Waste Surgical Masks as Precursors of Activated Carbon: A Circular Economy Approach to Mitigate the Impact of Microplastics and Emerging Dye Contaminants
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Carbonaceous Adsorbent Materials
2.3. Characterization of Starting Material and ACMs
2.3.1. Chemical Characterization
2.3.2. Morphological Characterization
2.3.3. Thermogravimetric Analysis
2.3.4. Textural Characterization
2.3.5. Chemical–Surface Characterization
2.4. Adsorption of Dyes
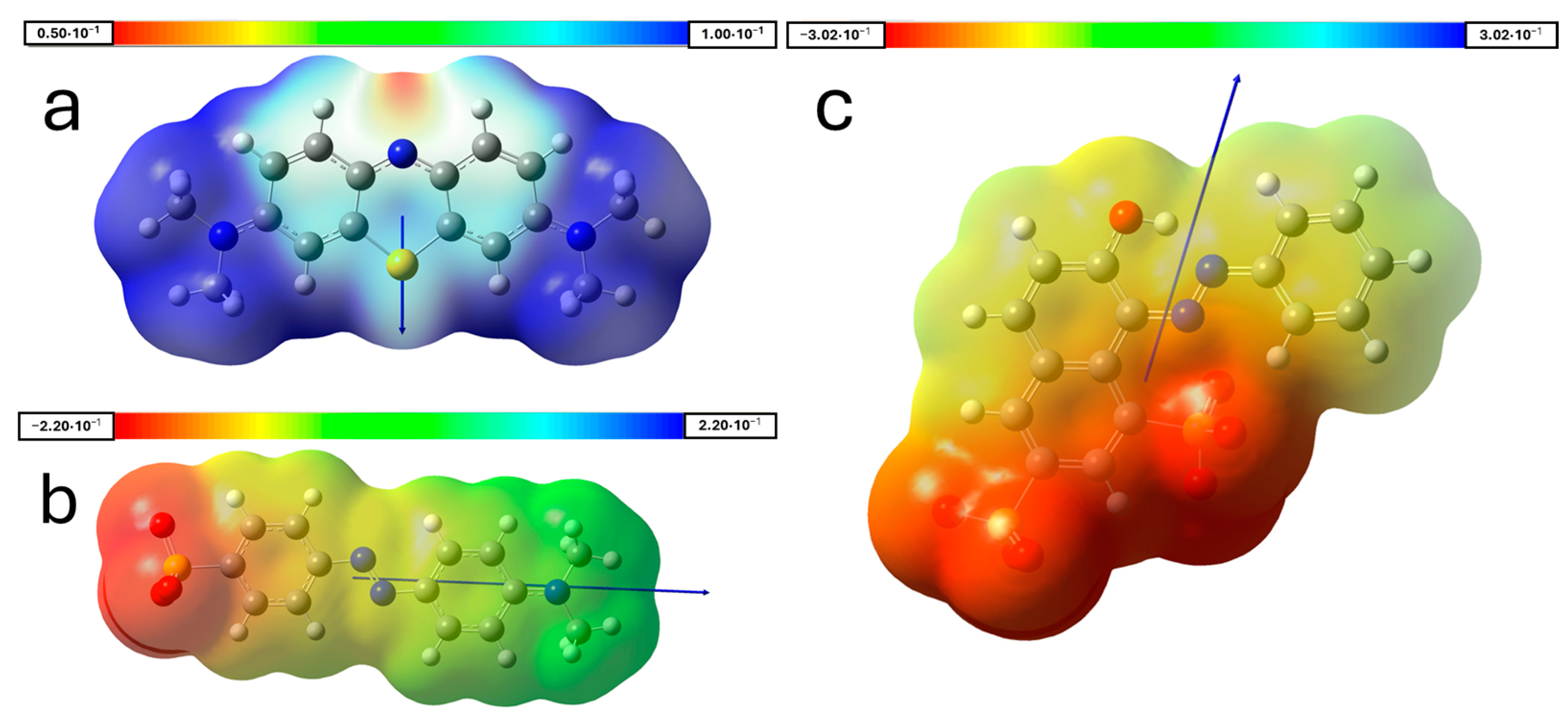
2.5. Computational Methods
3. Results and Discussion
3.1. Analysis of Starting Material
3.2. Analysis of Carbonaceous Adsorbent Materials: Chemical Treatment
3.2.1. TG-DTG Analysis and Scanning Electron Microscopy
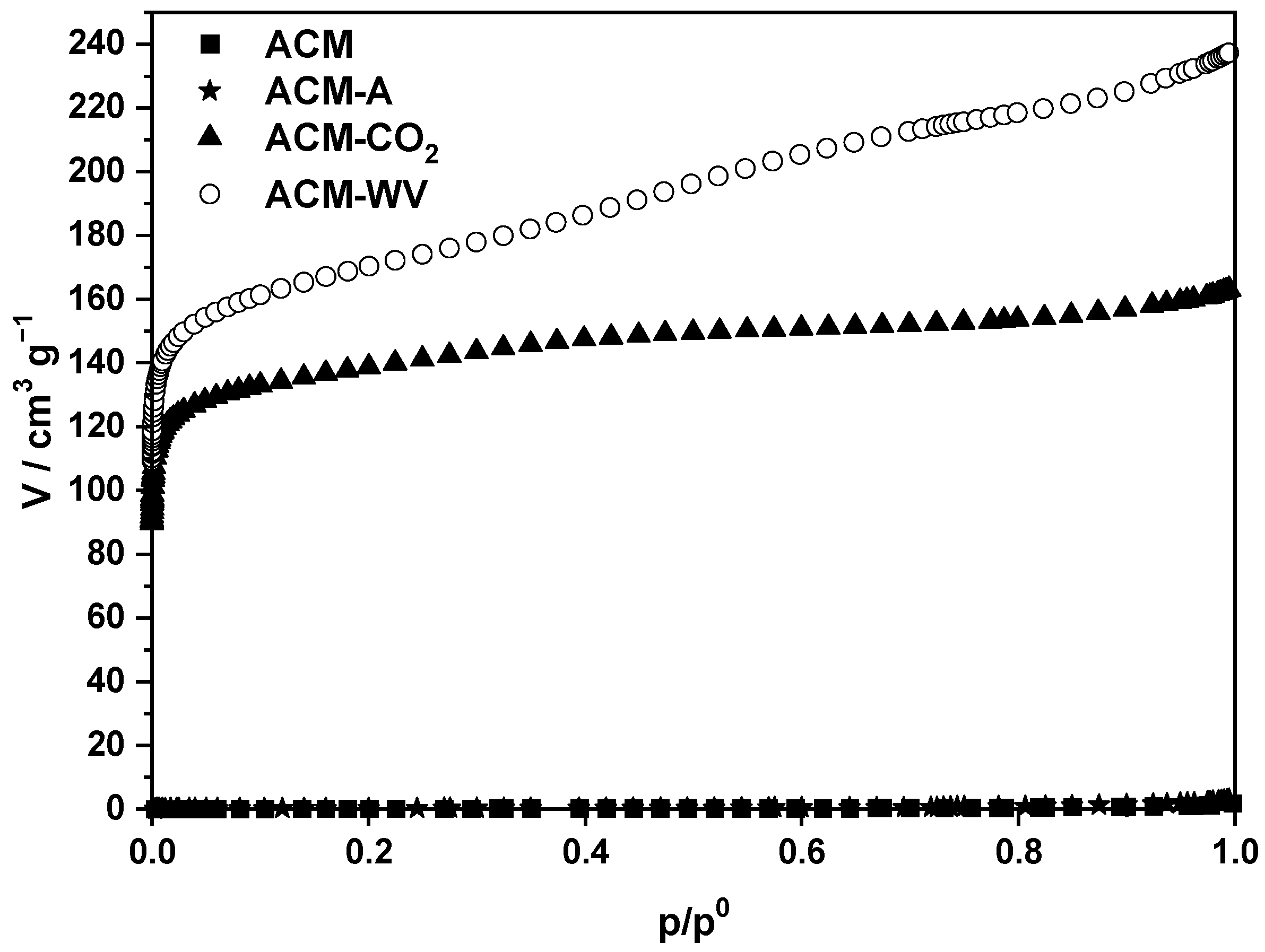
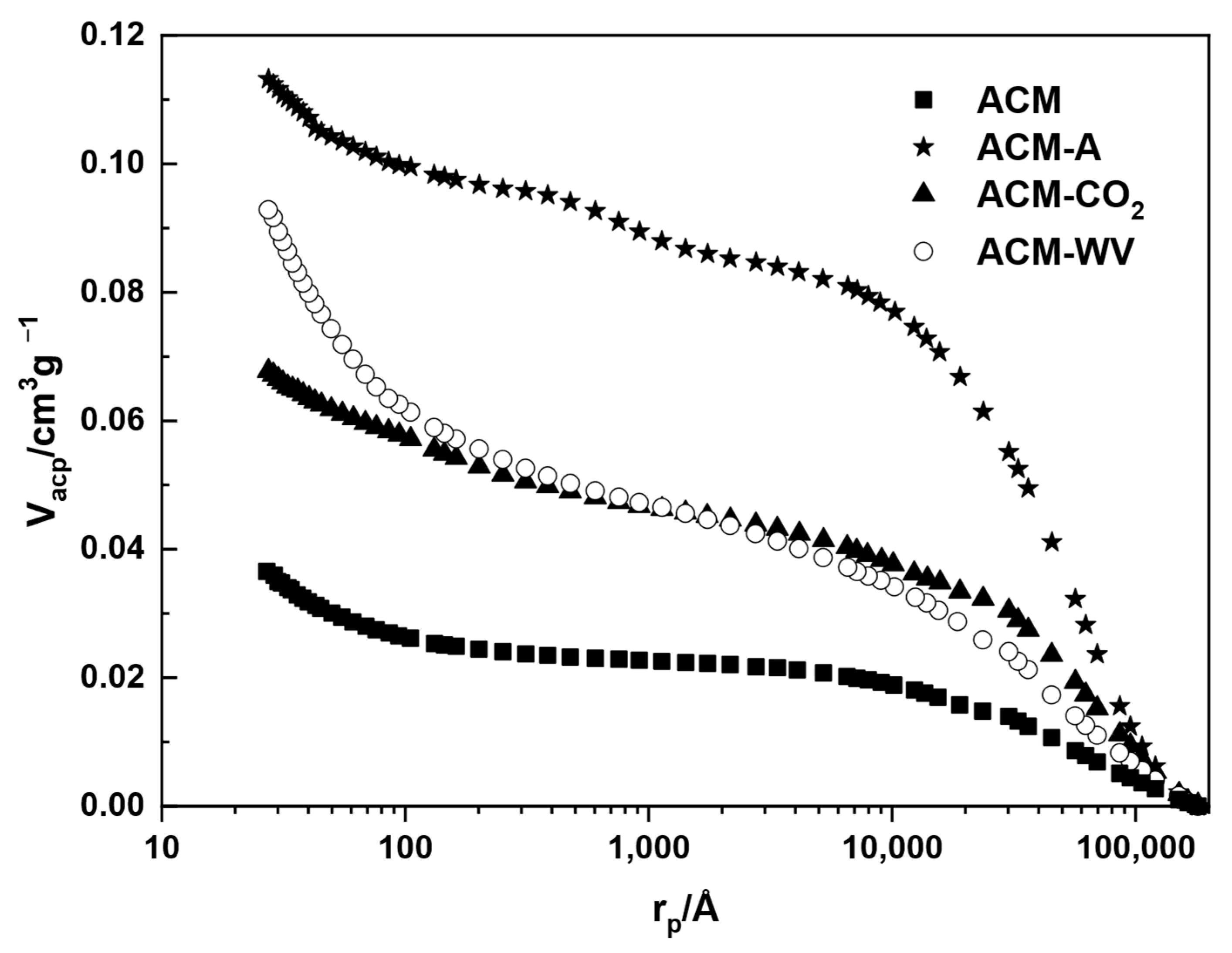
3.2.2. Textural Characterization
3.2.3. Chemical-Surface Characterization
3.3. Physical Treatment
