3.1.1. Analysis of Thermodynamic Calculation Results
Table 7 presents the Gibbs energy change (ΔG) of reactions between [Al] and [Ti] of IF steel and the main composition of the stuffing sand. The calculated ΔG values for Groups A and B indicate that both [Al] and [Ti] in IF steel can be oxidized by Cr
2O
3, SiO
2, and FeO in the Cr
2O
3-based stuffing sand, with these components accounting for 76% of the total content. This means that most components of the stuffing sand can cause the reoxidation of [Al] and [Ti] in IF steel and thus lead to an adverse impact on the steel cleanliness. The absolute values of ΔG in
Table 7 are in the order of (c) > (a) > (b) for Group A and (h) > (e) > (f) for Group B. This indicates that the oxidizability of the Cr
2O
3-based stuffing sand composition is in the order of FeO > Cr
2O
3 > SiO
2. The absolute ΔG value of (j) is larger than that of (k), which indicates the [Al] in IF steel can be oxidized much more easily than [Ti].
In the practical production process, partial oxidation of [Al] and [Ti] inevitably occurs due to oxidizing agents such as atmospheric oxygen and oxidizing components within the slag, which leads to burning loss of [Al] and [Ti]. Massive inclusions of Al
2O
3 and TiO
2 form during this burning loss process and thus largely affect the steel cleanliness. Consequently, the burning loss amount of [Al] and [Ti] from the end of RH to the tundish is usually regarded as an important index indicating the pollution of the steel caused by reoxidation and can be calculated as follows:
