Photoelectrocatalytic Degradation of Rhodamine B in the Presence of TiO2-BiVO4
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TiO2-BiVO4 Composite and Layered Materials
2.3. Characterization of TiO2-BiVO4
2.4. Photoelectrocatalytic Degradation Experiment
3. Results and Discussion
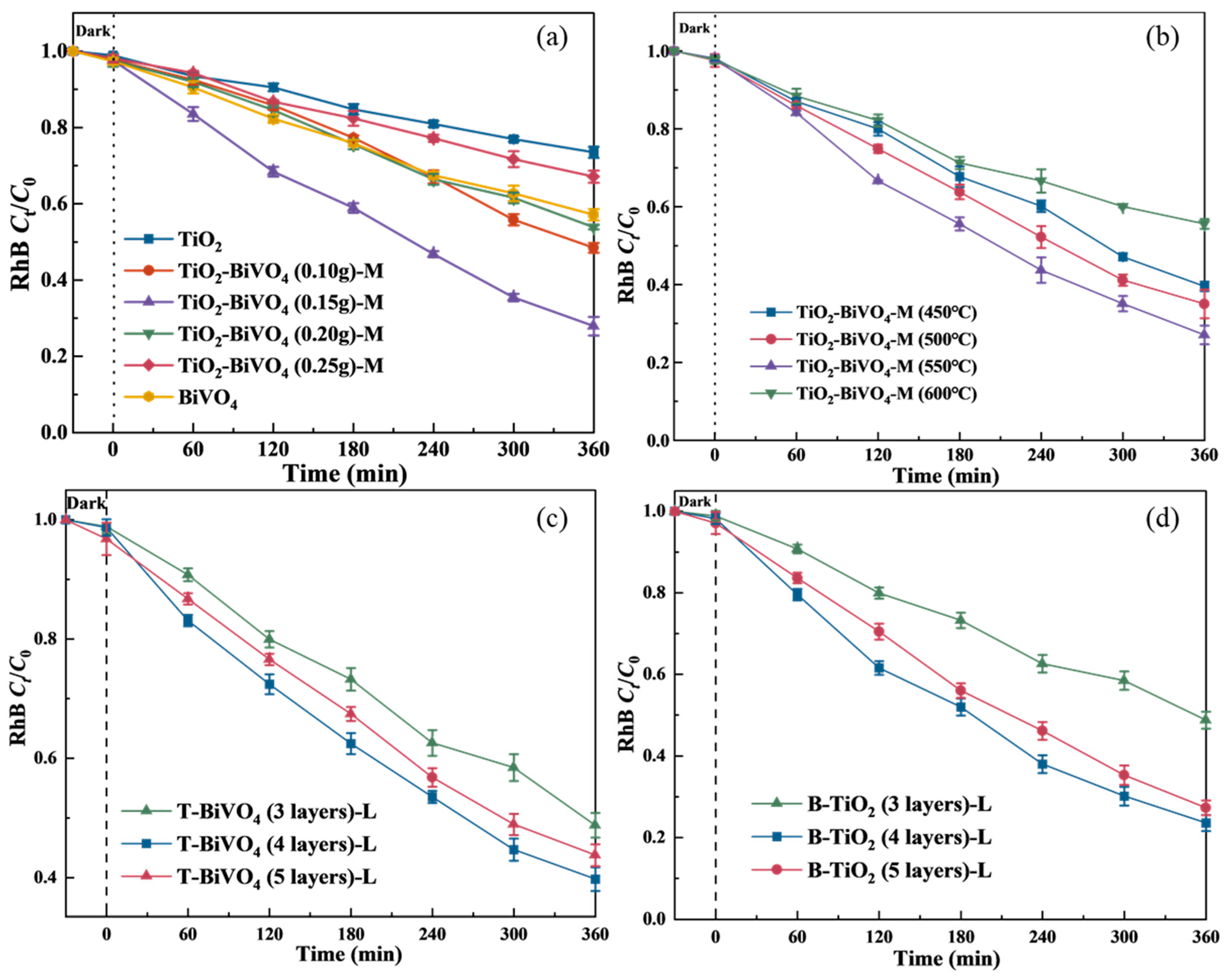
3.1. Optimization of Preparation Process of TiO2-BiVO4
3.1.1. TiO2-BiVO4 Composite Material
3.1.2. TiO2-BiVO4 Layered Material
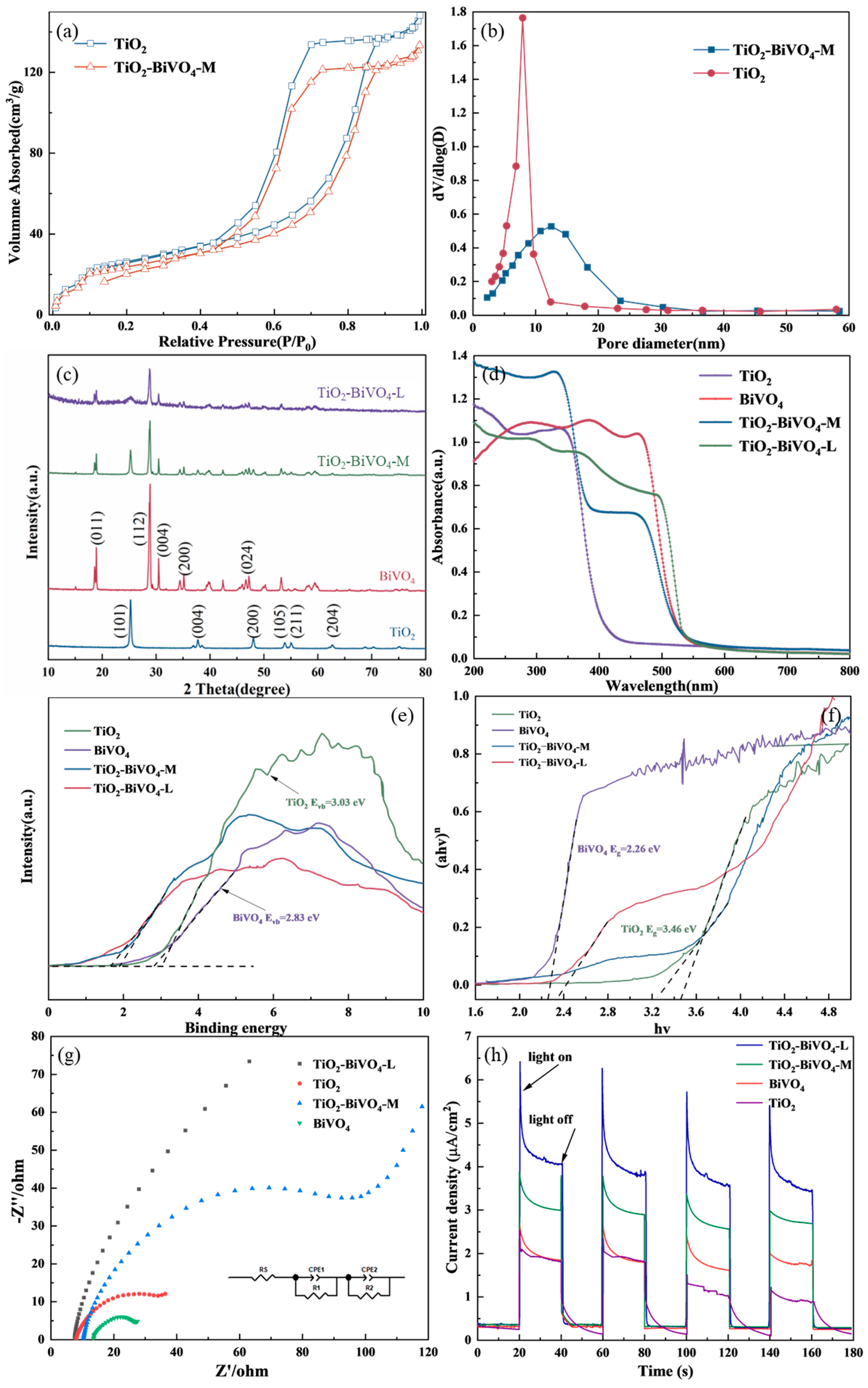
3.2. Characterization of the Catalysts
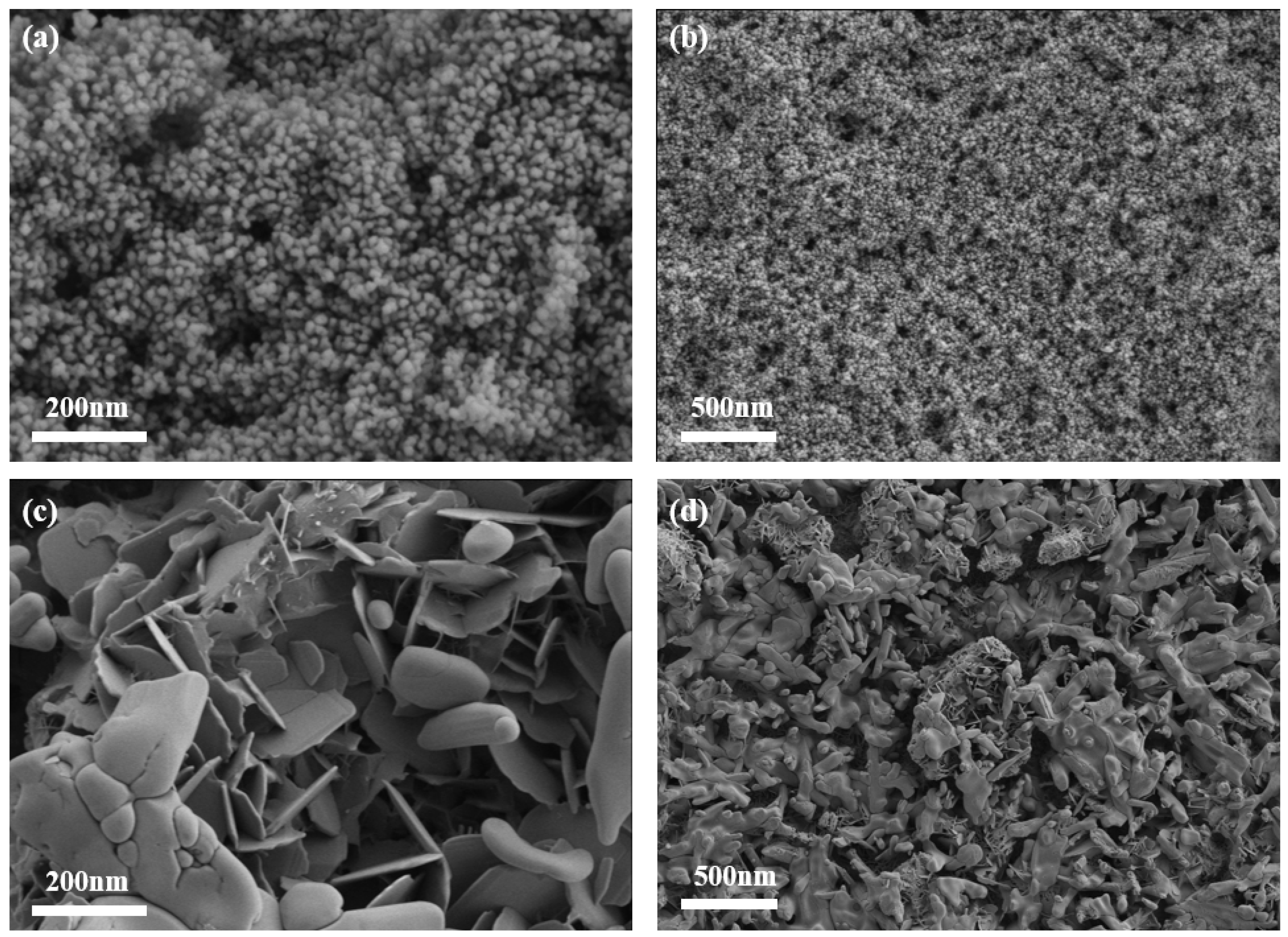
3.2.1. Morphological Analysis
3.2.2. Crystal Structure Analysis
3.2.3. Optical Properties
3.2.4. Photoelectrochemical Properties
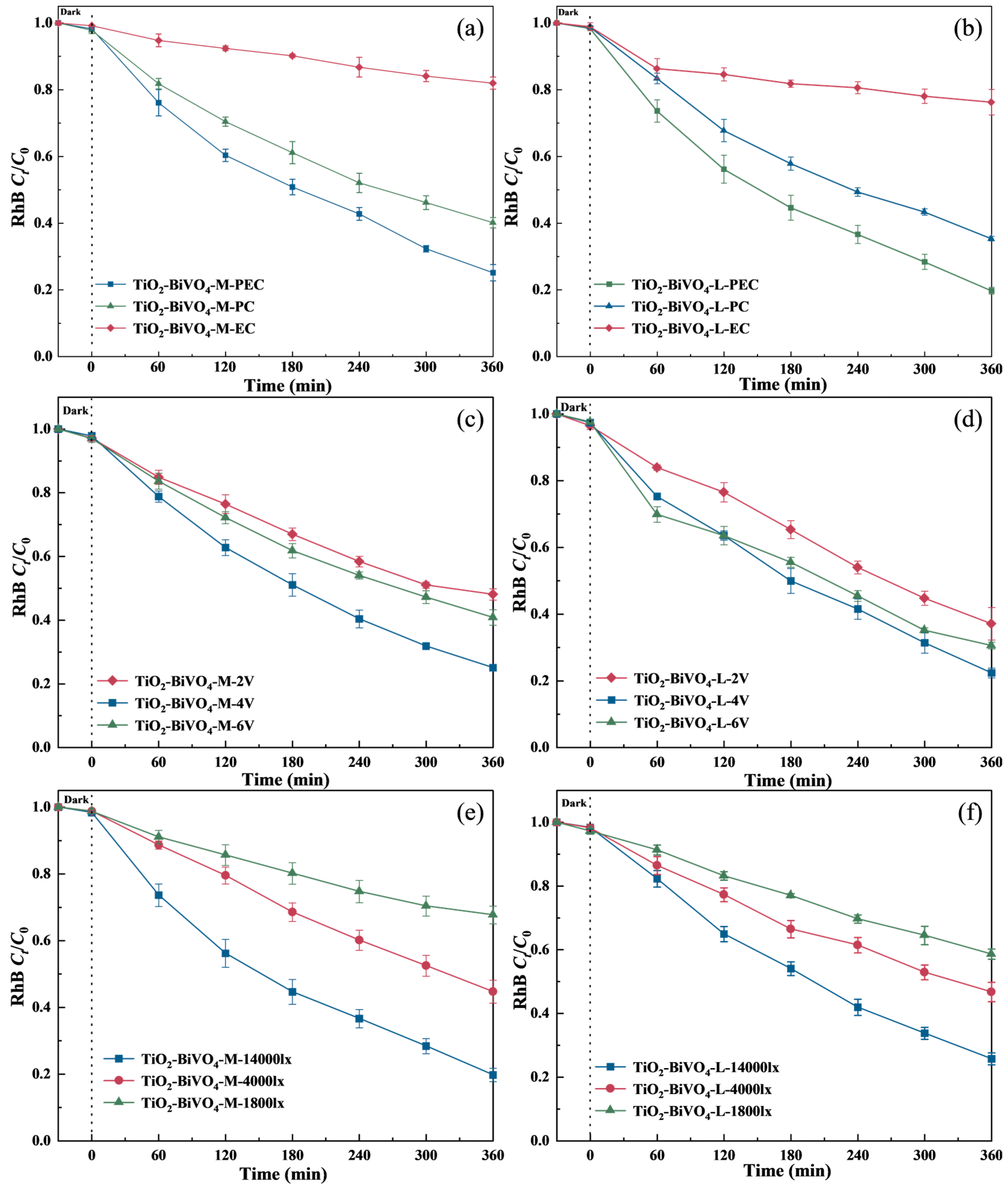
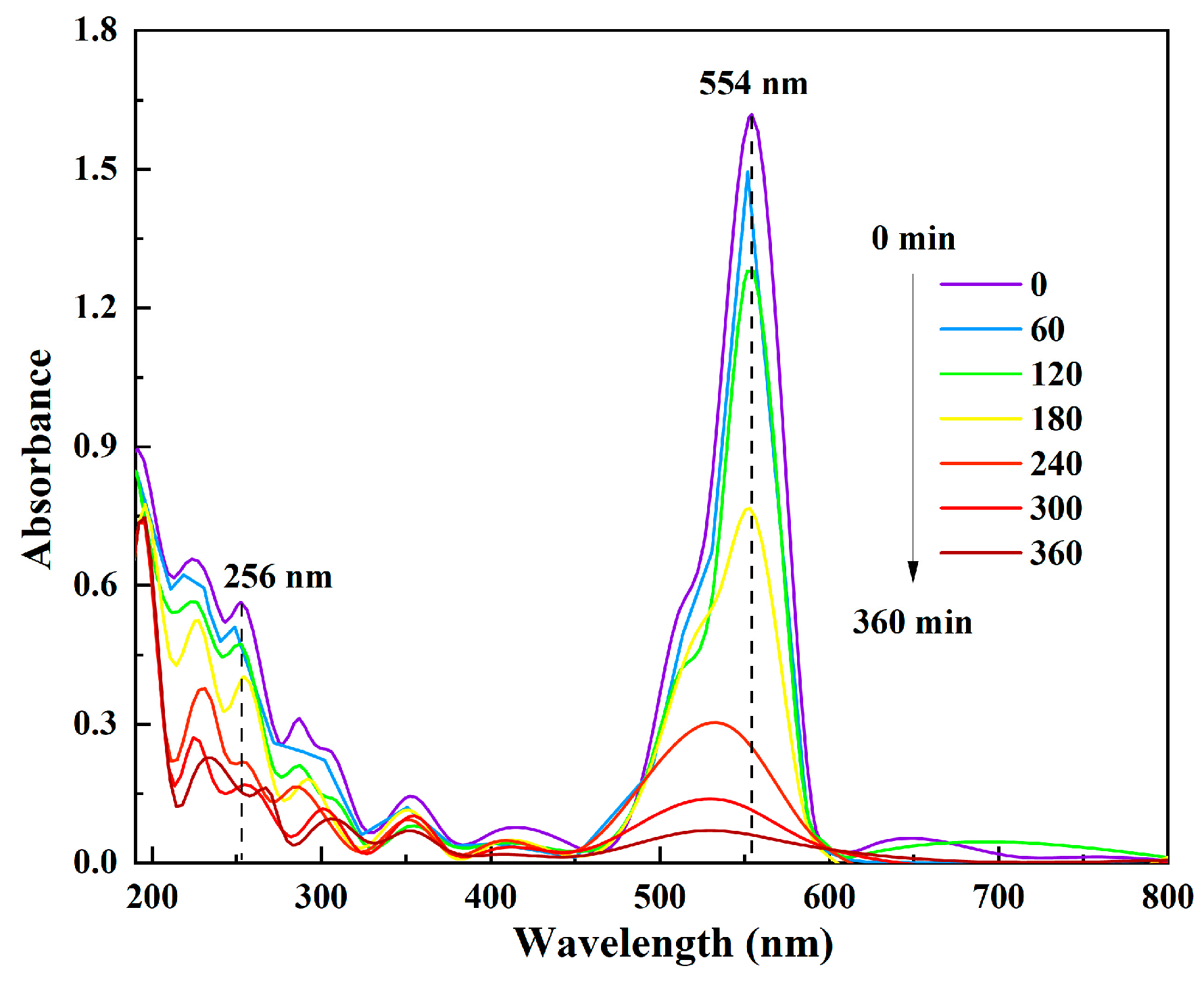
3.3. Photoelectrocatalytic Degradation of RhB
3.3.1. Optimization of Photoelectrocatalytic Conditions
3.3.2. Reutilization Performance of TiO2-BiVO4
3.3.3. Effects of Water Quality Factors
3.4. Catalytic Mechanisms of TiO2-BiVO4
3.4.1. Active Species During Photoelectrocatalyzing
3.4.2. Photoelectrocatalytic Mechanism of TiO2-BiVO4
3.5. Degradation Pathways of RhB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; An, X.; Gong, J.; Xu, Z.; Wang, L.; Xia, X.; Zhang, Q. Molecular response of Anoxybacillus sp. PDR2 under azo dye stress: An integrated analysis of proteomics and metabolomics. J. Hazard. Mater. 2022, 438, 129500. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Samsami, S.; Mohamadizaniani, M.; Sarrafzadeh, M.-H.; Rene, E.R.; Firoozbahr, M. Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Ding, S.; Li, X.; Qiao, X.; Liu, Y.; Wang, H.; Ma, C. Identification and screening of priority pollutants in printing and dyeing industry wastewater and the importance of these pollutants in environmental management in China. Environ. Pollut. 2024, 362, 124938. [Google Scholar] [CrossRef]
- Rafii, F.; Hall, J.D.; Cerniglia, C.E. Mutagenicity of azo dyes used in foods, drugs and cosmetics before and after reduction by Clostridium species from the human intestinal tract. Food Chem. Toxicol. 1997, 35, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Daghrir, R.; Drogui, P.; Robert, D. Photoelectrocatalytic technologies for environmental applications. J. Photochem. Photobiol. A Chem. 2012, 238, 41–52. [Google Scholar] [CrossRef]
- Zanoni, M.V.B.; Guaraldo, T. Photoelectrochemical hydrogen generation and concomitant organic dye oxidation under TiO2 nanotube. ECS Trans. 2013, 50, 63. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Y.; Zhou, Y.; Dong, F. Photoelectrocatalytic carbon dioxide reduction: Fundamental, advances and challenges. Nano Mater. Sci. 2021, 3, 344–367. [Google Scholar] [CrossRef]
