Corrosion Performance in 0.5 mol/L HF Solution of Cr-Cu-Mo-Ni Porous Alloys with Varying Cr Contents
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Cr-Cu-Mo-Ni Porous Alloys
2.2. Immersion Test
2.3. Electrochemical Measurement
2.3.1. Preparation of Corrosion Test Samples
2.3.2. Electrochemical Test Procedure
3. Results and Discussion
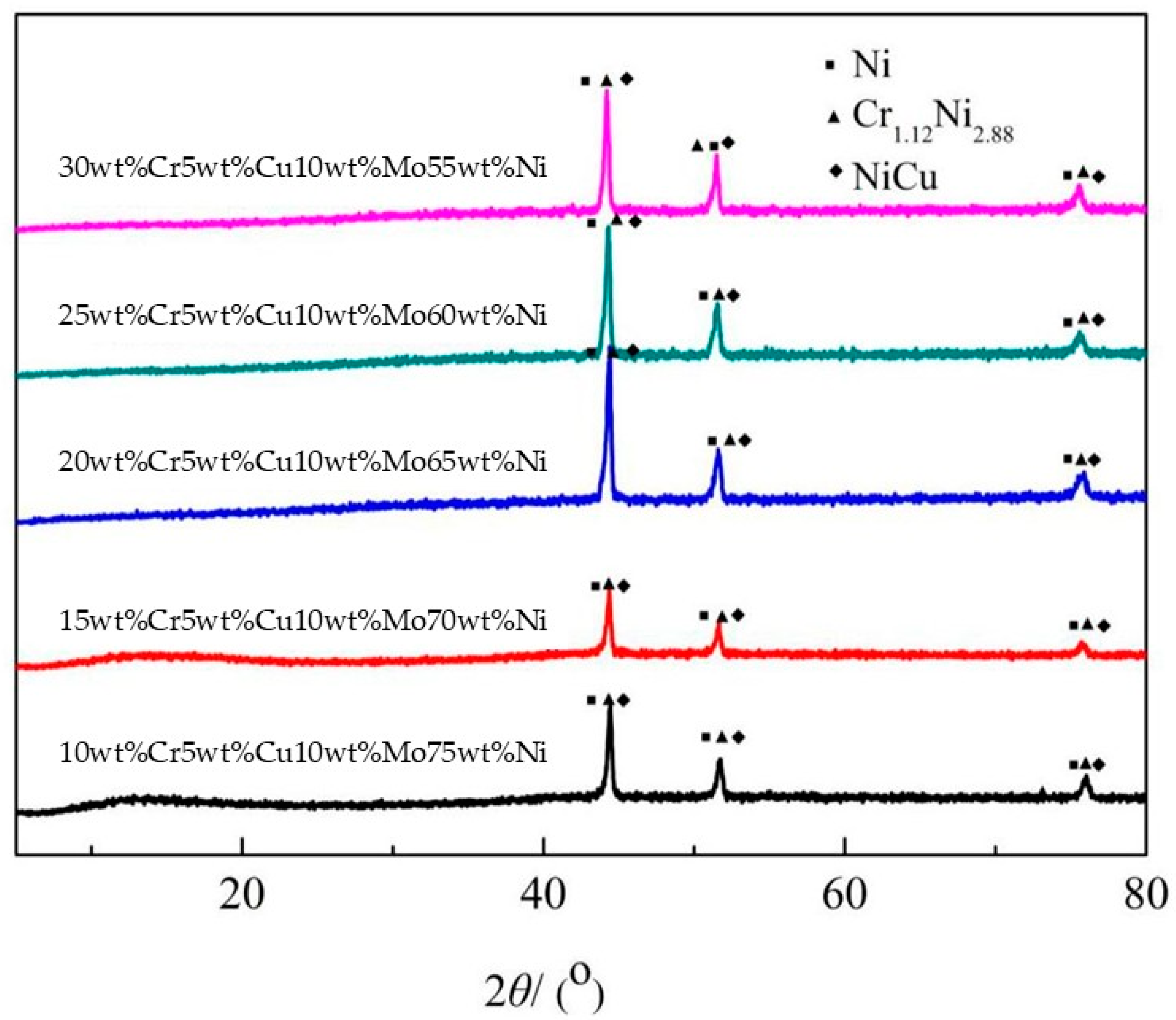
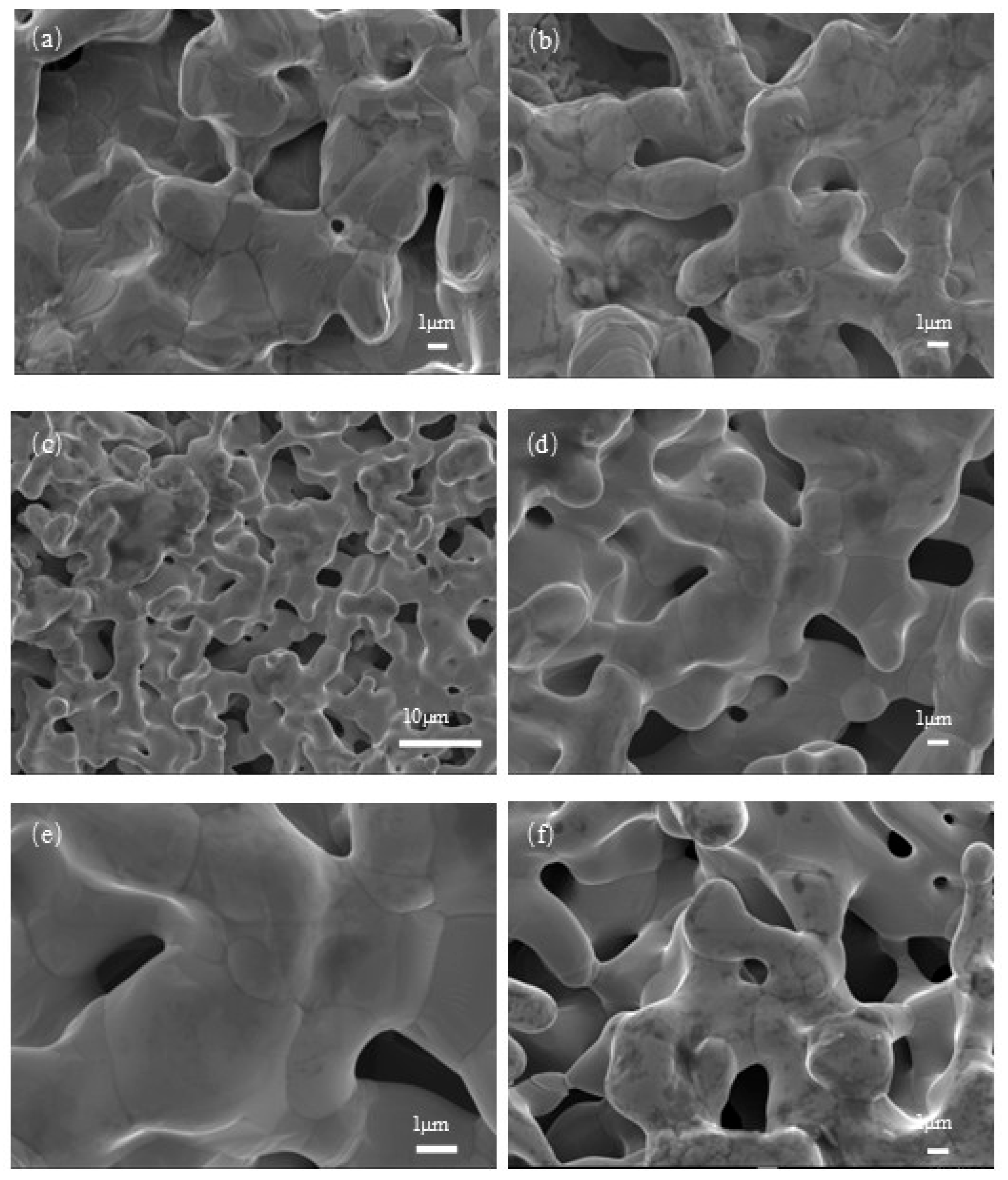
3.1. Characterization of Porous Cr-Cu-Mo-Ni Alloy
3.2. Immersion Test
