Abstract
In the context of circular economy, waste generated by fruit processing can be used to produce new materials with a wide range of uses. This study presents a method to synthesize biochar from peach kernel or grape pit waste. The adsorbents were tested in the removal of hexavalent chromium from synthetic wastewater with Cr6+ concentrations specific to plating processes. Characterization by BET, SEM, FTIR, and TG-DTG confirmed the formation of porous structures, and a well-functionalized surface. The effects of contact time, initial Cr6+ concentration, and adsorbent dose were investigated in static conditions. Both materials are efficient in hexavalent chromium removal, with sorption equilibrium achieved within 180 min. Kinetic studies indicated that the removal process follows a pseudo-second-order model. Equilibrium studies showed that optimal sorption occurred at pH = 6, with sorption capacities of 78.54 mg/g for biochar from peach kernels and 67.57 mg/g for biochar from grape pits. Hexavalent chromium followed a Sips adsorption isotherm for both biochars. Following the reusability study, it can be concluded that biochar from peach kernels maintains removal efficiency higher than 75% after four cycles.
1. Introduction
Water is one of the basic natural resources. Its geographical and seasonal distribution is uneven. Moreover, access to water resources is becoming problematic due to climate change. To remediate the water shortage, reclaimed water is used wherever possible, for example in industrial processes [1], agriculture [1], groundwater recharge [2], various urban uses and reintegration into water bodies used for drinking water production [3,4].
According to the WHO (World Health Organization), almost all hexavalent chromium sources are anthropogenic [5]. Depending on their provenance (e.g., plating for protection against corrosion [6], leather processing and manufacturing [7], textile dyeing [8], steel industry [9]), wastewater can be contaminated by hexavalent chromium. Chromium is an element that is found in the environment in its more stable forms: Cr3+ and Cr6+. While the trivalent form is a micronutrient for humans, hexavalent chromium is known to be toxic, affecting both humans and the environment [5,10].
The plating process consists of successive steps of surface chemical cleaning, washing and coating; these steps generate several types of wastewater with high concentrations of zinc, iron, grease or hexavalent chromium [11,12]. The present study focuses on the treatment of wastewater generated by chromium galvanizing baths.
In plating processes, the concentrations of Cr6+ range from 0.5 to 800 mg/L [6,11,13,14,15]; this is well above the limits for total chromium in water established by standards (e.g., 0.1 mg/L by EPA, 0.05 mg/L by the European Commission and WHO) [16,17,18]. The European Union plans to restrict the use of Cr6+ to prevent an estimated quantity of 17 tonnes of Cr6+ from being released in the environment [19].
The treatment of wastewater from plating consists of successive steps of pH adjustment, precipitation, sludge separation, sludge thickening and dewatering. All these steps use supplementary chemical reagents (e.g., FeCl3 ferric chloride, Na2S sodium sulfide, NaHSO3 sodium bisulfite, H2O2 hydrogen peroxide, etc.) [20]. The removal of pollutants by adsorption has several advantages compared with the previous methods: it is efficient and can achieve the fulfillment of concentration limits, does not require the addition of supplementary chemical reagents and has a low cost when biosorbents are used [21].
The common adsorbent, activated carbon, has been tested for the removal of hexavalent chromium from wastewater and has proven high adsorption capacity, but it has a high price [7]. To comply with the latest standards regarding waste management, researchers have started to investigate the manufacturing of valuable products from waste resulting from different activities. Biochar has been used in the formulation of sorbents tested for the removal of various pollutants from wastewater (e.g., pharmaceuticals [22], Cr6+ [23,24], heavy metals [25], lead [26], ammonia [27], Cd2+ and Ni2+ [28]). The Cr6+ removal efficiency for biochar materials was found to range between 80 and 99.79% [23,24]. In the case of Pb2+ removal, reusability studies indicated that the biochar removal efficiency decreases from 99.14% to 94.5% after four adsorption–desorption cycles, which makes biochar materials good candidates as sorbents [26]. Waste materials used in the production of sorbents offer multiple advantages: (i) waste minimization; (ii) obtaining a value-added product; (iii) replacement of high-cost adsorbents with cheap and efficient materials; (iv) the sorbent has low environmental risk. One of the biochar materials that has been tested for Cr6+ removal is obtained from coffee grounds [29]. A composite made from coffee ground biochar and alginate was found to be rich in oxygen-containing functional groups; it was evidenced that the material is not only a sorbent but also contributes to a toxicity decrease by hexavalent chromium’s reduction to trivalent chromium [24]. In regions where sugarcane bagasse by-product is produced in large quantities (e.g., India), research studies have proposed its transformation into biochar instead of dumping it on fields or burning [30]. In some cases, the biochar derived from agricultural waste was used for soil bioremediation [31]. Iron-enriched biochar was made from the sludge resulting from a wastewater treatment facility in the steel industry; the inclusion of iron in the formulation helps reduce hexavalent chromium to trivalent chromium [32]. However, because of its origin, the biochar is not suitable for producing high-purity water.
This study addresses a gap that exists in the circular economy in the agrifood branch by transforming waste into a valuable product that can be used for wastewater treatment. To the authors’ knowledge, waste from fruit processing has not been previously valorized in Cr6+ removal for plating wastewater treatment. Experimental and theoretical approaches have evidenced that biochars from peach kernels or grape pits are alternative sorbents for commercial materials and contribute to a reduction in agrifood waste generated by local businesses.
2. Materials and Methods
2.1. Chemicals and Reagents
K2Cr2O7 was used for the preparation of Cr6+ solution. Sulfuric acid was used in the preparation of the sorbent. Sodium hydroxide, hydrochloric acid, and sodium chloride were used for pH adjustment and pHPZC determination. All chemical reagents were purchased from Merck/Sigma-Aldrich Chemical (Darmstadt, Germany). Ultrapure water used in the experiments was produced by the Milli-Q Integral system (Merck, Bucharest, Romania).