- Comer, B.M.; Lenk, M.H.; Rajanala, A.P.; Flynn, E.L.; Medford, A.J. Computational study of transition-metal substitutions in rutile TiO2 (110) for photoelectrocatalytic ammonia synthesis. Catal. Lett. 2021, 151, 1142–1154. [Google Scholar] [CrossRef]
- Song, R.; Chi, H.; Ma, Q.; Li, D.; Wang, X.; Gao, W.; Wang, H.; Wang, X.; Li, Z.; Li, C. Highly efficient degradation of persistent pollutants with 3D nanocone TiO2-based photoelectrocatalysis. J. Am. Chem. Soc. 2021, 143, 13664–13674. [Google Scholar] [CrossRef]
- Bessegato, G.G.; Cardoso, J.C.; Zanoni, M.V.B. Enhanced photoelectrocatalytic degradation of an acid dye with boron-doped TiO2 nanotube anodes. Catal. Today 2015, 240, 100–106. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Brillas, E. Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters. J. Photochem. Photobiol. C Photochem. Rev. 2017, 31, 1–35. [Google Scholar] [CrossRef]
- Ahmadpour, N.; Nowrouzi, M.; Madadi Avargani, V.; Sayadi, M.H.; Zendehboudi, S. Design and optimization of TiO2-based photocatalysts for efficient removal of pharmaceutical pollutants in water: Recent developments and challenges. J. Water Process Eng. 2024, 57, 104597. [Google Scholar] [CrossRef]
- Li, D.; Zhang, N.; Yuan, R.; Chen, H.; Wang, F.; Zhou, B. Effect of wavelengths on photocatalytic oxidation mechanism of sulfadiazine and sulfamethoxazole in the presence of TiO2. J. Environ. Chem. Eng. 2021, 9, 106243. [Google Scholar] [CrossRef]
- Li, Z.; Luo, W.; Zhang, M.; Feng, J.; Zou, Z. Photoelectrochemical cells for solar hydrogen production: Current state of promising photoelectrodes, methods to improve their properties, and outlook. Energy Environ. Sci. 2013, 6, 347–370. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.-X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting—A critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Tayebi, M.; Lee, B.-K. Recent advances in BiVO4 semiconductor materials for hydrogen production using photoelectrochemical water splitting. Renew. Sustain. Energy Rev. 2019, 111, 332–343. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Selvaraj, S.; Kim, D.-H. Construction of heterojunction photoelectrode via atomic layer deposition of Fe2O3 on Bi2WO6 for highly efficient photoelectrochemical sensing and degradation of tetracycline. Appl. Catal. B Environ. 2019, 244, 11–24. [Google Scholar] [CrossRef]
