Geopolymer Chemistry and Composition: A Comprehensive Review of Synthesis, Reaction Mechanisms, and Material Properties—Oriented with Sustainable Construction
Abstract
1. Introduction
1.1. Supplementary Cementitious Materials and the Rise of Geopolymers
1.2. Applications of Geopolymers: Terrestrial, Space, and Extraterrestrial
1.3. Material Enhancement and Technology Integration
1.4. Differentiating Geopolymer Systems
1.5. Material Variability and Testing Standards
1.6. Alignment with Key SDG’s
1.7. Sector Specific Contributions
1.8. Biomedical and Environmental Applications
1.9. The Way Forward: Adoption and Integration
2. Understanding Geopolymers: Origins and Chemistry
2.1. Historical Origins and Definitions
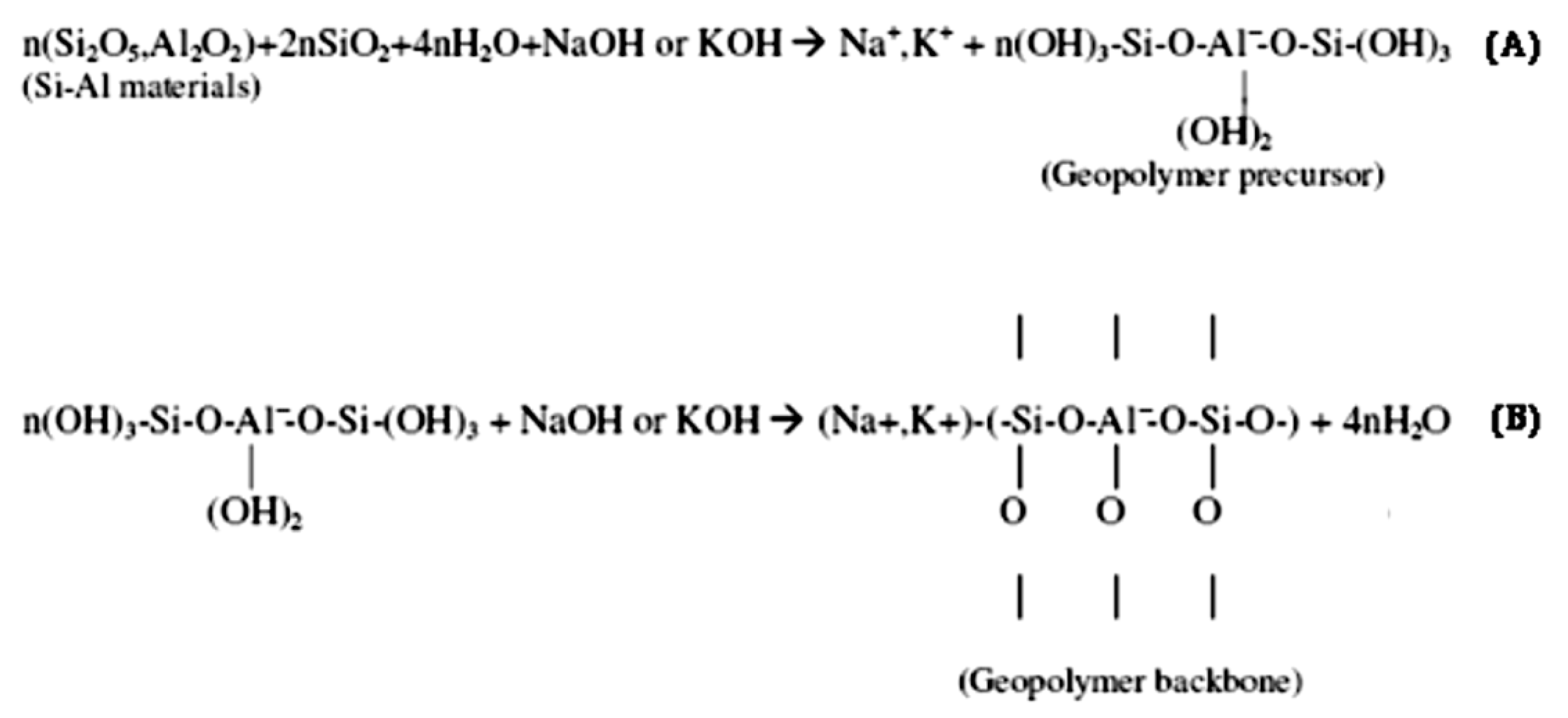
