Investigation of Low-Temperature Molten Oxide Electrolysis of a Mixture of Hematite and Zinc Oxide
Highlights
- CO2-free method for recycling electric arc furnace dust is proposed in this study
- Molten oxide electrolysis was conducted using hematite and zinc oxide.
- Thermodynamic calculations were performed to determine oxide decomposition voltages.
- Fe oxide was selectively reduced at 1.1 V and co-reduction with Zn occurred at 1.6 V.
- Fe–Zn alloy was separated by vacuum distillation, yielding high-purity metals.
Abstract
1. Introduction
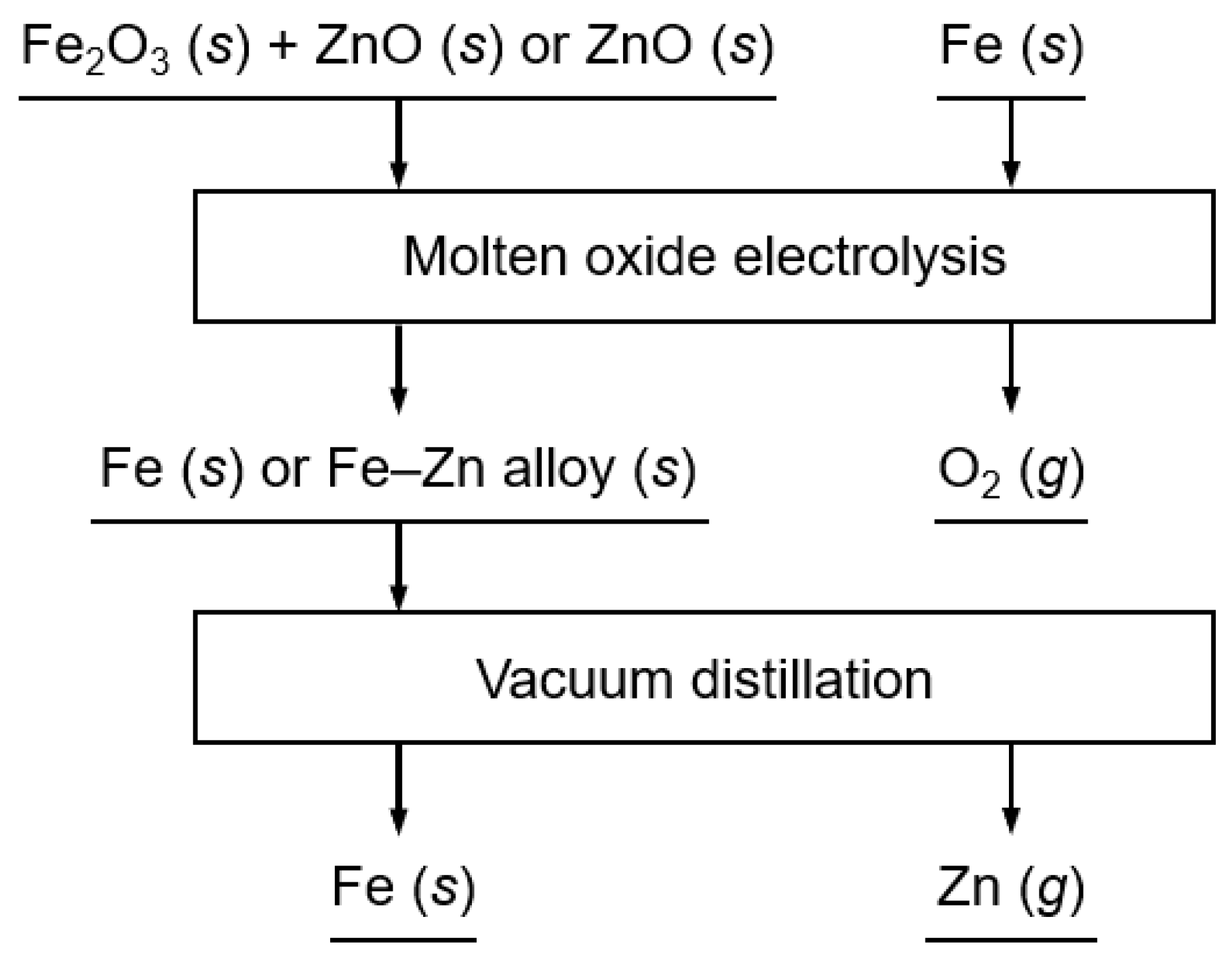
2. Materials and Methods
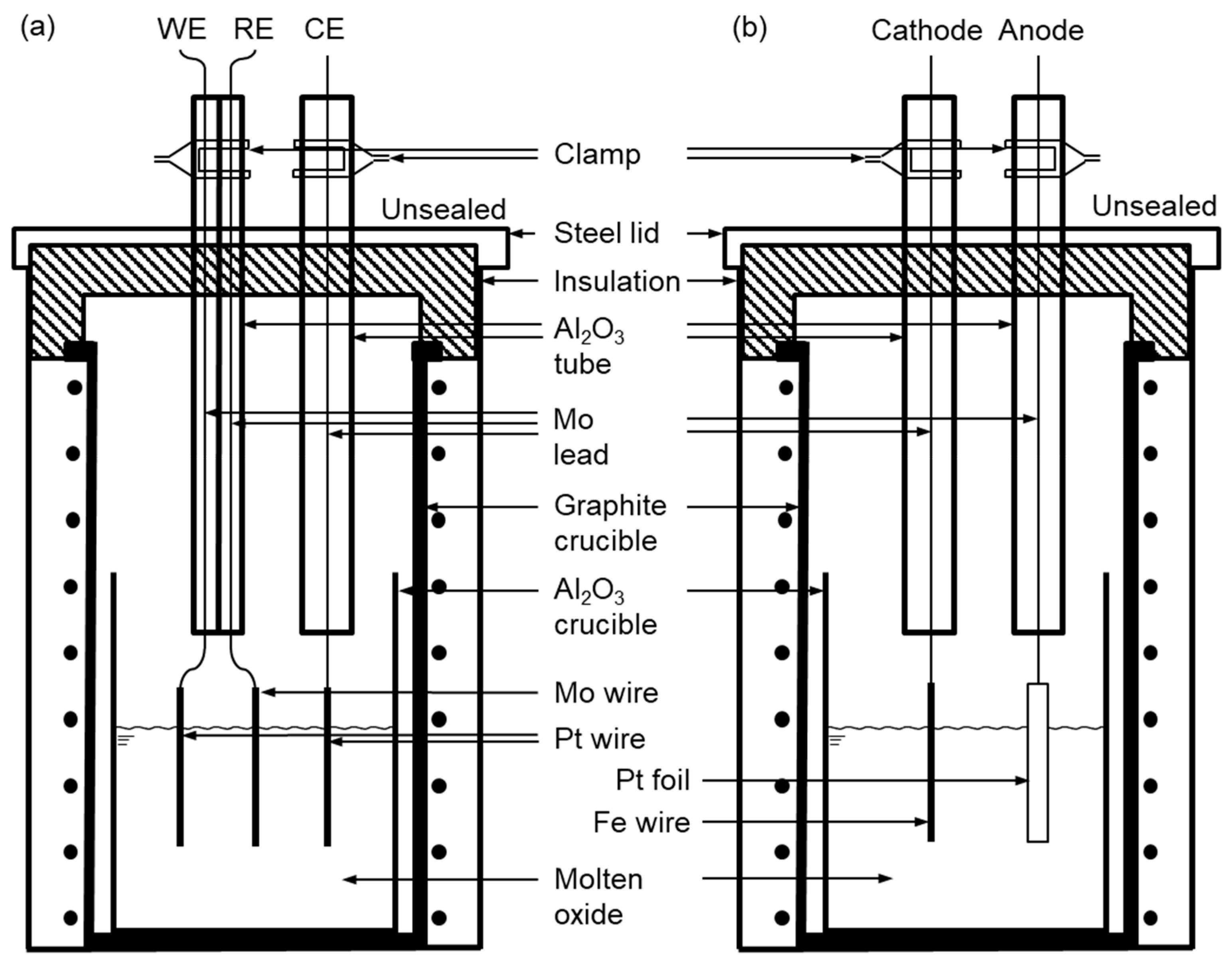
2.1. Cyclic Voltammetry (CV) of Fe2O3 and ZnO in B2O3–Na2O Molten Oxide
2.2. Molten Oxide Electrolysis of Fe2O3 and ZnO Using the Fe Cathode
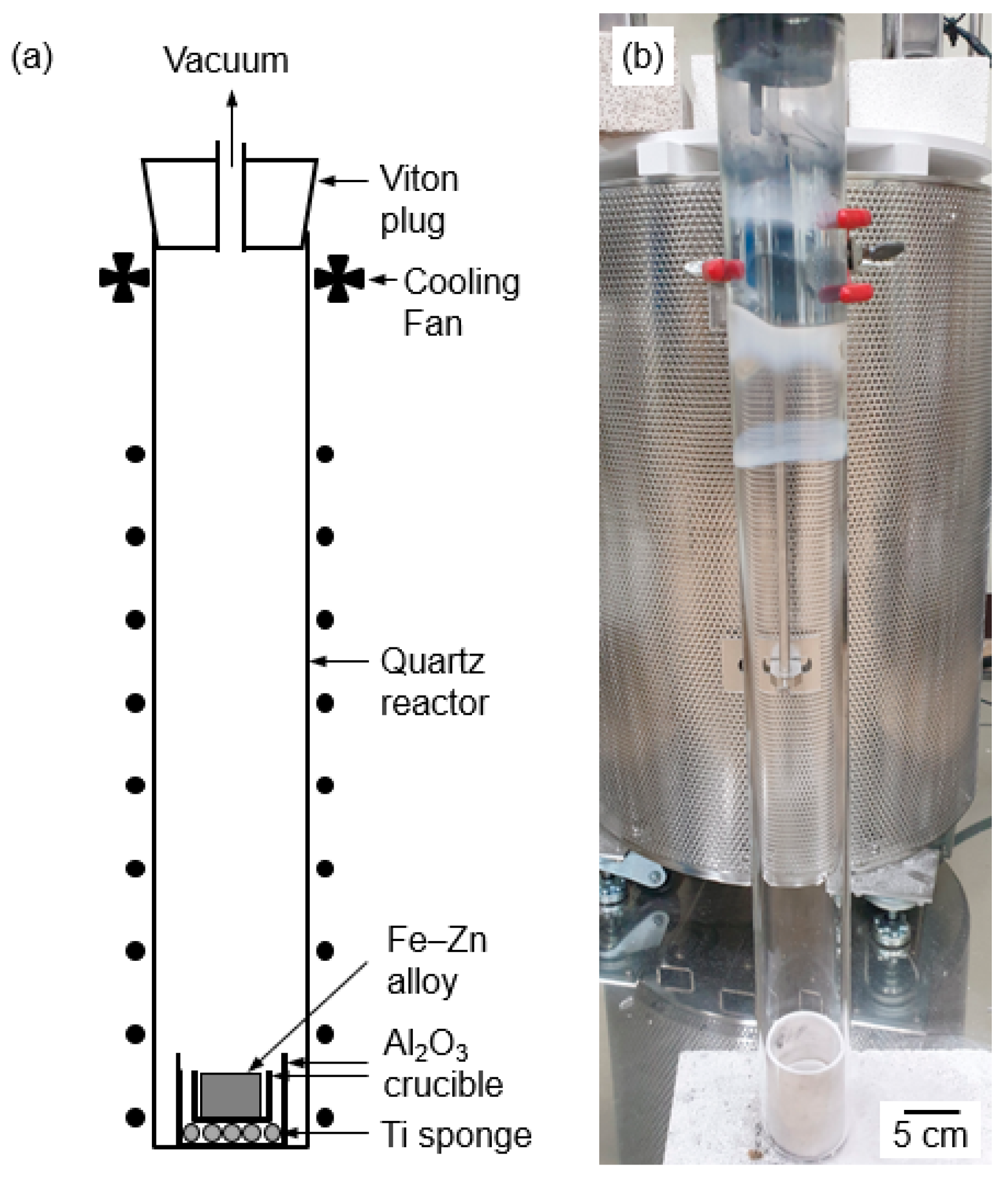