- Wu, S.; Hu, Y.H. A comprehensive review on catalysts for electrocatalytic and photoelectrocatalytic degradation of antibiotics. Chem. Eng. J. 2021, 409, 127739. [Google Scholar] [CrossRef]
- Ye, S.; Chen, Y.; Yao, X.; Zhang, J. Simultaneous removal of organic pollutants and heavy metals in wastewater by photoelectrocatalysis: A review. Chemosphere 2021, 273, 128503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, H.; Chen, X.; Wang, W.; Li, F.; Qiang, T.; Shen, Y.; Cong, Y. CQDs improved the photoelectrocatalytic performance of plasma assembled WO3/TiO2-NRs for bisphenol A degradation. J. Hazard. Mater. 2023, 443, 130250. [Google Scholar] [CrossRef]
- Hu, Y.; Niu, Q.; Wang, Y.; Zhang, Y.; Zhao, G. Highly efficient removal mechanism of dimethyl phthalate over an economical 3D {001}TiO2/Ti photoelectrode with enhanced photoelectrocatalytic activity and long service life. Appl. Catal. B Environ. 2021, 285, 119812. [Google Scholar] [CrossRef]
- Yu, J.; Zou, J.; Xu, P.; He, Q. Three-dimensional photoelectrocatalytic degradation of the opaque dye acid fuchsin by Pr and Co co-doped TiO2 particle electrodes. J. Clean. Prod. 2020, 251, 119744. [Google Scholar] [CrossRef]
- Gong, Q.; Zhou, Y.; Wang, R.; Jiao, W. Enhanced photocatalytic pure water splitting of porous g-C3N4/CdS composite by the bimetallic phosphide. J. Environ. Chem. Eng. 2022, 10, 108046. [Google Scholar] [CrossRef]
- Shen, H.; Ni, D.; Niu, P.; Zhou, Y.; Zhai, T.; Ma, Y. Enhancing photocatalytic H2 evolution from water on CuO-Co3O4/TiO2: The key roles of Co3O4 loading amounts. Int. J. Hydrogen Energy 2017, 42, 30559–30568. [Google Scholar] [CrossRef]
- Long, L.; Li, J.; Wu, L.; Li, X. Enhanced photocatalytic performance of platinized CdS/TiO2 by optimizing calcination temperature of TiO2 nanotubes. Mater. Sci. Semicond. Process. 2014, 26, 107–111. [Google Scholar] [CrossRef]
- Jiang, D.; Zhao, L.; Shao, Y.; Wang, D. Preparation and characterization of layered LiNi0.9Co0.05Mn0.025Mg0.025O2 cathode material by a sol–gel method for lithium-ion batteries. RSC Adv. 2015, 5, 40779–40784. [Google Scholar] [CrossRef]
- Miao, J.; Geng, W.; Alvarez, P.J.J.; Long, M. 2D N-doped porous carbon derived from polydopamine-coated graphitic carbon nitride for efficient nonradical activation of peroxymonosulfate. Environ. Sci. Technol. 2020, 54, 8473–8481. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Y. Phase change and crystal growth of TiO2 in metatitanic acid. Ceram. Int. 2023, 49, 4607–4613. [Google Scholar] [CrossRef]
