Heat Treatment-Assisted Optimization of the Water Splitting Performance of CoCrNi0.5Ti0.3V0.2Al0.4 Eutectic High-Entropy Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Alloy Preparation and Heat Treatment Process
2.2. Dealloying Process
2.3. Material Characterization
2.4. Electrochemical Testing
3. Results and Discussion
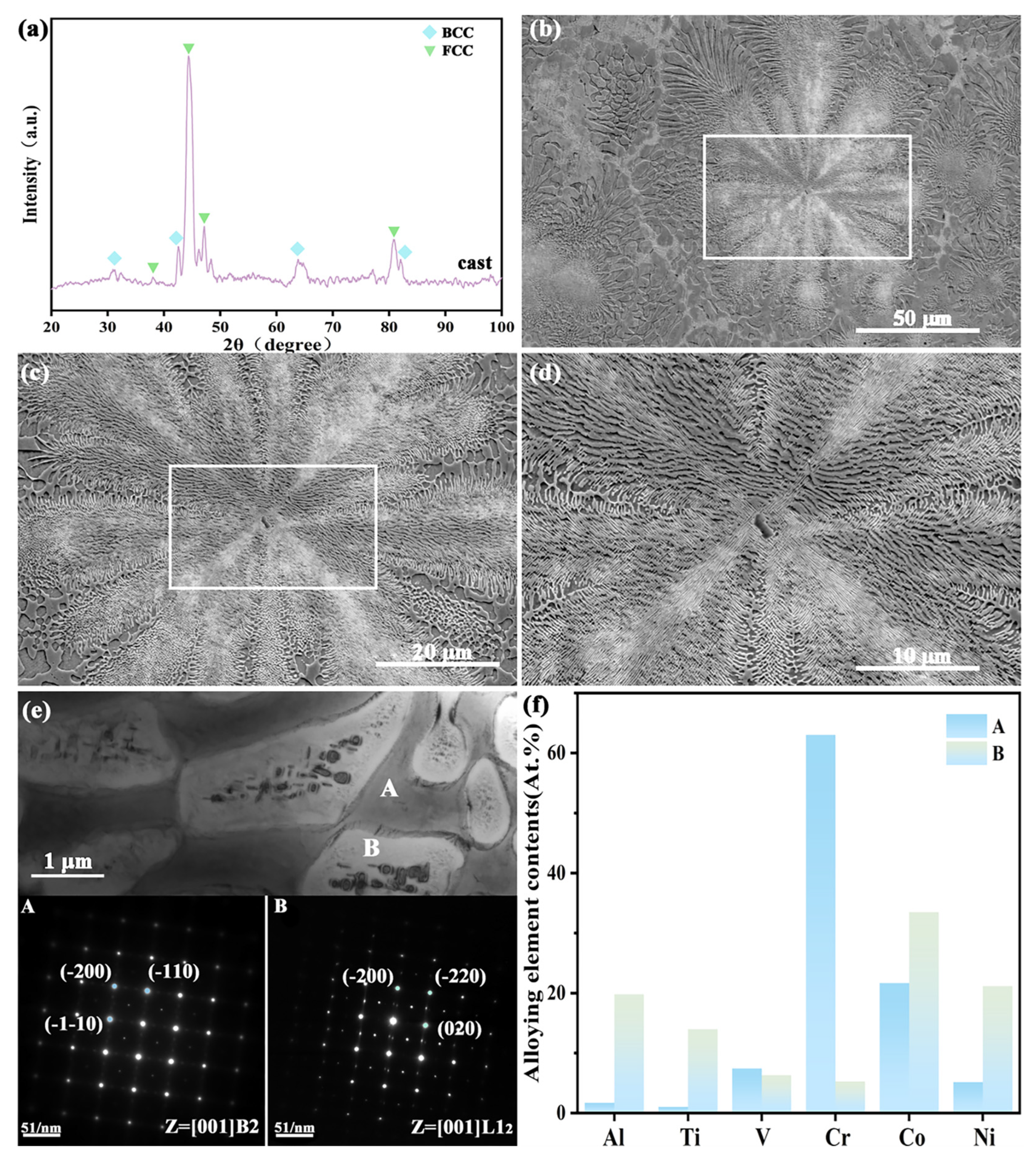
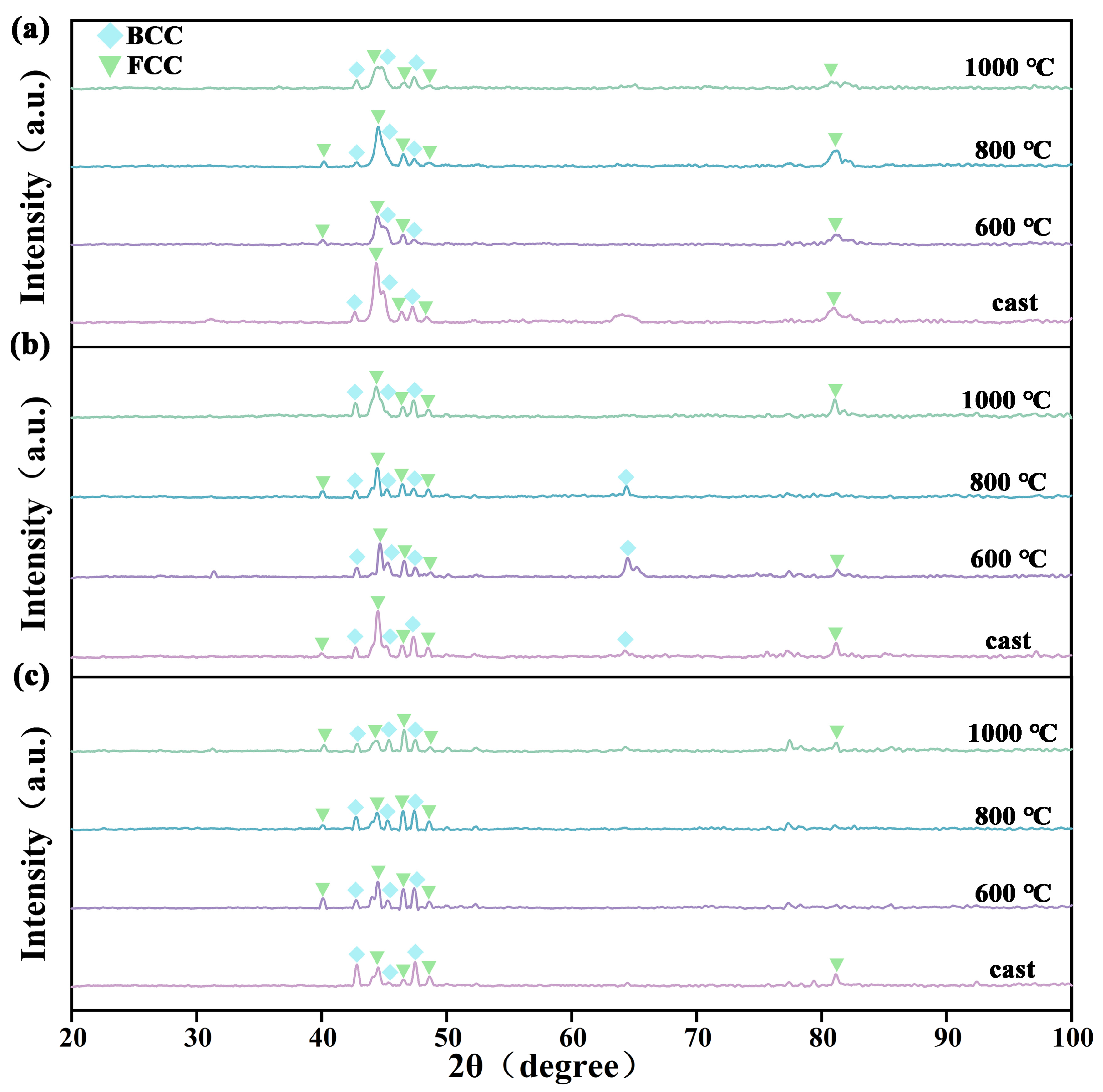
3.1. Effect of Heat Treatment on the Microstructure of the As-Cast CoCrNi0.5Ti0.3V0.2Al0.4 EHEA
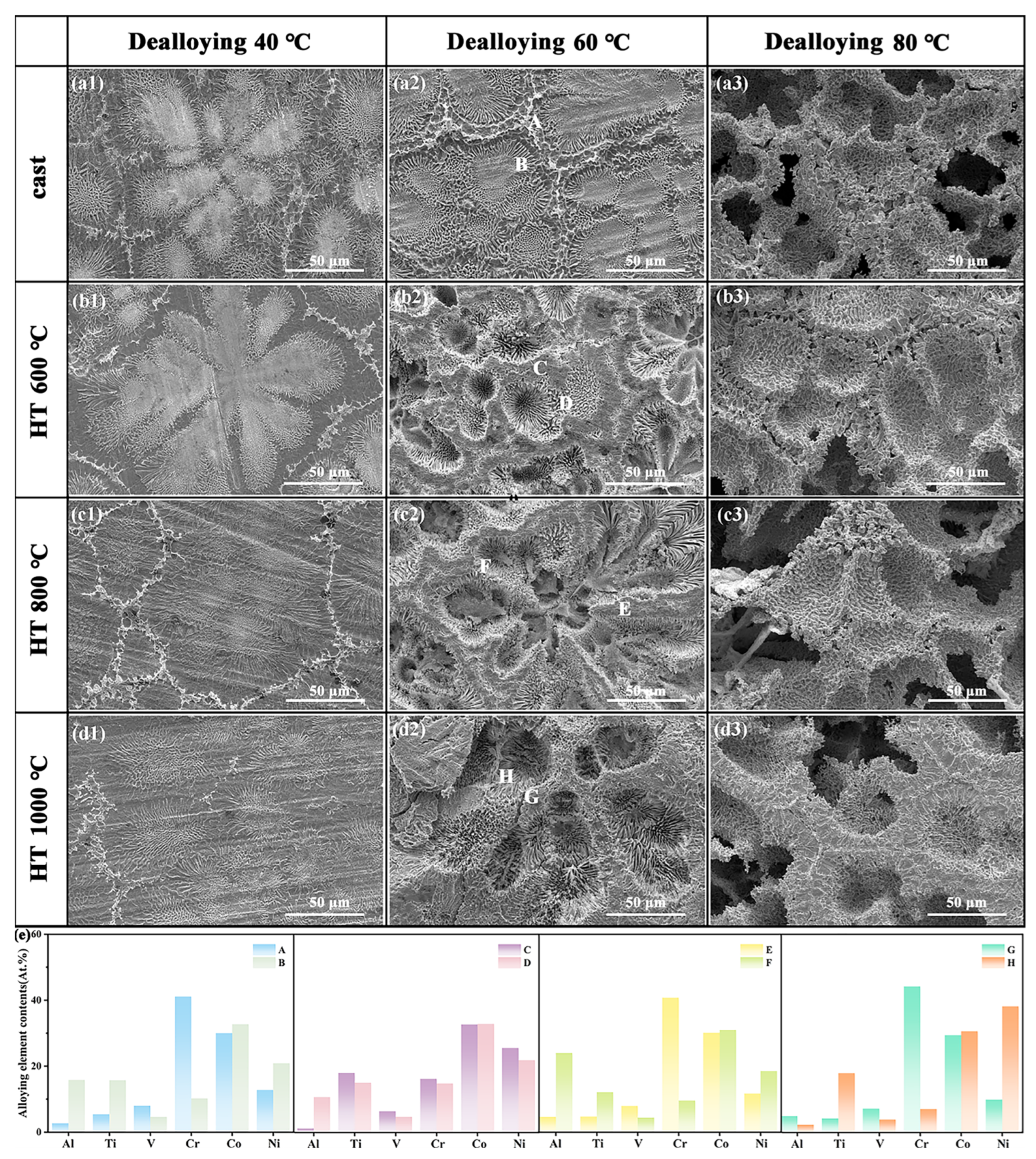
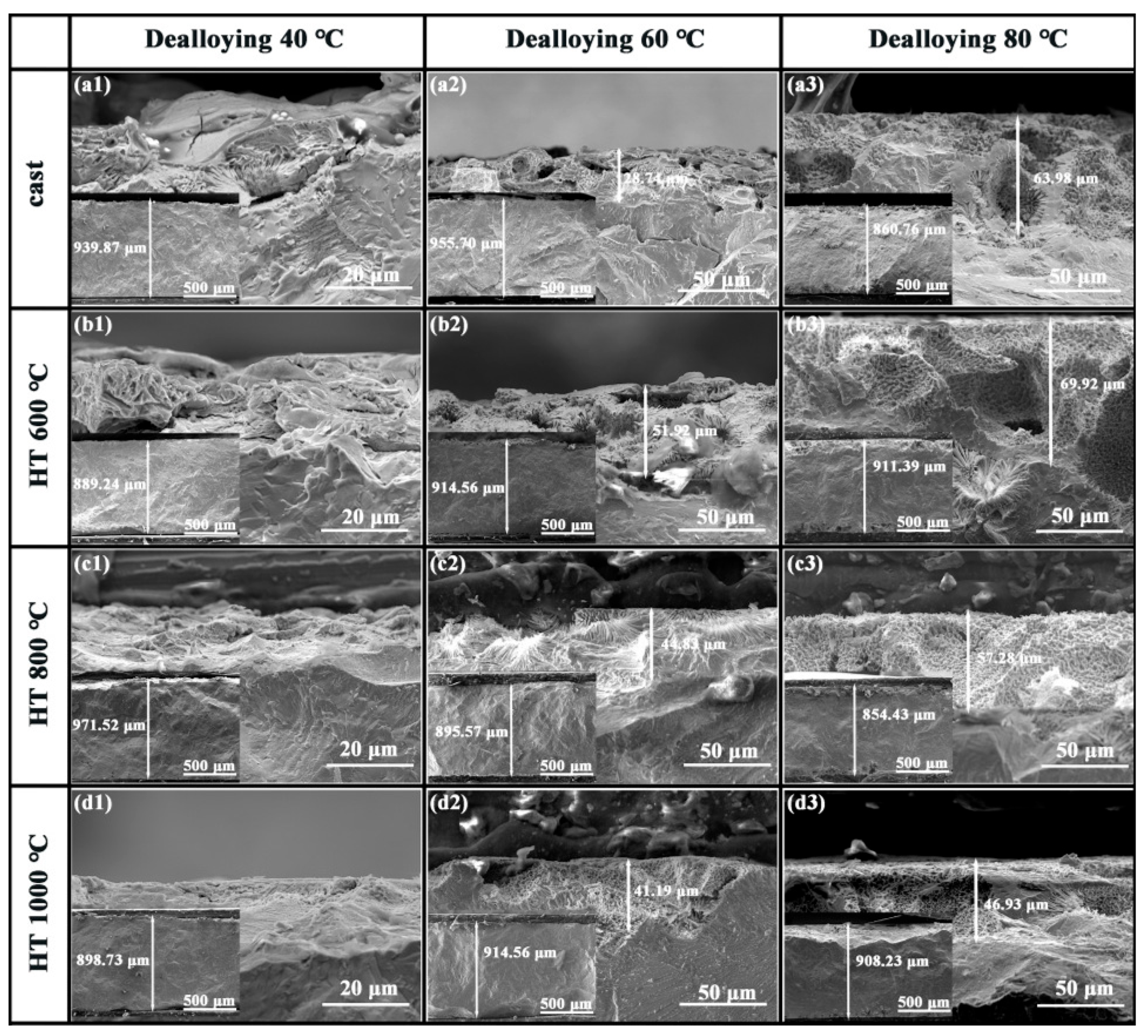
3.2. Effect of Dealloying on the Porous Structure Formatting of Mathematical Components
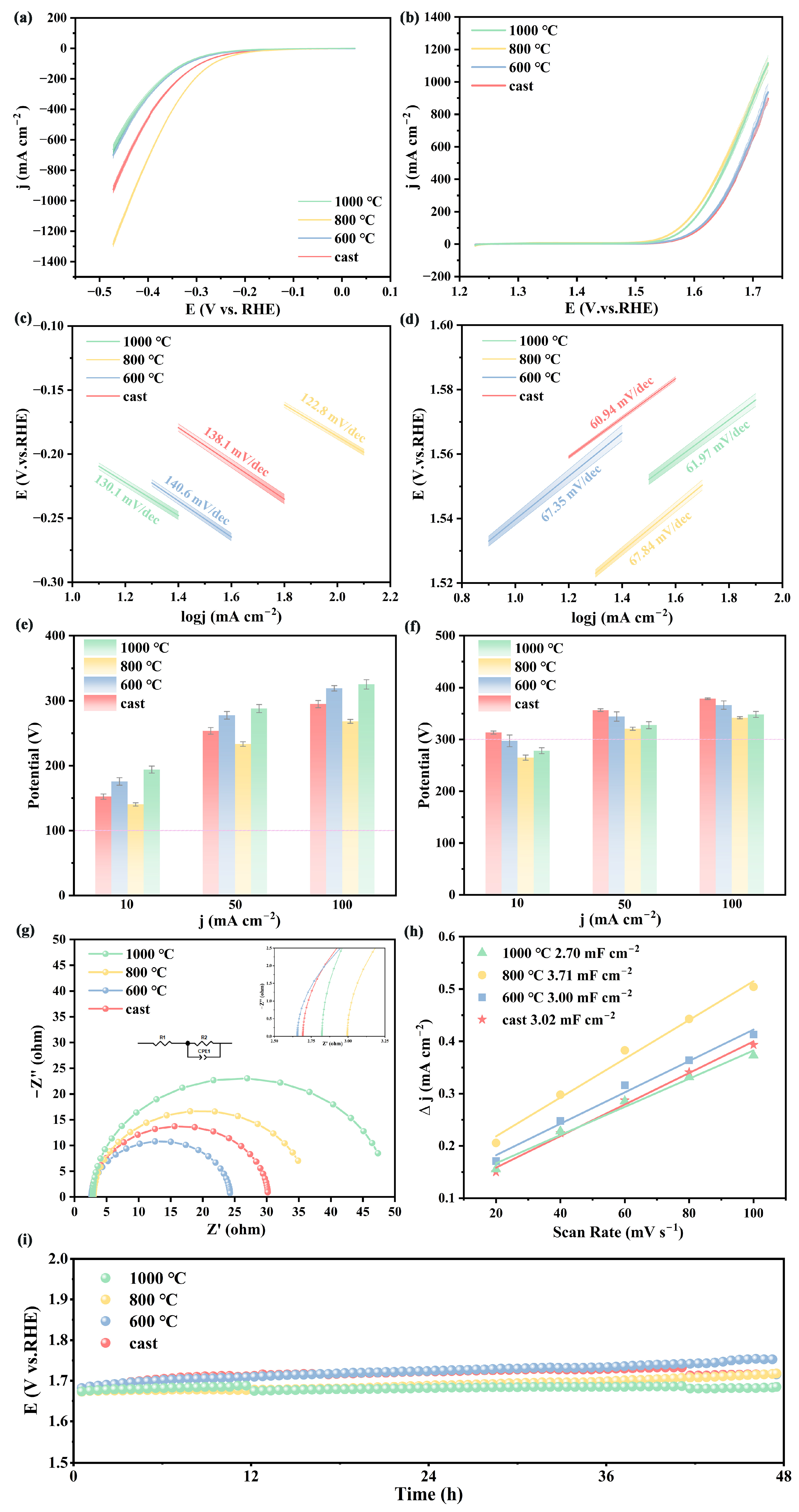
3.3. Electrocatalytic Performance
3.4. Effect of the Electrocatalytic Reaction on the Microstructure of CoCrNi0.5Ti0.3V0.2Al0.4 EHEA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, W.T.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Shao-Horn, Y. Toward the Rational Design of Non-Precious Transition Metal Oxides for Oxygen Electrocatalysis. Energy Environ. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef]
- Chen, D.; Chen, C.; Baiyee, Z.M.; Shao, Z.; Ciucci, F. Nonstoichiometric Oxides as Low-Cost and Highly-Efficient Oxygen Reduction/Evolution Catalysts for Low-Temperature Electrochemical Devices. Chem. Rev. 2015, 115, 9869–9921. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Jeon, K.-J.; Show, P.L.; Lee, I.H.; Jung, S.-C.; Choi, Y.J.; Rhee, G.H.; Lin, K.-Y.A.; Park, Y.-K. Mini Review on H2 Production from Electrochemical Water Splitting According to Special Nanostructured Morphology of Electrocatalysts. Fuel 2022, 308, 122048. [Google Scholar] [CrossRef]
- Saad, A.; Liu, D.; Wu, Y.; Song, Z.; Li, Y.; Najam, T.; Zong, K.; Tsiakaras, P.; Cai, X. Ag Nanoparticles Modified Crumpled Borophene Supported Co3O4 Catalyst Showing Superior Oxygen Evolution Reaction (OER) Performance. Appl. Catal. B Environ. 2021, 298, 120529. [Google Scholar] [CrossRef]
