Experimental and Simulation Investigation of Octadecyltriethoxysilane-Decorated Diatomaceous Earth Coatings with Enhanced Superhydrophobic and Self-Cleaning Properties
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
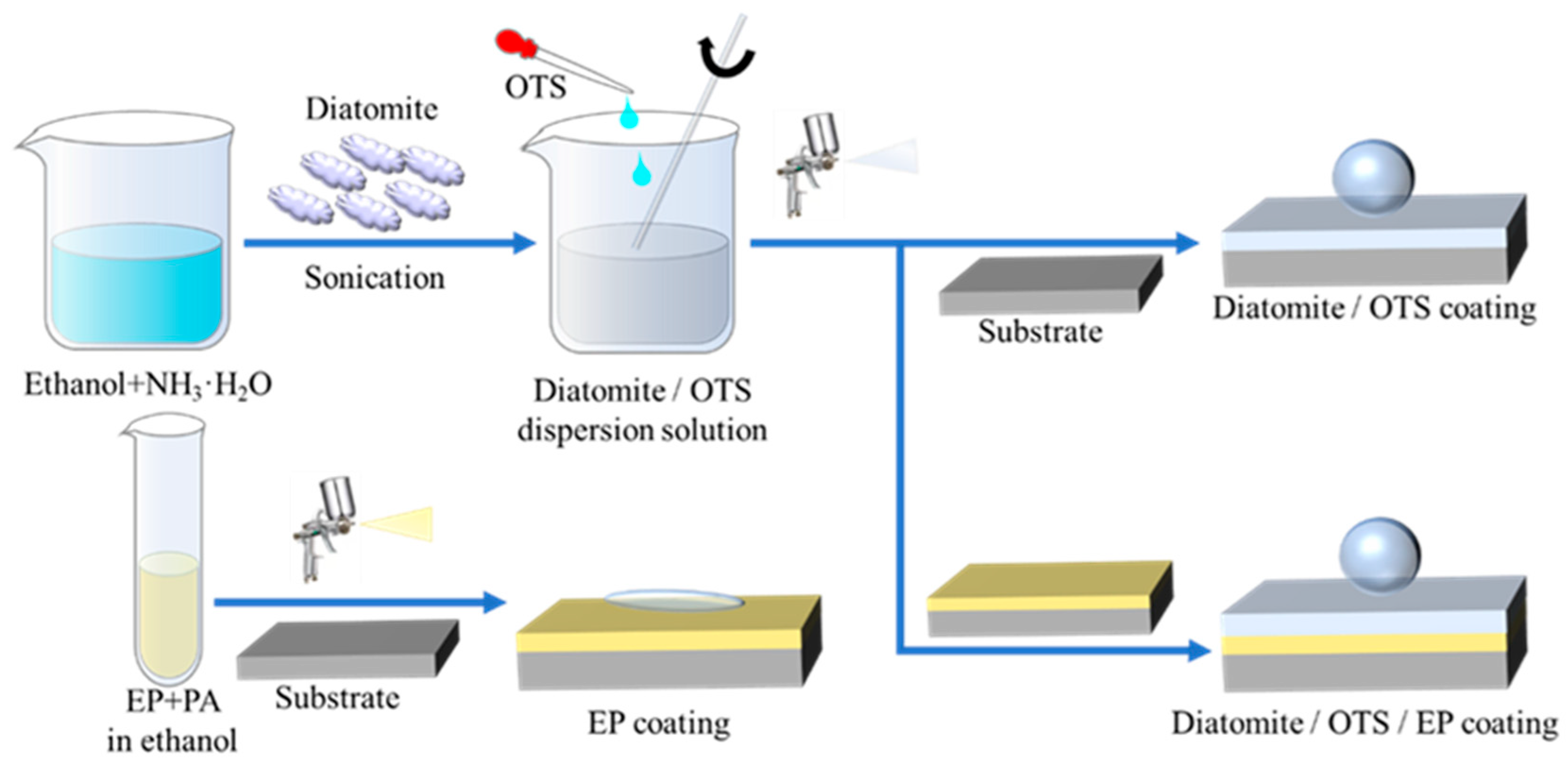
2.2. Synthesis of Dia/OTS/EP Coatings
2.3. Characterisation
3. Results and Discussion
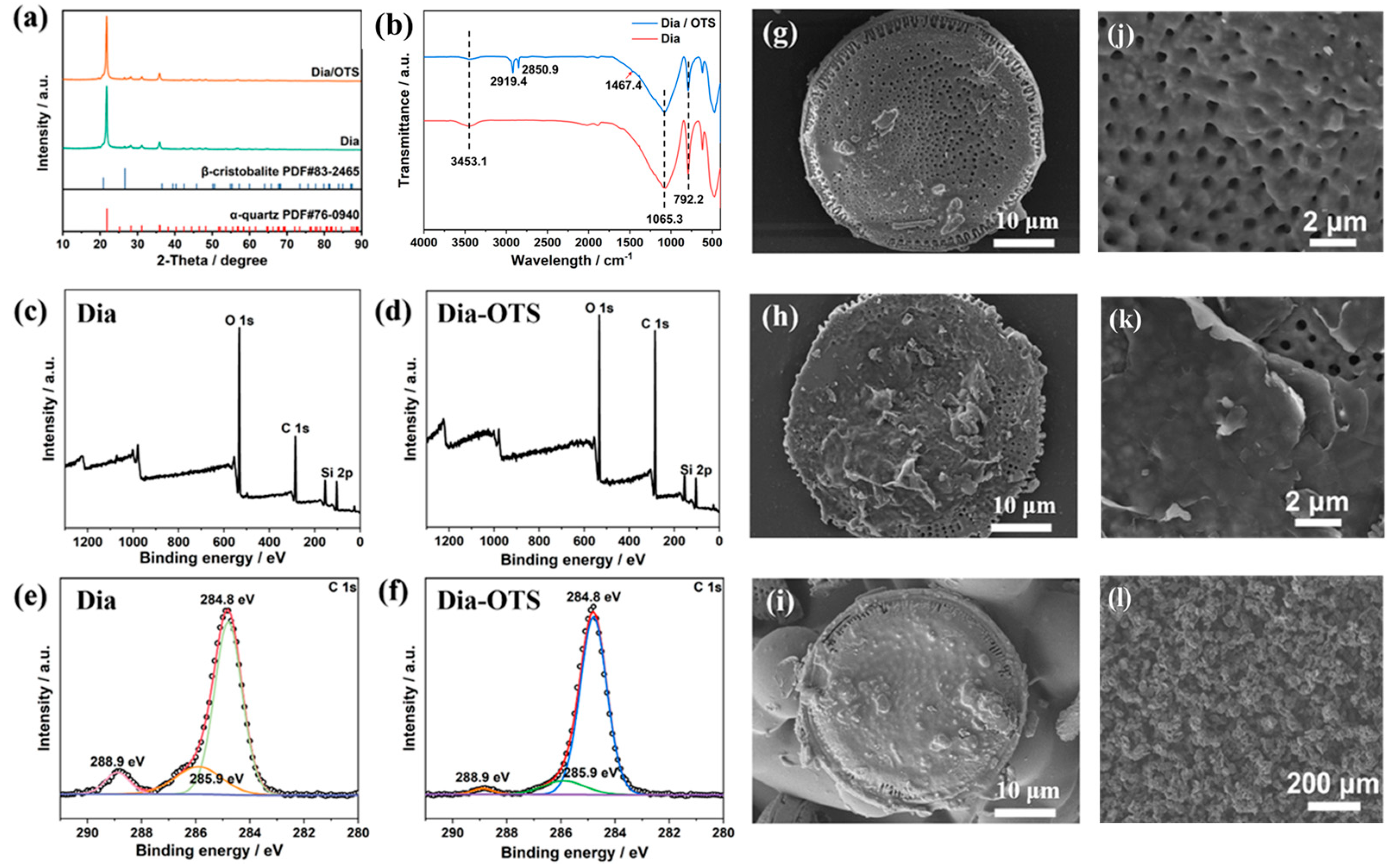
3.1. Microstructural and Morphological Analysis of Dia and Dia/OTS
3.1.1. Dia and Dia/OTS Surface Morphology Analysis
3.1.2. Physical Phase Analysis of Dia and Dia/OTS Powders
3.1.3. Surface Chemical State Analysis of Dia and Dia/OTS Materials
3.2. Application Performance Study of Dia/OTS/EP Materials
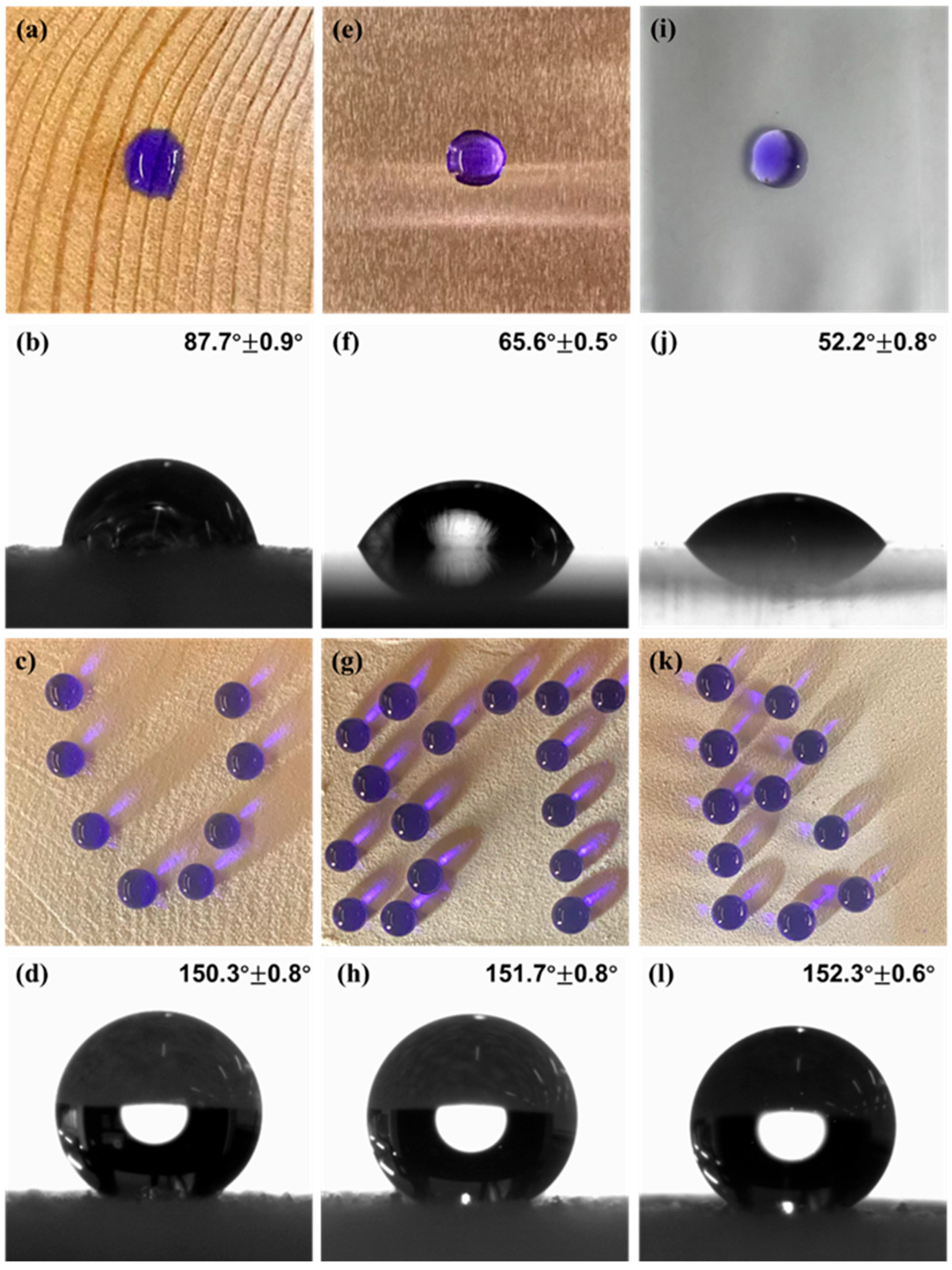
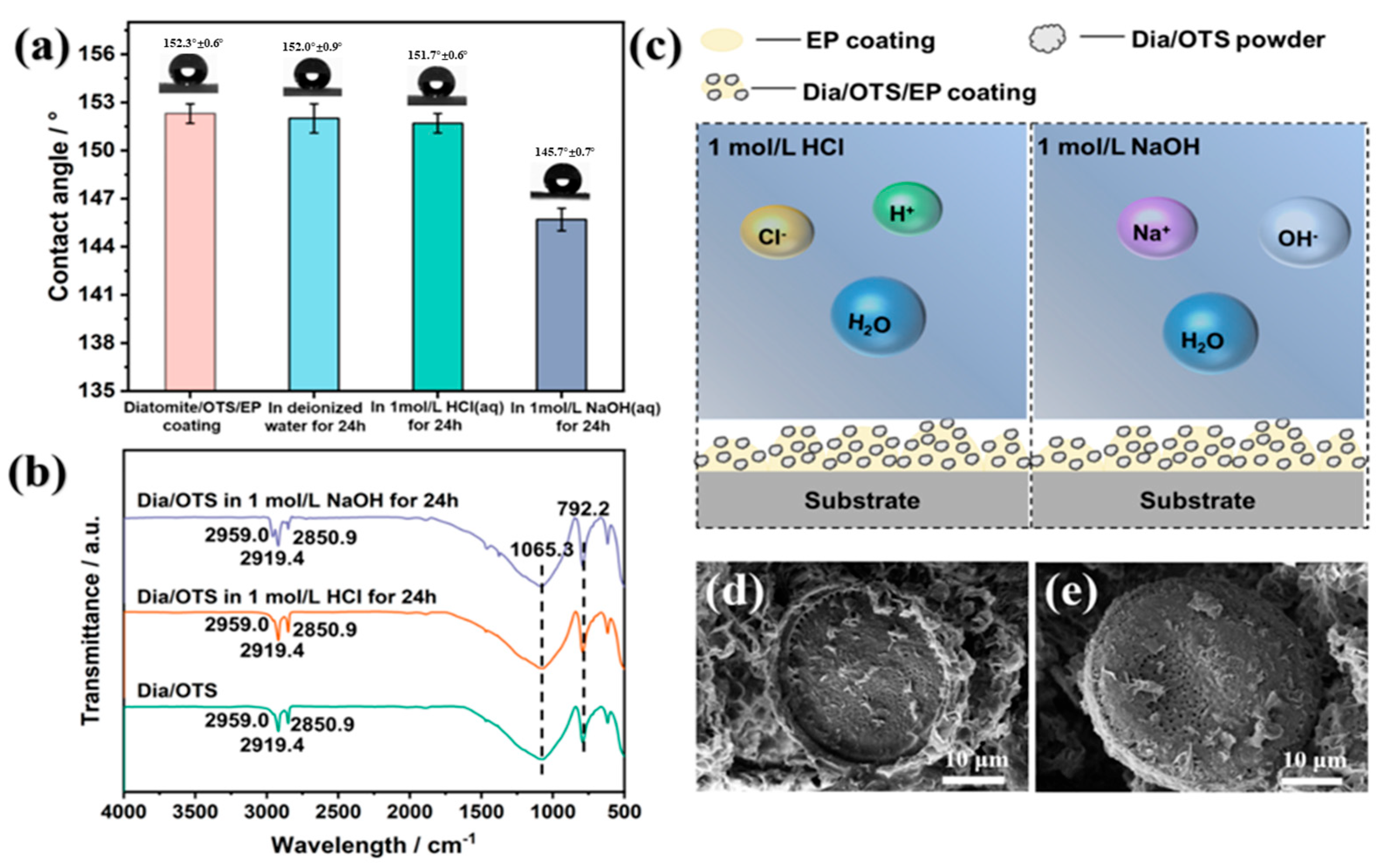
3.2.1. Hydrophobicity Study of Dia/OTS/EP Materials
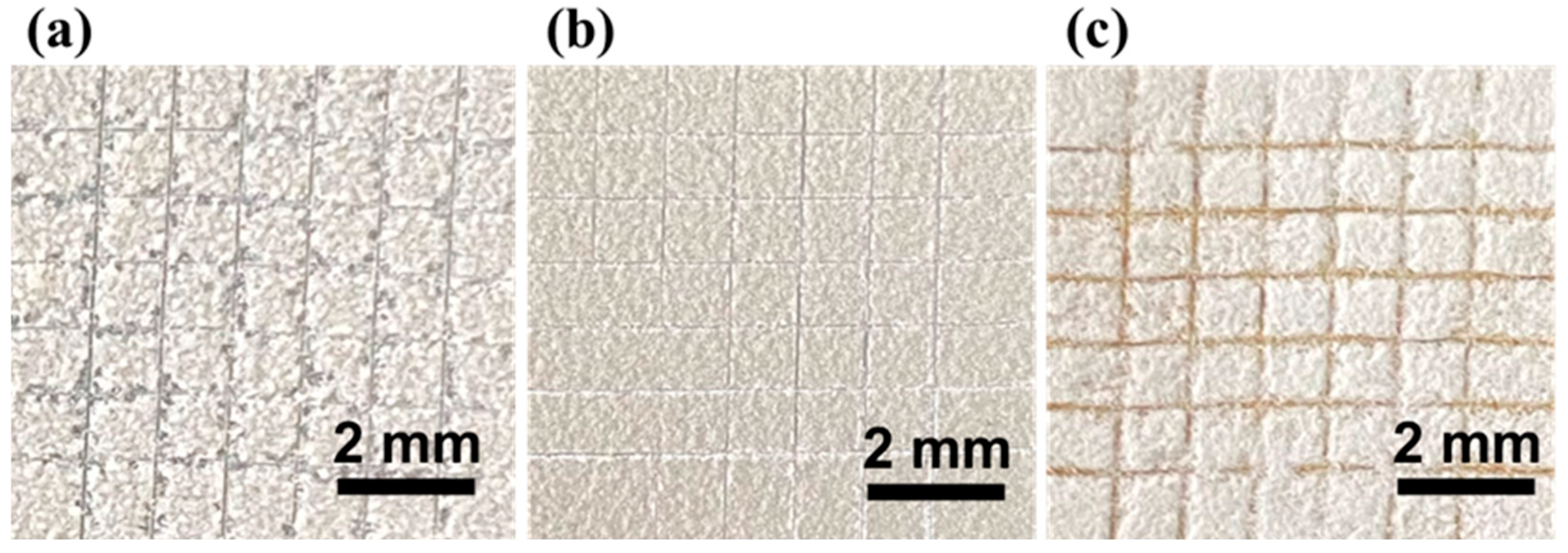
3.2.2. Dia/OTS/EP Coating Adhesion Study
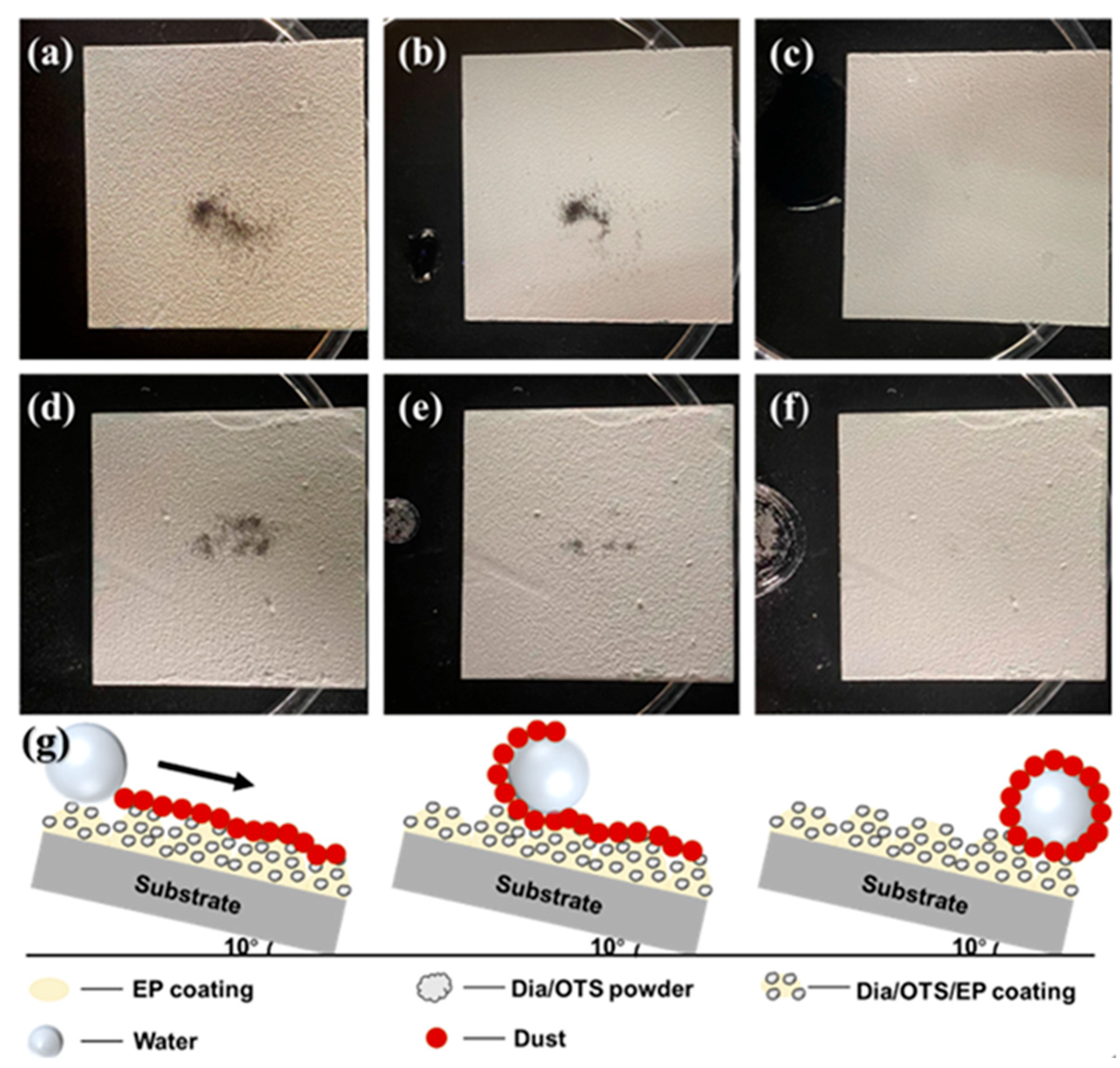
3.2.3. Study of Self-Cleaning Properties of Dia/OTS/EP Coatings
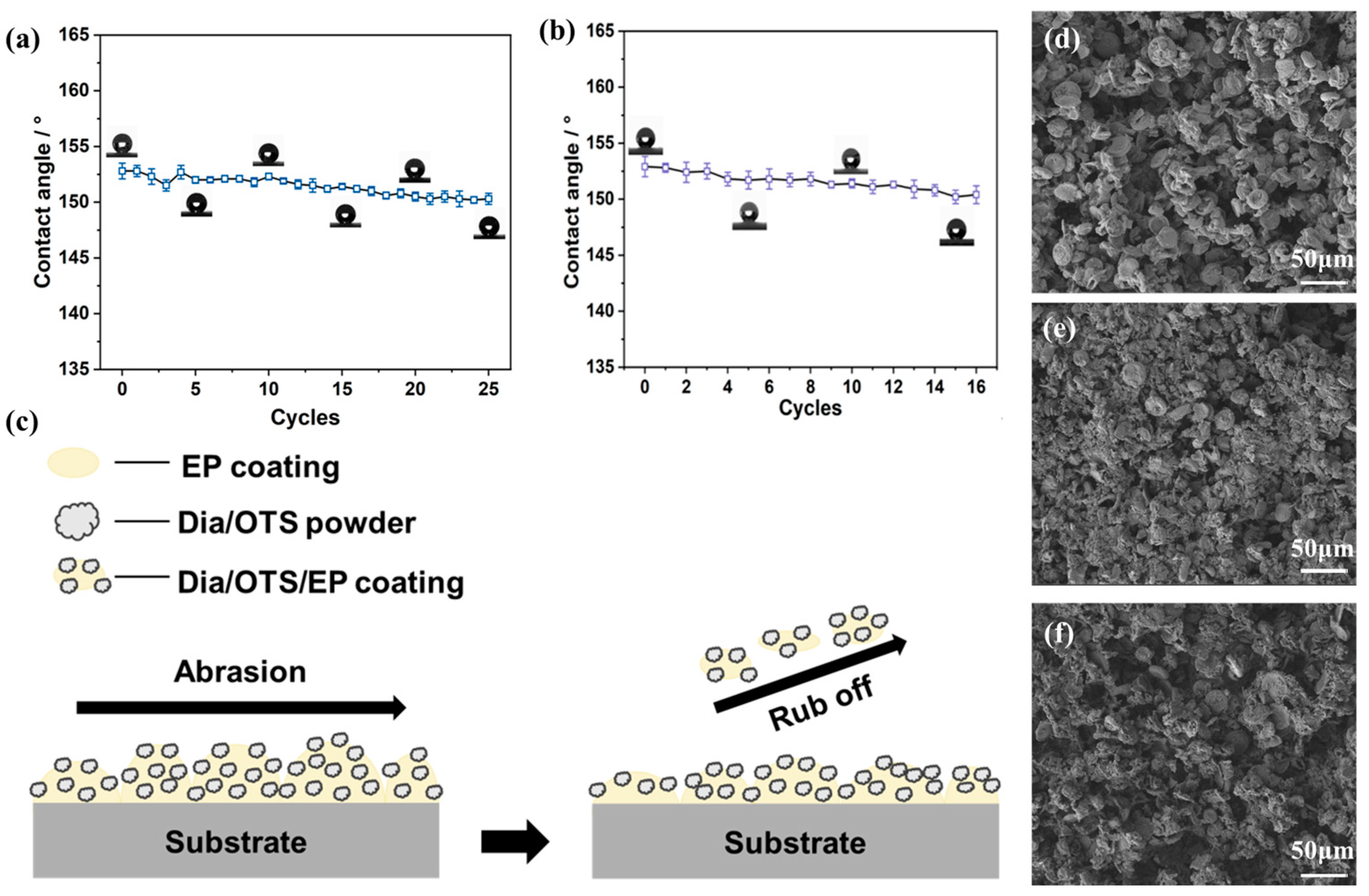
3.2.4. Dia/OTS/EP Coating Application Stability Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sakhvidi, M.J.Z.; Lequy, E.; Goldberg, M.; Jacquemin, B. Air pollution exposure and bladder, kidney and urinary tract cancer risk: A systematic review. Environ. Pollut. 2020, 267, 115328. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, B.; Yang, M.; Ma, J.; Ye, Z.; Xie, L.; Zhou, M.; Chen, W. microRNAs expression in relation to particulate matter exposure: A systematic review. Environ. Pollut. 2020, 260, 113961. