Zirconium Phosphonate Sorbent Materials—Synthesis, Characterization, and Application for Copper Removal from Acidic Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Solutions Employed in Sorption Studies
- Nine solutions with different concentrations of Cu2+, i.e., 5 mg/L, 10 mg/L, 20 mg/L (designated as Solution 1), 50 mg/L, 100 mg/L, 200 mg/L, 500 mg/L, 1000 mg/L, and 2000 mg/L;
- A solution designated as Solution 2 containing 20 mg/L Cu2+, 1 g/L Na+, and 1 g/L K+;
- A solution designated as Solution 3 containing 20 mg/L Cu2+, 1 g/L Na+, 1 g/L K+, 200 mg/L Mg2+, and 20 mg/L Ca2+;
- A solution designated as Solution 4 simulating acid wastewater from copper mining with the following composition: 20 mg/L Cu2+, 1 g/L Na+, 1 g/L K+, 200 mg/L Mg2+, 20 mg/L Ca2+, 20 mg/L Cd2+, 20 mg/L Mn2+, 20 mg/L Ni2+, 20 mg/L Zn2+, and 10 mg/L Fe3+.
2.2. Synthesis of Zirconium Phosphonate Sorbents
2.3. Physicochemical Characterization
2.4. Sorption Studies
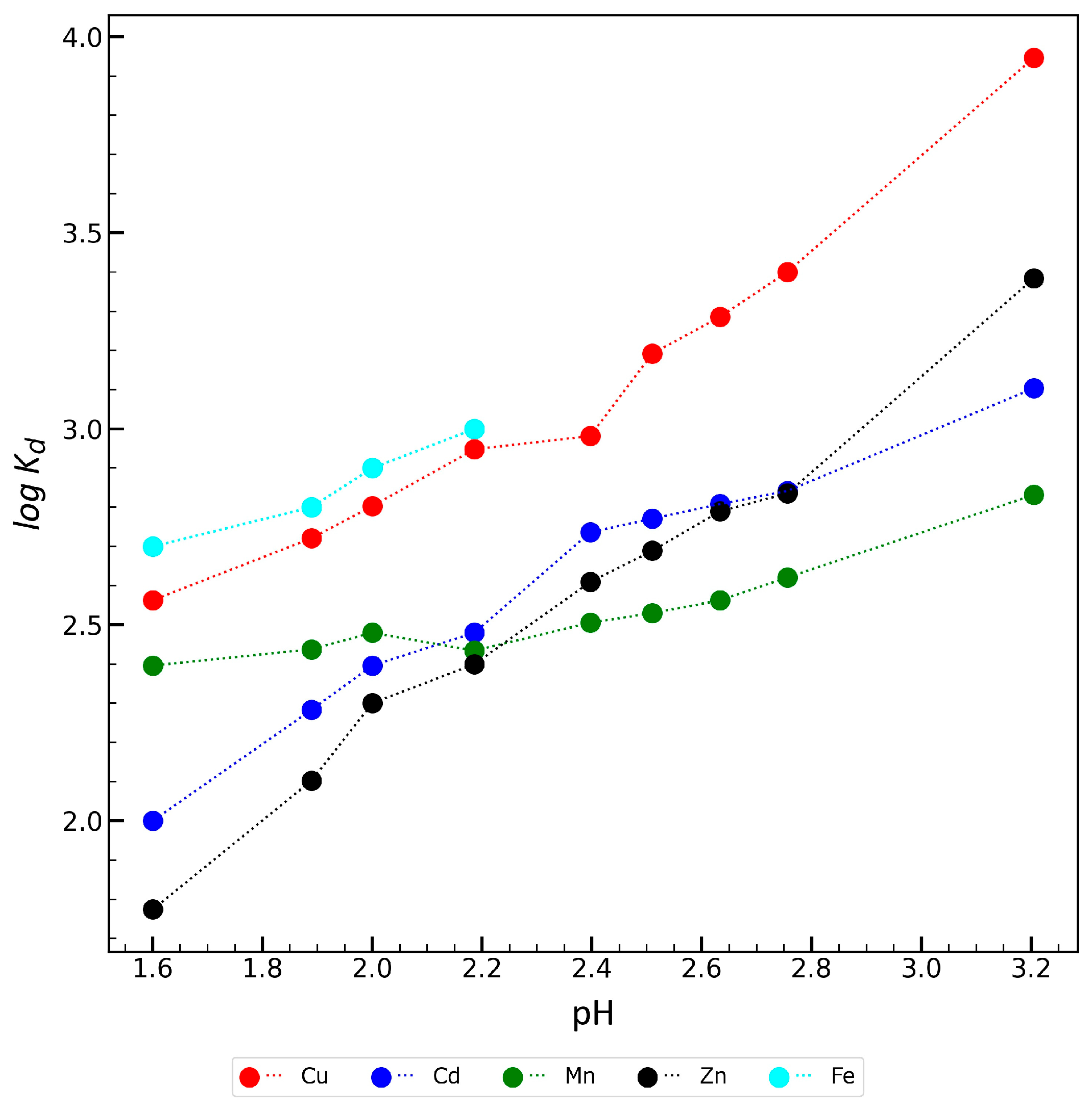
2.5. Studies on the Effect of pH on the Sorption Properties of Zr-ATMP 7 Sorbent
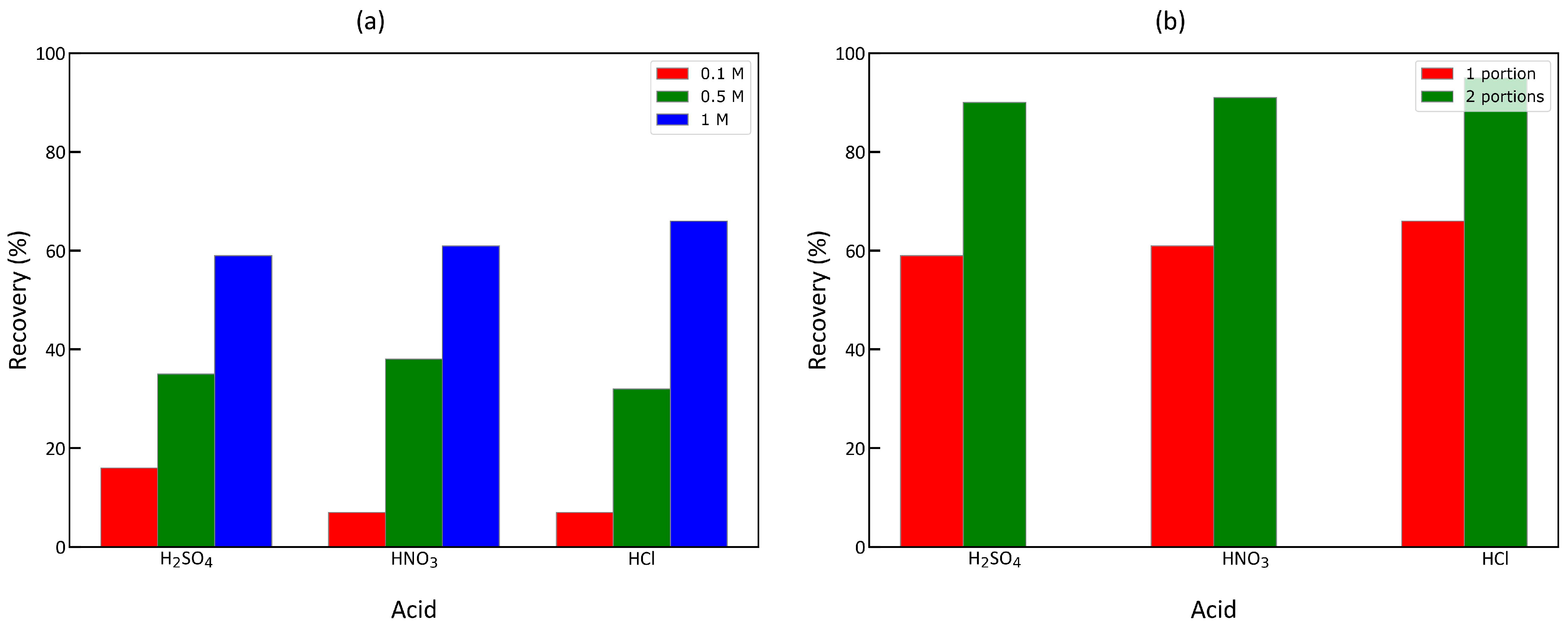
2.6. Regeneration Studies
3. Results and Discussion
3.1. Synthesis and Characterization of Zirconium Aminotris(methylenephosphonate) Sorbents
3.2. Sorption Properties of Zr-ATMP Materials and Selected Reference Resins in Pure Copper Ions Solution—Study of Sorption Isotherms and Kinetics
3.3. Sorption Properties of Zr-ATMP Materials and Selected Reference Resins in Solutions of Increasing Complexity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATMP | aminotris(methylenephosphonic acid) |
| Zr-ATMP | zirconium aminotris(methylenephosphonate) |
| ATR | attenuated total reflection |
| FTIR | Fourier transform infrared spectroscopy |
| SEM | scanning electron microscope |
| EDS | energy dispersive X-ray spectroscopy |
| XRD | X-ray diffraction |
| WHO | World Health Organization |
| IUPAC | International Union of Pure and Applied Chemistry |
| AAS | atomic absorption spectrometry |
| BET | Brunauer–Emmett–Teller |
| BJH | Barrett–Joyner–Halenda |
| ICP-MS | inductively coupled plasma mass spectrometry |