where Δ
w[Al] and Δ
w[Ti] represent the burning loss amount of [Al] and [Ti];
w[Al]
RH and
w[Ti]
RH represent the content of [Al] and [Ti] of steel at end of RH; and
w[Al]
Tun and
w[Ti]
Tun represent the content of [Al] and [Ti] of steel in the tundish. Larger values of Δ
w[Al] and Δ
w[Ti] represent a more severe pollution degree caused by reoxidation.
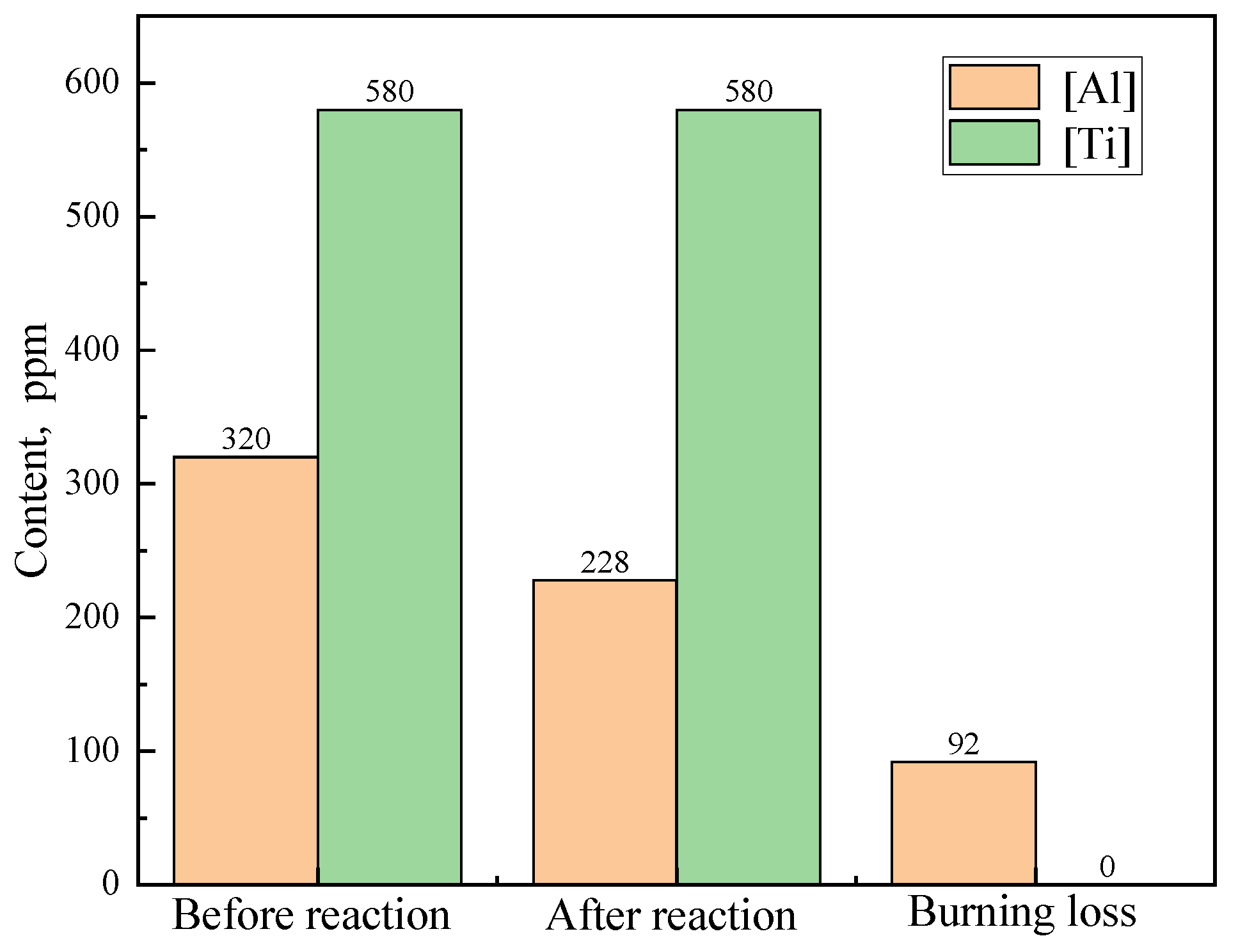
Figure 3 presents the calculated variation in [Al] and [Ti] before and after the reaction of 70 kg of stuffing sand with 220 t of IF steel for one heat (the specific reaction system is shown in
Table 3 and
Table 4) and simultaneously shows the corresponding burning loss amount. The contents before and after the reaction in
Figure 3 correspond to the contents of steel at end of RH and in the tundish in Equations (1) and (2). So, the burning loss in
Figure 3 is equal to that the content before reaction minus the content after the reaction. Due to the oxidability of the stuffing sand, Δ
w[Al] reaches 92 ppm. However, Δ
w[Ti] is 0 ppm. This is because [Al] can be oxidized much easier than [Ti], as mentioned above, and the content of [Al] is still high (228 ppm) after the reaction between stuffing sand and steel. As a result, [Ti] can hardly be oxidized by the stuffing sand.
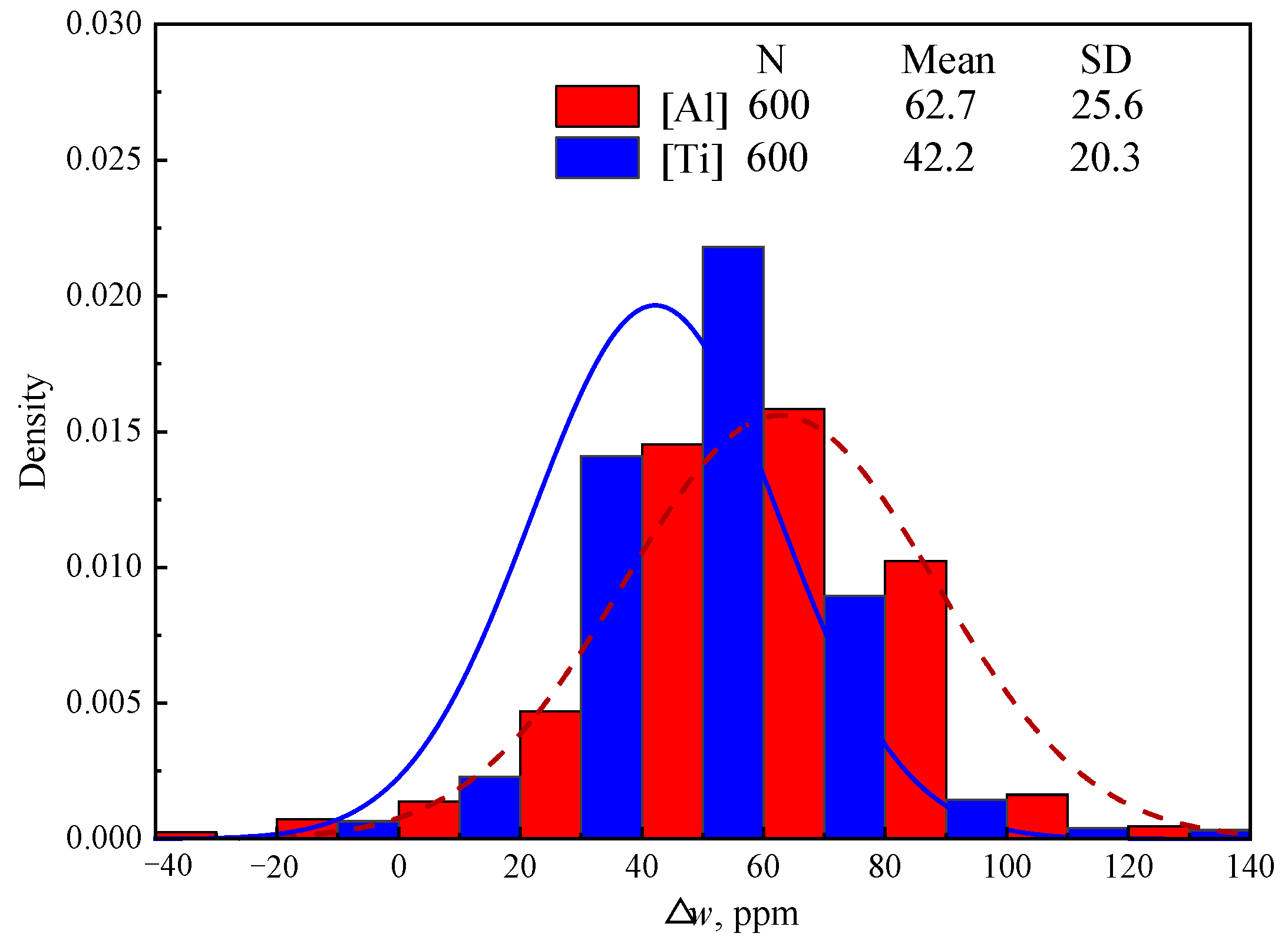