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, D.; Zhao, C.; Kwan, M.-P.; Cai, J.; Zhuang, Y.; Zhao, B.; Wang, X.; Chen, B.; Yang, J.; et al. Influence of meteorological conditions on PM 2.5 concentrations across China: A review of methodology and mechanism. Environ. Int. 2020, 139, 105558. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Bai, J.; He, J.; Ding, C.; Dai, X.; Ci, W.; Zhu, T.; Liao, R.; Yuan, Y. A Review on Superhydrophobic Surface with Anti-Icing Properties in Overhead Transmission Lines. Coatings 2023, 13, 301. [Google Scholar] [CrossRef]
- Peng, F.; Zhang, D.; Liu, X.; Zhang, Y. Recent progress in superhydrophobic coating on Mg alloys: A general review. J. Magnes. Alloys 2021, 9, 1471–1486. [Google Scholar] [CrossRef]
- Wang, L.; Guo, X.; Zhang, H.; Liu, Y.; Wang, Y.; Liu, K.; Liang, H.; Ming, W. Recent Advances in Superhydrophobic and Antibacterial Coatings for Biomedical Materials. Coatings 2022, 12, 1469. [Google Scholar] [CrossRef]
- Ji, K.; Gao, Y.; Zhang, L.; Wang, S.; Yue, Q.; Xu, X.; Kong, W.; Gao, B.; Cai, Z.; Chen, Y. A tunable amphiphilic Enteromorpha-modified graphene aerogel for oil/water separation. Sci. Total Environ. 2020, 763, 142958. [Google Scholar] [CrossRef]
- Rabajczyk, A.; Zielecka, M.; Klapsa, W.; Dziechciarz, A. Self-Cleaning Coatings and Surfaces of Modern Building Materials for the Removal of Some Air Pollutants. Materials 2021, 14, 2161. [Google Scholar] [CrossRef]