2.3. Vacuum Distillation of the Fe–Zn Alloys
2.4. Analysis
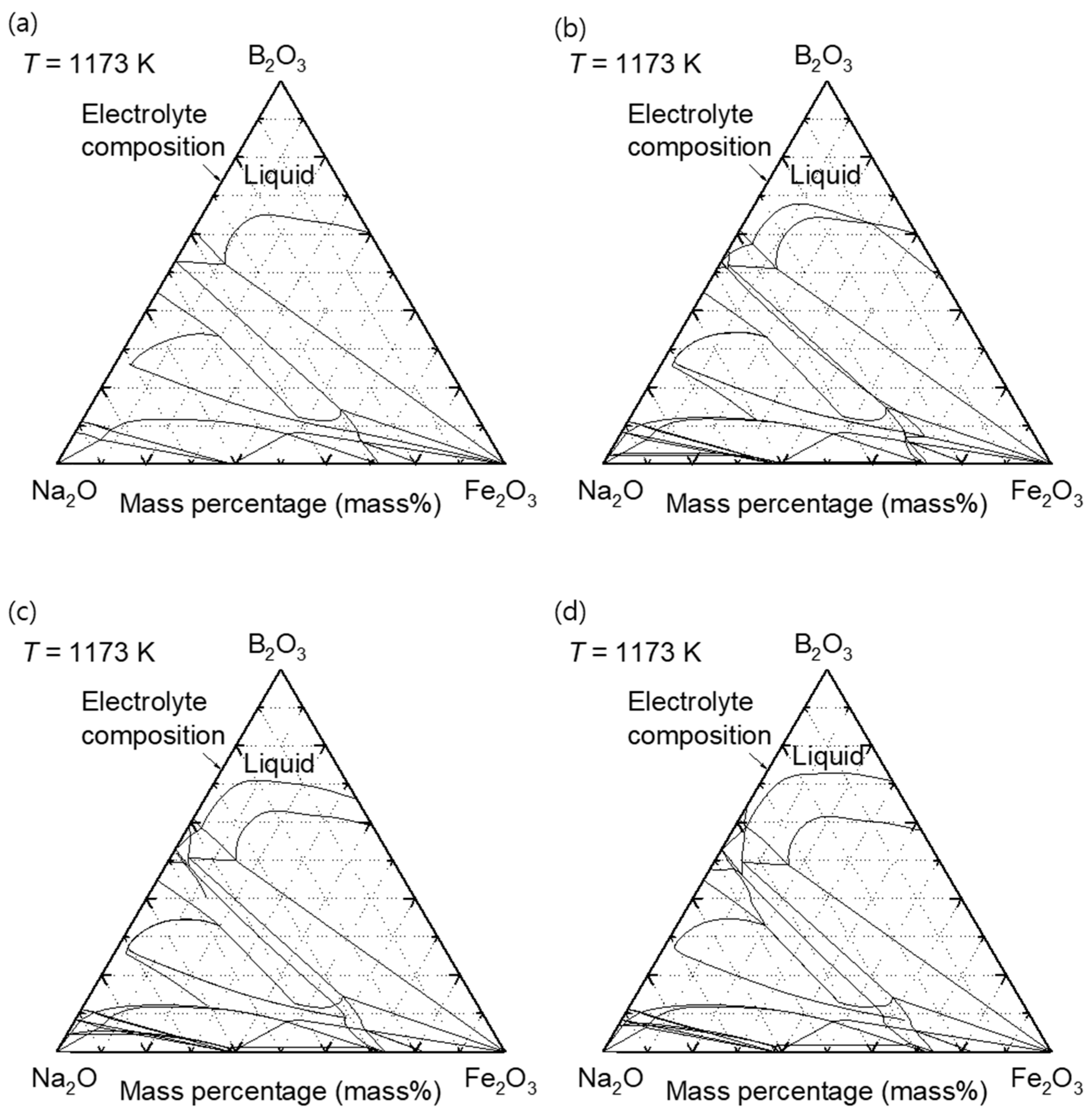
3. Electrolysis Mechanism of Fe2O3 and ZnO in Molten B2O3–Na2O
4. Results and Discussion
4.1. CV Measurements of Fe2O3 and the Mixture of Fe2O3 and ZnO in the Molten Oxide
4.2. Electrolysis of ZnO and the Mixture of Fe2O3 and ZnO in the Molten Oxide
4.2.1. Selective Reduction of the Fe Oxide from B2O3–Na2O–Fe2O3–ZnO Melt
4.2.2. Reduction of ZnO from B2O3–Na2O–ZnO Melt
4.2.3. Co-Reduction of Fe Oxide and ZnO from B2O3–Na2O–Fe2O3–ZnO Melt
4.3. Vacuum Distillation of the Fe–Zn Alloys
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Steel Association. World Steel in Figures 2025. Available online: https://worldsteel.org/data/world-steel-in-figures/world-steel-in-figures-2025/ (accessed on 7 July 2025).
