Topographic, Thermal and Chemical Characterization of Oxidized Cu and Cu-Ag Thin Films
Abstract
1. Introduction
2. Materials and Methods
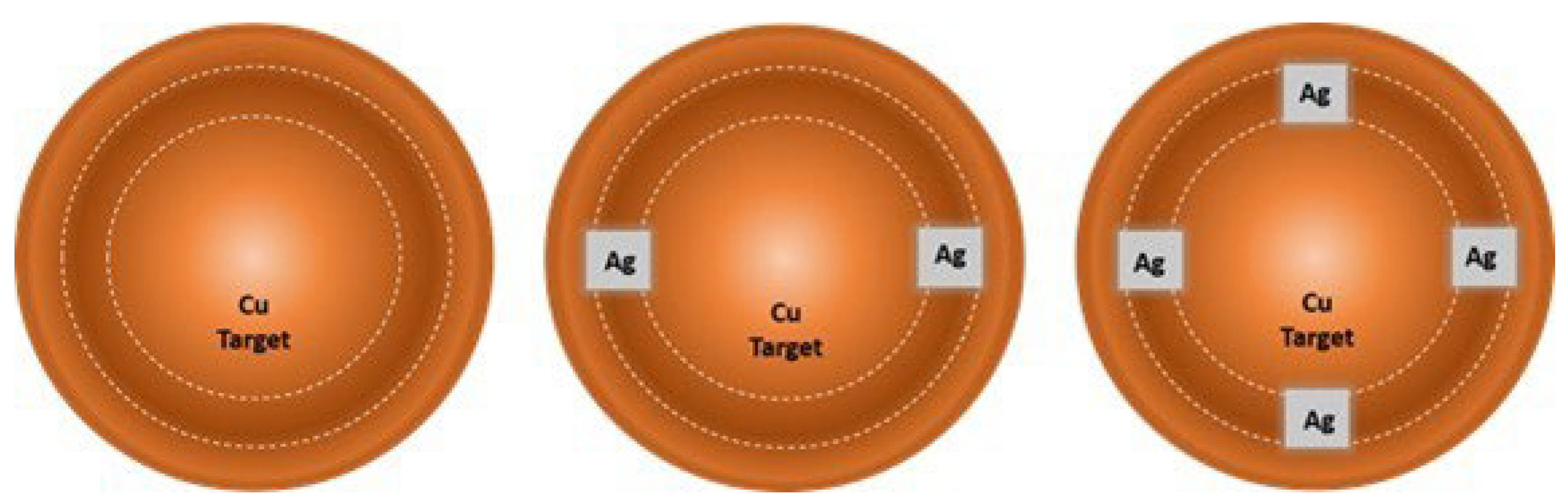
2.1. Depositions and Oxidation of Thin Films
2.2. Characterization Techniques
2.2.1. X-Ray Photoelectron Spectroscopy (XPS)
2.2.2. Atomic Force Microscopy (AFM)
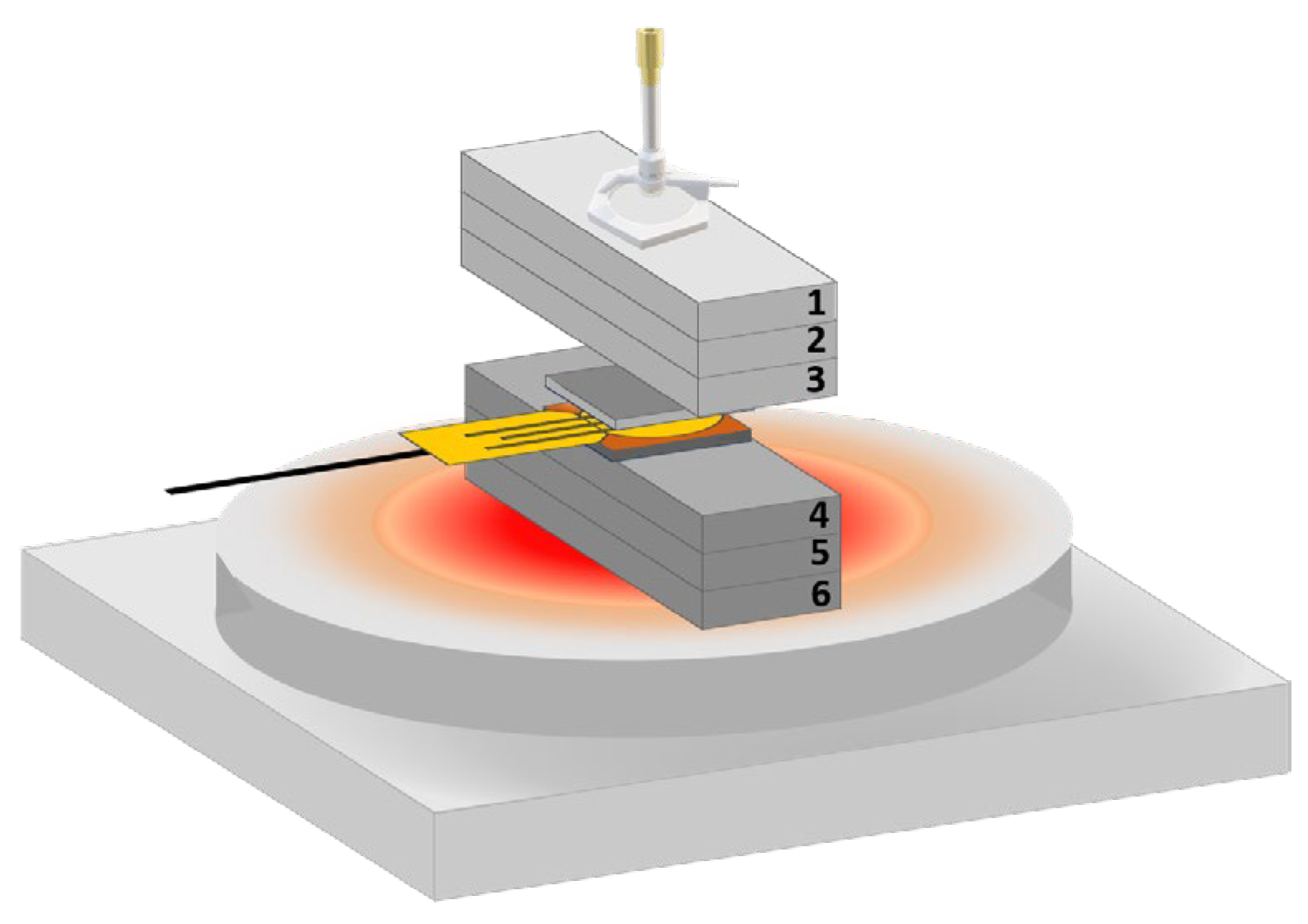
2.2.3. Thermal Constants Analysis (Hot Disk®)
3. Results
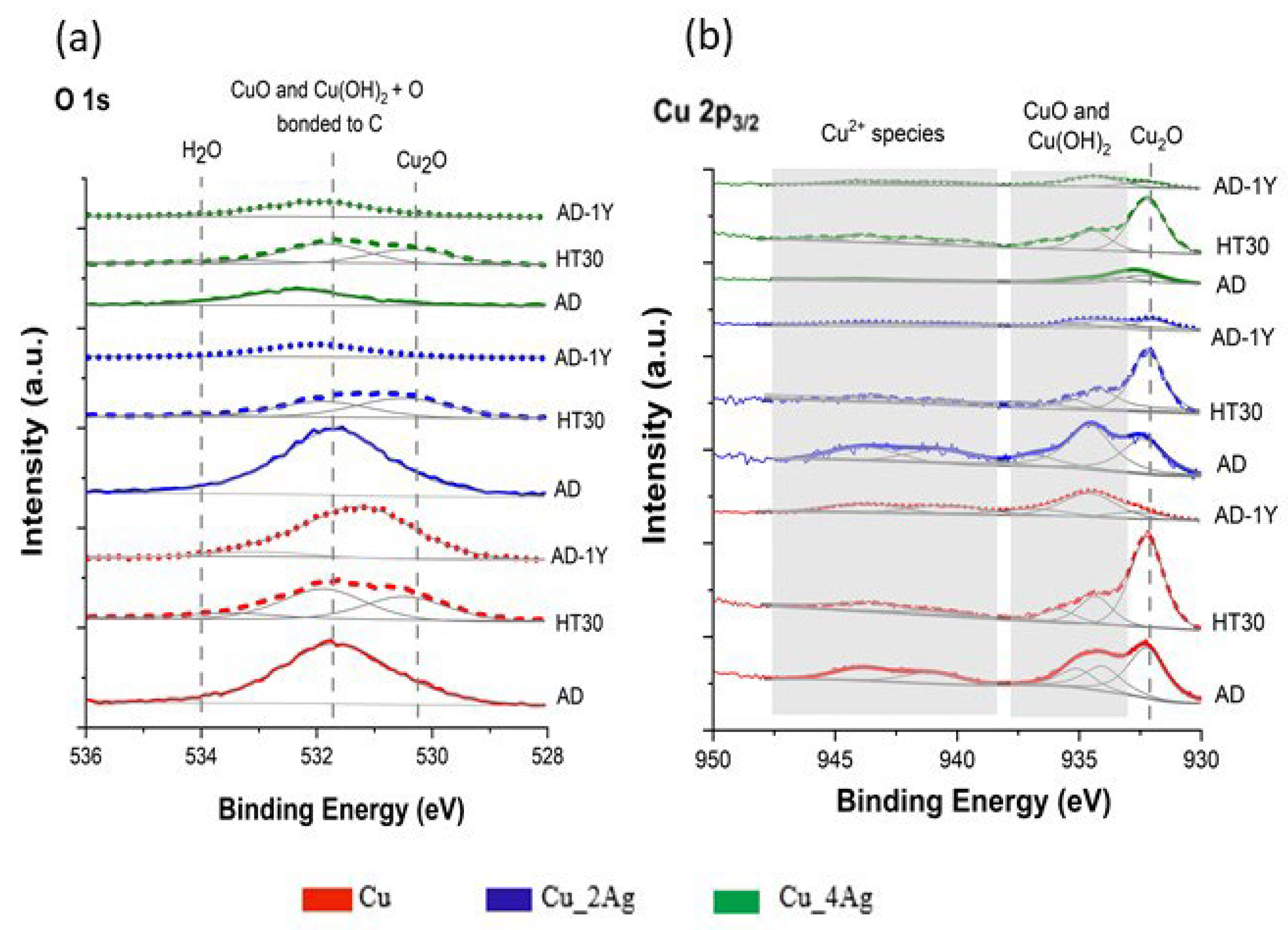
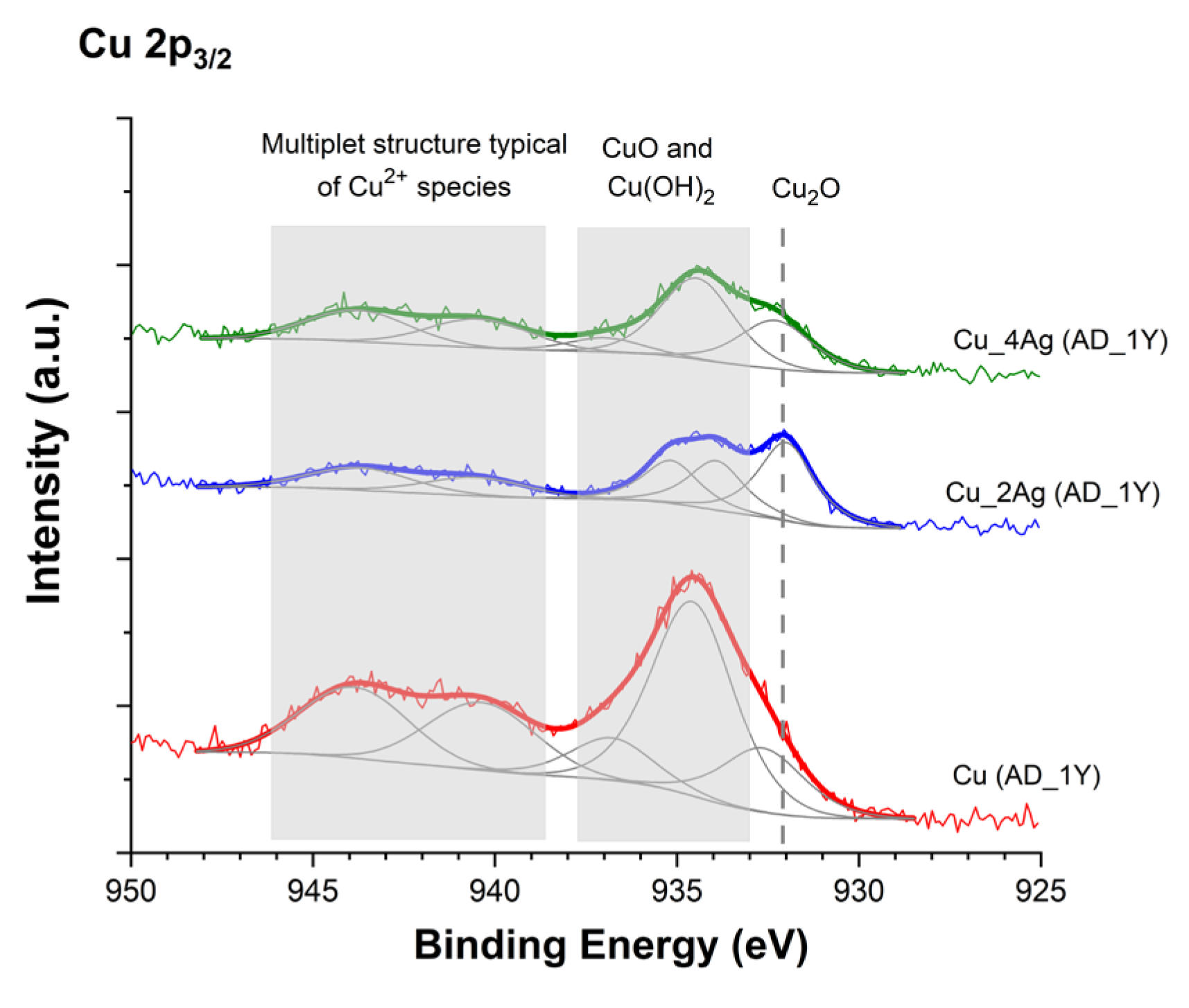
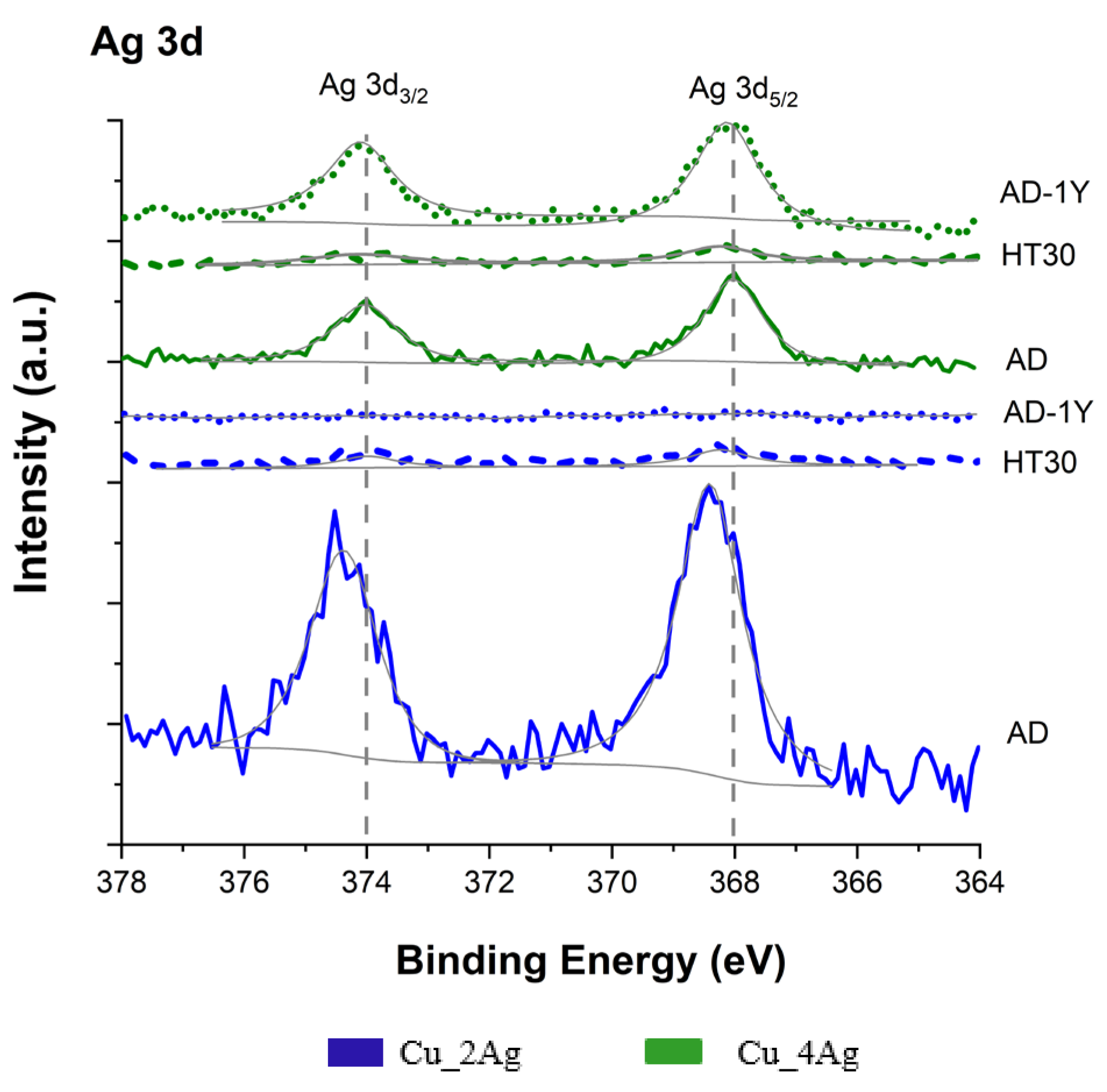
3.1. XPS Analysis
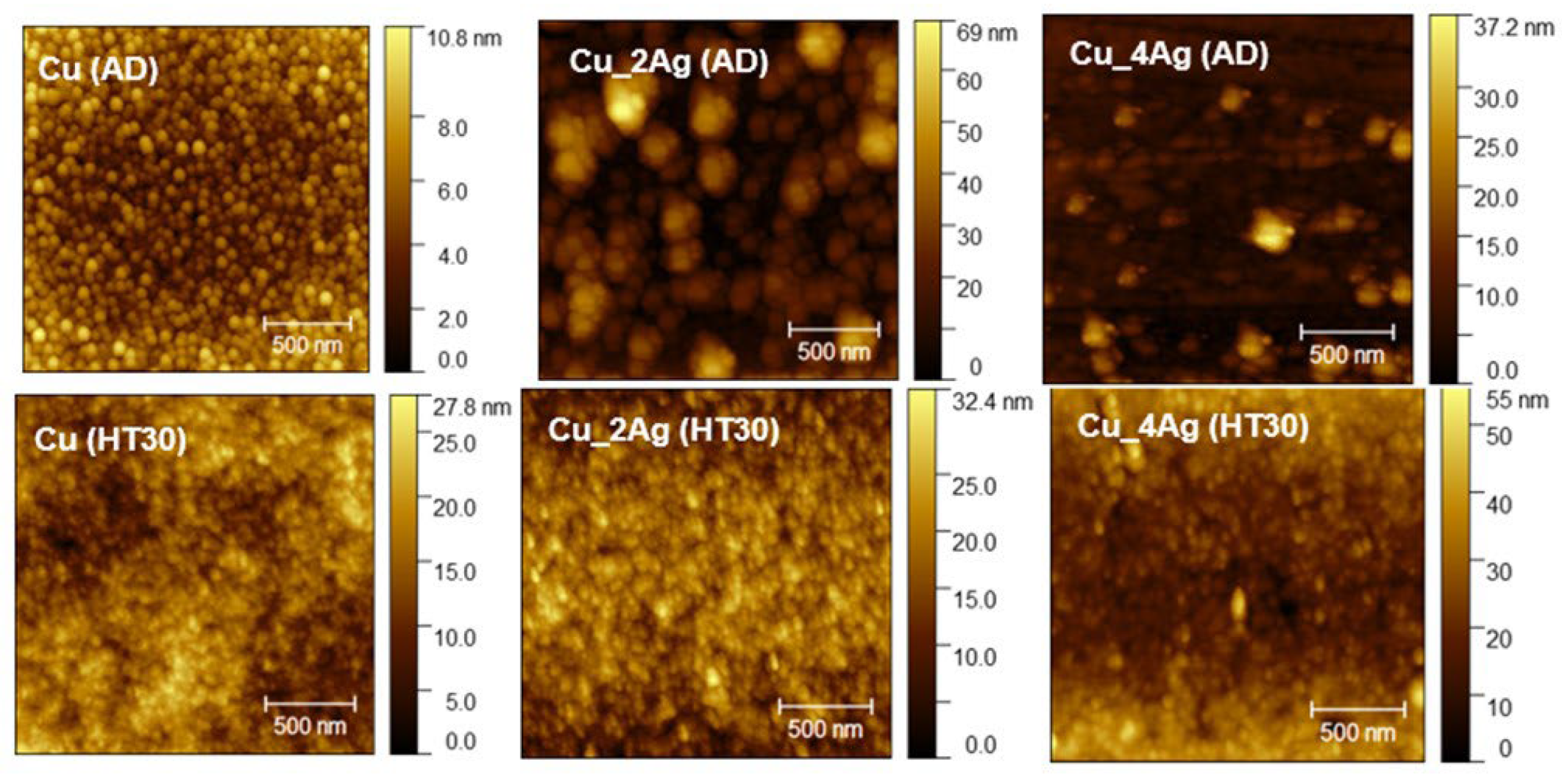
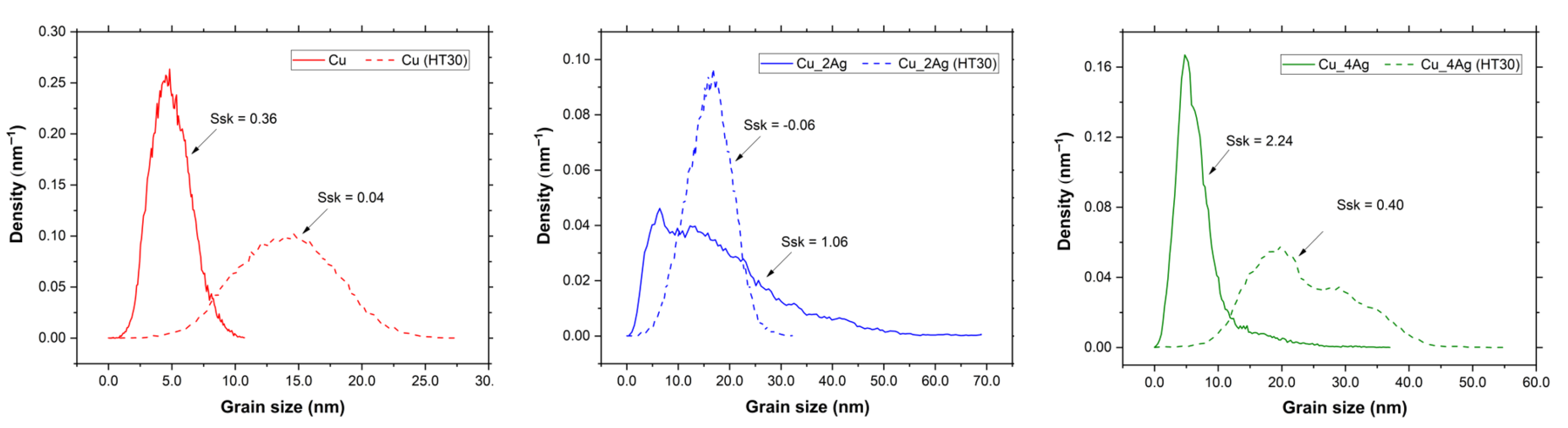
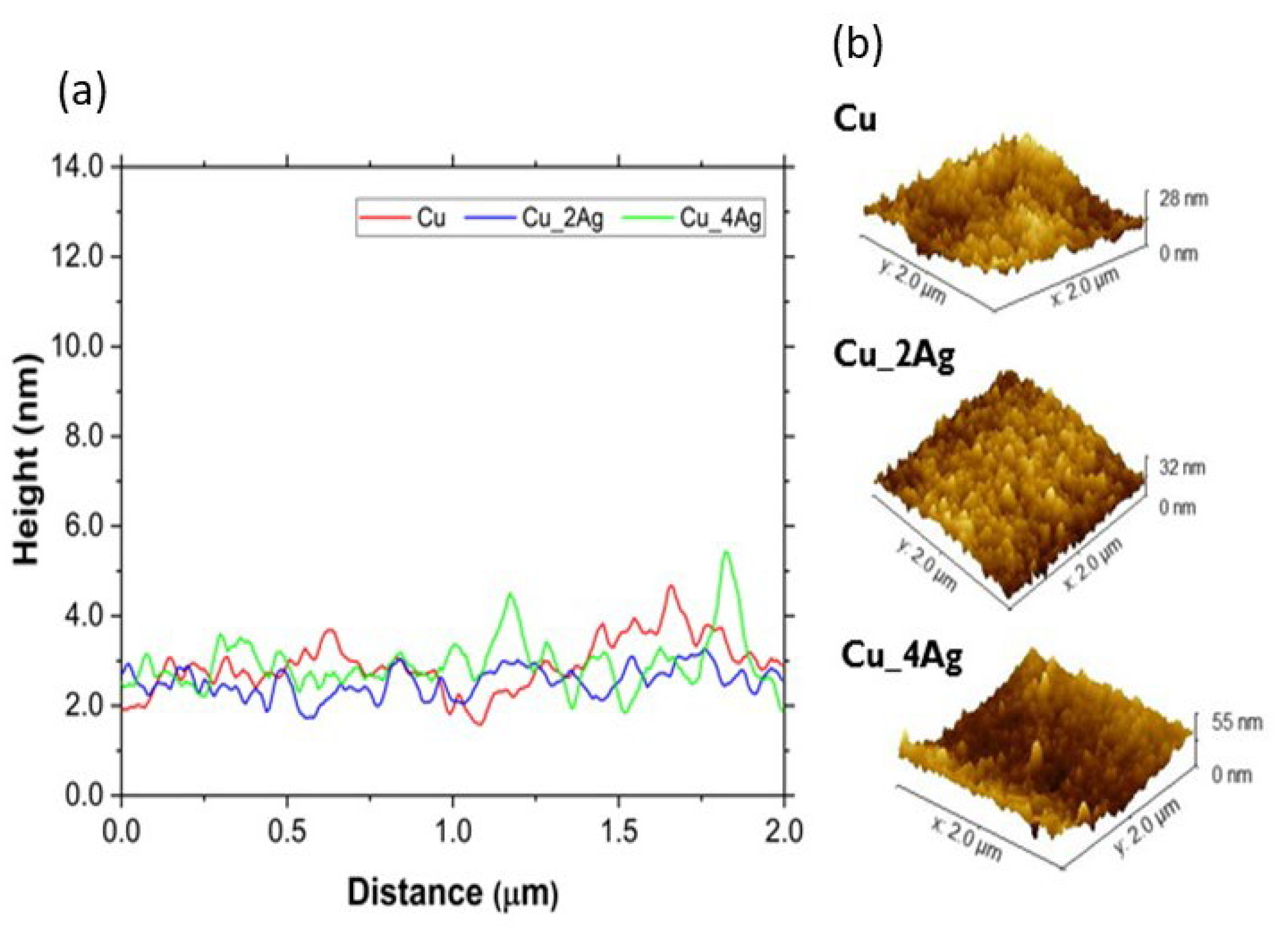
3.2. AFM Characterization
3.3. Thermal Constants Analysis (Hot Disk®)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvalho, D.; Sousa, T.; Morais, P.V.; Piedade, A.P. Polymer/metal nanocomposite coating with antimicrobial activity against hospital isolated pathogen. Appl. Surf. Sci. 2016, 379, 489–496. [Google Scholar] [CrossRef]
- Oliveira, B.M.C.; Santos, R.F.; Piedade, A.P.; Ferreira, P.J.; Vieira, M.F. Co-W Barrier Layers for Metallization of Copper Interconnects: Thermal Performance Analysis. Nanomaterials 2022, 12, 1752. [Google Scholar] [CrossRef]
- Piedade, A.P.; Vieira, M.T.; Martins, A.; Silva, F. In vitro behaviour of nanocrystalline silver-sputtered thin films. Nanotechnology 2007, 18, 105103. [Google Scholar] [CrossRef]
