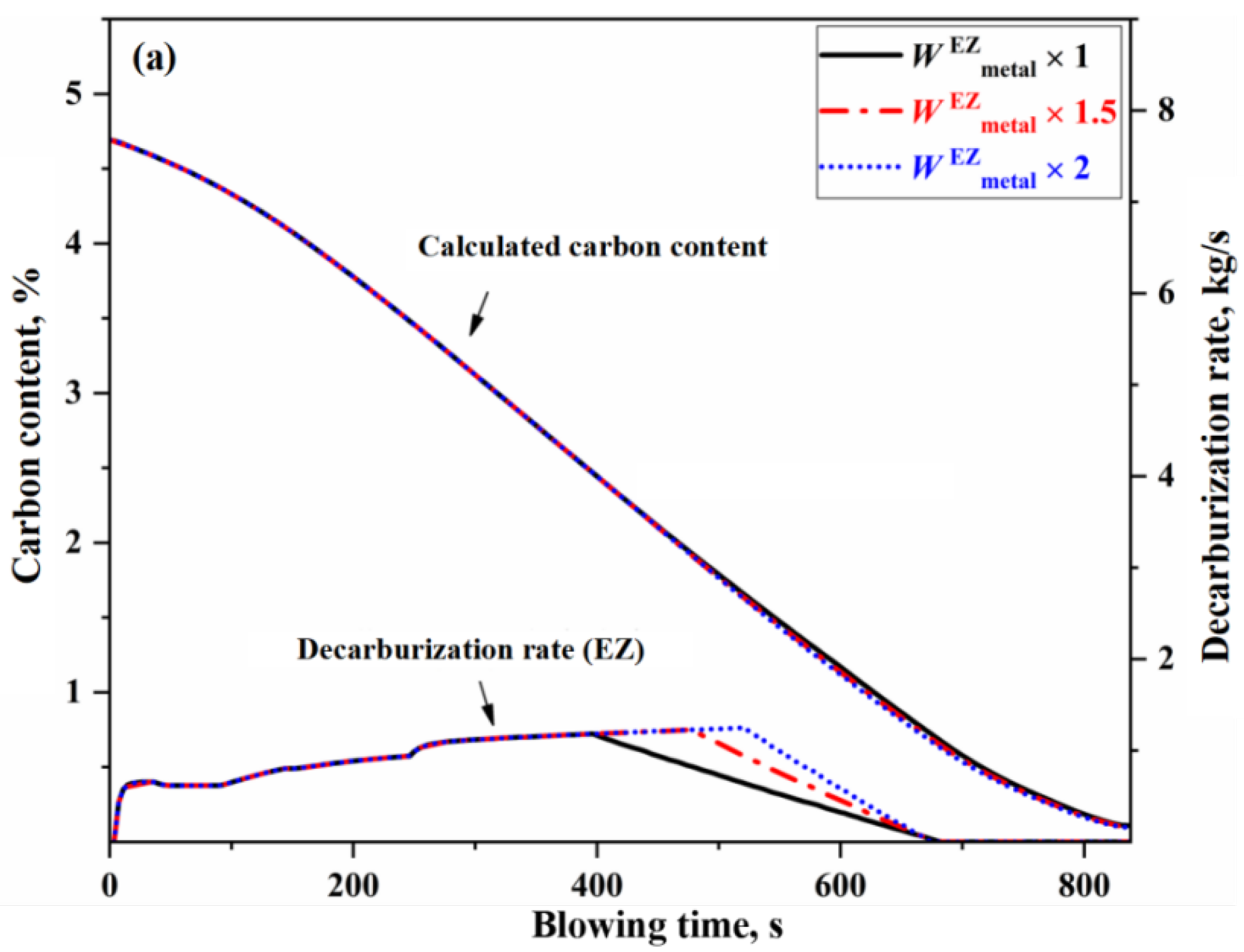
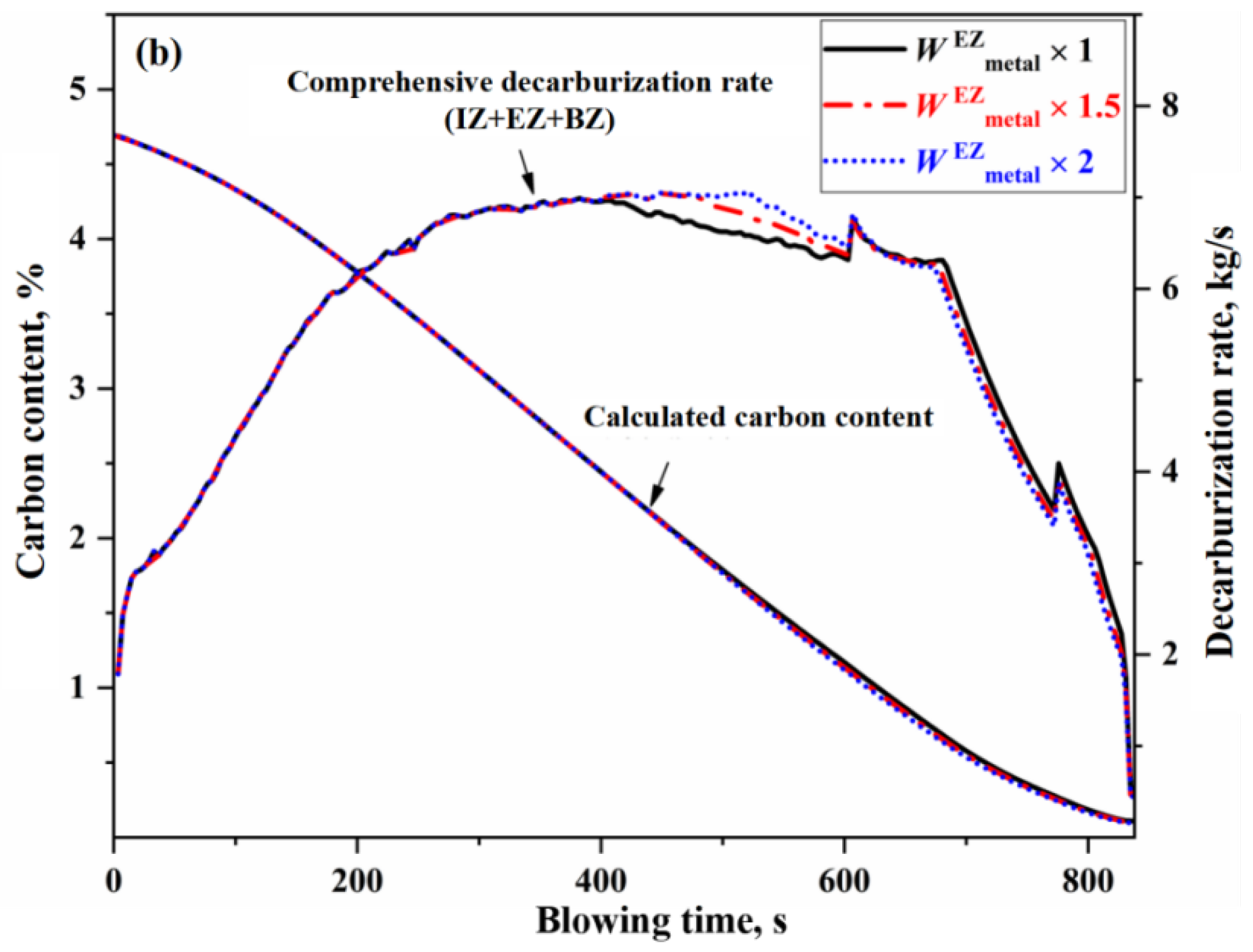
3.2. Effect of Auxiliary Reaction Zone on Decarburization Process
4. Conclusions
- (1)
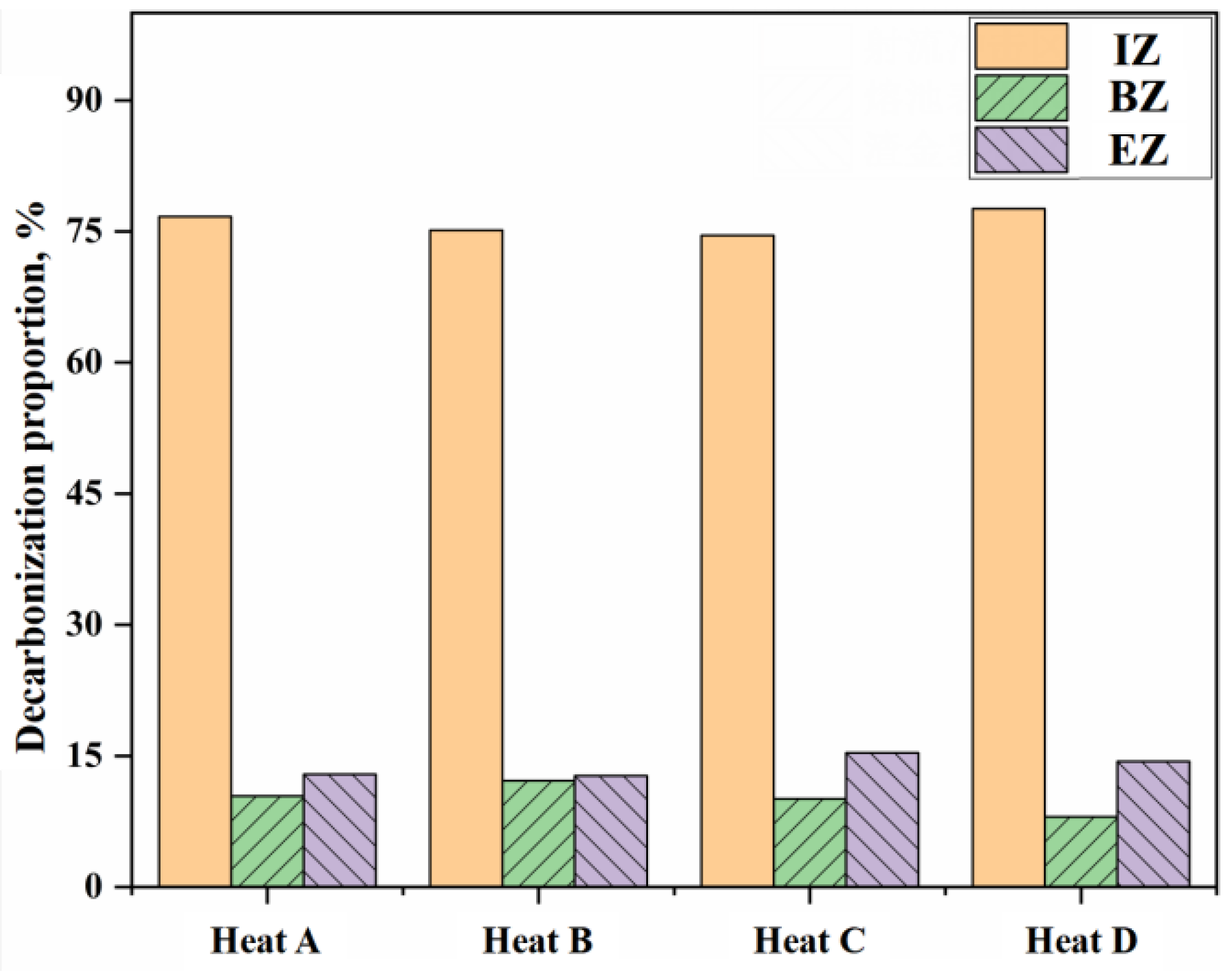
- The decarburization reaction mainly occurs in IZ, BZ, and EZ. Their contributions to overall decarburization decrease in the order IZ > EZ > BZ, with each zone accounting for 76%, 14%, and 10% of the total decarburization, respectively.