- US Geological Survey. Mineral Commodity Summaries 2025: Iron and Steel. Available online: https://pubs.usgs.gov/periodicals/mcs2025/mcs2025-iron-steel.pdf (accessed on 14 July 2025).
- Sohn, H. Recycling of Common Metals; KNU Press: Daegu, Republic of Korea, 2020; pp. 120–121. [Google Scholar]
- World Steel Association. Steel’s Contribution to a Low Carbon Future and Climate Resilient Societies. Available online: https://www.acero.org.ar/wp-content/uploads/2020/02/Position_paper_climate_2020_vfinal.pdf (accessed on 14 July 2025).
- Pistorius, P.C. Energy requirements for electrification of ironmaking and steelmaking. In Proceedings of the Southern African Pyrometallurgy, Misty Hills Conference Centre, Johannesburg, South Africa, 12–14 March 2024. [Google Scholar]
- Patisson, F.; Mirgaux, O. Hydrogen ironmaking: How it works. Metals 2020, 10, 922. [Google Scholar] [CrossRef]
- IEAGHG. Clean Steel: An Environmental and Technoeconomic Outlook of a Disruptive Technology. In IEAGHG Technical Report; IEAGHG: Cheltenham, UK, 2024. [Google Scholar]
- Allanore, A.; Yin, L.; Sadoway, D.R. A new anode material for oxygen evolution in molten oxide electrolysis. Nature 2013, 497, 353–356. [Google Scholar] [CrossRef]
- Zhang, K.; Jiao, H.; Zhou, Z.; Jiao, S.; Zhu, H. Electrochemical behavior of Fe (III) ion in CaO–MgO–SiO2–Al2O3–NaF–Fe2O3 melts at 1673 K. J. Electrochem. Soc. 2016, 163, D710. [Google Scholar] [CrossRef]
- Wiencke, J.; Lavelaine, H.; Panteix, P.-J.; Petitjean, C.; Rapin, C. Electrolysis of iron in a molten oxide electrolyte. J. Appl. Electrochem. 2018, 48, 115–126. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y. Electroreduction mechanism and electrodeposition of ferric ions in CaO–SiO2–Al2O3–Fe2O3 slags at 1773 K. Can. Metall. Q. 2023, 62, 119–124. [Google Scholar] [CrossRef]
- Kim, H.; Paramore, J.D.; Allanore, A.; Sadoway, D.R. Stability of iridium anode in molten oxide electrolysis for ironmaking: Influence of slag basicity. ECS Trans. 2010, 33, 219. [Google Scholar] [CrossRef]