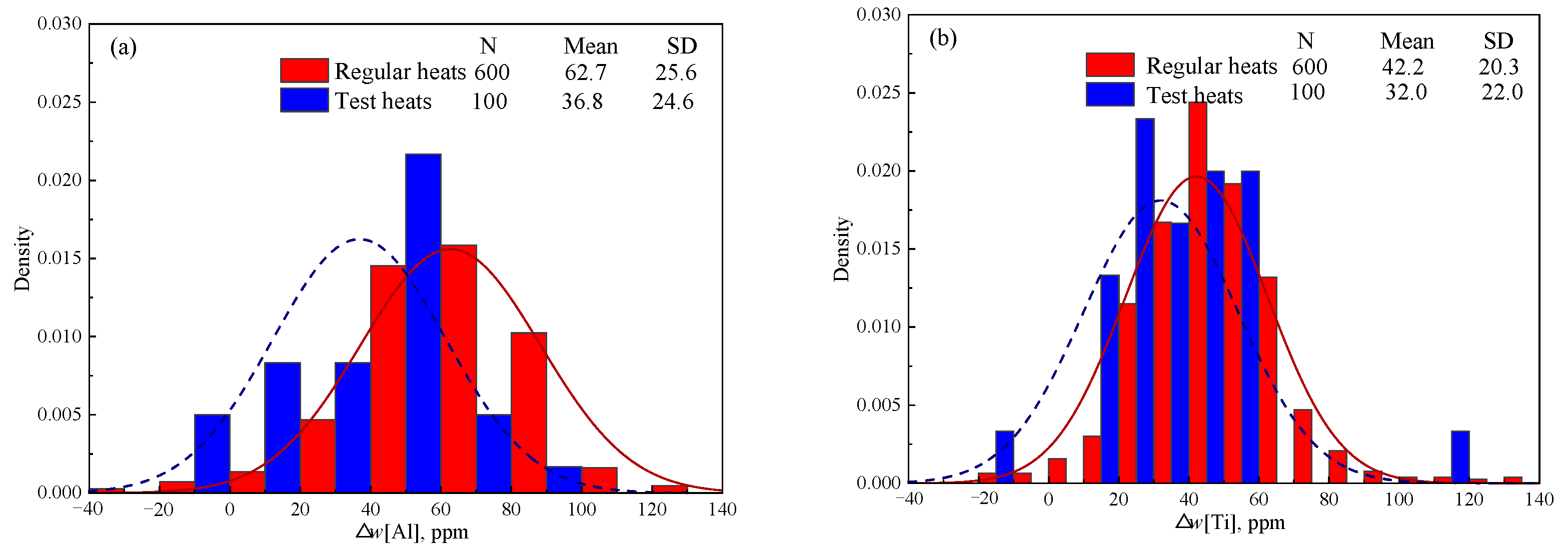
Figure 4 shows the detected Δ
w[Al] and Δ
w[Ti] of 600 heats in industry production, which was calculated based on the detected
w[Al]
RH,
w[Ti]
RH,
w[Al]
Tun, and
w[Ti]
Tun of steel samples collected at the end of RH and in the tundish. “N”, “Mean” and “SD” in
Figure 4 represent the number of samples, mean value of samples, and standard deviation of samples, respectively. Although the content of [Ti] in IF steel is twice than that of [Al] as shown in
Table 1, Δ
w[Ti] (average 42.2 ppm) is significantly lower than Δ
w[Al] (average 62.7 ppm). This proves that [Al] can be oxidized much more easily than [Ti], which is consistent with the calculated results above in Group C in
Table 7.
The calculated Δ
w[Al] (shown in
Figure 1) reaches 92 ppm, which is obviously larger than the detected result of 62.7 ppm in industry production (shown in
Figure 4). This can be attributed to three reasons. Firstly, the calculated results in
Figure 3 are at a thermodynamic equilibrium state, and all the filled stuffing sand reacts with IF steel completely. However, in industry production, some of the filled stuffing sand inevitably floats to ladle slag during BOF tapping, and some others adheres to the inside of the upper nozzle or shroud during steel ladle teeming. Consequently, only partially filled stuffing sand can enter the tundish and react with the liquid steel. Secondly, some of the