- Ahmad, A.; Nairan, A.; Feng, Z.; Zheng, R.; Bai, Y.; Khan, U.; Gao, J. Unlocking the Potential of High Entropy Alloys in Electrochemical Water Splitting: A Review. Small 2024, 20, 2311929. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, P.; Han, J.; Cheng, C.; Ning, S.; Hirata, A.; Fujita, T.; Chen, M. Engineering the Internal Surfaces of Three-Dimensional Nanoporous Catalysts by Surfactant-Modified Dealloying. Nat. Commun. 2017, 8, 1066. [Google Scholar] [CrossRef]
- Li, M.; Duanmu, K.; Wan, C.; Cheng, T.; Zhang, L.; Dai, S.; Chen, W.; Zhao, Z.; Li, P.; Fei, H.; et al. Single-Atom Tailoring of Platinum Nanocatalysts for High-Performance Multifunctional Electrocatalysis. Nat. Catal. 2019, 2, 495–503. [Google Scholar] [CrossRef]
- Forty, A.J. Corrosion Micromorphology of Noble Metal Alloys and Depletion Gilding. Nature 1979, 282, 597–598. [Google Scholar] [CrossRef]
- Lu, Q.; Hutchings, G.S.; Yu, W.; Zhou, Y.; Forest, R.V.; Tao, R.; Rosen, J.; Yonemoto, B.T.; Cao, Z.; Zheng, H.; et al. Highly Porous Non-Precious Bimetallic Electrocatalysts for Efficient Hydrogen Evolution. Nat. Commun. 2015, 6, 6567. [Google Scholar] [CrossRef]
- Dong, C.; Kou, T.; Gao, H.; Peng, Z.; Zhang, Z. Eutectic-Derived Mesoporous Ni-Fe-O Nanowire Network Catalyzing Oxygen Evolution and Overall Water Splitting. Adv. Energy Mater. 2018, 8, 1701347. [Google Scholar] [CrossRef]
- Li, M.; Lin, F.; Zhang, S.; Zhao, R.; Tao, L.; Li, L.; Li, J.; Zeng, L.; Luo, M.; Guo, S. High-Entropy Alloy Electrocatalysts Go to (Sub-)Nanoscale. Sci. Adv. 2024, 10, eadn2877. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural Development in Equiatomic Multicomponent Alloys. