Assessment of the Surface Characteristics of ISO 5832-1 Stainless Steel for Biomaterial Applications
Abstract
1. Introduction
2. Experimental
2.1. Materials
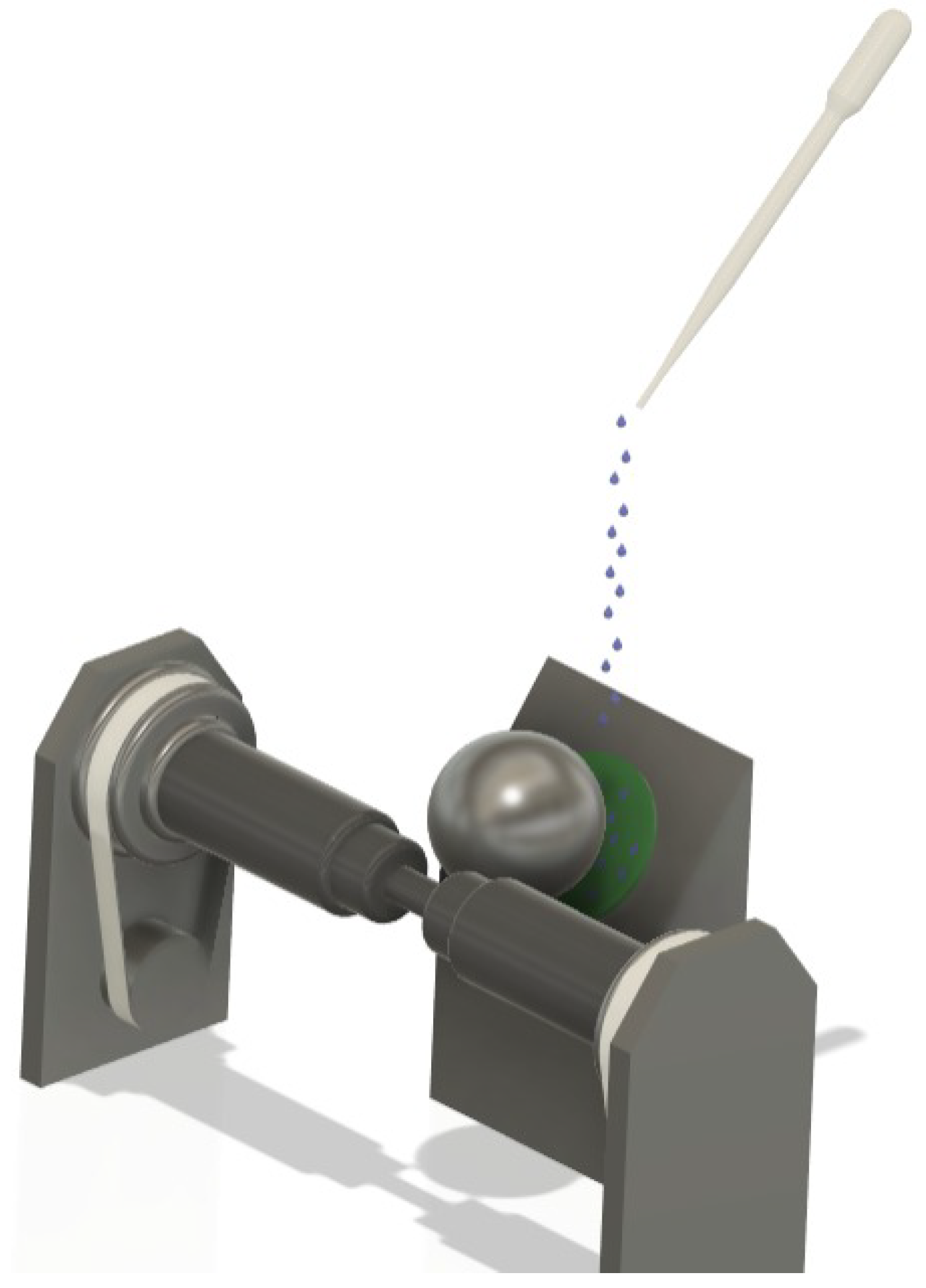
2.2. Ball-Cratering Wear Test Equipment and Data Acquisition
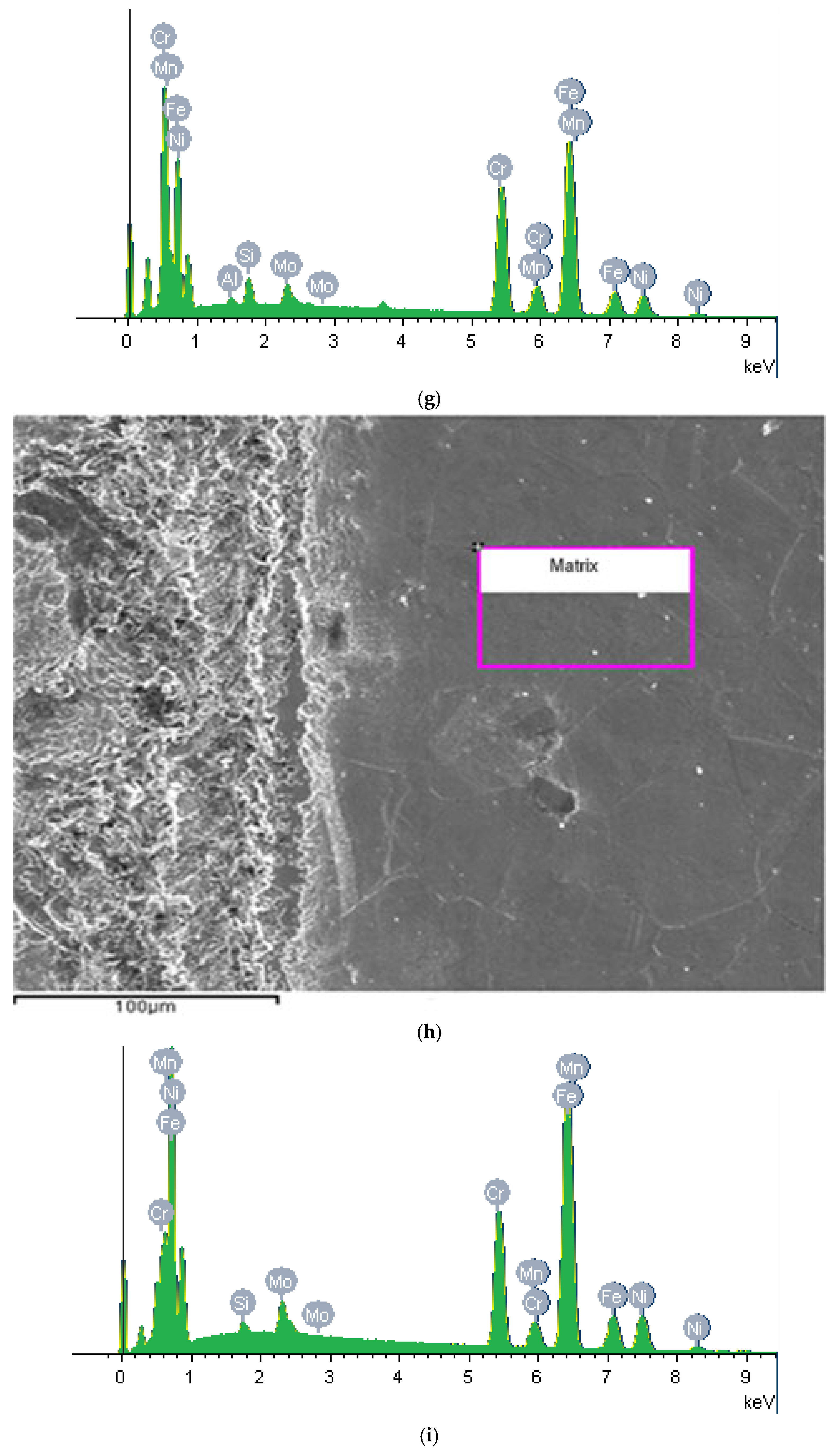
2.3. Surface Characterizations
2.4. Cytotoxicity Analysis
- Solution of MTS/PMS prepared with PBS at a concentration of 20:1;
- Solution of MTS/PMS culture medium with serum: 20 μL was added to the solution of MTS/PMS, and an additional 80 μL of culture medium was added to each test well.
2.5. Electrochemical Experiments
3. Results and Discussion
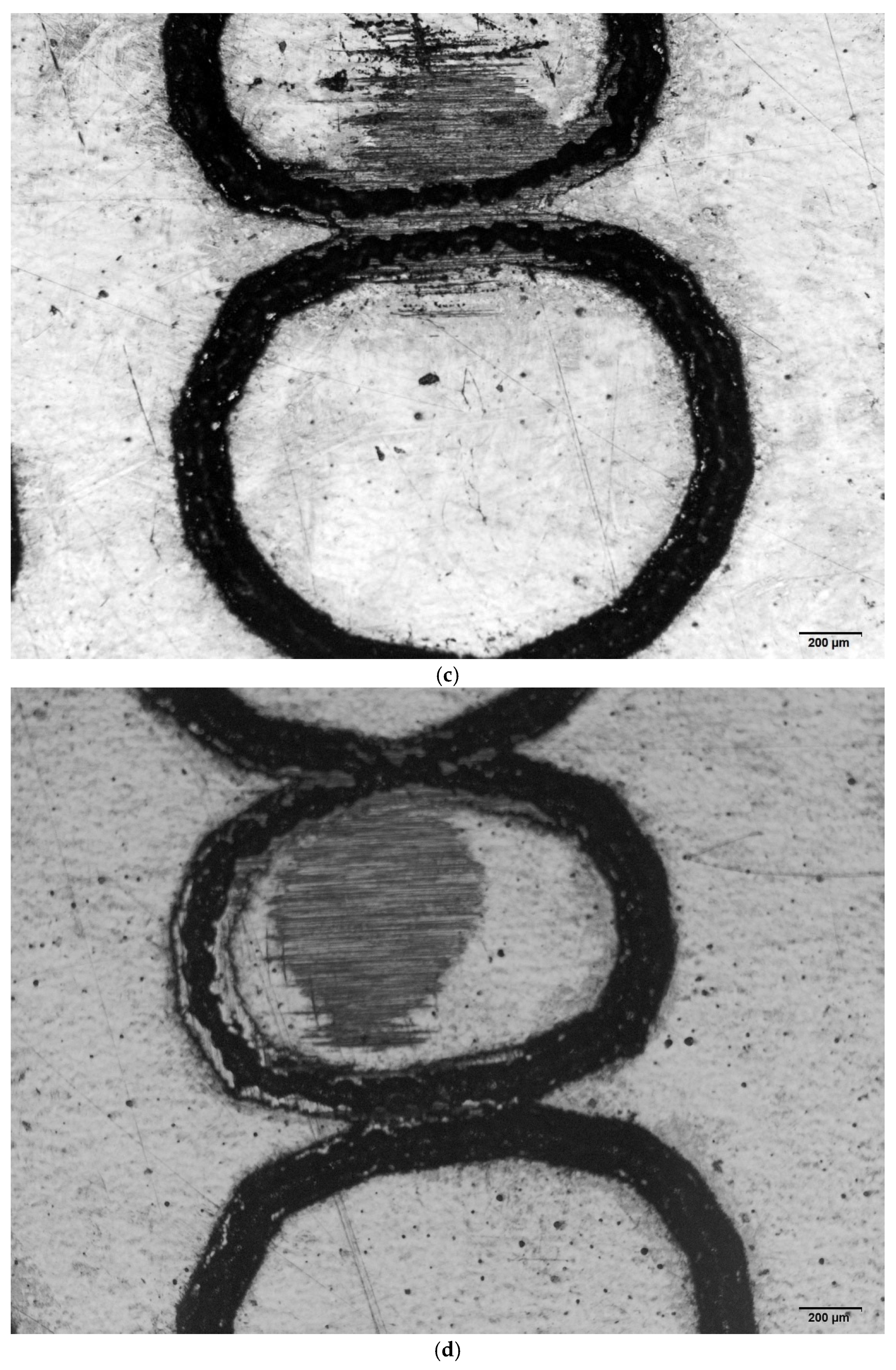
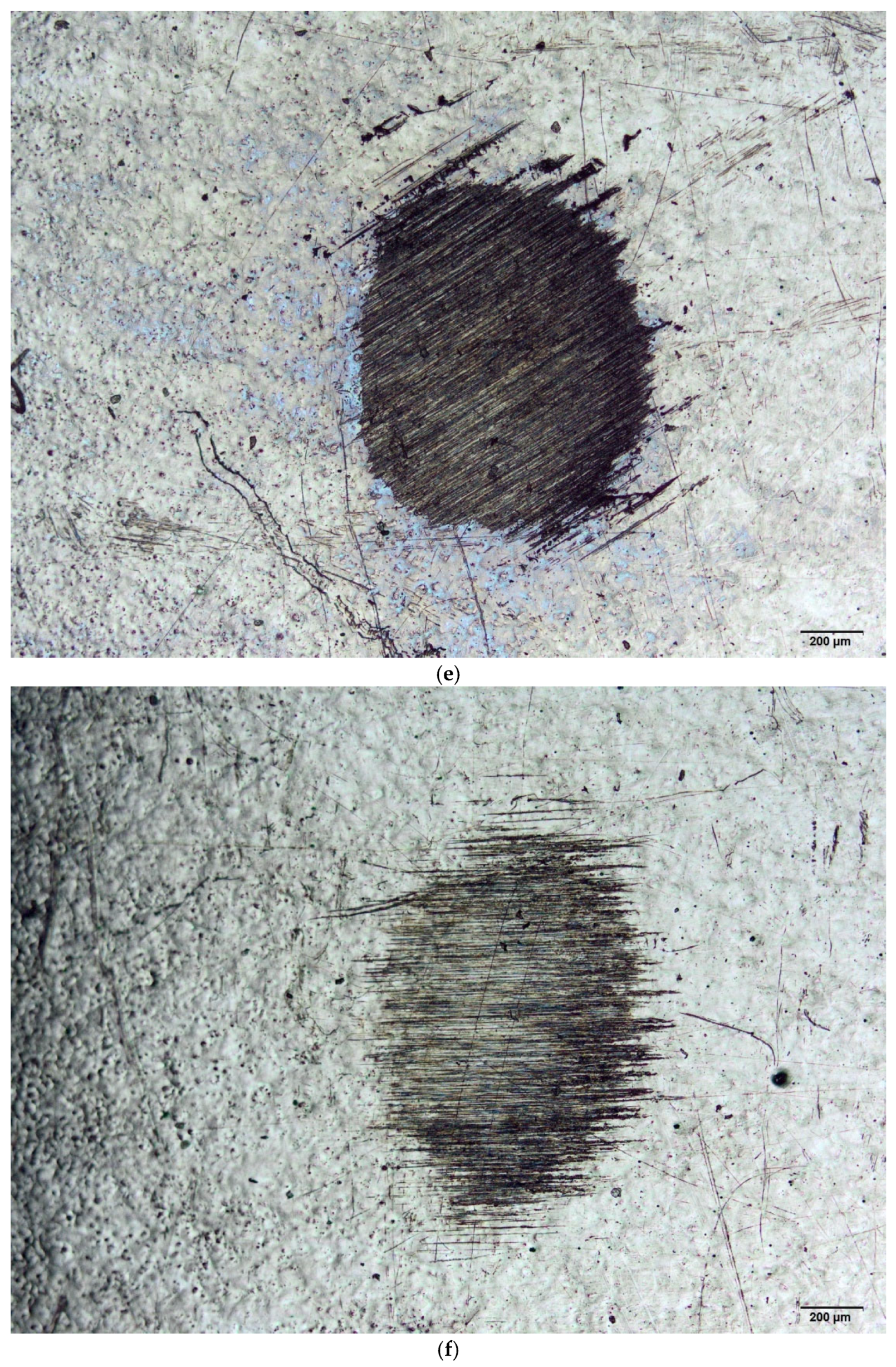
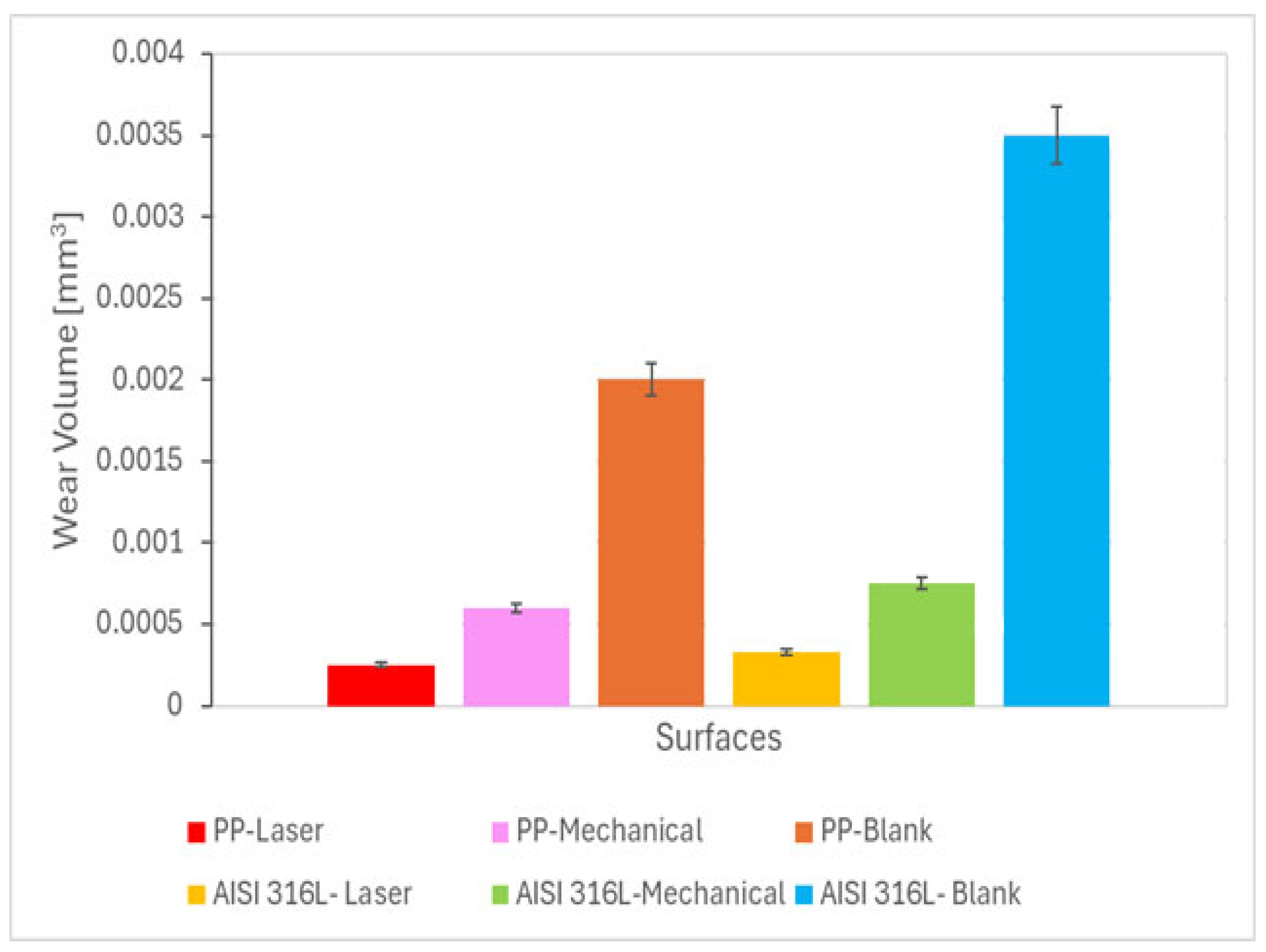
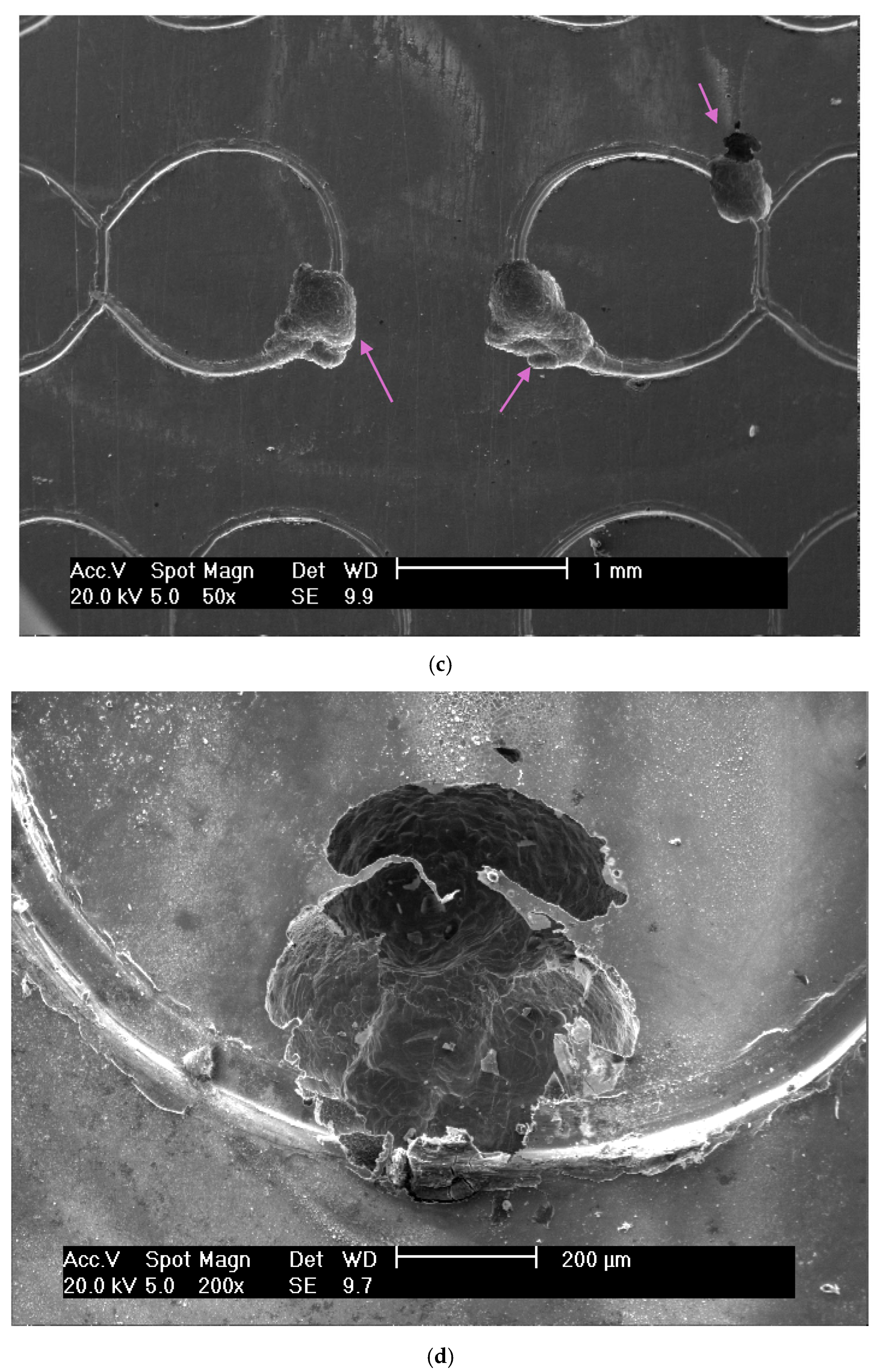
3.1. Wear Volume
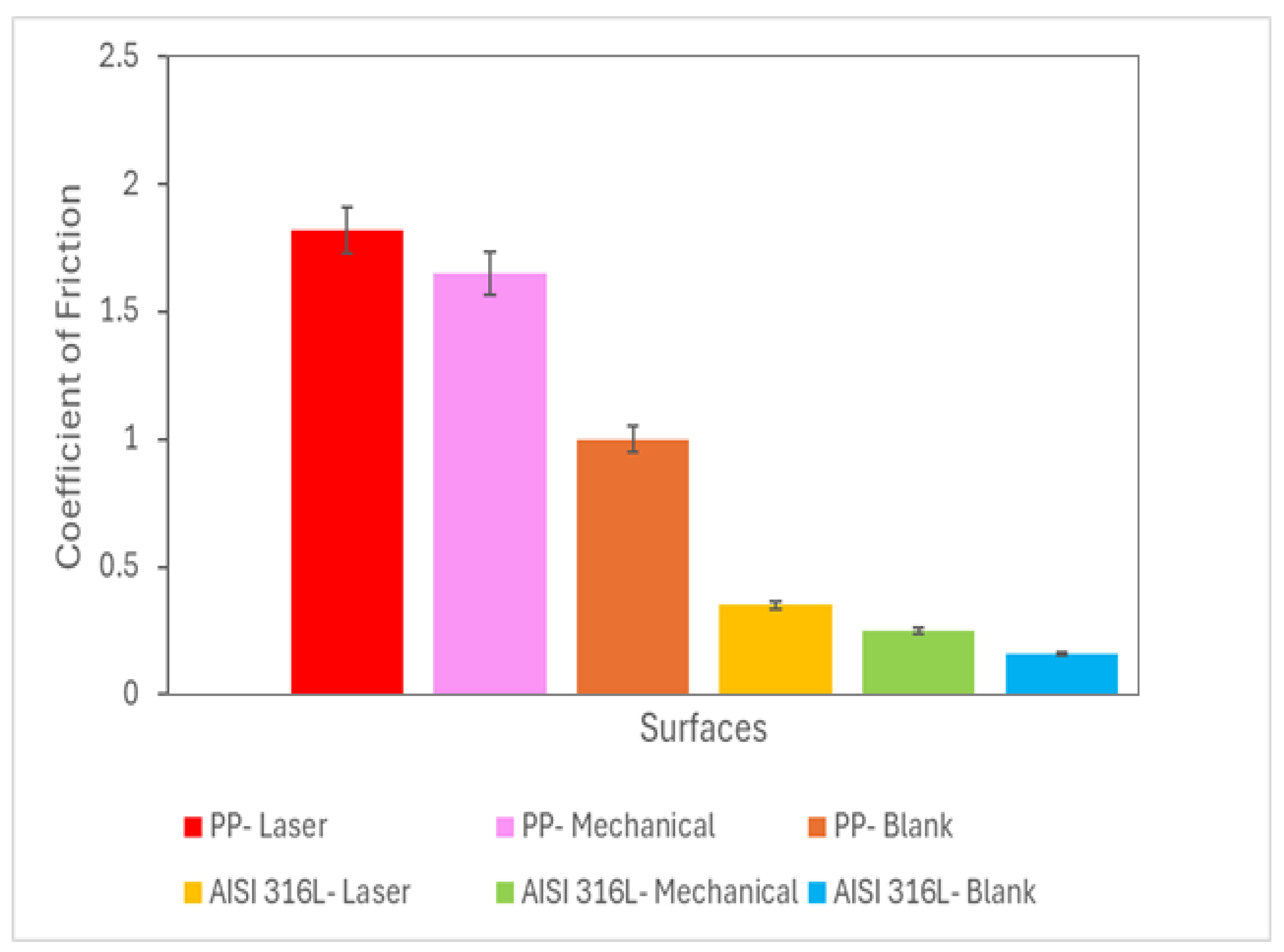
3.2. Analysis of Coefficient of Friction
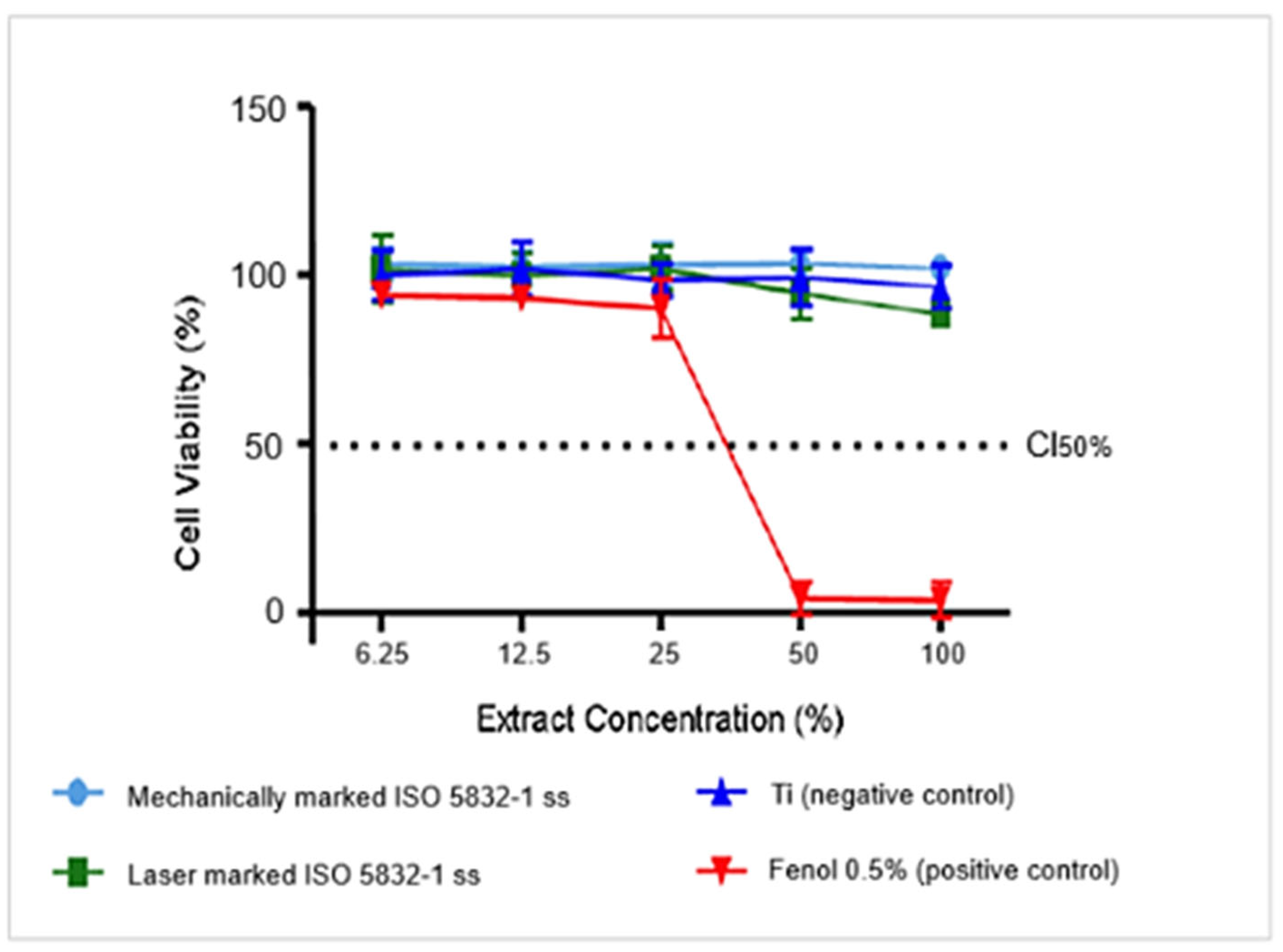
3.3. Cytotoxicity Analysis
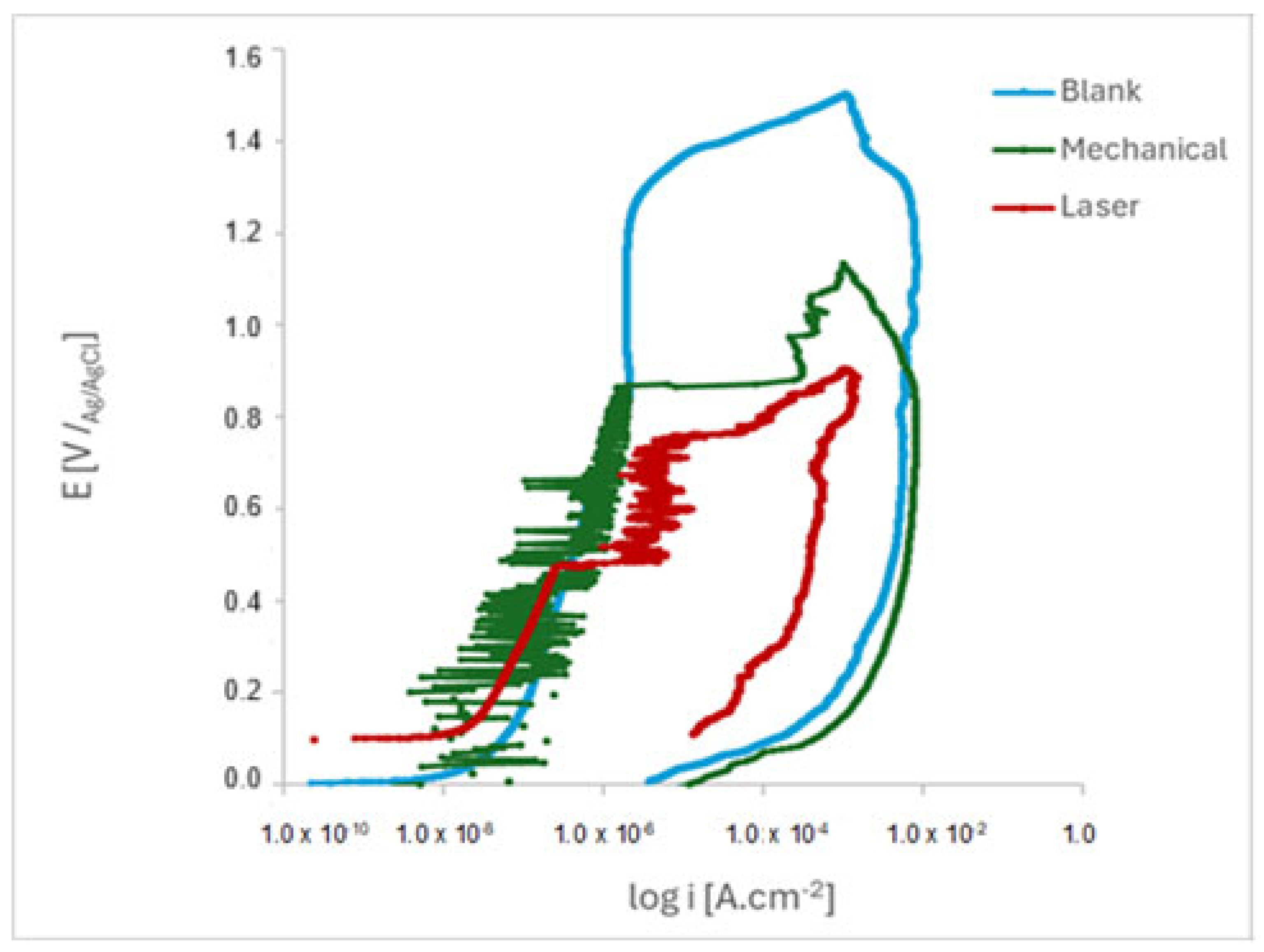
3.4. Electrochemical Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okasaki, Y. Effect of friction on anodic polarization properties of metallic biomaterials. Biomaterials 2002, 23, 2071–2077. [Google Scholar] [CrossRef]