stuffing sand that enters the tundish can easily float to the tundish slag due to its large particle size (0.15~1 mm), which depresses the reaction between stuffing sand and IF steel. Thirdly, the tundish slag is a CaO-Al
2O
3 type, and the Al
2O
3 content is more than 30%. The high content of Al
2O
3 in the tundish inhibits the reoxidation process of (M
xO
y) + [Al] → [M] + (Al
2O
3) (M
xO
y denotes FeO, Cr
2O
3, SiO
2 of the Cr
2O
3-based stuffing sand) between stuffing sand and [Al] of the IF steel, which is helpful for decreasing Δ
w[Al].
The calculated results in
Figure 3 indicate that the [Ti] in IF steel cannot be oxidized by the Cr
2O
3-based stuffing sand. However, the detected results in industry production in
Figure 4 show that the average practical Δ
w[Ti] reaches 42.2 ppm. This significant difference between the detected and the calculated results can be explained by two reasons. Firstly, although the high content of Al
2O
3 in tundish slag inhibits the reaction of (M
xO
y) + [Al] → [M] + (Al
2O
3), the reaction of (M
xO
y) + [Ti] → [M] + (TiO
2) resulting in Δ
w[Ti] is influenced little due to the small content of TiO
2 (<0.5%) in tundish slag. As a result, a small amount of [Ti] is inevitably oxidized by the stuffing sand along with massive [Al] oxidation. Secondly, [Ti] can be oxidized by oxygen in air and Fe
xO
y in ladle slag and tundish slag during steel transit from the end of RH to the tundish.
