1. Introduction
In recent years, the rapid expansion of industrial activities has significantly increased global energy demand and the depletion of non-renewable natural resources. This trend has raised critical environmental concerns, particularly regarding the exponential growth of pollution and waste associated with the extraction, processing, and production of materials. In response, governments and industries are under mounting pressure to adopt more sustainable practices. Transitioning toward a sustainable economic and societal model requires the implementation of comprehensive strategies aimed at minimizing environmental impact and energy consumption, notably through the reduction of CO
2 emissions and other greenhouse gases (GHGs). To achieve these goals, it is imperative to promote the recycling, revalorization, and reuse of waste, by-products, and end-of-life materials [
1]. The cement industry is investigating alternative raw materials and fuels to reduce GHGs [
2]. The use of industrial by-products in construction [
3] and the development of new materials can lower CO
2 emissions and enhance building energy efficiency [
4]. Durability remains a key consideration in adopting these alternative binders [
5]. Despite extensive data on Portland cement production [
6], there is a clear need for sustainable binders capable of competing in terms of environmental performance [
7].
Among these, chemically bonded phosphate ceramics (CBPCs), and specifically magnesium phosphate cements (MPC), have garnered increasing attention. MPCs are typically synthesized from magnesium oxide (MgO), which acts as the basic component, and an acidic phosphate source. The preparation of MPC varies depending on the specific raw materials employed. For instance, when ammonium dihydrogen phosphate (ADP) is used, ammonia is released into the environment during the reaction. In contrast, monopotassium phosphate (MKP) is a preferred alternative, as it eliminates the need for retarders and offers additional processing advantages [
8]. Although MKP is generally less economical than other phosphate sources, it is widely adopted due to its beneficial properties, including lower solubility, improved workability, and slower hardening kinetics, which facilitate handling and casting [
9]. However, an excessive amount of MKP can detrimentally affect the mechanical performance of the final product [
10]. The MgO required for magnesium potassium phosphate cement (MKPC) synthesis can be derived from various sources, including thermal decarbonation of magnesite (MgCO
3) or dolomite (CaMg(CO
3)
2), as well as from seawater-derived magnesium chloride (MgCl
2) via electrolysis. To achieve the appropriate level of reactivity for MKPC formation, MgO must undergo thermal treatment, typically at 1500 °C for 5 h, to produce dead-burned MgO [
8]. In this study, a sustainable MKPC (sust-MKPC) was developed using a low-grade MgO (LG-MgO) obtained as a by-product from the air pollution control system of an industrial magnesite calcination process. This LG-MgO is a dead-burned material containing approximately 50–60 wt.% MgO, along with inert phases that did not fully decompose during processing, such as residual MgCO
3, CaMg(CO
3)
2, and CaCO
3 [
11].
The exothermic acid–base reaction between phosphate sources and MgO in aqueous media proceeds slowly enough to allow phosphate gel formation, yielding K-struvite (KMgPO
4·6H
2O) as the primary crystalline product [
12]. MKPC also contains an amorphous phase that contributes to its distinctive microstructure [
13]. This dual-phase composition distinguishes MKPC from conventional cements and supports its use as a matrix for reinforcing agents. MKPC offers excellent thermal stability, chemical resistance, and long-term durability under aggressive conditions [
14]. Furthermore, MKPC is a non-toxic, inorganic cement. Due to its phosphate-based composition, it also shows high biocompatibility, making it suitable for biomedical and environmental applications [
15].
Given the advantageous properties of MKPC, its application has expanded into diverse fields, including its use as a structural repair material for bridges, roads, and airport pavements [
16,
17]. Moreover, MKPC has shown significant potential in the nuclear industry, particularly for the stabilization and encapsulation of radioactive and hazardous waste, due to its chemical durability and containment capabilities [
18,
19]. Beyond their practical applications, ongoing research into CBPCs and MPCs is also driven by their environmental benefits compared to OPC. For instance, the production of one ton of OPC typically results in approximately 900 kg of CO
2 emissions and requires around 5 GJ of energy, whereas MPC production can reduce CO
2 emissions by up to 40% for the same amount of material [
20]. Nevertheless, a recent study indicates that the production of one ton of MKPC results in 1160 kg of CO
2-eq. However, if the source of MgO is a secondary resource, the emissions are significantly reduced to 430 kg of CO
2-eq. This means that the production-related emissions of MKPC can be reduced by more than 60% when secondary resources are used as the raw material for MgO, and by nearly 50% when compared to the emissions associated with producing 1 ton of OPC [
21]. The use of LG-MgO further enhances the environmental credentials of MKPC. As a by-product of the magnesite calcination process, LG-MgO not only diverts waste from industrial processes but also eliminates the need for high-temperature thermal treatment, thereby reducing energy consumption and associated emissions. Consequently, the development of a sustainable MKPC (sust-MKPC) aligns closely with the European Union’s environmental and sustainability goals by promoting circular resource use and mitigating high energy demands and pollution.
In addition to reducing the carbon footprint of construction materials, this study seeks to enhance the energy efficiency of buildings by minimizing reliance on heating, ventilation, and air conditioning (HVAC) systems to maintain thermal comfort. Specifically, the focus is on developing a sust-MKPC with tailored porosity that can contribute to passive thermal regulation. By optimizing its porous structure, the proposed material aims to reduce the operational energy demand of buildings, thereby lowering both CO2 emissions and total energy consumption.
To develop sust-MKPC with thermal insulation properties from an LG-MgO by-product while meeting environmental sustainability criteria, an air-entraining agent (AEA) is typically used to reduce the thermal conductivity of the sust-MKPC matrix [
22]. A wide range of foaming agents is commonly employed in porous cementitious systems, including surfactants, proteins, synthetic compounds, metal powders, and hydrogen peroxide (H
2O
2) [
14]. Among these, H
2O
2 is widely used due to its effectiveness as a chemical pore-forming agent. It is an unstable compound that decomposes exothermically into oxygen and water, thereby generating gas bubbles that contribute to pore formation within the cement matrix. The decomposition rate of H
2O
2 is strongly influenced by both temperature and pH, increasing under higher values of either parameter [
23]. With respect to pH, H
2O
2 behaves as a weak acid and undergoes ionization according to Equation (1). In alkaline conditions, it further reacts as shown in Equation (2), while under strongly alkaline environments, it can decompose rapidly through the reaction described in Equation (3). These pH-dependent reactions are critical in controlling the gas generation rate, and thus the porosity and microstructure of the final sust-MKPC composite.