2.2. Preparation of Adsorbents
To prepare the peach kernel-derived biochar, peach kernels from the Peach Tree Black Boy variety were used. Grape seed-derived biochar was prepared from waste generated from the use of two grape varieties: large-seeded table grape seeds of the “Italia” variety and the “Afuz-Ali” variety. The raw material was purchased from a local market. The peach kernels or grape pits were washed thoroughly with tap water to remove impurities and dust and dried at room temperature for 48 h. Then they were ground to a particle size in the range of 1–3 mm using a BB51 jaw crusher (manufactured by Retsch, Haan, Germany). In a porcelain capsule, about 100 g of the shredded material was mixed with concentrated sulfuric acid at a mass ratio of 1:1, under slow stirring for 24 h. Then the solid material was recovered by filtration, it was washed with distilled water until the pH value was 6 and was placed in an oven at 140 °C for 8 h. The resulting powder was ground and stored in hermetically sealed containers.
2.3. Sample Characterization
FTIR spectra were recorded using a Bruker Vertex 70 spectrometer (Bruker, Billerica, MA, USA) equipped with a diamond ATR (attenuated total reflectance) device. The spectra were recorded in the range 600 to 4000 cm−1, as an average of 32 successive measurements, eliminating bands of noise and atmospheric carbon dioxide and water vapor.
TG and DTG analyses were carried out using STA 449 C Jupiter equipment from Netzsch (Gottinger, Germany), with a heating range from 25 to 800 °C at a constant heating rate of 10 °C/min and continuous air flow.
To understand the morphological structure of the biochars, scanning electron microscopy (SEM) was employed to visualize the sample surfaces. High-resolution images were recorded on a Hitachi S2600N (Chiyoda, Tokyo, Japan), which uses secondary electron imaging analysis (SEI) with a resolution of up to 4.0 nm (at 25 kV in high vacuum), and an energy-dispersive X-ray spectrometer (EDS) for qualitative and quantitative microanalysis.
The specific surface area measurements were performed by a nitrogen adsorption–desorption technique using a Micromeritics TriStar II Plus BET surface area analyzer (Malvern Panalytical, Malvern, Worcestershire, UK). For that, the samples were outgassed at 40 °C, 17 h before recording nitrogen adsorption–desorption isotherms. The specific surface area was determined in the relative pressure range P/P0 of 0.08–0.25 using the Brunauer–Emmett–Teller (BET) physical adsorption model.
2.4. Batch Experiments
Batch experiments were performed with synthetic solutions that contain hexavalent chromium in the concentration ranges specific to those found in plating wastewater. A volume of 50 mL of Cr6+ solution at the initial concentration C0 was mixed with various weights of biochar. The mixture was stirred at 200 rpm for a predetermined amount of time with a magnetic stirrer and placed in a thermostatic enclosure at 25 °C. Solutions of 0.1 M NaOH and 0.1 M HCl were used for pH adjustment. Kinetic experiments were made by taking a small volume of clear solution (0.1 mL) at regular intervals and determining the residual Cr6+ concentration. The method used for Cr6+ quantification consisted of supernatant analysis by flame atomic absorption spectroscopy (Analytik Jena ContrAA 300, Jena, Germany) at a wavelength of 357.9 nm.
The retention of Cr6+ from the aqueous solution was analyzed by calculating the sorption capacity qt, defined by
where C0 (mg·L−1) is the Cr6+ initial concentration; Ct (mg·L−1) is the Cr6+ residual concentration at the time t; qt (mg·g−1) is the sorption capacity of biochar at time t; W (g) is the mass of the biochar; and V (L) is the volume of the Cr6+ solution.
The removal rate of Cr6+ ions was determined by calculating
The determination of the point of zero charge was carried out according to the salt addition method [33]. A 0.1 M NaCl solution was used as a background electrolyte solution. Equal volumes (50 mL) of this solution were added to a series of flasks, and the pH was adjusted in the interval 1–11 using 0.1 M HCl and NaOH solutions. Afterwards, a constant weight of the sorbent (50 mg) was added to each flask. The final pH value was read after the samples were shaken at 200 rpm for 24 h in a temperature-controlled environment.
2.5. Stability Tests and Reusability
HCl 1M was used as a Cr6+ desorbent to explore the regeneration of the biochars over 4 adsorption–desorption cycles. For that, 50 mg of spent biochar was placed in a beaker with 50 mL of HCl 1 M; the mixture was slowly shaken at 50 rpm for 24 h. The biochar was separated by filtration and washed with deionized water until the pH of the filtrate was slightly acidic to neutral. The solid was then dried and reused.
To ensure experimental data reproducibility, every experiment was carried out three times, and the averages of the results were used in the further processing of the data.
3. Results
3.1. Characterization of Biochars
3.1.1. Thermogravimetric Analysis of Adsorbent Materials
The thermal behavior of the sorbents was studied using TG-DTG analyses. The total mass loss for peach kernel-derived biochar was 52.28% at 699.6 °C (Figure 1a), while grape pit-derived biochar had a total mass loss of 50.07% at 699.6 °C (Figure 1b). DTG graphs indicate that the decomposition of sorbents took place in three stages between 35 and 699.6 °C and between 32.5 and 699.6 °C, respectively. The first zone (35–132.5 °C and 32.5–140 °C, respectively) is characterized by an initial mass loss of 4.6% and 7.3%, respectively, corresponding to the evaporation of free water (endothermic peak in the DTG profiles). The main mass loss of 27% and 27.7%, respectively, was recorded during the second temperature interval (132.5–500 °C and 140–500 °C, respectively) and was attributed to the decomposition of less stable hemicellulose at temperatures below 350 °C [34] and cellulose at temperatures below 500 °C [35,36], and initiation of lignin decomposition [37]. The third temperature interval (500–699.6 °C) corresponds to a mass loss of 20.68% and 15.07%, respectively, due to the second-stage decomposition of the more stable polymer lignin [37,38].
