Degradation Law and Experimental Study of High- Performance Shotcrete Under the Coupling Effect of Sulfate and Chloride Salt
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Mix of HPSC
2.2. Preparation of HPSC Specimens
2.3. Experimental Methods
2.3.1. Long-Term Immersion Test of High-Performance Shotcrete
2.3.2. SO42− Erosion Experiment
3. Results and Discussion
3.1. Durability Degradation Law of HPSC
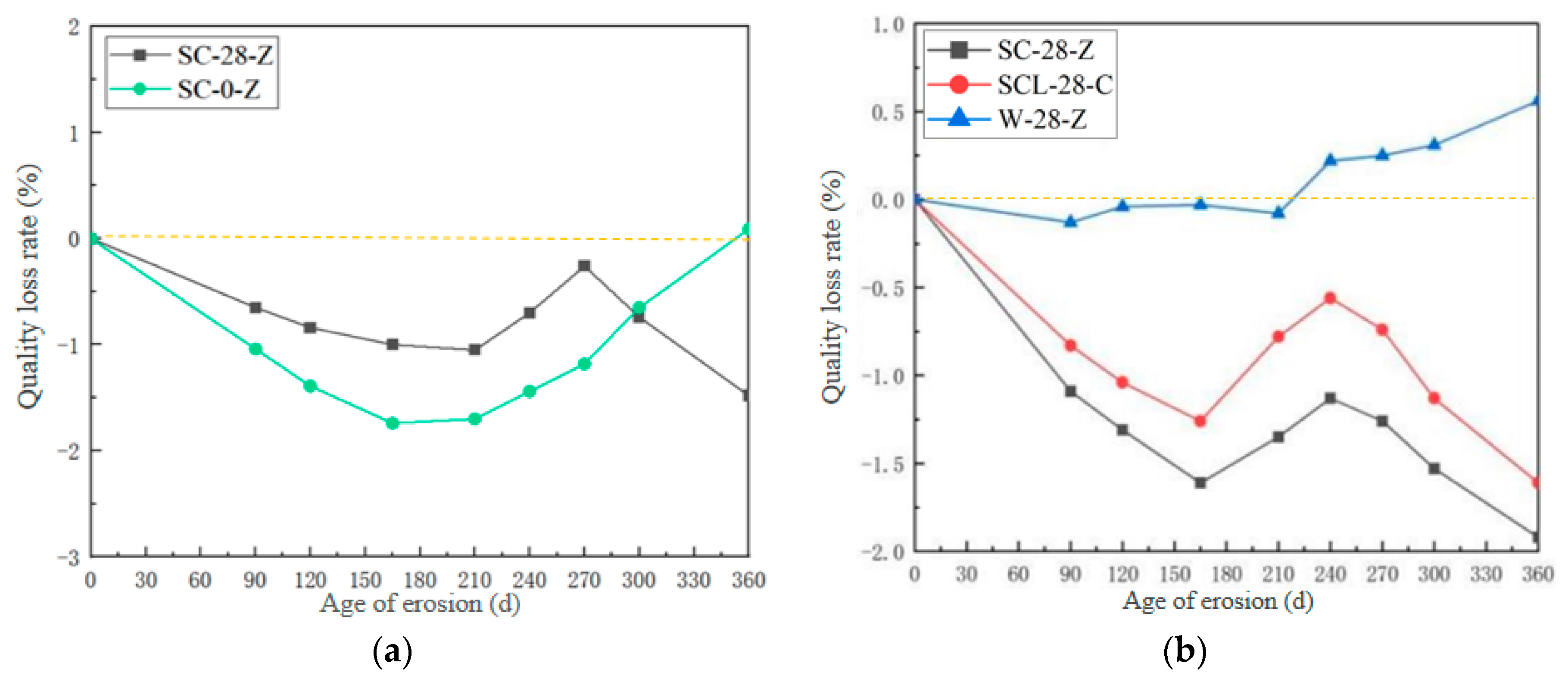
3.1.1. Quality Loss Rate
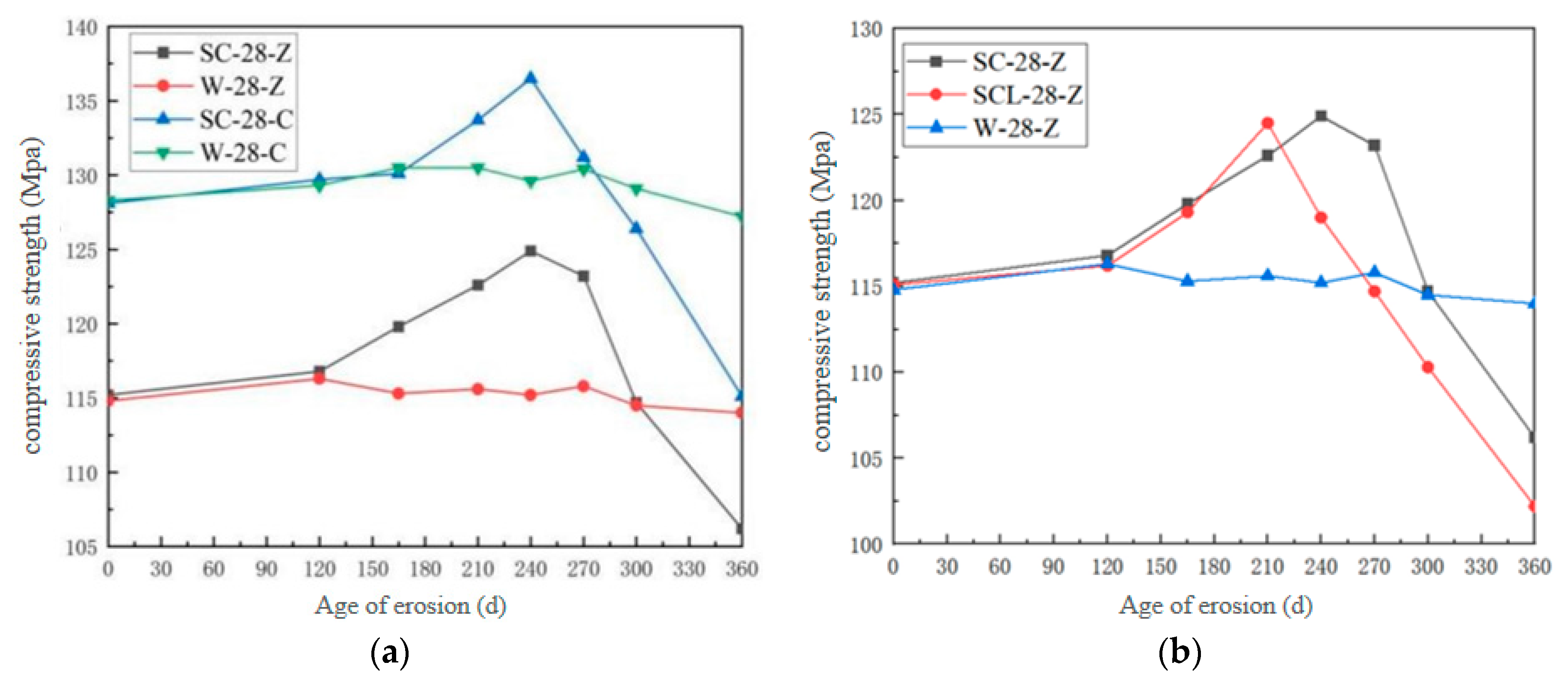
3.1.2. Compressive Strength
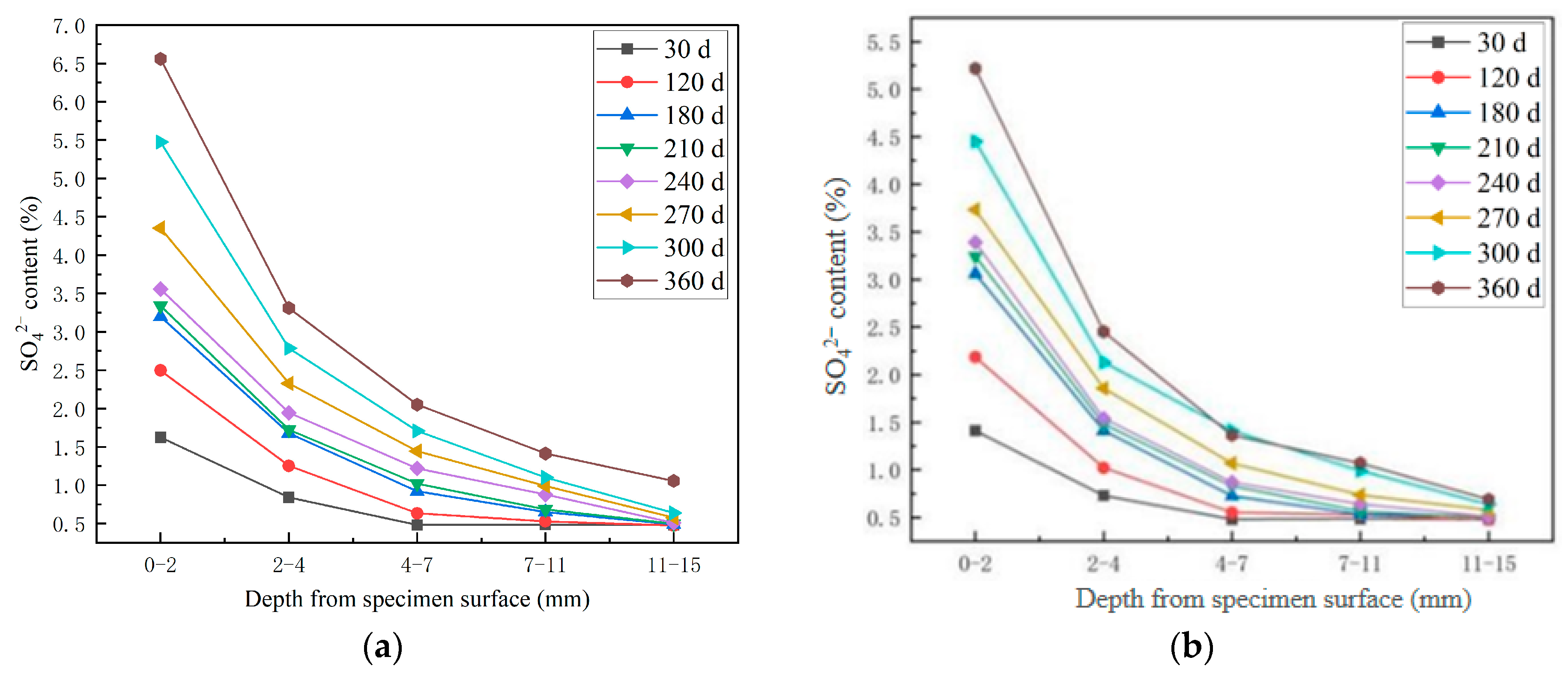