| ROI | residue on ignition |
References
- Regulation (EU) 2024/1252 of the European Parliament and of the Council of 11 April 2024 Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) No 168/2013, (EU) 2018/858, (EU) 2018/1724 and (EU) 2019/1020, OJ L, 2024/1252, 3.5.2024. Available online: https://eur-lex.europa.eu/eli/reg/2024/1252/oj/eng (accessed on 24 March 2025).
- Copper. Outlook for Key Energy Transition Minerals. Available online: https://www.iea.org/reports/copper (accessed on 24 March 2025).
- As Copper Demand Surges, Urban Mining May Save The Day. Available online: https://www.forbes.com/sites/kevinomarah/2024/04/25/as-copper-demand-surges-urban-mining-may-save-the-day/ (accessed on 24 March 2025).
- International Copper Association. Urban Mining. Available online: https://internationalcopper.org/policy-focus/climate-environment/urban-mining/ (accessed on 24 March 2025).
- Waste Mission. Scrap Copper Recycling 101: Everything You Need to Know. Available online: https://wastemission.com/blog/scrap-copper-recycling/ (accessed on 24 March 2025).
- Loibl, A.; Espinoza, T.L.A. Current Challenges in Copper Recycling: Aligning Insights from Material Flow Analysis with Technological Research Developments and Industry Issues in Europe and North America. Resour. Conserv. Recycl. 2021, 169, 105462. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, W.; Li, J.; Wang, X.; Liu, L.; Wang, J. A Critical Review on the Recycling of Copper and Precious Metals from Waste Printed Circuit Boards Using Hydrometallurgy. Front. Environ. Sci. Eng. 2017, 11, 8. [Google Scholar] [CrossRef]
- Jadoun, S.; Chinnam, S.; Jabin, S.; Upadhyay, Y.; Jangid, N.K.; Zia, J. Recovery of Metals from E-waste: Facts, Methods, Challenges, Case Studies, and Sustainable Solutions. Environ. Sci. Technol. Lett. 2025, 12, 8–24. [Google Scholar] [CrossRef]
- Emew Clean Technologies. Recovery and Recycling of Metals From Spent Lithium-Ion Batteries. Available online: https://emew.com/blog/recovery-and-recycling-of-metals-from-spent-lithium-ion-batteries (accessed on 24 March 2025).
