Electrochemical Reduction of CO2 to C2 Hydrocarbons Using Cu 3D Nanostructures
Highlights
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrode Preparation
2.2. Electrochemical Measurements
2.3. Morphological Characterisation Using Scanning Electron Microscopy (SEM)
2.4. CO2 Reduction Gaseous Product Analysis
3. Results and Discussion
3.1. Cu Foam Microstructural Characterization
3.2. Activity and Selectivity of Electrocatalytic CO2 Reduction on Cu Foam Electrodes
3.3. The Structure–Properties Relationship
3.3.1. Crystal Facet Effect
3.3.2. Grain Boundaries and Defects
3.3.3. Effect of Crystallite Dimensions and Geometry on C2 Yields
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BE | Base Electrode |
| CO2ER | Carbon Dioxide Electrochemical Reduction |
| CV | Cyclic Voltammetry |
| FE | Faradaic Efficiency |
| GC | Gas Chromatography |
| HER | Hydrogen Evolution Reaction |
| SEM | Scanning Electron Microscopy |
| SHE | Standard Hydrogen Electrode |
| UPD | Underpotential Deposition |
| XRD | X-ray Diffraction |
References
- Hussain, N.; Abdelkareem, M.A.; Alawadhi, H.; Olabi, A.-G. Electrochemical Reduction of CO2 on Cu-Based Heterogeneous Catalysts. In Encyclopedia of Smart Materials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 807–815. [Google Scholar]
- Li, X.; Chen, Y.; Zhan, X.; Xu, Y.; Hao, L.; Xu, L.; Li, X.; Umer, M.; Tan, X.; Han, B.; et al. Strategies for Enhancing Electrochemical CO2 Reduction to Multi-Carbon Fuels on Copper. Innov. Mater. 2023, 1, 100014. [Google Scholar] [CrossRef]
- Chico-Mesa, L.; Herrero, E.; Arán-Ais, R.M. Tuning Carbon Dioxide Electroreduction through Selective Facet Exposure. Curr. Opin. Chem. Eng. 2024, 43, 100997. [Google Scholar] [CrossRef]
- Girichandran, N.; Saedy, S.; Kortlever, R. Electrochemical CO2 Reduction on a Copper Foam Electrode at Elevated Pressures. Chem. Eng. J. 2024, 487, 150478. [Google Scholar] [CrossRef]
- Tabassum, H.; Yang, X.; Zou, R.; Wu, G. Surface Engineering of Cu Catalysts for Electrochemical Reduction of CO2 to Value-Added Multi-Carbon Products. Chem Catal. 2022, 2, 1561–1593. [Google Scholar] [CrossRef]