As the main component of Cr
2O
3-based stuffing sand, Cr
2O
3 reacts with [Al] as 1/2Cr
2O
3 + [Al] → 1/2(Al
2O
3) + [Cr], which causes an increase in [Cr] content in IF steel from the end of RH to the tundish. Therefore, the increase in the amount of [Cr] (Δ
w[Cr]) from the end of RH to the tundish can validate the reoxidation process of IF steel due to the Cr
2O
3-based stuffing sand.
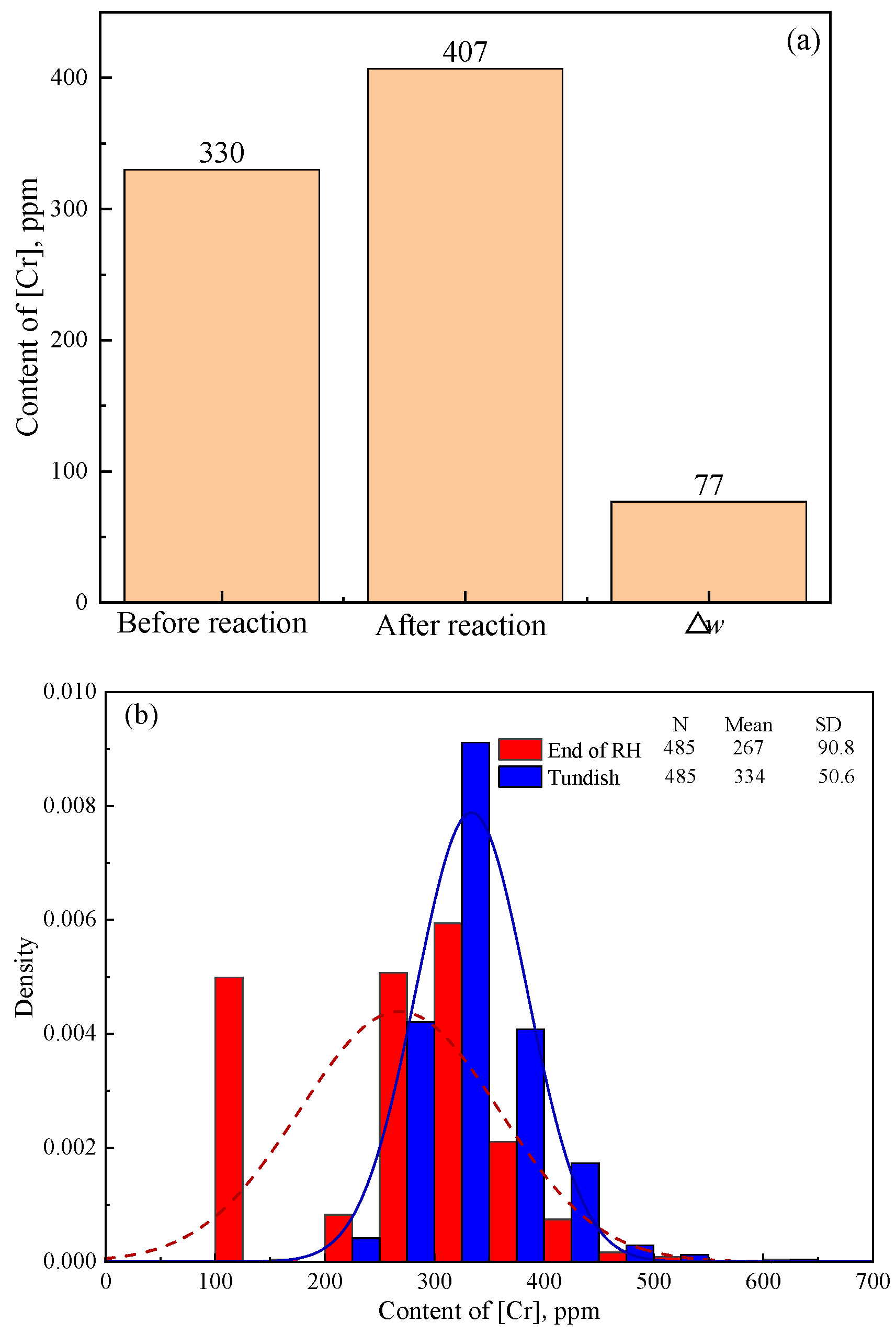
Figure 5a shows the calculated Δ
w[Cr] using FactSage 8.0 based on the reaction system in
Table 3 and
Table 4, and
Figure 5b shows the detected [Cr] content of steel samples taken at the end of RH and in the tundish during industry production. The calculated Δ
w[Cr] in
Figure 5a and the average detected Δ
w[Cr] in practical production in
Figure 5b are 77 ppm (407 − 330 = 77) and 67 ppm (334 − 267 = 67), respectively.