- Lishchynskyi, O.; Shymborska, Y.; Stetsyshyn, Y.; Raczkowska, J.; Skirtach, A.G.; Peretiatko, T.; Budkowski, A. Passive antifouling and active self-disinfecting antiviral surfaces. Chem. Eng. J. 2022, 446, 137048. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Tran, H.N.; Plamondon, C.O.; Tuduri, L.; Vo, D.-V.N.; Nanda, S.; Mishra, A.; Chao, H.-P.; Bajpai, A.K. Recent progress in the preparation, properties and applications of superhydrophobic nano-based coatings and surfaces: A review. Prog. Org. Coat. 2019, 132, 235–256. [Google Scholar] [CrossRef]
- Luo, J.; Yu, H.; Lu, B.; Wang, D.; Deng, X. Superhydrophobic Biological Fluid-Repellent Surfaces: Mechanisms and Applications. Small Methods 2022, 6, 2201106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gao, J.; Deng, Y.; Peng, L.; Yi, P.; Lai, X.; Lin, Z. Tunable Superhydrophobicity from 3D Hierarchically Nano-Wrinkled Micro-Pyramidal Architectures. Adv. Funct. Mater. 2021, 31, 2101068. [Google Scholar] [CrossRef]
- Zhang, B.; Bai, X.; Wang, S.; Li, L.; Li, X.; Fan, F.; Wang, T.; Zhang, L.; Zhang, X.; Li, Y.; et al. Preparation of Superhydrophobic Metal–Organic Framework/Polymer Composites as Stable and Efficient Catalysts. ACS Appl. Mater. Interfaces 2021, 13, 32175–32183. [Google Scholar] [CrossRef]
- Lin, J.; Cai, X.; Liu, Z.; Liu, N.; Xie, M.; Zhou, B.; Wang, H.; Guo, Z. Anti-liquid-Interfering and Bacterially Antiadhesive Strategy for Highly Stretchable and Ultrasensitive Strain Sensors Based on Cassie-Baxter Wetting State. Adv. Funct. Mater. 2020, 30, 2000398. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Q.; Hokkanen, M.J.; Zhang, C.; Lin, F.Y.; Liu, Q.; Zhu, S.P.; Zhou, T.; Chang, Q.; He, B.; et al. Design of robust superhydrophobic surfaces. Nature 2020, 582, 55–59. [Google Scholar] [CrossRef]