3.3.1. Elemental Analysis
3.3.2. Scanning Electron Microscopy (SEM)
3.3.3. Textural Characterization
3.3.4. Chemical-Surface Characterization: FT-IR Spectroscopy and pHSus
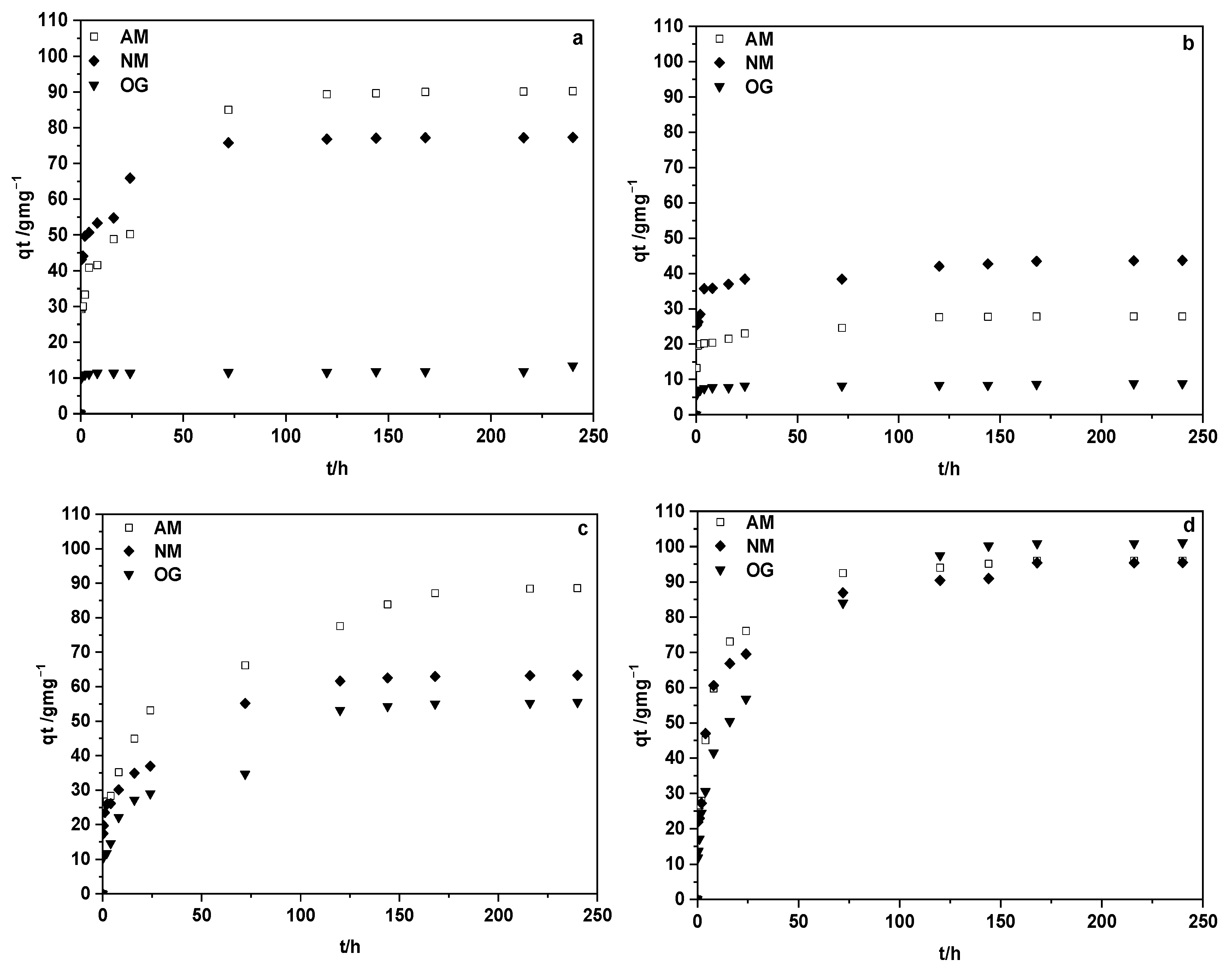
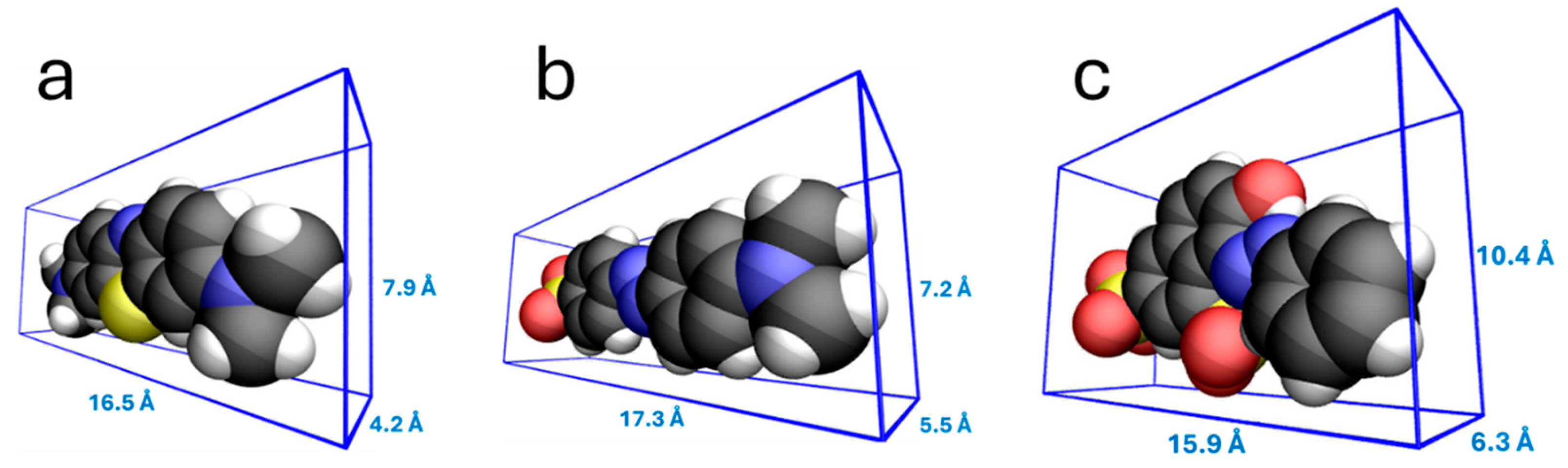
3.4. Adsorption of Dyes
4. Conclusions
- Disposable surgical masks, largely composed of polypropylene, represent a growing source of microplastic pollution but also a promising carbon-rich precursor.