- Torrubia, J.; Parvez, A.M.; Sajjad, M.; Paz, F.A.G.; Boogart, K.G.v.d. Recovery of Copper from Electronic Waste: An Energy Transition Approach to Decarbonise the Industry. J. Clean. Prod. 2024, 485, 144349. [Google Scholar] [CrossRef]
- Ullah, M.; Ippolito, N.M.; Spera, L.; Vegliò, F. Treatment of Wastewater Produced During the Hydrometallurgical Extraction of Silver from In-mold Structural Electronics. Case Stud. Chem. Environ. Eng. 2024, 10, 100916. [Google Scholar] [CrossRef]
- Iannicelli-Zubiani, E.M.; Giani, M.I.; Recanati, F.; Dotelli, G.; Puricelli, S.; Cristiani, C. Environmental Impacts of a Hydrometallurgical Process for Electronic Waste Treatment: A Life Cycle Assessment Case Study. J. Clean. Prod. 2017, 140, 1204–1216. [Google Scholar] [CrossRef]
- Sabia, G.; Tammaro, M.; Cerchier, P.; Salluzzo, A.; Brunelli, K. Treatment and Management of the Effluents Generated By Hydrometallurgical Processes Applied to End-of-Life Photovoltaic Panels. J. Water Process Eng. 2022, 47, 102814. [Google Scholar] [CrossRef]
- Moran, E.S.; Prodius, D.; Nlebedim, I.C.; Wright, M.M. Rare-Earth Elements Recovery from Electronic Waste: Techno-Economic and Life Cycle Analysis. ACS Sustain. Chem. Eng. 2024, 12, 14164–14172. [Google Scholar] [CrossRef]
- Milićević, S.; Vlahović, M.; Kragović, M.; Martinović, S.; Milošević, V.; Jovanović, I.; Stojmenović, M. Removal of Copper from Mining Wastewater Using Natural Raw Material—Comparative Study Between the Synthetic and Natural Wastewater Samples. Minerals 2020, 10, 753. [Google Scholar] [CrossRef]
- Balintova, M.; Junakova, N.; Chernysh, Y. The Influence of Acidic Mine Waters on Physico-Chemical Processes in the Aquatic Environment. Eng. Proc. 2023, 57, 4. [Google Scholar] [CrossRef]
- Sasmaz Kislioglu, M. Removal of Ag, Au, and As from Acid Mine Water Using Lemna gibba and Lemna minor—A Performance Analysis. Water 2023, 15, 1293. [Google Scholar] [CrossRef]
- Ab Hamid, N.H.; bin Mohd Tahir, M.I.H.; Chowdhury, A.; Nordin, A.H.; Alshaikh, A.A.; Suid, M.A.; Nazaruddin, N.‘I.; Nozaizeli, N.D.; Sharma, S.; Rushdan, A.I. The Current State-of-Art of Copper Removal from Wastewater: A Review. Water 2022, 14, 3086. [Google Scholar] [CrossRef]
- Bui, T.K.L.; Do-Hong, L.C.; Dao, T.S.; Hoang, T.C. Copper Toxicity and the Influence of Water Quality of Dongnai River and Mekong River Waters on Copper Bioavailability and Toxicity to Three Tropical Species. Chemosphere 2016, 144, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Latorre, M.; Troncoso, R.; Uauy, R. Chapter 4—Biological Aspects of Copper. In Clinical and Translational Perspectives on WILSON DISEASE; Academic Press Cambridge: Cambridge, MA, USA, 2019; pp. 25–31. [Google Scholar]
- Olivares, M.; Uauy, R. Limits of Metabolic Tolerance to Copper and Biological Basis for Present Recommendations and Regulations. Am. J. Clin. Nutr. 1996, 63, 846S–852S. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017; pp. 340–342.
- Regulation of the Minister of Maritime Economy and Inland Navigation of July 12, 2019 on Substances Particularly Harmful to the Aquatic Environment and the Conditions to be Met When Discharging Wastewater into Waters or Ground, as Well as When Discharging Rainwater or Meltwater into Waters or Water Facilities. Journal of Laws 2019, Item 1311 (In Polish). Available online:https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20190001311 (accessed on 24 March 2025).
- Al-Saydeh, S.A.; El-Naas, M.H.; Zaidi, S.J. Copper Removal from Industrial Wastewater: A Comprehensive Review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Rodríguez, C.; Tapia, C.; Leiva-Aravena, E.; Leiva, E. Graphene Oxide–ZnO Nanocomposites for Removal of Aluminum and Copper Ions from Acid Mine Drainage Wastewater. Int. J. Environ. Res. Public Health 2020, 17, 6911. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Cui, Y.; Chen, N. Removal of Copper Ions From Wastewater: A Review. Int. J. Environ. Res. Public Health 2023, 20, 3885. [Google Scholar] [CrossRef]
- Simate, G.S.; Ndlovu, S. Acid Mine Drainage: Challenges and Opportunities. J. Environ. Chem. Eng. 2014, 2, 1785–1803. [Google Scholar] [CrossRef]
- Esmaeili, A.; Mobini, M.; Eslami, H. Removal of Heavy Metals from Acid Mine Drainage by Native Natural Clay Minerals, Batch and Continuous Studies. Appl. Water Sci. 2019, 9, 97. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Bouafif, H.; Neculita, C.M.; Koubaa, A. Performance of Physically and Chemically Activated Biochars in Copper Removal from Contaminated Mine Effluents. Water Air Soil Pollut. 2019, 230, 178. [Google Scholar] [CrossRef]
- Park, S.; Lee, M. Removal of Copper and Cadmium in Acid Mine Drainage Using Ca-Alginate Beads as Biosorbent. Geosci. J. 2017, 21, 373–383. [Google Scholar] [CrossRef]
- Trublet, M.; Scukins, E.; Carabante, I.; Rusanova, D. Competitive Sorption of Metal Ions on Titanium Phosphate Sorbent (TiP1) in Fixed-Bed Columns: A Closed-Mine Waters Study. ACS Sustain. Chem. Eng. 2019, 7, 8145–8154. [Google Scholar] [CrossRef]
- Trublet, M.; Maslova, M.V.; Rusanova, D.; Antzutkin, O.N. Sorption Performances of TiO(OH)(H2PO4)·H2O in Synthetic and Mine Waters. RSC Adv. 2017, 7, 1989. [Google Scholar] [CrossRef]
- Marszałek, M.; Piotrowski, M.; Dziełak, B.; Blicharz, M.; Malarska, W.; Wzorek, Z. Titanium(IV), Zirconium(IV), and Cerium(IV) Phosphates Synthesized Under Mild Conditions—Composition Characteristics and Evaluation of Sorption Properties Towards Copper Ions in Comparison to Commercially Available Ion-Exchange Resins. Materials 2024, 17, 6226. [Google Scholar] [CrossRef]
- Purolite. Metals Removal and Recovery Products. Available online: https://www.purolite.com/index/core-technologies/application/metal-removal-and-recovery (accessed on 24 March 2025).
- Dupont. Separation of Copper from Liquid Media. Available online: https://www.dupont.com/water/periodic-table/copper.html (accessed on 24 March 2025).
