Selective Recovery of Molybdenum over Nickel and Cobalt from Simulated Secondary Sources Using Bifunctional Ionic Liquid [TOA][Cy272]
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Bifunctional Ionic Liquid
2.3. Extraction and Stripping Experiments
3. Results
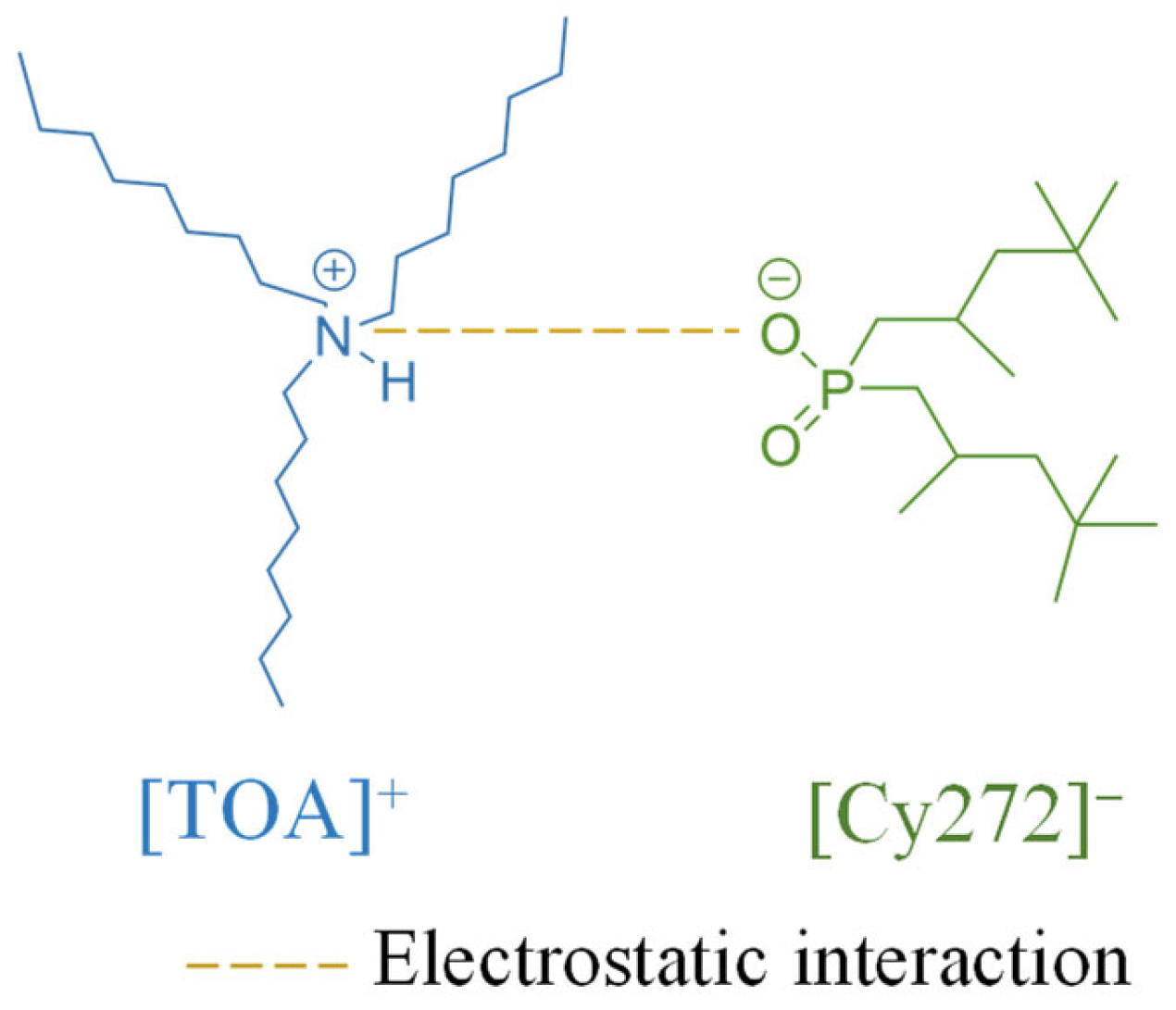
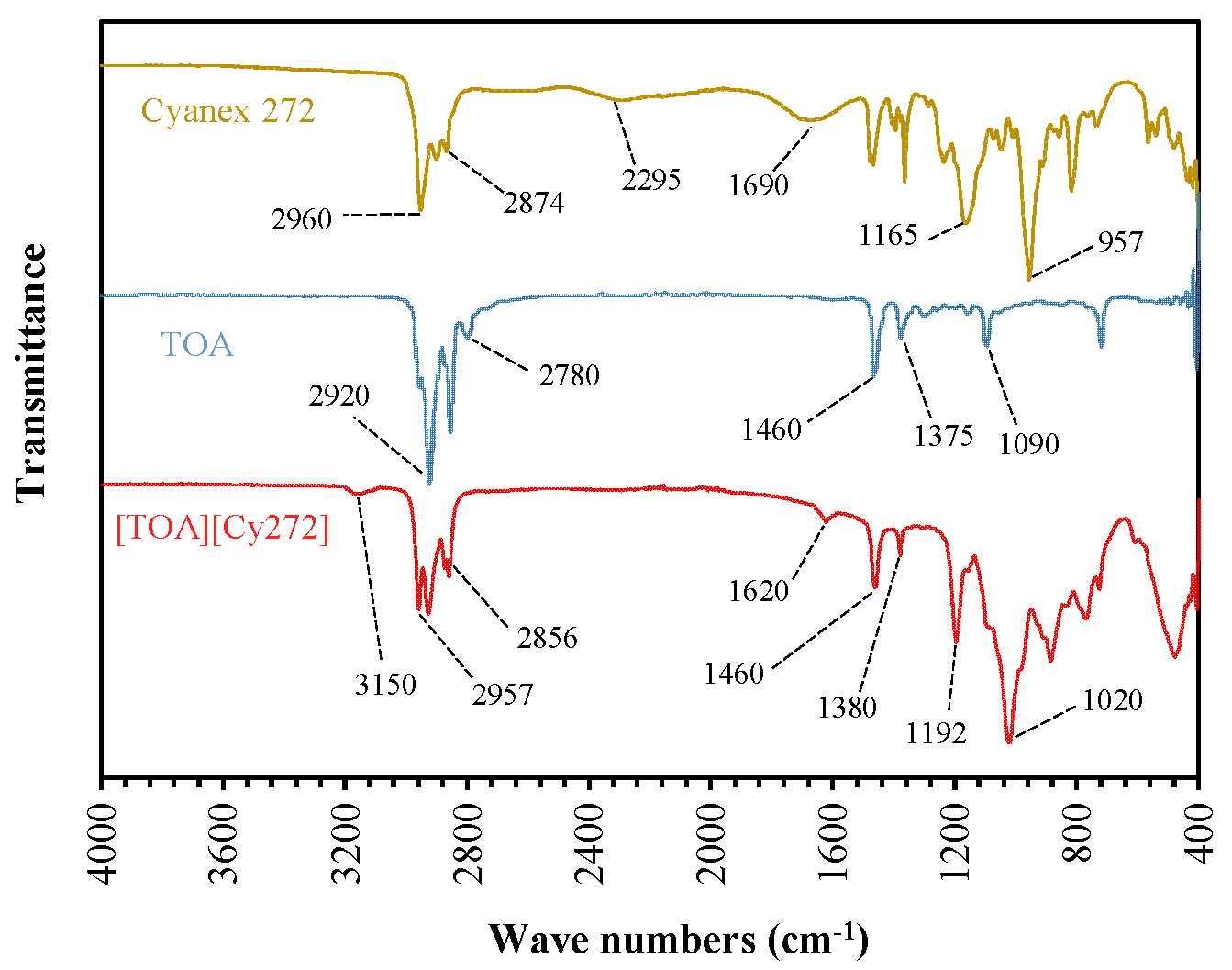
3.1. Confirmation of [TOA][Cy272] IL Formation
3.2. Assessment of Extraction Efficiency
3.2.1. [TOA][Cy272] vs. Precursors
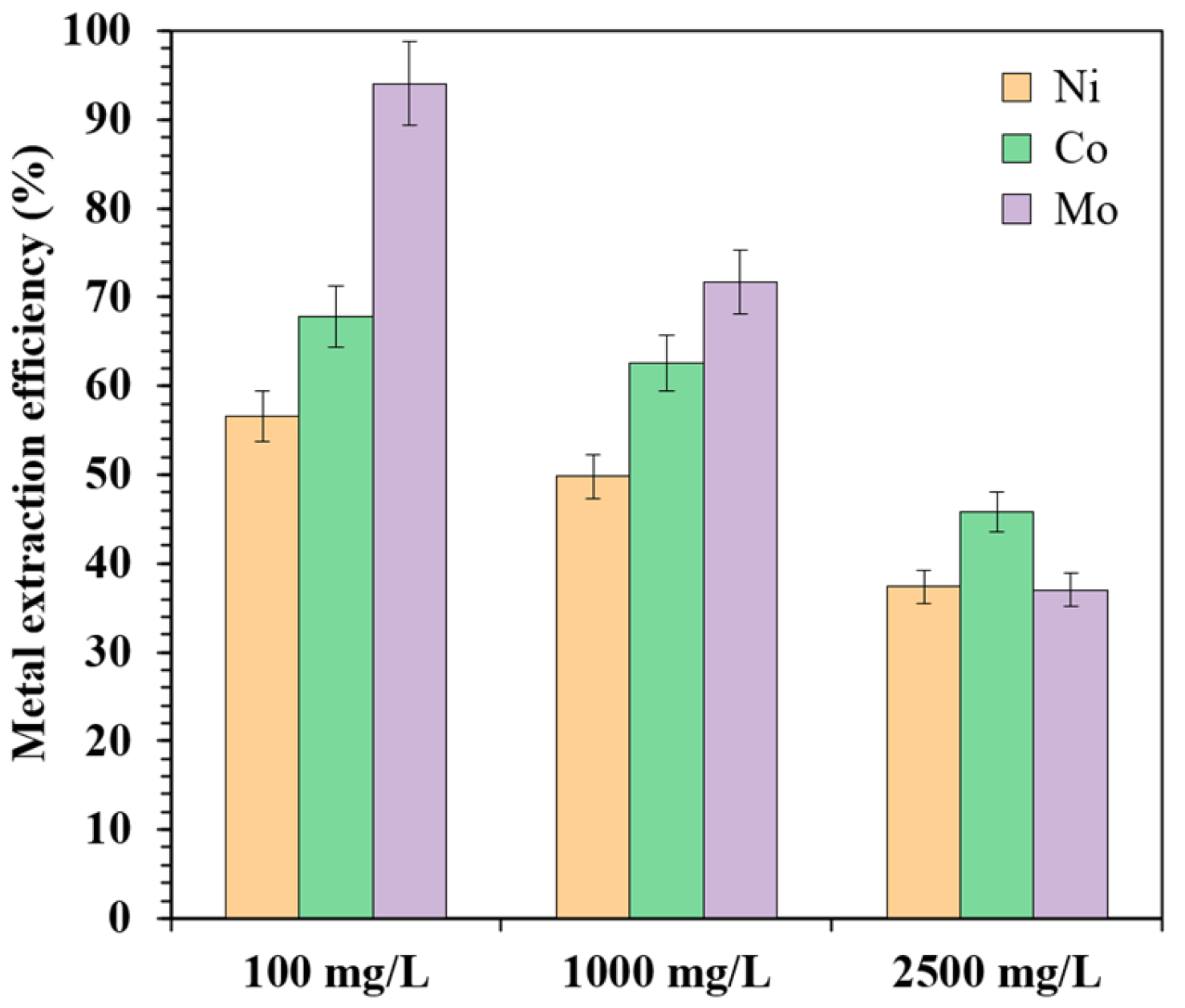
3.2.2. Effect of Metal Concentration
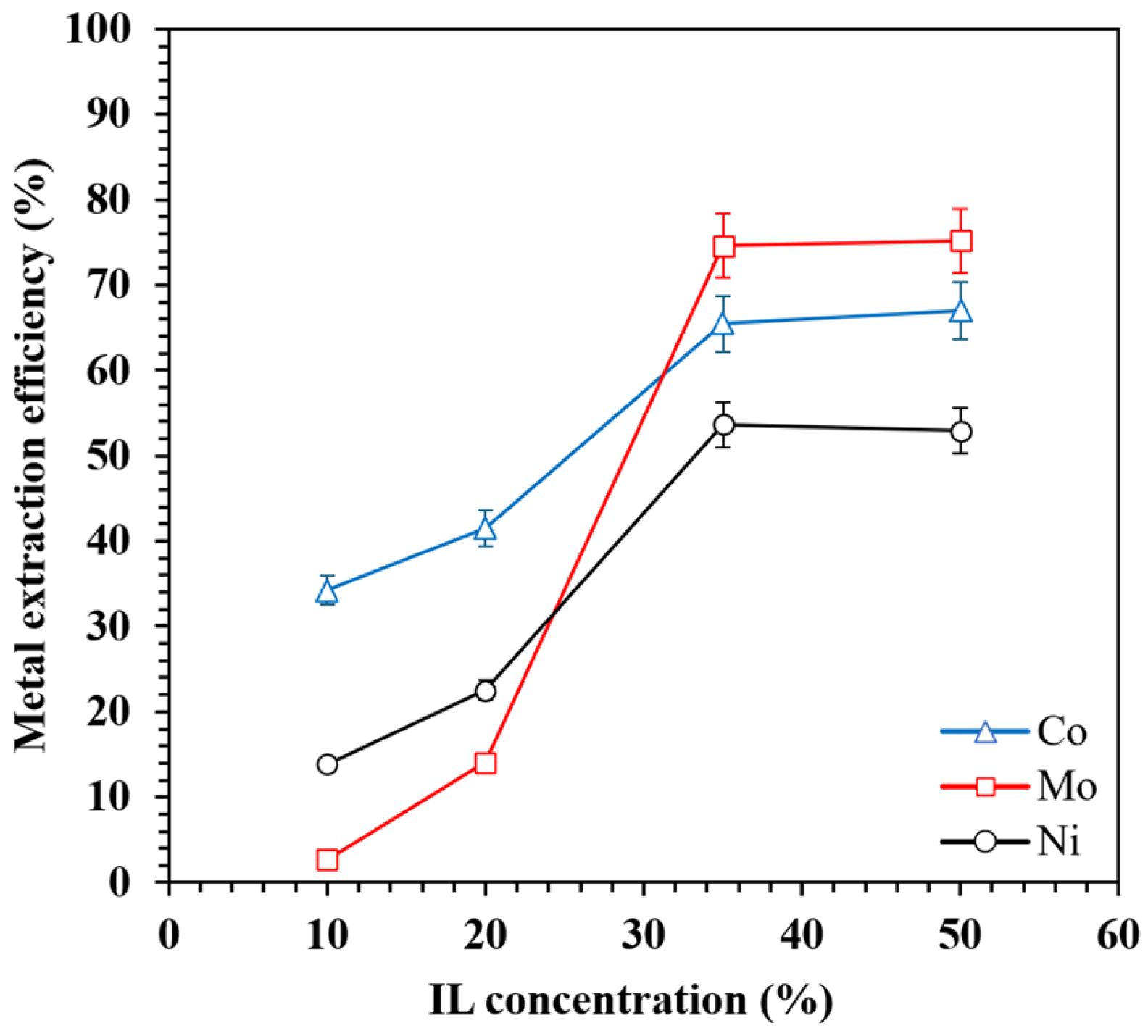
3.2.3. Effect of IL Concentration
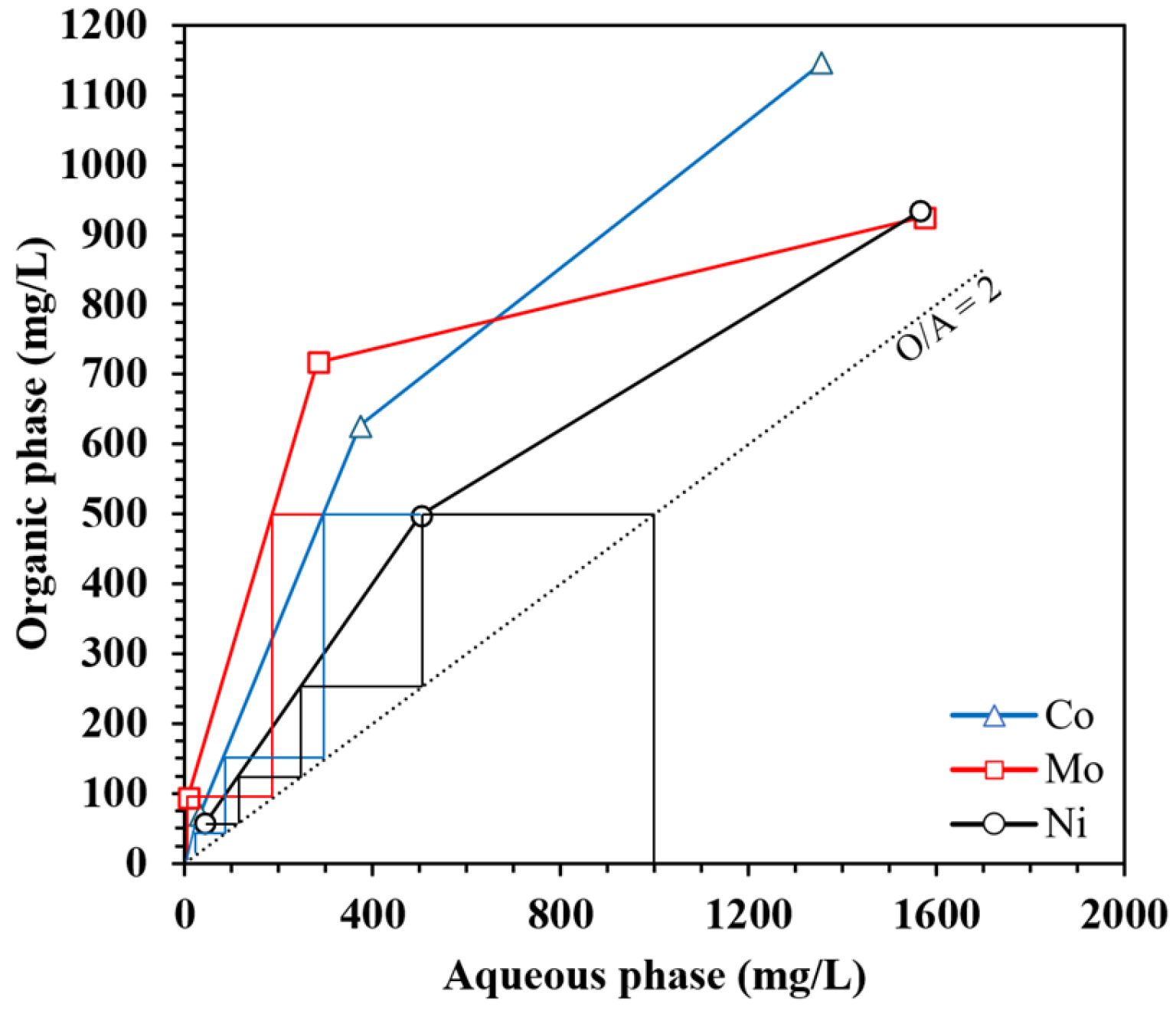
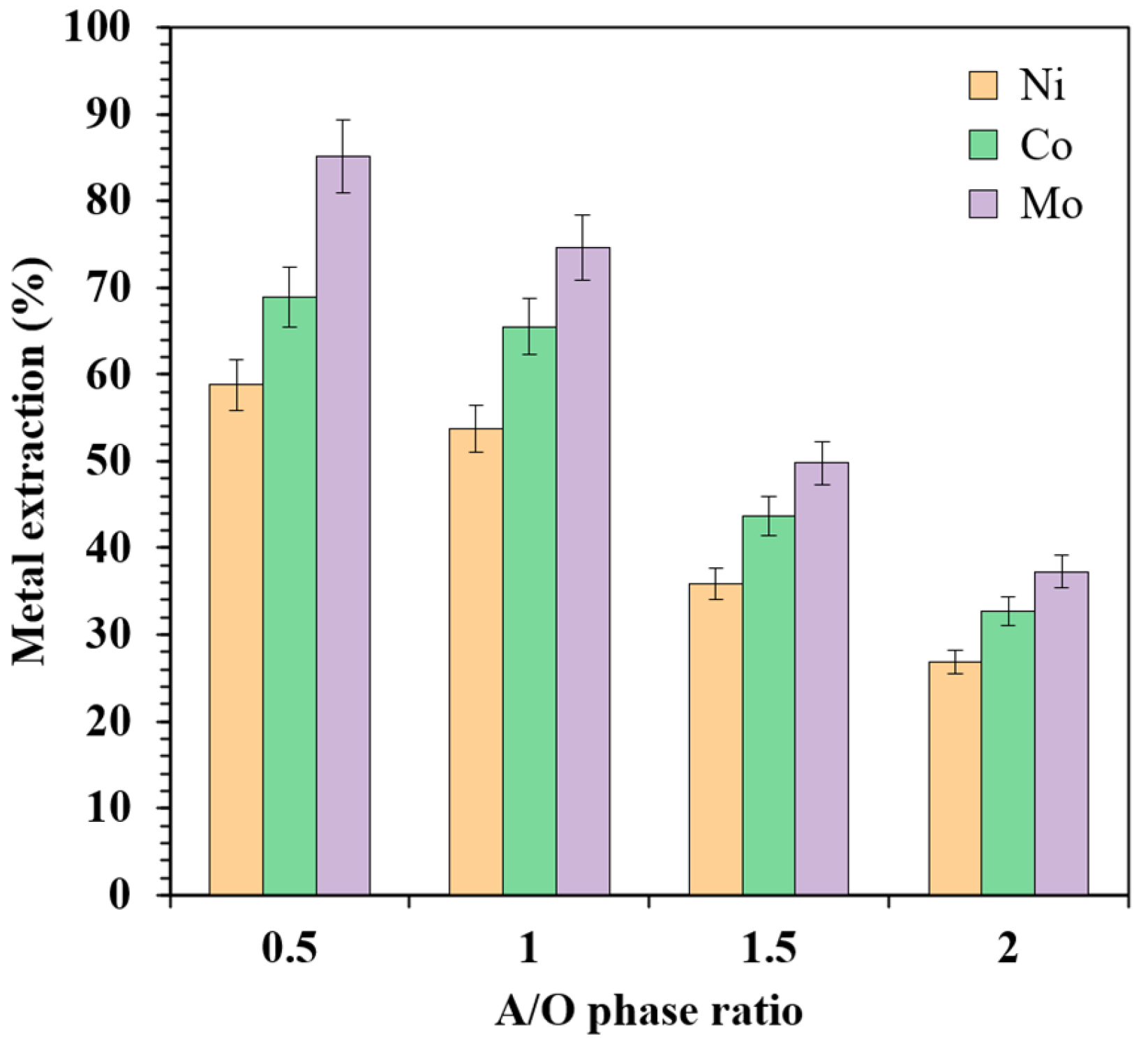
3.2.4. Effect of A/O Phase Ratio
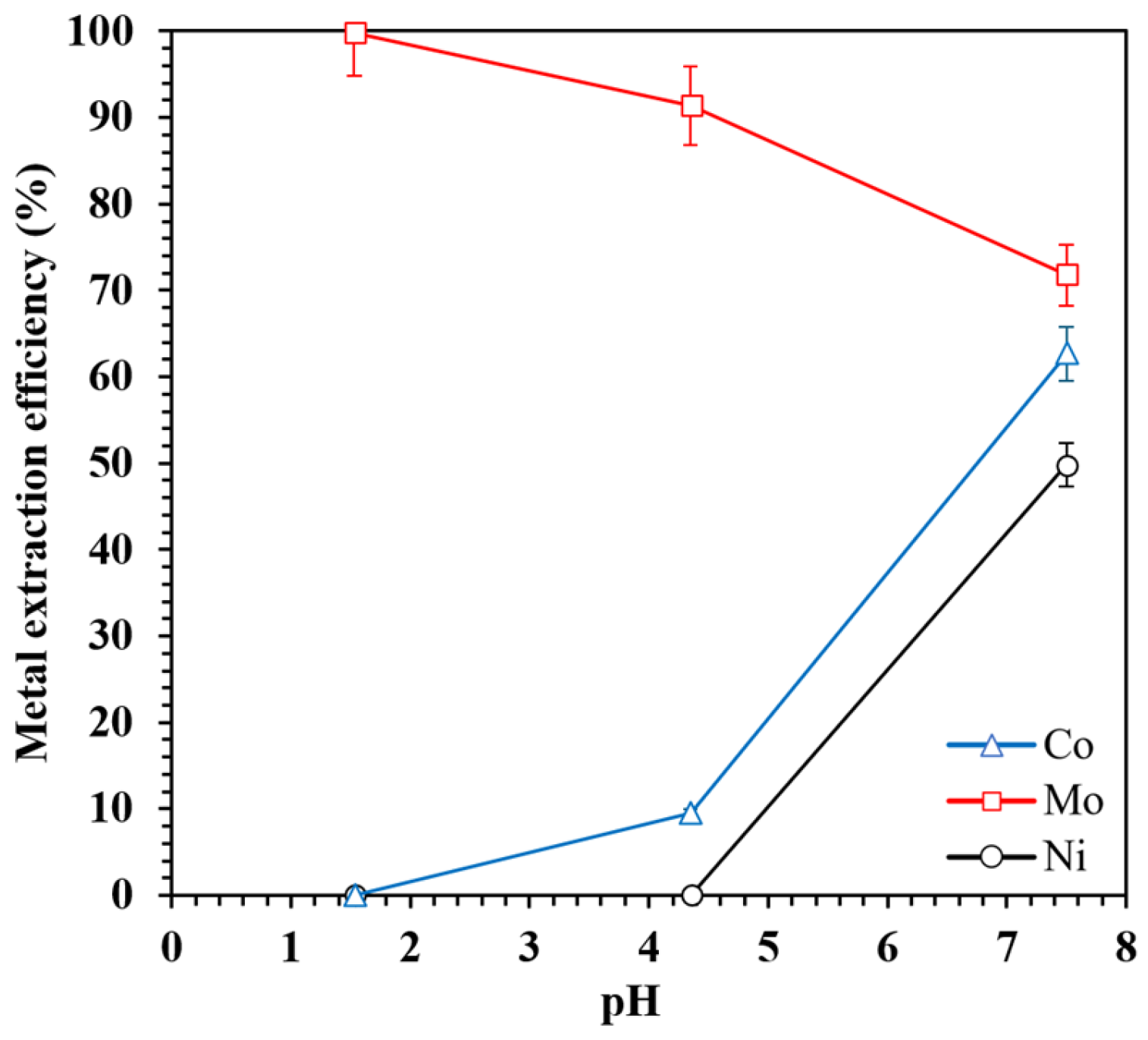
3.2.5. Effect of pH
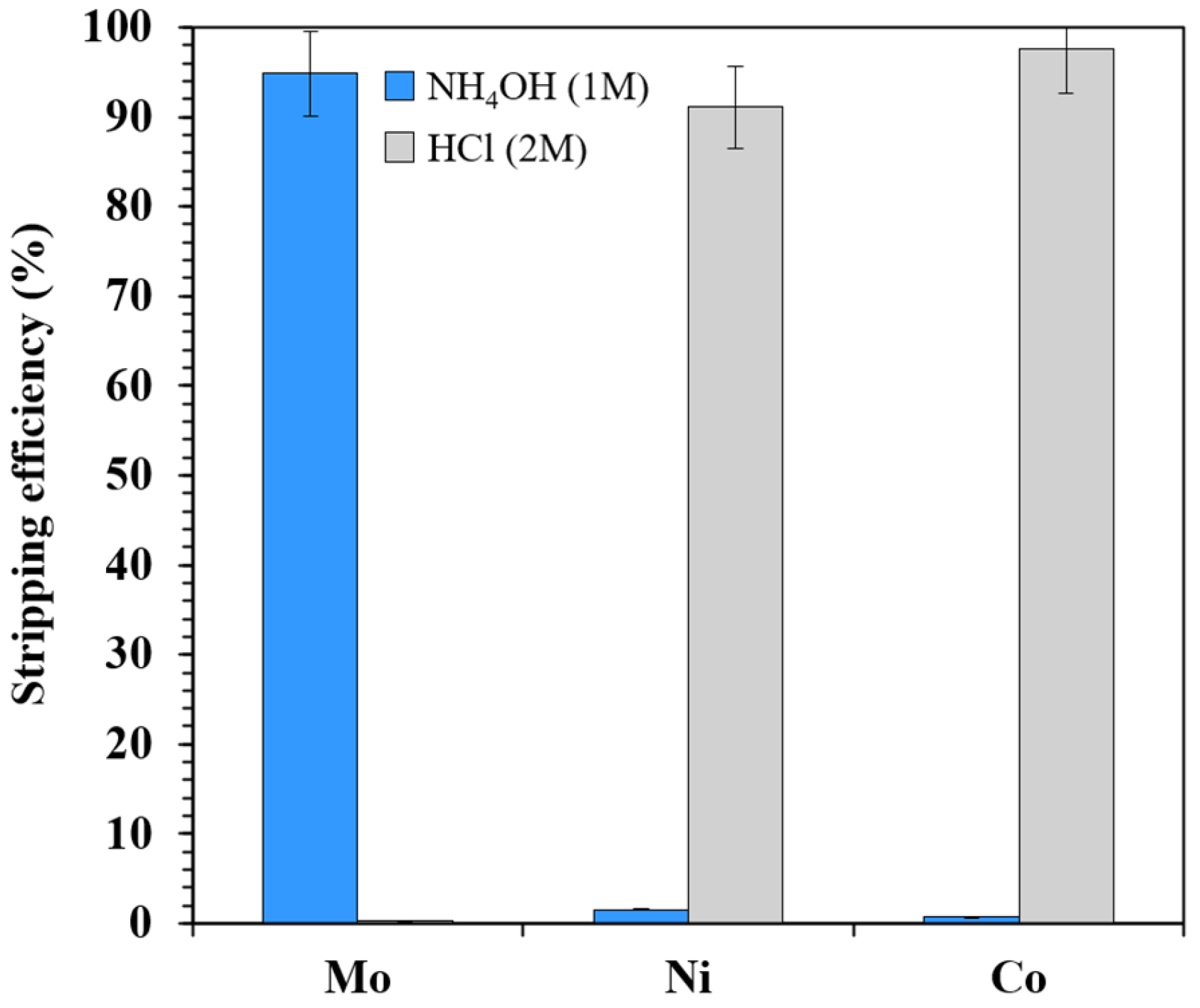
3.3. Stripping
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Srour, H.; Devers, E.; Mekki-Berrada, A.; Toufaily, J.; Hamieh, T.; Batiot-Dupeyrat, C.; Pinard, L. Regeneration of an aged hydrodesulfurization catalyst: Conventional thermal vs non-thermal plasma technology. Fuel 2021, 306, 121674. [Google Scholar] [CrossRef]
- Adavodi, R.; Romano, P.; Rahmati, S.; Birloaga, I.; Mancini, A.; Vegliò, F. Solvent washing strategy for optimal vanadium and molybdenum recovery from spent hydrodesulfurization catalysts without thermal pre-treatment. Sep. Purif. Technol. 2025, 363, 132020. [Google Scholar] [CrossRef]
- D’Adamo, I.; Ippolito, N.M.; Shalchian, H.; Innocenzi, V.; Ferella, F.; Vegliò, F. A feasibility study for a circular approach in oil refining: Metals recovery from hydrodesulphurization catalysts. Sustain. Mater. Technol. 2023, 36, e00634. [Google Scholar] [CrossRef]