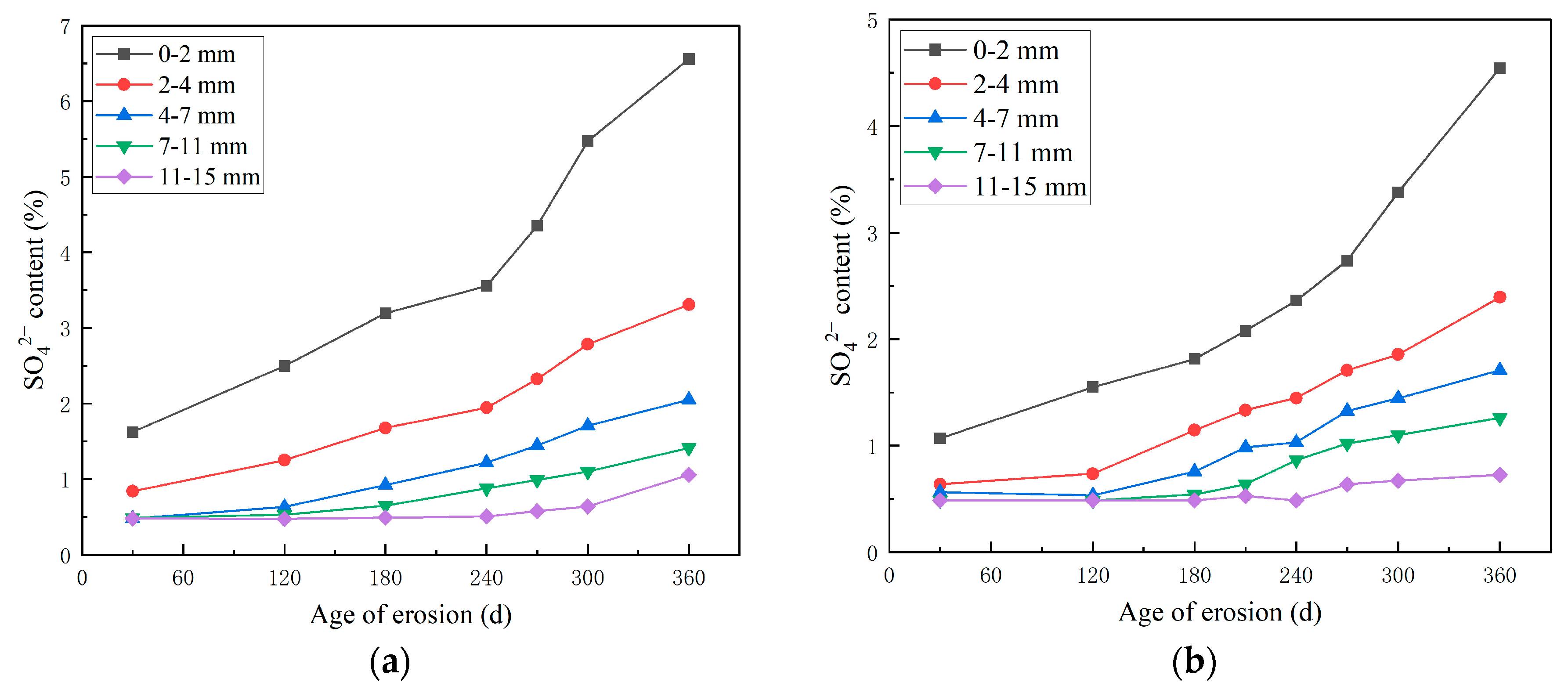
3.2. Study on the Erosion Law of SO42− in HPSC
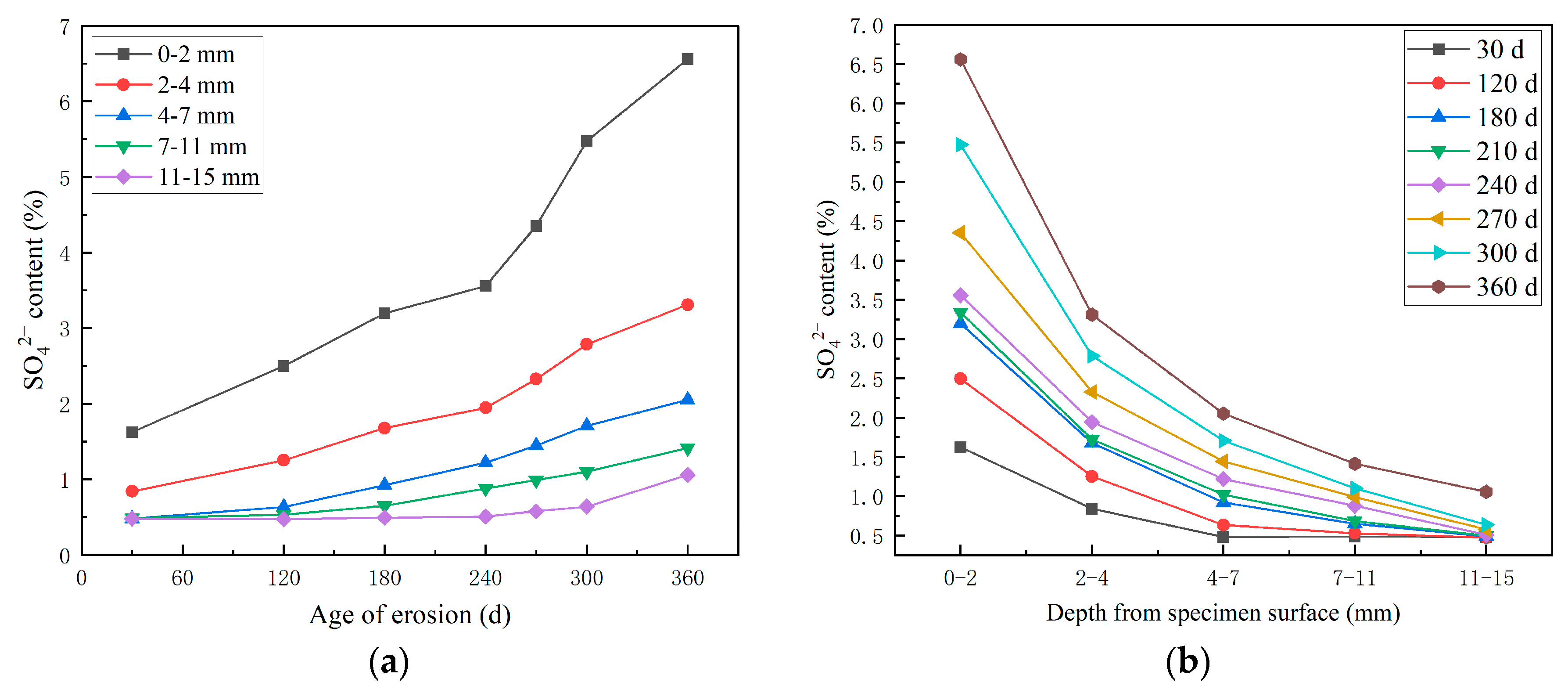
3.2.1. Study on the SO42− Erosion Law of HPSC Along the Direction of Shotcrete and Perpendicularly to the Direction of Shotcrete
3.2.2. Study on the Erosion Pattern of SO42− in a Complex Salt Solution Environment