- Pachchigar, V.; Ranjan, M.; Sooraj, K.; Augustine, S.; Kumawat, D.; Tahiliani, K.; Mukherjee, S. Self-cleaning and bouncing behaviour of ion irradiation produced nanostructured superhydrophobic PTFE surfaces. Surf. Coat. Technol. 2021, 420, 127331. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, N.; Yu, X.; Yang, C.; Chu, H. Preparation of superhydrophobic coatings with excellent mechanical and chemical stability by one-step spraying method with selected fluorine-free modifiers. Appl. Surf. Sci. 2024, 642, 158635. [Google Scholar] [CrossRef]
- Yu, X.; Liu, X.; Shi, X.; Zhang, Z.; Wang, H.; Feng, L. SiO2 nanoparticle-based superhydrophobic spray and multi-functional surfaces by a facile and scalable method. Ceram. Int. 2019, 45, 15741–15744. [Google Scholar] [CrossRef]
- Qing, Y.; Shi, S.; Lv, C.; Zheng, Q. Microskeleton-Nanofiller Composite with Mechanical Super-Robust Superhydrophobicity against Abrasion and Impact. Adv. Funct. Mater. 2020, 30, 1910665. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Salim, A.; Seeger, S. Hierarchical Structured Multifunctional Self-Cleaning Material with Durable Superhydrophobicity and Photocatalytic Functionalities. Small 2019, 15, 1901822. [Google Scholar] [CrossRef] [PubMed]
- Luong, D.; Yang, K.; Yoon, J.; Singh, S.P.; Wang, T.; Arnusch, C.J.; Tour, J.M. Laser-Induced Graphene Composites as Multifunctional Surfaces. ACS Nano 2019, 13, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, G.; Li, H.; Cui, Y.; Zhang, Y. Superamphiphobic surfaces with robust self-cleaning, abrasion resistance and anti-corrosion. Chem. Eng. J. 2021, 406, 126753. [Google Scholar] [CrossRef]