- Baek, J.H.; Kim, B.J.; Han, G.S.; Hwang, S.W.; Kim, D.R.; Cho, I.S.; Jung, H.S. BiVO4/WO3/SnO2 double-heterojunction photoanode with enhanced charge separation and visible-transparency for bias-free solar water-splitting with a perovskite solar cell. ACS Appl. Mater. Interfaces 2017, 9, 1479–1487. [Google Scholar] [CrossRef]
- Polo, A.; Nomellini, C.; Marra, G.; Selli, E.; Dozzi, M.V. WO3/BiVO4 heterojunction photoanodes: Optimized photoelectrochemical performance in relation to both oxides layer thickness. Catal. Today 2025, 446, 115137. [Google Scholar] [CrossRef]
- Hwang, S.W.; Kim, J.U.; Baek, J.H.; Kalanur, S.S.; Jung, H.S.; Seo, H.; Cho, I.S. Solution-processed TiO2/BiVO4/SnO2 triple-layer photoanode with enhanced photoelectrochemical performance. J. Alloys Compd. 2019, 785, 1245–1252. [Google Scholar] [CrossRef]
- Qadri, M.A.A.; Nurfani, E. Effect of TiO2:Zn layer thickness on the performance of MAPbI3-based perovskite solar cells fabricated under open-air condition. Next Mater. 2025, 8, 100537. [Google Scholar] [CrossRef]
- Izmirli, S.; Cavdar, S. Effect of film thickness on photodiode and photodetector performance of titanium dioxide (TiO2) prepared via solution assisted spin coating method. Appl. Surf. Sci. 2025, 688, 162187. [Google Scholar] [CrossRef]
- Mohamed, R.M. UV-assisted photocatalytic synthesis of TiO2-reduced graphene oxide with enhanced photocatalytic activity in decomposition of sarin in gas phase. Desalin. Water Treat. 2012, 50, 147–156. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Huang, L.; Yuan, Y.; Wang, Y.; Yılmaz, M.; Zhang, T.C.; Yuan, S. Visible-light-driven photocatalytic oxidation of H2S by boron-doped TiO2/LDH heterojunction: Synthesis, performance, and reaction mechanism. Chem. Eng. J. 2022, 448, 137607. [Google Scholar] [CrossRef]
- Eghbali, S.; Boroujerdnia, M.; Haghighatzadeh, A. Visible-light-active Ag-doped BiVO4 nanostructures: Preparation and optical and photocatalytic studies. Phys. B Condens. Matter 2025, 703, 416990. [Google Scholar] [CrossRef]
- Jubeer, E.M.; Manthrammel, M.A.; Subha, P.A.; Shkir, M.; Biju, K.P.; AlFaify, S.A. Defect engineering for enhanced optical and photocatalytic properties of ZnS nanoparticles synthesized by hydrothermal method. Sci. Rep. 2023, 13, 16820. [Google Scholar] [CrossRef]
- Kaplan, R.; Erjavec, B.; Dražić, G.; Grdadolnik, J.; Pintar, A. Simple synthesis of anatase/rutile/brookite TiO2 nanocomposite with superior mineralization potential for photocatalytic degradation of water pollutants. Appl. Catal. B Environ. 2016, 181, 465–474. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Feng, K.; Hu, X.; Fan, J.; Liu, E. A carbon membrane-mediated CdSe and TiO2 nanofiber film for enhanced photoelectrochemical degradation of methylene blue. RSC Adv. 2021, 11, 11872–11881. [Google Scholar] [CrossRef]