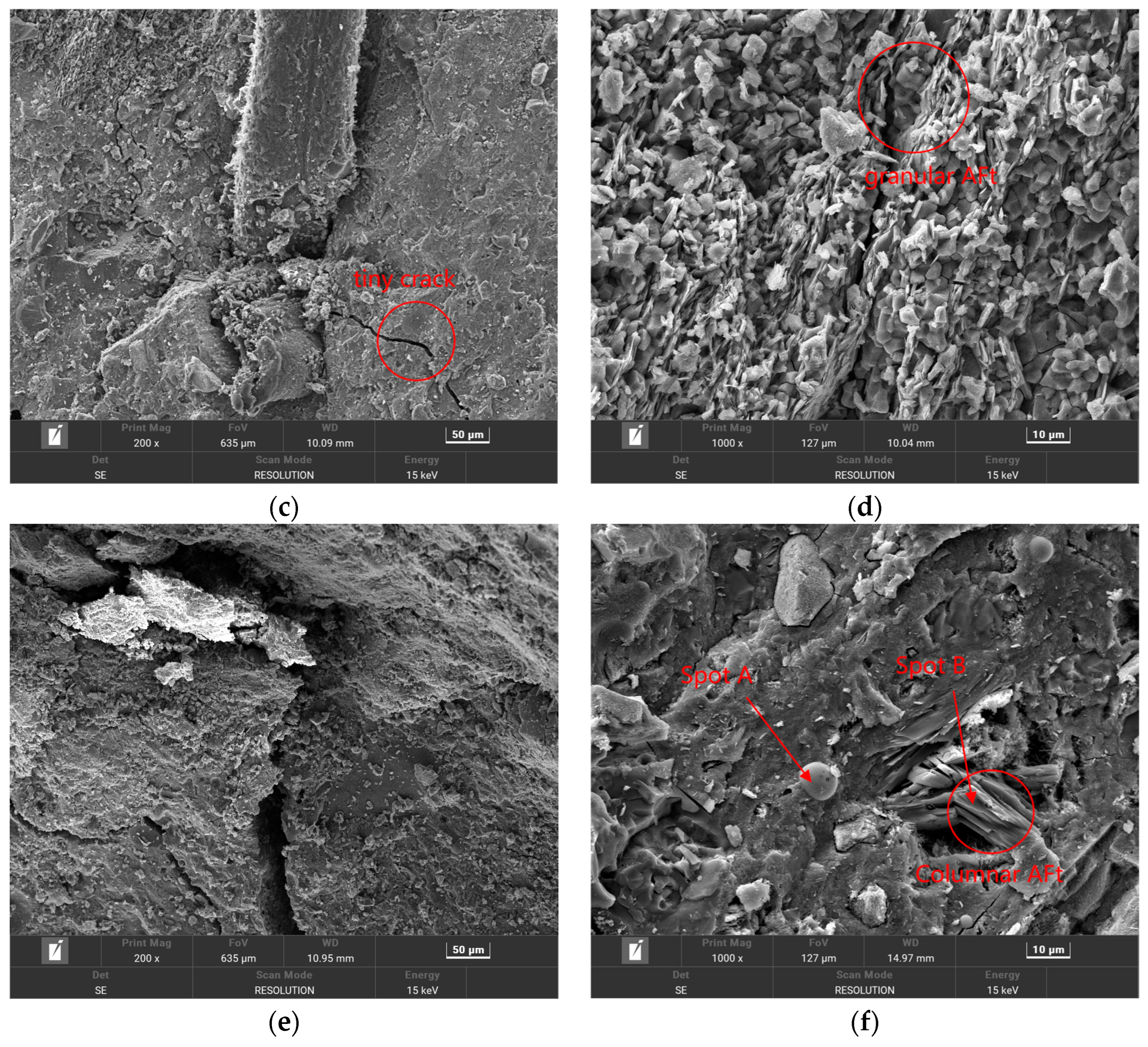
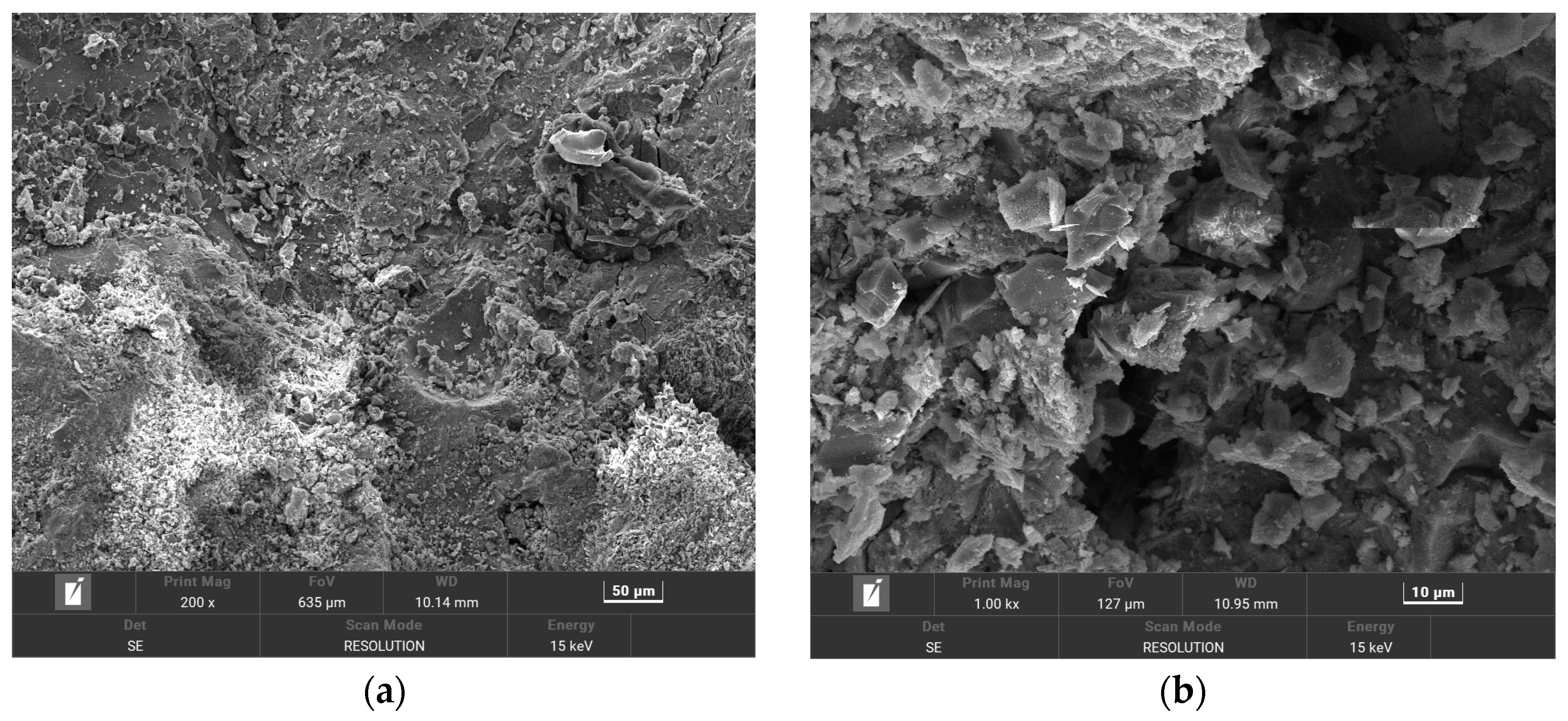
3.3. SEM Scanning Electron Microscope Analysis and EDS Energy Spectrum Analysis
3.3.1. Analysis of Microstructure of HPSC Under Sulfate Solution Erosion
3.3.2. Micromorphology Analysis of HPSC Under Sulfate–Chloride Composite Solution Erosion