- Chen, H.; Wang, F.; Fan, H.; Hong, R.; Li, W. Construction of MOF-based superhydrophobic composite coating with excellent abrasion resistance and durability for self-cleaning, corrosion resistance, anti-icing, and loading-increasing research. Chem. Eng. J. 2020, 408, 127343. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Yu, L.; Duan, Q.; Ji, Z.; Chen, L. Superhydrophobic modification on starch film using PDMS and ball milled MMT coating. ACS Sustain. Chem. Eng. 2020, 8, 10423–10430. [Google Scholar] [CrossRef]
- Peng, X.; Yuan, Z.; Zhao, H.; Wang, H.; Wang, X. Preparation and mechanism of hydrophobic modified diatomite coatings for oil-water separation. Sep. Purif. Technol. 2022, 288, 120708. [Google Scholar] [CrossRef]
- Chen, B.; Jia, Y.; Zhang, M.; Li, X.; Yang, J.; Zhang, X. Facile modification of sepiolite and its application in superhydrophobic coatings. Appl. Clay Sci. 2019, 174, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, A.; Peng, M.; Tan, D.; Liu, X.; Shang, C.; Luo, S.; Peng, L. Preparation and characterization of a fluorizated kaolin–modified melamine sponge as an absorbent for efficient and rapid oil/water separation. J. Clean. Prod. 2019, 217, 308–316. [Google Scholar] [CrossRef]
- Razavi, S.M.R.; Oh, J.; Haasch, R.T.; Kim, K.; Masoomi, M.; Bagheri, R.; Slauch, J.M.; Miljkovic, N. Environment-Friendly Antibiofouling Superhydrophobic Coatings. ACS Sustain. Chem. Eng. 2019, 7, 14509–14520. [Google Scholar] [CrossRef]