- Aboulfadl, H.; Sopiha, K.V.; Keller, J.; Larsen, J.K.; Scragg, J.J.S.; Persson, C.; Thuvander, M.; Edo, M. Alkali Dispersion in (Ag, Cu)(In, Ga) Se2 Thin Film Solar Cells—Insight from Theory and Experiment. ACS Appl. Mater. Interfaces 2021, 13, 7188–7199. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Tian, Q.; Meng, Y.; Kou, D.; Zhou, Z.; Zhou, W.; Wu, S. Elemental Precursor Solution Processed (Cu1−). ACS Appl. Mater. Interfaces 2017, 9, 21243–21250. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Kawaguchi, T.; Chantana, J.; Minemoto, T.; Harada, T.; Nakanishi, S.; Ikeda, S. Structural and Solar Cell Properties of a Ag-Containing Cu2 ZnSnS4 Thin Film Derived from Spray Pyrolysis. ACS Appl. Mater. Interfaces 2018, 10, 5455–5463. [Google Scholar] [CrossRef]
- Kangsabanik, M.; Gayen, R.N. A Comprehensive Review on the Recent Strategy of Cation Substitution in CZTS (Se) Thin Films to Achieve Highly Efficient Kesterite Solar Cells. Sol. RRL 2023, 7, 2300670. [Google Scholar] [CrossRef]
- Huang, J.; Liu, X.; Lu, Y.; Zhou, Y.; Xu, J.; Li, J.; Wang, H. Seed-layer-free growth of ultra-thin Ag transparent conductive fi lms imparts fl exibility to polymer solar cells. Sol. Energy Mater. Sol. Cells 2018, 184, 73–81. [Google Scholar] [CrossRef]
- Hooijer, R.; Weis, A.; Kaiser, W.; Biewald, A.; Patrick, D.; Arsatiants, O.; Helminger, D.; Dyakonov, V.; Hartschuh, A.; Mosconi, E.; et al. Cu/Ag–Sb–I Rudorffite Thin Films for Photovoltaic Applications. Chem. Mater. 2023, 35, 9988–10000. [Google Scholar] [CrossRef]
- Nihad, A.; Haneen, K.; Arif, M.; Agam, B. Efficiency enhancement of nano structured Cu2O:Ag/laser etched silicon-thin films fabricated via vacuum thermal evaporation technique for solar cell application. Optik 2021, 247, 167980. [Google Scholar] [CrossRef]
