An S-Infused/S, F-Codoped PVDF-Derived Carbon as a High-Performance Anode for Sodium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.1.1. Preparation of the S-Infused/S, F-Codoped Carbon Material (SFC5)
2.1.2. Preparation of the F-Doped Carbon Material (FC5)
2.1.3. Preparation of the S-Doped Fluorine Carbon Material (FC5-S5)
2.2. Materials Characterization
2.3. Electrochemical Testing
3. Results and Discussion
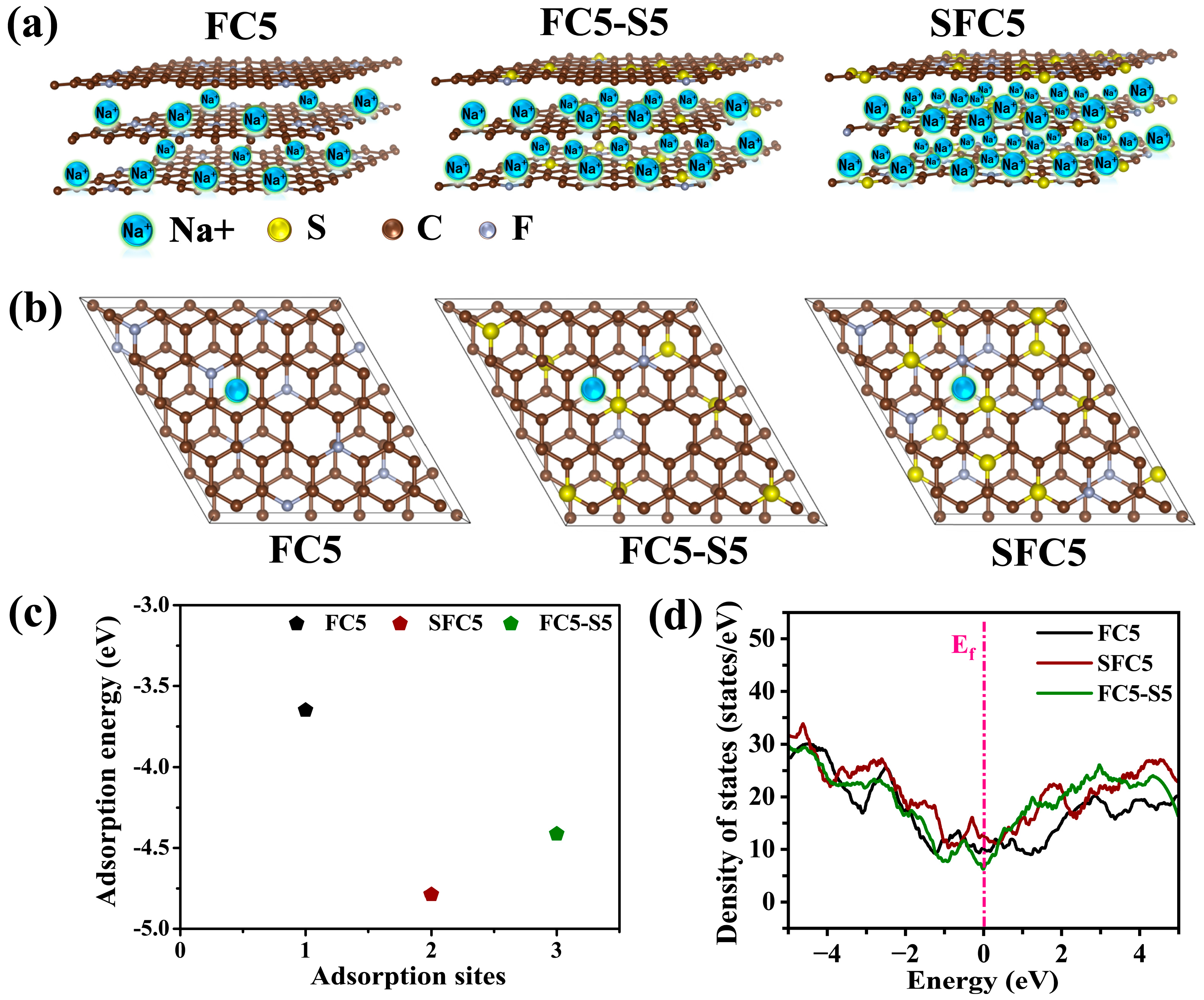
3.1. Synthesis and Characterization
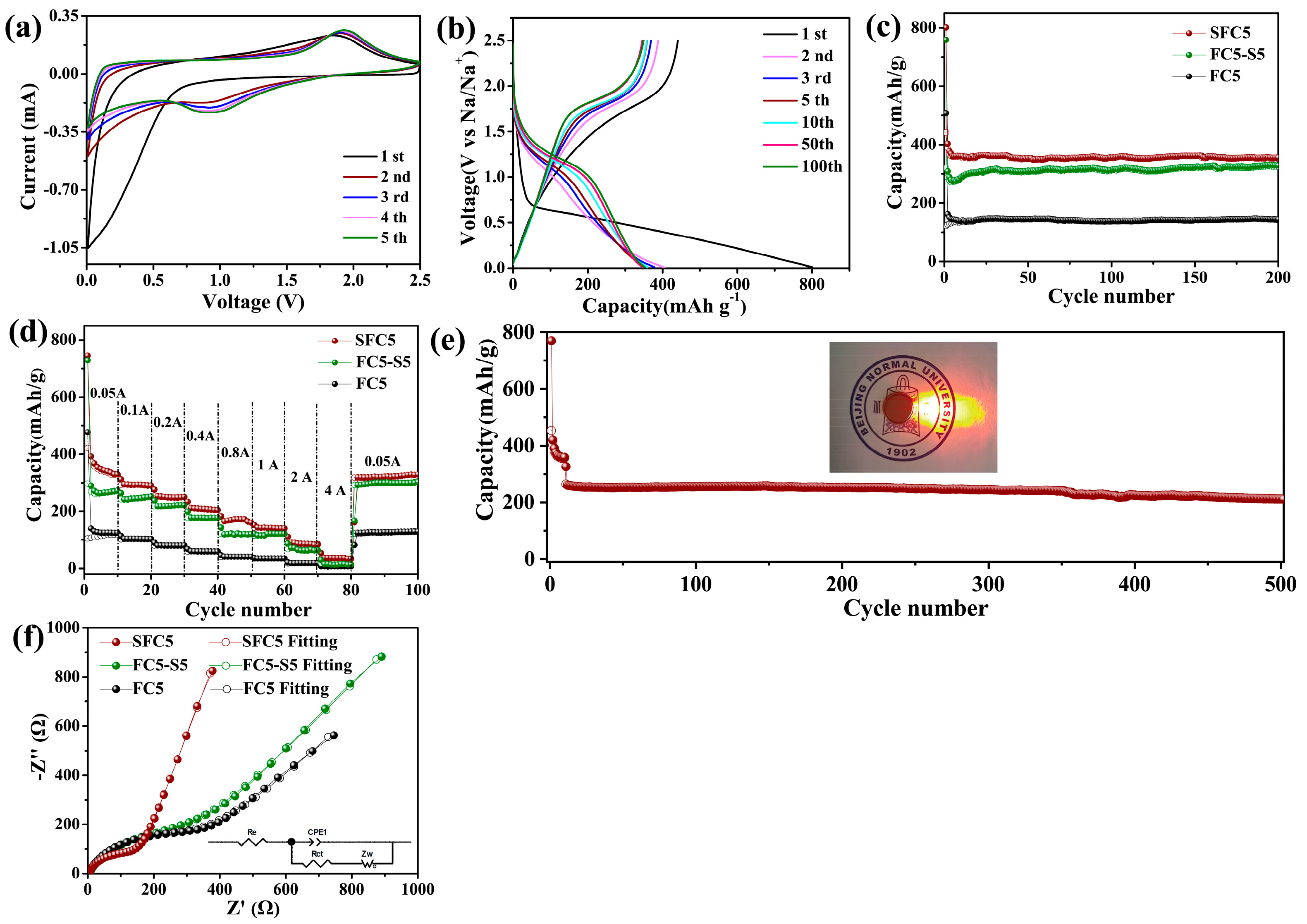
3.2. Electrochemical Performance Evaluation
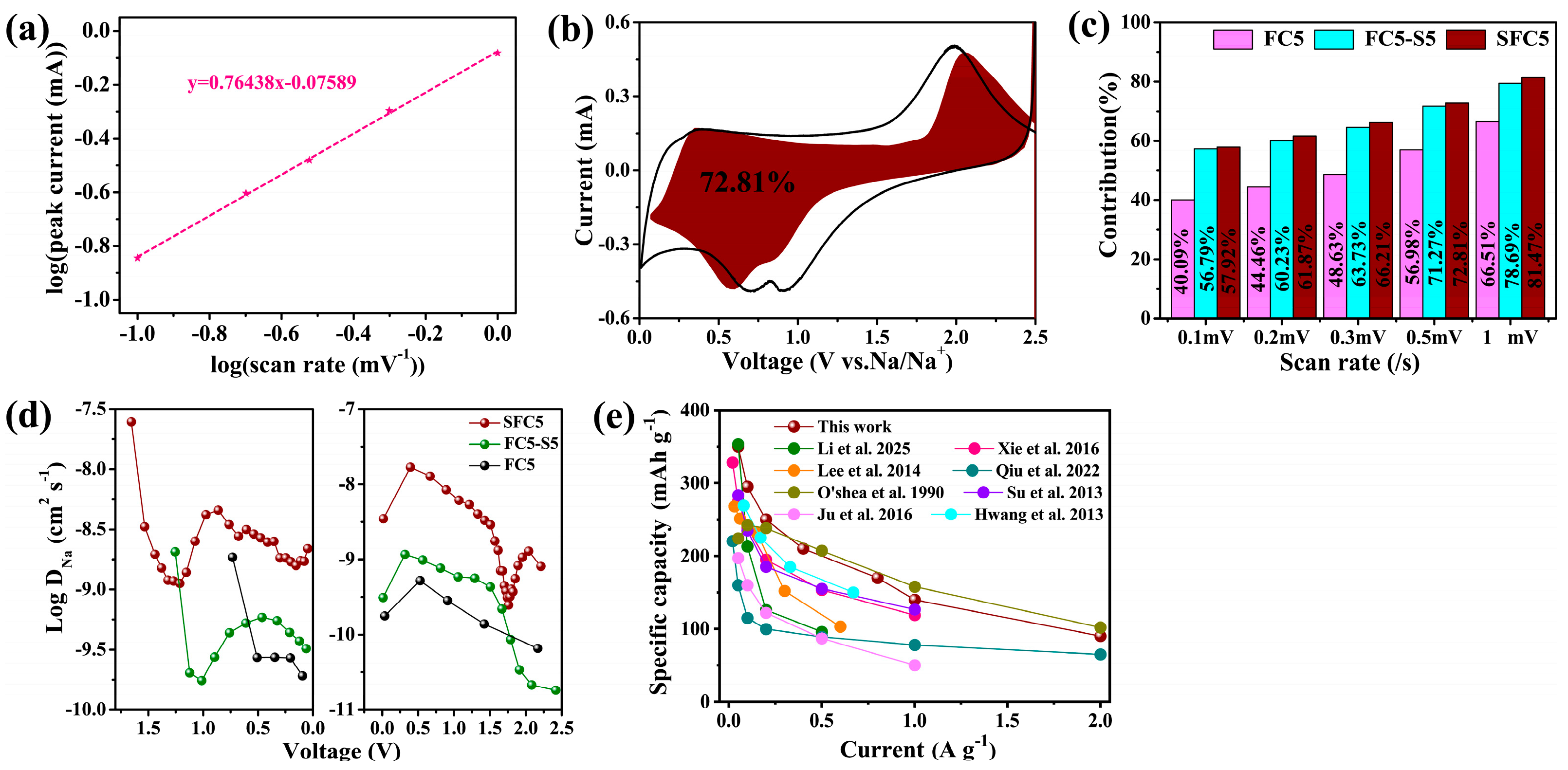
3.3. Quantitative Analysis of the Electrode Reaction Kinetics of SFC5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Wadia, C.; Albertus, P.; Srinivasan, V. Resource constraints on the battery energy storage potential for grid and transportation applications. J. Power Sources 2011, 196, 1593–1598. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-Ion Batteries. Adv. Funct. Mater. 2012, 23, 947–958. [Google Scholar] [CrossRef]
- Sawicki, M.; Shaw, L.L. Advances and challenges of sodium ion batteries as post lithium ion batteries. RSC Adv. 2015, 5, 53129–53154. [Google Scholar] [CrossRef]
- Komaba, S.; Murata, W.; Ishikawa, T.; Yabuuchi, N.; Ozeki, T.; Nakayama, T.; Ogata, A.; Gotoh, K.; Fujiwara, K. Electrochemical Na Insertion and Solid Electrolyte Interphase for Hard-Carbon Electrodes and Application to Na-Ion Batteries. Adv. Funct. Mater. 2011, 21, 3859–3867. [Google Scholar] [CrossRef]
- Ponrouch, A.; Goñi, A.; Palacín, M.R. High capacity hard carbon anodes for sodium ion batteries in additive free electrolyte. Electrochem. Commun. 2013, 27, 85–88. [Google Scholar] [CrossRef]