As temperature increases, so does the decomposition rate of hydrogen peroxide (H2O2), along with the pH of the surrounding medium. For optimal stability, H2O2 requires a pH below 4.5. However, when the pH exceeds five, its decomposition accelerates significantly. Moreover, the presence of impurities, particularly transition metals such as Fe, Cu, Mn, Ni, and Cr, can strongly catalyze the decomposition reaction. In the context of formulating a high-porosity sust-MKPC for thermal insulation panels, the rapid decomposition of H2O2 is desirable, as it promotes effective pore formation. Notably, the use of LG-MgO as the magnesium source inherently contributes to three key factors that enhance the decomposition kinetics of H2O2: (i) the initial pH of the system is highly alkaline due to the solubility equilibrium of MgO, though it becomes near-neutral upon completion of the MKPC setting reaction; (ii) an exothermic neutralization reaction between MKPC precursors results in a notable increase in temperature; (iii) the LG-MgO by-product contains catalytically active metal ions such as Fe and Mn, which further promote H2O2 decomposition. Collectively, these conditions facilitate the controlled decomposition of H2O2, leading to the formation of a porous structure in the sust-MKPC matrix, an essential feature for improving its thermal insulation performance.
The primary objective of this research is to evaluate the potential of H2O2 as a pore-forming agent in sust-MKPC formulations, aiming to enhance their thermal insulation properties for use in passive conditioning systems. The use of LG-MgO, an industrial by-product, aligns with key environmental sustainability criteria by promoting waste valorization, reducing the extraction of natural MgO resources, and lowering the CO2 emissions associated with cement production. Given that critical material properties such as mechanical strength, permeability, diffusivity, and shrinkage are closely linked to porosity and pore size distribution, various H2O2 dosages were explored to optimize the balance between thermal and mechanical performance. Accordingly, a series of sust-MKPC formulations was developed incorporating H2O2 as an AEA, with the goal of decreasing thermal conductivity while preserving structural integrity.
3. Results and Discussion
3.1. Physico-Chemical Characterization of LG-MgO
The particle size of the powder is a critical parameter, as it can significantly influence the reaction rate of MKPC formation. In general, larger particle sizes are associated with slower reaction kinetics [
25].
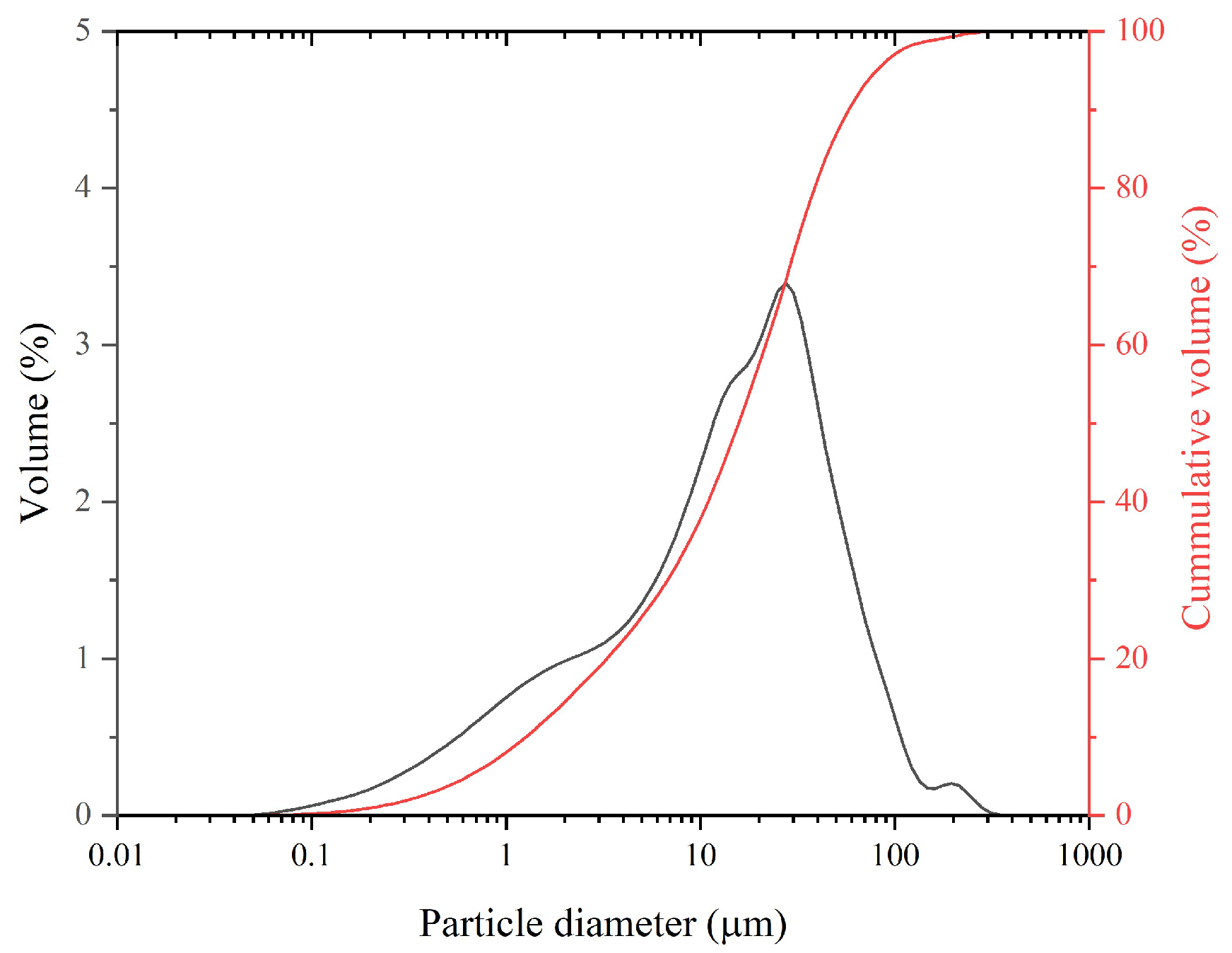
Figure 1 shows the particle size distribution of LG-MgO, which exhibits a mean particle size (d
50) of 25.13 μm.
The chemical composition of LG-MgO, determined by XRF analysis, is presented in
Table 2, which describes the most stable oxides of each element detected in the sample. As expected, Mg was the major element, followed by Ca, S, Si, Fe, and Al oxides with lower percentages. The 10% loss on ignition (LOI) was attributable to the possible carbonates and hydrated phases in LG-MgO.