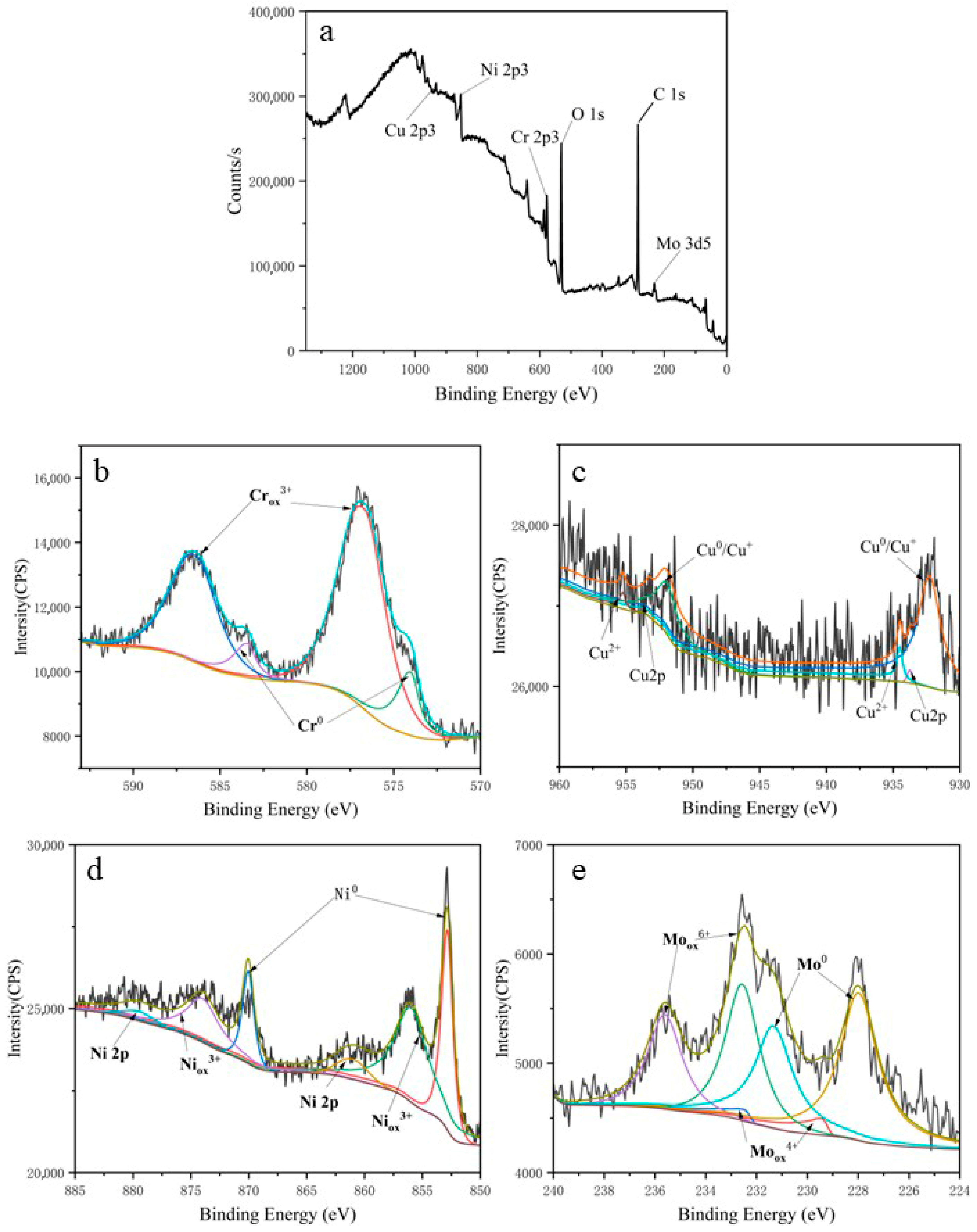
3.3. X-Ray Photoelectron Spectroscopy
3.4. Electrochemical Measurements
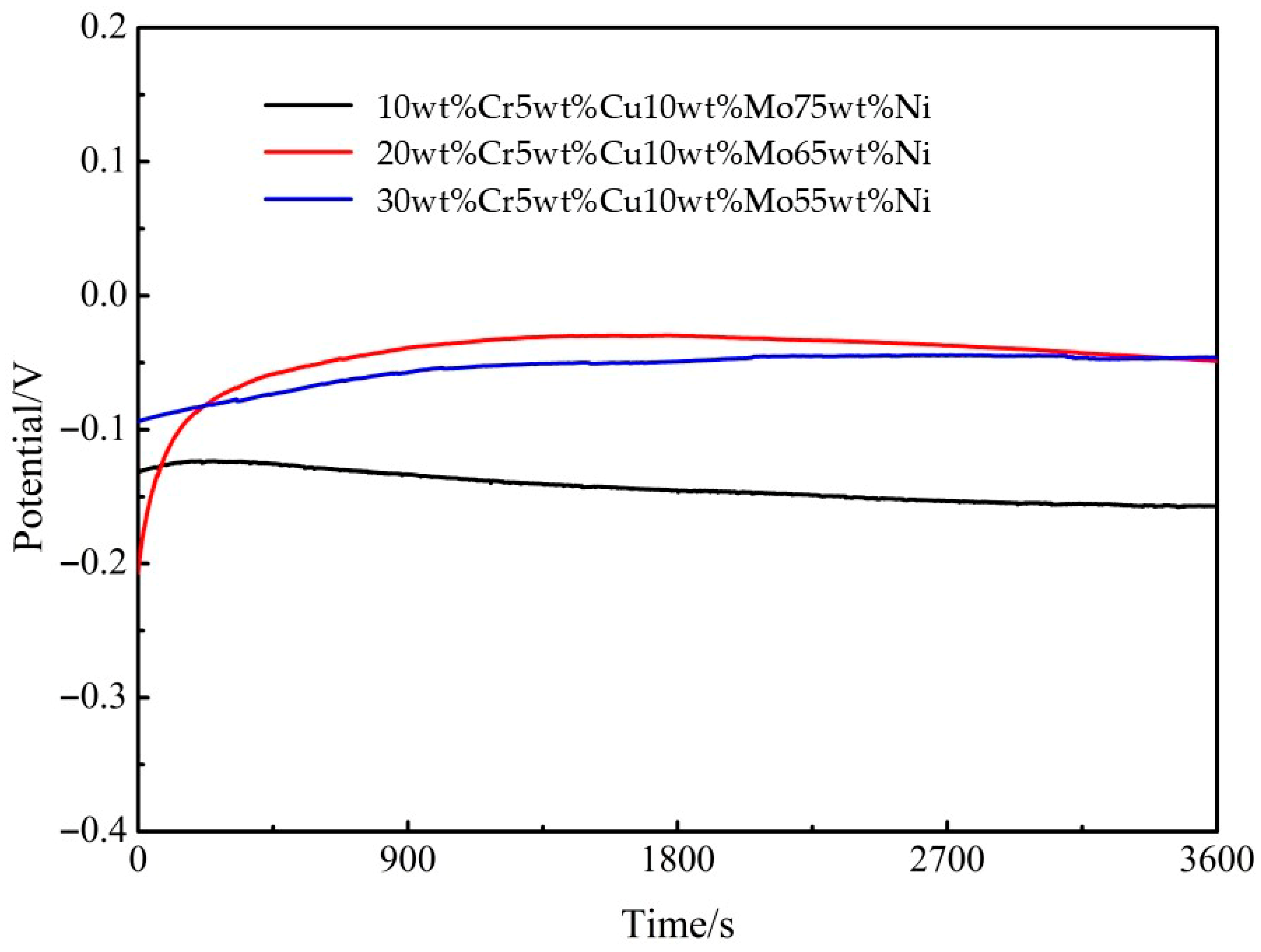
3.4.1. Open Circuit Potential
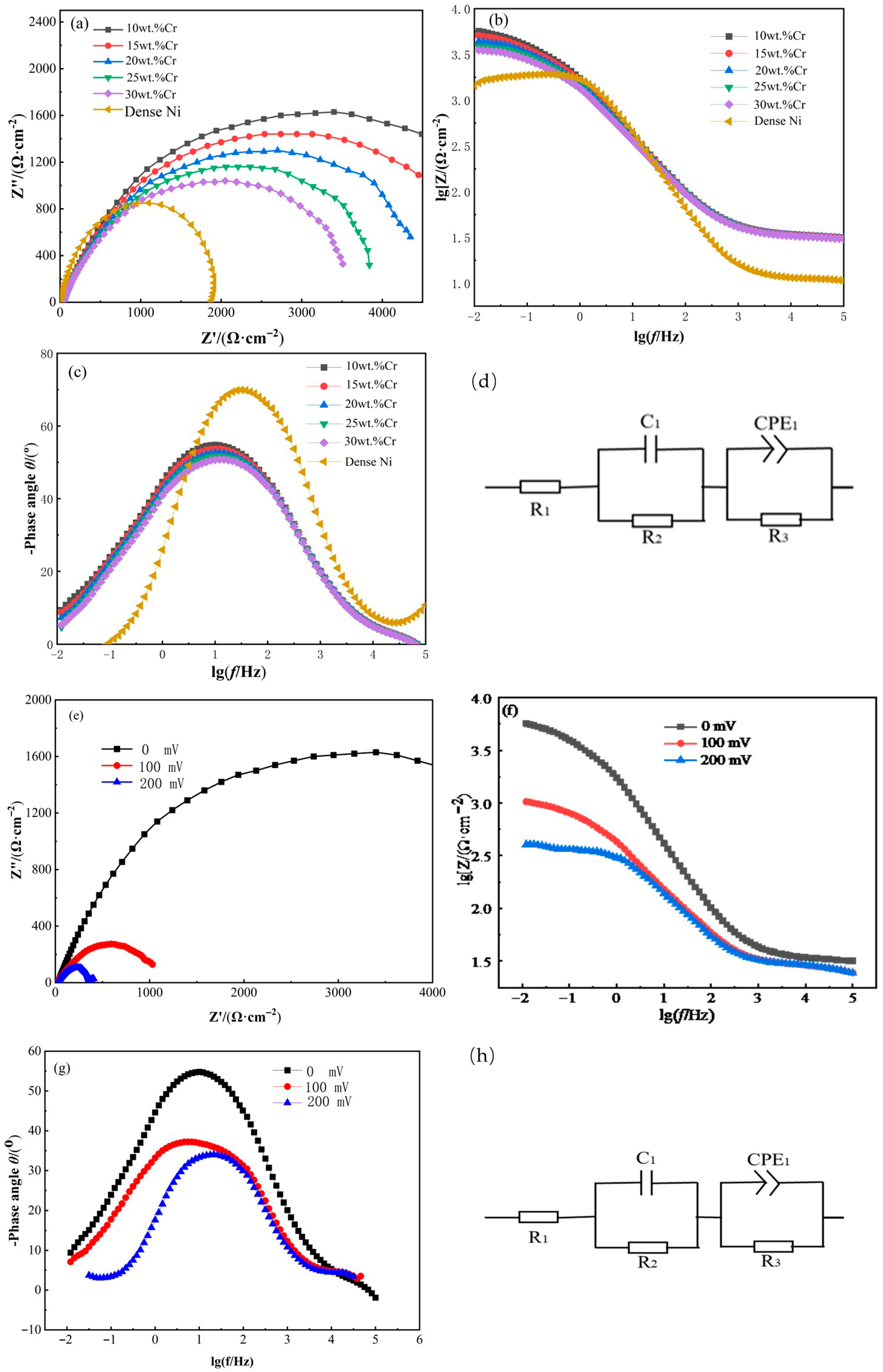
3.4.2. Electrochemical Impedance Spectroscopy (EIS)
3.4.3. Polarization Test
4. Conclusions
- (1)
- The corrosion products obtained after the corrosion of Cr-Cu-Mo-Ni porous alloys in a 0.5 mol/L hydrofluoric acid solution adhere to the inner surface of the pores, and the corrosion resistance is improved through the combined action of chromium and other elements.
- (2)
- Cr-Cu-Mo-Ni porous alloys with different Cr contents all exhibit good corrosion resistance in a 0.5 mol/L hydrofluoric acid solution.