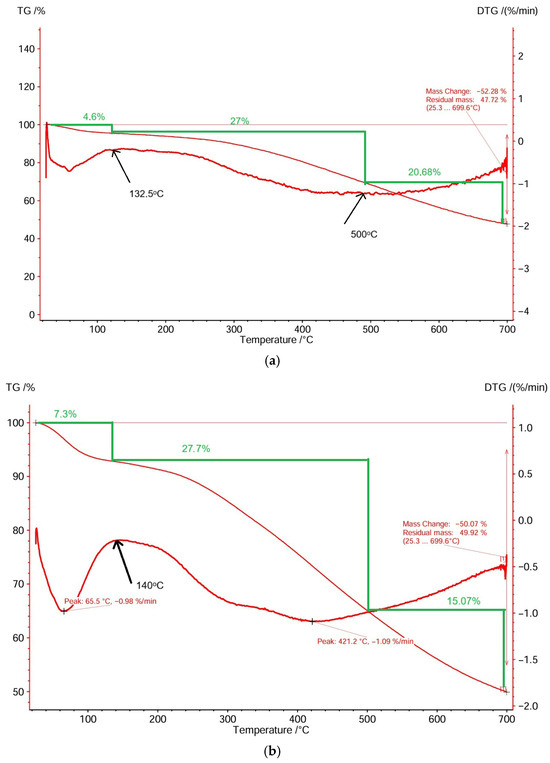
Figure 1.
TG-DTG of biosorbents: (a) peach kernel biochar; (b) grape pit biochar.
Based on the thermogravimetric analysis of the samples, the biochars underwent evaporation of free water and only slight thermal decomposition of hemicellulose.
3.1.2. Fourier Transform Infrared Spectroscopy
The effect of thermal treatment on the surface functional groups of the sorbents was observed by FT-IR spectroscopy (Figure 2). The peaks at 2929 cm−1 (biochar from peach kernels) and 3011 cm−1 (biochar from grape pits) correspond to hydroxyl functional groups –OH (associated with alcohol, phenol, and water). Peaks at 1712 cm−1 and 1702 cm−1 correspond to carbonyl bond C=O stretching found in ketones, aldehydes, and esters. Peaks at 1598 cm−1 and 1594 cm−1 are attributed to C=C stretching ring vibrations in lignin. The bands at 1175 cm−1 and 1156 cm−1 indicate vibrations of C–O groups (ether or alcohol bonds), while vibrations at 1044 cm−1 and 1021 cm−1 indicate a C–O–C group (ether bond) or C–OH (alcohol group), respectively. The 858 cm−1 and 845 cm−1 peaks are generally associated with vibrations of C–H bonds in aromatic compounds.
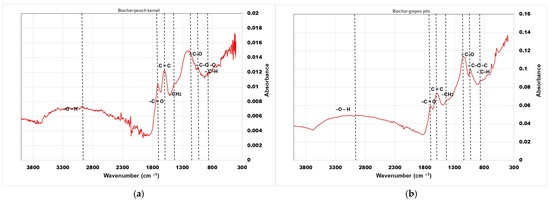
Figure 2.
FT-IR results of biosorbents: (a) peach kernel biochar; (b) grape pit biochar.
3.1.3. SEM and EDX Analysis
The micrographs obtained by SEM analysis show that the biochar prepared from peach kernels has a spongy uniform surface and well-defined pores, while the second biochar presents smaller but less uniform pores (Figure 3).

Figure 3.
SEM results of biosorbents: (a) peach kernel biochar; (b) grape pit biochar.
The composition given by EDX analysis (Table 1) shows that the sorbents contain mainly carbon and oxygen and traces of other elements like sulfur, potassium, and calcium. The O to C ratio is 0.31 for biochar derived from peach kernels and 0.27 for the biochar derived from grape pits; this ratio is used to evaluate the degree of maturation of biochars [39], and indicates a higher content of aromatic compounds and enhanced carbonization for the second sorbent.

Table 1.
EDX analysis results of biochars.
3.1.4. Morphology Analysis and Specific Surface Area
Specific surface area is an important physical parameter; it was found that the composition (carbon percentage and the content of inorganic compounds) of biomass is a key factor that influences the specific surface area [40]. A comparison with data reported in the literature showed that the prepared materials have values of specific surface area comparable with those previously reported in the literature (Table 2).

Table 2.
Specific surface area and porosity of prepared samples and values from the literature.
3.1.5. pH of Point of Zero Charge (pHPZC)
The pH of the point of zero charge (pHPZC) of the tested sorbents was found to be 2.4 for biochar from peach kernels and 2.1 for biochar from grape pits (Figure 4). These values indicate that at a pH less than pHPZC, the biochar surface is positively charged, while at a pH higher than pHPZC, the surface is negatively charged. Thus, at pH values lower than 2.4 and 2.1, respectively, repulsive forces will appear between the positively charged surface of the sorbents and the hexavalent chromium ions. This is not favorable for the sorption process. These results are in agreement with data reported in the literature for biochar sorbents [42].
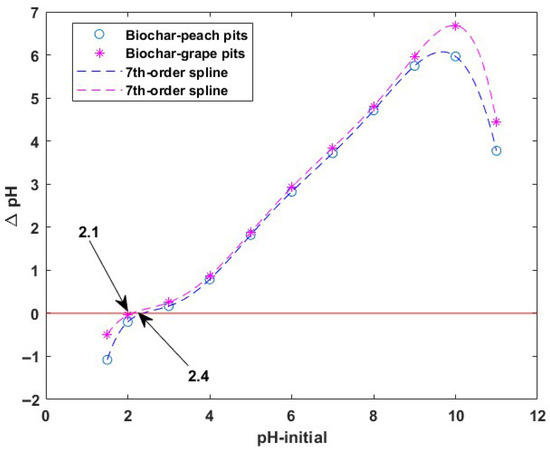
Figure 4.
pH of zero charge (pHPZC) of the biochars.
During this experiment it was noticed that at pH values higher than 11, chemical degradation of materials starts.