- Jache, B.; Adelhelm, P. Use of Graphite as a Highly Reversible Electrode with Superior Cycle Life for Sodium-Ion Batteries by Making Use of Co-Intercalation Phenomena. Angew. Chem. Int. Ed. 2014, 53, 10169–10173. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Sushko, M.L.; Wang, W.; Schwenzer, B.; Xiao, J.; Nie, Z.; Saraf, L.V.; Yang, Z.; Liu, J. Sodium Ion Insertion in Hollow Carbon Nanowires for Battery Applications. Nano Lett. 2012, 12, 3783–3787. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; He, K.; Zhu, Y.; Han, F.; Xu, Y.; Matsuda, I.; Ishii, Y.; Cumings, J.; Wang, C. Expanded graphite as superior anode for sodium-ion batteries. Nat. Commun. 2014, 5, 4033. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, W.; Yuan, F.; Zhang, D.; Li, Z.; Wang, Q.; Li, W.; Wang, H.; Wang, B. Hierarchical porous carbon nanofibers with enhanced capacitive behavior as a flexible self-supporting anode for boosting potassium storage. J. Power Sources 2022, 523, 231043. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Wang, X.; Gao, C.; Yin, J.; Wen, Q.; Wang, G. N/S Co-Doped Carbon-Coated Micro-Expanded Graphite for High-Performance Lithium-Ion Battery Anodes. Materials 2025, 18, 2477. [Google Scholar] [CrossRef]
- Li, N.; Li, H.; Huang, H. Synergistic Nitrogen-Doping and Defect Engineering in Hard Carbon: Unlocking Ultrahigh Rate Capability and Long-Cycling Stability for Sodium-Ion Battery Anodes. Materials 2025, 18, 2397. [Google Scholar] [CrossRef]
- An, H.; Li, Y.; Feng, Y.; Cao, Y.; Cao, C.; Long, P.; Li, S.; Feng, W. Reduced graphene oxide doped predominantly with CF2 groups as a superior anode material for long-life lithium-ion batteries. Chem. Commun. 2018, 54, 2727–2730. [Google Scholar] [CrossRef]
- Kong, L.; Li, Y.; Feng, W. Fluorine-Doped Hard Carbon as the Advanced Performance Anode Material of Sodium-Ion Batteries. Trans. Tianjin Univ. 2022, 28, 123–131. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, L.; Ma, C.; Han, G. One-Step Fabrication of Fluorine-Doped Graphite Derived from a Low-Grade Microcrystalline Graphite Ore for Potassium-Ion Batteries. Energy Fuels 2020, 34, 8993–9001. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X.; Wu, D.; Zhao, X.; Zhou, Z. S-Doped N-Rich Carbon Nanosheets with Expanded Interlayer Distance as Anode Materials for Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1604108. [Google Scholar] [CrossRef]