The significant increase in the detected [Cr] content from the end of RH to the tundish in
Figure 5b validates the reoxidation process of IF steel caused by Cr
2O
3-based stuffing sand. The detected Δ
w[Cr] in
Figure 5b is slightly lower than but close to the calculated result in
Figure 5a. The detected average Δ
w[Cr] reaches 87.0% of the calculated one. This indicates that most of the filled stuffing sand reacts with the steel, which results in burning loss of [Al] and [Ti] and thus the pollution of the steel. Meanwhile, the calculated Δ
w[Al] (92 ppm in
Figure 3) is higher than the detected result (62.7 ppm in
Figure 2) in industry production. This indicates that the reoxidation of IF steel is primarily caused by the Cr
2O
3-based stuffing sand, which is detrimental to the steel cleanliness. The adverse effect of Cr
2O
3-based stuffing sand on the steel cleanliness should be even more serious than other factors, like oxygen in air and slag carryover in the last stage of ladle teeming.
3.1.2. Analysis of Melting Test Results
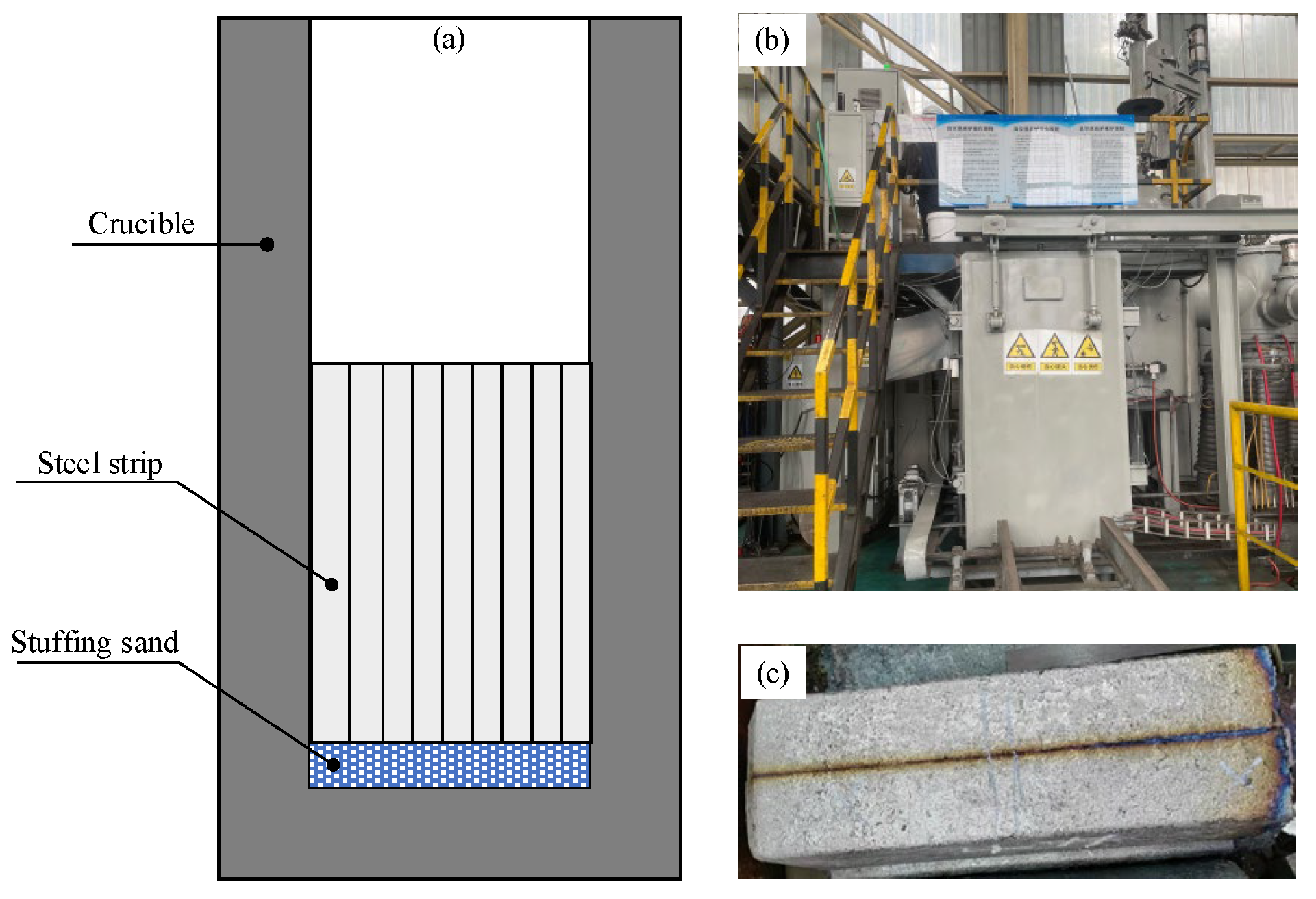
To further validate the influence of the conventional Cr
2O
3-based stuffing sand on the reoxidation of IF steel, a melting test using a vacuum induction furnace was conducted. During the test, 37.2 g of stuffing sand and 117 kg of steel strips were filled in the crucible. The detailed procedure of the melting test has been described in