- Li, X.; Kang, B.; Dong, F.; Zhang, Z.; Luo, X.; Han, L.; Huang, J.; Feng, Z.; Chen, Z.; Xu, J.; et al. Enhanced photocatalytic degradation and H2/H2O2 production performance of S-pCN/WO2.72 S-scheme heterojunction with appropriate surface oxygen vacancies. Nano Energy 2021, 81, 105671. [Google Scholar] [CrossRef]
- Kim, Y.I.; Atherton, S.J.; Brigham, E.S.; Mallouk, T.E. Sensitized layered metal oxide semiconductor particles for photochemical hydrogen evolution from nonsacrificial electron donors. J. Phys. Chem. 1993, 97, 11802–11810. [Google Scholar] [CrossRef]
- Fidalgo, A.; Letichevsky, S.; Santos, B.F. Assessment of TiO2 band gap from structural parameters using artificial neural networks. J. Photochem. Photobiol. A-Chem. 2021, 405, 112870. [Google Scholar] [CrossRef]
- Wang, X.; Huo, P.; Liu, Y.; Xiang, Y.; Jia, C.; Yan, Z. High-throughput screening of ternary vanadate photoanodes for efficient oxygen evolution reactions: A review of band-gap engineering. Appl. Catal. A Gen. 2021, 616, 118073. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.; Kumar Joshi, M.; Cheng, Y.; Zhang, P.; Tan, M.; Yu, R.; Mao, Z.; Li, X. In-situ and ex-situ preparation of Bi2S3 on the BiVO4/TiO2 to construct Bi2S3/BiVO4/TiO2 heterojunction for efficient Cr(VI) reduction. Chem. Eng. J. 2024, 500, 156640. [Google Scholar] [CrossRef]
- Ma, Q.; Yan, C.; Lv, W.; Mei, Y.; Peng, H.; Du, J.; Zheng, B.; Guo, Y. Coexisting chloride ion for boosting the photoelectrocatalytic degradation efficiency of organic dyes. Catal. Lett. 2023, 153, 378–387. [Google Scholar] [CrossRef]
- Xie, G.; Chang, X.; Adhikari, B.R.; Thind, S.S.; Chen, A. Photoelectrochemical degradation of acetaminophen and valacyclovir using nanoporous titanium dioxide. Chin. J. Catal. 2016, 37, 1062–1069. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, Y. Synergetic degradation of rhodamine B at a porous ZnWO4 film electrode by combined electro-oxidation and photocatalysis. Environ. Sci. Technol. 2006, 40, 3367–3372. [Google Scholar] [CrossRef]
- Orimolade, B.O.; Zwane, B.N.; Koiki, B.A.; Rivallin, M.; Bechelany, M.; Mabuba, N.; Lesage, G.; Cretin, M.; Arotiba, O.A. Coupling cathodic electro-fenton with anodic photo-electrochemical oxidation: A feasibility study on the mineralization of paracetamol. J. Environ. Chem. Eng. 2020, 8, 104394. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Z.; Duan, J.; Li, J.; Lin, Q.; Jiang, Y.; Zhu, K.; Fang, Z.; Ji, B.; Yang, Z. Optimize the preparation process of m-TiO2@CdS by response surface methodology to enhance the light intensity adaptability of photocatalytic degradation of tetracycline. J. Alloys Compd. 2025, 1010, 177979. [Google Scholar] [CrossRef]