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A Critical Review of High Entropy Alloys and Related Concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Zhen, P.; Jian, S.; Luan, H.W.; Na, C.; Yao, K.F. Effect of Mo on the high temperature oxidation behavior of Al19Fe20−xCo20−xNi41Mo2x high entropy alloys. Intermetallics 2023, 155, 107845. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, Z.; Hao, J.; Sun, S.; Lu, S.; Wang, C.; Ma, P.; Dong, W.; Du, M. High-Entropy Alloy Stabilized Active Ir for Highly Efficient Acidic Oxygen Evolution. Chem. Eng. J. 2022, 431, 133251. [Google Scholar] [CrossRef]
- Louis, M.P.; Peter, F. A synthesis of the Brewer-Engel and Samsonov-Pryadko-Pryadko electron correlations for metals. J. Solid State Chem. 1979, 27, 239–253. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, W.; Gao, Z.; Liang, Y.; Jiang, H.; Li, Z.; Wu, S.; Cui, Z.; Sun, H.; Zhang, H.; et al. Self-Supporting High-Entropy Co-Cr-Fe-Ni-Nb Oxide Electrocatalyst with Nanoporous Structure for Oxygen Evolution Reaction. Chem. Eng. J. 2024, 489, 151233. [Google Scholar] [CrossRef]
- Chongjun, Z.; Wenlei, C.; Nan, S.; Shi, C.; Wenbin, J.; Chunhua, Z. Facile preparation of porous high-entropy alloy FeCoNiCuMn and its OER performance. J. Phys. Chem. Solids 2024, 184, 111668. [Google Scholar] [CrossRef]
- Zhu, J.; Bian, P.; Sun, G.; Zhang, J.; Lou, G.; Song, X.; Zhao, R.; Liu, J.; Xu, N.; Li, A.; et al. Practical High-Voltage Lithium Metal Batteries Enabled by the In-Situ Fabrication of Main-Chain Fluorinated Polymer Electrolytes. Angew. Chem. Int. Ed. 2025, 64, e202424685. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, S.; Han, G.; Bian, H.; Zhao, X.; Wang, L.; Xie, G. Hierarchical Porous Nonprecious High-entropy Alloys for Ultralow Overpotential in Hydrogen Evolution Reaction. Small Methods 2024, 8, 2301691. [Google Scholar] [CrossRef]