3.2. Batch Adsorption Tests
3.2.1. Effect of m/V Ratio
The ratio of adsorbent to adsorbate influences the pollutant removal efficiency. Various amounts of adsorbent were mixed with a fixed volume of adsorbate (50 mL) of 100 mg/L hexavalent chromium solution. The mixture was shaken for 10 h at a constant temperature. By increasing the adsorbent dose, the percentage of hexavalent chromium removed from aqueous solution increases from about 52% to 83% for grape pit biochar, and 57% to 87% for peach kernel biochar (Figure 5). This is because with increasing adsorbent quantity, the number of active sites available for adsorption increases. However, at higher adsorbent doses, a substantial portion of the adsorption sites remains unavailable because of the coalescence of solid particles.
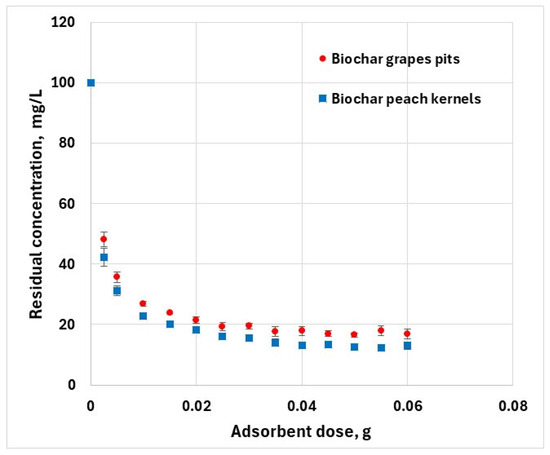
Figure 5.
Effect of adsorbent dose on the removal of Cr6+ (C0 = 100 mg·L−1, T = 25 ± 2 °C; error bars show means ± standard error of the mean of triplicate experiments).
3.2.2. Effect of Contact Time
The effect of contact time for an initial hexavalent chromium concentration of 200 mg·L−1 (Figure 6) indicates that initially, the sorption process is rapid and slows as equilibrium is approached. This is because at the beginning of the adsorption, a large number of adsorption sites were available while towards the equilibrium stage, this number reduces simultaneously with the appearance of repulsive forces between chromium ions in the external layer of the adsorbent particles.
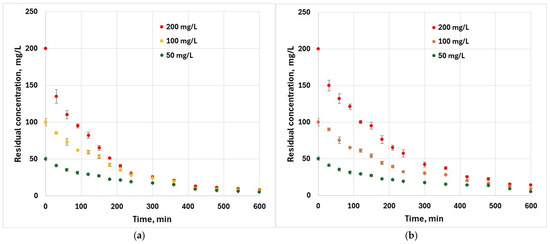
Figure 6.
Effect of contact time on the removal of Cr6+ (T = 25 ± 2 °C, m = 0.05 g, V = 50 mL): (a) peach kernel biochar; (b) grape pit biochar.
3.2.3. Effect of Temperature
The representation of temperature influence on hexavalent chromium removal (Figure 7) shows a slight increase in the adsorption capacity of both biochars in the range of 25 to 40 °C, with a maximum adsorption capacity of 76.9 mg·g−1 for grape pit biochar and 80 mg·g−1 for peach kernel biochar. This suggests an endothermic adsorption process, which is confirmed by other studies conducted on carbon-based adsorbents [30].
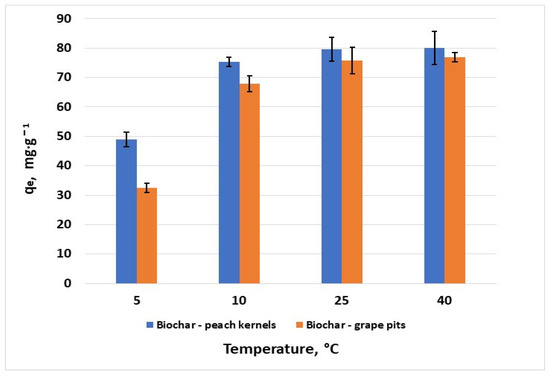
Figure 7.
Effect of temperature on the removal of Cr6+ (C0 = 100 mg·L−1; error bars show means ± standard error of the mean of triplicate experiments).
3.2.4. Effect of pH
Experiments at different pH values of the solutions indicated that increasing the pH leads to an increase in the adsorption capacity (Figure 8). This is attributed to the existence of electrostatic attractions between the hexavalent chromium ions and the negatively charged surface of the sorbents at pH values higher than pHPZC. The pH at which maximum hexavalent chromium adsorption capacity was observed was considered the optimum pH. Further experiments were performed at pH 6 for both biochar materials.
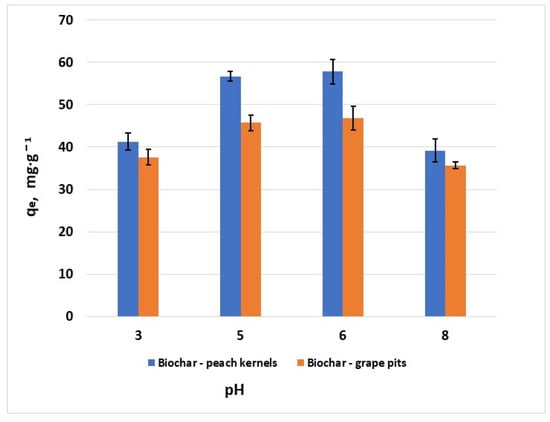
Figure 8.
Effect of pH on the removal of Cr6+ (C0 = 100 mg·L−1, T = 25 ± 2 °C; error bars show means ± standard error of the mean of triplicate experiments).
3.2.5. Adsorption and Kinetic Models
Theoretical Backgrounds—Adsorption Models
The models usually used to describe the interaction between the sorbent and sorbate, at equilibrium and constant temperature, are Langmuir, Freundlich, Sips, Temkin, and Dubinin–Radushkevich. Reviewing the literature data, it was found that the best agreement between experimental and calculated data for biochar-type sorbents was obtained for chemical adsorption using the Langmuir isotherm and empirical Freundlich isotherm [43,44]. Additionally, Sips and Temkin models have been tested.