- Pu, N.; Hu, A.; Yang, Q.; Zheng, A.; Chen, L.; Wang, Z.; Huang, Y.; Wang, S.; Hu, D.; Nie, H. Atomic decoration of MoS2 using Fe, Co or Ni for highly efficient and selective hydrodesulfurization. Chem. Eng. J. 2024, 499, 156100. [Google Scholar] [CrossRef]
- Gao, B.; Jiang, H.; Zeng, M.; Peng, M.; Hu, L.; Zhang, W.; Mao, L. High-efficiency recycling method for Mo and Ni from spent catalyst via soda roasting and solvent extraction. J. Clean. Prod. 2022, 367, 132976. [Google Scholar] [CrossRef]
- Arslanoğlu, H.; Yaraş, A. Recovery of molybdenum, cobalt and nickel from spent hydrodesulphurization catalyst through oxidizing roast followed by sodium persulfate leaching. Sustain. Mater. Technol. 2021, 28, e00286. [Google Scholar] [CrossRef]
- Parhi, P.; Misra, P. Environmental friendly approach for selective extraction and recovery of molybdenum (Mo) from a sulphate mediated spent Ni–Mo/Al2O3 catalyst baked leach liquor. J. Environ. Manag. 2022, 306, 114474. [Google Scholar] [CrossRef]
- Xhaferaj, N.; Ferella, F. Extraction and recovery of metals from spent HDS catalysts: Lab-and pilot-scale results of the overall process. Metals 2022, 12, 2162. [Google Scholar] [CrossRef]
- Rouhani, S.H.R.; Davarkhah, R.; Zaheri, P.; Mousavian, S.M.A. Separation of molybdenum from spent HDS catalysts using emulsion liquid membrane system. Chem. Eng. Process.-Process Intensif. 2020, 153, 107958. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Du, H.; Olayiwola, A.; Liu, B.; Gao, F.; Jia, M.-L.; Wang, M.-H.; Gao, M.-L.; Wang, X.-D.; Wang, S.-N. Recent advances in the recovery of transition metals from spent hydrodesulfurization catalysts. Tungsten 2021, 3, 305–328. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, C.; Yuan, S.; Liu, M.; Chen, R.; Hu, H.; Hu, J. Sustainable recovery of surface-deposited oils and valuable metals from uncrushed spent hydroprocessing catalysts. J. Clean. Prod. 2022, 338, 130564. [Google Scholar] [CrossRef]