- Huang, K.; Xia, J.; Lu, Y.; Zhang, B.; Shi, W.; Cao, X.; Zhang, X.; Woods, L.M.; Han, C.; Chen, C.; et al. Self-Reconstructed Spinel Surface Structure Enabling the Long-Term Stable Hydrogen Evolution Reaction/Oxygen Evolution Reaction Efficiency of FeCoNiRu High-Entropy Alloyed Electrocatalyst. Adv. Sci. 2023, 10, 2300094. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Zong, L.; Zhang, G.; Li, P.; Fan, K.; Jiang, S.; Chen, X.; Liu, P.; Wang, L. Ultrafine high entropy alloys with Ru activated sites for highly durable and industrial grade electrocatalytic water splitting. Compos. Part B Eng. 2025, 301, 112501. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Guo, S.; Jiang, L.; Kang, H.; Wang, T.; Wen, B.; Wang, Z.; Jie, J.; Cao, Z.; et al. A Promising New Class of High-Temperature Alloys: Eutectic High-Entropy Alloys. Sci. Rep. 2014, 4, 6200. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, Y.T.; Yao, R.Q.; Wan, W.B.; Zhang, Q.H.; Gu, L.; Wen, Z.; Lang, X.Y.; Jiang, Q. Intermetallic Cu5Zr Clusters Anchored on Hierarchical Nanoporous Copper as Efficient Catalysts for Hydrogen Evolution Reaction. Research 2020, 2020, 2987234. [Google Scholar] [CrossRef]
- Luo, M.; Peng, W.; Zhao, Y.; Lan, J.; Peng, M.; Han, J.; Li, H.; Tan, Y. Dilute molybdenum atoms embedded in hierarchical nanoporous copper accelerate the hydrogen evolution reaction. Scr. Mater. 2021, 191, 56–61. [Google Scholar] [CrossRef]
- Chen, Q.; Han, X.; Xu, Z.; Chen, Q.; Wu, Q.; Zheng, T.; Wang, P.; Wang, Z.; Wang, J.; Li, H.; et al. Atomic phosphorus induces tunable lattice strain in high entropy alloys and boosts alkaline water splitting. Nano Energy. 2023, 110, 108380. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, Q.; Zhang, Y.; He, Y.; Gong, W.; Liu, W.; Liu, X.; Li, R. Architecting Gradient Hierarchically Porous Catalyst via Negative Mixing Enthalpy High-Entropy Alloy for Durable Water Splitting at Ampere-Level Current Density. Adv. Funct. Mater. 2025, 35, 2414446. [Google Scholar] [CrossRef]