- Chen, Y.; Miao, R.K.; Yu, C.; Sinton, D.; Xie, K.; Sargent, E.H. Catalyst Design for Electrochemical CO2 Reduction to Ethylene. Matter 2024, 7, 25–37. [Google Scholar] [CrossRef]
- Ye, W.; Guo, X.; Ma, T. A Review on Electrochemical Synthesized Copper-Based Catalysts for Electrochemical Reduction of CO2 to C2+ Products. Chem. Eng. J. 2021, 414, 128825. [Google Scholar] [CrossRef]
- Maniam, K.K.; Maniam, M.; Diaz, L.A.; Kukreja, H.K.; Papadopoulos, A.I.; Kumar, V.; Seferlis, P.; Paul, S. Progress in Electrodeposited Copper Catalysts for CO2 Conversion to Valuable Products. Processes 2023, 11, 1148. [Google Scholar] [CrossRef]
- Ooka, H.; Figueiredo, M.C.; Koper, M.T.M. Competition between Hydrogen Evolution and Carbon Dioxide Reduction on Copper Electrodes in Mildly Acidic Media. Langmuir 2017, 33, 9307–9313. [Google Scholar] [CrossRef]
- Rashid, N.; Bhat, M.A.; Ingole, P.P. Dendritic Copper Microstructured Electrodeposits for Efficient and Selective Electrochemical Reduction of Carbon Dioxide into C1 and C2 Hydrocarbons. J. CO2 Util. 2020, 38, 385–397. [Google Scholar] [CrossRef]
- Xie, M.S.; Xia, B.Y.; Li, Y.; Yan, Y.; Yang, Y.; Sun, Q.; Chan, S.H.; Fisher, A.; Wang, X. Amino Acid Modified Copper Electrodes for the Enhanced Selective Electroreduction of Carbon Dioxide towards Hydrocarbons. Energy Environ. Sci. 2016, 9, 1687–1695. [Google Scholar] [CrossRef]
- Li, M.; Hu, Y.; Wu, T.; Sumboja, A.; Geng, D. How to Enhance the C2 Products Selectivity of Copper-Based Catalysts towards Electrochemical CO2 Reduction?—A Review. Mater. Today 2023, 67, 320–343. [Google Scholar] [CrossRef]
- Ni, Z.; Liang, H.; Yi, Z.; Guo, R.; Liu, C.; Liu, Y.; Sun, H.; Liu, X. Research Progress of Electrochemical CO2 Reduction for Copper-Based Catalysts to Multicarbon Products. Coord. Chem. Rev. 2021, 441, 213983. [Google Scholar] [CrossRef]
- Li, G.; Liu, H.; Yang, H.; Chen, X.; Ji, K.; Yang, D.; Zhang, S.; Ma, X. Tuning Product Distributions of CO2 Electroreduction over Copper Foil through Cathodic Corrosion. Chem. Eng. Sci. 2022, 263, 118142. [Google Scholar] [CrossRef]
- Gonçalves, M.R.; Gomes, A.; Condeço, J.; Fernandes, T.R.C.; Pardal, T.; Sequeira, C.A.C.; Branco, J.B. Electrochemical Conversion of CO2 to C2 Hydrocarbons Using Different Ex Situ Copper Electrodeposits. Electrochim. Acta 2013, 102, 388–392. [Google Scholar] [CrossRef]
