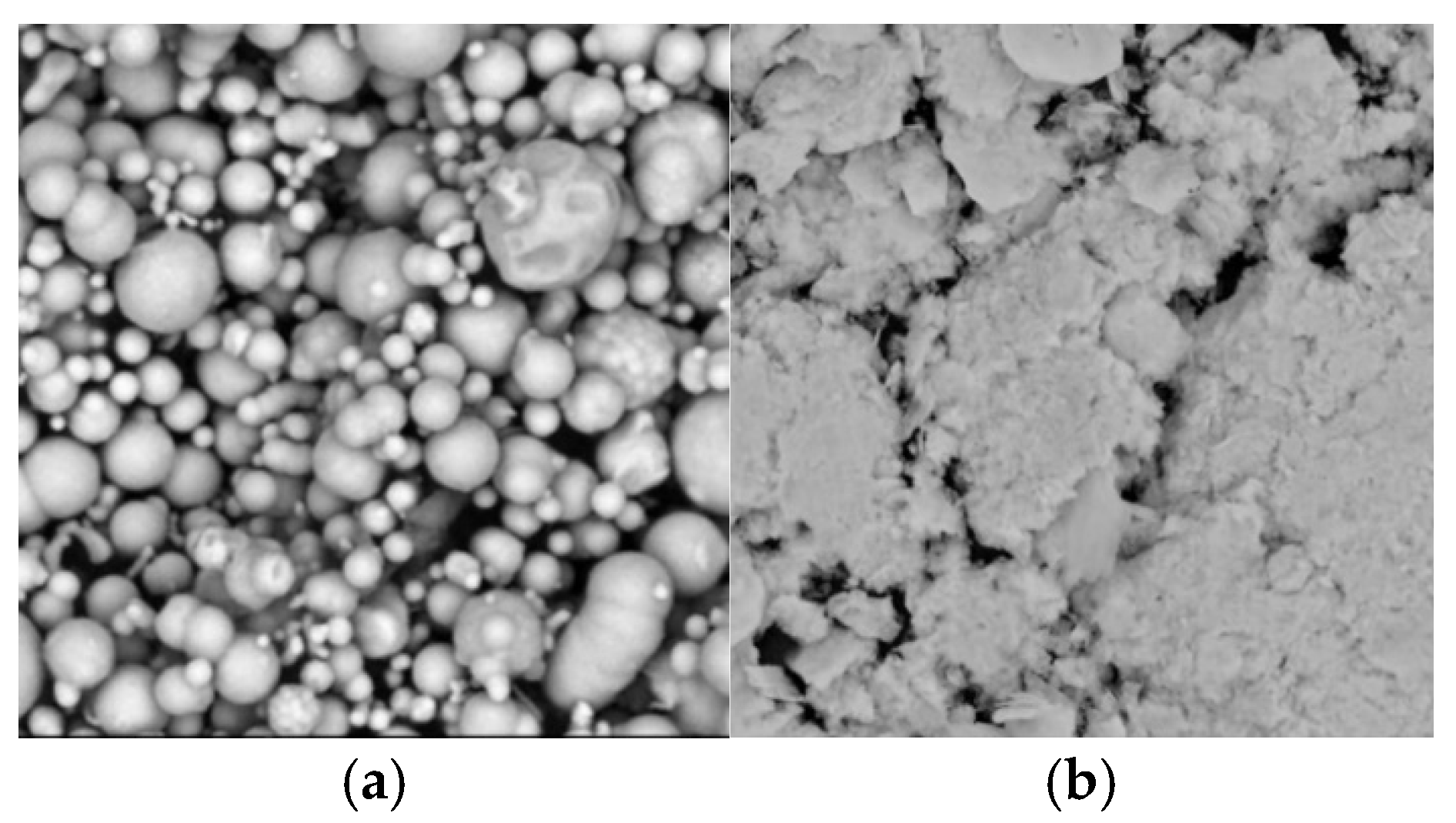
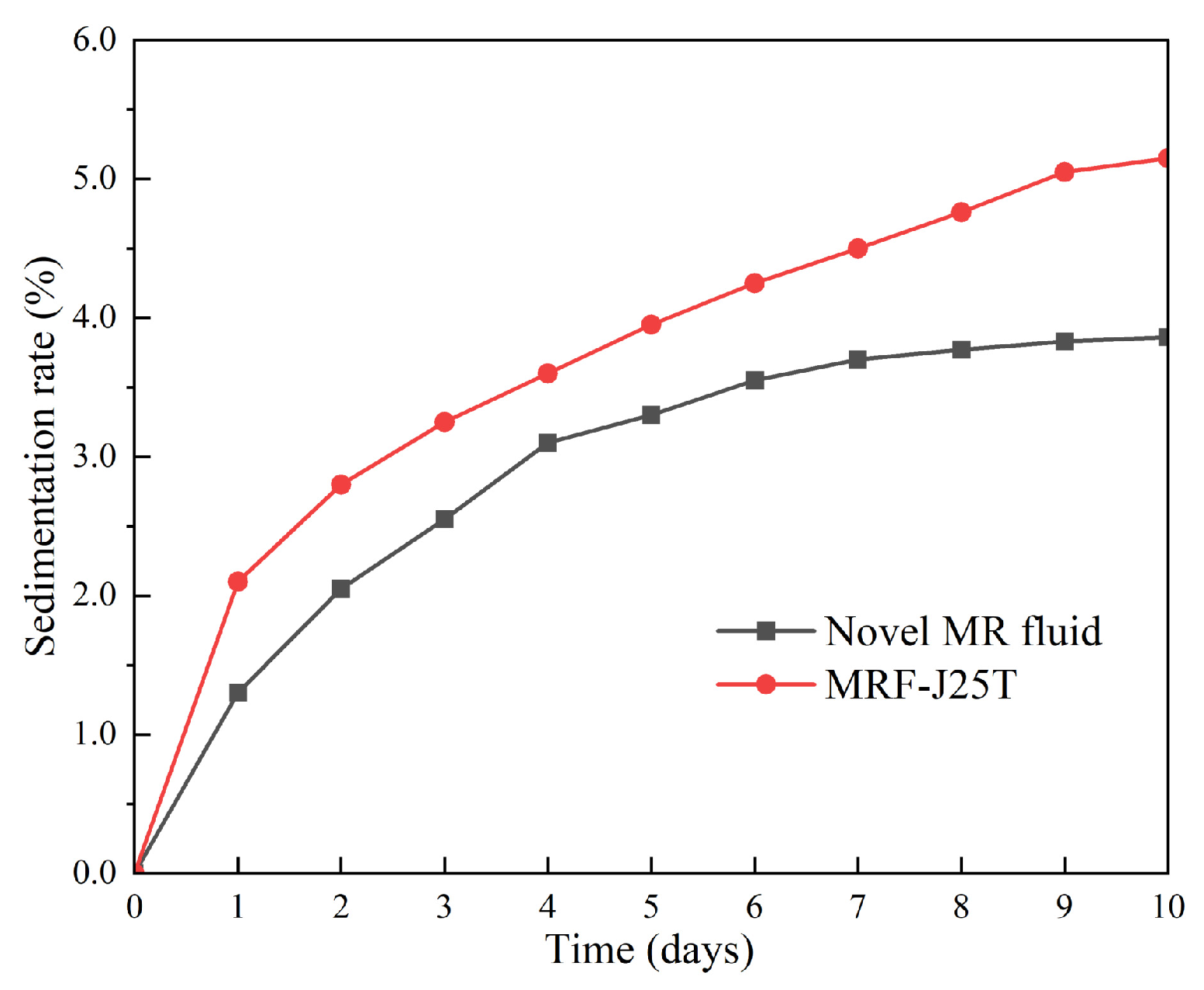
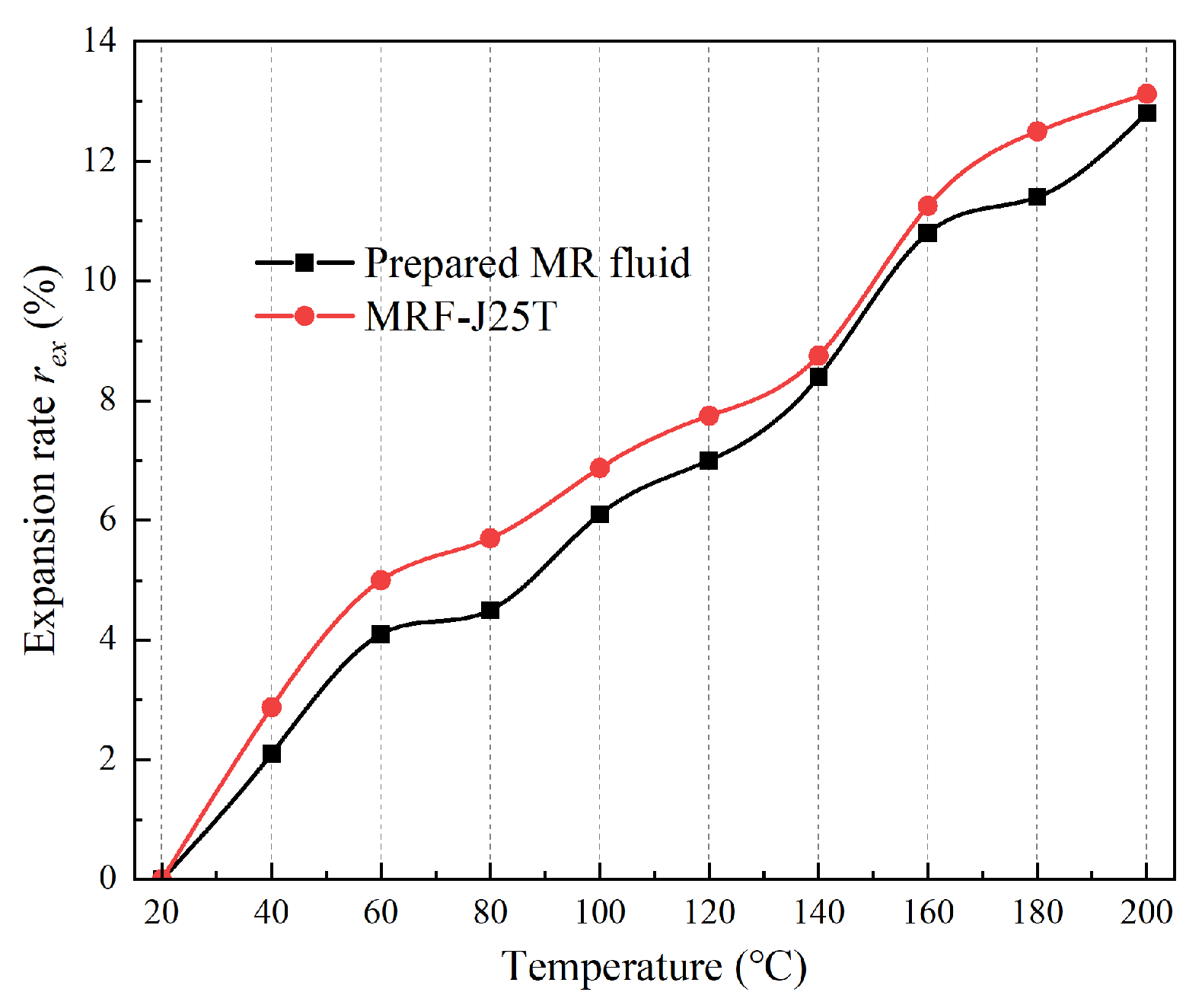
3.1. Soft Magnetic Particles
(1) Magnetic permeability
The MR effect relies on the magnetization of particles under an applied magnetic field, making magnetic permeability a critical property for soft magnetic particles. Previous research [
29] has demonstrated that CIP exhibits no significant reduction in permeability or shear yield stress at temperatures up to 200 °C, indicating excellent thermal stability. Therefore, CIP is suitable as a soft magnetic particle material for MR fluids with high-temperature and shear-thinning resistance.
(2) Shear-thinning resistance
To evaluate the shear-thinning resistance, two types of MR fluids with a mass fraction of 60 wt% (18.3 vol%) were prepared by dispersing spherical and flake-shaped CIPs in dimethyl silicone oil, respectively. The rotational rheometer MCR 302 equipped with an MRD 170/1 T MR module was employed to measure the viscosity across a shear rate range from 0.01 s
−1 to 100 s
−1. The tests were conducted at room temperature. The apparent viscosity of each MR fluid was recorded under magnetic field strengths of 161 mT, 325 mT, 489 mT, 653 mT, and 817 mT. The shear-thinning behavior was quantified using the ratio
rs of maximum apparent viscosity
ηmax (0.01 s
−1) to minimum apparent viscosity
ηmin (100 s
−1), as defined in Equation (1).
Figure 2 compares the shear-thinning performance of MR fluids formulated with CIPs of different morphologies.