Langmuir Isotherm
The Langmuir model assumes equilibrium between the sorbate and the solid surface during adsorption, using hypotheses of monolayer sorption of the pollutant on the homogeneous surface of the biochar. The Langmuir isotherm’s non-linear form is described by Equation (3):
where qe (mg·g−1) is the amount adsorbed at equilibrium concentration Ce (mg·L−1), qm (mg·g−1) is the maximum amount of hexavalent chromium adsorbed per unit mass of biochar, and KL (L·mg−1) is the Langmuir constant.
The ease of sorption can be analyzed by calculating the separation factor, RL:
The value of RL indicates the type of isotherm: favorable (RL < 1), unfavorable (RL > 1), reversible (RL = 0) and linear (RL = 1).
Freundlich Isotherm
The Freundlich model is a non-linear, empirical model that is used when the Langmuir model fails to adequately describe the isothermal sorption process. This mechanism assumes that the sorbent surface is heterogeneous, which is closer to the characteristics of biochar materials, especially the biochar derived from grape pits (Figure 3b). It is represented by the following equation:
where KF (g−1·mg(1−1/n)·L1/n) and n (dimensionless) are the Freundlich constants. n also indicates the nature of the adsorption process: when the value of 1/n is between 0 and 1, the adsorption is considered favorable, while a value of 1 simplifies the model to a simple linear one (qe = KF·Ce).
Sips Isotherm
Temkin Isotherm
Theoretical Backgrounds—Kinetic Models
The rate at which the hexavalent chromium ions are removed from the aqueous solution was investigated using the following models: pseudo-first-order, pseudo-second-order, Elovich, and intra-particle diffusion models, given by Equations (8)–(11).
Pseudo-First-Order Model
Pseudo-Second-Order Model
Elovich Model
Because of computational simplicity, it is common to use the linearized form of the models described by Equations (3)–(10) [43]. However, linearization of these equations contributes to error propagation. Therefore, in this study, the estimation of adsorption parameters was made using the non-linear regression in Matlab R2024b. The goodness of the fitting was determined by calculating R2 (coefficient of determination), RMSE (root mean square error), and χ2 (chi-square).
3.2.6. Thermodynamic Study
The thermodynamic study allows us to better understand the influence of temperature. The thermodynamic parameters were calculated by the following equations:
where (J·mol−1) is the Gibbs free energy used to predict the spontaneity of the process, is the adsorption enthalpy (J·mol−1), and (J·mol−1) is used to determine the level of disorder of the adsorbate–adsorbent system. K is a non-dimensional equilibrium constant derived from the isotherm models (Equations (3)–(7)). Some studies report the direct use of equilibrium constants of one of the isotherms. However, this is not entirely correct because these have units. We have opted to correct the value of K with the equation proposed by Zhou et al. (2014) [45]; given the good fitting of experimental results by the Langmuir model, KL was chosen as the equilibrium constant for the thermodynamic study:
where M is the molecular weight. The isotherms were derived for three temperatures: 10, 25, and 40 °C.
4. Discussion
The isotherm study gives information about the distribution of pollutant molecules between the liquid and solid phases. This study was performed by using initial hexavalent chromium concentrations in the range 100 to 250 mg/L, at 25 °C, pH = 6. The fitting results obtained for Langmuir, Freundlich, Sips, and Temkin isotherms (Figure 9) indicated strong sorption capacity of biochars for hexavalent chromium ions. Experimental data was fitted best to the Sips isotherm than to the other models, as indicated by the RMSE and χ 2 values in Table 3. The R2 criterion is accurate only for linear models. Hence, it was regarded only for orientation because the models were solved in their non-linear form. The RL parameter is 0.78 for biochar from grape waste, and 0.68 for biochar from peach waste, indicating that the adsorption process is favorable.
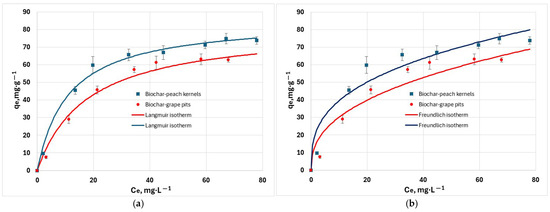
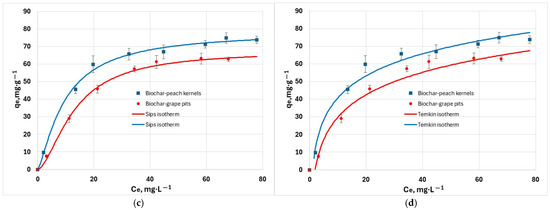
Figure 9.
Adsorption isotherm of Cr6+ cations at 25 °C: (a) non-linearized Langmuir isotherm; (b) non-linearized Freundlich isotherm; (c) non-linearized Sips isotherm; (d) non-linearized Temkin isotherm.

Table 3.
Parameters of isothermal adsorption models for hexavalent chromium removal at 25 °C.
Sips adsorption capacities were compared with data from the literature for low-cost adsorbents; as shown in Table 4, the synthesised biochars have comparable adsorption capacities or better.

Table 4.
Adsorption capacity of several adsorbents for Cr6+ removal.
Kinetic experimental data was obtained by the variation of interaction time (Figure 10a). Data was fitted against the pseudo-first-order, pseudo-second-order, and Elovich kinetic models (Figure 10). Determination coefficients for the kinetic models have values above 0.9, indicating a good correlation between experimental and calculated data (Table 5). However, R2 alone is not enough to discriminate between the kinetic models. In the case of both adsorbents, considering the values of RMSE and χ2, the kinetic experimental data match the pseudo-second-order model well (Figure 10b).
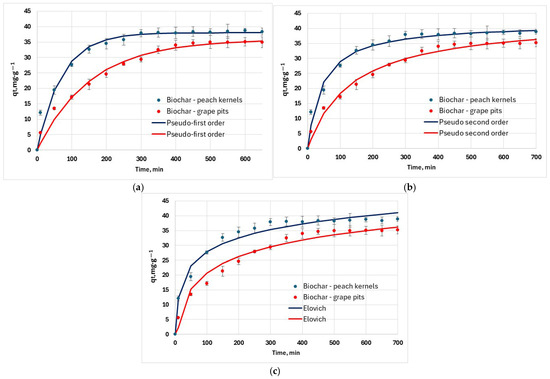
Figure 10.