- Yao, R.Q.; Zhou, Y.T.; Shi, H.; Wan, W.B.; Zhang, Q.H.; Gu, L.; Zhu, Y.F.; Wen, Z.; Lang, X.Y.; Jiang, Q. Nanoporous Surface High-Entropy Alloys as Highly Efficient Multisite Electrocatalysts for Nonacidic Hydrogen Evolution Reaction. Adv. Funct. Mater. 2021, 31, 200961. [Google Scholar] [CrossRef]
- Li, R.; Liu, X.J.; Wang, H.; Zhou, D.Q.; Wu, Y.; Lu, Z.P. Formation mechanism and characterization of nanoporous silver with tunable porosity and promising capacitive performance by chemical dealloying of glassy precursor. Acta Mater. 2016, 105, 367–377. [Google Scholar] [CrossRef]
- Munitz, A.; Salhov, S.; Hayun, S.; Frage, N. Heat treatment impacts the micro-structure and mechanical properties of AlCoCrFeNi high entropy alloy. J. Alloys Compd. 2016, 683, 221–230. [Google Scholar] [CrossRef]
- Zahra, Z.; Milad, Z.; Amir, M.; Saeed, S.; Mahesh, S. Effect of heat treatment regime on microstructure and phase evolution of AlMo0.5NbTa0.5TiZr refractory high entropy alloy. J. Alloys Compd. 2023, 949, 169818. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Duan, H.; Ma, Z.; Wu, C.; Bai, Y.; Sun, C.; Wang, P.; Li, J. Microstructure and mechanical properties of Al0.4Co0.5V0.6FeNi high-entropy alloys processed by homogenization treatment. Intermetallics 2023, 159, 107941. [Google Scholar] [CrossRef]
- Sun, H.; Liu, T.; Hashimoto, N.; Oka, H. Effects of vacuum heat treatment on novel Co-free Al0.9Cr0.8FeMn0.8Ni2.0 eutectic high entropy alloy: Microstructure evolution and mechanical properties. Vacuum 2024, 222, 113038. [Google Scholar] [CrossRef]
- Zhang, G.; Ming, K.; Kang, J.; Huang, Q.; Zhang, Z.; Zheng, X.; Bi, X. High entropy alloy as a highly active and stable electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2018, 279, 19–23. [Google Scholar] [CrossRef]