- Chemical treatment with H2SO4 followed by physical activation enabled the preparation of activated carbons (ACMs) with enhanced surface chemistry and porosity.
- Steam activation (ACM-WV) yielded the highest surface area and adsorption capacity, achieving nearly complete removal of methylene blue and high efficiencies for methyl orange and orange G.
- Adsorption followed pseudo-second-order kinetics and was strongly influenced by pore structure, surface functional groups, and dye molecular properties.
- The ACMs proved effective not only in ultrapure water but also in natural river water, confirming their potential for real-world wastewater treatment.
- Future work will address scaling up the process, extending adsorption studies to a wider range of emerging contaminants (pharmaceuticals, pesticides, heavy metals), and assessing regeneration and reuse for industrial applications.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Activated carbon |
| ACM | Carbonaceous adsorbent material |
| ACM-A | Air-activated carbon sample |
| ACM-CO2 | CO2-activated carbon sample |
| ACM-WV | Water vapor-activated carbon sample |
| APW | Average pore width |
| ASPT | Área de la superficie polar topológica |
| BDDT | Brunauer, Deming, Deming, and Teller |
| ρHg | Apparent density of the sample |
| DSC | Differential Scanning Calorimetry |
| p/p0 | Relative pressure |
| pHSus | pH at the point of the aqueous suspension |
| SBET | Specific surface area |
| SCAI-UMA | Research Support Center of the University of Málaga |
| SEM | Scanning Electron Microscopy |
| SM | Surgical mask |
| TG | Thermogravimetry |
| TPSA | Topological polar surface area |
| Vma-p | Macropore volume (mercury porosimetry) |
| Vme-p | Mesopore volume (mercury porosimetry) |
| Vme | Mesopore volume (N2 adsorption isotherm) |
| Vmi | Micropore volume (N2 adsorption isotherm) |
References
- Türkeli, S.; Kemp, R.; Huang, B.; Bleischwitz, R.; McDowall, W. Circular Economy Scientific Knowledge in the European Union and China: A Bibliometric, Network and Survey Analysis (2006–2016). J. Clean. Prod. 2018, 197, 1244–1261. [Google Scholar] [CrossRef]
- Katz-Gerro, T.; López Sintas, J. Mapping Circular Economy Activities in the European Union: Patterns of Implementation and Their Correlates in Small and Medium-sized Enterprises. Bus. Strategy Environ. 2019, 28, 485–496. [Google Scholar] [CrossRef]
- Goworek, H. Social and Environmental Sustainability in the Clothing Industry: A Case Study of a Fair Trade Retailer. Soc. Responsib. J. 2011, 7, 74–86. [Google Scholar] [CrossRef]
- Textile Exchange. Materials Market Report 2024; Textile Exchange: Lubbock, TX, USA, 2024; Available online: https://textileexchange.org/knowledge-center/reports/materials-market-report-2024/ (accessed on 25 July 2025).
- Leal Filho, W.; Ellams, D.; Han, S.; Tyler, D.; Boiten, V.J.; Paco, A.; Moora, H.; Balogun, A.L. A Review of the Socio-Economic Advantages of Textile Recycling. J. Clean. Prod. 2019, 218, 10–20. [Google Scholar] [CrossRef]
- Biyada, S.; Urbonavičius, J. Circularity in Textile Waste: Challenges and Pathways to Sustainability. Clean. Eng. Technol. 2025, 24, 100905. [Google Scholar] [CrossRef]
- Das, A.K.; Hossain, M.F.; Khan, B.U.; Rahman, M.M.; Asad, M.A.Z.; Akter, M. Circular Economy: A Sustainable Model for Waste Reduction and Wealth Creation in the Textile Supply Chain. SPE Polym. 2025, 6, e10171. [Google Scholar] [CrossRef]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Dyes and Pigments: Their Structure and Properties. In Dyes and Pigments; Springer: Cham, Switzerland, 2016; pp. 13–29. ISBN 978-3-319-33892-7. [Google Scholar]
- Ayadi, I.; Souissi, Y. Chemical Synonyms, Molecular Structure and Toxicological Risk Assessment of Synthetic Textile Dyes: A Critical Review. J. Dev. Drugs 2015, 5, 151. [Google Scholar] [CrossRef]
- Kiernan, J.A. Classification and Naming of Dyes, Stains and Fluorochromes. Biotech. Histochem. 2001, 76, 261–278. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Zou, M.; Jia, Z.; Zhou, S.; Li, Y. Microplastics in Soils: A Review of Methods, Occurrence, Fate, Transport, Ecological and Environmental Risks. Sci. Total Environ. 2020, 748, 141368. [Google Scholar] [CrossRef]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M.A. The Potential of Microplastics as Carriers of Metals. Environ. Pollut. 2019, 255, 113363. [Google Scholar] [CrossRef]
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic Transfer of Microplastics and Mixed Contaminants in the Marine Food Web and Implications for Human Health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of Microplastics in Human Lung Tissue Using ΜFTIR Spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Huang, S.; Wang, J. Environmental Risks of Polymer Materials from Disposable Face Masks Linked to the COVID-19 Pandemic. Sci. Total Environ. 2022, 815, 152980. [Google Scholar] [CrossRef] [PubMed]
- Galli, P.; Vecellio, G. Polyolefins: The Most Promising Large-Volume Materials for the 21st Century. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 396–415. [Google Scholar] [CrossRef]
- Ju, J.T.J.; Boisvert, L.N.; Zuo, Y.Y. Face Masks against COVID-19: Standards, Efficacy, Testing and Decontamination Methods. Adv. Colloid Interface Sci. 2021, 292, 102435. [Google Scholar] [CrossRef]
- Bansal, R.C.; Donnet, J.B.; Stoeckli, F. Active Carbon; Marcel Dekker: New York, NY, USA, 1988. [Google Scholar]