- Wang, D.; Gmitter, A.J.; Sadoway, D.R. Production of oxygen gas and liquid metal by electrochemical decomposition of molten iron oxide. J. Electrochem. Soc. 2011, 158, E51. [Google Scholar] [CrossRef]
- Choi, H.-G.; Choi, S.; Kim, M.-K.; Jang, J.; Nam, K.T.; Jung, I.-H.; Yi, K.-W. Electrolysis of iron with oxygen gas evolution from molten sodium borate electrolytes. Ironmak. Steelmak. 2021, 48, 1030–1037. [Google Scholar] [CrossRef]
- Park, K.W.; Sohn, I. Electrochemical reduction of K2MoO4–Fe2O3 binary melts using a consumable steel electrode to produce ferromolybdenum alloys. J. Sustain. Metall. 2023, 9, 753–762. [Google Scholar] [CrossRef]
- Zhou, Z.; Jiao, H.; Tu, J.; Zhu, J.; Jiao, S. Direct production of Fe and Fe–Ni alloy via molten oxides electrolysis. J. Electrochem. Soc. 2017, 164, E113. [Google Scholar] [CrossRef]
- Haarberg, G.M.; Kvalheim, E. Electrochemical behavior of dissolved Fe2O3 in molten CaCl2–KF. J. Electrochem. Soc. 2017, 164, E113. [Google Scholar] [CrossRef]
- Haarberg, G.M.; Kvalheim, E.; Rolseth, S.; Murakami, T.; Pietrzyk, S.; Wang, S. Electrodeposition of iron from molten mixed chloride/fluoride electrolytes. ECS Trans. 2007, 3, 341. [Google Scholar] [CrossRef]
- Li, H.; Jia, L.; Liang, J.-l.; Yan, H.-y.; Cai, Z.-y.; Reddy, R.G. Study on the direct electrochemical reduction of Fe2O3 in NaCl–CaCl2 melt. Int. J. Electrochem. Sci. 2019, 14, 11267–11278. [Google Scholar] [CrossRef]
- Xie, K.; Kamali, A.R. Molten salt electrochemical production and in situ utilization of hydrogen for iron production. Int. J. Hydrogen Energy 2019, 44, 24353–24359. [Google Scholar] [CrossRef]
- Li, G.; Wang, D.; Chen, Z. Direct reduction of solid Fe2O3 in molten CaCl2 by potentially green process. J. Mater. Sci. Technol. 2009, 25, 767. [Google Scholar]
- Liu, C.; Liang, J.; Li, H.; Yan, H.; Zheng, S.; Cao, W.; Wang, L. The electrochemical reduction mechanism of ZnFe2O4 in NaCl–CaCl2 melts. Crystals 2021, 11, 925. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, H.; Jing, Z.; Qi, X.; Li, H.; Liang, J. Effect of Fe2O3 on electro-deoxidation in Fe2O3–Al2O3–NaCl–KCl system. Crystals 2021, 11, 1026. [Google Scholar] [CrossRef]