- Gao, Y.; Han, J.; Ge, F.; Zhang, X.; Cai, Y.; Cui, Y. Design of eutectic high-entropy alloys in the Co-Cr-Ni-V-Ti-Al system using Scheil solidification path optimization. J. Alloys Compd. 2024, 1002, 175327. [Google Scholar] [CrossRef]
- Chen, X.; Xie, W.; Zhu, J.; Wang, Z.; Wang, Y.; Ma, Y.; Yang, M.; Jiang, W.; Yu, H.; Wu, Y.; et al. Influences of Ti additions on the microstructure and tensile properties of AlCoCrFeNi2.1 eutectic high entropy alloy. Intermetallics 2021, 128, 107024. [Google Scholar] [CrossRef]
- He, J.Y.; Liu, W.H.; Wang, H.; Wu, Y.; Liu, X.J.; Nieh, T.G.; Lu, Z.P. Effects of Al addition on structural evolution and tensile properties of the FeCoNiCrMn high-entropy alloy system. Acta Mater. 2014, 62, 105–113. [Google Scholar] [CrossRef]
- Yan, P.X.; Chang, J.; Wang, W.L.; Zhu, X.N.; Lin, M.J.; Wei, B.J.A.M. Eutectic growth kinetics and microscopic mechanical properties of rapidly solidified CoCrFeNiMo0.8 high entropy alloy. Acta Mater. 2022, 237, 118149. [Google Scholar] [CrossRef]
- Kyoungdoc, K.; Peter, W.V. Ostwald ripening of spheroidal particles in multicomponent alloys. Acta Mater. 2018, 152, 327–337. [Google Scholar] [CrossRef]
- Zhao, L.; Zhisong, C.; Lingyu, W.; Zhou, W.; Qi, L.; Jianfeng, W.; Wei, X. Effect of Cr on high-temperature oxidation resistance of Cr–Si–Mn alloyed press-hardened steel during press hardening. J. Mater. Res. Technol. 2024, 32, 1552–1564. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Wang, J.; Wang, W.Y.; Kou, H.; Beaugnon, E. Temperature dependent deformation mechanisms of Al0.3CoCrFeNi high-entropy alloy, starting from serrated flow behavior. J. Alloys Compd. 2018, 757, 39–43. [Google Scholar] [CrossRef]
- Song, L.; Hu, W.; Liao, B.; Wan, S.; Guo, X. Corrosion behavior of AlCoCrFeNi2.1 eutectic high-entropy alloy in Cl--containing solution. J. Alloys Compd. 2023, 938, 168609. [Google Scholar] [CrossRef]