- Wang, Z.; Qiao, W.; Yuan, M.; Li, N.; Chen, J. Light-intensity-dependent semiconductor–cocatalyst interfacial electron transfer: A dilemma of sunlight-driven photocatalysis. J. Phys. Chem. Lett. 2020, 11, 2369–2373. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.T.; Li, X.; Chen, J.F.; Tao, X. Enhanced Visible-Light Photoelectrocatalytic Degradation of Organic Contaminants at Iodine-Doped Titanium Dioxide Film Electrode. Ind. Eng. Chem. Res. 2012, 51, 218–224. [Google Scholar] [CrossRef]
- Jayaraman, V.; Ayappan, C.; Mani, A. Facile preparation of bismuth vanadate-sheet/carbon nitride rod-like interface photocatalyst for efficient degradation of model organic pollutant under direct sunlight irradiation. Chemosphere 2022, 287, 132055. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, R.; Mittal, A.; Saleh, T.A.; Nayak, A.; Agarwal, S.; Sikarwar, S. Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Mater. Sci. Eng. C 2012, 32, 12–17. [Google Scholar] [CrossRef]
- Leng, H.; Jia, Y.; Lv, Z.; You, H.; Fu, L. Enhanced quinoline degradation by 3D stack Z-scheme photoelectrocatalytic system with Ag-TNTs photoanode and CN-CNWs photocathode. J. Environ. Chem. Eng. 2022, 10, 106826. [Google Scholar] [CrossRef]
- Rao, W.; Piliouras, P.; Wang, X.; Guido, A.; Kugler, K.; Sieren, B.; Wang, L.; Lv, G.; Li, Z. Zwitterionic dye rhodamine B (RhB) uptake on different types of clay minerals. Appl. Clay Sci. 2020, 197, 105790. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Shen, W.-H. Preparation and properties of stable nanocrystalline anatase TiO2 colloids. Chem. Eng. Technol. 2008, 31, 1277–1281. [Google Scholar] [CrossRef]
- Ghazal, N.; Mohamed, S.A.; Hameed, M.F.O.; Obayya, S.S.A.; El Nazer, H.A.; Madkour, M. Surface and optoelectronic impacts of ZnO/BiVO4/MWCNT nanoheterostructure toward photodegradation of water contaminants. Surf. Interfaces 2022, 33, 102278. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Wong, P.K. Quantitative characterization of hydroxyl radicals produced by various photocatalysts. J. Colloid Interface Sci. 2011, 357, 163–167. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Wei, X.-P.; Yang, Y.-T.; Zheng, Z.-Y.; Yuan, W.-B.; Ni, H.-G. A simple preparation method of Ti/TiO2/BiVO4 and implications for enhanced photoelectrocatalytic performance under visible light illumination. Inorg. Chem. Commun. 2025, 171, 113602. [Google Scholar] [CrossRef]