- Selvam, K. Recent Trends in Agro-Waste Based Activated Carbons for the Removal of Emerging Textile Pollutants. Int. J. Environ. Anal. Chem. 2023, 103, 5142–5158. [Google Scholar] [CrossRef]
- Doczekalska, B.; Ziemińska, N.; Kuśmierek, K.; Zwia’tkowski, A. Activated Carbons Prepared from Stump Wood of Various Tree Species by Chemical Activation and Their Application for Water Purification. Eur. J. Wood Wood Prod. 2024, 82, 2121–2135. [Google Scholar] [CrossRef]
- Ojha, A.; Tiwary, D.; Oraon, R.; Singh, P. Degradations of Endocrine-Disrupting Chemicals and Pharmaceutical Compounds in Wastewater with Carbon-Based Nanomaterials: A Critical Review. Environ. Sci. Pollut. Res. 2021, 28, 30573–30594. [Google Scholar] [CrossRef]
- Alvez-Tovar, B.; Scalize, P.S.; Angiolillo-Rodríguez, G.; Albuquerque, A.C.; Ebang, M.N.; Ferreira de Oliveira, T.F. Agro-Industrial Waste Upcycling into Activated Carbons: A Sustainable Approach for Dye Removal and Wastewater Treatment. Sustainability 2025, 17, 2036. [Google Scholar] [CrossRef]
- Micheletti, D.H.; da Silva Andrade, J.G.; Porto, C.E.; Alves, B.H.M.; de Carvalho, F.R.; Sakai, O.A.; Batistela, V.R. A Review of Adsorbents for Removal of Yellow Tartrazine Dye from Water and Wastewater. Bioresour. Technol. Rep. 2023, 24, 101598. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, W.; Dong, X.; Wang, F.; Yang, W.; Liu, C.; Chen, D. A Review on Recent Advances of Biochar from Agricultural and Forestry Wastes: Preparation, Modification and Applications in Wastewater Treatment. J. Environ. Chem. Eng. 2024, 12, 111638. [Google Scholar] [CrossRef]
- Dada, A.O.; Inyinbor, A.A.; Atunwa, B.T.; Gonuguntla, S.; Bello, O.S.; Adekola, F.A.; Pal, U. Agrowaste-Carbon and Carbon-Based Nanocomposites for Endocrine Disruptive Cationic Dyes Removal: A Critical Review. Biotechnol. Rep. 2024, 44, e00860. [Google Scholar] [CrossRef]
- Troca-Torrado, C.; Alexandre-Franco, M.F.; Fernández-González, C.; Alfaro-Domínguez, M.; Gómez-Serrano, V. Carbonaceous Adsorbents from Polymers-Rubber and Plastic Wastes for Adsorption of Methylene Blue. Express Polym. Lett. 2022, 16, 1280–1303. [Google Scholar] [CrossRef]
- Yuwen, C.; Liu, B.; Rong, Q.; Zhang, L.; Guo, S. Porous Carbon Materials Derived from Discarded COVID-19 Masks via Microwave Solvothermal Method for Lithium-sulfur Batteries. Sci. Total Environ. 2022, 817, 152995. [Google Scholar] [CrossRef]
- Satyam, S.; Patra, S. The Evolving Landscape of Advanced Oxidation Processes in Wastewater Treatment: Challenges and Recent Innovations. Processes 2025, 13, 987. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Singh, K.; Choudhary, A.; Brighu, U.; Singh, S.K.; Bhattacharya, S. Combined Advanced Oxidation Dye-Wastewater Treatment Plant: Design and Development with Data-Driven Predictive Performance Modeling. npj Clean Water 2024, 7, 15. [Google Scholar] [CrossRef]
- Azmi, L.S.; Jabit, N.A.; Ismail, S.; Ku Ishak, K.M.; Abdullah, T.K. Membrane Filtration Technologies for Sustainable Industrial Wastewater Treatment: A Review of Heavy Metal Removal. Desalination Water Treat. 2025, 323, 101321. [Google Scholar] [CrossRef]
- Aziz, S.; Mazhar, A.R.; Ubaid, A.; Shah, S.M.H.; Riaz, Y.; Talha, T.; Jung, D.W. A Comprehensive Review of Membrane-Based Water Filtration Techniques. Appl. Water Sci. 2024, 14, 169. [Google Scholar] [CrossRef]
- Husen, A.K.; Bidira, F.; Bekel, E.A.; Tegegn, M.; Desta, W.M.; Asaithambi, P. Color, COD, and Turbidity Removal from Surface Water by Using Linseed and Alum Coagulants: Optimization through Response Surface Methodology. Appl. Water Sci. 2024, 14, 203. [Google Scholar] [CrossRef]
- Rahmoun, H.B.; Boumediène, M.; Ghenim, A.N.; da Silva, E.F.; António Labrincha, J.A. Coupling Coagulation–Flocculation–Sedimentation with Adsorption on Biosorbent (Corncob) for the Removal of Textile Dyes from Aqueous Solutions. Environments 2025, 12, 201. [Google Scholar] [CrossRef]
- Sadhwani, N.; Adhikari, S.; Eden, M.R. Biomass Gasification Using Carbon Dioxide: Effect of Temperature, CO2/C Ratio, and the Study of Reactions Influencing the Process. Ind. Eng. Chem. Res. 2016, 55, 2883–2891. [Google Scholar] [CrossRef]
- Zhang, J.; Jones, I.M.; Zhu, M.; Zhang, Z.; Preciado-Hernandez, J.; Zhang, D. Pore Development During CO2 and Steam Activation of a Spent Tyre Pyrolysis Char. Waste Biomass Valorization 2021, 12, 2097–2108. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Dubinin, M.M. Adsorption in Micropores. J. Colloid Interface Sci. 1967, 23, 487–499. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView; Semichem. Inc.: Shawnee, KS, USA, 2016. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Realization of Conceptual Density Functional Theory and Information-Theoretic Approach in Multiwfn Program. In Conceptual Density Functional Theory; Wiley: Hoboken, NJ, USA, 2022; pp. 631–647. ISBN 9783527829941. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van Der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie Der Sogenannten Adsorption Gelöster Stoffe. Z. Chem. Ind. Kolloide 1907, 2, 15. [Google Scholar] [CrossRef]
- Ho, Y.; Gordon, M. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast Calculation of Molecular Polar Surface Area as a Sum of Fragment-Based Contributions and Its Application to the Prediction of Drug Transport Properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef] [PubMed]
- Caron, G.; Ermondi, G. Molecular Descriptors for Polarity: The Need for Going Beyond Polar Surface Area. Future Med. Chem. 2016, 8, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 1 July 2025).