Adsorption kinetic models used to fit experimental data (25 °C): (a) pseudo-first-order kinetic model; (b) pseudo-second-order kinetic model; (c) Elovich model.

Table 5.
Kinetic parameters for hexavalent chromium removal by biochars at 25 °C.
The thermodynamic parameters are presented in Table 6. The negative values of the Gibbs free energy demonstrate the spontaneous nature of the adsorption on the two sorbents. It decreases with increasing temperature; this indicates the feasibility of adsorption at increasing temperature. The positive values of the enthalpy indicate that the adsorption process is endothermic, and the interactions between the adsorbate and the adsorbent are predominantly through weak van der Waals forces (physical adsorption). The positive values of the entropy indicate increased disorder at the adsorbent–solution interface containing hexavalent chromium.

Table 6.
Thermodynamic parameters for hexavalent chromium adsorption at C0=100 mg/L.
5. Regeneration and Reusability
From the practical point of view, the regeneration and reusability of an adsorbent are decisive factors that determine the long-term applicability of the material. Adsorption–desorption experiments showed that biochar from peach kernels maintains a removal efficiency over 75% after four cycles (Figure 11), while biochar from grape pits showed a faster aging of the structure and a removal efficiency of 77.99% after the second cycle. The decrease in removal efficiency can be attributed to the gradual occupation of active sites with hexavalent chromium ions that are strongly bound, as well as to the structural changes. Nevertheless, the biochar from peach kernels has the ability to maintain high Cr6+ removal efficiency over multiple cycles.
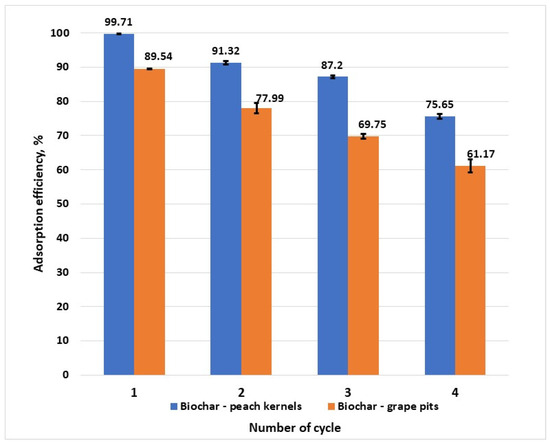
Figure 11.
Adsorbent adsorption–desorption cycles and regeneration experiment (C0 =100 mg/L, T = 25 ± 2 °C).
Regarding the possible secondary contamination from the sorbent, carbon-based adsorbents are used worldwide in water and wastewater treatment applications. They are well known for their stability and for not releasing dangerous compounds in the normal operating conditions (neutral pH, slightly acidic or basic, ambient temperature, etc).
6. Conclusions
Biochar materials were synthesized from agricultural waste (peach kernels or grape pits). The obtained materials were characterized by BET, SEM, FTIR, and TG-DTG. The results indicated a uniform porous surface for biochar from peach kernels while the surface of biochar from grape pits was found to be uneven. FT-IR data indicated a good functionalization of the adsorbents’ surfaces.
The adsorption equilibrium of hexavalent chromium was investigated by Langmuir, Freundlich, Sips, and Temkin adsorption isotherm models. The Sips model was best for representing the equilibrium adsorption of hexavalent chromium on both biochars. The maximum adsorption capacities were found to be 78.54 mg/g for biochar from peach kernels and 67.57 mg/g for biochar from grape pits, according to the Sips model. According to data reported in the literature, these values are in the upper range for low-cost sorbent materials used for hexavalent chromium removal.
The pseudo-second-order kinetic model was found to be the best option for interpreting the kinetics of hexavalent chromium removal by sorption on both biochars.
The reusability study indicates that the biochars can be reused for at least four adsorption–desorption cycles.
Author Contributions
Conceptualization, E.R.C., O.D.O. and C.M.; methodology, C.M. and A.M.D.; software, M.B.; validation, O.D.O., C.M. and A.M.D.; formal analysis, M.B. and O.D.O.; investigation, E.R.C., O.D.O., E.T. and G.G.; resources, C.M.; writing—original draft preparation, E.R.C., M.B. and O.D.O.; writing—review and editing, M.B. and C.M.; visualization, E.T. and G.G.; supervision, C.M.; funding acquisition, C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Palumbo, M.; Carbone, V.; Ricci, I.; Pace, B.; Cefola, M.; Minasi, P.; Garofalo, S.P.; Camposeo, S.; Tallou, A.; Vivaldi, G.A. Qualitative and biochemical characteristics of pomegranate fruit grown using reclaimed water and low input fertigation treatments at harvest and during storage. Heliyon 2024, 10, e34430. [Google Scholar] [CrossRef]
- Sifi, S.; Aydi, A.; Zaghdoudi, S.; Gasmi, M.; Abdo, H.G. Geospatial technique and multi-criteria evaluation to select suitable sites for groundwater recharge with reclaimed water in arid and semi-arid regions. Water Cycle 2025, 6, 213–228. [Google Scholar] [CrossRef]
- Dogan, S.; Azarm, S.A.S. Cost analysis and site selection for reclaimed water injection to enhance coastal aquifer sustainability. J. Water Process Eng. 2024, 68, 106551. [Google Scholar] [CrossRef]
- Recycled Water for Drinking: An Overview 2024. Available online: https://www.cdc.gov/drinking-water/about/recycled-water-for-drinking-an-overview.html (accessed on 5 May 2025).
- WHO. Environmental Health Criteria 61: Chromium. Available online: https://iris.who.int/bitstream/handle/10665/40419/9241542616-eng.pdf?sequence=1&isAllowed=y (accessed on 5 May 2025).