- (3)
- Compared with dense Ni alloys and Cu alloys, the Cr-Cu-Mo-Ni alloys with a porous structure have a slower corrosion rate. With the increase in Cr content, the weight loss rate of the porous alloy first decreases and then increases. Among them, the corrosion resistance of the Cr-Cu-Mo-Ni porous alloy containing 20wt%Cr is significantly improved compared with those containing 10wt%Cr and 30wt%Cr.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vida-Simiti, I.; Jumate, N.; Thalmaier, G.; Sechel, N.; Moldovan, V. Study of gradual porous metallic membranes obtained by powder sedimentation. J. Porous Mater. 2012, 19, 21–27. [Google Scholar] [CrossRef]
- Nabovati, A.; Llewellin, E.W.; Sousa, A.C.M. A general model for the permeability of fibrous porous media based on fluid flow simulations using the lattice Boltzmann method. Compos. Part A Appl. Sci. Manuf. 2009, 40, 860–869. [Google Scholar] [CrossRef]
- Myasnikov, V.M.; Sazhin, S.G.; Kostikov, E.S. The distribution of test-gas leaks in the medium of a material with an open-pore structure. Russ. J. Nondestruct. Test. 2012, 48, 291–295. [Google Scholar] [CrossRef]
- Meng, Y.; Xu, K.; Li, S.; Cao, H.; Chen, C.; Wei, Y.; Wu, S.; Tian, J. Slippery liquid-infused porous surfaces with long-term durable antifouling and anti-corrosion performance via bimodal structure design. Surf. Coat. Technol. 2025, 498, 131850. [Google Scholar] [CrossRef]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef]
- Campoli, G.; Borleffs, M.S.; Amin Yavari, S.; Wauthle, R.; Weinans, H.; Zadpoor, A.A. Mechanical properties of open-cell metallic biomaterials manufactured using additive manufacturing. Mater. Des. 2013, 49, 957–965. [Google Scholar] [CrossRef]
- Gorgin Karaji, Z.; Hedayati, R.; Pouran, B.; Apachitei, I.; Zadpoor, A.A. Effects of plasma electrolytic oxidation process on the mechanical properties of additively manufactured porous biomaterials. Mater. Sci. Eng. C 2017, 76, 406–416. [Google Scholar] [CrossRef]
- Mousavi, A.; Zhao, B.; Wang, T.; Wang, P.; Pan, S. Electrochemistry-informed dealloying for strong and crack-free porous Cu with Sc-induced intermetallic phase modification. Mater. Today Commun. 2025, 43, 111726. [Google Scholar] [CrossRef]
- Yu, L.; Jiang, Y.; He, Y.; Liu, C.T. The corrosion behavior of sintering micro-porous Ni–Cu alloy in hydrofluoric acid solution. J. Alloys Compd. 2015, 638, 7–13. [Google Scholar] [CrossRef]
- Amin, M.I.; Ali, M.M.; Kamal, H.M.; Youssef, A.M.; Akl, M.A. Recovery of high grade phosphoric acid from wet process acid by solvent extraction with aliphatic alcohols. Hydrometallurgy 2010, 105, 115–119. [Google Scholar] [CrossRef]
- Rebak, R.B.; Dillman, J.R.; Crook, P.; Shawber, C.V.V. Corrosion behavior of nickel alloys in wet hydrofluoric acid. Mater. Corros. 2001, 52, 289–297. [Google Scholar] [CrossRef]
- Yang, R.; Shu, J.; Chen, K.; Wang, K. Valence electron structures and properties of Ni-based corrosion resistant alloy. Trans. Nonferrous Met. Soc. China Engl. Ed. 2007, 17, 84–87. [Google Scholar]
- Li Hua, P.; Yang, R.C. The Dependence of the Corrosion Resistance of Ni-Cr-Mo-Cu Alloy to HCL Solution on the 4Cr/ (2Mo+Cu). Adv. Mater. Res. 2011, 194–196, 1920–1923. [Google Scholar] [CrossRef]