- Gerold, C.T.; Henry, C.S. Observation of Dynamic Surfactant Adsorption Facilitated by Divalent Cation Bridging. Langmuir 2018, 34, 1550–1556. [Google Scholar] [CrossRef]
- Guo, Y.; Rockstraw, D.A. Physical and Chemical Properties of Carbons Synthesized from Xylan, Cellulose, and Kraft Lignin by H3PO4 Activation. Carbon 2006, 44, 1464–1475. [Google Scholar] [CrossRef]
- El-Hendawy, A.N.A. Influence of HNO3 Oxidation on the Structure and Adsorptive Properties of Corncob-Based Activated Carbon. Carbon 2003, 41, 713–722. [Google Scholar] [CrossRef]
- Ioannidou, O.A.; Zabaniotou, A.A. Agricultural Residues as Precursors for Activated Carbon Production—A Review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Serban, G.V.; Iancu, V.I.; Dinu, C.; Tenea, A.G.; Vasilache, N.; Cristea, I.N.; Niculescu, M.; Ionescu, A.I.; Chiriac, L.F. Removal Efficiency and Adsorption Kinetics of Methyl Orange from Wastewater by Commercial Activated Carbon. Sustainability 2023, 15, 12939. [Google Scholar] [CrossRef]
- Taquieteu, I.K.; Tamaguelon, H.D.; Shikuku, V.O.; Banenzoué, C.; Joh Dina, D.D. Fixed-Bed Adsorption of an Azo Dye (Methyl Orange) onto Chemically and Thermally Regenerated Activated Carbons. J. Chem. 2023, 2023, 6677710. [Google Scholar] [CrossRef]
- Abbas, M.; Trari, M. Adsorption Behavior of Methylene Blue Onto Activated Coconut Shells: Kinetic, Thermodynamic, Mechanism and Regeneration of the Adsorbent. Dose-Response 2024, 22, 15593258241290708. [Google Scholar] [CrossRef] [PubMed]
- Paluch, D.; Bazan-Wozniak, A.; Nosal-Wiercińska, A.M.; Pietrzak, R. Removal of Methylene Blue and Methyl Red from Aqueous Solutions Using Activated Carbons Obtained by Chemical Activation of Caraway Seed. Molecules 2023, 28, 6306. [Google Scholar] [CrossRef] [PubMed]
- Sayed, N.S.M.; Ahmed, A.S.A.; Abdallah, M.H.; Gouda, G.A. ZnO@ Activated Carbon Derived from Wood Sawdust as Adsorbent for Removal of Methyl Red and Methyl Orange from Aqueous Solutions. Sci. Rep. 2024, 14, 5384. [Google Scholar] [CrossRef] [PubMed]
- Pongwiwanna, D.; Tangsathitkulchai, C. The Use of High Surface Area Mesoporous-Activated Carbon from Longan Seed Biomass for Increasing Capacity and Kinetics of Methylene Blue Adsorption from Aqueous Solution. Molecules 2021, 26, 6521. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Bahrudin, N.N.; Hum, N.N.M.F.; Surip, S.N.; Syed-Hassan, S.S.A.; Yousif, E.A.; Sabar, S. Microporous Activated Carbon Developed from KOH Activated Biomass Waste: Surface Mechanistic Study of Methylene Blue Dye Adsorption. Water Sci. Technol. 2021, 84, 1858–1872. [Google Scholar] [CrossRef]
- El-Bery, H.M.; Saleh, M.; El-Gendy, R.A.; Saleh, M.R.; Thabet, S.M. High Adsorption Capacity of Phenol and Methylene Blue Using Activated Carbon Derived from Lignocellulosic Agriculture Wastes. Sci. Rep. 2022, 12, 5499. [Google Scholar] [CrossRef]
- Dolaş, H. Activated Carbon Synthesis and Methylene Blue Adsorption from Pepper Stem Using Microwave Assisted Impregnation Method: Isotherm and Kinetics. J. King Saud Univ. Sci. 2023, 35, 102559. [Google Scholar] [CrossRef]
- Mariana, M.; Mistar, E.M.; Alfatah, T.; Supardan, M.D. High-Porous Activated Carbon Derived from Myristica Fragrans Shell Using One-Step KOH Activation for Methylene Blue Adsorption. Bioresour. Technol. Rep. 2021, 16, 100845. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, X.; Zhou, S.Q. Adsorption of Methylene Blue in Water onto Activated Carbon by Surfactant Modification. Water 2020, 12, 587. [Google Scholar] [CrossRef]
- Zhu, W.; He, J.; Wang, Q.; Zhang, D.; Qi, G.; Cai, X.; Li, P.; Zhang, J. Activated Carbon Based on Recycled Epoxy Boards and Their Adsorption toward Methyl Orange. Polymers 2024, 16, 1648. [Google Scholar] [CrossRef]