- Tran, T.T.; Liu, Y.; Lee, M.S. Recovery of pure molybdenum and vanadium compounds from spent petroleum catalysts by treatment with ionic liquid solution in the presence of oxidizing agent. Sep. Purif. Technol. 2021, 255, 117734. [Google Scholar] [CrossRef]
- Zhang, M.; Song, H.; Zheng, C.; Lin, Z.; Liu, Y.; Wu, W.; Gao, X. Highly efficient recovery of molybdenum from spent catalyst by an optimized process. J. Air Waste Manag. Assoc. 2020, 70, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Z.; Jung, S.M. Green and selective recovery process of Mo, V, and Ni from spent hydrodesulfurization catalysts via novel ionic liquids and deep eutectic solvents technology. Sep. Purif. Technol. 2024, 346, 127450. [Google Scholar] [CrossRef]
- Mohapatra, A.; Behera, S.; Tripathy, S.; Parhi, P.; Sanjay, K. Extensive investigation on extraction behaviour of organo-phosphrous based bi-functional ionic liquids for separation of molybdenum (Mo) from spent Co-Mo/Al2O3 leach liquor. J. Mol. Liq. 2022, 366, 120087. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Ferella, F.; De Michelis, I.; Vegliò, F. Adsorption onto activated carbon for molybdenum recovery from leach liquors of exhausted hydrotreating catalysts. Hydrometallurgy 2011, 110, 67–72. [Google Scholar] [CrossRef]
- Cai, Y.; Ma, L.; Xi, X.; Nie, Z.; Yang, Z. Comprehensive recovery of metals in spent Ni–Mo/γ–Al2O3 hydrofining catalyst. Hydrometallurgy 2022, 208, 105800. [Google Scholar] [CrossRef]
- Shakib, B.; Torab-Mostaedi, M.; Outokesh, M.; Asadollahzadeh, M. Mass transfer coefficients of extracting Mo (VI) and W (VI) in a stirred tank by solvent extraction using mixture of Cyanex272 and D2EHPA. Sep. Sci. Technol. 2020, 55, 3140–3150. [Google Scholar] [CrossRef]