To complement the XRF analysis, the crystallographic mineral phases were identified by XRD. The LG-MgO diffractogram revealed periclase (MgO) as the predominant crystalline phase, resulting from the decarbonization of natural magnesite during the thermal process. Several minor phases were also identified, including calcite (CaCO3), dolomite (CaMg (CO3)2), quartz (SiO2), magnesite (MgCO3), anhydrite (CaSO4), brucite (Mg(OH)2), and calcium oxide (CaO).
The specific surface area of LG-MgO was evaluated through a BET analyzer, yielding a value of 5.2 m2·g−1. In comparison with other commercial caustic magnesia products (e.g., 70 m2·g−1), this significantly lower value corroborates the reduced reactivity of the LG-MgO by-product.
3.2. FT-IR
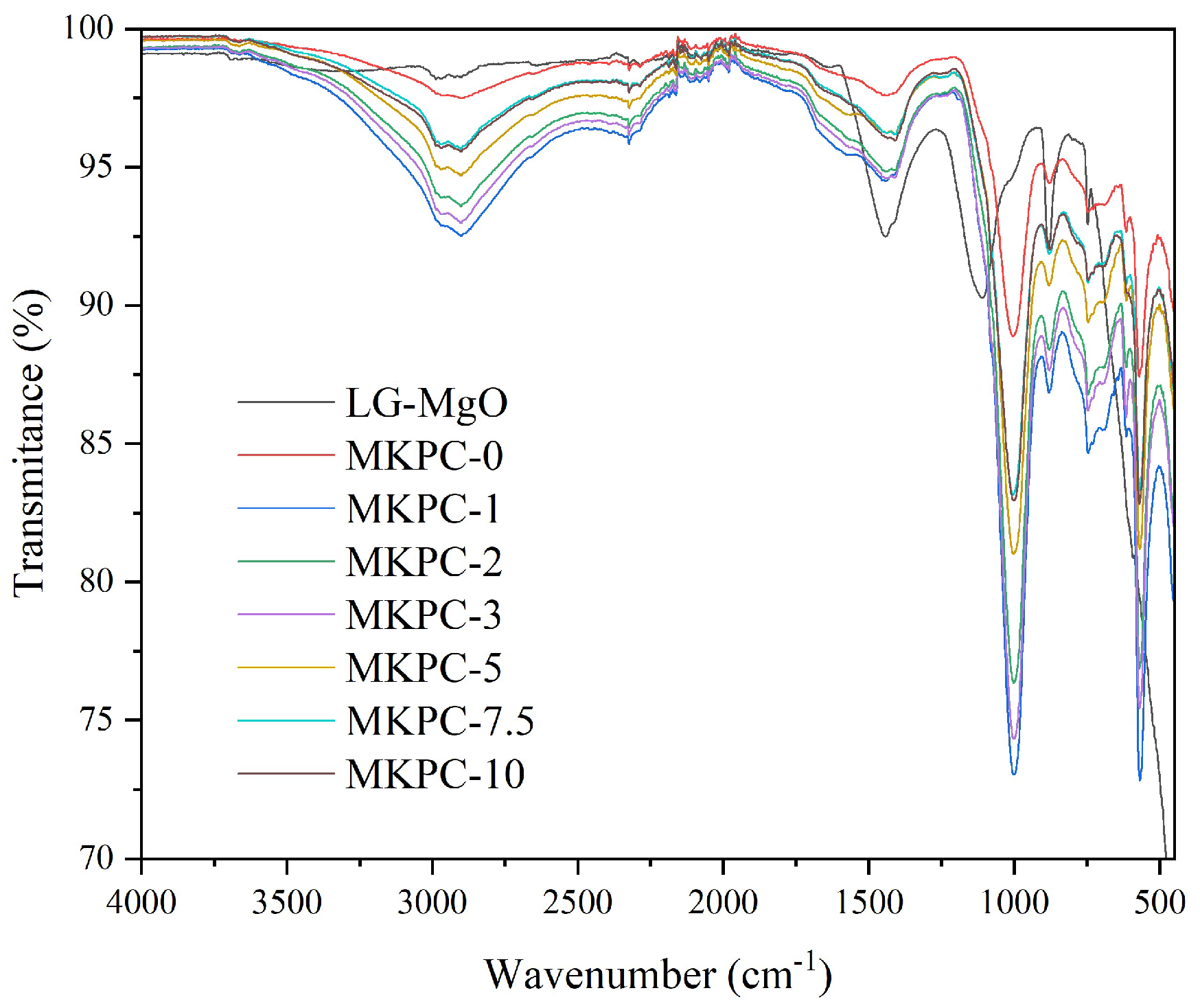
The most relevant peak in the LG-MgO spectrum was observed at 740 cm
−1, corresponding to the Mg-O bond. The compositional differences among the various sust-MKPC formulations were analyzed and are presented in
Figure 2. The spectrum of MKPC-0 primarily exhibited the characteristic bands of K-struvite [
26], the main crystalline mineral phase in MKPC. All formulations showed consistent spectral profiles, with no evidence of compositional changes or the formation of new chemical bonds, indicating that K-struvite remained the principal hydration product across all samples.