- Veliscek-Carolan, J.; Hanley, T.L.; Luca, V. Zirconium Organophosphonates as High Capacity, Selective Lanthanide Sorbents. Sep. Purif. Technol. 2014, 129, 150–158. [Google Scholar] [CrossRef]
- Veliscek-Carolan, J.; Rawal, A.; Luca, V.; Hanley, T.L. Zirconium Phosphonate Sorbents with Tunable Structure and Function. Microporous Mesoporous Mater. 2017, 252, 90–104. [Google Scholar] [CrossRef]
- Xiong, L.; Lv, K.; Gu, M.; Yang, C.; Wu, F.; Han, J.; Hu, S. Efficient Capture of Actinides from Strong Acidic Solution by Hafnium Phosphonate Frameworks with Excellent Acid Resistance and Radiolytic Stability. Chem. Eng. J. 2019, 355, 159–169. [Google Scholar] [CrossRef]
- Qi, S.; Xiong, S.; Xiong, L.; Li, H.; Liu, B.; Liu, Y.; Xiong, K.; Yan, H.; Lv, K.; Liu, H.; et al. Crystalline versus Amorphous: High-Performance Hafnium Phosphonate Framework for the Separation of Uranium and Transuranium Elements. Inorg. Chem. 2023, 62, 10881–10886. [Google Scholar] [CrossRef]
- Luca, V.; Hanna, J.V. A versatile Zr(IV)-Organophosphonate Coordination Polymer Platform for the Selective Adsorption of Lanthanides and Actinides. Hydrometallurgy 2015, 154, 118–128. [Google Scholar] [CrossRef]
- Mu, W.; Chen, B.; Li, X. A Comparative Study of Adsorption Properties of Zirconium (IV) Phosphonates for Removal of 90Sr. Nucl Anal. 2023, 2, 100072. [Google Scholar] [CrossRef]
- Mu, W.; Chen, B.; Li, X.; Wei, H.; Yang, Y.; Peng, S. Preparation of Novel Zirconium Phosphonates for Adsorption of Lutetium. Nucl Anal. 2022, 1, 100010. [Google Scholar] [CrossRef]
- Veliscek-Carolan, J.; Rawal, A.; Oldfield, D.T.; Thorogood, G.J.; Bedford, N.M. Nanoporous Zirconium Phosphonate Materials with Enhanced Chemical and Thermal Stability for Sorbent Applications. ACS Appl. Nano Mater. 2020, 3, 3717–3729. [Google Scholar] [CrossRef]
- Shah, B.; Chudasama, U. Synthesis and Characterization of a Novel Hybrid Material Titanium Amino Tris(methylenephosphonic acid) and Its Application as a Cation Exchanger. Ind. Eng. Chem. Res. 2014, 53, 17454–17467. [Google Scholar] [CrossRef]
- Shah, B.; Chudasama, U. Kinetics, Thermodynamics and Metal Separation Studies of Transition (Co2+, Ni2+, Cu2+, Zn2+) and Heavy Metal Ions (Cd2+, Hg2+, Pb2+) Using Novel Hybrid Ion Exchanger—Zirconium Amino Tris Methylene Phosphonic Acid. Sep. Sci. Technol. 2019, 54, 1560–1572. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, Y.; Wang, R.; Yi, J.; Feng, X.; Xu, O. Mesoporous Zirconium Phosphonate Hybrid Material as Adsorbent to Heavy Metal Ions. Ind. Eng. Chem. Res. 2012, 51, 12266–12273. [Google Scholar] [CrossRef]
- Shah, B.; Chudasama, U. Application of Zirconium Phosphonate—A Novel Hybrid Material as an Ion Exchanger. Desalin. Water Treat. 2012, 38, 276–284. [Google Scholar] [CrossRef]
- Nitrilotrimethylenetris(phosphonic Acid) Registration Dossier. Available online: https://echa.europa.eu/pl/registration-dossier/-/registered-dossier/15198 (accessed on 24 March 2025).
- Ataman Chemicals. ATMP Product Application. Available online: https://atamankimya.com/sayfalar.asp?LanguageID=3&cid=3&id=8&id2=278 (accessed on 24 March 2025).
- PurometTM MTS9300. Product Data Sheet. Available online: https://www.purolite.com/product-pdf/MTS9300.pdf (accessed on 24 March 2025).
- AmberSepTM M4195. Product Data Sheet. Available online: https://www.dupont.com/content/dam/dupont/amer/us/en/water-solutions/public/documents/en/IER-AmberSep-M4195-and-M4195-UPS-PDS-45-D00810-en.pdf (accessed on 24 March 2025).