- Strehle, S.; Menzel, S.; Bartha, J.W.; Wetzig, K. Microelectronic Engineering Electroplating of Cu (Ag) thin films for interconnect applications. Microelectron. Eng. 2010, 87, 180–186. [Google Scholar] [CrossRef]
- Han, S.; Ju, S.; Jung, D.; Hoon, S.; Lee, H.; Yang, C. Highly flexible and transparent electrodes for high-performance thin-film heaters with nanostructured micromesh Cu—Ag ultrathin films. Thin Solid Film. 2023, 787, 140141. [Google Scholar] [CrossRef]
- Pinho, A.C.; Morais, P.V.; Pereira, M.F.; Piedade, A.P. Changes in the Antibacterial Performance of Polymer-Based Nanocomposites Induced by Additive Manufacturing Processing. Polymers 2025, 17, 171. [Google Scholar] [CrossRef] [PubMed]
- Baburin, A.S.; Moskalev, D.O.; Lotkov, E.S.; Sorokina, O.S.; Baklykov, D.A.; Avdeev, S.S.; Buzaverov, K.A.; Yankovskii, G.M.; Baryshev, A.V.; Rodionov, I.A. Evolutionary selection growth of silver films for low-loss nanophotonic devices. Surf. Interfaces 2023, 39, 102897. [Google Scholar] [CrossRef]
- Kim, D.; Lee, Y.J.; Ahn, K.H. Interconnected network of Ag and Cu in bioplastics for ultrahigh electromagnetic interference shielding efficiency with high thermal conductivity. Compos. Commun. 2022, 30, 101093. [Google Scholar] [CrossRef]
- Niti, N.; Kumar, Y.; Seema, S.; Reddy, V.R.; Vas, J.V.; Gupta, S.; Stahn, J.; Gupta, A.; Gupta, M. Stabilizing effects of Ag doping on structure and thermal stability of FeN thin films. J. Phys. Condens. Matter 2022, 34, 115702. [Google Scholar] [CrossRef]