3.3. Setting Time and Temperature
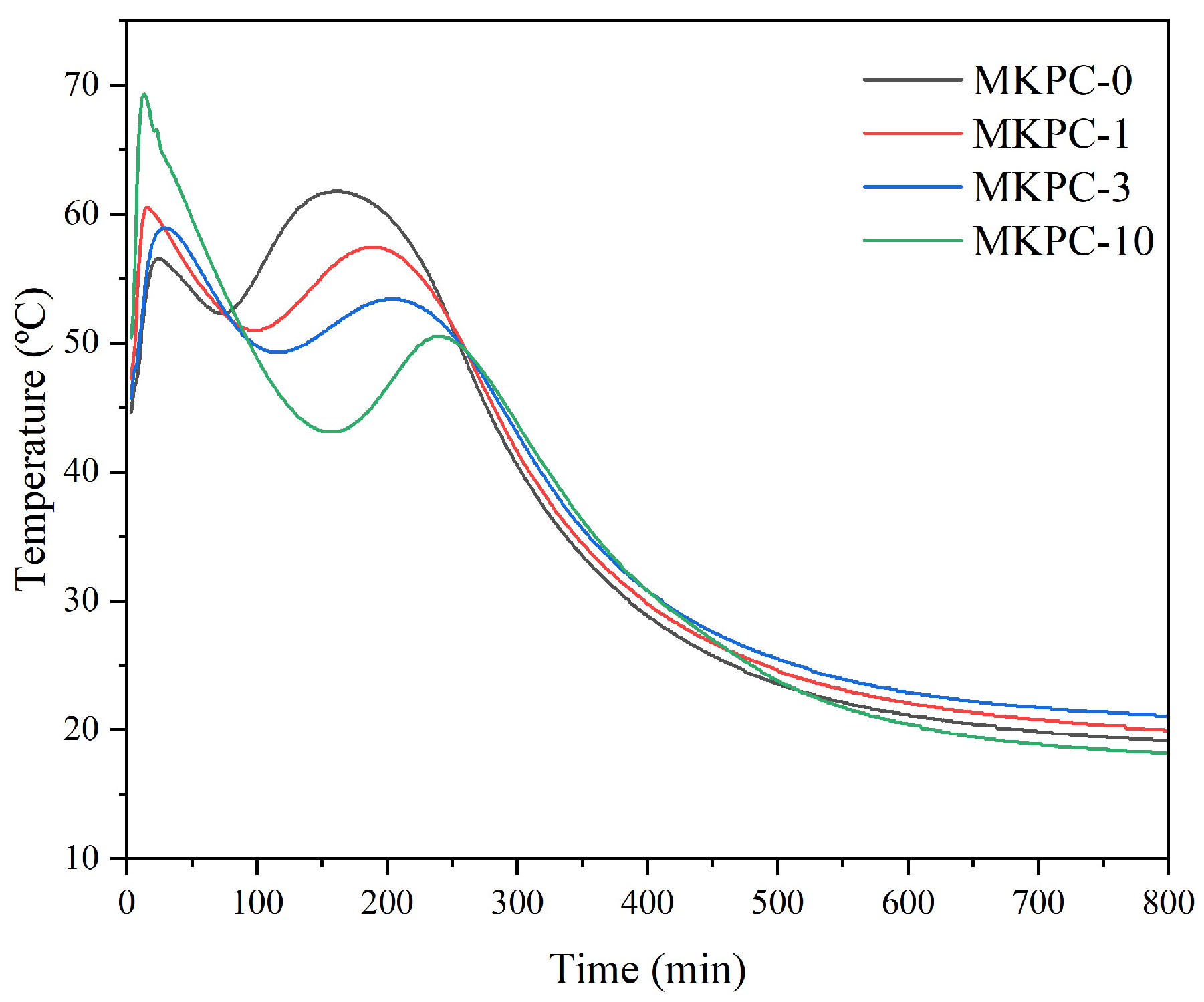
Figure 3 displays the temperature–time profile during the setting of sust-MKPC. The maximum temperature increased with higher H
2O
2 content, as the reaction becomes more exothermic due to the decomposition of H
2O
2 into H
2O and O
2. The first peak can be attributed to the setting time of each sust-MKPC formulation, marking the point at which the fresh paste hardened and lost its plasticity as a result of the chemical reaction between water and the precursors in the mixture. For MKPC-10, the maximum temperature reached 80 °C, while MKPC-0 peaked at approximately 60 °C. After the first sharp exothermic peak, associated with the acid–base reaction (chemical setting reaction), a second broad peak due to cement crystallization is detected. The second peak was delayed with increasing H
2O
2 content, also reducing its maximum temperature, suggesting that the incorporation of H
2O
2 hinders the formation and crystallization of K-struvite.
3.4. Density and Porosity
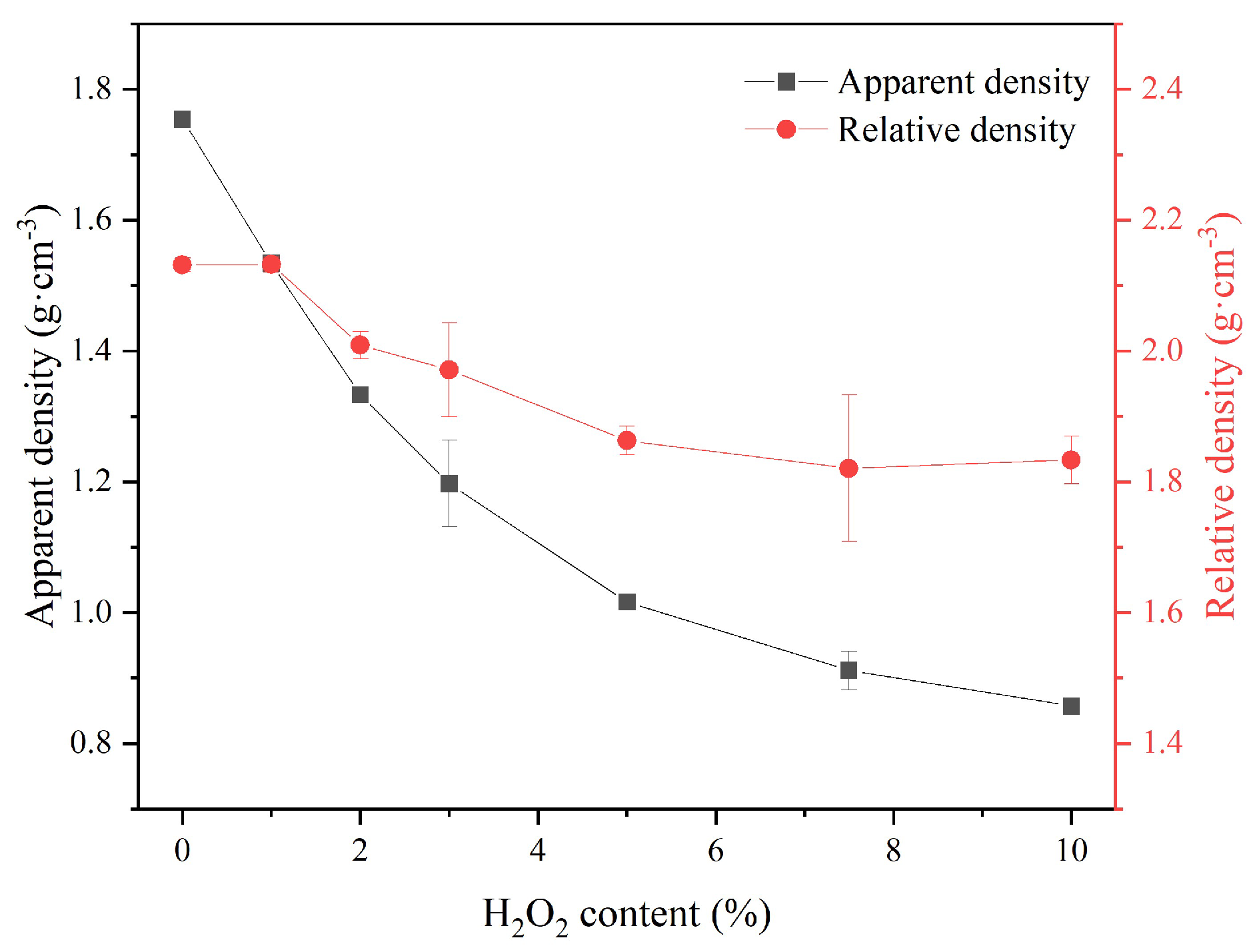
As shown in
Figure 4, both the apparent density (ρ
app) and relative density (ρ
rel) at 28 days significantly decreased with the increasing H
2O
2 content. MKPC-10 exhibited values of ρ
app as low as 0.8 g·cm
−3. The densities of the sust-MKPC formulations were significantly lower than that of OPC (3.15 g·cm
−3), confirming that sust-MKPCs can be considered as lightweight materials.