- Knapik, E.; Rotko, G.; Marszałek, M.; Piotrowski, M. Comparative Study on Lithium Recovery with Ion-Selective Adsorbents and Extractants: Results of Multi-Stage Screening Test with the Use of Brine Simulated Solutions with Increasing Complexity. Energies 2023, 16, 3149. [Google Scholar] [CrossRef]
- Choël, M.; Deboudt, K.; Flament, P. Evaluation of quantitative procedures for X-ray microanalysis of environmental particles. Microsc. Res. Tech. 2007, 70, 996. [Google Scholar] [CrossRef]
- Clearfield, A.; Stynes, J.A. The preparation of crystalline zirconium phosphate and some observations on its ion exchange behaviour. J. Inorg. Nucl. Chem. 1964, 26, 117. [Google Scholar] [CrossRef]
- Glover, T.G.; Peterson, G.W.; DeCoste, J.B.; Browe, M.A. Adsorption of Ammonia by Sulfuric Acid Treated Zirconium Hydroxide. Langmuir 2012, 28, 10478. [Google Scholar] [CrossRef]

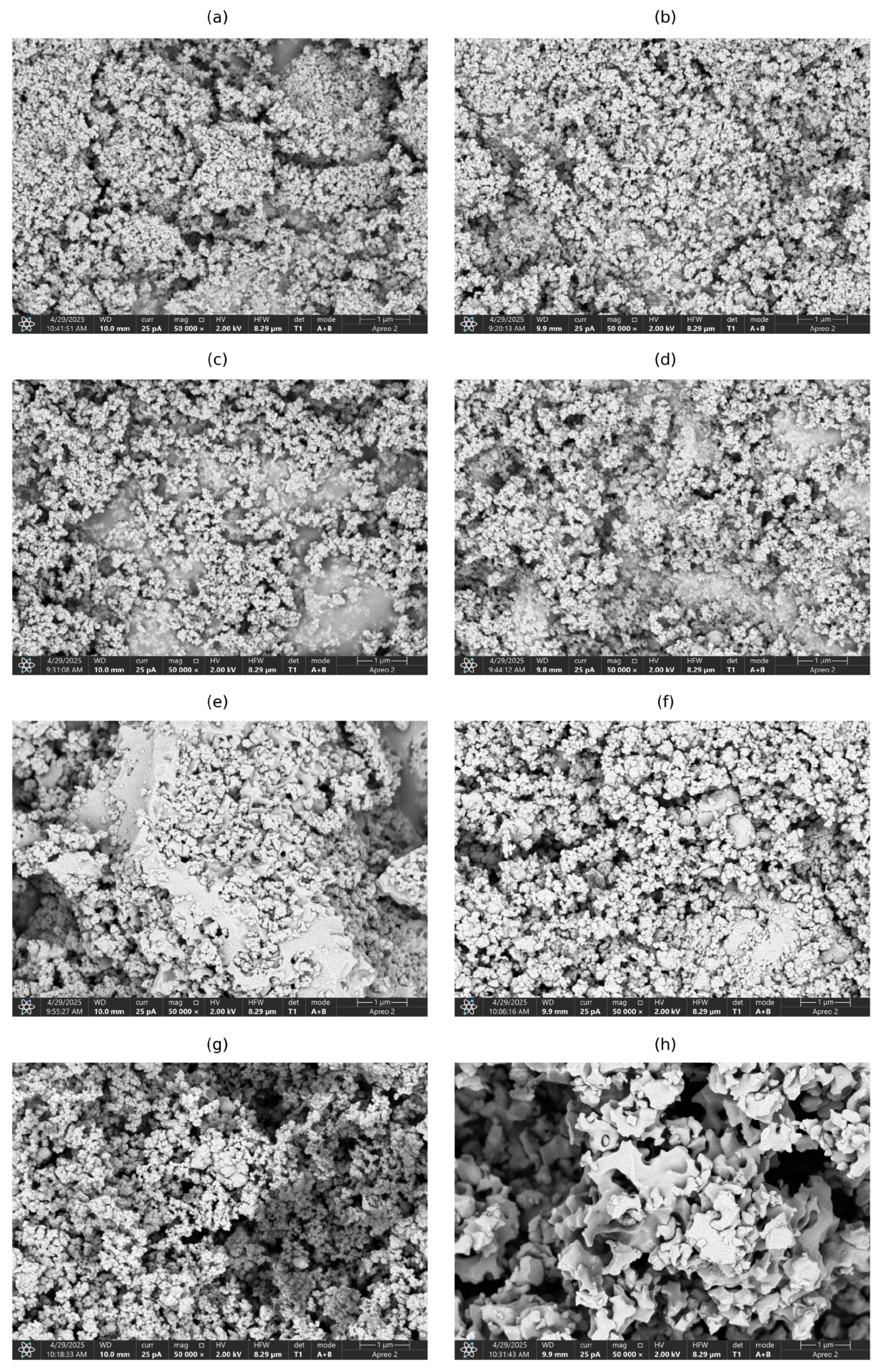
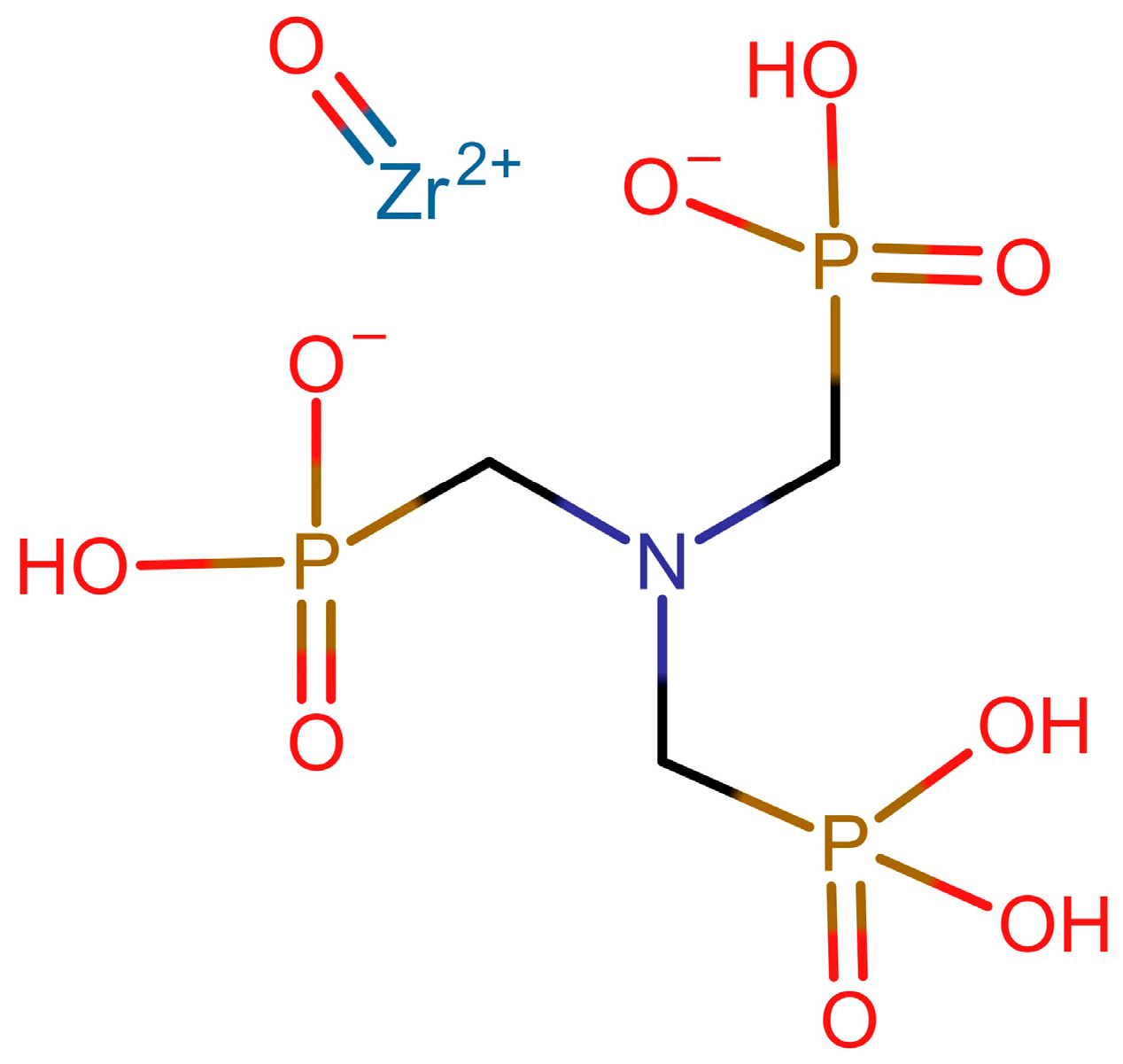


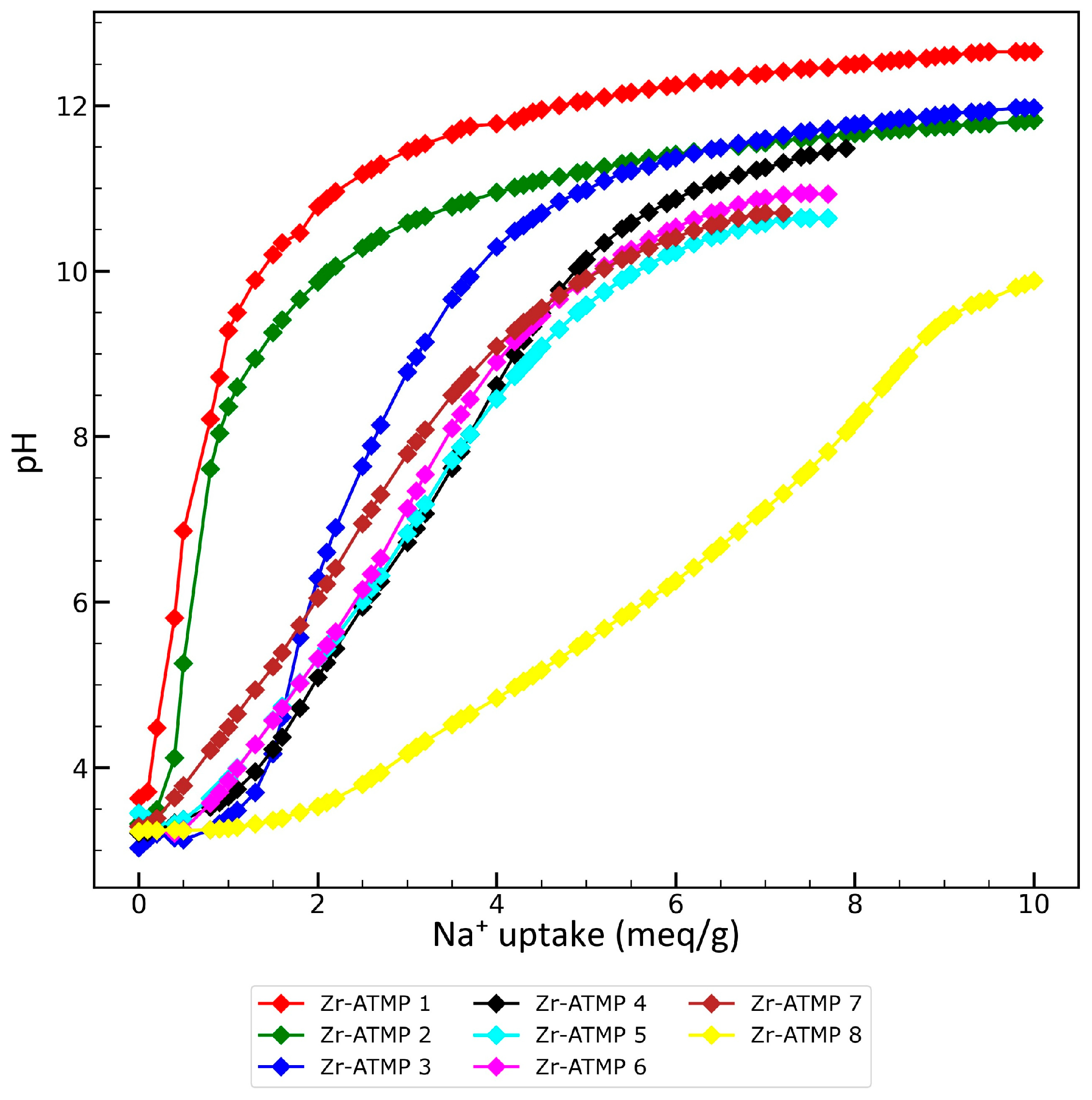

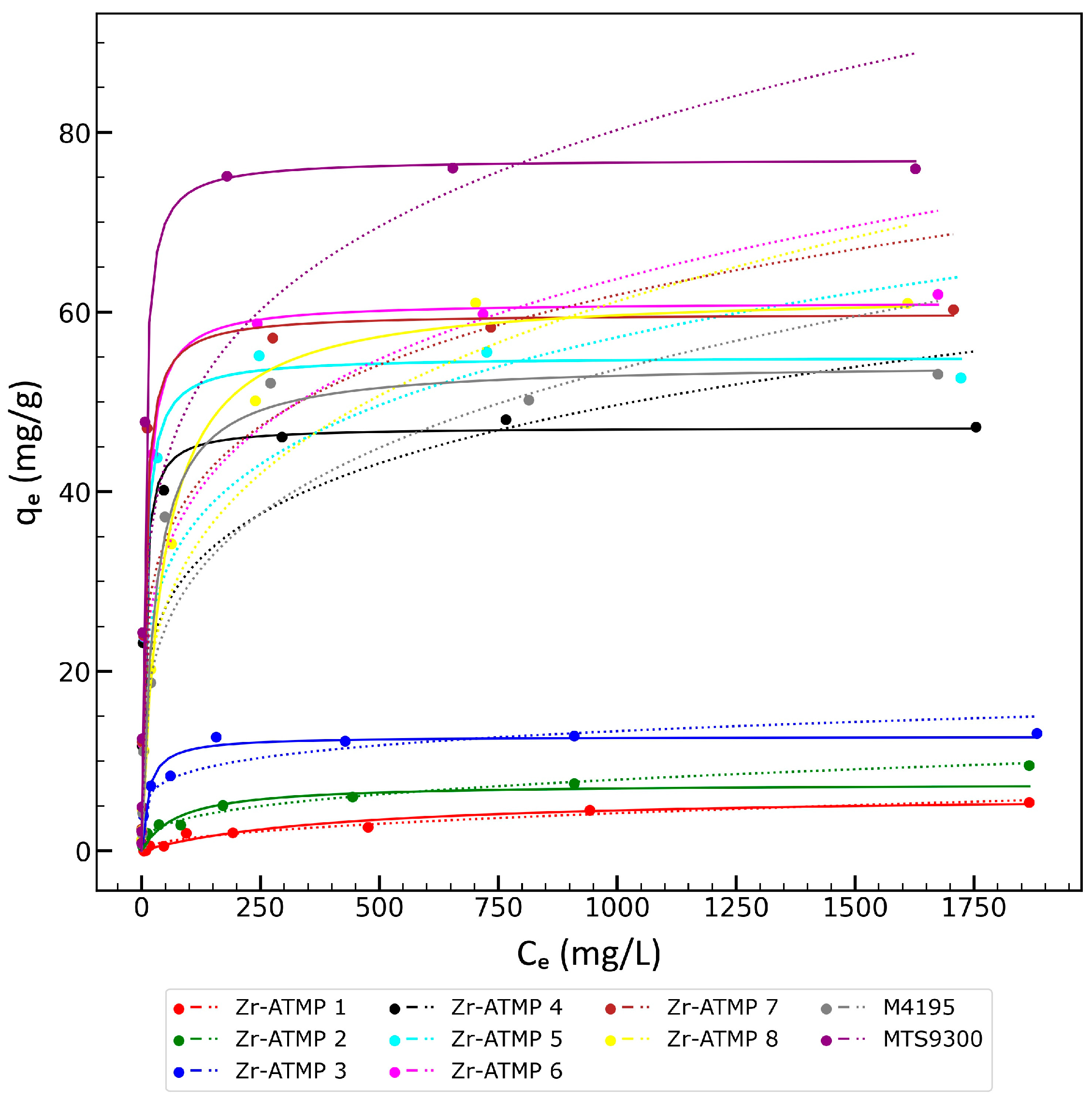


| Sorbent Name | P Source | Zr Source | P:Zr Molar Ratio | Yield (%) a |
|---|---|---|---|---|
| Zr-ATMP 1 | 50% ATMP solution | zirconium carbonate basic hydrate reacted with 65% HNO3 | 0.5:1 | 45 |
| Zr-ATMP 2 | 1:1 | 67 | ||
| Zr-ATMP 3 | 2:1 | 87 | ||
| Zr-ATMP 4 | 5:1 | >99 | ||
| Zr-ATMP 5 | 10:1 | >99 | ||
| Zr-ATMP 6 | 25:1 | >99 | ||
| Zr-ATMP 7 | 50:1 | >99 | ||
| Zr-ATMP 8 | 100:1 | >99 |
| Sorbent Name | Zr(IV) [atomic%] | P [atomic%] | O [atomic%] | N [atomic%] | C [atomic%] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range a (rel. Range b) | Mean | Range a (rel. Range b) | Mean | Range a (rel. Range b) | Mean | Range a (rel. Range b) | Mean | Range a (rel. Range b) | |