- (2)
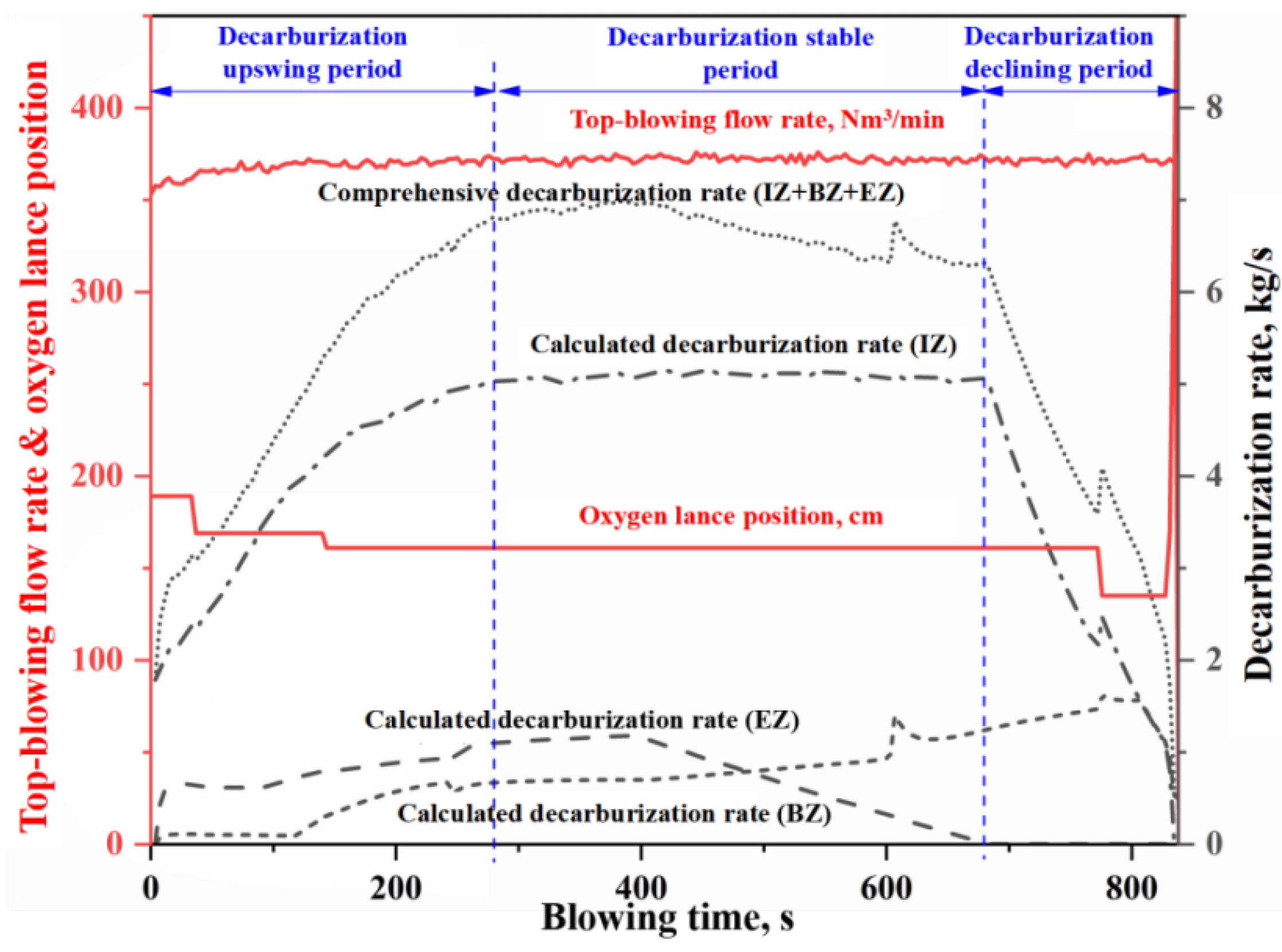
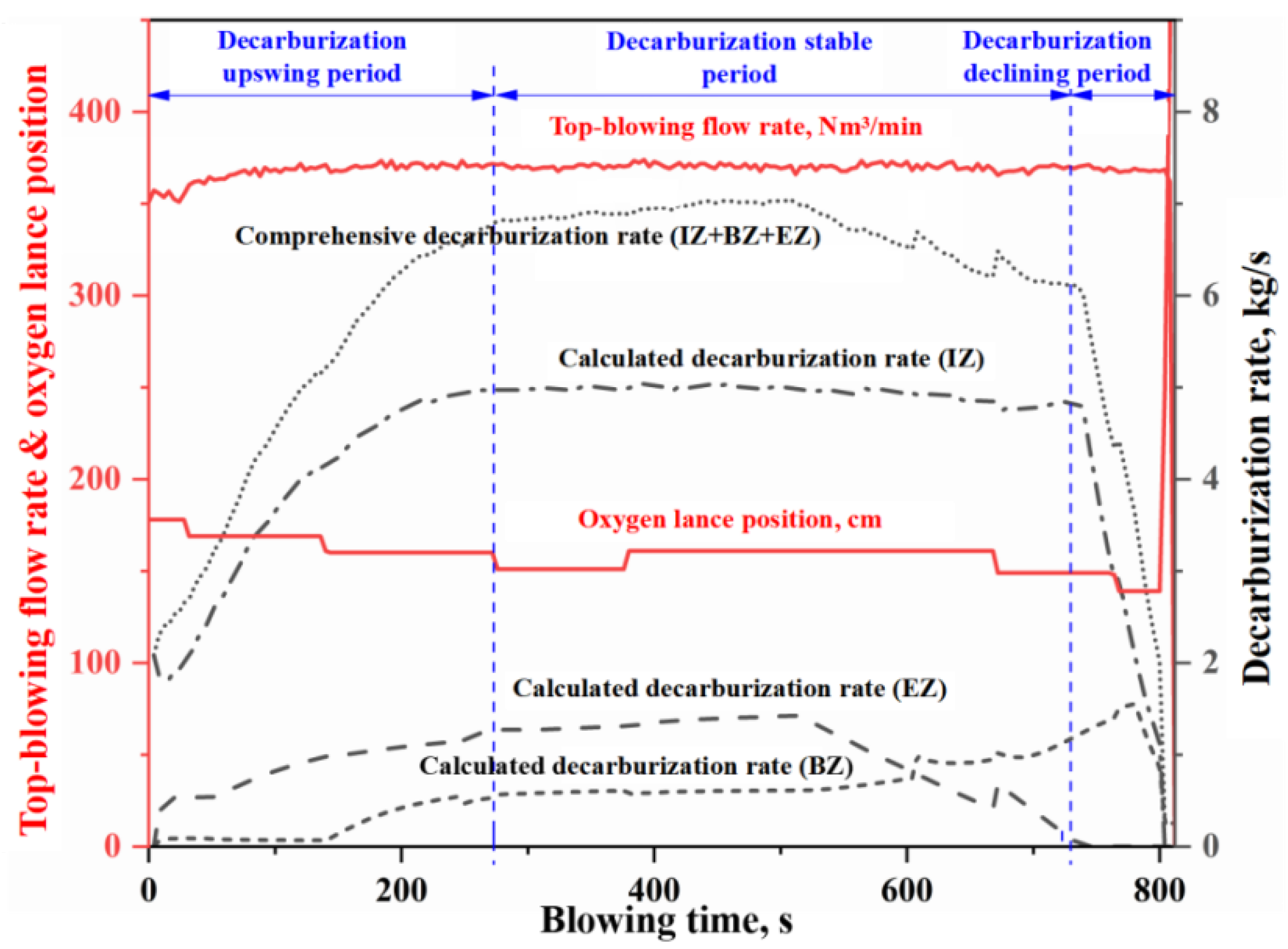
- The morphology of the decarburization rate curve in the IZ determines the overall profile of the comprehensive decarburization rate curve. Under the same oxygen blowing conditions, the larger the effective reaction amount in the IZ, the steeper the decarburization rate curve in the early and late stages of blowing, while having almost no effect on the decarburization rate curve in the stable period. When the effective reaction amount in the IZ is increased by one time, the endpoint carbon content decreases by 0.066%.
- (3)
- The changes of effective reaction amount in the EZ (i.e., the amount of metal droplets) primarily affect decarburization during the latter half of the stable period, while has no effect on the upswing period and the first half of the stable period. The metal droplets in the emulsion phase no longer undergo decarburization during the declining period. When the effective reaction amount in the EZ is increased by one time, the endpoint carbon content decreases by 0.013%.