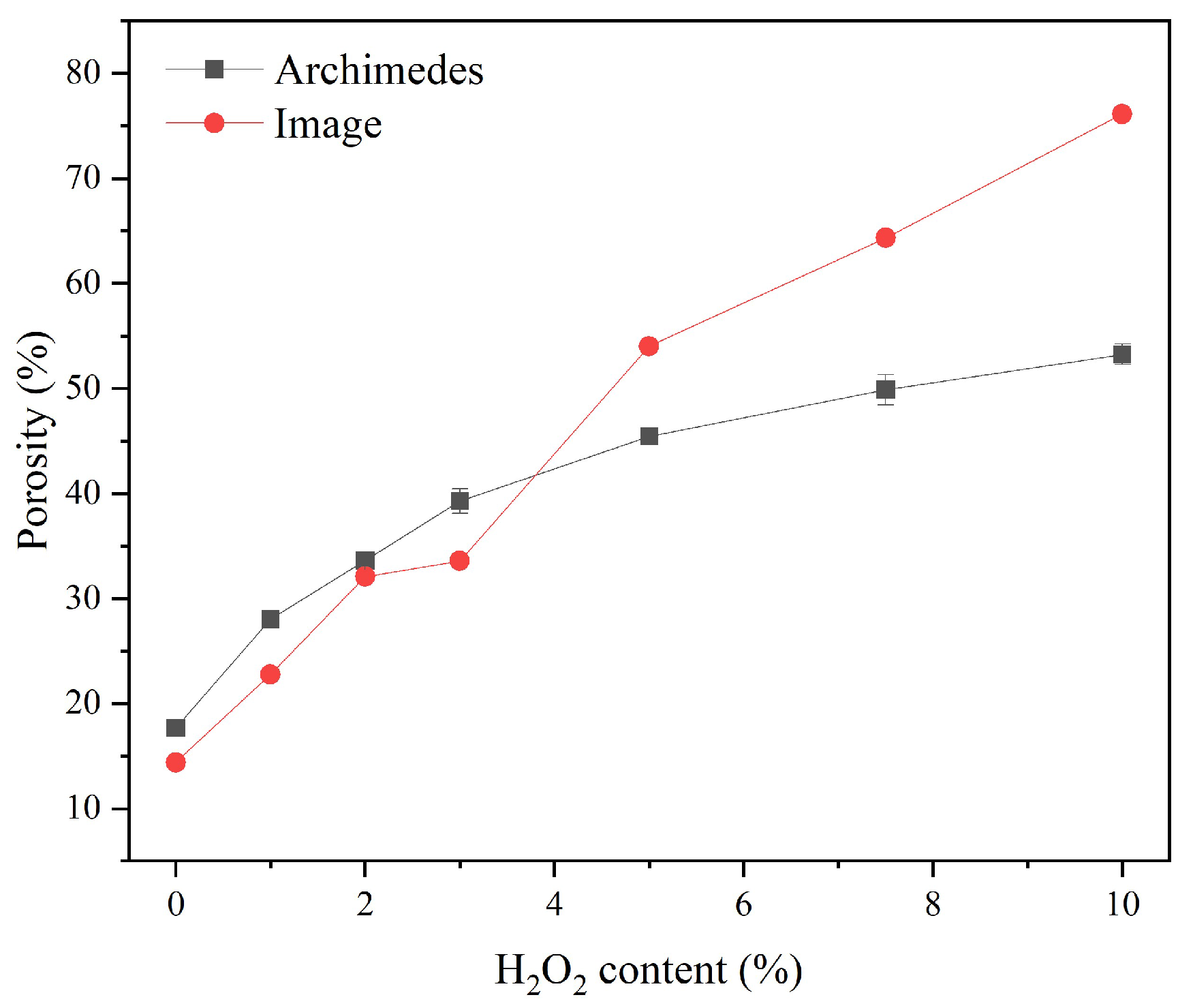
Figure 5 displays the porosity of the sust-MKPC samples, as determined by the Archimedes principle and image analysis techniques. Porosity increased with the increasing AEA content. This trend can be attributed to the decomposition of H
2O
2, which acts as an air-entraining agent and promotes pore formation within the sust-MKPC formulations. Moreover, as seen in
Figure 3, the maximum temperature during cement hydration was significantly higher with H
2O
2 incorporation, promoting H
2O evaporation. It is significant to point out that the results were exceptionally high for MKPC-10, as the sample reached over 50% of porosity through Archimedes’ measurements and almost 80% through image analysis. Therefore, considering the high porosity values obtained, formulations exhibiting porosity levels above 40%, corresponding to H
2O
2 contents greater than 3 wt.%, can be considered suitable for thermal insulation applications. The observed increase in porosity with higher H
2O
2 content also accounts for the significant reduction in the density of the sust-MKPC formulations.
Notably, at low H2O2 contents, the values of porosity measured through both methods were comparable. However, at high percentages, the porosity measured through image analysis was significantly higher. This discrepancy may be attributed to the presence of enclosed pores, which are not filled with water during Archimedes-based measurements but are observed in a cross-section image of the sample.