- Panigrahi, M.; Iizuka, A.; Shibata, E.; Nakamura, T. Fe–Ti alloy production by electrolytic reduction of (Fe, Ti) oxide electrodes in molten calcium chloride. In TT Chen Honorary Symposium on Hydrometallurgy, Electrometallurgy and Materials Characterization; TMS: Warrendale, PA, USA, 2012; pp. 273–284. [Google Scholar]
- Qiu, G.; Wang, D.; Ma, M.; Jin, X.; Chen, G.Z. Electrolytic synthesis of TbFe2 from Tb4O7 and Fe2O3 powders in molten CaCl2. J. Electroanal. Chem. 2006, 589, 139–147. [Google Scholar] [CrossRef]
- Tang, D.; Yin, H.; Xiao, W.; Zhu, H.; Mao, X.; Wang, D. Reduction mechanism and carbon content investigation for electrolytic production of iron from solid Fe2O3 in molten K2CO3–Na2CO3 using an inert anode. J. Electroanal. Chem. 2013, 689, 109–116. [Google Scholar] [CrossRef]
- Cox, A.; Fray, D.J. Mechanistic investigation into the electrolytic formation of iron from iron (III) oxide in molten sodium hydroxide. J. Appl. Electrochem. 2008, 38, 1401–1407. [Google Scholar] [CrossRef]
- Wang, S.; Ge, J.; Hu, Y.; Zhu, H.; Jiao, S. Electrochemical reduction of iron oxide in molten sodium hydroxide based on a Ni0.94Si 0.04Al0.02 metallic inert anode. Electrochim. Acta 2013, 87, 148–152. [Google Scholar] [CrossRef]
- Kim, M.-K.; Jung, I.-H. Coupled experimental phase diagram study and thermodynamic optimization of the Na2O–B2O3–Fe2O3 system in air. Calphad 2022, 76, 102364. [Google Scholar] [CrossRef]
- Kim, M.-K.; Jung, I.-H. Coupled phase diagram study and thermodynamic modeling of the Na2O–B2O3–ZnO system. J. Eur. Ceram. Soc. 2024, 44, 7370–7382. [Google Scholar] [CrossRef]
- Claes, P.; Coq, J.; Glibert, J. Electrical conductivity of molten B2O3–Na2O mixtures. Electrochim. Acta 1988, 33, 347–352. [Google Scholar] [CrossRef]
- Al-Zaibani, M.; Althobiti, R.; El Agammy, E.; Alzahrani, E.; El-Damrawi, G.; Doweidar, H.; Al-Muntaser, A. Electrical conduction in ternary Na2O–ZnO–B2O3 glasses; a unique dependence on the mobility of Na+ ions as main charge carriers. J. Non-Crystal. Solids 2025, 650, 123367. [Google Scholar] [CrossRef]