- (4)
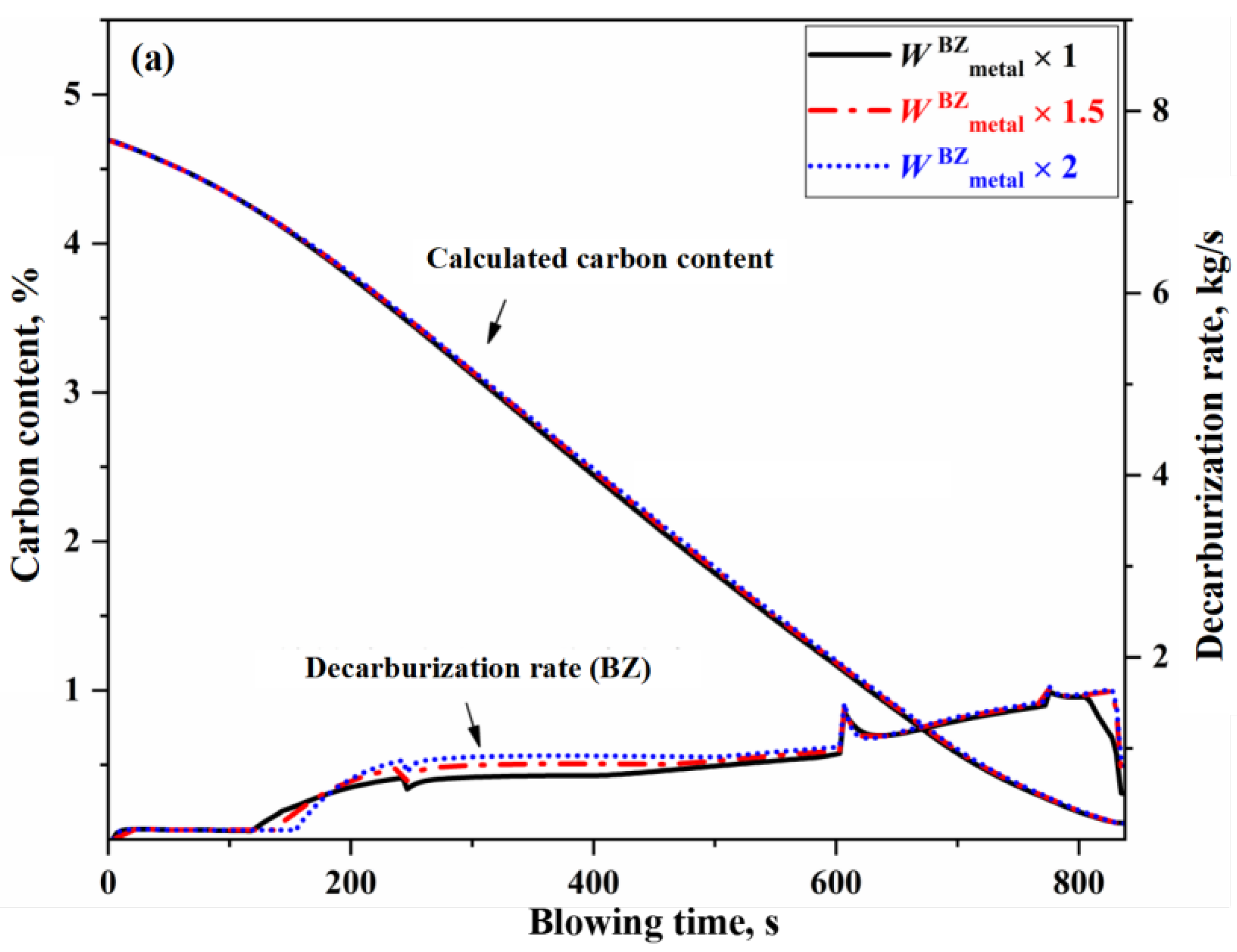
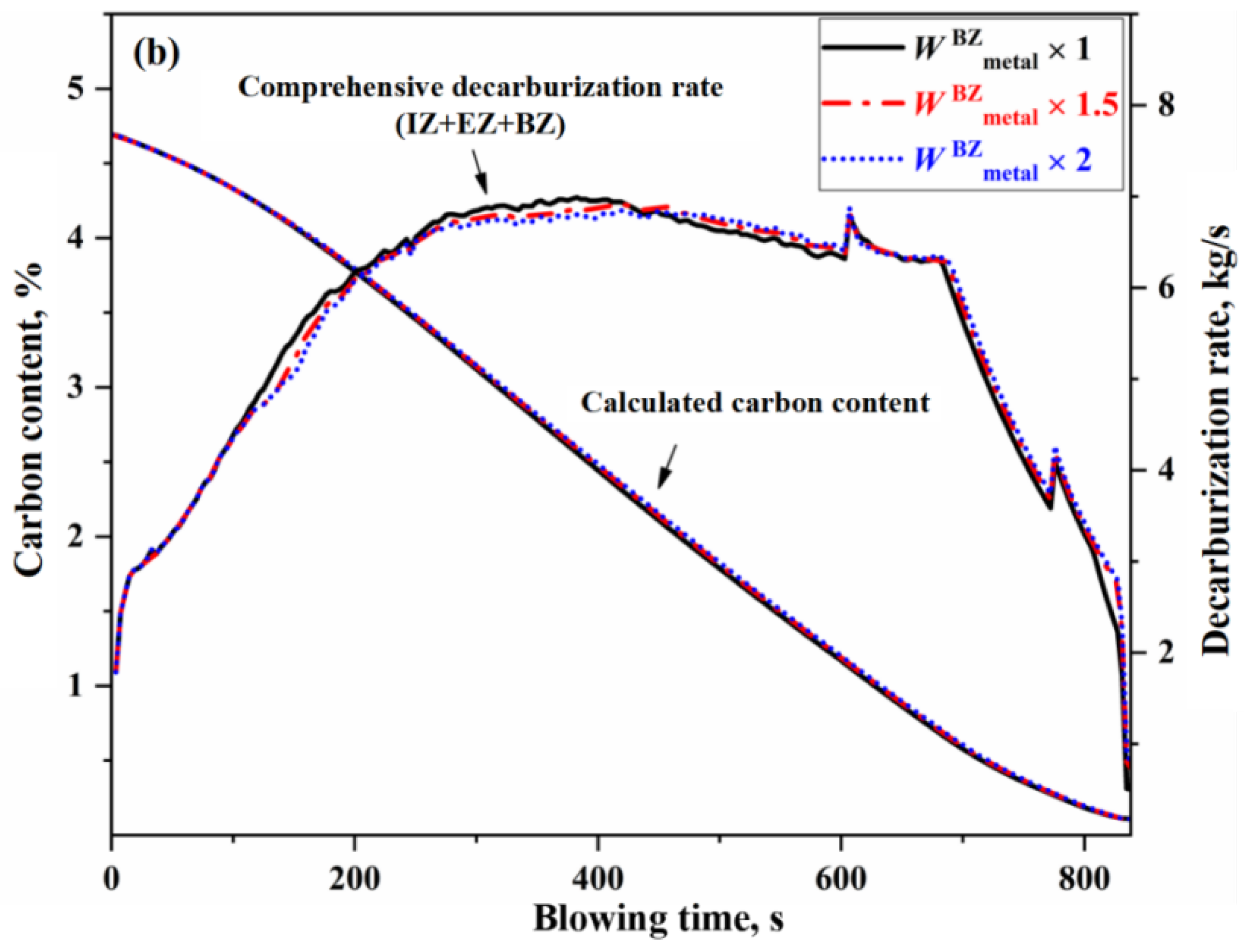
- The variation in the effective reaction amount in the BZ affects decarburization throughout almost the entire blowing process, but the overall impact is relatively small. When the effective reaction amount in the BZ is increased by one time, the endpoint carbon content decreases by 0.011%.
- (5)
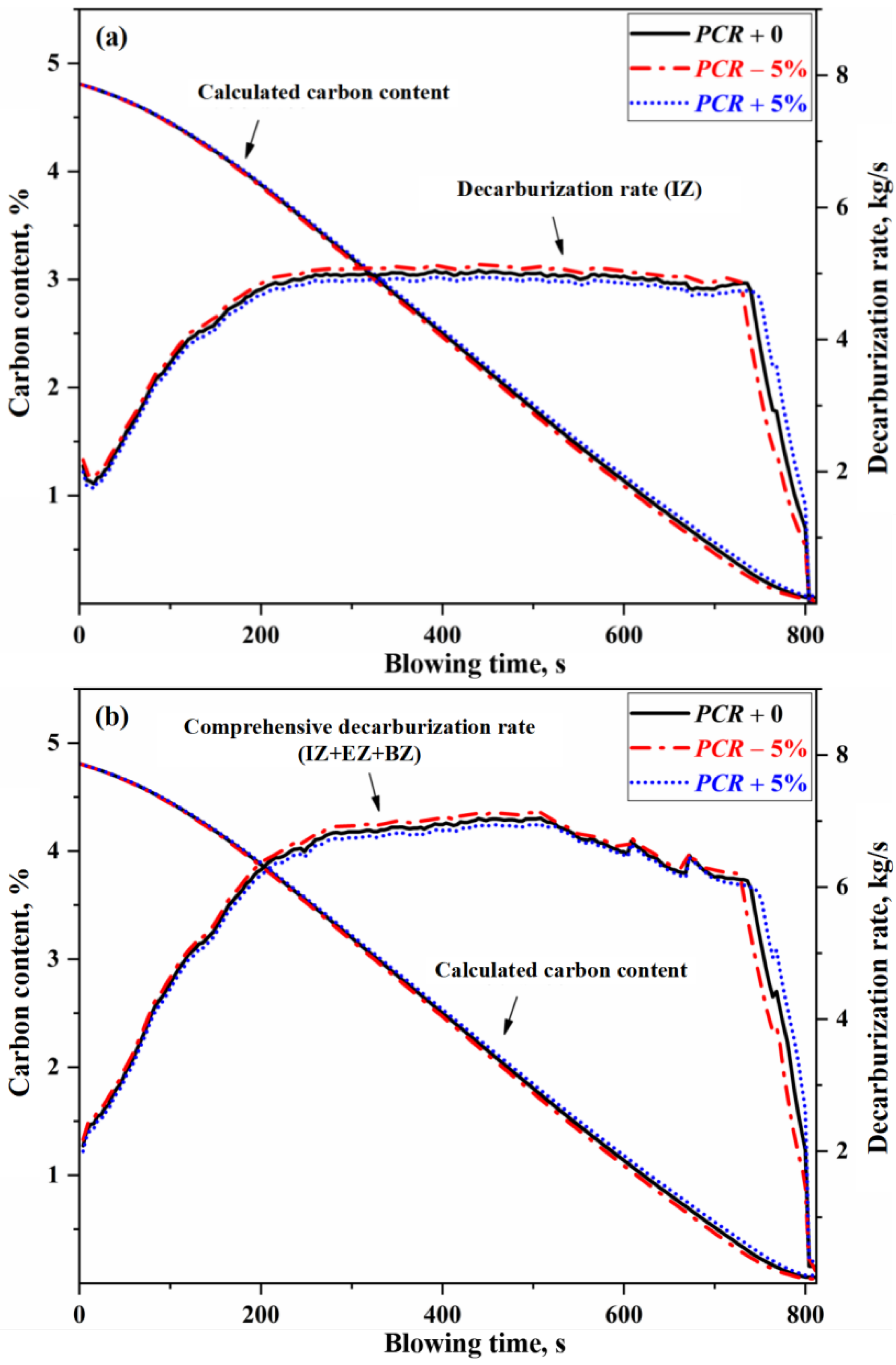
- When the PCR in the GHZ fluctuates by ±5%, the decarburization rate in the IZ and the overall decarburization rate vary by approximately ±0.09 kg/s, while the endpoint carbon content fluctuates between −0.013% and 0.016%. When the amount of unmelted scrap steel (high carbon scrap steel) in the MHZ at the end of blowing exceeds 1.4 t, the carbon content in the molten bath increases by more than 0.01%.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Q.; Wang, B. Multi-Scale Modeling and Collaborative Manufacturing for Steelmaking Plant; Metallurgical Industry Press: Beijing, China, 2025. [Google Scholar]
- Liu, Y.J.; Zhang, X.G.; Peng, Q.; Xia, Y.J.; Wang, H.C.; Deng, A.J. Evaluation of feature selection methods for oxygen supply prediction in BOF steelmaking. ISIJ Int. 2025, 65, 1454–1462. [Google Scholar] [CrossRef]
- Zhang, J.-B.; Ghalati, M.K.; Fu, J.; Yang, X.-A.; El-Fallah, G.; Dong, H.-B. Boosting algorithms for predicting end-point temperature in BOF steelmaking using big industrial datasets. J. Iron Steel Res. Int. 2025, 32, 1856–1868. [Google Scholar] [CrossRef]
- Worldsteel Association. Total Production of Crude Steel World Total 2024. Available online: https://worldsteel.org/data/annual-production-steel-data/?ind=P1_crude_steel_total_pub/CHN/IND (accessed on 27 August 2025).
- Peng, K.X.; Zhang, X.Y.; Hu, X.Y. An endpoint prediction method for BOF steelmaking based on just-in-time learning and local model integration. Metall. Ind. Autom. 2022, 46, 110–117. [Google Scholar]