- Reller, C.; Krause, R.; Volkova, E.; Schmid, B.; Neubauer, S.; Rucki, A.; Schuster, M.; Schmid, G. Selective Electroreduction of CO2 toward Ethylene on Nano Dendritic Copper Catalysts at High Current Density. Adv. Energy Mater. 2017, 7, 1602114. [Google Scholar] [CrossRef]
- Ou, L.; Chen, Y.; Jin, J. The Origin of CO2 Electroreduction into C1 and C2 Species: Mechanistic Understanding on the Product Selectivity of Cu Single-Crystal Faces. Chem. Phys. Lett. 2018, 710, 175–179. [Google Scholar] [CrossRef]
- Gao, D.; Sinev, I.; Scholten, F.; Arán-Ais, R.M.; Divins, N.J.; Kvashnina, K.; Timoshenko, J.; Cuenya, B.R. Selective CO2 Electroreduction to Ethylene and Multicarbon Alcohols via Electrolyte-Driven Nanostructuring. Angew. Chem. 2019, 131, 17203–17209. (In German) [Google Scholar] [CrossRef]
- Dutta, A.; Rahaman, M.; Luedi, N.C.; Mohos, M.; Broekmann, P. Morphology Matters: Tuning the Product Distribution of CO2 Electroreduction on Oxide-Derived Cu Foam Catalysts. ACS Catal. 2016, 6, 3804–3814. [Google Scholar] [CrossRef]
- Christensen, O.; Zhao, S.; Sun, Z.; Bagger, A.; Lauritsen, J.V.; Pedersen, S.U.; Daasbjerg, K.; Rossmeisl, J. Can the CO2 Reduction Reaction Be Improved on Cu: Selectivity and Intrinsic Activity of Functionalized Cu Surfaces. ACS Catal. 2022, 12, 15737–15749. [Google Scholar] [CrossRef]
- Khan, R.R.M.; Saleem, R.; Batool, S.S.; Rasheed, S.; Saeed, Z.; Pervaiz, M.; Younas, U.; Summer, M.; Liaqat, M. Electrochemical Reduction of CO2 to Liquid Products: Factors Influencing Production and Selectivity. Int. J. Hydrogen Energy 2025, 128, 800–832. [Google Scholar] [CrossRef]
- Nam, S.; Jo, H.; Choe, H.; Ahn, D.; Choi, H. Development of Nanoporous Copper Foams by Chemical Dealloying of Mechanically Alloyed Al–Cu Compounds. Mater. Trans. 2014, 55, 1414–1418. [Google Scholar] [CrossRef]
- Klingan, K.; Kottakkat, T.; Jovanov, Z.P.; Jiang, S.; Pasquini, C.; Scholten, F.; Kubella, P.; Bergmann, A.; Cuenya, B.R.; Roth, C.; et al. Reactivity Determinants in Electrodeposited Cu Foams for Electrochemical CO2 Reduction. ChemSusChem 2018, 11, 3449–3459. [Google Scholar] [CrossRef]