| Zr-ATMP 1 | 3.7 | 1.1 (30%) | 6.4 | 2.2 (34%) | 62.0 | 12.0 (19%) | 8.3 | 1.3 (16%) | 19.6 | 12.6 (64%) |
| Zr-ATMP 2 | 3.7 | 1.6 (43%) | 8.3 | 2.5 (30%) | 60.3 | 7.5 (12%) | 8.1 | 1.1 (14%) | 19.7 | 3.2 (16%) |
| Zr-ATMP 3 | 2.9 | 0.7 (24%) | 7.4 | 1.6 (22%) | 59.4 | 3.7 (6%) | 7.1 | 0.4 (6%) | 23.3 | 4.1 (18%) |
| Zr-ATMP 4 | 2.7 | 0.9 (33%) | 11.4 | 2.3 (20%) | 58.0 | 8.3 (14%) | 7.6 | 1.0 (13%) | 20.3 | 4.2 (21%) |
| Zr-ATMP 5 | 2.9 | 2.7 (93%) | 11.5 | 4.2 (37%) | 56.3 | 6.5 (12%) | 8.3 | 2.1 (25%) | 21.0 | 2.2 (10%) |
| Zr-ATMP 6 | 2.0 | 0.8 (40%) | 7.8 | 3.0 (38%) | 61.3 | 2.8 (5%) | 7.1 | 0.5 (7%) | 21.8 | 1.3 (6%) |
| Zr-ATMP 7 | 4.4 | 5.1 (116%) | 12.3 | 14 (114%) | 49.0 | 30.9 (63%) | 4.8 | 3.5 (73%) | 29.4 | 52.1 (177%) |
| Zr-ATMP 8 | 1.5 | 0.4 (27%) | 10.7 | 2.1 (20%) | 61.7 | 6.0 (10%) | 7.1 | 0.2 (3%) | 19.0 | 3.8 (20%) |
| Sorbent Name | P:Zr(IV) Molar Ratio a | N:P Molar Ratio a | O:P Molar Ratio a | C:P Molar Ratio a |
|---|---|---|---|---|
| Zr-ATMP 1 | 1.71 (1.41–1.94) | 1.30 (1.15–1.39) | 9.68 (8.30–11.17) | 3.06 (2.00–5.07) |
| Zr-ATMP 2 | 2.24 (2.09–2.52) | 0.97 (0.88–1.04) | 7.26 (5.68–8.48) | 2.37 (2.15–2.74) |
| Zr-ATMP 3 | 2.57 (2.42–2.71) | 0.96 (0.85–1.12) | 8.07 (7.49–9.32) | 3.15 (2.63–3.57) |
| Zr-ATMP 4 | 4.22 (3.90–4.50) | 0.67 (0.64–0.71) | 5.09 (4.50–6.34) | 1.78 (1.67–1.84) |
| Zr-ATMP 5 | 3.97 (3.07–5.21) | 0.72 (0.68–0.79) | 4.90 (3.69–5.90) | 1.83 (1.40–2.22) |
| Zr-ATMP 6 | 3.82 (3.74–3.88) | 0.91 (0.77–1.04) | 7.90 (6.20–9.44) | 2.79 (2.20–3.39) |
| Zr-ATMP 7 | 2.82 (2.57–3.04) | 0.39 (0.33–0.72) | 3.98 (3.33–8.00) | 2.39 (0.65–17.67) |
| Zr-ATMP 8 | 7.32 (7.00–7.54) | 0.67 (0.59–0.74) | 5.75 (4.87–6.52) | 1.78 (1.69–1.82) |