- Tijaria, M.; Sharma, Y.; Kumar, V.; Dahiya, S.; Dalal, J. Effect of Na2O on physical, structural and electrical properties of borate glasses. Mater. Today Proc. 2021, 45, 3722–3725. [Google Scholar] [CrossRef]
- Kim, H.; Paramore, J.; Allanore, A.; Sadoway, D.R. Electrolysis of molten iron oxide with an iridium anode: The role of electrolyte basicity. J. Electrochem. Soc. 2011, 158, E101. [Google Scholar] [CrossRef]
- Asai, K.; Yokokawa, T. Thermodynamic activity of Na2O in Na2O–B2O3–SiO2 melt. Trans. Jpn Inst. Met. 1982, 23, 571–577. [Google Scholar] [CrossRef]
- Konakov, V. A study of the acid-base properties of melts in the Na2O–B2O3–SiO2 system: II. Composition Joins with Sodium Oxide Contents of 25, 30, and 35 mol%. Glass Phys. Chem. 2002, 28, 135–138. [Google Scholar] [CrossRef]
- Park, J.H.; Min, D.J. Thermodynamic behavior of Na2O–B2O3 melt. Metall. Mater. Trans. B 2001, 32, 297–303. [Google Scholar] [CrossRef]
- Popov, K.I.; Nikolić, N.D. General theory of disperse metal electrodeposits formation. In Electrochemical Production of Metal Powders; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–62. [Google Scholar] [CrossRef]


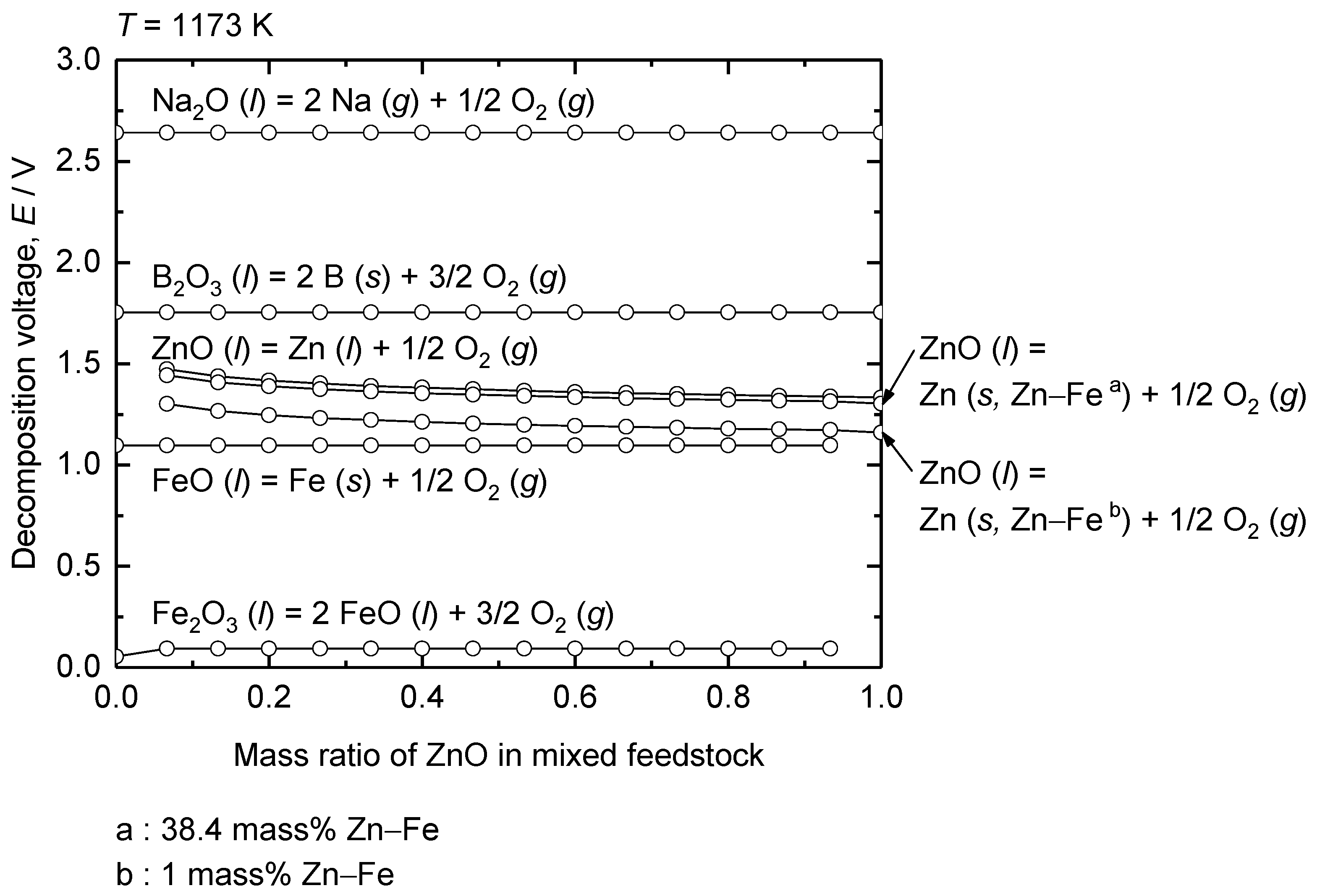
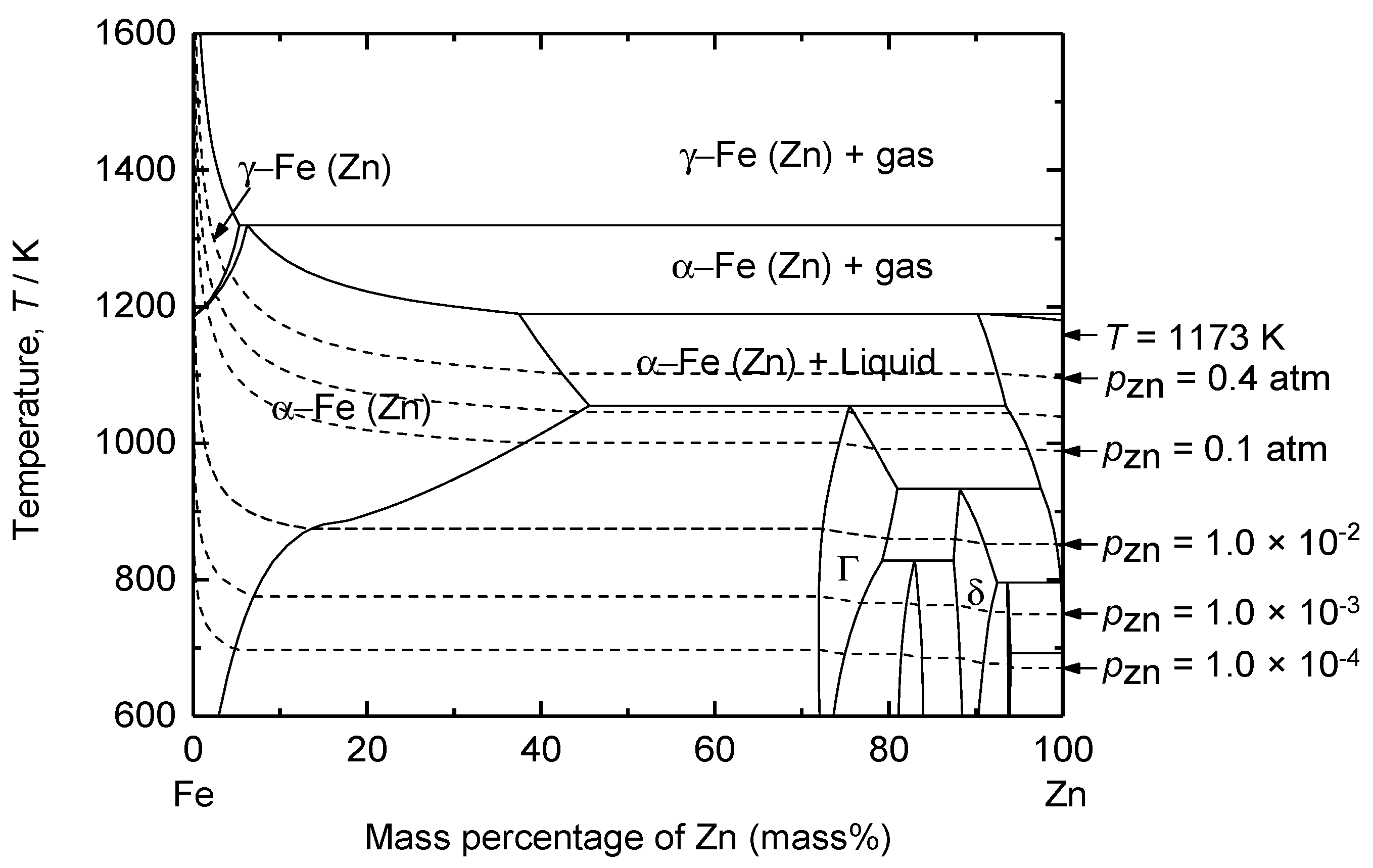
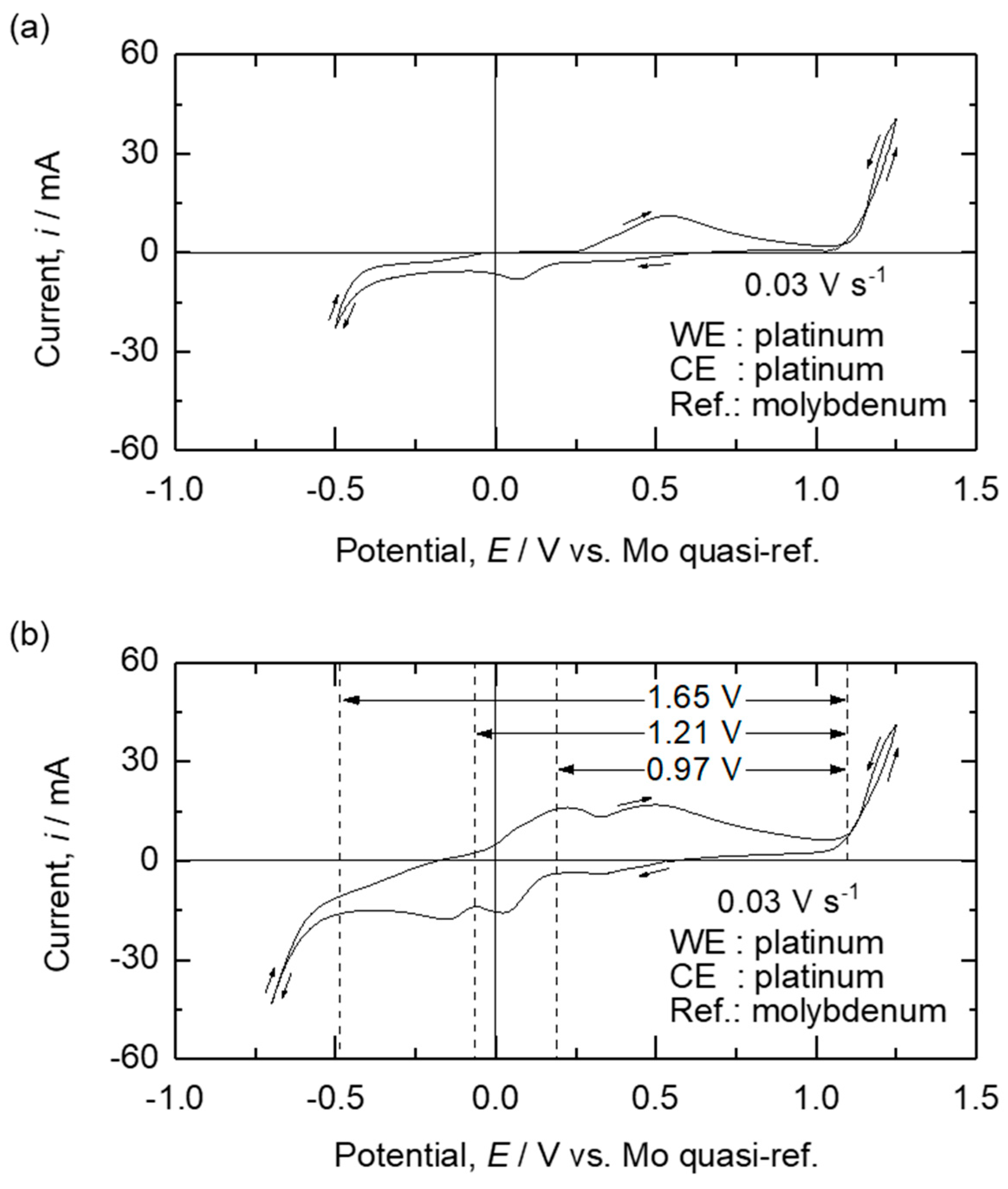
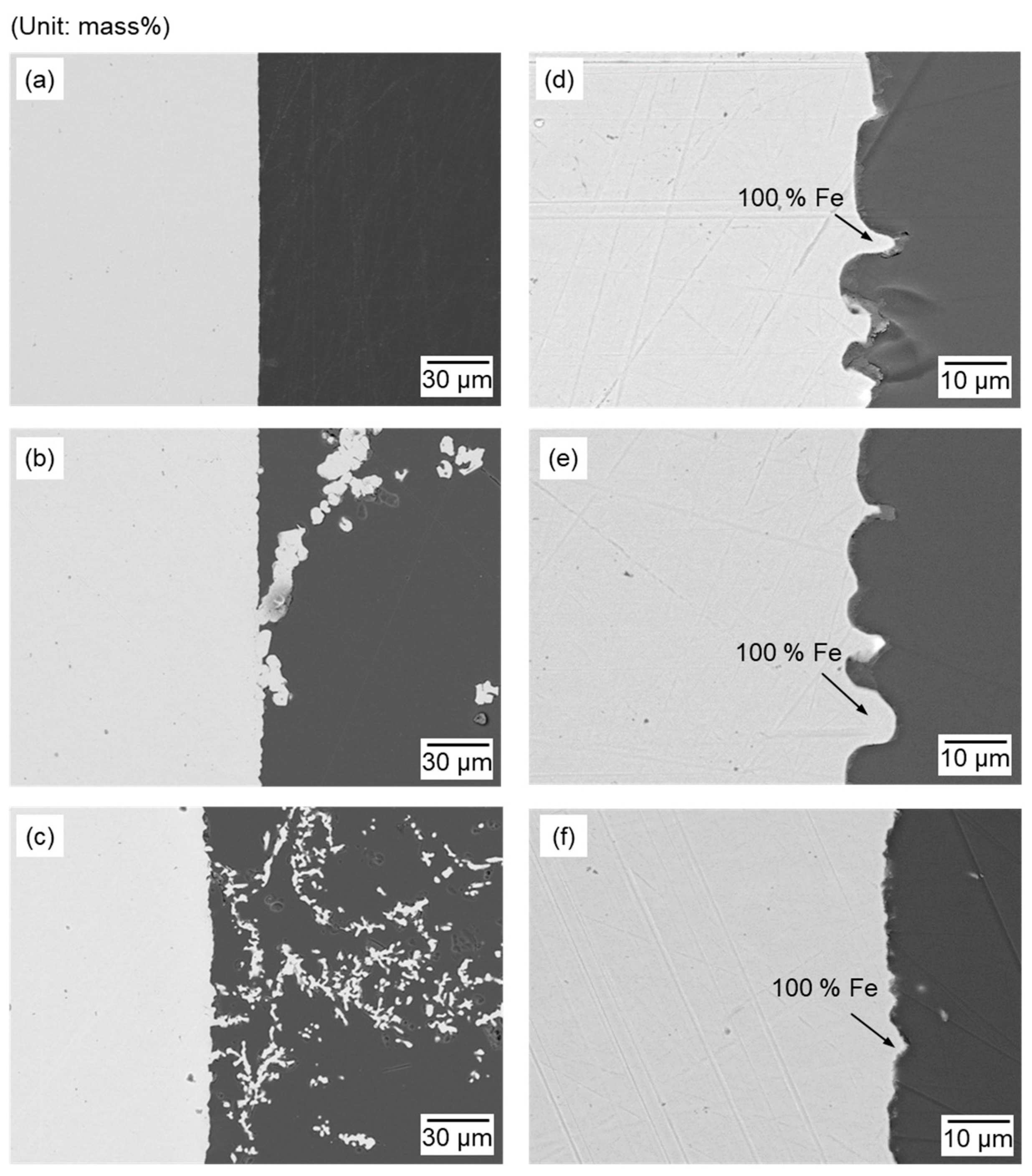


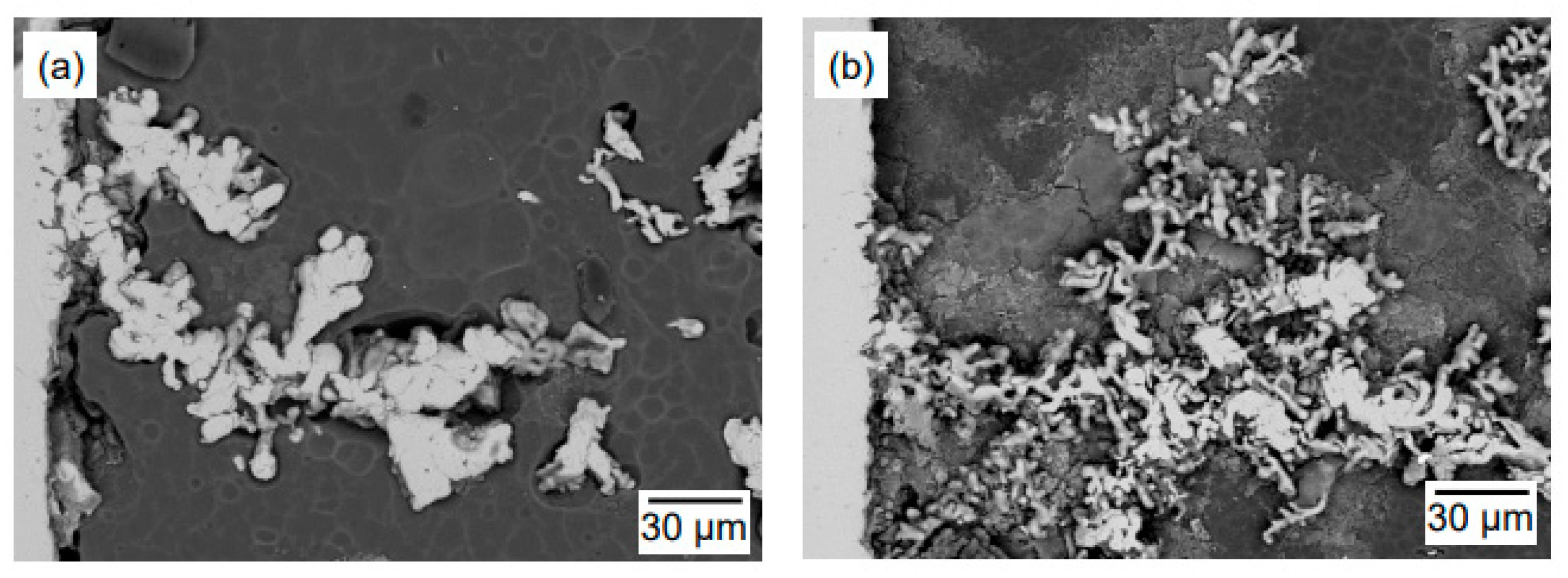
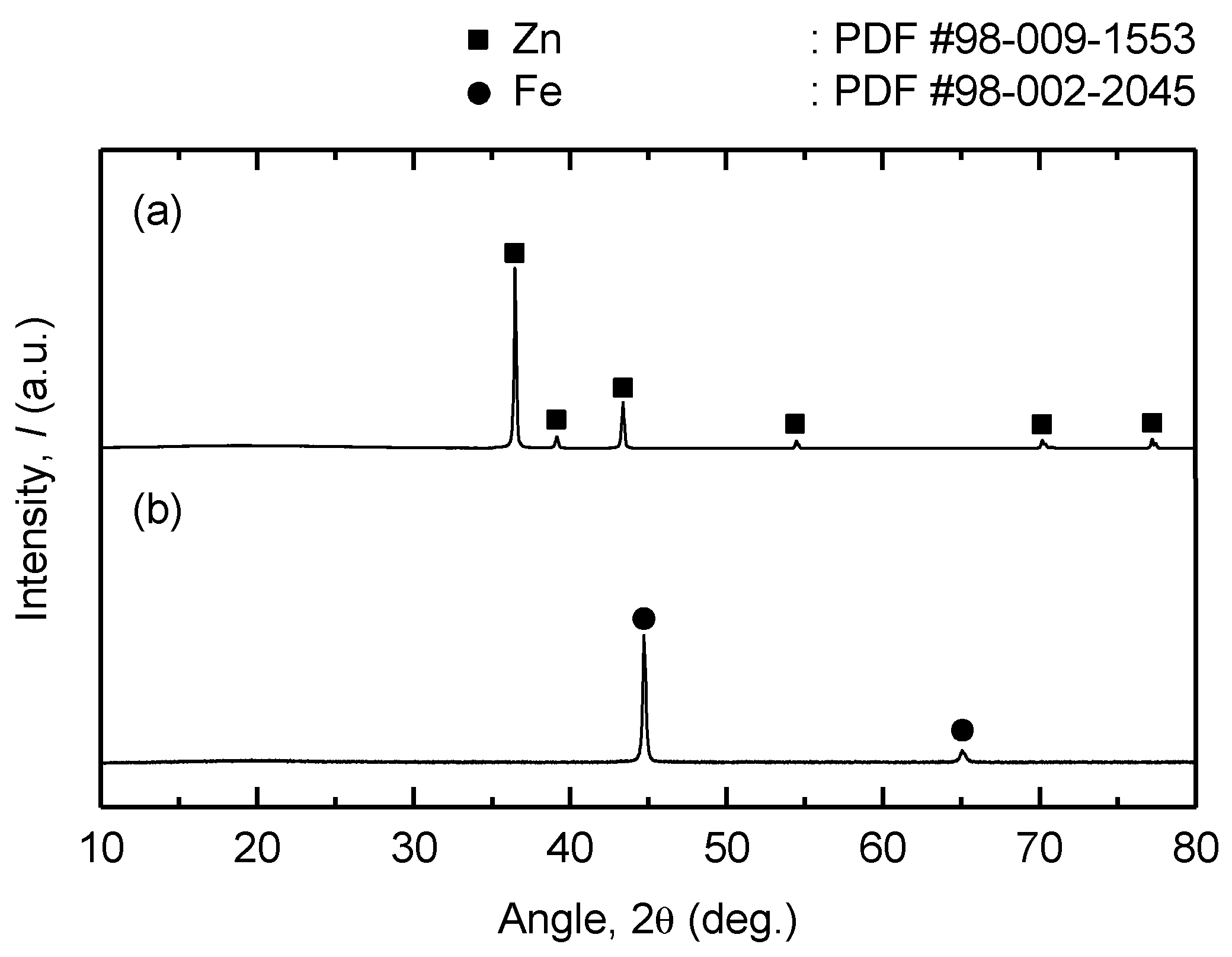
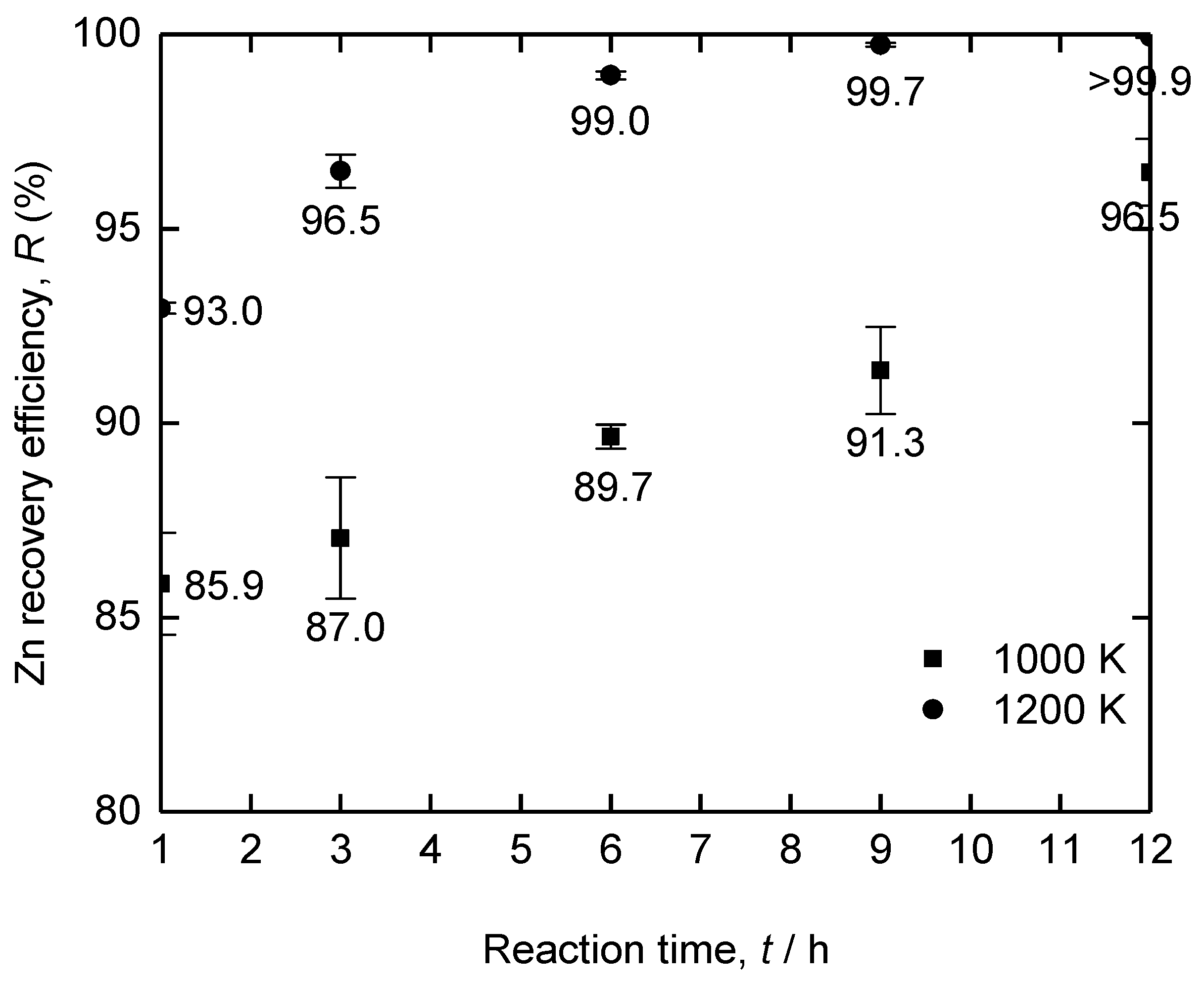
| Method | Electrolyte | Feedstock | Temp., T/K | Electrode for Electrolysis | Cathode Product | Cell Voltage, E/V | Faradaic Efficiency, (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | FeOx Conc., (mass%) | Cathode | Anode | |||||||
| Molten oxide electrolysis | Al2O3–CaO–MgO | Fe3O4 | 10 | 1838 | Mo | Cr90Fe10 | Fe | 3.8 | 34 | [8] |
| Al2O3–CaO–MgO–SiO2 | Fe2O3 | 10 | 1873 | Mo | Graphite | Fe | 2 | 32 | [9] | |
| Al2O3–MgO–SiO2 | Fe3O4 | 15 | 1823 | Pt–Rh | Pt | Fe | 3 | 36 | [10] | |
| Al2O3–CaO–SiO2 | Fe2O3 | 5 | 1773–1873 | Mo | Graphite | Fe | – | 64–83 | [11] | |
| Al2O3–CaO–MgO–SiO2 | Fe2O3 | 9.1 | 1823 | Mo | Ir | Fe | 2 | 25 a | [12] | |
| Al2O3–CaO–MgO–SiO2 | Fe2O3 | 5 | 1848 | Mo | Ir | Fe | – | – | [13] | |