- Qie, L.; Chen, W.; Xiong, X.; Hu, C.; Zou, F.; Hu, P.; Huang, Y. Sulfur-Doped Carbon with Enlarged Interlayer Distance as a High-Performance Anode Material for Sodium-Ion Batteries. Adv. Sci. 2015, 2, 1500195. [Google Scholar] [CrossRef]
- Li, W.; Zhou, M.; Li, H.; Wang, K.; Cheng, S.; Jiang, K. A high performance sulfur-doped disordered carbon anode for sodium ion batteries. Energy Environ. Sci. 2015, 8, 2916–2921. [Google Scholar] [CrossRef]
- Ye, J.; Zang, J.; Tian, Z.; Zheng, M.; Dong, Q. Sulfur and nitrogen co-doped hollow carbon spheres for sodium-ion batteries with superior cyclic and rate performance. J. Mater. Chem. A 2016, 4, 13223–13227. [Google Scholar] [CrossRef]
- Huang, S.; Li, Y.; Feng, Y.; An, H.; Long, P.; Qin, C.; Feng, W. Nitrogen and fluorine co-doped graphene as a high-performance anode material for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 23095–23105. [Google Scholar] [CrossRef]
- Yan, J.; Li, W.; Feng, P.; Wang, R.; Jiang, M.; Han, J.; Cao, S.; Wang, K.; Jiang, K. Enhanced Na+ pseudocapacitance in a P, S co-doped carbon anode arising from the surface modification by sulfur and phosphorus with C–S–P coupling. J. Mater. Chem. A 2020, 8, 422–432. [Google Scholar] [CrossRef]
- Shi, L.; Miao, C.L.; Chen, G.R.; Xu, B.; Chen, S. Effect of Fluorine Content on the Electrochemical Properties of PVDF-Derived Carbons for Lithium Ion Battery. Adv. Mater. Res. 2012, 463–464, 730–733. [Google Scholar] [CrossRef]