| Sorbent Name | C [wt%] | H [wt%] | N [wt%] | Residue on Ignition [wt%] | N:C Molar Ratio | H:C Molar Ratio |
|---|---|---|---|---|---|---|
| Zr-ATMP 1 | 5.60 | 2.45 | 2.87 | 78.7 | 0.44 | 5.20 |
| Zr-ATMP 2 | 5.49 | 2.62 | 2.77 | 78.1 | 0.43 | 5.67 |
| Zr-ATMP 3 | 6.72 | 2.32 | 3.73 | 74.0 | 0.48 | 4.09 |
| Zr-ATMP 4 | 7.81 | 2.75 | 4.41 | 55.8 | 0.48 | 4.18 |
| Zr-ATMP 5 | 7.90 | 2.58 | 4.35 | 59.7 | 0.47 | 3.88 |
| Zr-ATMP 6 | 8.23 | 3.20 | 3.32 | 54.7 | 0.35 | 4.62 |
| Zr-ATMP 7 | 8.97 | 2.99 | 3.61 | 54.4 | 0.34 | 3.96 |
| Zr-ATMP 8 | 9.96 | 3.24 | 4.35 | 33.0 | 0.37 | 3.86 |
| Sorbent Name | Langmuir Isotherm Model | Freundlich Isotherm Model | ||||
|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | n | KF ((mg/g)·(L/mg)1/n) | R2 | |
| Zr-ATMP 1 | 6.39 | 0.0024 | 0.95 | 2.11 | 0.158 | 0.96 |
| Zr-ATMP 2 | 7.48 | 0.0132 | 0.88 | 2.99 | 0.789 | 0.98 |
| Zr-ATMP 3 | 12.74 | 0.0744 | 0.95 | 5.49 | 3.788 | 0.88 |
| Zr-ATMP 4 | 47.66 | 0.1811 | 0.98 | 4.87 | 12.18 | 0.83 |
| Zr-ATMP 5 | 55.02 | 0.1406 | 0.99 | 4.88 | 13.9 | 0.81 |
| Zr-ATMP 6 | 61.12 | 0.1208 | 0.99 | 4.58 | 14.09 | 0.85 |
| Zr-ATMP 7 | 59.82 | 0.1521 | 0.99 | 5.15 | 16.19 | 0.83 |
| Zr-ATMP 8 | 62.25 | 0.0228 | 0.99 | 3.68 | 9.359 | 0.94 |
| M4195 | 54.33 | 0.037 | 0.99 | 3.89 | 9.083 | 0.85 |
| MTS9300 | 77.01 | 0.197 | 0.99 | 4.81 | 19.14 | 0.84 |
| Sorbent/Resin Name | Time Required to Reach 90% of the Equilibrium Capacity (min) | |||||
|---|---|---|---|---|---|---|
| 5 (mg/L) | 10 (mg/L) | 20 (mg/L) | 50 (mg/L) | 100 (mg/L) | 200 (mg/L) | |
| Zr-ATMP 1 | >60 | >60 | >60 | >60 | >60 | >60 |
| Zr-ATMP 2 | >60 | >60 | >60 | >60 | >60 | >60 |
| Zr-ATMP 3 | 30 | 45 | 60 | >60 | >60 | >60 |
| Zr-ATMP 4 | <3 | <3 | <3 | <3 | <3 | 15 |