- Qi, L.; Guan, J. Electronic structure modulation of high entropy materials for advanced electrocatalysis. Green Energy Environ. 2025, 10, 917–936. [Google Scholar] [CrossRef]
- Munzir, S.; Abdullah, A.G.; Turki, B.Q.D.; Mohd, R.Z.Y.; Mohammad, Q. Growth of ultrathin nanosheets of nickel iron layered double hydroxide for the oxygen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 23498–23507. [Google Scholar] [CrossRef]
- Rokhsareh, A.; Ghasem, B.D. Interfacial surface engineering of Co-Mn-P ultrathin nanosheets on Ni-Co hierarchical nanostructure for boosting electrochemical active sites in overall water splitting. J. Power Sources 2025, 641, 236840. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, W.; Lou, Y.; Yao, Z.; Ying, H.; Dong, M.; Tan, L.; Zeng, J.; Ji, H.; Zhu, H.; et al. Ultrafast joule-heating synthesis of FeCoMnCuAl high-entropy-alloy nanoparticles as efficient OER electrocatalysts. Prog. Nat. Sci. Mater. Int. 2024, 34, 880–887. [Google Scholar] [CrossRef]
- Li, Z.; Liu, M.; Yan, J.; Lee, L.Y.S. A “doping–interfacing” Strategy Enables Efficient Alkaline Freshwater and Seawater Oxidation by NiFe-layered Double Hydroxides. Chem. Eng. J. 2023, 473, 145293. [Google Scholar] [CrossRef]
- Abdul, H.; Muhammad, Y.S.; Muhammad, N.L.; Abdulaziz, A.; Muhammad, A.S.; Abdul, J.L.; Imtiaz, A.S.; Muhammad, I.A.; Mukesh, K.; Umair, A. CoSe2@Co3O4 nanostructures: A promising catalyst for oxygen evolution reaction in alkaline media. Catal. Commun. 2024, 186, 106830, ISSN 1566-7367. [Google Scholar] [CrossRef]
- Frydendal, R.; Paoli, E.A.; Knudsen, B.P.; Wickman, B.; Malacrida, P.; Stephens, I.E.L.; Chorkendorff, I. Benchmarking the Stability of Oxygen Evolution Reaction Catalysts: The Importance of Monitoring Mass Losses. ChemElectroChem 2014, 1, 2075–2081. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, T.; Zhang, Y.; Du, J.; Han, X.; Chen, J. Enhancing Electrocatalytic Oxygen Reduction on MnO2 with Vacancies. Angew. Chem. Int. Ed. 2013, 52, 2474–2477. [Google Scholar] [CrossRef] [PubMed]