As shown in
Figure 2, the shear-thinning effect of MR fluids prepared using spherical and flake-shaped particles decreases with increasing magnetic field strength. This occurs because a stronger magnetic field generates greater interparticle forces, which prevent the particles from dislodging. Under the same magnetic field conditions, the
rs value of flake-shaped particles is lower than that of spherical particles, indicating that MR fluids formulated with flake particles exhibit higher resistance to shear-thinning. The particle chains formed by flake-shaped particles are more tightly packed under the magnetic field, making it more difficult for the particles to detach when the chain rotates with the shear disk.
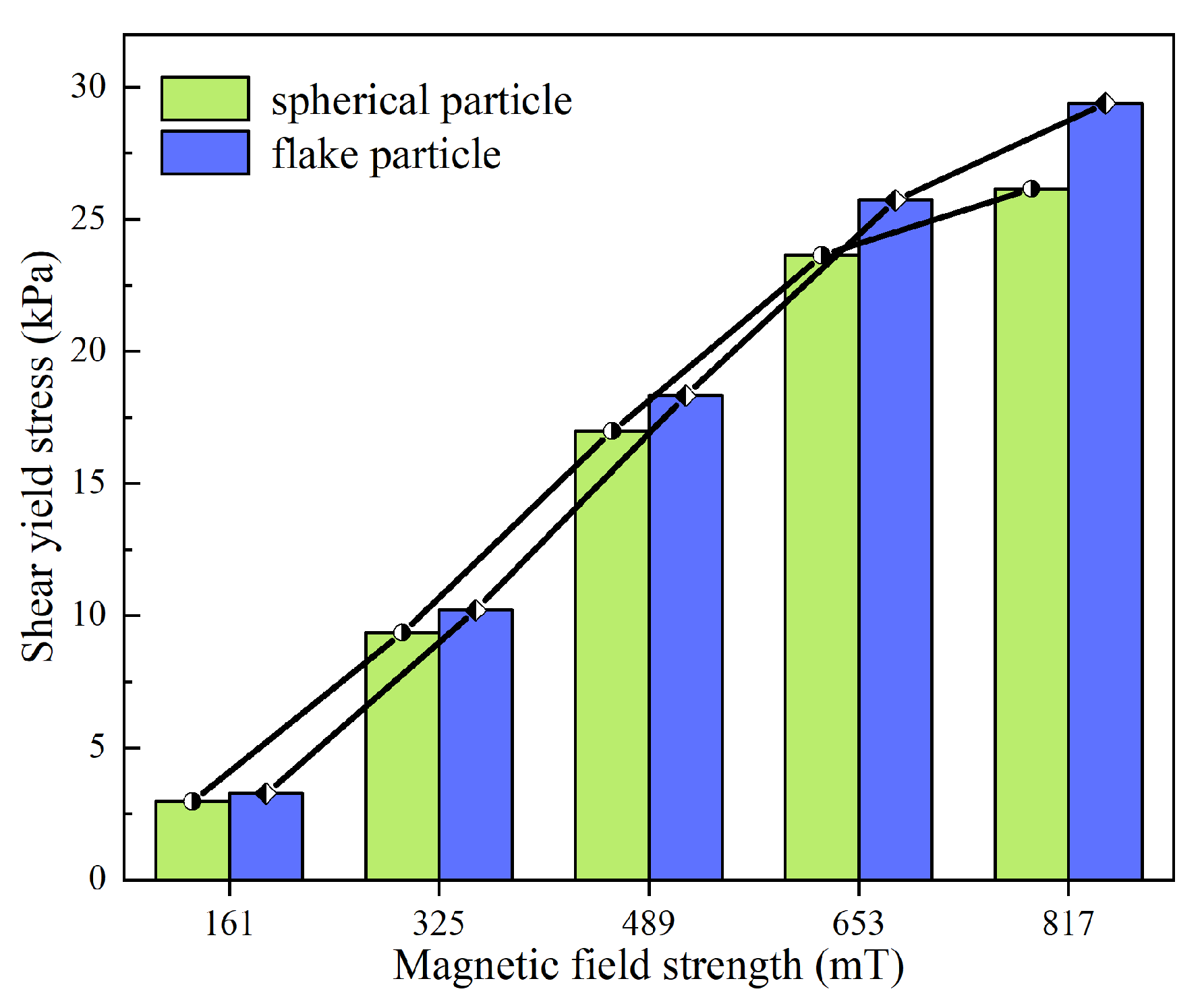
(3) Shear yield stress
The testing temperature was maintained at room temperature by the circulating water within the module. The yield stress of the MR fluid under each magnetic field strength (61 mT, 325 mT, 489 mT, 653 mT, and 817 mT) was subsequently measured and recorded.