- Wu, Q.; Barkey, D. Faceting and Roughening Transitions on Copper Single Crystals in Acid Sulfate Plating Baths with Chloride. J. Electrochem. Soc. 2000, 147, 1038–1045. [Google Scholar] [CrossRef]
- Sandberg, R.B.; Montoya, J.H.; Chan, K.; Nørskov, J.K. CO-CO Coupling on Cu Facets: Coverage, Strain and Field Effects. Surf. Sci. 2016, 654, 56–62. [Google Scholar] [CrossRef]
- Bagger, A.; Ju, W.; Varela, A.S.; Strasser, P.; Rossmeisl, J. Electrochemical CO2 Reduction: Classifying Cu Facets. ACS Catal. 2019, 9, 7894–7899. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, Z.J.; Cheng, D.; Li, H.; Yu, J.; Wang, Q.; Gao, H.; Guo, J.; Wang, H.; Ozin, G.A.; et al. Efficient CO2 Electroreduction on Facet-Selective Copper Films with High Conversion Rate. Nat. Commun. 2021, 12, 5745. [Google Scholar] [CrossRef]
- Zhang, H.; He, C.; Han, S.; Du, Z.; Wang, L.; Yun, Q.; Cao, W.; Zhang, B.; Tian, Y.-H.; Lu, Q. Crystal Facet-Dependent Electrocatalytic Performance of Metallic Cu in CO2 Reduction Reactions. Chin. Chem. Lett. 2022, 33, 3641–3649. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, J.; Shi, D.; Zhang, W.; Tang, Y.; Gao, Q. Chlorine-Mediated Electrodeposition of Hierarchical and Hydrophobic Copper Electrocatalysts for Efficient CO2 Electroreduction to Ethylene. Chem. Commun. 2023, 59, 10428–10431. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.M.; Kwon, Y.; Won, J.H.; Lum, Y.; Cheng, M.J.; Kim, K.H.; Head-Gordon, M.; Kang, J.K. Atomic-Scale Spacing between Copper Facets for the Electrochemical Reduction of Carbon Dioxide. Adv. Energy Mater. 2020, 10, 1903423. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, G.; Zhang, Z.; Jin, M.; Yin, Y. Selectivity on Etching: Creation of High-Energy Facets on Copper Nanocrystals for CO2 Electrochemical Reduction. ACS Nano 2016, 10, 4559–4564. [Google Scholar] [CrossRef]
- Serafini, M.; Mariani, F.; Fasolini, A.; Scavetta, E.; Basile, F.; Tonelli, D. Nanostructured Copper-Based Electrodes Electrochemically Synthesized on a Carbonaceous Gas Diffusion Membrane with Catalytic Activity for the Electroreduction of CO2. ACS Appl. Mater. Interfaces 2021, 13, 57451–57461. [Google Scholar] [CrossRef]