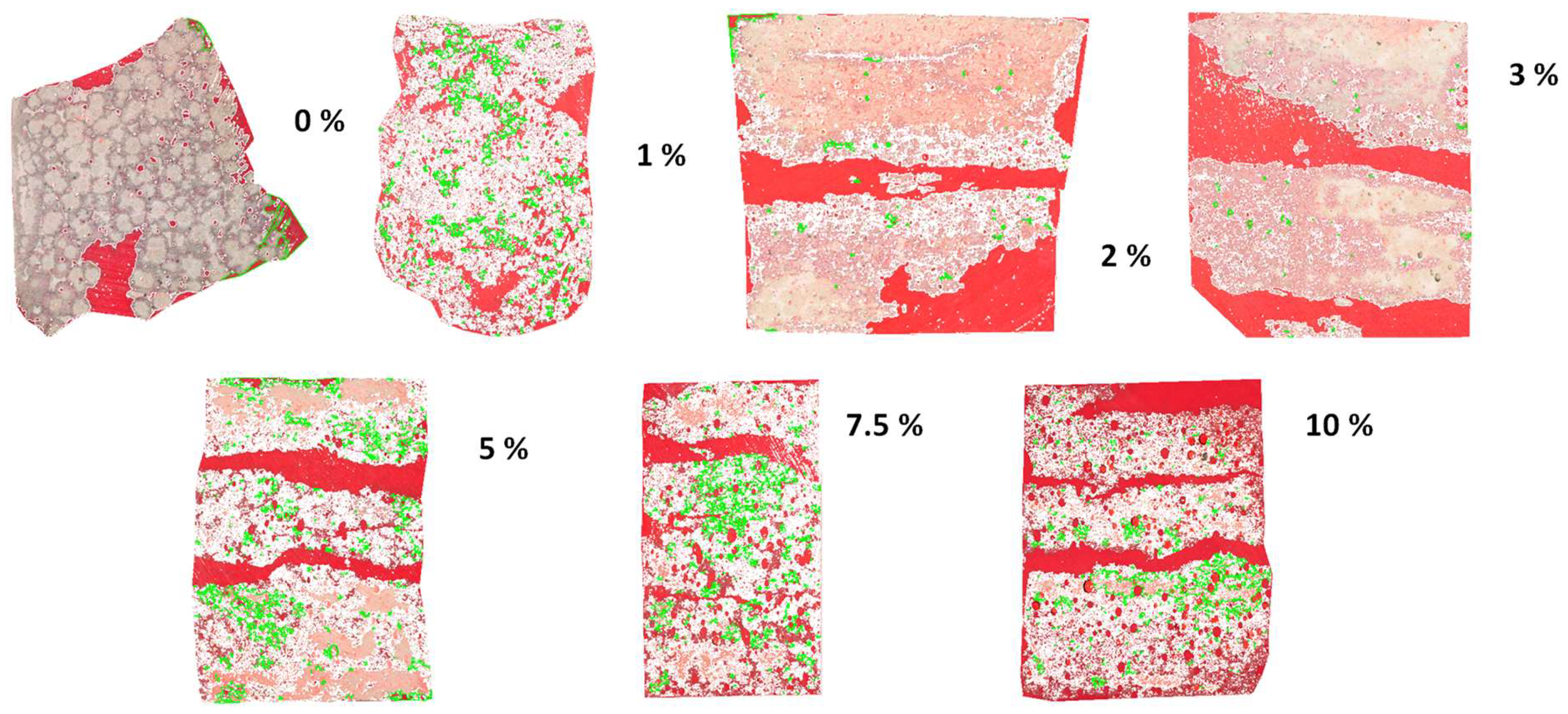
Figure 6 presents 2D cross-section images of sust-MKPC formulations (MKPC-0 to MKPC-10), with a progressively increasing H
2O
2 content analyzed using ImageJ software. The corresponding H
2O
2 content (wt.%) is indicated for each specimen. For the porosity assessment, the samples were precision-sectioned into thin slices and embedded in red resin, which infiltrated and visually highlighted the pore spaces. It should be noted that the absolute thickness of the thin slices and the size of the image are not considered in this analysis; the images are intended solely to provide a rapid visual comparison of porosity trends as a function of AEA content. In the images, the MKPC matrix appears white, the resin-filled pores appear red, and green outlines have been superimposed to delineate the boundaries of pores with poorly defined edges, thereby improving surface definition and measurement reproducibility. In addition to the overall increase in total porosity observed in
Figure 5,
Figure 6 reveals a progressive enlargement of pore size with a higher AEA content, culminating in the formation of large cracks. These cracks are likely the result of coalescence between adjacent large pores. This behavior can be partially attributed to the extended setting time, which reduces the viscosity of the binder phases — primarily due to the formation of K-struvite. Lower viscosity decreases the resistance of the paste to internal gas expansion, enabling the growth of larger pores. Additionally, the simultaneous decrease in surface tension amplifies the influence of gas expansion forces within the matrix, thereby promoting pore enlargement and, in some cases, the merging of pores into microcracks.
3.5. Thermal Properties
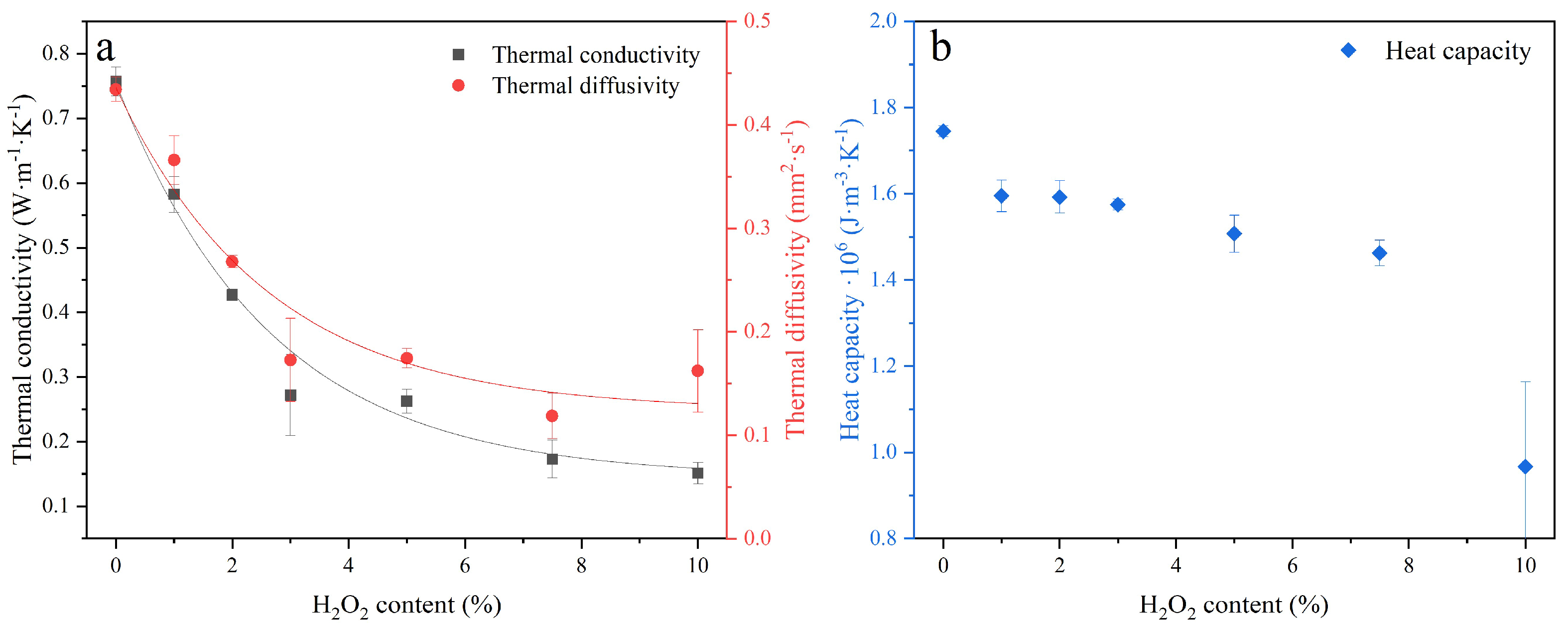
The conductivity, thermal diffusivity, and heat capacity were measured to assess the impact of increased porosity on the insulating properties of sust-MKPC formulations. The thermal conductivity and thermal diffusivity are shown in
Figure 7a, and the volumetric heat capacity is plotted in
Figure 7b. The increase in AEA content contributed to a significant reduction in the thermal conductivity of the sust-MKPC formulations. This is strongly related to the porosity generated by the decomposition of H
2O
2, triggered by the exothermic reaction in sust-MKPC, as well as the evaporation of the mixing water due to the temperature rise during the hydration of binder phases. Accordingly, the increased porosity in these samples resulted in diminished heat transport, which is expected to lead to lower thermal inertia. The developed sust-MKPC cannot be considered insulating materials since their thermal conductivity exceeds 0.09 W·m
−1·K
−1 [
27]. However, the thermal conductivity values are substantially lower than conventional building materials, such as OPC [
28]. The thermal conductivity values obtained are consistent with those reported in previous studies on MPC-based foamed concretes formulated with H
2O