- Black, J. Systemic effects of biomaterials. Biomaterials 1984, 5, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M. Biological response to materials. Mater. Res. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Pieretti, E.F.; Costa, I. Surface characterisation of ASTM F139 stainless steel marked by laser and mechanical techniques. Electrochim. Acta 2013, 114, 838–843. [Google Scholar] [CrossRef]
- Pieretti, E.F.; Manhabosco, S.M.; Dick, L.F.P.; Hinder, S.; Costa, I. Localized corrosion evaluation of the ASTM F139 stainless steel marked by laser using scanning vibrating electrode technique, X-ray photoelectron spectroscopy and Mott-Schottky techniques. Electrochim. Acta 2014, 124, 150–155. [Google Scholar] [CrossRef]
- Pieretti, E.F.; Palatnic, R.P.; Leivas, T.P.; Costa, I.; Neves, M.D.M. Evaluation of Laser Marked ASTM F 139 Stainless Steel in Phosphate Buffer Solution with Albumin. Int. J. Electrochem. Sci. 2014, 9, 2435–2444. [Google Scholar] [CrossRef]
- Pieretti, E.F.; Costa, I.; Marques, R.A.; Leivas, T.P.; Neves, M.D.M. Electrochemical Study of a Laser Marked Biomaterial in Albumin Solution. Int. J. Electrochem. Sci. 2014, 9, 3828–3836. [Google Scholar] [CrossRef]
- Adachi, K.; Hutchings, I.M. Wear-mode mapping for the micro-scale abrasion test. Wear 2003, 255, 23–29. [Google Scholar] [CrossRef]
- Stachowiak, G.B.; Stachowiak, G.W.; Celliers, O. Ball-cratering abrasion tests of high-Cr white cast irons. Tribol. Int. 2005, 38, 1076–1087. [Google Scholar] [CrossRef]
- Stachowiak, G.B.; Stachowiak, G.W.; Brandt, J.M. Ball-cratering abrasion tests with large abrasive particles. Tribol. Int. 2006, 39, 1–11. [Google Scholar] [CrossRef]
- Rutherford, K.L.; Hutchings, I.M. Theory and application of a micro-scale abrasive wear test. J. Test. Eval.—JTEVA 1997, 25, 250–260. [Google Scholar] [CrossRef]
- Günen, A.; Keddam, M.; Alkan, S.; Erdoğan, A.; Çetin, M. Microstructural characterization; boriding kinetics, and tribo-wear behavior of borided Fe-based A286 superalloy. Mater. Charact. 2022, 186, 111778. [Google Scholar] [CrossRef]
- Krelling, A.P.; da Costa, C.E.; Milan, J.C.G.; Almeida, E.A.S. Micro-abrasive wear mechanisms of borided AISI 1020 steel. Tribol. Int. 2017, 111, 234–242. [Google Scholar] [CrossRef]