- Yang, J.; Zou, H.; Li, X.; Chen, J.; Lv, L.; Wen, Y.; Fan, Y.; Xiong, L.; Liu, Y. Effects of Cr content on the corrosion behavior of porous Ni–Cr–Mo–Cu alloys in H3PO4 solution. Mater. Res. Express 2021, 8, 096522. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Lv, L.; Liu, X.; Zou, H.; Zhang, C.; Xiong, L.; Liu, Y. Porous Ni-Cr-Mo-Cu alloys fabricated by elemental powder reactive synthesis. Mater. Res. Express 2021, 8, 096527. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Hu, Y.; Zou, H.; Zhang, C.; Liu, Y.; Xiong, L.; Ye, J. Effects of Cr content on the corrosion behaviour of porous Ni–Cr–Mo–Cu alloys. Corros. Eng. Sci. Technol. 2021, 56, 594–603. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Feng, X.; Hu, Y.; Zou, H.; Zhang, C.; Xiong, L.; Zheng, X.; Liu, Y. Electrochemical performance of porous Ni–Cr–Mo–Cu alloys for hydrogen evolution reactions in alkali solution. Mater. Res. Express 2020, 7, 095505. [Google Scholar] [CrossRef]
- Raza, A.; Abdulahad, S.; Kang, B.; Ryu, H.J.; Hong, S.H. Corrosion resistance of weight reduced AlxCrFeMoV high entropy alloys. Appl. Surf. Sci. 2019, 485, 368–374. [Google Scholar] [CrossRef]
- Chen, T.-F.; Tiwari, G.P.; Iijima, Y.; Yamauchi, K. Volume and Grain Boundary Diffusion of Chromium in Ni-Base Ni-Cr-Fe Alloys. Mater. Trans. 2003, 44, 40–46. [Google Scholar] [CrossRef]
- Neumann, G.; Tuijn, C. Self-Diffusion and Impurity Diffusion in Pure Metals: Handbook of Experimental Data; Elsevier: Amsterdam, The Netherlands, 2011; Volume 14. [Google Scholar]
- Cross, A.; Roslyakov, S.; Manukyan, K.V.; Rouvimov, S.; Rogachev, A.S.; Kovalev, D.; Wolf, E.E.; Mukasyan, A.S. In Situ Preparation of Highly Stable Ni-Based Supported Catalysts by Solution Combustion Synthesis. J. Phys. Chem. C 2014, 118, 26191–26198. [Google Scholar] [CrossRef]
- Rodriguez, A.A.; Tylczak, J.H.; Gao, M.C.; Jablonski, P.D.; Detrois, M.; Ziomek-Moroz, M.; Hawk, J.A. Effect of Molybdenum on the Corrosion Behavior of High-Entropy Alloys CoCrFeNi2 and CoCrFeNi2Mo0.25 under Sodium Chloride Aqueous Conditions. Adv. Mater. Sci. Eng. 2018, 2018, 3016304. [Google Scholar] [CrossRef]
- Chou, Y.L.; Yeh, J.W.; Shih, H.C. The effect of molybdenum on the corrosion behaviour of the high-entropy alloys Co1.5CrFeNi1.5Ti0.5Mox in aqueous environments. Corros. Sci. 2010, 52, 2571–2581. [Google Scholar] [CrossRef]
- Wu, L.; He, Y.-h.; Jiang, Y.; Zeng, Y.; Xiao, Y.-f.; Nan, B. Effect of pore structures on corrosion resistance of porous Ni3Al intermetallics. Trans. Nonferrous Met. Soc. China 2014, 24, 3509–3516. [Google Scholar] [CrossRef]
- Rammelt, U.; Koehler, S.; Reinhard, G. Synergistic effect of benzoate and benzotriazole on passivation of mild steel. Corros. Sci. 2008, 50, 1659–1663. [Google Scholar] [CrossRef]
- Martinez, S.; Metikoš-Huković, M.; Valek, L. Electrocatalytic properties of electrodeposited Ni–15Mo cathodes for the HER in acid solutions: Synergistic electronic effect. J. Mol. Catal. A Chem. 2006, 245, 114–121. [Google Scholar] [CrossRef]
- Antozzi, A.L.; Bargioni, C.; Iacopetti, L.; Musiani, M.; Vázquez-Gómez, L. EIS study of the service life of activated cathodes for the hydrogen evolution reaction in the chlor–alkali membrane cell process. Electrochim. Acta 2008, 53, 7410–7416. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Gao, Y.; Xue, Q.; Hu, L.; Xu, T. Grain size effect in corrosion behavior of electrodeposited nanocrystalline Ni coatings in alkaline solution. Scr. Mater. 2006, 55, 657–660. [Google Scholar] [CrossRef]