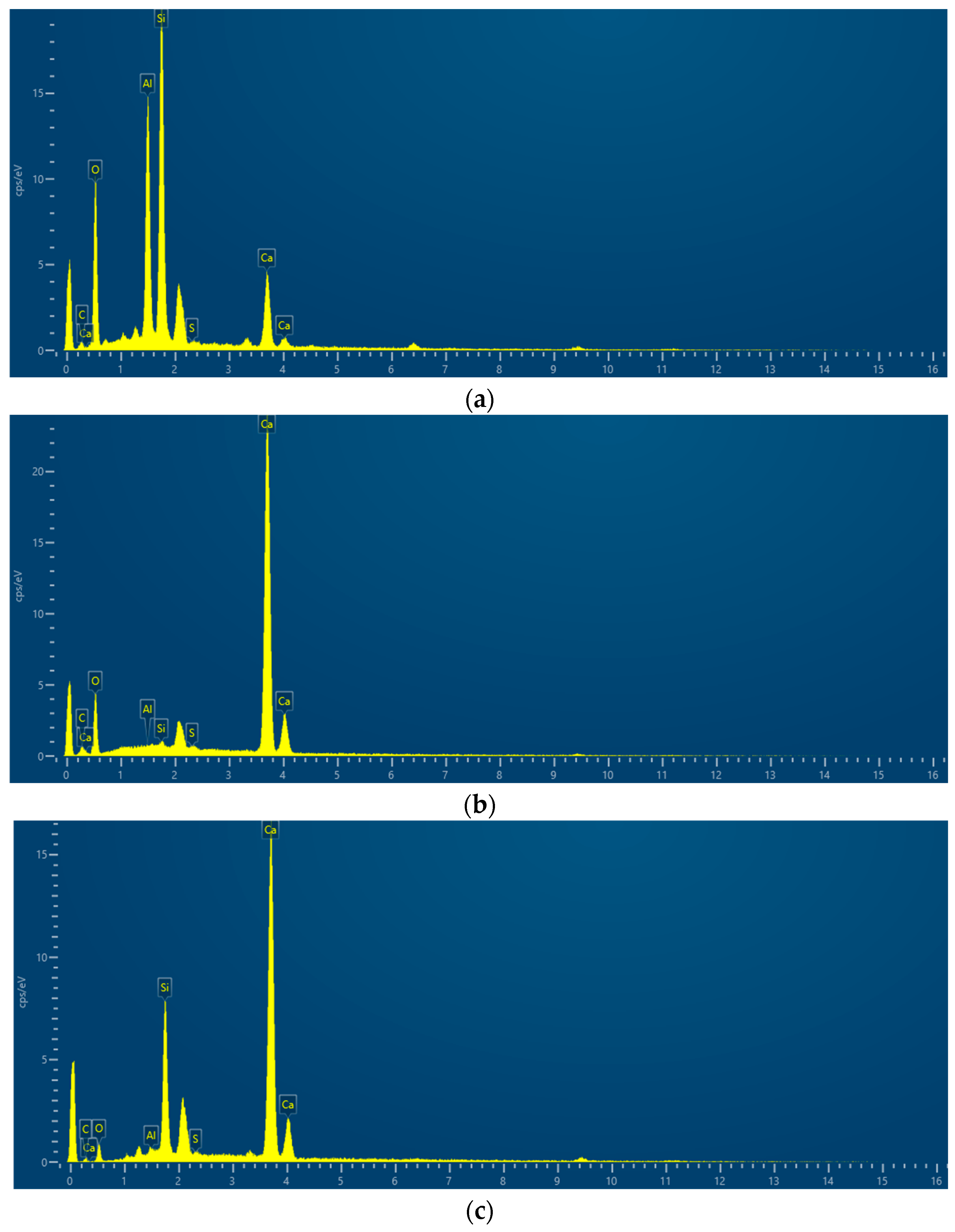
3.3.3. EDS Analysis
4. Conclusions
- (1)
- The mass change rate of high-performance shotcrete increases first, then decreases, and then increases with the increase in erosion time. Under different jetting surfaces, the compressive strength of the vertical jetting direction surface is better than that along the jetting direction surface, but the superiority of the vertical jetting direction surface gradually weakens in the later stage of erosion, and the chloride salt will improve the resistance to sulfate attack.