- Kozhevnikova, A.V.; Zinov’eva, I.V.; Milevskii, N.A.; Zakhodyaeva, Y.A.; Voshkin, A.A. Complex extraction of rare earth elements from nitrate solutions with a tri-n-octylamine-octanoic acid bifunctional ionic liquid. J. Mol. Liq. 2023, 390, 123073. [Google Scholar] [CrossRef]
- Rahmati, S.; Adavodi, R.; Hosseini, M.R.; Veglio’, F. Efficient metal extraction from dilute solutions: A review of novel selective separation methods and their applications. Metals 2024, 14, 605. [Google Scholar] [CrossRef]
- Tran, T.T.; Azra, N.; Iqbal, M.; Lee, M.S. Synthesis of succinimide based ionic liquids and comparison of extraction behavior of Co (II) and Ni (II) with bi-functional ionic liquids synthesized by Aliquat336 and organophosphorus acids. Sep. Purif. Technol. 2020, 238, 116496. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, Q.; Yan, J.-X.; Sha, L.-T.; Li, Y.; Li, X.-X.; Huang, Q.-G.; Yan, Z.-Y. One-step extraction and solidification of uranium (VI) from sulfate leaching liquor using bifunctional ionic liquids. J. Environ. Chem. Eng. 2023, 11, 109279. [Google Scholar] [CrossRef]
- Zeng, Z.; Su, X.; Gao, Y.; Yu, G.; Ni, S.; Su, J.; Sun, X. Separation of lutetium using a novel bifunctional ionic liquid based on phosphonate functionalization. Sep. Purif. Technol. 2021, 264, 118439. [Google Scholar] [CrossRef]
- Emam, S.S.; El-Hefny, N. Bi-functional ionic liquid based on Aliquat 336 and Cyanex 572 for effective separation of Nd (III) and Ni (II) from chloride solution and recover Nd2O3 from waste Nd-Ni-Fe magnets. Hydrometallurgy 2023, 219, 106064. [Google Scholar] [CrossRef]
- Meshram, P.; Agarwal, N. A review on assessment of ionic liquids in extraction of lithium, nickel, and cobalt vis-à-vis conventional methods. RSC Adv. 2025, 15, 8321–8334. [Google Scholar] [CrossRef]