- Bratovcic, A.; Buksek, H.; Helix-Nielsen, C.; Petrinic, I. Concentrating hexavalent chromium electroplating wastewater for recovery and reuse by forward osmosis using underground brine as draw solution. Chem. Eng. J. 2022, 431, 133918. [Google Scholar] [CrossRef]
- Jimenez-Paz, J.; Lozada-Castro, J.J.; Lester, E.; Wiliams, O.; Stevens, L.; Barraza-Burgos, J. Solutions to hazardous wastes issues in the leather industry: Adsorption of Chromium iii and vi from leather industry wastewaters using activated carbons produced from leather industry solid wastes. J. Environ. Chem. Eng. 2023, 11, 109715. [Google Scholar] [CrossRef]
- Singha, K.; Pandit, P.; Maity, S.; Sharma, S.R. Harmful environmental effects for textile chemical dyeing practice. In Green Chemistry for Sustainable Textiles; Woodhead Publishing: Amsterdam, The Netherlands, 2021; pp. 153–164. [Google Scholar]
- Rawat, A.; Srivastava, A.; Bhatnagar, A.; Gupta, A.K. Technological advancements for the treatment of steel industry wastewater: Effluent management and sustainable treatment strategies. J. Clean. Prod. 2023, 383, 135382. [Google Scholar] [CrossRef]
- Vaiopoulou, E.; Gikas, P. Regulations for chromium emissions to the aquatic environment in Europe and elsewhere. Chemosphere 2020, 254, 126876. [Google Scholar] [CrossRef] [PubMed]
- Hackbarth, F.V.; Maass, D.; de Souza, A.A.U.; Vilar, V.J.P.; de Souza, S.M.A.G.U. Removal of hexavalent chromium from electroplating wastewaters using marine macroalga Pelvetia canaliculata as natural electron donor. Chem. Eng. J. 2016, 290, 477–489. [Google Scholar] [CrossRef]
- Santis, A.; Arbeláez, O.; Cardenas, L.A.; Castellanos, J.; Velasquez, P. Optimizing Cr(VI) Reduction in Plastic Chromium Plating Wastewater: Particle Size, Irradiation, Titanium Dose. Emerg. Sci. J. 2024, 8, 17–27. [Google Scholar] [CrossRef]
- Erkabaev, F.; Muhammadieva, D.; Rabbimkulova, S. Composition and properties of industrial wastewater and its electrochemical treatment. E3S Web Conf. 2024, 563, 01002. [Google Scholar] [CrossRef]
- Kobya, M.; Erdem, N.; Demirbas, E. Treatment of Cr, Ni and Zn from galvanic rinsing wastewater by electrocoagulation process using iron electrodes. Desalination Water Treat. 2015, 56, 1191–1201. [Google Scholar] [CrossRef]
- Rodríguez, R.; Espada, J.J.; Gallardo, M.; Molina, R.; Lopez-Munoz, M.J. Life cycle assessment and techno-economic evaluation of alternatives for the treatment of wastewater in a chrome-plating industry. J. Clean. Prod. 2018, 172, 2351–2362. [Google Scholar] [CrossRef]
- EPA. Chromium in Drinking Water. Available online: https://www.epa.gov/sdwa/chromium-drinking-water#what-are-regs (accessed on 5 May 2025).
- Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. Available online: https://eur-lex.europa.eu/eli/dir/1998/83/oj/eng (accessed on 5 May 2025).
- WHO. Chromium in Drinking-Water 2003. Available online: https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/chromium.pdf?sfvrsn=37abd598_6 (accessed on 5 May 2025).
- ECHA. Proposes Restrictions on Chromium (VI) Substances to Protect Health. Available online: https://echa.europa.eu/-/echa-proposes-restrictions-on-chromium-vi-substances-to-protect-health (accessed on 5 May 2025).
- Tumolo, M.; Ancona, V.; De Paola, D.; Losacco, D.; Campanale, C.; Massarelli, C.; Uricchio, V.F. Chromium Pollution in European Water, Sources, Health Risk, and Remediation Strategies: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 5438. [Google Scholar] [PubMed]
- Kerur, S.S.; Bandekar, S.; Hanagadakar, M.S.; Nandi, S.S.; Ratnamala, G.M.; Hegde, P.G. Removal of hexavalent Chromium-Industry treated water and Wastewater: A review. Mater. Today Proc. 2021, 42, 1112–1121. [Google Scholar] [CrossRef]
- Ndankou, C.S.D.; Ștefan, D.S.; Nsami, N.J.; Daouda, K.; Bosomoiu, M. Evaluation of Phenobarbital Adsorption Efficiency on Biosorbents or Activated Carbon Obtained from Adansonia Digitata Shells. Materials 2024, 17, 1591. [Google Scholar] [CrossRef]
- Sun, Y.; Lyu, H.; Gai, L.; Sun, P.; Shen, B.; Tang, J. Biochar-anchored low-cost natural iron-based composites for durable hexavalent chromium removal. Chem. Eng. J. 2023, 476, 146604. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, X.; Chen, N.; Cui, X.; Yu, H.; Feng, Y.; Xing, D.; He, W. Efficient removal of hexavalent chromium from wastewater using a novel sodium alginate-biochar composite adsorbent. J. Water Process Eng. 2024, 64, 105655. [Google Scholar] [CrossRef]
- Burlacu, I.F.; Deák, G.; Favier, L.; Serre, I.P.; Balloy, D. Advanced Catalytic Materials Obtained From Waste For Wastewater Treatment Applications. Univ. Polytech. Buchar. Sci. Bull. Ser. B 2021, 83, 161–166. [Google Scholar]
- Tan, G.; Wu, Y.; Liu, Y.; Xiao, D. Removal of Pb(II) ions from aqueous solution by manganese oxide coated rice straw biochar—A low-cost and highly effective sorbent. J. Environ. Chem. Eng. 2025, 13, 117662. [Google Scholar] [CrossRef]
- Yu, Y.; An, Q.; Li, Z.; Zhao, B. Synergistic removal of ammonia nitrogen and hexavalent chromium by the hybrid-system of Acinetobacter baumannii AL-6 and original walnut shell biochar in solution: Mechanism and application. J. Environ. Chem. Eng. 2024, 12, 114969. [Google Scholar] [CrossRef]