| Catalyst Composition | Electrolyte Type | Overpotentials(mV) 10 mA/cm2 | Tafel Slopes (mV/dec) |
|---|---|---|---|
| CoCrNi0.5Ti0.3V0.2Al0.4 EHEA | 1 M KOH | 265 | 67.84 |
| Ni2Fe1Al97 | 1 M KOH | 244 | 39 |
| P-Ni30Co30Fe10Cr10Al18W2 | 1 M KOH | 211 | 41.3 |
| FeCoNiCrZr0.25 HEA | 1 M KOH | 215 | 44.9 |
| NiFe LDH | 1 M KOH | 270 | 48.6 |
| Co-Mn-P@Ni-Co | 1 M KOH | 309 | 56 |
| FeCoMnCuAl HEA [48] | 1 M KOH | 280 | 76.13 |
| Co-Cr-Fe-Ni-Nb | 1 M KOH | 251 | 42.7 |
| Li-NiFe-LDH/g-C3N4 [49] | 1 M KOH | 276 | 51.1 |
| CoSe2@Co3O4 [50] | 1 M KOH | 252 | 69 |
| RuO2 | 1 M KOH | 290 | 82.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Yin, Z.; Liu, S.; Cai, Y.; Zhang, Y. Heat Treatment-Assisted Optimization of the Water Splitting Performance of CoCrNi0.5Ti0.3V0.2Al0.4 Eutectic High-Entropy Alloy. Materials 2025, 18, 4015. https://doi.org/10.3390/ma18174015
Sun M, Yin Z, Liu S, Cai Y, Zhang Y. Heat Treatment-Assisted Optimization of the Water Splitting Performance of CoCrNi0.5Ti0.3V0.2Al0.4 Eutectic High-Entropy Alloy. Materials. 2025; 18(17):4015. https://doi.org/10.3390/ma18174015
Chicago/Turabian StyleSun, Mingran, Zixiang Yin, Shuai Liu, Yangchuan Cai, and Yu Zhang. 2025. "Heat Treatment-Assisted Optimization of the Water Splitting Performance of CoCrNi0.5Ti0.3V0.2Al0.4 Eutectic High-Entropy Alloy" Materials 18, no. 17: 4015. https://doi.org/10.3390/ma18174015
APA StyleSun, M., Yin, Z., Liu, S., Cai, Y., & Zhang, Y. (2025). Heat Treatment-Assisted Optimization of the Water Splitting Performance of CoCrNi0.5Ti0.3V0.2Al0.4 Eutectic High-Entropy Alloy. Materials, 18(17), 4015. https://doi.org/10.3390/ma18174015