- Prasenjit, S.; Ajay, K.S. Contribution of hot-spot zone in decarburization of BOF steelmaking: Fundamental analysis based upon the FactSage-Macro program. Metals 2022, 12, 638. [Google Scholar]
- Gu, M.Q.; Xu, A.J.; Yuan, F.; He, X.M.; Cui, Z.F. An improved CBR model using time-series data for predicting the end-point of a converter. ISIJ Int. 2021, 61, 2564–2570. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Wan, X.F.; Lin, D.; Peng, F.; Zou, Z. Carbon content prediction at blowing end-point of converter with off-gas analysis. J. Mater. Metall. 2007, 6, 3–6. [Google Scholar]
- Gao, C.; Shen, M.; Liu, X.; Wang, L.; Chu, M. End-point static control of basic oxygen furnace (BOF) steelmaking based on wavelet transform weighted twin support vector regression. Complexity 2019, 2019, 7408725. [Google Scholar] [CrossRef]
- Yang, X.M.; Zhao, Y.; Zhong, L.C.; Geng, Y.F. Endpoint prediction of converter steelmaking based on XGBoost algorithm. Steelmaking 2021, 37, 1–8. [Google Scholar]
- Han, M.; Zhao, Y.; Yang, X.L.; Lin, D. Endpoint prediction model of basic oxygen furnace steelmaking based on robust relevance-vector-machines. Control Theory Appl. 2011, 28, 343–348. [Google Scholar]
- Yan, L.T.; Li, M.; Yang, D.Y. Prediction of carbon content at end point based on GA-KPLSR in converters. Control Eng. China 2017, 5, 923–926. [Google Scholar]
- Zhang, Q.; Yang, Y.; Dai, Y.X.; Zhang, Q.; Yang, Y.; Dai, Y.; Lin, L. BOF smelting model based on coupling reaction mechanism. Iron Steel 2024, 59, 40–49. [Google Scholar]
- Liu, K.; Liu, L.; He, P.; Liu, W. A new algorithm of endpoint carbon content of BOF based on off-gas analysis. Steelmaking 2009, 25, 33–37. [Google Scholar]
- Yue, F.; Bao, Y.P.; Cui, H.; Gao, S.Y.; Li, B.H.; Zhang, J. Sub-lance control-based predication model for BOF end-point. Steelmaking 2009, 25, 38–40. [Google Scholar]