- Bian, Y.; Zhang, F.; Liu, Q.; Mo, X.; Xu, T.; Yi, W.; Xu, Y.; Bai, S.; Liu, L. Simultaneous removal capacity and selectivity of Cd(II) and Ni(II) by KMnO4 modified coconut shell and peach kernel biochars. J. Water Process Eng. 2024, 65, 105862. [Google Scholar] [CrossRef]
- Campbell, R.; Xiao, B.; Mangwandi, C. Production of activated carbon from spent coffee grounds (SCG) for removal of hexavalent chromium from synthetic wastewater solutions. J. Environ. Manag. 2024, 366, 121682. [Google Scholar] [CrossRef] [PubMed]
- Mondal, G.; Sahoo, P.; Banerjee, S.; Nandi, R.; Ghosh, C.; Mandal, J.; Bhattacharyya, P. Utilizing nano zero-valent iron impregnated biochar for removal of hexavalent chromium from water: An assessment through Box-Behnken optimization, kinetics, and isotherm studies. Groundw. Sustain. Dev. 2025, 29, 101446. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, R.; Wu, H.; Jia, X.; Liu, Y.; Zhou, G.; Chen, S.; Zhao, F.; Li, L.; Hu, S. Enhanced bioremediation of hexavalent chromium via Stenotrophomonas acidaminiphila 4–1 assisted with agricultural wastes-derived biochar. Biochem. Eng. J. 2024, 208, 109355. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhou, l.; Wang, X.; Zhang, G.; Bao, X.; Yan, Z.; Ma, W. One-step synthesis of iron-rich biochar for efficient hexavalent chromium removal: Adsorption-reduction performance, mechanism and column experiments. J. Environ. Chem. Eng. 2025, 13, 115701. [Google Scholar] [CrossRef]
- Mehrabi, N.; Soleimani, M.; Yeganeh, M.M.; Sharififard, H. Parameter optimization for nitrate removal from water using activated carbon and composite of activated carbon and Fe2O3 nanoparticles. RSC Adv. 2015, 5, 51470–51482. [Google Scholar] [CrossRef]
- Waters, C.L.; Janupala, R.R.; Mallinson, R.G.; Lobban, L.L. Staged thermal fractionation for segregation of lignin and cellulose pyrolysis products: An experimental study of residence time and temperature effects. J. Anal. Appl. Pyrolysis 2017, 126, 380–389. [Google Scholar] [CrossRef]
- Bernardino, C.A.R.; Mahler, C.F.; Veloso, M.C.C.; Romeiro, G.A. Preparation of Biochar from Sugarcane By-product Filter Mud by Slow Pyrolysis and Its Use Like Adsorbent. Waste Biomass Valorization 2017, 8, 2511–2521. [Google Scholar] [CrossRef]
- Wang, J.; Minami, E.; Asmadi, M.; Kawamoto, H. Effect of delignification on thermal degradation reactivities of hemicellulose and cellulose in wood cell walls. J. Wood Sci. 2021, 67, 19. [Google Scholar] [CrossRef]
- López-Beceiro, J.; Díaz-Díaz, A.M.; Álvarez-García, A.; Tarrío-Saavedra, J.; Naya, S.; Artiaga, R. The Complexity of Lignin Thermal Degradation in the Isothermal Context. Processes 2021, 9, 1154. [Google Scholar] [CrossRef]
- Jagnade, P.; Panwar, N.L.; Agarwal, C. Experimental Investigation of Kinetic Parameters of Bamboo and Bamboo Biochar Using Thermogravimetric Analysis Under Non-Isothermal Conditions. Bioenergy Res. 2023, 16, 1143–1155. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, B.; Budai, A.; Jeng, A.; Hao, X.; Wei, D.; Zhang, Y.; Rasse, D. Study of Biochar Properties by Scanning Electron Microscope—Energy Dispersive X-Ray Spectroscopy (SEM-EDX). Commun. Soil Sci. Plant Anal. 2016, 47, 593–601. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Dudło, A.; Michalska, J.; Turek-Szytow, J.; Kobyłecki, R.; Zarzycki, R.; Wichlinski, M.; Surmacz-Gorska, J. Humic substances sorption from wastewater on the biochar produced from the waste materials. J. Environ. Manag. 2024, 370, 122366. [Google Scholar] [CrossRef]
- Kasera, N.; Augoustides, V.; Kolar, P.; Hall, S.G.; Vicente, B. Effect of Surface Modification by Oxygen-Enriched Chemicals on the Surface Properties of Pine Bark Biochars. Processes 2022, 10, 2136. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Saleh, T.A. Isotherm models of adsorption processes on adsorbents and nanoadsorbents. Interface Sci. Technol. 2022, 34, 99–126. [Google Scholar]
- Zhou, X.; Zhou, X. The unit problem in the thermodynamic calculation of adsorption using the Langmuir equation. Chem. Eng. Commun. 2014, 201, 1459–1467. [Google Scholar] [CrossRef]
- Owlad, M.; Aroua, M.K.; Daud, W.A.W. Hexavalent chromium adsorption on impregnated palm shell activated carbon with polyethyleneimine. Bioresour. Technol. 2010, 101, 5098–5103. [Google Scholar] [CrossRef] [PubMed]
- Anandkumar, J.; Mandal, B. Removal of Cr(VI) from aqueous solution using Bael fruit (Aegle marmelos correa) shell as an adsorbent. J. Hazard. Mater. 2009, 168, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.K. Hexavalent chromium [Cr(VI)] removal by acid modified waste activated carbons. J. Hazard. Mater. 2009, 171, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Yang, J.; Liang, S.; Li, M.; Gan, Q.; Xiao, K.; Hu, J. Enhanced Cr(VI) removal from acidic solutions using biochar modified by Fe3O4@SiO2-NH2 particles. Sci. Total Environ. 2018, 628–629, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fang, X.; Yuan, W.; Zhang, X.; Yu, J.; Chen, J.; Qiu, X. Preparing of layered double hydroxide-alginate microspheres for Cr(VI)-contaminated soil remediation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130655. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).