Section 2.2.1. Additionally, the thermodynamic calculation for the melting test was conducted based on Group A of the calculated reaction system in
Table 5 and
Table 6. The calculation method has been described in
Section 2.3.2.
Table 8 shows the detected and the calculated element contents of the original steel strips (before reaction between stuffing sand and steel) and the casting ingots (after the reaction between stuffing sand and steel). The detected and calculated results in
Table 8 were obtained based on the melting test and thermodynamic calculation, respectively. Based on the results in
Table 8, the burning loss of [Al] and [Ti] (Δ
w[Al] and Δ
w[Ti]) and the increase in the amount of [Cr] and [Si] (Δ
w[Cr] and Δ
w[Si]) can be determined as follows:
where Δ
w[Al]
Det/Cal and Δ
w[Al]
Det/Cal represent the detected/calculated burning loss of [Al] and [Ti]; Δ
w[Cr]
Det/Cal and Δ
w[Si]
Det/Cal represent the detected/calculated increase in the amount of [Cr] and [Si]; [Al]
str, [Ti]
str, [Cr]
str, and [Si]
str represent the element content of the original steel strip; and [Al]
Det/Cal, [Ti]
Det/Cal, [Cr]
Det/Cal, and [Si]
Det/Cal represent the detected/calculated element content of the casting ingot.