- Carrupt, M.C.; Serro, A.P.; Piedade, A.P. Influence of the Ag Content on the Natural and Thermal Induced Oxidation of Cu Thin Films. Materials 2024, 17, 5974. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. (Eds.) CRC Handbook of Chemistry and Physics, 95th ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2014; ISBN 9781482208689. [Google Scholar]
- Platzman, I.; Brener, R.; Haick, H.; Tannenbaum, R. Oxidation of polycrystalline copper thin films at ambient conditions. J. Phys. Chem. C 2008, 112, 1101–1108. [Google Scholar] [CrossRef]
- Pal, A.K.; Bharathi Mohan, D. SERS enhancement, sensitivity and homogeneity studies on bi-metallic Ag-Cu films through tuning of broad band SPR towards red region. J. Alloys Compd. 2017, 698, 460–468. [Google Scholar] [CrossRef]
- Timalsina, Y.P.; Washington, M.; Wang, G.C.; Lu, T.M. Slow oxidation kinetics in an epitaxial copper(1 0 0) film. Appl. Surf. Sci. 2016, 363, 209–216. [Google Scholar] [CrossRef]
- Ramsey, J.A.; Garlick, G.F.J.; Roberts, J.K. Theory of the oxidation of metals Some interactions of gases with metals and crystalline solids. Rep. Prog. Phys. 1949, 12, 163. [Google Scholar]
- Lim, J.W.; Mimura, K.; Miyake, K.; Yamashita, M.; Isshiki, M. Effect of substrate bias voltage on the purity of Cu films deposited by non-mass separated ion beam deposition. Thin Solid Film. 2003, 434, 30–33. [Google Scholar] [CrossRef]
- Mohanty, B.C.; Malar, P.; Osipowicz, T.; Murty, B.S.; Varma, S.; Kasiviswanathan, S. Characterization of silver selenide thin films grown on Cr-covered Si substrates. Surf. Interface Anal. 2009, 41, 170–178. [Google Scholar] [CrossRef]