- Qu, M.; Ma, X.; He, J.; Feng, J.; Liu, S.; Yao, Y.; Hou, L.; Liu, X. Facile Selective and Diverse Fabrication of Superhydrophobic, Superoleophobic-Superhydrophilic and Superamphiphobic Materials from Kaolin. ACS Appl. Mater. Interfaces 2017, 9, 1011–1020. [Google Scholar] [CrossRef]
- Nine, M.J.; Cole, M.A.; Johnson, L.; Tran, D.N.H.; Losic, D. Robust Superhydrophobic Graphene-Based Composite Coatings with Self-Cleaning and Corrosion Barrier Properties. ACS Appl. Mater. Interfaces 2015, 7, 28482–28493. [Google Scholar] [CrossRef]
- Wei, H.; Feng, J.; Ma, C.; Li, Z.; He, M.; Wang, J.; You, X.; Li, L. Effect of iron doping on the hydrophobicity of titanium dioxide film: Experiment and simulation. Mol. Phys. 2020, 118, e1696477. [Google Scholar] [CrossRef]
- Yaphary, Y.L.; Yu, Z.; Lam, R.H.; Hui, D.; Lau, D. Molecular dynamics simulations on adhesion of epoxy-silica interface in salt environment. Compos. Part B Eng. 2017, 131, 165–172. [Google Scholar] [CrossRef]
- He, X.; Lou, T.; Cao, P.; Bai, X.; Yuan, C.; Wang, C.; Neville, A. Experimental and molecular dynamics simulation study of chemically stable superhydrophobic surfaces. Surf. Coat. Technol. 2021, 418, 127236. [Google Scholar] [CrossRef]
- Issa, A.A.; El-Azazy, M.; Luyt, A.S. Kinetics of alkoxysilanes hydrolysis: An empirical approach. Sci. Rep. 2019, 9, 17624. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Sun, C.; Bai, B. Molecular Dynamics Study on the Effect of Surface Hydroxyl Groups on Three-Phase Wettability in Oil-Water-Graphite Systems. Polymers 2017, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Kausar, A.; Mehmood, M.; Muhammad, B.; Jabeen, S. Progress on Epoxy/Polyamide and Inorganic Nanofiller-Based Hybrids: Introduction, Application, and Future Potential. Polym.