- Liang, L.; Gao, S.; Zhu, J.; Wang, L.; Xiong, Y.; Xia, X.; Yang, L. The enhanced photocatalytic performance toward carbamazepine by nitrogen-doped carbon dots decorated on BiOBr/CeO2: Mechanism insight and degradation pathways. Chem. Eng. J. 2020, 391, 123599. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Zhang, W.; Yu, C.; Zheng, S. Highly efficient g-C3N4/TiO2/kaolinite composite with novel three-dimensional structure and enhanced visible light responding ability towards ciprofloxacin and S. aureus. Appl. Catal. B Environ. 2018, 220, 272–282. [Google Scholar] [CrossRef]
- Zheng, Q.; Liu, X.; Tian, L.; Zhou, Y.; Liao, L.; Lv, G. Mechanism of Fenton catalytic degradation of Rhodamine B induced by microwave and Fe3O4. Chin. Chem. Lett. 2025, 36, 109771. [Google Scholar] [CrossRef]
- Hussein, A.; Scholz, M. Dye wastewater treatment by vertical-flow constructed wetlands. Ecol. Eng. 2017, 101, 28–38. [Google Scholar] [CrossRef]
- Law, M.; Goldberger, J.; Yang, P. Semiconductor nanowires and nanotubes. Annu. Rev. Mater. Res. 2004, 34, 83–122. [Google Scholar] [CrossRef]
- Hu, Y.; Li, D.; Zheng, Y.; Chen, W.; He, Y.; Shao, Y.; Fu, X.; Xiao, G. BiVO4/TiO2 nanocrystalline heterostructure: A wide spectrum responsive photocatalyst towards the highly efficient decomposition of gaseous benzene. Appl. Catal. B Environ. 2011, 104, 30–36. [Google Scholar] [CrossRef]
- Tokunaga, S.; Kato, H.; Kudo, A. Selective preparation of monoclinic and tetragonal BiVO4 with scheelite structure and their photocatalytic properties. Chem. Mater. 2001, 13, 4624–4628. [Google Scholar] [CrossRef]
- Guang, L.; Hui, W.; Xuejun, Z. Effect of drying temperatures on structural performance and photocatalytic activity of BiOCl synthesized by a soft chemical method. J. Solid State Chem. 2016, 239, 259–264. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Z. Ag3VO4/AgI composites for photocatalytic degradation of dyes and tetracycline hydrochloride under visible light. Mater. Lett. 2018, 216, 216–219. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, N.; Luo, M.; Fan, L.; Zhao, K.; Qu, J.; Guan, J.; Yuan, X. Enhancement mechanism of fiddlehead-shaped TiO2-BiVO4 type II heterojunction in SPEC towards RhB degradation and detoxification. Appl. Surf. Sci. 2019, 463, 234–243. [Google Scholar] [CrossRef]
- Shafaee, M.; Goharshadi, E.K.; Mashreghi, M.; Sadeghinia, M. TiO2 nanoparticles and TiO2@graphene quantum dots nancomposites as effective visible/solar light photocatalysts. J. Photochem. Photobiol. A Chem. 2018, 357, 90–102. [Google Scholar] [CrossRef]