- Allsopp, D.N.; Trezona, R.I.; Hutchings, I.M. The effects of ball surface condition in the micro-scale abrasive wear test. Tribol. Lett. 1998, 5, 259–264. [Google Scholar] [CrossRef]
- Trezona, R.I.; Allsopp, D.N.; Hutchings, I.M. Transitions between two-body and three-body abrasive wear: Influence of test conditions in the microscale abrasive wear test. Wear 1999, 225–229, 205–214. [Google Scholar] [CrossRef]
- Neville, A.; Rafailidi, V.K. A comparison of boundary wear film formation on steel and a thermal sprayed Co/Cr/Mo coating under sliding conditions. Wear 2002, 252, 227–239. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Huang, W.; Chen, X.; He, H. Micro-abrasion–corrosion behaviour of a biomedical Ti–25Nb–3Mo–3Zr–2Sn alloy in simulated physiological fluid. J. Mech. Behav. Biomed. Mater. 2016, 63, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Bose, K.; Wood, R.J.K. Optimum test conditions for attaining uniform rolling abrasion in ball cratering tests on hard coatings. Wear 2005, 258, 322–332. [Google Scholar] [CrossRef]
- Axén, N.; Jacobson, S.; Hogmark, S. Influence of hardness of the counterbody in three-body abrasive wear—An overlooked hardness effect. Tribol. Int. 1994, 27, 233–241. [Google Scholar] [CrossRef]
- Gee, M.G.; Wicks, M.J. Ball crater testing for the measurement of the unlubricated sliding wear of wear-resistant coatings. Surf. Coat. Technol. 2000, 133–134, 376–382. [Google Scholar] [CrossRef]
- Colaço, R.; Vilar, R. Abrasive wear of metallic matrix reinforced materials. Wear 2003, 255, 643–650. [Google Scholar] [CrossRef]
- Pieretti, E.F.; Leivas, T.P.; Pillis, M.F.; Das Neves, M.D.M. Failure Analysis of Metallic Orthopedic Implant for Total Knee Replacement. Mater. Sci. Forum 2020, 1012, 471–476. [Google Scholar] [CrossRef]
- Pieretti, E.F.; Corrêa, O.V.; Das Neves, M.D.M.; Antunes, R.A.; Pillis, M.F. Tribology analysis on anodized aluminum surfaces for biomedical purposes. Braz. J. Mot. Behav. 2023, 17, 196–197. [Google Scholar]
- Feng, J.; Wang, Z.M.; Zheng, D.; Song, G.-L. Influence of dissolved oxygen on the corrosion of mild steel in a simulated cement pore solution under supercritical carbon dioxide. Constr. Build. Mater. 2021, 311, 125270. [Google Scholar] [CrossRef]