- Kadrolkar, A.; Dogan, N. The decarburization kinetics of metal droplets in emulsion zone. Metall. Mater. Trans. B 2019, 50, 2912–2929. [Google Scholar] [CrossRef]
- Cicutti, C.; Valdez, M.; Pérez, T.; Donayo, R.; Petroni, J. Analysis of slag foaming during the operation of an industrial converter. Lat. Am. Appl. Res. 2002, 32, 237–240. [Google Scholar]
- Cicutti, C.; Valdez, M.; Pérez, T.; Petroni, J.; Gómez, A.; Donayo, R.; Ferro, L. Study of slag-metal reactions in an LD-LBE converter. In Proceedings of the 6th International Conference on Molten Slags, Fluxes and Salts, Helsinki, Finland, 12–17 June 2000; pp. 367–384. [Google Scholar]
- Rout, B.K.; Brooks, G.; Akbar Rhamdhani, M.; Li, Z.; Schrama, F.N.H.; Overbosch, A. Dynamic model of basic oxygen steelmaking process based on multizone reaction kinetics: Modeling of decarburization. Metall. Mater. Trans. B 2018, 49, 1022–1033. [Google Scholar] [CrossRef]
- Dogan, N.; Brooks, G.A.; Rhamdhani, M.A. Comprehensive model of oxygen steelmaking Part 1: Model development and validation. ISIJ Int. 2011, 51, 1086–1092. [Google Scholar] [CrossRef]
- Dogan, N.; Brooks, G.A.; Rhamdhani, M.A. Comprehensive model of oxygen steelmaking Part 2: Application of bloated droplet theory for decarburization in emulsion zone. ISIJ Int. 2011, 51, 1093–1101. [Google Scholar] [CrossRef]
- Dogan, N.; Brooks, G.A.; Rhamdhani, M.A. Comprehensive model of oxygen steelmaking Part 3: Decarburization in impact zone. ISIJ Int. 2011, 51, 1102–1109. [Google Scholar] [CrossRef]
- Dumont-Fillon, J.; Vayssiere, P.; Trentini, B. Continuous carbon determination in the basic oxygen processes. JOM 1964, 16, 508–511. [Google Scholar] [CrossRef]
- Meyer, H.W.; Dukelow, D.A.; Fischer, M.M. Static and dynamic control of the basic oxygen process. JOM 1964, 16, 501–507. [Google Scholar] [CrossRef]
- Glasgow, J.A.; Porter, W.F.; Morrill, J. Development and operation of BOF dynamic control. JOM 1967, 19, 81–87. [Google Scholar] [CrossRef]