| B2O3–Na2O | Fe2O3 | 5 | 1273 | Pt | Pt | Fe | 1.4 | 33.2 | [14] | |
| K2MoO4–Fe2O3 | Fe2O3 | – | 1273 | Steel | Steel | Fe–Mo | – | 70.77 | [15] | |
| Al2O3–CaO–MgO–SiO2 | Fe2O3, NiO | 15 | 1723 | W | Graphite | Fe–Ni | – | 46 | [16] | |
| Molten salt electrolysis | CaCl2–KF | Fe2O3 | 1.7 | 1100 | Fe | Fe3O4 | Fe | – | – | [17] |
| CaCl2–CaF2 | Fe2O3 | 1.5 | 1163 | Fe | Fe3O4 | Fe | – | 92 | [18] | |
| NaCl–CaCl2 | Fe2O3 b | – | 1073 | Fe2O3 | Graphite | Fe | 1.2 | 95.3 | [19] | |
| LiCl | Fe2O3 b | – | 933 | Fe2O3 | Graphite | Fe | 0.97 | 97 | [20] | |
| CaCl2 | Fe2O3 b | – | 1073 | Fe2O3 | Graphite | Fe | 1.8 | 90 | [21] | |
| NaCl–CaCl2 | ZnFe2O4 b | – | 1073 | ZnFe2O4 | Graphite | Fe c | 1.8 | – | [22] | |
| NaCl–KCl | Fe2O3, Al2O3 b | – | 1123 | Fe2O3– Al2O3 | Graphite | FeO–Al2O3 | 2.3 | – | [23] | |
| CaCl2 | Fe2O3, TiO2 b | – | 1223 | Fe2O3– TiO2 | Graphite | Fe–Ti | 3 | – | [24] | |
| CaCl2 | Fe2O3, Tb4O7 b | – | 1173 | Fe2O3– Tb4O7 | Graphite | Fe–Tb | 2.6 | 97 | [25] | |
| Molten carbonate electrolysis | Na2CO3–K2CO3 | Fe2O3 b | – | 1023 | Fe2O3 | Ni–Cu–Fe | Fe | 2 | 93.6 | [26] |
| Molten hydroxide electrolysis | NaOH | Fe2O3 b | – | 803 | Fe2O3 | Ni | Fe | 1.7 | 89 | [27] |
| NaOH | Fe2O3 b | – | 773 | Fe2O3 | Ni–Si–Al | Fe | 1.7 | 30 | [28] | |
| Exp. No. a | Weight of Feed, wfeed/g | Mass Ratio of Oxide to Feed, roxide/feed | Applied Cell Voltage, E/V | Time, t/h | ||
|---|---|---|---|---|---|---|
| Fe2O3 | ZnO | Fe2O3 | ZnO | |||
| 1-1 | 2.25 | 0.75 | 0.75 | 0.25 | 1.10 | 1 |
| 1-2 | 1.50 | 1.50 | 0.50 | 0.50 | 1.10 | 1 |
| 1-3 | 0.75 | 2.25 | 0.25 | 0.75 | 1.10 | 1 |
| 2-1 | 2.25 | 0.75 | 0.75 | 0.25 | 1.60 | 1 |
| 2-2 | 1.50 | 1.50 | 0.50 | 0.50 | 1.60 | 1 |
| 2-3 | 0.75 | 2.25 | 0.25 | 0.75 | 1.60 | 1 |
| 3-1 | 0.00 | 3.00 | 0.00 | 1.00 | 1.60 | 1 |
| 3-2 | 0.00 | 3.00 | 0.00 | 1.00 | 1.60 | 2 |
| 3-3 | 0.00 | 3.00 | 0.00 | 1.00 | 1.60 | 3 |
| Reaction | Decomposition Voltage, E/V | Phase Transformation | ||
|---|---|---|---|---|
| 1173 K | 1273 K | 1373 K | ||
| FeO (s) = Fe (s) + 1/2 O2 (g) | 0.98 | 0.94 | 0.91 | Fe (BCC) → Fe (FCC) at 1184.81 K |