- Han, X.; Yang, D.Y.; Frangopol, D.M. Optimum maintenance of deteriorated steel bridges using corrosion resistant steel based on system reliability and life-cycle cost. Eng. Struct. 2021, 243, 112633. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, G.; Tang, J.; Zhang, L.; Shen, G.; Gu, Z.; Jie, X. Enhanced corrosion and wear resistance of Zn–Ni/Cu–Al2O3 composite coating prepared by cold spray. J. Solid State Electrochem. 2023, 27, 439–453. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D.; Chastain, J. (Eds.) Handbook of X-Ray Photoelectron Spectroscopy; PerkinElmer Corporation, Physical Electronics Division: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Tang, H.; Chen, X.; Deng, W.; Luo, J. Comprehensive review: Advances and critical mechanisms in liquid superlubricity. Adv. Colloid Interface Sci. 2025, 344, 103585. [Google Scholar] [CrossRef]
- Bian, Y. Bone tissue engineering for treating osteonecrosis of the femoral head. Exploration 2023, 3, 20210105. [Google Scholar] [CrossRef]
- Han, T.; Zhang, C.; Luo, J. Macroscale superlubricity enabled by hydrated alkali metal ions. Langmuir 2018, 34, 11281–11291. [Google Scholar] [CrossRef]