- Al-Hazeef, M.S.F.; Aidi, A.; Hecini, L.; Osman, A.I.; Hasan, G.G.; Althamthami, M.; Ziad, S.; Otmane, T.; Rooney, D.W. Valorizing Date Palm Spikelets into Activated Carbon-Derived Composite for Methyl Orange Adsorption: Advancing Circular Bioeconomy in Wastewater Treatment—A Comprehensive Study on Its Equilibrium, Kinetics, Thermodynamics, and Mechanisms. Environ. Sci. Pollut. Res. 2024, 31, 50493–50512. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Zheng, Y.; Fagbohun, E.O.; Cui, Y. Magnetic Activated Carbon for the Removal of Methyl Orange from Water via Adsorption and Fenton-like Degradation. Particuology 2024, 94, 314–326. [Google Scholar] [CrossRef]
- Raji, Y.; Nadi, A.; Rouway, M.; Jamoudi Sbai, S.; Yassine, W.; Elmahbouby, A.; Cherkaoui, O.; Zyade, S. Efficient Adsorption of Methyl Orange on Nanoporous Carbon from Agricultural Wastes: Characterization, Kinetics, Thermodynamics, Regeneration and Adsorption Mechanism. J. Compos. Sci. 2022, 6, 385. [Google Scholar] [CrossRef]
- Zuhara, S.; Pradhan, S.; Gordon, M. Investigating Mixed Biosolids and Cardboard for Methylene Blue Adsorption: Activation, Adsorption Modelling and Thermodynamics. Environ. Res. 2023, 225, 115534. [Google Scholar] [CrossRef]
- Mohamed, F.; Shaban, M.S.; Zaki, S.K.; Abd-Elsamie, M.S.; Sayed, R.; Zayed, M.K.; Khalid, N.; Saad, S.; Omar, S.; Ahmed, A.M. Activated Carbon Derived from Sugarcane and Modified with Natural Zeolite for Efficient Adsorption of Methylene Blue Dye: Experimentally and Theoretically Approaches. Sci. Rep. 2022, 12, 18031. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, N.; Shi, H.; Wang, H.; Ruan, F.; Wang, H.; Feng, Q. Biomass Activated Carbon Derived from Golden Needle Mushroom Root for the Methylene Blue and Methyl Orange Adsorption from Wastewater. Ind. Crops Prod. 2025, 223, 120051. [Google Scholar] [CrossRef]
- Wang, S.; Dou, J.; Zhang, T.; Li, S.; Chen, X. Selective Adsorption of Methyl Orange and Methylene Blue by Porous Carbon Material Prepared From Potassium Citrate. ACS Omega 2023, 8, 35024–35033. [Google Scholar] [CrossRef]



| Sample | C | H | N | S | O | Ashes |
|---|---|---|---|---|---|---|
| SM | 85.72 | 14.76 | 0.00 | 0.90 | 1.87 | 0.00 |
| ACM | 40.10 | 2.26 | 0.00 | 6.09 | 47.90 | 1.67 |
| Sample | C | H | N | S | O | Ashes |
|---|---|---|---|---|---|---|
| ACM | 40.10 | 2.26 | 0.00 | 6.09 | 47.90 | 1.67 |
| ACM-A | 58.29 | 1.82 | 0.00 | 1.89 | 30.15 | 3.42 |
| ACM-CO2 | 78.93 | 0.72 | 0.00 | 1.22 | 3.37 | 5.74 |
| ACM-WV | 79.36 | 1.03 | 0.00 | 1.41 | 6.96 | 5.88 |
| Sample | SBET/m2·g−1 | W0/cm3·g−1 | Vmi/cm3·g−1 | Vme/cm3·g−1 | APW/Å |
|---|---|---|---|---|---|
| ACM | <1 | 0.00 | 0.00 | 0.00 | n.a. |
| ACM-A | 1 | 0.00 | 0.00 | 0.01 | n.a. |
| ACM-CO2 | 525 | 0.21 | 0.21 | 0.04 | 28.5 |
| ACM-WV | 633 | 0.25 | 0.25 | 0.11 | 37.8 |
| Sample | Vme-p (cm3·g−1) | Vma-p (cm3·g−1) | ρHg (g·cm−3) |
|---|---|---|---|
| ACM | 0.012 | 0.024 | 1.690 |
| ACM-A | 0.017 | 0.096 | 1.421 |
| ACM-CO2 | 0.015 | 0.052 | 1.310 |
| ACM-WV | 0.039 | 0.054 | 1.182 |
| Sample | pHSus | Surface Net Charge at pH 7 |
|---|---|---|
| ACM | 1.96 | Negative |
| ACM-A | 5.25 | Negative |
| ACM-CO2 | 8.25 | Positive |
| ACM-WV | 9.25 | Positive |
| Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Material | Sample | te (h) | qe (Experimental mg·g−1) | qe (mg·g−1) | k1 (h−1) | R2 | qe (mg·g−1) | k2 (g·mg−1 ·h−1) | R2 |
| ACM | MB | 100 | 90.18 | 65.43 | 0.0355 | 0.9899 | 92.93 | 0.0016 | 0.9963 |
| MO | 100 | 77.32 | 30.88 | 0.0338 | 0.9904 | 78.08 | 0.0055 | 0.9996 | |
| OG | 120 | 13.45 | 2.13 | 0.0016 | 0.8853 | 11.85 | 0.1878 | 0.9999 | |
| ACM-A | MB | 120 | 27.88 | 10.30 | 0.0302 | 0.9654 | 28.10 | 0.0139 | 0.9991 |
| MO | 130 | 43.72 | 13.67 | 0.0207 | 0.9423 | 43.73 | 0.0103 | 0.9991 | |
| OG | 6 | 8.89 | 1.34 | 0.0092 | 0.9511 | 8.81 | 0.0677 | 0.9991 | |
| ACM-CO2 | MB | 200 | 88.55 | 65.53 | 0.0192 | 0.9547 | 90.32 | 0.0011 | 0.9924 |
| MO | 130 | 63.31 | 44.06 | 0.0273 | 0.9969 | 64.96 | 0.0022 | 0.9967 | |