- Adams, D.; Alford, T.L.; Mayer, J.W. Silver Metallization: Stability and Reliability; Springer Science & Business Media, Ed.; Springer Sciene + Business Media, LLC: London, UK, 2007; ISBN 1848000278/9781848000278. [Google Scholar]
- Hsieh, J.; Hung, S. The effect of cu: Ag atomic ratio on the properties of sputtered cu-ag alloy thin films. Materials 2016, 9, 914. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Alford, T.L. Texture and surface morphology evolution of Ag(Cu) layers on indium tin oxide thin films. J. Phys. D Appl. Phys. 2008, 41, 155306. [Google Scholar] [CrossRef]
- Han, H.; Zoo, Y.; Mayer, J.W.; Alford, T.L. Improved surface morphology and texture of Ag films on indium tin oxide via Cu additions. J. Appl. Phys. 2007, 102, 036101. [Google Scholar] [CrossRef]
- Ţălu, Ş.; Matos, R.S.; Pinto, E.P.; Rezaee, S.; Mardani, M. Stereometric and fractal analysis of sputtered Ag-Cu thin films. Surf. Interfaces 2020, 21, 100650. [Google Scholar] [CrossRef]
- Warren, A.P.; Sun, T.; Yao, B.; Barmak, K.; Toney, M.F.; Coffey, K.R. Evolution of nanoscale roughness in Cu/SiO2 and Cu/Ta interfaces. Appl. Phys. Lett. 2012, 100, 024106. [Google Scholar] [CrossRef]
- Kang, S.H.; Obeng, Y.S.; Decker, M.A.; Oh, M.; Merchant, S.M.; Karthikeyan, S.K.; Seet, C.S.; Oates, A.S. Effect of annealing on the surface microstructural evolution and the electromigration reliability of electroplated Cu films. J. Electron. Mater. 2001, 30, 1506–1512. [Google Scholar] [CrossRef]