- Han, T.; Zhang, S.; Zhang, C. Unlocking the secrets behind liquid superlubricity: A state-of-the-art review on phenomena and mechanisms. Friction 2022, 10, 1137–1165. [Google Scholar] [CrossRef]
- Kumar, P.S.; Jegadheesan, C.; Somasundaram, P.; Kumar, S.P.; Anand, A.V.; Singh, A.P.; Jeyaprakash, N. State of art: Review on laser surface hardening of alloy metals. Mater. Today Proc. 2023, in press. [Google Scholar]
- Gant, A.J.; Gee, M.G. A review of micro-scale abrasion testing. J. Phys. D: Appl. Phys. 2011, 44, 073001. [Google Scholar] [CrossRef]
- Adachi, K.; Hutchings, I.M. Sensitivity of wear rates in the micro-scale abrasion test to test conditions and material hardness. Wear 2005, 258, 318–321. [Google Scholar] [CrossRef]
- Allsopp, D.N.; Hutchings, I.M. Micro-scale abrasion and scratch response of PVD coatings at elevated temperatures. Wear 2001, 251, 1308–1314. [Google Scholar] [CrossRef]
- Stachowiak, G.B.; Stachowiak, G.W. Wear mechanisms in ball-cratering tests with large abrasive particles. Wear 2004, 256, 600–607. [Google Scholar] [CrossRef]
- ISO 10993; Biological Evaluation of Medical Devices—Part 5: Tests for Cytotoxicity: In Vitro Methods. ISO: Geneva, Switzerland, 2009; pp. 1–7.