- Boudellioua, H.; Hamlaoui, Y.; Tifouti, L.; Pedraza, F. Effect of the temperature of cerium nitrate–NaCl solution on corrosion inhibition of mild steel. Mater. Corros. 2020, 71, 1300–1309. [Google Scholar] [CrossRef]
- Abdallah, M. Antibacterial drugs as corrosion inhibitors for corrosion of aluminium in hydrochloric solution. Corros. Sci. 2004, 46, 1981–1996. [Google Scholar] [CrossRef]
- Muniandy, M.T.; Rahim, A.A.; Osman, H.; Shah, A.M.; Yahya, S.; Raja, P.B. INVESTIGATION OF SOME SCHIFF BASES AS CORROSION INHIBITORS FOR ALUMINIUM ALLOY IN 0.5 M HYDROCHLORIC ACID SOLUTIONS. Surf. Rev. Lett. 2011, 18, 127–133. [Google Scholar] [CrossRef]



| Samples | Average Aperture (μm) | Permeability (%) |
|---|---|---|
| 10wt%Cr5wt%Cu10wt%Mo75wt%Ni | 3.12 | 7.2 |
| 15wt%Cr5wt%Cu10wt%Mo70wt%Ni | 2.03 | 6.6 |
| 20wt%Cr5wt%Cu10wt%Mo65wt%Ni | 0.68 | 5.2 |
| 25wt%Cr5wt%Cu10wt%Mo60wt%Ni | 0.85 | 5.8 |
| 30wt%Cr5wt%Cu10wt%Mo55wt%Ni | 1.63 | 6.4 |
| Porous Cr-Cu-Mo-Ni | Cr Content (wt%) | ||||
|---|---|---|---|---|---|
| 10 | 15 | 20 | 25 | 30 | |
| R1 (Ω) | 30.63 | 30.95 | 31.99 | 31.59 | 31.73 |
| C1 (mF) | 2.52 × 10−5 | 2.70 × 10−5 | 1.58 × 10−5 | 1.64 × 10−5 | 0.000219 |
| R2 (Ω) | 2.90 × 10−5 | 1.86 × 10−5 | 1.30 × 10−5 | 5.53 × 10−5 | 0.132 |
| CPE1-T | 0.000178 | 0.000169 | 0.000145 | 0.000154 | 0.000150 |
| CPE1-P | 0.66502 | 0.66959 | 0.68314 | 0.67698 | 0.68571 |
| R3 (Ω) | 3684 | 4068 | 5757 | 5172 | 4093 |
| Porous Cr-Cu-Mo-Ni Alloys | External Voltages (mV) | ||
|---|---|---|---|
| 0 | 100 | 200 | |
| R1 (Ω) | 31.99 | 21.03 | 26.71 |
| C1 (mF) | 1.58 × 10−5 | 2.37 × 10−7 | 5.70 × 10−5 |
| R2 (Ω) | 1.30 × 10−5 | 5.272 | 0.4834 |
| CPE1-T | 0.000145 | 0.00065016 | 0.00046128 |
| CPE1-P | 0.68314 | 0.5705 | 0.63847 |
| R3 (Ω) | 5757 | 1108 | 258.8 |
| Porous Cr-Cu-Mo-Ni Alloys | φcorr/V | Jcorr/(A·cm−2) | Rp/Ω | Ea/(kJ/mol) |
|---|---|---|---|---|
| 10wt%Cr5wt%Cu10wt%Mo75wt%Ni | −0.232 | 4.113 × 10−5 | 833 | 14.55 |
| 20wt%Cr5wt%Cu10wt%Mo65wt%Ni | −0.112 | 5.066 × 10−5 | 8241 | 23.74 |
| 30wt%Cr5wt%Cu10wt%Mo55wt%Ni | −0.21 | 3.131 × 10−5 | 1198 | 21.43 |
| Temperature/K | φcorr/V | Jcorr/(A·cm−2) | Rp/Ω | Passivation Range/V |
|---|---|---|---|---|
| 303 | −0.123 | 6.339 × 10−6 | 6021 | 0.10 |
| 323 | −0.137 | 6.673 × 10−6 | 4430 | 0.06 |
| 343 | −0.198 | 1.376 × 10−5 | 2718 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Feng, Y.; Li, X.; Yang, J.; Jiang, W. Corrosion Performance in 0.5 mol/L HF Solution of Cr-Cu-Mo-Ni Porous Alloys with Varying Cr Contents. Materials 2025, 18, 4012. https://doi.org/10.3390/ma18174012
Wang J, Feng Y, Li X, Yang J, Jiang W. Corrosion Performance in 0.5 mol/L HF Solution of Cr-Cu-Mo-Ni Porous Alloys with Varying Cr Contents. Materials. 2025; 18(17):4012. https://doi.org/10.3390/ma18174012
Chicago/Turabian StyleWang, Jiefeng, Yulong Feng, Xide Li, Junsheng Yang, and Wenkai Jiang. 2025. "Corrosion Performance in 0.5 mol/L HF Solution of Cr-Cu-Mo-Ni Porous Alloys with Varying Cr Contents" Materials 18, no. 17: 4012. https://doi.org/10.3390/ma18174012
APA StyleWang, J., Feng, Y., Li, X., Yang, J., & Jiang, W. (2025). Corrosion Performance in 0.5 mol/L HF Solution of Cr-Cu-Mo-Ni Porous Alloys with Varying Cr Contents. Materials, 18(17), 4012. https://doi.org/10.3390/ma18174012