- Lopez, M.B.; Ustarroz, J. Electrodeposition of Nanostructured Catalysts for Electrochemical Energy Conversion: Current Trends and Innovative Strategies. Curr. Opin. Electrochem. 2021, 27, 100688. [Google Scholar] [CrossRef]
- Shin, H.C.; Dong, J.; Liu, M. Nanoporous Structures Prepared by an Electrochemical Deposition Process. Adv. Mater. 2003, 15, 1610–1614. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Popov, K.I.; Pavlović, L.J.; Pavlović, M.G. Morphologies of Copper Deposits Obtained by the Electrodeposition at High Overpotentials. Surf. Coat. Technol. 2006, 201, 560–566. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, R.-H.; Kwon, H.-S. Preparation of Copper Foam with 3-Dimensionally Interconnected Spherical Pore Network by Electrodeposition. Electrochem. Commun. 2008, 10, 1148–1151. [Google Scholar] [CrossRef]
- Sen, S.; Liu, D.; Palmore, G.T.R. Electrochemical Reduction of CO2 at Copper Nanofoams. ACS Catal. 2014, 4, 3091–3095. [Google Scholar] [CrossRef]
- Qiao, Y.; Seger, B. Recent Advances in Single Crystal and Facet Dependency of Copper Electrodes on Electrochemical CO2 Reduction. Curr. Opin. Chem. Eng. 2024, 43, 100999. [Google Scholar] [CrossRef]
- Serapinienė, B.; Gudavičiūtė, L.; Tutlienė, S.; Grigucevičienė, A.; Selskis, A.; Juodkazytė, J.; Ramanauskas, R. On the Electrochemically Active Surface Area Determination of Electrodeposited Porous Cu 3D Nanostructures. Coatings 2023, 13, 1335. [Google Scholar] [CrossRef]
- Serapinienė, B.; Staišiūnas, L.; Selskis, A.; Juškėnas, R.; Gudavičiūtė, L.; Juodkazytė, J.; Ramanauskas, R. Electrodeposition of Cu 3D Structures Suitable for CO2 Reduction. Chemija 2024, 35, 25–34. [Google Scholar] [CrossRef]
- Zhu, P.; Zhao, Y. Effects of Electrochemical Reaction and Surface Morphology on Electroactive Surface Area of Porous Copper Manufactured by Lost Carbonate Sintering. RSC Adv. 2017, 7, 26392–26400. [Google Scholar] [CrossRef]
- Couce, P.M.; Madsen, T.K.; Plaza-Mayoral, E.; Kristoffersen, H.H.; Chorkendorff, I.; Dalby, K.N.; van der Stam, W.; Rossmeisl, J.; Escudero-Escribano, M.; Sebastián-Pascual, P. Tailoring the Facet Distribution on Copper with Chloride. Chem. Sci. 2024, 15, 1714–1725. [Google Scholar] [CrossRef]