| OG | 130 | 55.46 | 48.73 | 0.0253 | 0.9704 | 57.68 | 0.0018 | 0.9955 | |
| ACM-WV | MB | 130 | 95.93 | 55.60 | 0.0302 | 0.9607 | 97.58 | 0.0026 | 0.9997 |
| MO | 180 | 95.45 | 57.35 | 0.0226 | 0.9156 | 96.71 | 0.0021 | 0.9989 | |
| OG | 150 | 101.12 | 85.32 | 0.0285 | 0.9757 | 105.28 | 0.0010 | 0.9961 | |
| Retention (%) (Ultrapure Water) | Retention (%) (River Water) | |||||
|---|---|---|---|---|---|---|
| Sample | Methylene Blue | Methyl Orange | Orange G | Methylene Blue | Methyl Orange | Orange G |
| ACM | 94 | 79 | 10 | 91 | 83 | 12 |
| ACM-A | 29 | 45 | 5 | 26 | 42 | 4 |
| ACM-CO2 | 92 | 64 | 41 | 59 | 60 | 21 |
| ACM-WV | 100 | 97 | 74 | 100 | 97 | 94 |
| Adsorbent (Type/Precursor) | Dye(s) | Performance (Capacity/Removal) | Key Notes | Reference |
|---|---|---|---|---|
| ACM-WV (this work) | MB, MO, Orange G | MB ≈ 98 mg·g−1; MO ≈ 98 mg·g−1; OG ≈ 110 mg·g−1 | - | This work |
| Commercial AC (powder) | MO | qmax = 129.8 mg·g−1; removal 97.8% | Batch; Langmuir; pH ≈ 3 | [57] |
| Commercial AC (fixed-bed) | MO | q ≈ 16.9 mg·g−1 | Continuous column | [58] |
| Coconut shell AC | MB | qmax = 30.3 mg·g−1 | Batch; Langmuir; pH ~6 | [59] |
| Caraway seed AC (K2CO3) | MB, MR | MB = 296 mg·g−1; MR = 203 mg·g−1 | Batch | [60] |
| Wood sawdust AC | MO | 31.93 mg·g−1 | pH 3; 60 min | [61] |
| Wood sawdust ZnO@AC | MO | 42.61 mg·g−1 | Improved vs. AC | [61] |
| Mesoporous AC | MB | ≈1000 mg·g−1 | Very high SSA | [62] |
| Microporous KOH-AC | MB | 136.5 mg·g−1 | Micropore-rich | [63] |
| Water-vapor AC | MB | 148.8 mg·g−1 | Fast removal | [64] |
| Pepper-stem AC | MB | ≈75 mg·g−1 | RSM optimized | [65] |
| Nutmeg shell AC/K2CO3 | MB | 346.9 mg·g−1 | High porosity | [66] |
| Surfactant-modified AC (SLS-C) | MB | Virgin AC = 153.8; SLS-C = 232.5 mg·g−1 | Surfactant effect | [67] |
| Recycled epoxy-board AC | MO | 23.1–37.2 mg·g−1 | Temp-dependent | [68] |
| Date-palm ZnO@DPS-AC | MO | 227 mg·g−1 | Rapid uptake | [69] |
| Magnetic AC (MAC) | MO | ≈101 to 108 mg·g−1 | Langmuir fits | [70] |
| Nanoporous carbon (ZnCl2) | MO | 367.8 mg·g−1 | Recyclable | [71] |
| Biosolids/cardboard KOH-AC | MB | ≈191 mg·g−1 | Batch | [72] |
| Sugarcane AC/zeolite | MB | ≈51 mg·g−1 | Composite | [73] |
| Golden-needle mushroom AC/KOH | MB, MO | MB = 816 mg·g−1; MO = 287 mg·g−1 | Biomass self-activation | [74] |
| Porous carbon (PC-900)/KOH | MB, MO | MB = 1853.6 mg·g−1; MO = 927 mg·g−1 | Very high capacity | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Galán, M.d.M.; Fernández-Blanco, C.A.; Cuerda-Correa, E.M.; Garrido-Zoido, J.M.; Alexandre-Franco, M.F. Waste Surgical Masks as Precursors of Activated Carbon: A Circular Economy Approach to Mitigate the Impact of Microplastics and Emerging Dye Contaminants. Materials 2025, 18, 4115. https://doi.org/10.3390/ma18174115
García-Galán MdM, Fernández-Blanco CA, Cuerda-Correa EM, Garrido-Zoido JM, Alexandre-Franco MF. Waste Surgical Masks as Precursors of Activated Carbon: A Circular Economy Approach to Mitigate the Impact of Microplastics and Emerging Dye Contaminants. Materials. 2025; 18(17):4115. https://doi.org/10.3390/ma18174115
Chicago/Turabian StyleGarcía-Galán, María del Mar, Carlos A. Fernández-Blanco, Eduardo M. Cuerda-Correa, Juan M. Garrido-Zoido, and María F. Alexandre-Franco. 2025. "Waste Surgical Masks as Precursors of Activated Carbon: A Circular Economy Approach to Mitigate the Impact of Microplastics and Emerging Dye Contaminants" Materials 18, no. 17: 4115. https://doi.org/10.3390/ma18174115
APA StyleGarcía-Galán, M. d. M., Fernández-Blanco, C. A., Cuerda-Correa, E. M., Garrido-Zoido, J. M., & Alexandre-Franco, M. F. (2025). Waste Surgical Masks as Precursors of Activated Carbon: A Circular Economy Approach to Mitigate the Impact of Microplastics and Emerging Dye Contaminants. Materials, 18(17), 4115. https://doi.org/10.3390/ma18174115