As shown in
Figure 3, the shear yield stress of MR fluids prepared using flake-shaped and spherical particles increases with the strengthening of the magnetic field; however, the rate of increase gradually diminishes. The MR fluid formulated with flake-shaped particles consistently exhibits a higher shear yield stress compared to that with spherical particles across all tested magnetic field strengths, and the disparity becomes more pronounced as the field strength increases. Under the same magnetic field, the particle chains formed by flake-shaped particles are more closely aligned than those formed by spherical particles [
36], resulting in stronger interparticle forces that enhance resistance to chain disruption under shear.
In summary, flake-shaped CIPs demonstrate excellent thermal stability, superior shear-thinning resistance, and higher shear yield stress, making them well-suited for use as soft magnetic particles in MR fluids designed for high-temperature and shear-thinning environments.
3.3. Base Carrier Fluid
(1) Expansion characteristic
When MR fluid operates in a high-temperature environment, the amplitude of particle vibration increases with rising temperature, leading to volumetric expansion of the MR fluid. Significant expansion can exert pressure on other components within MR devices, potentially causing mechanical deformation and MR fluid leakage. The base carrier fluid typically constitutes more than 70% of the MR fluid by volume, and its thermal expansibility significantly influences the overall expansion behavior of the MR fluid. To evaluate this characteristic, the base carrier fluid was heated from 20 °C to 200 °C, and its volume was recorded at 20 °C intervals. The expansion rate,
rex, was then calculated using Equation (2).
where
Vrex is the recorded volume after the heat, and
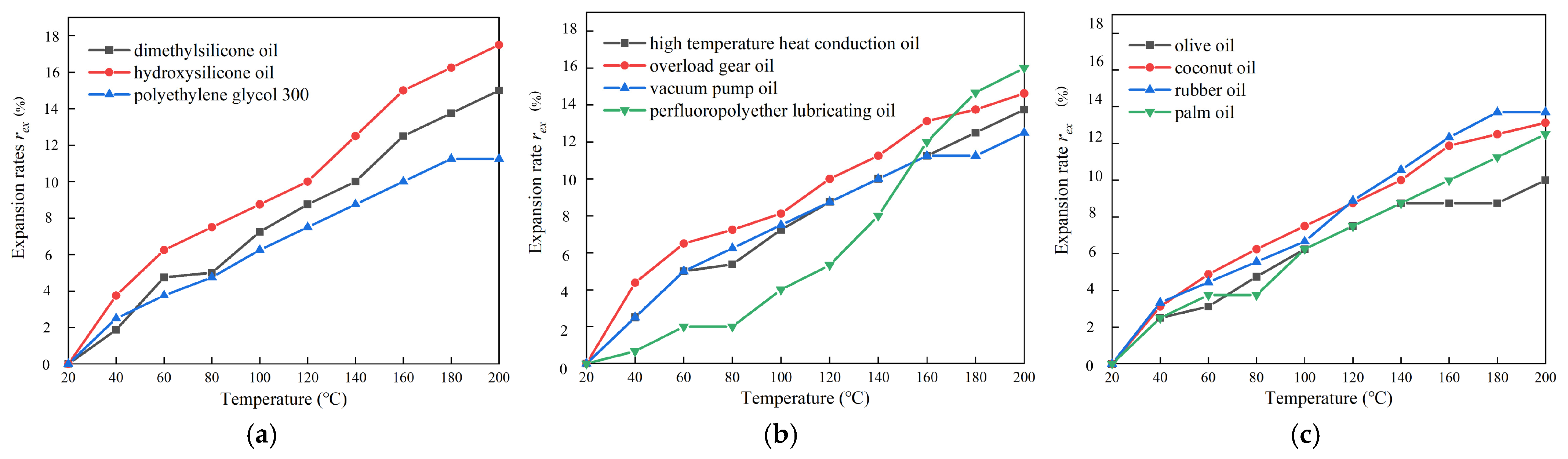
Vo is the original volume before heating. The expansion-temperature curves of the eleven kinds of base carrier fluids are shown in
Figure 6.