- Uemura, T. Process computer system at the No.3 BOF shop in Wakayama steel works. Sumitomo Met. 1973, 25, 71–88. [Google Scholar]
- Lin, W.-H.; Jiao, S.-Q.; Sun, J.-K.; Zhou, K.-X.; Liu, M.; Su, X.; Liu, Q. Modified exponential model for carbon prediction in the end blowing stage of basic oxygen furnace converter. Chin. J. Eng. 2020, 42, 854–861. [Google Scholar]
- Xin, Z.; Liu, Q.; Zhang, J.; Lin, W. Analysis of Multi-Zone Reaction Mechanisms in BOF Steelmaking and Comprehensive Simulation. Materials 2025, 18, 1038. [Google Scholar] [CrossRef]
- Sun, J.; Lin, W.; Zhang, J.; Feng, X.; Liu, Q. Theoretical analysis of supersonic jet attenuation and impact cavity morphology in BOF steelmaking process. Ironmak. Steelmak 2025. Available online: https://webofscience.clarivate.cn/wos/alldb/full-record/WOS:001519855200001 (accessed on 27 August 2025). [CrossRef]
- Xin, Z.-C.; Liu, Q.; Zhang, J.-S.; Lin, W.-H.; Peng, K.-X. Splashing behavior of metal droplets in basic oxygen furnace steelmaking process. J. Iron Steel Res. Int. 2025. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, Z.; Lin, W.; Zhang, J.; Liu, Q. Study on Decarburization Behavior in BOF Steelmaking Based on Multi-Zone Reaction Mechanism. Materials 2025, 18, 4599. https://doi.org/10.3390/ma18194599
Xin Z, Lin W, Zhang J, Liu Q. Study on Decarburization Behavior in BOF Steelmaking Based on Multi-Zone Reaction Mechanism. Materials. 2025; 18(19):4599. https://doi.org/10.3390/ma18194599
Chicago/Turabian StyleXin, Zicheng, Wenhui Lin, Jiangshan Zhang, and Qing Liu. 2025. "Study on Decarburization Behavior in BOF Steelmaking Based on Multi-Zone Reaction Mechanism" Materials 18, no. 19: 4599. https://doi.org/10.3390/ma18194599
APA StyleXin, Z., Lin, W., Zhang, J., & Liu, Q. (2025). Study on Decarburization Behavior in BOF Steelmaking Based on Multi-Zone Reaction Mechanism. Materials, 18(19), 4599. https://doi.org/10.3390/ma18194599