2 as a foaming agent, although using ADP instead of MKP [
29].
Since thermal diffusivity represents the rate at which heat propagates through a material, its reduction with increasing H2O2 content followed a similar exponential trend to that of thermal conductivity. This behavior may be influenced by pore morphology. Specifically, larger pores tend to disrupt heat transfer more significantly than smaller ones, leading to a more pronounced reduction in thermal diffusivity. Moreover, at up to 3 wt.% AEA content, the thermal diffusivity decreases substantially, after which it remains relatively stable.
Regarding the volumetric heat capacity of the different sust-MKPC formulations, it exhibited a generally decreasing trend, with particularly low values observed in the MKPC-10 sample. However, this sample also showed significant variability in the measurements, likely due to its highly porous, microcracked, and irregular structure.
Thermal cycling tests were conducted to evaluate the influence of H
2O
2 content, used as AEA, on the thermal inertia of sust-MKPC formulations.
Figure 8 depicts the ambient, internal, and surface temperature of representative specimens (MKPC-1 and MKPC-10) during a heating–cooling cycle. The addition of the AEA agent improves the thermal inertia of sust-MKPCs, due to its lower thermal conductivity. Therefore, the higher content of H
2O
2 enhanced the gap between the inner and the outer measured temperature, attributed to the higher porosity of the samples. This higher thermal inertia could help maintain a comfortable temperature in buildings.
As observed, the evolution of the internal and surface temperatures in sust-MKPC specimens during the heating–cooling cycle reveals distinct thermal responses associated with their porosity levels. MKPC-10, characterized by a higher content of AEA and consequently greater porosity, exhibits a pronounced temperature gradient between the internal and surface regions at the onset of each thermal transition. In some instances, this gradient exceeds 7 °C. Conversely, MKPC-1, with lower porosity, shows negligible differences between the internal and surface temperatures, indicating a more uniform thermal response. As expected, the temperature gradients observed in MKPC-10 tend to diminish over time as thermal equilibrium is progressively reached within the specimen. This behavior highlights the significant influence of porosity on the thermal inertia of sust-MKPCs, as higher porosity enhances the material’s capacity to delay internal temperature changes and thereby modulates the heat transfer dynamics.
3.6. Mechanical Properties
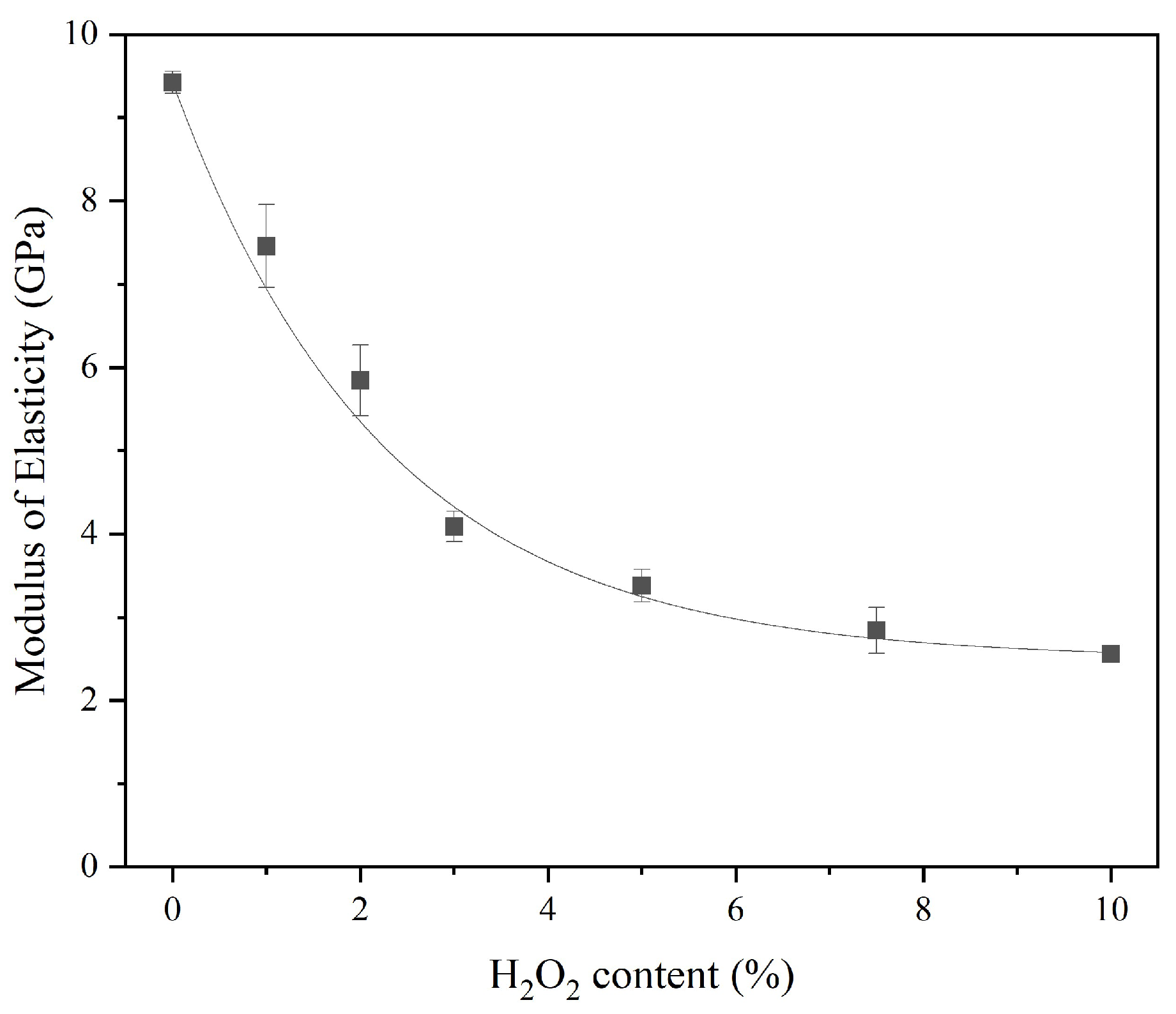
An increase in porosity leads to a reduction in density, which implies less compactness of the sust-MKPC formulations and results in a lower MOE. Moreover, both ultrasound wave propagation through the material and stiffness decrease as porosity increases [
5]. This trend is observed in
Figure 9, where an exponential decrease in the MOE of the samples can be observed with an increasing AEA content.