As can be seen from
Figure 6, the expansion rate of the three types of oil-based liquids increases gradually with the temperature rising, in which the expansion rate of polymer oil and synthetic oil is higher, while the expansion rate of natural oil is lower. Among the three polymer oils, the expansion rate of hydroxy-silicone oil is the highest (16.0%), and that of polyethylene glycol 300 is the lowest (11.3%). Among the four synthetic oils, the expansion rate of perfluoropolyether lubricating oil is the highest (16.0%), and that of vacuum pump oil is the lowest (12.5%). Among the four natural oils, the expansion rate of rubber oil is the highest (13.7%), and the expansion rate of olive oil is the lowest (10.0%). The expansion rate of the most commonly used dimethyl silicone oil is 15%. Among the 11 kinds of oil-based carrier fluids proposed above, except for hydroxyl silicone oil and perfluoropolyether lubricant, the expansion rate of the 8 kinds of oil-based carrier fluids is lower than that of dimethyl silicone oil, with good high-temperature stability. At temperatures below 150 °C, the expansion rate of perfluorinated polyether lubricating oil is lower than that of other 10 oil-based carrier fluids, so its other characteristics can be further explored.
(2) Evaporation characteristic
As temperature increases, the Brownian motion of particles in the magnetorheological fluid becomes more intense. When this motion reaches a critical threshold, particles overcome interparticle forces and escape, resulting in a macroscopic phase transition from liquid to gas. Similarly, the base carrier liquid typically constitutes more than 70% of the magnetorheological fluid by volume, making it essential to evaluate its evaporation characteristics. Eleven types of base carrier liquids listed in
Table 4 were placed in a high-temperature drying oven, heated to 200 °C, and maintained at this temperature for two hours. After cooling to room temperature, the remaining volume of each base carrier liquid was measured, and the evaporation rate,
rev, at different temperatures was calculated using Equation (3).
where
Vrev is the recorded volume after the heat, and
Vo is the original volume before the heat.
Figure 7 shows the evaporation rates of eleven kinds of base carrier fluids rev at high temperatures.
As shown in
Figure 7, evaporation occurs in all eleven types of base carrier fluids after two hours of heating. Rubber oil exhibits the lowest evaporation rate (0.27%), whereas palm oil has the highest (3.75%). The evaporation rates of hydroxyl silicone oil, high-temperature heat conduction oil, perfluoroether lubricating oil, and palm oil exceed 1%. Repeated heating leads to a decrease in MR fluid volume and changes in particle concentration, which compromise the reliability of MR devices. Therefore, these fluids are not suitable as base carrier fluids for MR fluids. The remaining seven oil-based carrier fluids have evaporation rates below 1%, with polyethylene glycol 300 and rubber oil showing lower evaporation rates than dimethyl silicone oil, indicating superior high-temperature stability.
In addition, some base carrier fluids exhibit color changes after two hours of heating. Polyethylene glycol 300 changes from colorless to brown, high-temperature thermal oil changes from colorless to light yellow, and both rubber oil and palm oil also turn light yellow. These color changes suggest that these four base carrier fluids undergo structural or chemical alterations at elevated temperatures.
(3) Viscosity
The zero-field fluidity of MR fluid is primarily determined by the viscosity of the base carrier fluid, which also plays a critical role in sedimentation stability. Previous studies [
29] have demonstrated that the viscosity of base carrier fluids varies significantly with temperature; therefore, it is essential to investigate viscosity across different temperature conditions. Since carrier fluids can be classified as Newtonian or non-Newtonian, the Newtonian behavior of oil-based carrier fluids was first examined. Based on the characteristics of these fluids, an SNB-1 digital rotational viscometer (parameters listed in
Table 5) was employed to measure the viscosities of the eleven selected oil-based carrier fluids at shear rates of 1.3 s
−1, 2.6 s
−1, 6.5 s
−1, and 13.0 s
−1.
Figure 8 presents the viscosities of these eleven base carrier fluids under varying shear rates. All experiments were conducted at room temperature.
As shown in
Figure 8, the viscosity of base carrier fluids is almost constant at different shear rates, indicating that the above 11 base carrier fluids are all Newtonian fluids. Therefore, the viscosity at any shear rate can be selected to represent the viscosity of the base carrier fluid at the temperature. In the temperature range from 20 °C to 200 °C, the viscosities of the eleven kinds of base carrier fluids were measured with 20 °C as the temperature gradient, as shown in
Figure 9. The experiments were carried out at a shear rate of 13 s
−1. As the coconut oil is solid at room temperature, its viscosity data is missing.