- Zoo, Y.; Han, H.; Alford, T.L. Copper enhanced (111) texture in silver thin films on amorphous SiO2. J. Appl. Phys. 2007, 102, 083548. [Google Scholar] [CrossRef]
- Filoti, D.I.; Bedell, A.R.; Harper, J.M.E. Synergistic Ag (111) and Cu (111) texture evolution in phase-segregated Cu1−xAgx magnetron sputtered composite thin films. J. Vac. Sci. Technol. A Vac. Surf. Film. 2010, 28, 838–841. [Google Scholar] [CrossRef]
- Purswani, J.M.; Gall, D. Surface morphological evolution during annealing of epitaxial Cu(001) layers. J. Appl. Phys. 2008, 104, 044305. [Google Scholar] [CrossRef]
- Noorbakhsh, R.; Rezaee, S.; Nia, B.A.; Boochani, A. Influence of deposition time on the optical and morphological properties of silver–copper thin films: Experimental and statistical studies. Opt. Quantum Electron. 2021, 53, 1–12. [Google Scholar] [CrossRef]
- Swiatkowska-Warkocka, Z.; Shakeri, M.S.; Polit, O.; Gurgul, J.; Biesiadecka, M.; Dziedzic, A.; Pawlik, P.; Kot, J. Surface Modification of CuO/Cu2O/Cu Composite Particles with Ag by Pulsed Laser Irradiation of Suspension and Their Antimicrobial Potentia. J. Phys. Chem. C 2025, 129, 12953–12965. [Google Scholar] [CrossRef]
- Das, S.; Alford, T.L. Structural and optical properties of Ag-doped copper oxide thin films on polyethylene napthalate substrate prepared by low temperature microwave annealing. J. Appl. Phys. 2013, 113, 244905. [Google Scholar] [CrossRef]