- Xu, B.; Hou, S.; Chu, M.; Cao, G.; Yang, Y. An activation-free method for preparing microporous carbon by the pyrolysis of poly(vinylidene fluoride). Carbon 2010, 48, 2812–2814. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, D.; Xiong, D.; Chen, L.; Feng, Z.; Wen, K.; He, M. Red phosphorus confined in porous structures formed by PVDF-Derived carbon as superior anode material for sodium-ion batteries. J. Electroanal. Chem. 2024, 957, 118122. [Google Scholar] [CrossRef]
- Son, I.-S.; Oh, Y.; Yi, S.-H.; Bin Im, W.; Chun, S.-E. Facile fabrication of mesoporous carbon from mixed polymer precursor of PVDF and PTFE for high-power supercapacitors. Carbon 2020, 159, 283–291. [Google Scholar] [CrossRef]
- Park, H.-Y.; Lee, C.H.; Cho, D.-W.; Park, J.-H. Synthesis of porous carbon derived from poly(vinylidenefluoride) and its adsorption characteristics for CO2 and CH4. Microporous Mesoporous Mater. 2020, 299, 110121. [Google Scholar] [CrossRef]
- Quan, B.; Jin, A.; Yu, S.H.; Kang, S.M.; Jeong, J.; Abruña, H.D.; Jin, L.; Piao, Y.; Sung, Y.E. Solvothermal-Derived S-Doped Graphene as an Anode Material for Sodium-Ion Batteries. Adv. Sci. 2018, 5, 1700880. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Park, S.-J. Carbon dioxide adsorption performance of ultramicroporous carbon derived from poly(vinylidene fluoride). J. Anal. Appl. Pyrolysis 2014, 106, 147–151. [Google Scholar] [CrossRef]
- O’shea, M.L.; Morterra, C.; Low, M.J.D. Spectroscopic studies of carbons. XVII. Pyrolysis of polyvinylidene fluoride. Mater. Chem. Phys. 1990, 26, 193–209. [Google Scholar] [CrossRef]
- Ju, Z.; Zhang, S.; Xing, Z.; Zhuang, Q.; Qiang, Y.; Qian, Y. Direct Synthesis of Few-Layer F-Doped Graphene Foam and Its Lithium/Potassium Storage Properties. ACS Appl. Mater. Interfaces 2016, 8, 20682–20690. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, X.; Wu, M.; Li, Q.; Wang, Y.; Yao, J. Metal-Free Fluorine-Doped Carbon Electrocatalyst for CO2 Reduction Outcompeting Hydrogen Evolution. Angew. Chem. Int. Ed. Engl. 2018, 57, 9640–9644. [Google Scholar] [CrossRef]