- (2)
- With the progress of the erosion process, the content of SO42− and Cl− in concrete increases. Compared with different erosion directions, the SO42− diffusion depth of the ion invasion surface along the jet direction is larger, and the SO42− content is larger at the same depth. Compared with different erosion solutions, the presence of Cl− delays the diffusion of SO42− to a certain extent, and the final diffusion depth of SO42− is smaller under the erosion of composite salt.
- (3)
- The deterioration process of the durability of high-performance shotcrete can be divided into the formation stage of ettringite and gypsum, the filling stage of corrosion products, and the generation stage of new cracks. In the early stage of erosion, the external SO42− diffuses into the concrete to generate corrosion expansion products to fill the original pores and cracks and enhance the durability of high-performance shotcrete. In the middle and late stages of erosion, the accumulation of corrosion products increases, resulting in the expansion of original cracks and the generation of new cracks, which accelerates the deterioration of the durability of high-performance shotcrete.
- (4)
- For high-performance shotcrete eroded by sulfate solution and sulfate–chloride solution, the concentration of erosion solution also has a great influence on the erosion operation of concrete. This study mainly considers a 10% Na2SO4 solution and a 10% Na2SO4 + 5% NaCl composite solution as the erosion solutions and does not set different erosion medium concentration gradient combinations. Subsequent research can set different gradient erosion solution concentrations to study the durability degradation law in more detail. In addition, the service environment of HPSC is often more complex, such as sulfate, chloride, magnesium salt, carbonization, and other multi-factor coupling erosion. In the follow-up study, the influence of various factors on the durability degradation of concrete can be considered. In the follow-up study, laboratory immersion and field exposure, the dry–wet cycle test, and other tests can be designed to systematically study the durability of HPSC in a complex environment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Won, J.-P.; Hwang, U.-J.; Kim, C.-K.; Lee, S.-J. Mechanical Performance of Shotcrete Made with a High-Strength Cement-Based Mineral Accelerator. Constr. Build. Mater. 2013, 49, 175–183. [Google Scholar] [CrossRef]
- Wang, J.; Niu, D.; Ding, S.; Mi, Z.; Luo, D. Microstructure, Permeability and Mechanical Properties of Accelerated Shotcrete at Different Curing Age. Constr. Build. Mater. 2015, 78, 203–216. [Google Scholar] [CrossRef]
- Franzen, T.; Garshol, K.F.; Tomisawa, N. Sprayed Concrete for Final Linings: ITA Working Group Report. Tunn. Undergr. Space Technol. 2001, 16, 295–309. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, X.; Zhou, X.; Du, L. Diffusion Model of Sulfate Ions in Concrete Based on Pore Change of Cement Mortar and Its Application in Mesoscopic Numerical Simulation. Struct. Concr. 2022, 23, 3786–3803. [Google Scholar] [CrossRef]
- Romer, M. Steam Locomotive Soot and the Formation of Thaumasite in Shotcrete. Cem. Concr. Compos. 2003, 25, 1173–1176. [Google Scholar] [CrossRef]
- Chen, F.; Gao, J.; Qi, B.; Shen, D.; Li, L. Degradation Progress of Concrete Subject to Combined Sulfate-Chloride Attack under Drying-Wetting Cycles and Flexural Loading. Constr. Build. Mater. 2017, 151, 164–171. [Google Scholar] [CrossRef]
- Luo, D.; Zhou, M.; Li, F.; Niu, D. Chloride Ion Transport in Coral Aggregate Concrete Subjected to Coupled Erosion by Sulfate and Chloride Salts in Drying-Wetting Cycles. J. Mater. Res. Technol. 2024, 30, 3251–3267. [Google Scholar] [CrossRef]
- Li, T.; Zhu, P.; Zhang, B.; Ding, K.; Sun, Z. Experimental Study on Corrosion Reaction-Diffusion Process of Concrete under Sulfate Attack. Bull. Chin. Ceram. Soc. 2020, 39, 50–55. [Google Scholar] [CrossRef]