- Ilyas, S.; Srivastava, R.R.; Kim, H. Selective separation of cobalt versus nickel by split-phosphinate complexation using a phosphonium-based ionic liquid. Environ. Chem. Lett. 2023, 21, 673–680. [Google Scholar] [CrossRef]
- Abbas, Z.; Jung, S.M. A Comprehensive Comparison Analysis between Ammonium-Based and Phosphonium-Based Bifunctional Ionic Liquids for Metal Extraction and Separation Processes. ACS Omega 2024, 9, 44304–44311. [Google Scholar] [CrossRef]
- Mahandra, H.; Singh, R.; Gupta, B. Development of a hydrometallurgical route for the recovery of molybdenum from spent hydrodesulphurization catalyst using Cyphos IL 104. J. Ind. Eng. Chem. 2018, 65, 213–223. [Google Scholar] [CrossRef]
- Brzęczek-Szafran, A.; Więcławik, J.; Barteczko, N.; Szelwicka, A.; Byrne, E.; Kolanowska, A.; Kwaśny, M.S.; Chrobok, A. Protic ionic liquids from di-or triamines: Even cheaper Brønsted acidic catalysts. Green Chem. 2021, 23, 4421–4429. [Google Scholar] [CrossRef]
- Inman, G.; Nlebedim, I.C.; Prodius, D. Application of ionic liquids for the recycling and recovery of technologically critical and valuable metals. Energies 2022, 15, 628. [Google Scholar] [CrossRef]
- İnan, S.; Tel, H.; Sert, Ş.; Çetinkaya, B.; Sengül, S.; Özkan, B.; Altaş, Y. Extraction and separation studies of rare earth elements using Cyanex 272 impregnated Amberlite XAD-7 resin. Hydrometallurgy 2018, 181, 156–163. [Google Scholar] [CrossRef]
- Li, J.; Wu, R.; Tian, S.; Liu, R.; Wei, H.; Yang, Y. Cyanex 272 and 8-HQ synergistic system for the extraction and recovery of Nd (III) from waste-NdFeB magnets. Hydrometallurgy 2025, 236, 106512. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Staszak, K.; Wieszczycka, K.; Masalska, A. Removal of cobalt (II) and zinc (II) from sulphate solutions by means of extraction with sodium bis (2, 4, 4-trimethylpentyl) phosphinate (Na-Cyanex 272). Clean Technol. Environ. Policy 2016, 18, 1961–1970. [Google Scholar] [CrossRef]