- Qiu, D.; Zhang, B.; Zhang, T.; Shen, T.; Zhao, Z.; Hou, Y. Sulfur-Doped Carbon for Potassium-Ion Battery Anode: Insight into the Doping and Potassium Storage Mechanism of Sulfur. ACS Nano 2022, 16, 21443–21451. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, Y.; Zhuang, X.; Li, S.; Wu, D.; Zhang, F.; Feng, X. Low-temperature synthesis of nitrogen/sulfur co-doped three-dimensional graphene frameworks as efficient metal-free electrocatalyst for oxygen reduction reaction. Carbon 2013, 62, 296–301. [Google Scholar] [CrossRef]
- Hwang, T.H.; Jung, D.S.; Kim, J.-S.; Kim, B.G.; Choi, J.W. One-Dimensional Carbon–Sulfur Composite Fibers for Na–S Rechargeable Batteries Operating at Room Temperature. Nano Lett. 2013, 13, 4532–4538. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Hassan, F.M.; Li, J.; Fan, X.; Batmaz, R.; Xiao, X.; Chen, Z. Sulfur covalently bonded graphene with large capacity and high rate for high-performance sodium-ion batteries anodes. Nano Energy 2015, 15, 746–754. [Google Scholar] [CrossRef]
- de Tomas, C.; Alabidun, S.; Chater, L.; Darby, M.T.; Raffone, F.; Restuccia, P.; Au, H.; Titirici, M.M.; Cucinotta, C.S.; Crespo-Ribadenyra, M. Doping carbon electrodes with sulfur achieves reversible sodium ion storage. J. Phys. Energy 2023, 5, 024006. [Google Scholar] [CrossRef]
- Wang, P.; Qiao, B.; Du, Y.; Li, Y.; Zhou, X.; Dai, Z.; Bao, J. Fluorine-Doped Carbon Particles Derived from Lotus Petioles as High-Performance Anode Materials for Sodium-Ion Batteries. J. Phys. Chem. C 2015, 119, 21336–21344. [Google Scholar] [CrossRef]
- An, H.; Li, Y.; Gao, Y.; Cao, C.; Han, J.; Feng, Y.; Feng, W. Free-standing fluorine and nitrogen co-doped graphene paper as a high-performance electrode for flexible sodium-ion batteries. Carbon 2017, 116, 338–346. [Google Scholar] [CrossRef]
- Sun, M.; Qu, Y.; Zeng, F.; Yang, Y.; Xu, K.; Yuan, C.; Lu, Z.-H. Hierarchical Porous and Sandwich-like Sulfur-Doped Carbon Nanosheets as High-Performance Anodes for Sodium-Ion Batteries. Ind. Eng. Chem. Res. 2022, 61, 2126–2135. [Google Scholar] [CrossRef]