| Zr-ATMP 5 | <3 | <3 | <3 | <3 | 5 | 45 |
| Zr-ATMP 6 | <3 | <3 | <3 | <3 | <3 | <3 |
| Zr-ATMP 7 | <3 | <3 | <3 | <3 | <3 | <3 |
| Zr-ATMP 8 | <3 | <3 | <3 | <3 | <3 | <3 |
| M4195 | >60 | >60 | >60 | >60 | >60 | >60 |
| MS9300 | 30 | 30 | 30 | 30 | 30 | 30 |
| Sorbent Name | log Kd | |||
|---|---|---|---|---|
| Solution 1 | Solution 2 | Solution 3 | Solution 4 | |
| Zr-ATMP 6 | 3.9 | 4.0 | 2.9 | 2.7 |
| Zr-ATMP 7 | 3.8 | 4.0 | 2.9 | 2.8 |
| Sorbent Name | SFCu/Cd | SFCu/Ni | SFCu/Mn | SFCu/Zn | SFCu/Fe |
|---|---|---|---|---|---|
| Zr-ATMP 6 | 2.5 | 330 | 2.1 | 3.0 | 0.9 |
| Zr-ATMP 7 | 2.6 | 340 | 2.0 | 3.2 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marszałek, M.; Piotrowski, M.; Druzgała, B.; Wzorek, Z. Zirconium Phosphonate Sorbent Materials—Synthesis, Characterization, and Application for Copper Removal from Acidic Wastewater. Materials 2025, 18, 2333. https://doi.org/10.3390/ma18102333
Marszałek M, Piotrowski M, Druzgała B, Wzorek Z. Zirconium Phosphonate Sorbent Materials—Synthesis, Characterization, and Application for Copper Removal from Acidic Wastewater. Materials. 2025; 18(10):2333. https://doi.org/10.3390/ma18102333
Chicago/Turabian StyleMarszałek, Marta, Marcin Piotrowski, Bożena Druzgała, and Zbigniew Wzorek. 2025. "Zirconium Phosphonate Sorbent Materials—Synthesis, Characterization, and Application for Copper Removal from Acidic Wastewater" Materials 18, no. 10: 2333. https://doi.org/10.3390/ma18102333
APA StyleMarszałek, M., Piotrowski, M., Druzgała, B., & Wzorek, Z. (2025). Zirconium Phosphonate Sorbent Materials—Synthesis, Characterization, and Application for Copper Removal from Acidic Wastewater. Materials, 18(10), 2333. https://doi.org/10.3390/ma18102333