- Sebastián-Pascual, P.; Escudero-Escribano, M. Surface Characterization of Copper Electrocatalysts by Lead Underpotential Deposition. J. Electroanal. Chem. 2021, 896, 115446. [Google Scholar] [CrossRef]
- Giri, S.D.; Sarkar, A. Estimating Surface Area of Copper Powder: A Comparison between Electrochemical, Microscopy and Laser Diffraction Methods. Adv. Powder Technol. 2018, 29, 3520–3526. [Google Scholar] [CrossRef]
- Chen, C.S.; Handoko, A.D.; Wan, J.H.; Ma, L.; Ren, D.; Yeo, B.S. Stable and Selective Electrochemical Reduction of Carbon Dioxide to Ethylene on Copper Mesocrystals. Catal. Sci. Technol. 2015, 5, 161–168. [Google Scholar] [CrossRef]
- Zeng, J.; Bejtka, K.; Ju, W.; Castellino, M.; Chiodoni, A.; Sacco, A.; Farkhondehfal, M.A.; Hernández, S.; Rentsch, D.; Battaglia, C.; et al. Advanced Cu-Sn Foam for Selectively Converting CO2 to CO in Aqueous Solution. Appl. Catal. B Environ. 2018, 236, 475–482. [Google Scholar] [CrossRef]
- Wu, B.; Chen, J.; Qian, L. Recent Advances in Heterogeneous Electroreduction of CO2 on Copper-Based Catalysts. Catalysts 2022, 12, 860. [Google Scholar] [CrossRef]
- Zarandi, R.F.; Rezaei, B.; Ghaziaskar, H.S.; Ensafi, A.A. Electrochemical Reduction of CO2 to Ethanol Using Copper Nanofoam Electrode and 1-Butyl-3-Methyl-Imidazolium Bromide as the Homogeneous Co-Catalyst. J. Environ. Chem. Eng. 2019, 7, 103141. [Google Scholar] [CrossRef]
- Levin, E.E.; Morozov, D.A.; Frolov, V.V.; Arkharova, N.A.; Khmelenin, D.N.; Antipov, E.V.; Nikitina, V.A. Roughness Factors of Electrodeposited Nanostructured Copper Foams. Nanomaterials 2023, 13, 3011. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Ye, K.; Gao, Y.; Long, Z.; Cheng, K.; Zhang, W.; Wang, G.; Cao, D. Preparation of Three-Dimensional Porous Cu Film Supported on Cu Foam and Its Electrocatalytic Performance for Hydrazine Electrooxidation in Alkaline Medium. Mater. Sci. Eng. B 2016, 210, 51–56. [Google Scholar] [CrossRef]
- Creus, A.H.; Souto, R.M.; Gonz~lez, S.; Laz, M.M.; Salvarezza, R.C.; Arvia, A.J. The Electrochemical Faceting of Copper in 85% Aqueous O-Phosphoric Acid by Using a Potential Reversal Technique. Appl. Surf. Sci. 1994, 81, 387–398. [Google Scholar] [CrossRef]