- Horikoshi, S.; Hidaka, H.; Serpone, N. Environmental remediation by an integrated microwave/UV-illumination technique: IV. Non-thermal effects in the microwave-assisted degradation of 2,4-dichlorophenoxyacetic acid in UV-irradiated TiO2/H2O dispersions. J. Photochem. Photobiol. A Chem. 2003, 159, 289–300. [Google Scholar] [CrossRef]
- Yu, K.; Yang, S.; He, H.; Sun, C.; Gu, C.; Ju, Y. Visible light-driven photocatalytic degradation of rhodamine B over NaBiO3: Pathways and mechanism. J. Phys. Chem. A 2009, 113, 10024–10032. [Google Scholar] [CrossRef]
- He, Z.; Sun, C.; Yang, S.; Ding, Y.; He, H.; Wang, Z. Photocatalytic degradation of rhodamine B by Bi2WO6 with electron accepting agent under microwave irradiation: Mechanism and pathway. J. Hazard. Mater. 2009, 162, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Li, D.; Chen, Y.; Chen, W.; Li, W.; He, Y.; Fu, X. Synthesis and photocatalytic activity of calcium antimony oxide hydroxide for the degradation of dyes in water. J. Phys. Chem. C 2009, 113, 13825–13831. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Ma, W.; Zhao, J.; Hidaka, H.; Serpone, N. Effect of transition metal ions on the TiO2-assisted photodegradation of dyes under visible irradiation: A probe for the interfacial electron transfer process and reaction mechanism. J. Phys. Chem. B 2002, 106, 318–324. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Thomas, M.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Study on UV-LED/TiO2 process for degradation of Rhodamine B dye. Chem. Eng. J. 2011, 169, 126–134. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, A.; Kong, C.; Wang, J.; Zhou, B.; Chen, H.; Yuan, R.; Bai, Z. Photoelectrocatalytic Degradation of Rhodamine B in the Presence of TiO2-BiVO4. Materials 2025, 18, 4253. https://doi.org/10.3390/ma18184253
Sun A, Kong C, Wang J, Zhou B, Chen H, Yuan R, Bai Z. Photoelectrocatalytic Degradation of Rhodamine B in the Presence of TiO2-BiVO4. Materials. 2025; 18(18):4253. https://doi.org/10.3390/ma18184253
Chicago/Turabian StyleSun, Anli, Chao Kong, Jie Wang, Beihai Zhou, Huilun Chen, Rongfang Yuan, and Zhiming Bai. 2025. "Photoelectrocatalytic Degradation of Rhodamine B in the Presence of TiO2-BiVO4" Materials 18, no. 18: 4253. https://doi.org/10.3390/ma18184253
APA StyleSun, A., Kong, C., Wang, J., Zhou, B., Chen, H., Yuan, R., & Bai, Z. (2025). Photoelectrocatalytic Degradation of Rhodamine B in the Presence of TiO2-BiVO4. Materials, 18(18), 4253. https://doi.org/10.3390/ma18184253