- Yasir, N.; Khan, A.S.; Akbar, N.; Hassan, M.F.; Ibrahim, T.H.; Khamis, M.; Siddiqui, R.; Khan, N.A.; Nancarrow, P. Amine-based deep eutectic solvents for alizarin extraction from aqueous media. Processes 2022, 10, 794. [Google Scholar] [CrossRef]
- Nayak, S.; Servis, M.J.; Combs, D.; Szeliga, K.; Seifert, S. Speciation and organic phase structure in nitric acid extraction with trioctylamine. J. Phys. Chem. B 2024, 128, 3236–3248. [Google Scholar] [CrossRef] [PubMed]
- Ramjhan, Z.; Lokhat, D.; Alshammari, M.B.; Joy, M.N.; Ahmad, A. Trioctylammonium-based Ionic liquids for metal ions Extraction: Synthesis, characterization and application. J. Mol. Liq. 2021, 342, 117534. [Google Scholar] [CrossRef]
- Adavodi, R.; Rahmati, S.; Vegliò, F. Towards efficient and selective boron recovery from end-of-life rare earth permanent magnets using an ionic liquid. J. Clean. Prod. 2025, 512, 145694. [Google Scholar] [CrossRef]
- Torkaman, R.; Asadollahzadeh, M.; Torab-Mostaedi, M.; Ghanadi Maragheh, M. Recovery of cobalt from spent lithium ion batteries by using acidic and basic extractants in solvent extraction process. Sep. Purif. Technol. 2017, 186, 318–325. [Google Scholar] [CrossRef]
- Janiszewska, M.; Markiewicz, A.; Regel-Rosocka, M. Hydrometallurgical separation of Co (II) from Ni (II) from model and real waste solutions. J. Clean. Prod. 2019, 228, 746–754. [Google Scholar] [CrossRef]
- Cholico-Gonzalez, D.; Chagnes, A.; Cote, G.; Avila-Rodriguez, M. Separation of Co (II) and Ni (II) from aqueous solutions by bis (2, 4, 4-trimethylpentyl) phosphinic acid (Cyanex 272) using trihexyl (tetradecyl) phosphonium chloride (Cyphos IL 101) as solvent. J. Mol. Liq. 2015, 209, 203–208. [Google Scholar] [CrossRef]