- De Carlo, I.; Baudino, L.; Klapetek, P.; Serrapede, M.; Michieletti, F.; De Leo, N.; Pirri, F.; Boarino, L.; Lamberti, A.; Milano, G. Electrical and Thermal Conductivities of Single CuxO Nanowires. Nanomaterials 2023, 13, 2822. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Y.; Chen, Y.; Wang, C. Study on the Anharmonic Interaction in Negative Thermal Expansion Compounds Ag2O and Cu2O by Three-Phonon Scattering. J. Phys. Chem. C 2024, 128, 1534–1545. [Google Scholar] [CrossRef]
- Tanguy, A. Vibrations and Heat Transfer in Glasses: The Role Played by Disorder. Comptes Rendus Phys. 2024, 24, 73–97. [Google Scholar] [CrossRef]
- Giri, A.; King, S.W.; Lanford, W.A.; Mei, A.B.; Merrill, D.; Li, L.; Oviedo, R.; Richards, J.; Olson, D.H.; Braun, J.L.; et al. Interfacial Defect Vibrations Enhance Thermal Transport in Amorphous Multilayers with Ultrahigh Thermal Boundary Conductance. Adv. Mater. 2018, 30, e1804097. [Google Scholar] [CrossRef]
- Han, D.-G.; Yoon, J.-W. Effects of the grain size and orientation of Cu on the formation and growth behavior of intermetallic compounds in Sn-Ag-Cu solder joints. J. Alloys Compd. 2025, 1010, 177801. [Google Scholar] [CrossRef]


| Thickness of Thin Films (nm) | ||
|---|---|---|
| Thin Films | AD | HT30 |
| Cu | 210 ± 1 | 550 ± 3 |
| Cu_2Ag | 220 ± 3 | 480 ± 4 |
| Cu_4Ag | 240 ± 3 | 320 ± 4 |
| As-Deposited (AD) | Annealed (HT30) | |||||
|---|---|---|---|---|---|---|
| Cu | Cu_2Ag | Cu_4Ag | Cu | Cu_2Ag | Cu_4Ag | |
| Sa (nm) | 1.25 | 8.93 | 2.87 | 3.08 | 3.48 | 6.46 |
| RMS (nm) | 1.55 | 11.34 | 4.22 | 3.80 | 4.33 | 7.80 |
| Surface Energy (mJ/m2) | |||
|---|---|---|---|
| Surfaces | Cu | Cu_2Ag | Cu_4Ag |
| As-Deposited (AD) | 81.1 | 80.4 | 88.9 |
| Annealed (HT30) | 60.4 | 53.3 | 55.6 |
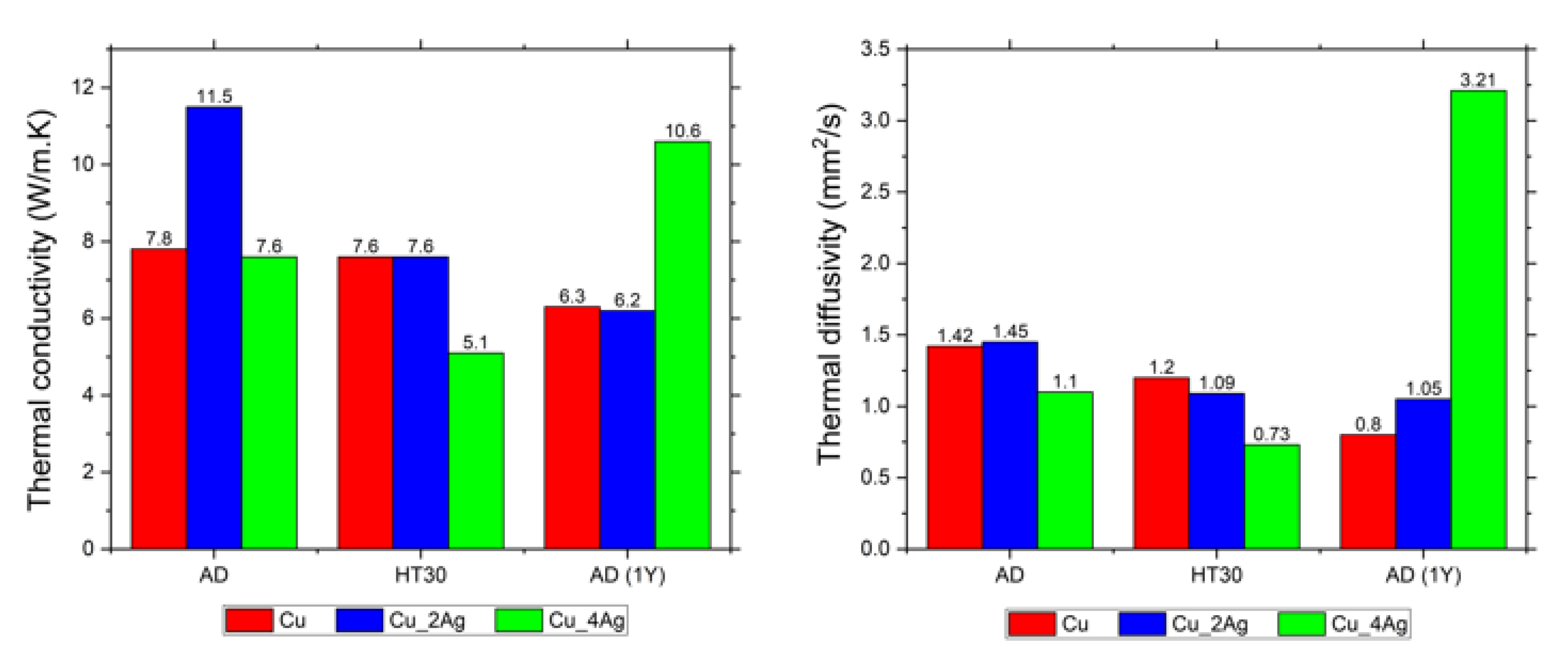