- Liu, Z.; Hou, L.; Deng, D.; Zhang, F.; Hu, W. Sodium Sulfate Salt Crystallization Distress on Carbonated Concrete. Kuei Suan Jen Hsueh Pao/J. Chin. Ceram. Soc. 2017, 45, 1621–1628. [Google Scholar] [CrossRef]
- Lu, F.; Wang, H.; Wang, L.; Zhao, K.; Zhang, J. Degradation Law and Service Life Prediction Model of Tunnel Lining Concrete Suffered Combined Effects of Sulfate Attack and Drying–Wetting Cycles. Materials 2022, 15, 4435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jin, X.; Lin, S.; Bi, J. Direct Shear Behavior of Sulfate-Exposed Shotcrete: Experimental and Modelling Research. Constr. Build. Mater. 2019, 210, 607–619. [Google Scholar] [CrossRef]
- Chen, S.; Ren, J.; Ren, X.; Li, Y. Deterioration Laws of Concrete Durability under the Coupling Action of Salt Erosion and Drying–Wetting Cycles. Front. Mater. 2022, 9. [Google Scholar] [CrossRef]
- Li, H.; Nie, Q.; Wang, C.; Wang, G.; Zhang, L.; Yuan, L. Durability Investigation of Fractured Coal-Gasified Ash Slag Concrete Eroded by Sulfate and Chlorine Salts. Case Stud. Constr. Mater. 2024, 20, e02745. [Google Scholar] [CrossRef]
- Xu, F.; Yang, Z.; Liu, W.; Wang, S.; Zhang, H. Experimental Investigation on the Effect of Sulfate Attack on Chloride Diffusivity of Cracked Concrete Subjected to Composite Solution. Constr. Build. Mater. 2020, 237, 117643. [Google Scholar] [CrossRef]
- Chen, L.; Chen, X.; Wang, L.; Ning, Y.; Ji, T. Compressive Strength, Pore Structure, and Hydration Products of Slag Foam Concrete under Sulfate and Chloride Environment. Constr. Build. Mater. 2023, 394, 132141. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Chen, Z.; Wang, C.; Ding, S.; Nie, Z.; Hou, T.; Zhao, G. The Improving Role of Basalt Fiber on the Sulfate–Chloride Multiple Induced Degradation of Cast-In-Situ Concrete. Materials 2024, 17, 4454. [Google Scholar] [CrossRef]
- Wang, J.; Niu, D.; Wang, Y.; Wang, B. Durability Performance of Brine-Exposed Shotcrete in Salt Lake Environment. Constr. Build. Mater. 2018, 188, 520–536. [Google Scholar] [CrossRef]
- Zhao, R.; Xu, L.; Yang, J.; Zou, Y.; Zhang, Z. Degradation of Shotcrete Materials Subjected to Sulfate and Chloride Attack in Varying Exposure Regimes. Adv. Civ. Eng. 2021, 2021. [Google Scholar] [CrossRef]
- El Inaty, F.; Marchetti, M.; Quiertant, M.; Omikrine Metalssi, O. Chemical Mechanisms Involved in the Coupled Attack of Sulfate and Chloride Ions on Low-Carbon Cementitious Materials: An In-Depth Study. Appl. Sci. 2023, 13, 11729. [Google Scholar] [CrossRef]
- Puppala, A.J.; Talluri, N.; Chittoori, B.C.S. Calcium-Based Stabiliser Treatment of Sulfate-Bearing Soils. Proc. Inst. Civ. Eng.—Ground Improv. 2014, 167, 162–172. [Google Scholar] [CrossRef]
- Vetter, T.W.; Pratt, K.W.; Turk, G.C.; Beck, C.M.; Butler, T.A. Using Instrumental Techniques to Increase the Accuracy of the Gravimetric Determination of Sulfate. Analyst 1995, 120, 2025. [Google Scholar] [CrossRef]




| HPSC Ingredients | Mix Proportion |
|---|---|
| Cement | 1.0 |
| Water | 0.2948 |
| Silica fume | 0.1282 |
| Coal ash | 0.1025 |
| Fine aggregate | 1.282 |
| Water reducing agent | 0.0051 |
| Polypropylene fiber | 0.1% |
| Thickener | 0.0769 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Deng, S.; Li, G.; Dai, X. Degradation Law and Experimental Study of High- Performance Shotcrete Under the Coupling Effect of Sulfate and Chloride Salt. Materials 2025, 18, 4505. https://doi.org/10.3390/ma18194505
Yang J, Deng S, Li G, Dai X. Degradation Law and Experimental Study of High- Performance Shotcrete Under the Coupling Effect of Sulfate and Chloride Salt. Materials. 2025; 18(19):4505. https://doi.org/10.3390/ma18194505
Chicago/Turabian StyleYang, Jianyu, Senrui Deng, Guanglin Li, and Xujun Dai. 2025. "Degradation Law and Experimental Study of High- Performance Shotcrete Under the Coupling Effect of Sulfate and Chloride Salt" Materials 18, no. 19: 4505. https://doi.org/10.3390/ma18194505
APA StyleYang, J., Deng, S., Li, G., & Dai, X. (2025). Degradation Law and Experimental Study of High- Performance Shotcrete Under the Coupling Effect of Sulfate and Chloride Salt. Materials, 18(19), 4505. https://doi.org/10.3390/ma18194505