- Zhang, H.-W.; Lu, J.-M.; Yang, L.; Hu, M.-X.; Huang, Z.-H.; Lu, R.-T.; Kang, F.-Y. N, S co-doped porous carbon nanospheres with a high cycling stability for sodium ion batteries. New Carbon Mater. 2017, 32, 517–526. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, Y.; Huang, Y.; Wang, X.; Sun, Y.; Guo, Y.; Jia, D.; Tang, X. Sulfur and phosphorus co-doped hard carbon derived from oak seeds enabled reversible sodium spheres filling and plating for ultra-stable sodium storage. J. Alloy Compd. 2021, 851, 156791. [Google Scholar] [CrossRef]
- Duan, M.; Zhu, F.; Zhao, G.; Hu, J.; Zhang, H.; Ren, G.; Meng, Y.; Fan, Z. Nitrogen and sulfur co-doped mesoporous carbon derived from ionic liquid as high-performance anode material for sodium ion batteries. Microporous Mesoporous Mater. 2020, 306, 110433. [Google Scholar] [CrossRef]
- Furthmüller, G.K.J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar]
- Kresse, D.J.G. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, Q.; Han, P.; Luo, J.; Peng, K.-Q. An S-Infused/S, F-Codoped PVDF-Derived Carbon as a High-Performance Anode for Sodium-Ion Batteries. Materials 2025, 18, 4018. https://doi.org/10.3390/ma18174018
Wang J, Zhang Q, Han P, Luo J, Peng K-Q. An S-Infused/S, F-Codoped PVDF-Derived Carbon as a High-Performance Anode for Sodium-Ion Batteries. Materials. 2025; 18(17):4018. https://doi.org/10.3390/ma18174018
Chicago/Turabian StyleWang, Jianjiao, Qian Zhang, Pengyu Han, Jiakun Luo, and Kui-Qing Peng. 2025. "An S-Infused/S, F-Codoped PVDF-Derived Carbon as a High-Performance Anode for Sodium-Ion Batteries" Materials 18, no. 17: 4018. https://doi.org/10.3390/ma18174018
APA StyleWang, J., Zhang, Q., Han, P., Luo, J., & Peng, K.-Q. (2025). An S-Infused/S, F-Codoped PVDF-Derived Carbon as a High-Performance Anode for Sodium-Ion Batteries. Materials, 18(17), 4018. https://doi.org/10.3390/ma18174018