As shown in
Figure 9, the viscosity of each base carrier fluid gradually decreases with increasing temperature. The trend can be described as follows: the viscosity drops rapidly when the temperature rises from 20 °C to 80 °C, and reaches a lower level at 160 °C. As the temperature continues to increase beyond this point, the viscosity remains low and only slightly decreases further. Since viscosity originates from intermolecular forces, the increase in intramolecular energy with rising temperature weakens these forces, resulting in a reduction in viscosity at the macroscopic level.
At room temperature, perfluoropolyether lubricating oil and rubber oil exhibit significantly higher viscosities, with perfluoropolyether lubricating oil exceeding 2 Pa·s—close to the viscosity of typical commercial MR fluids. When magnetic particles are dispersed in such high-viscosity base carrier fluids, the viscosity of the suspension increases substantially, which may impair the fluidity of the MR fluid. Consequently, perfluoropolyether lubricating oil and rubber oil are not suitable as base carrier fluids for MR fluids. Although overload gear oil and vacuum pump oil also show relatively high viscosities at room temperature, their values remain within an acceptable range, making them candidates for further evaluation of other performance properties.
(4) Shear-thinning resistance
To investigate the anti-shear thinning effect of base carrier fluid, the MCR 302 rotary rheometer and MRD 170/1 T rheological module by ANTON PAAR respectively obtained the apparent viscosity of MR fluids prepared by eleven kinds of base carrier fluids at different rates under the magnetic field of 817 mT, and calculated the ratio rs of the maximum apparent viscosity
ηmax and the minimum apparent viscosity
ηmin based on Equation (1), as shown in
Figure 10. The flake-shaped powder is used to prepare the MR fluid, and the experiments were carried out at room temperature.
As shown in
Figure 10, the shear-thinning behavior of MR fluids prepared with different base carrier fluids varies significantly under a magnetic field of 817 mT. A comparison of
Figure 9 and
Figure 10 reveals that the shear-thinning effect of the MR fluid is negatively correlated with the room-temperature viscosity of the base carrier fluid; higher room-temperature viscosity leads to a more pronounced shear-thinning effect. Among the eleven candidate base carrier fluids evaluated, perfluoropolyether lubricating oil, vacuum pump oil, rubber oil, palm oil, and overload gear oil exhibit a ratio of maximum apparent viscosity to minimum apparent viscosity exceeding 200. This large ratio indicates severe shear thinning during shearing under a magnetic field, making these fluids unsuitable as base carrier fluids for MR fluids. In contrast, dimethyl silicone oil, hydroxyl silicone oil, polyethylene glycol 300, high-temperature heat transfer oil, and olive oil show relatively low viscosity ratios and demonstrate good resistance to shear thinning, making them suitable candidates for use as base carrier fluids.
(5) Suitable base carrier fluid
Table 5 compares the expansion rate, evaporation rate, viscosity-temperature characteristics, and shear-thinning resistance characteristics of eleven kinds of base carrier fluids at high temperatures. In
Table 6, “√” indicates that the liquid has a good performance and is suitable to be used as the base carrier fluid. “×” indicates that the liquid has a poor performance and is not suitable for the base carrier fluid of MR fluid.
As shown in
Table 6, based on experimental results regarding the expansion rate, evaporation rate, sedimentation rate, and shear-thinning resistance of base carrier fluids, dimethyl silicone oil, polyethylene glycol 300, and olive oil exhibit favorable apparent and mechanical properties, making them suitable candidates for use as base carrier fluids in MR fluids with high-temperature and shear-thinning resistance.
Although polyethylene glycol 300 and olive oil demonstrate excellent performance in certain properties, such as low evaporation and expansion rates, polyethylene glycol 300 is prone to discoloration and thermal degradation at elevated temperatures. Olive oil, being a natural product, is susceptible to oxidation and lacks long-term thermal stability. In contrast, dimethyl silicone oil not only exhibits a low high-temperature evaporation rate (0.8%) and an acceptable expansion rate (15%), but also effectively mitigates shear thinning. Furthermore, its chemical inertness, well-established industrial application, and excellent compatibility with soft magnetic particles make it the most reliable base carrier fluid. This combination of properties ensures the long-term stability of MR fluids under high-temperature conditions.