- Durand, W.J.; Peterson, A.A.; Studt, F.; Abild-Pedersen, F.; Nørskov, J.K. Structure Effects on the Energetics of the Electrochemical Reduction of CO2 by Copper Surfaces. Surf. Sci. 2011, 605, 1354–1359. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, T.; Liu, B.; Cheng, D.; Hu, C.; Zhang, G.; Zhu, W.; Wang, H.; Zhao, Z.J.; Gong, J. Grain-Boundary-Rich Copper for Efficient Solar-Driven Electrochemical CO2 Reduction to Ethylene and Ethanol. J. Am. Chem. Soc. 2020, 142, 6878–6883. [Google Scholar] [CrossRef] [PubMed]
- Ron, J.; Robert, L.S. Introduction to X-Ray Powder Diffractometry; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Warren, B.E. X-Ray Diffraction; Dover Publications: New York, NY, USA, 1990. [Google Scholar]
- Gao, D.; Arán-Ais, R.M.; Jeon, H.S.; Cuenya, B.R. Rational Catalyst and Electrolyte Design for CO2 Electroreduction towards Multicarbon Products. Nat. Catal. 2019, 2, 198–210. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef] [PubMed]

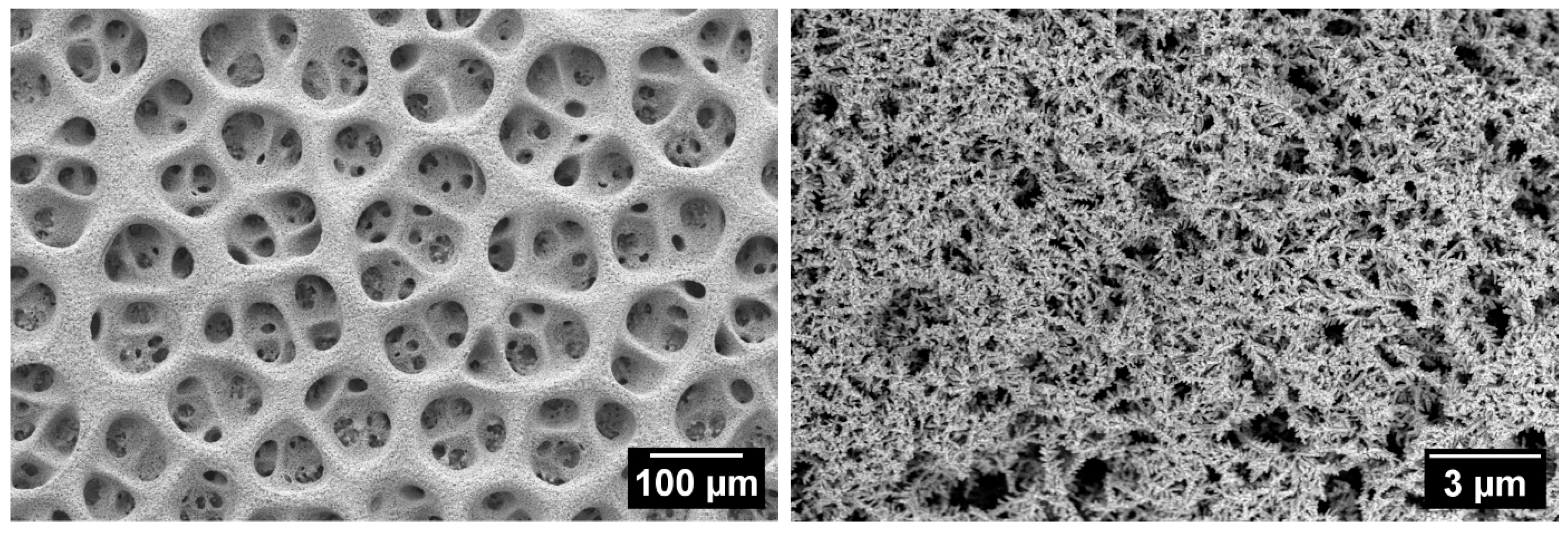
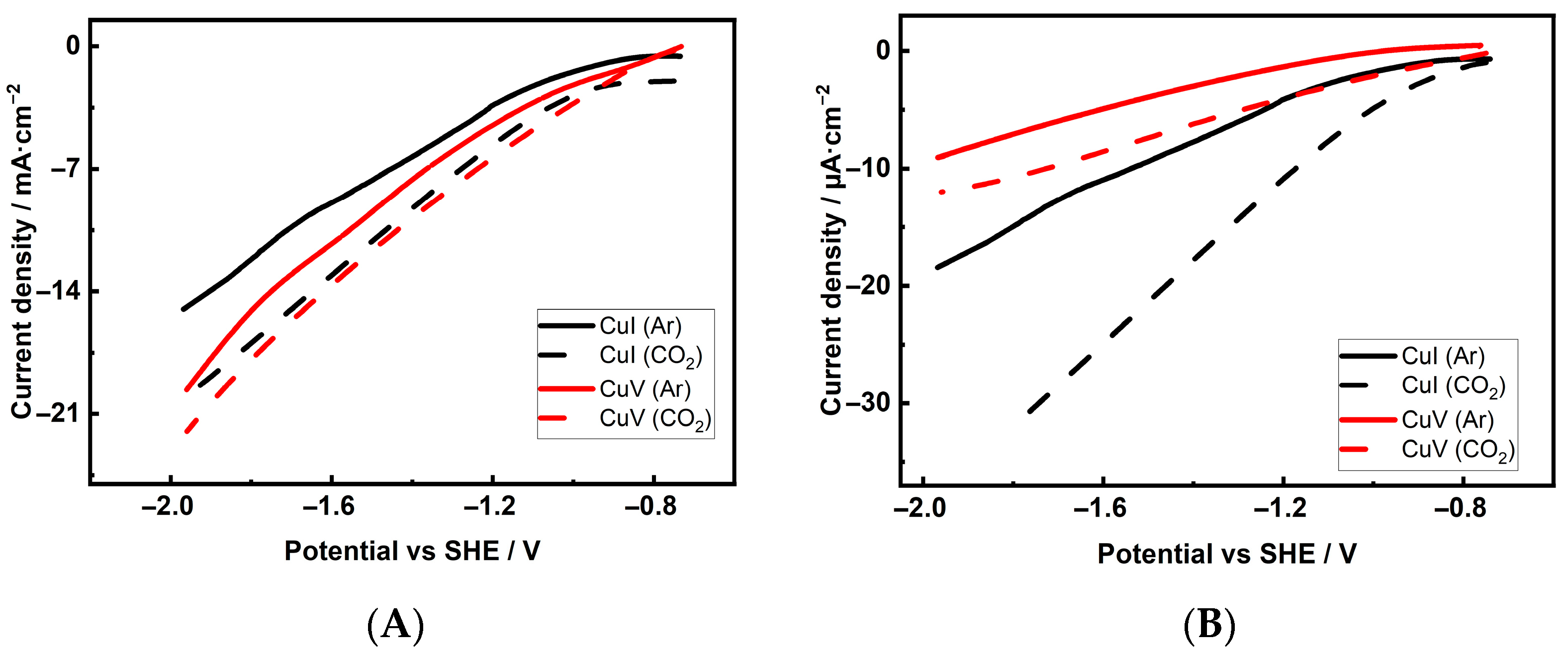
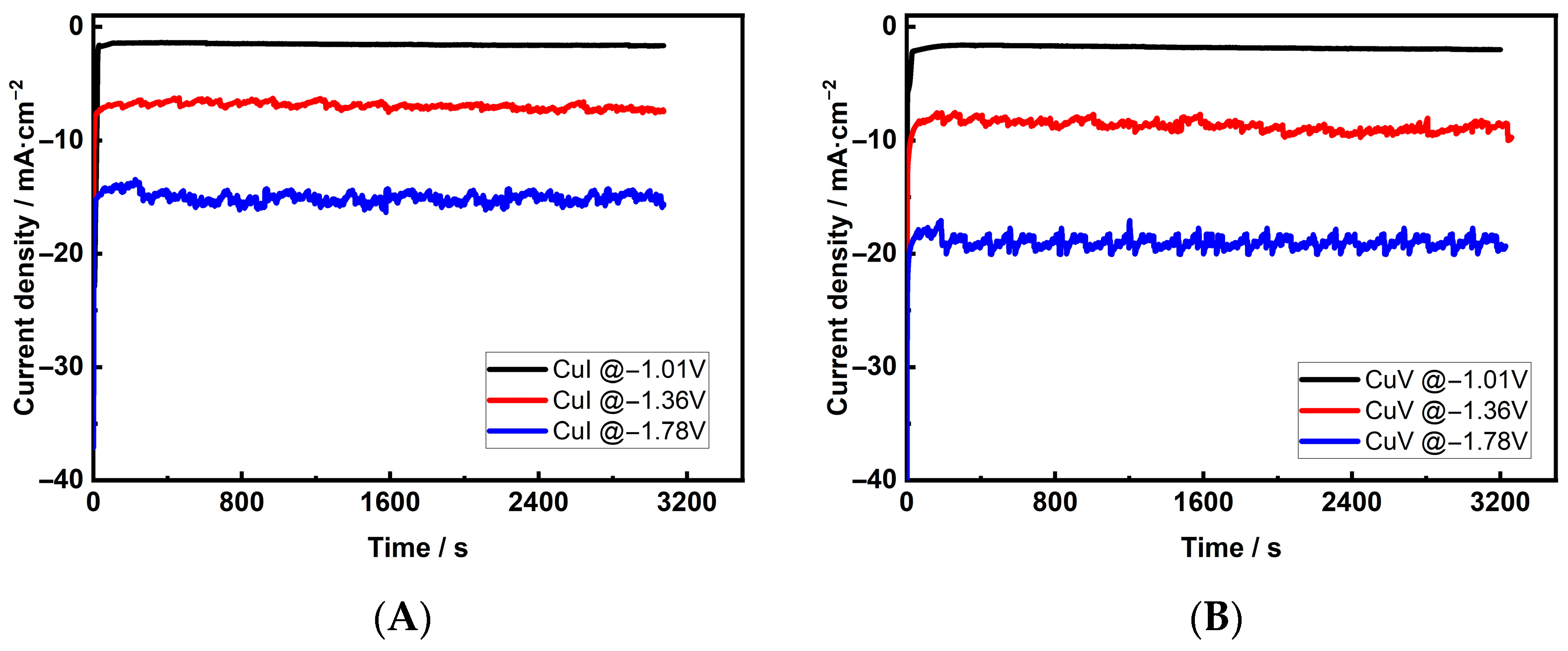
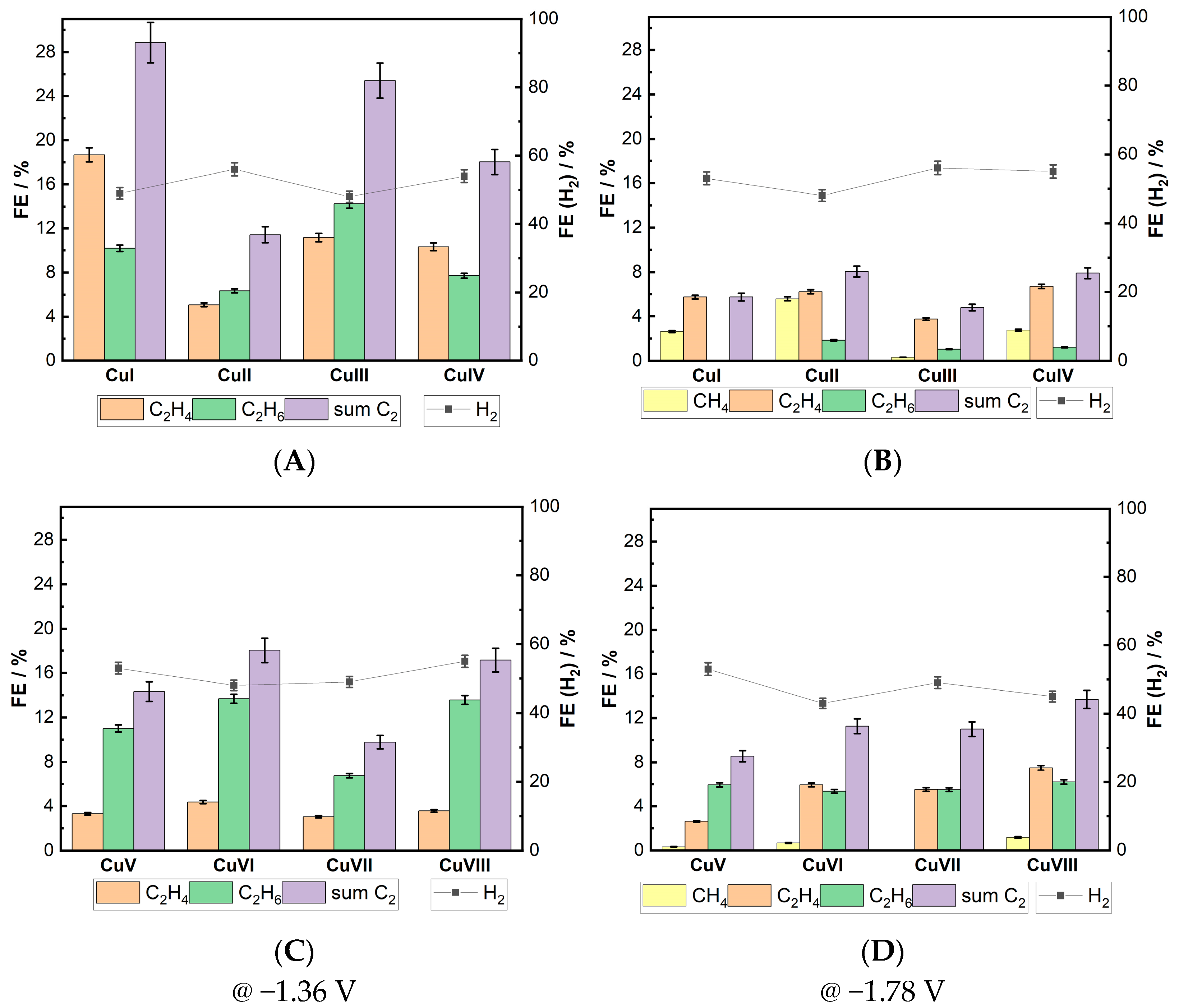
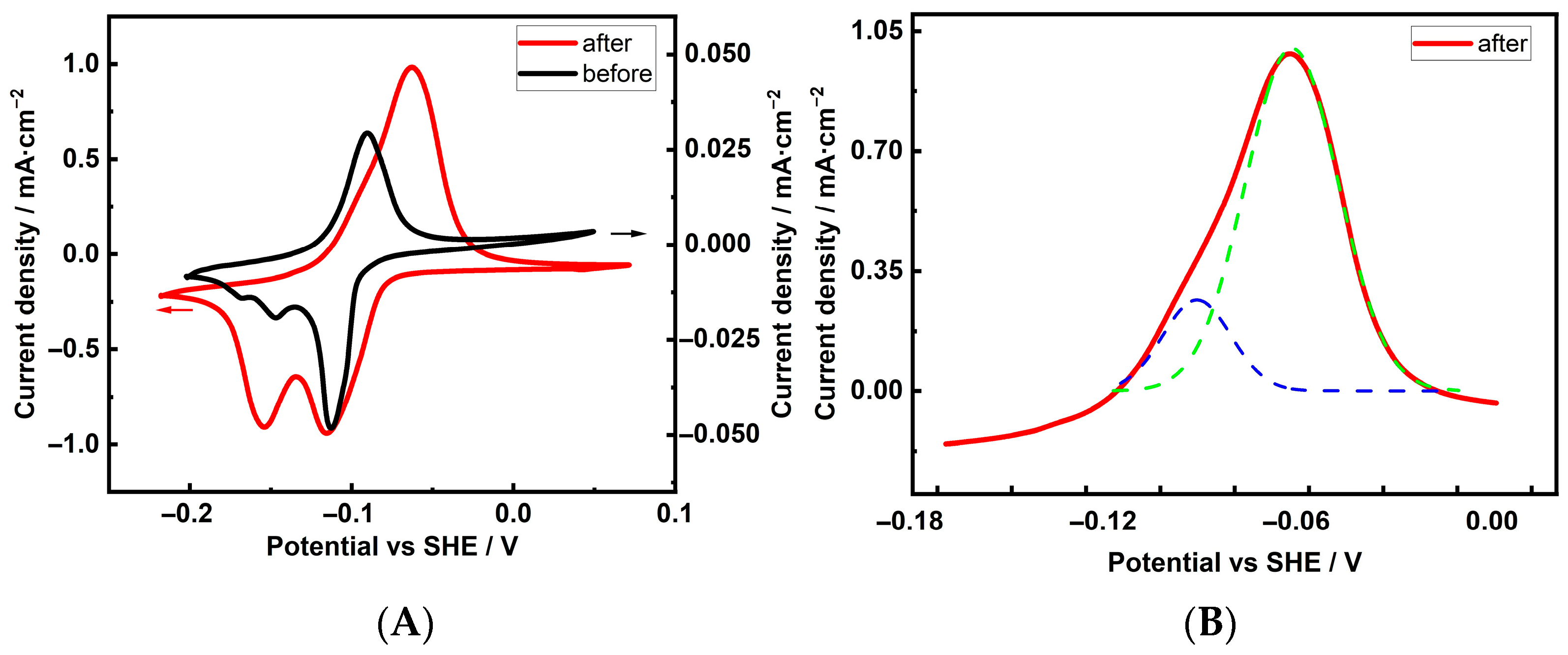
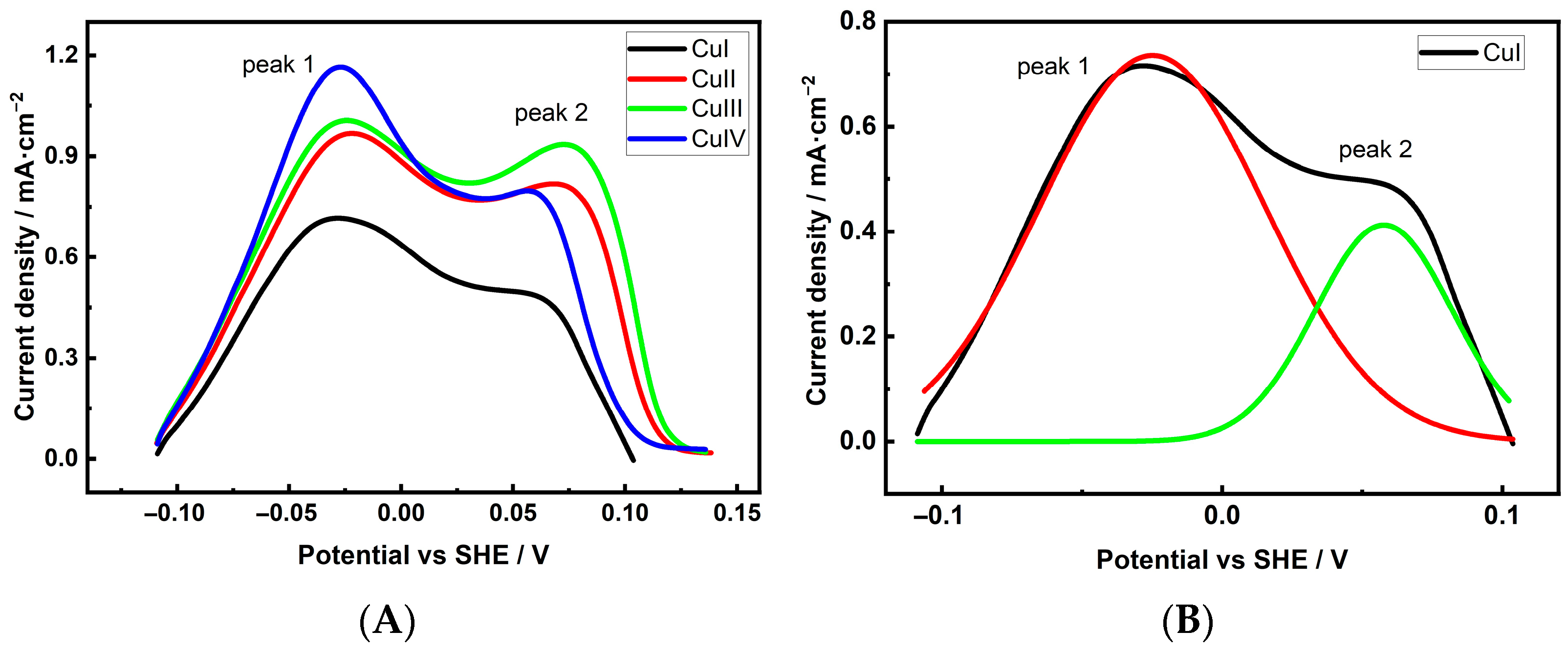
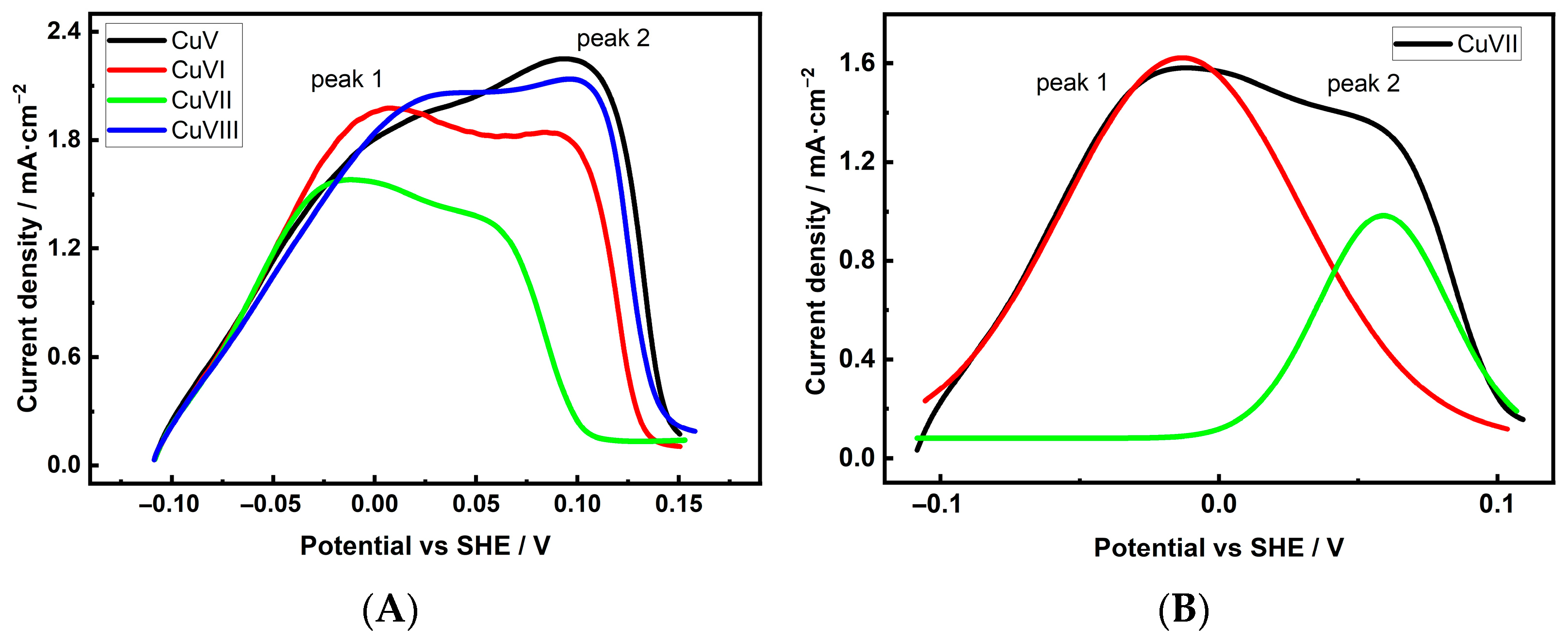
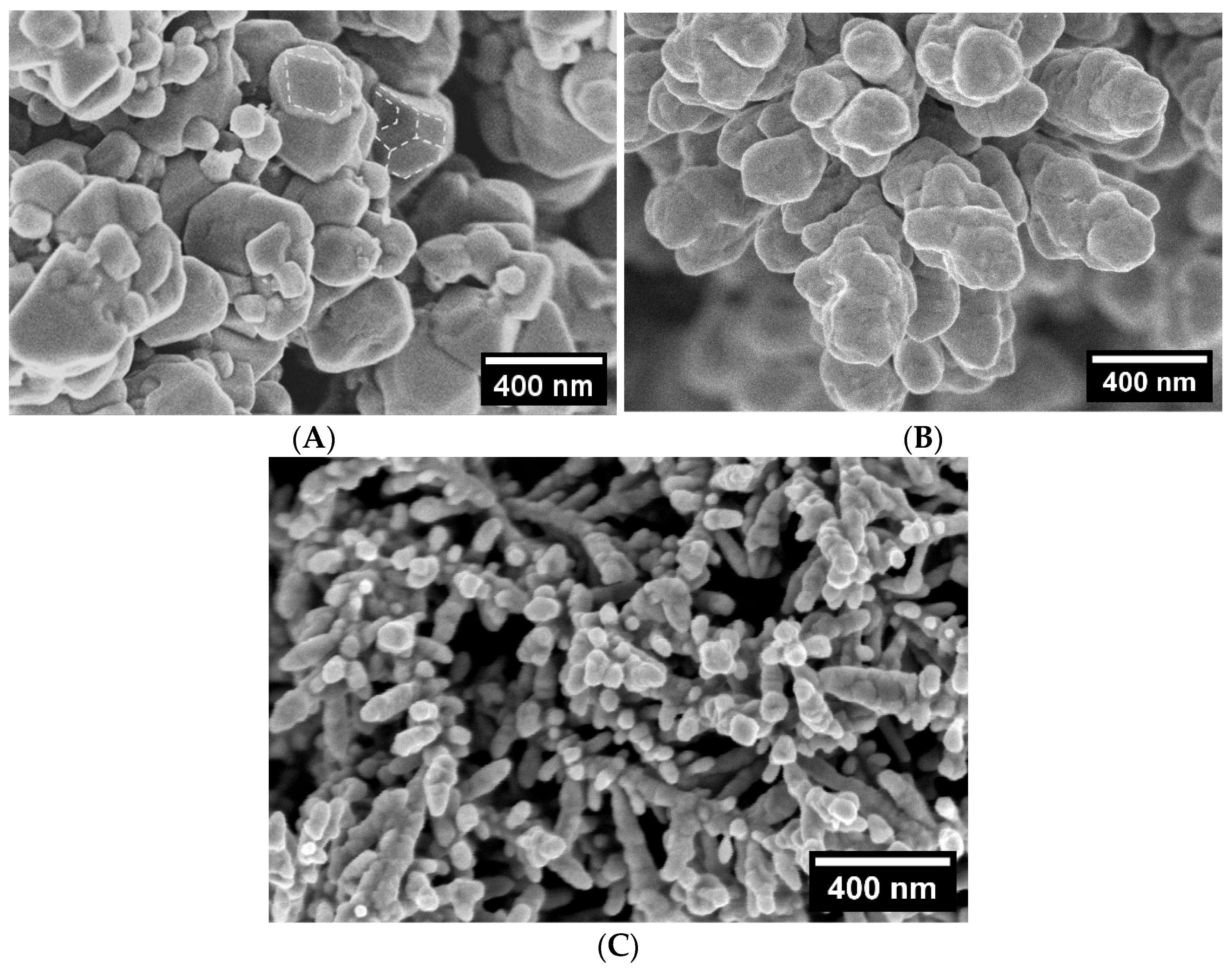
| Cu 3D Electrode | Composition of the Deposition Electrolyte | ||
|---|---|---|---|
| H2SO4, M | CuSO4, M | HCl, M | |
| CuI | 1.5 | 0.2 | - |
| CuII | 0.8 | 0.2 | |
| CuIII | 1.5 | 0.4 | |
| CuIV | 0.8 | 0.4 | |
| CuV | 1.5 | 0.2 | 0.05 |
| CuVI | 0.8 | 0.2 | |
| CuVII | 1.5 | 0.4 | |
| CuVIII | 0.8 | 0.4 | |
| Electrode | Average Pore Size, µm | Average Pore Density, cm−2 | |
|---|---|---|---|
| CuI | 25.3 ± 1.26 | 4.0 × 105 ± 1.4 × 104 | 814 ± 29 |
| CuII | 45.3 ± 1.81 | 1.0 × 104 ± 4.2 × 103 | 1035 ± 83 |
| CuIII | 40.0 ± 1.83 | 1.0 × 104 ± 4.1 × 103 | 817 ± 65 |
| CuIV | 45.0 ± 2.16 | 1.1 × 104 ± 3.8 × 103 | 911 ± 74 |
| CuV | 65.4 ± 2.88 | 1.7 × 104 ± 4.0 × 103 | 2512 ± 247 |
| CuVI | 73.0 ± 3.21 | 1.2 × 104 ± 7.2 × 103 | 1614 ± 154 |
| CuVII | 79.4 ± 3.73 | 1.7 × 104 ± 4.9 × 103 | 1786 ± 168 |
| CuVIII | 64.0 ± 2.98 | 1.6 × 104 ± 4.2 × 103 | 2444 ± 219 |