- Satyawirawan, S.A.; Cattrall, R.W.; Kolev, S.D.; Almeida, M.I.G. Improving Co (II) separation from Ni (II) by solvent extraction using phosphonium-based ionic liquids. J. Mol. Liq. 2023, 380, 121764. [Google Scholar] [CrossRef]
- Diabate, P.D.; Dupont, L.; Boudesocque, S.; Mohamadou, A. Novel Task Specific Ionic Liquids to Remove Heavy Metals from Aqueous Effluents. Metals 2018, 8, 412. [Google Scholar] [CrossRef]
- Azizi, D.; Larachi, F. Behavior of bifunctional phosphonium-based ionic liquids in solvent extraction of rare earth elements-quantum chemical study. J. Mol. Liq. 2018, 263, 96–108. [Google Scholar] [CrossRef]
- Zahraie, N. Study of Solvent Extraction for Metal Recovery during Lithium-Ion Batteries (LIBs) Recycling Process. Master’s Thesis, NTNU, Trondheim, Norway, 2021. [Google Scholar]
- Yudaev, P.A.; Chistyakov, E.M. Ionic liquids as components of systems for metal extraction. ChemEngineering 2022, 6, 6. [Google Scholar] [CrossRef]
- Asrami, M.R.; Tran, N.N.; Nigam, K.D.P.; Hessel, V. Solvent extraction of metals: Role of ionic liquids and microfluidics. Sep. Purif. Technol. 2021, 262, 118289. [Google Scholar] [CrossRef]
- Luo, Y.; Hu, H.; Wang, Y.; Hu, F.; Zhu, S.; Zhang, S.; Zhang, Y.; Li, S.; Wang, J. Phase separation in solvent extraction of copper or nickel from acidic solution using a sulfonic acid (HDNNS) and a carboxylate ester (4PC). J. Dispers. Sci. Technol. 2019, 40, 819–827. [Google Scholar] [CrossRef]
- Jimenez, Y.P.; Justel, F.; Casas, J.M. Model of speciation and solubility of sodium molybdate in water, 0–110 °C. J. Mol. Liq. 2022, 346, 117096. [Google Scholar] [CrossRef]
- Kordloo, M.; Khodadadmahmoudi, G.; Barati, M.R.; Rezaei, A. Effect of leaching media on selective precipitation of Al, Ni, Co, and Mn in the NMC battery recycling process. Sep. Purif. Technol. 2025, 363, 132093. [Google Scholar] [CrossRef]