| ZnO (s) = Zn (l,g) + 1/2 O2 (g) | 1.19 | 1.09 | 0.98 | Zn (l) → Zn (g) at 1181.47 K |
| B2O3 (l) = 2 B (s) + 3/2 O2 (g) | 1.70 | 1.66 | 1.62 | – |
| Na2O (s) = 2 Na (g) + 1/2 O2 (g) | 1.33 | 1.18 | 1.03 | Na2O (β) → Na2O (α) at 1243 K |
| Fe2O3 (s) = 2 Fe (s) + 3/2 O2 (g) | 0.90 | 0.86 | 0.81 | Fe (BCC) → Fe (FCC) at 1184.81 K |
| Fe3O4 (s) = 3 Fe (s) + 2 O2 (g) | 0.96 | 0.92 | 0.88 | Fe (BCC) → Fe (FCC) at 1184.81 K |
| Fe2O3 (s) = 2 FeO (s) + 1/2 O2 (g) | 0.74 | 0.68 | 0.62 | – |
| Sample | Temp., T/K | Time, t/h | Concentration of Element i, Ci (mass%) | |
|---|---|---|---|---|
| Fe | Zn | |||
| Fe–Zn feed | – | – | 72.894 | 27.105 |
| Zn deposit | 1200 | 12 | 0.003 | 99.996 |
| Residues of No. 1 | 1000 | 1 | 94.955 | 5.044 |
| Residues of No. 2 | 1000 | 3 | 95.380 | 4.619 |
| Residues of No. 3 | 1000 | 6 | 96.336 | 3.663 |
| Residues of No. 4 | 1000 | 9 | 96.764 | 3.235 |
| Residues of No. 5 | 1000 | 12 | 98.667 | 1.332 |
| Residues of No. 6 | 1200 | 1 | 97.407 | 2.592 |
| Residues of No. 7 | 1200 | 3 | 98.703 | 1.296 |
| Residues of No. 8 | 1200 | 6 | 99.610 | 0.389 |
| Residues of No. 9 | 1200 | 9 | 99.901 | 0.098 |
| Residues of No. 10 | 1200 | 12 | 99.978 | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Jung, I.-H.; Kang, J.; Yi, K.-W. Investigation of Low-Temperature Molten Oxide Electrolysis of a Mixture of Hematite and Zinc Oxide. Materials 2025, 18, 4116. https://doi.org/10.3390/ma18174116
Kim J, Jung I-H, Kang J, Yi K-W. Investigation of Low-Temperature Molten Oxide Electrolysis of a Mixture of Hematite and Zinc Oxide. Materials. 2025; 18(17):4116. https://doi.org/10.3390/ma18174116
Chicago/Turabian StyleKim, Joongseok, In-Ho Jung, Jungshin Kang, and Kyung-Woo Yi. 2025. "Investigation of Low-Temperature Molten Oxide Electrolysis of a Mixture of Hematite and Zinc Oxide" Materials 18, no. 17: 4116. https://doi.org/10.3390/ma18174116
APA StyleKim, J., Jung, I.-H., Kang, J., & Yi, K.-W. (2025). Investigation of Low-Temperature Molten Oxide Electrolysis of a Mixture of Hematite and Zinc Oxide. Materials, 18(17), 4116. https://doi.org/10.3390/ma18174116