-Plast. Technol. Eng. 2016, 55, 1842–1862. [Google Scholar] [CrossRef]
- Zeng, M.; Liu, J.; Lin, Q.; Kuang, W.; Kraithong, S.; Zhang, X.; Zhong, S.; Huang, R. Preparation, structural characterization, and potential applications of diatomaceous earth biomineralized with inorganic calcium and edible polysaccharide composite hydrogels. Int. J. Biol. Macromol. 2025, 319, 145369. [Google Scholar] [CrossRef]
- Xue, S.; Li, B.; Mu, P.; Li, J. Designing attapulgite-based self-healing superhydrophobic coatings for efficient corrosion protection of magnesium alloys. Prog. Org. Coat. 2022, 170, 106966. [Google Scholar] [CrossRef]
- Li, B.; Xue, S.; Mu, P.; Li, J. Robust Self-Healing Graphene Oxide-Based Superhydrophobic Coatings for Efficient Corrosion Protection of Magnesium Alloys. ACS Appl. Mater. Interfaces 2022, 14, 30192–30204. [Google Scholar] [CrossRef]
- Kohl, J.G.; Malicky, D.M.; Jones, A.M. Adhesion of Epoxy (Pseudobarnacles) to Glass that has been Treated with Hydrophobic Carbosilane-Based Coatings. Prog. Org. Coat. 2017, 107, 1–4. [Google Scholar] [CrossRef]
- Geyer, F.; D’Acunzi, M.; Sharifi-Aghili, A.; Saal, A.; Gao, N.; Kaltbeitzel, A.; Sloot, T.-F.; Berger, R.; Butt, H.-J.; Vollmer, D. When and how self-cleaning of superhydrophobic surfaces works. Sci. Adv. 2020, 6, 9727. [Google Scholar] [CrossRef] [PubMed]

| Atomic % | C | O | Si |
|---|---|---|---|