| E, V | jg/jSr | CuI | CuII | CuIII | CuIV | CuV | CuVI | CuVII | CuVIII |
|---|---|---|---|---|---|---|---|---|---|
| −1.01 | jg (Sg), mA·cm−2 | −2.16 | −2.00 | −2.05 | −2.43 | −3.29 | −3.52 | −4.87 | −3.22 |
| jSr (Sr), µA·cm−2 | −4.21 | −3.14 | −3.88 | −4.23 | −2.08 | −3.46 | −4.33 | −2.09 | |
| −1.36 | jg (Sg), mA·cm−2 | −8.38 | −7.19 | −7.51 | −7.86 | −8.86 | −10.62 | −13.02 | −10.51 |
| jSr (Sr), µA·cm−2 | −16.34 | −11.03 | −14.58 | −13.69 | −5.60 | −10.44 | −11.57 | −6.82 | |
| −1.78 | jg (Sg), mA·cm−2 | −16.57 | −15.27 | −15.29 | −15.81 | −17.10 | −20.84 | −24.71 | −18.11 |
| jSr (Sr), µA·cm−2 | −32.30 | −23.45 | −29.65 | −27.54 | −10.81 | −20.49 | −21.96 | −11.76 |
| Electrode | Integrated Peak 1 Area | Integrated Peak 2 Area | Peak1: Peak2 |
|---|---|---|---|
| @ −0.02 V | @ 0.06 V | ||
| CuI | 78.55 ± 3.14 | 21.35 ± 0.90 | 3.7: 1 |
| CuII | 71.84 ± 3.23 | 28.16 ± 1.30 | 2.6: 1 |
| CuIII | 69.00 ± 2.70 | 31.00 ± 1.21 | 2.2: 1 |
| CuIV | 72.45 ± 3.04 | 27.55 ± 1.16 | 2.6: 1 |
| @ 0.1V | @ 0.02 V | ||
| CuV | 82.17 ± 3.78 | 17.83 ± 0.82 | 4.6: 1 |
| CuVI | 82.78 ± 3.48 | 17.22 ± 0.73 | 4.8: 1 |
| CuVII | 75.31 ± 3.31 | 24.69 ± 1.09 | 3.0: 1 |
| CuVIII | 86.00 ± 3.87 | 14.00 ± 0.63 | 6.1: 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serapinienė, B.; Naujalis, E.; Selskis, A.; Juodkazytė, J.; Ramanauskas, R. Electrochemical Reduction of CO2 to C2 Hydrocarbons Using Cu 3D Nanostructures. Materials 2025, 18, 4210. https://doi.org/10.3390/ma18174210
Serapinienė B, Naujalis E, Selskis A, Juodkazytė J, Ramanauskas R. Electrochemical Reduction of CO2 to C2 Hydrocarbons Using Cu 3D Nanostructures. Materials. 2025; 18(17):4210. https://doi.org/10.3390/ma18174210
Chicago/Turabian StyleSerapinienė, Birutė, Evaldas Naujalis, Algirdas Selskis, Jurga Juodkazytė, and Rimantas Ramanauskas. 2025. "Electrochemical Reduction of CO2 to C2 Hydrocarbons Using Cu 3D Nanostructures" Materials 18, no. 17: 4210. https://doi.org/10.3390/ma18174210
APA StyleSerapinienė, B., Naujalis, E., Selskis, A., Juodkazytė, J., & Ramanauskas, R. (2025). Electrochemical Reduction of CO2 to C2 Hydrocarbons Using Cu 3D Nanostructures. Materials, 18(17), 4210. https://doi.org/10.3390/ma18174210