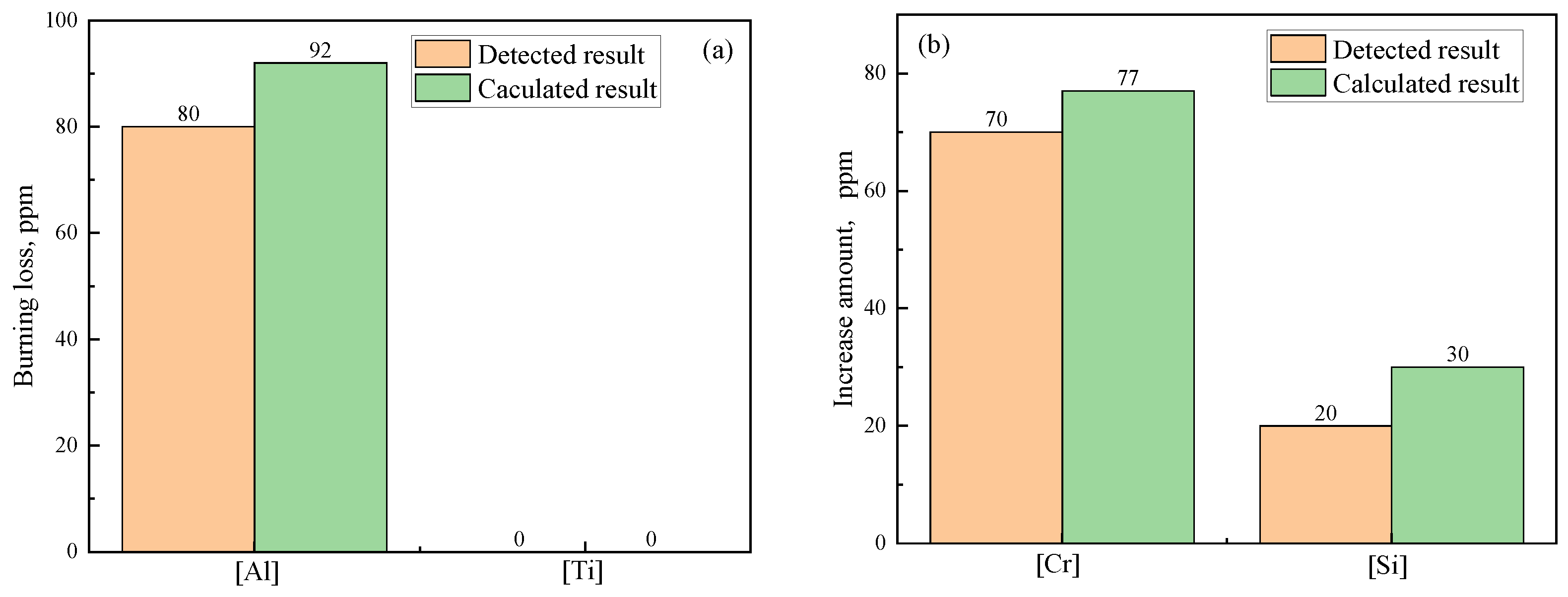
Figure 6 shows the variation in the element content of steel. It can be seen from
Figure 6a that obvious burning loss of [Al] occurs due to the Cr
2O
3-based stuffing sand and that Δ
w[Al]
Det reaches 80 ppm. However, no burning loss of [Ti] occurs (Δ
w[Ti]
Mes = 0). This is because [Al] can be oxidized by the Cr
2O
3-based stuffing sand much more easily than [Ti], as mentioned above in
Section 3.1.1. There is a still high content of [Al] (0.024%) in the casting ingot after the reaction. As a result, reoxidation of [Ti] caused by stuffing sand is very difficult. The [Cr] and [Si] content increase with burning loss of [Al] due to Reactions (a) and (b) in
Table 7, and Δ
w[Cr]
Det and Δ
w[Si]
Det reach 70 ppm and 20 ppm, respectively, as shown in
Figure 6b.
By comparing the calculated burning loss and increase in the amount of [Cr] and [Si] with the corresponding detected results in
Figure 6, it can be found that Δ
w[Al]
Det, Δ
w[Si]
Det, Δ
w[Cr]
Det are 87.0%, 90.9%, 66.7% of the Δ
w[Al]
Cal, Δ
w[Si]
Cal, Δ
w[Cr]
Cal, respectively. Although the detected results are lower than the calculated results, the variation trends of the detected and calculated results are similar. This not only validates the reliability of the calculated tendency but also confirms that Cr
2O
3-based stuffing sand is the main factor resulting in burning loss of [Al] and [Ti] during the continuous casting process of IF steel.