| Dia | 40.43 | 41.20 | 18.37 |
| Dia/OTS | 61.81 | 25.26 | 12.93 |
| Base Group | Combined Energy [38,39] |
|---|---|
| C-C/C-H | 284.8 eV |
| CH2-CO | 285.9 eV |
| O-C=O | 288.9 eV |
| Substrates | Adhesion Rating |
|---|---|
| glass | Level 2 (less than 15 per cent shedding) |
| aluminium sheet | Level 1 (less than 5 per cent shedding) |
| wood | Level 1 (less than 5 per cent shedding) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, A.; Xiao, N.; Yuan, K.; Cao, W. Experimental and Simulation Investigation of Octadecyltriethoxysilane-Decorated Diatomaceous Earth Coatings with Enhanced Superhydrophobic and Self-Cleaning Properties. Materials 2025, 18, 4209. https://doi.org/10.3390/ma18174209
Zhang A, Xiao N, Yuan K, Cao W. Experimental and Simulation Investigation of Octadecyltriethoxysilane-Decorated Diatomaceous Earth Coatings with Enhanced Superhydrophobic and Self-Cleaning Properties. Materials. 2025; 18(17):4209. https://doi.org/10.3390/ma18174209
Chicago/Turabian StyleZhang, Aijia, Nan Xiao, Kunjie Yuan, and Wenbin Cao. 2025. "Experimental and Simulation Investigation of Octadecyltriethoxysilane-Decorated Diatomaceous Earth Coatings with Enhanced Superhydrophobic and Self-Cleaning Properties" Materials 18, no. 17: 4209. https://doi.org/10.3390/ma18174209
APA StyleZhang, A., Xiao, N., Yuan, K., & Cao, W. (2025). Experimental and Simulation Investigation of Octadecyltriethoxysilane-Decorated Diatomaceous Earth Coatings with Enhanced Superhydrophobic and Self-Cleaning Properties. Materials, 18(17), 4209. https://doi.org/10.3390/ma18174209