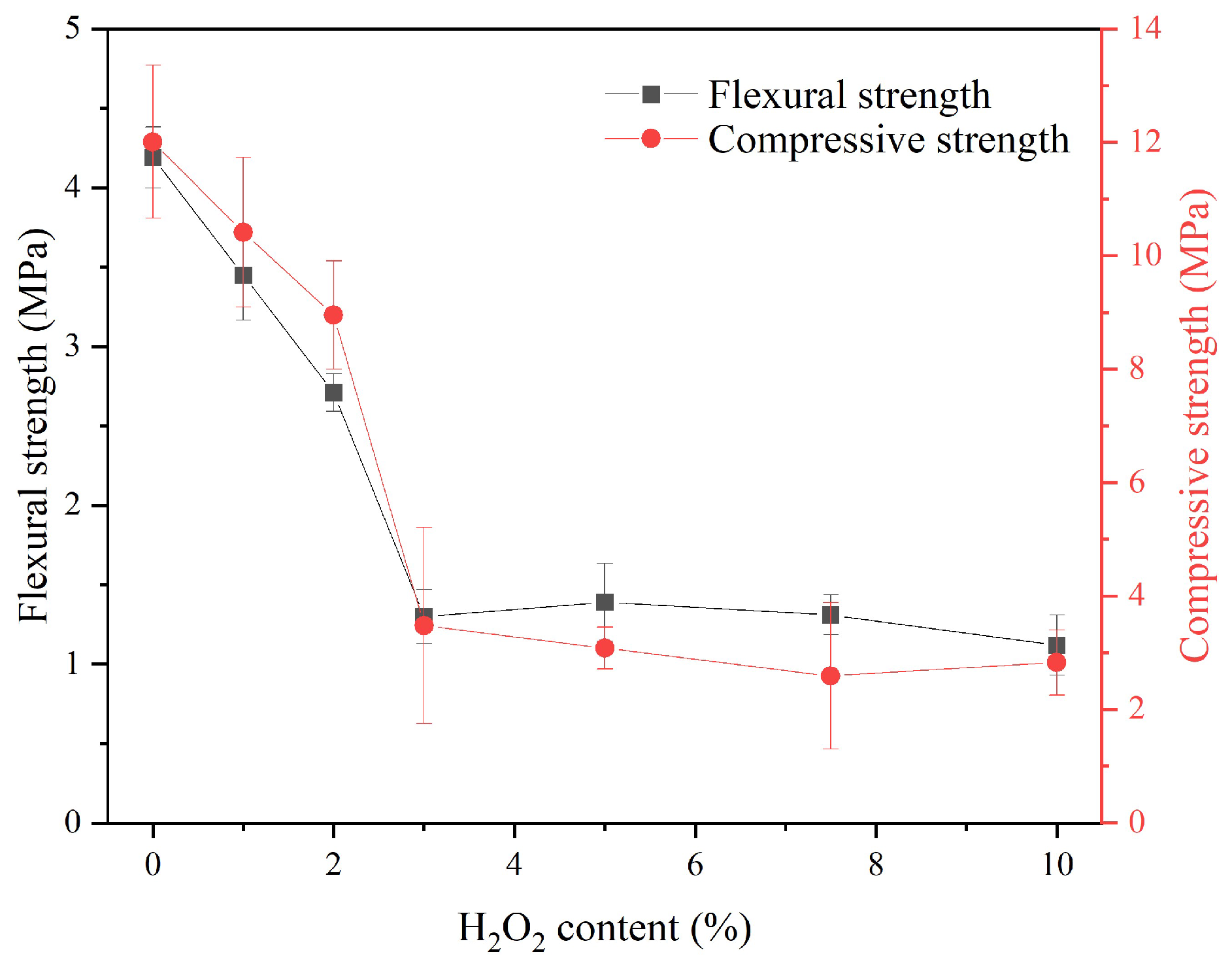
Figure 10 displays the flexural and compressive strength of the sust-MKPC formulations. Similar to the MOE results, the increase in the porosity led to a reduction in the strength of the binder. It is important to emphasize that the samples exhibited cracks caused by the neutralization exothermic reaction between the acid (MKP) and the base (LG-MgO), and significant pore generation (see
Figure 6). This formulation exhibited uncontrolled expansion during the rapid setting time. As a result, the samples displayed a decreasing trend in strength with an increasing H
2O
2 content, reaching a minimum at 3%. Beyond this point, strength remained stable, as the samples had already lost most of their structural integrity and exhibited significant cracking. Overall, as porosity increased, the mechanical properties declined, with a drastic reduction in strength observed in the MKPC-3 formulation. The compressive strength values obtained are consistent with those reported in previous studies on MPC-based foamed concretes formulated with H
2O
2 as a foaming agent, although using ADP instead of MKP [
29].