- Marques, R.A.; Rogero, S.O.; Terada, M.; Pieretti, E.F.; Costa, I. Localized Corrosion Resistance and Cytotoxicity Evaluation of Ferritic Stainless Steels for Use in Implantable Dental Devices with Magnetic Connections. Int. J. Electrochem. Sci. 2014, 9, 1340–1354. [Google Scholar] [CrossRef]
- Li, J.; Li, S.J.; Hao, Y.L.; Yang, R. Electrochemical characterization of nanostructured Ti–24Nb–4Zr–8Sn alloy in 3.5% NaCl solution. Int. J. Hydrogen Energy 2014, 39, 17452–17459. [Google Scholar] [CrossRef]
- Tang, J.; Luo, H.Y.; Zhang, H.B. Enhancing the surface integrity and corrosion resistance of Ti-6Al-4V titanium alloy through cryogenic burnishing. Int. J. Adv. Manuf. Technol. 2017, 88, 2785–2793. [Google Scholar] [CrossRef]
- Hodgson, A.W.E.; Mueller, Y.; Forster, D.; Virtanen, S. Electrochemical characterisation of Passive Films on Ti Alloys Under Simulated Biological Conditions. Electrochim. Acta 2002, 47, 1913–1923. [Google Scholar] [CrossRef]
- Hou, X.; Ren, Q.; Yang, Y.; Cao, X.; Hu, J.; Zhang, C.; Deng, H.; Yu, D.; Li, K.; Lan, W. Effect of temperature on the electrochemical pitting corrosion behavior of 316L stainless steel in chloride-containing MDEA solution. J. Nat. Gas Sci. Eng. 2021, 86, 103718. [Google Scholar] [CrossRef]
- Jafarzadegan, M.; Ahmadian, F.; Salarvand, V.; Kashkooli, S. Investigation of microstructure and corrosion resistance of AISI 304 stainless steel joint with ER308 and ERNiCr-3 filler metals by GTAW. Metall. Res. Technol. 2020, 117, 507. [Google Scholar] [CrossRef]
- Swayne, M.; Perumal, G.; Padmanaban, D.B.; Mariotti, D.; Brabazon, D. Exploring the impact of laser surface oxidation parameters on surface chemistry and corrosion behaviour of AISI 316L stainless steel. Appl. Surf. Sci. Adv. 2024, 22, 100622. [Google Scholar] [CrossRef]
- ISO 5832-1:2016; Implants for Surgery—Metallic Materials—Part 1: Wrought Stainless Steel. ISO: Geneva, Switzerland, 2016; pp. 1–9.
- Gui, Y.; Meng, X.B.; Zheng, Z.J.; Gao, Y. Critical temperature determination of detectable Cr diffusion enhancement by nanostructure through structural evolution analysis of the oxide films at 25–450 °C on 304 stainless steel. Appl. Surf. Sci. 2017, 419, 512–521. [Google Scholar] [CrossRef]
- Pfaffenberger, Z.J.; Liu, L.; Kim, T.K.J.; Venkataramani, V.; Kisley, L. Electrochemically-Assisted Low Power Density Laser Writing on Stainless Steel via Enrichment of Chromium Oxides. Adv. Mater. Interfaces 2025, 12, 2500240. [Google Scholar] [CrossRef]
- Suslov, R.; Bogdanov, V.P.R.; Vorkel, V.; Filatov, I.; Grishina, A.; Sokolov, M.; Khubezhov, S.; Davydova, E.; Romanova, G. Effect of chemical composition on pitting corrosion resistance of AISI 430 stainless steel after laser surface structuring. Opt. Laser Technol. 2025, 183, 112286. [Google Scholar] [CrossRef]
- Feyzi, M.; Fallahnezhad, K.; Taylor, M.; Hashemi, R. The Tribocorrosion Behaviour of Ti-6Al-4 V Alloy: The Role of Both Normal Force and Electrochemical Potential. Tribol. Lett. 2022, 70, 83. [Google Scholar] [CrossRef]





| Material | Hardness | |
|---|---|---|
| Sample | ISO 5832-1 SS | 88 HRB |
| Ball | AISI 316L SS | 25–39 HRC |
| Ball | Polypropylene | R85 |
| Ball-Cratering Parameters | |
|---|---|
| (N) Normal Force [N]—Ball of AISI 316L SS | 0.40 |
| (N) Normal force [N]—ball of PP | 0.05 |
| (S) Sliding distance [m] | 60.00 |
| (n) Ball rotational speed—[rpm] | 75.00 |
| (v) Tangential sliding speed—[m/s] | 0.10 |
| (t) Test duration—[min] | 10 |
| Extracts | pH |
|---|---|
| CP (Phenol 0.5%) | 7.50 |
| CN (Pure Ti) | 7.50 |
| Mechanically marked | 7.88 |
| Laser-marked | 7.88 |
| Element (Atomic%)/Selected Region | 1 | 2 | 3 |
|---|---|---|---|
| C | 9.67 | 3.75 | 3.99 |
| Cr | 13.68 | 16.87 | 21.62 |
| Fe | 14.86 | 14.31 | 11.28 |
| Mn | 0.62 | 1.32 | 2.07 |
| Mo | 1.23 | 0.27 | 0.34 |
| N | 3.38 | 3.51 | 3.39 |
| Ni | 4.06 | 5.56 | 5.88 |
| O | 50.29 | 51.00 | 47.80 |
| P | 2.21 | 3.41 | 3.63 |
| Cr/Fe | 0.92 | 1.18 | 1.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieretti, E.F.; Piaggio, D.; Costa, I. Assessment of the Surface Characteristics of ISO 5832-1 Stainless Steel for Biomaterial Applications. Materials 2025, 18, 4020. https://doi.org/10.3390/ma18174020
Pieretti EF, Piaggio D, Costa I. Assessment of the Surface Characteristics of ISO 5832-1 Stainless Steel for Biomaterial Applications. Materials. 2025; 18(17):4020. https://doi.org/10.3390/ma18174020
Chicago/Turabian StylePieretti, Eurico Felix, Davide Piaggio, and Isolda Costa. 2025. "Assessment of the Surface Characteristics of ISO 5832-1 Stainless Steel for Biomaterial Applications" Materials 18, no. 17: 4020. https://doi.org/10.3390/ma18174020
APA StylePieretti, E. F., Piaggio, D., & Costa, I. (2025). Assessment of the Surface Characteristics of ISO 5832-1 Stainless Steel for Biomaterial Applications. Materials, 18(17), 4020. https://doi.org/10.3390/ma18174020