- Wilson, A.M.; Bailey, P.J.; Tasker, P.A.; Turkington, J.R.; Grant, R.A.; Love, J.B. Solvent extraction: The coordination chemistry behind extractive metallurgy. Chem. Soc. Rev. 2014, 43, 123–134. [Google Scholar] [CrossRef]
- Sastre, A.; Alguacil, F. Co-extraction and selective stripping of copper (II) and molybdenum (VI) using LIX 622. Chem. Eng. J. 2001, 81, 109–112. [Google Scholar] [CrossRef]
| Component | Extraction (%) | Selectivity | ||||
|---|---|---|---|---|---|---|
| Mo | Ni | Co | βMo/Ni | βMo/Co | βCo/Ni | |
| TOA | ~0.0 | ~0.0 | ~0.0 | - | - | - |
| Cyanex 272 | 86.3 ± 4.3 | 12.1 ± 0.5 | 98.5 ± 4.1 | 45.65 | 0.01 | 475.73 |
| [TOA][Cy272] IL | 71.8 ± 3.2 | 49.8 ± 2.5 | 62.6 ± 3.3 | 2.58 | 1.53 | 1.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adavodi, R.; Zuffranieri, A.; Romano, P.; Rahmati, S.; Vegliò, F. Selective Recovery of Molybdenum over Nickel and Cobalt from Simulated Secondary Sources Using Bifunctional Ionic Liquid [TOA][Cy272]. Materials 2025, 18, 3826. https://doi.org/10.3390/ma18163826
Adavodi R, Zuffranieri A, Romano P, Rahmati S, Vegliò F. Selective Recovery of Molybdenum over Nickel and Cobalt from Simulated Secondary Sources Using Bifunctional Ionic Liquid [TOA][Cy272]. Materials. 2025; 18(16):3826. https://doi.org/10.3390/ma18163826
Chicago/Turabian StyleAdavodi, Roshanak, Adriana Zuffranieri, Pietro Romano, Soroush Rahmati, and Francesco Vegliò. 2025. "Selective Recovery of Molybdenum over Nickel and Cobalt from Simulated Secondary Sources Using Bifunctional Ionic Liquid [TOA][Cy272]" Materials 18, no. 16: 3826. https://doi.org/10.3390/ma18163826
APA StyleAdavodi, R., Zuffranieri, A., Romano, P., Rahmati, S., & Vegliò, F. (2025). Selective Recovery of Molybdenum over Nickel and Cobalt from Simulated Secondary Sources Using Bifunctional Ionic Liquid [TOA][Cy272]. Materials, 18(16), 3826. https://doi.org/10.3390/ma18163826