| Thermal Conductivity (W/m.K) | Volumetric Thermal Capacity (MJ/m3 K) | Thermal Diffusivity (mm2/s) | |
|---|---|---|---|
| Cu | |||
| AD | 7.8 ± 0.014 | 5.5 ± 0.026 | 1.42 ± 0.009 |
| HT30 | 7.6 ± 0.009 | 6.4 ± 0.020 | 1.20 ± 0.004 |
| AD-1Y | 6.3 ± 0.082 | 7.8 ± 0.060 | 0.80 ± 0.004 |
| Cu_2Ag | |||
| AD | 11.5 ± 0.010 | 7.9 ± 0.020 | 1.45 ± 0.003 |
| HT30 | 7.6 ± 0.008 | 6.9 ± 0.007 | 1.09 ± 0.002 |
| AD-1Y | 6.2 ± 0.067 | 5.8 ± 0.084 | 1.05 ± 0.026 |
| Cu_4Ag | |||
| AD | 7.6 ± 0.010 | 6.8 ± 0.023 | 1.10 ± 0.003 |
| HT30 | 5.1 ± 0.008 | 6.9 ± 0.022 | 0.73 ± 0.003 |
| AD-1Y | 10.6 ± 0.010 | 3.3 ± 0.006 | 3.21 ± 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrupt, M.C.; Ferraria, A.M.; Serro, A.P.; Piedade, A.P. Topographic, Thermal and Chemical Characterization of Oxidized Cu and Cu-Ag Thin Films. Materials 2025, 18, 4562. https://doi.org/10.3390/ma18194562
Carrupt MC, Ferraria AM, Serro AP, Piedade AP. Topographic, Thermal and Chemical Characterization of Oxidized Cu and Cu-Ag Thin Films. Materials. 2025; 18(19):4562. https://doi.org/10.3390/ma18194562
Chicago/Turabian StyleCarrupt, Maria C., Ana M. Ferraria, Ana P. Serro, and Ana P. Piedade. 2025. "Topographic, Thermal and Chemical Characterization of Oxidized Cu and Cu-Ag Thin Films" Materials 18, no. 19: 4562. https://doi.org/10.3390/ma18194562
APA StyleCarrupt, M. C., Ferraria, A. M., Serro, A. P., & Piedade, A. P. (2025). Topographic, Thermal and Chemical Characterization of Oxidized Cu and Cu-Ag Thin Films. Materials, 18(19), 4562. https://doi.org/10.3390/ma18194562