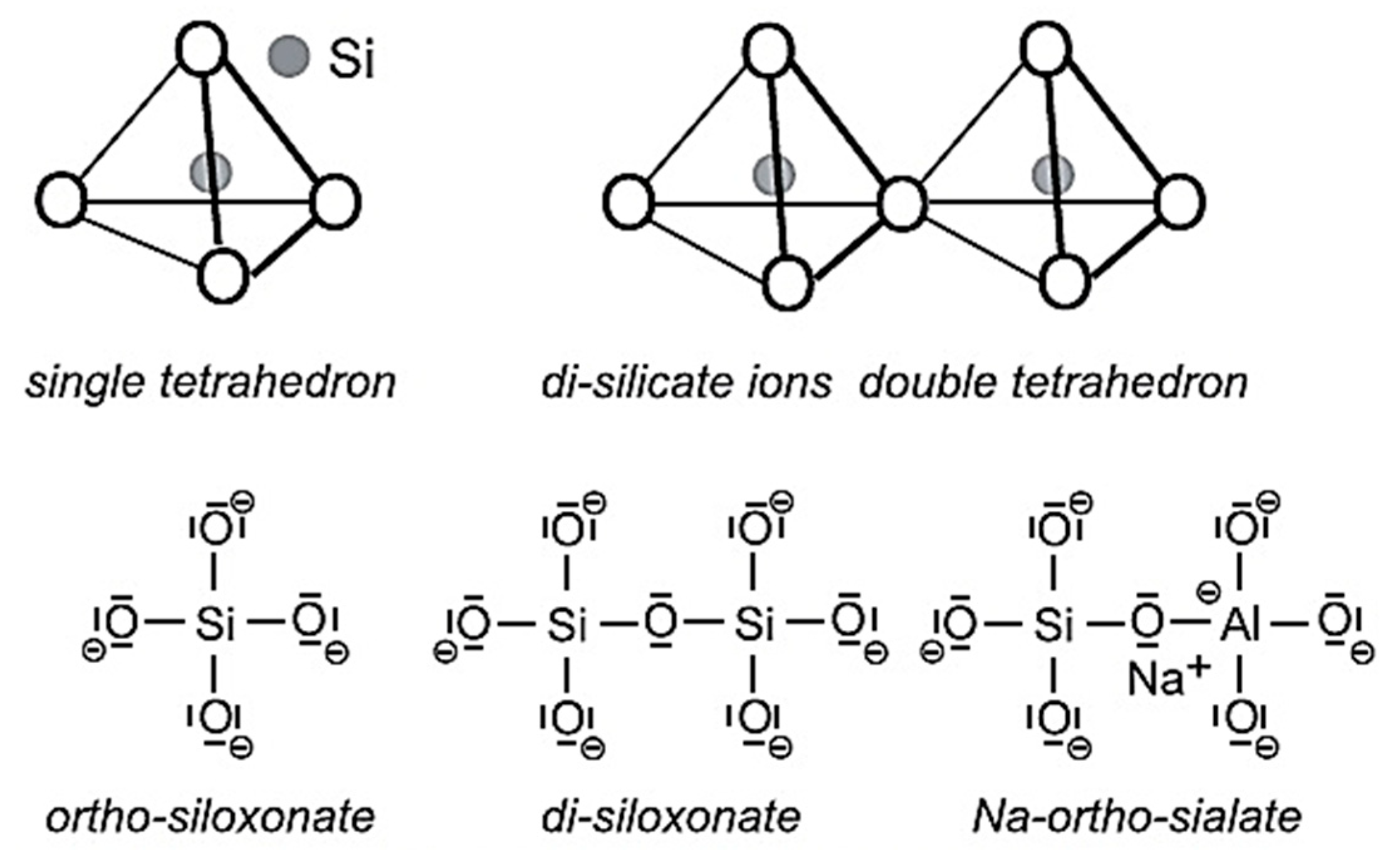
2.2. Molecular Structure and Polymerization Process
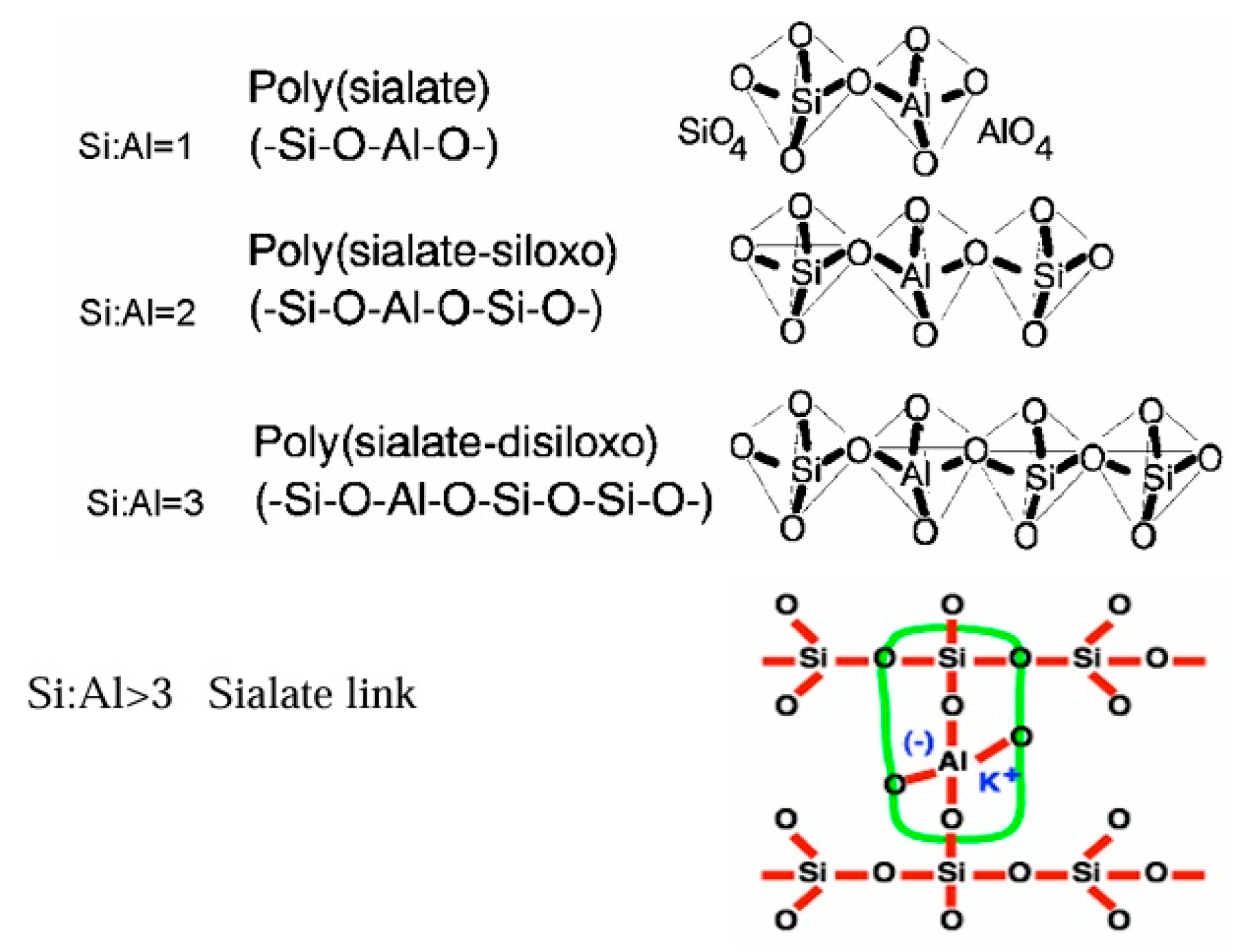
2.3. Types and Structural Variants of Geopolymers
2.3.1. Classification by Hardening Agents and Network Structure
2.3.2. Classification by Precursor Type
2.3.3. Functional Classification by Application
2.3.4. Hybrid and Composite Variants
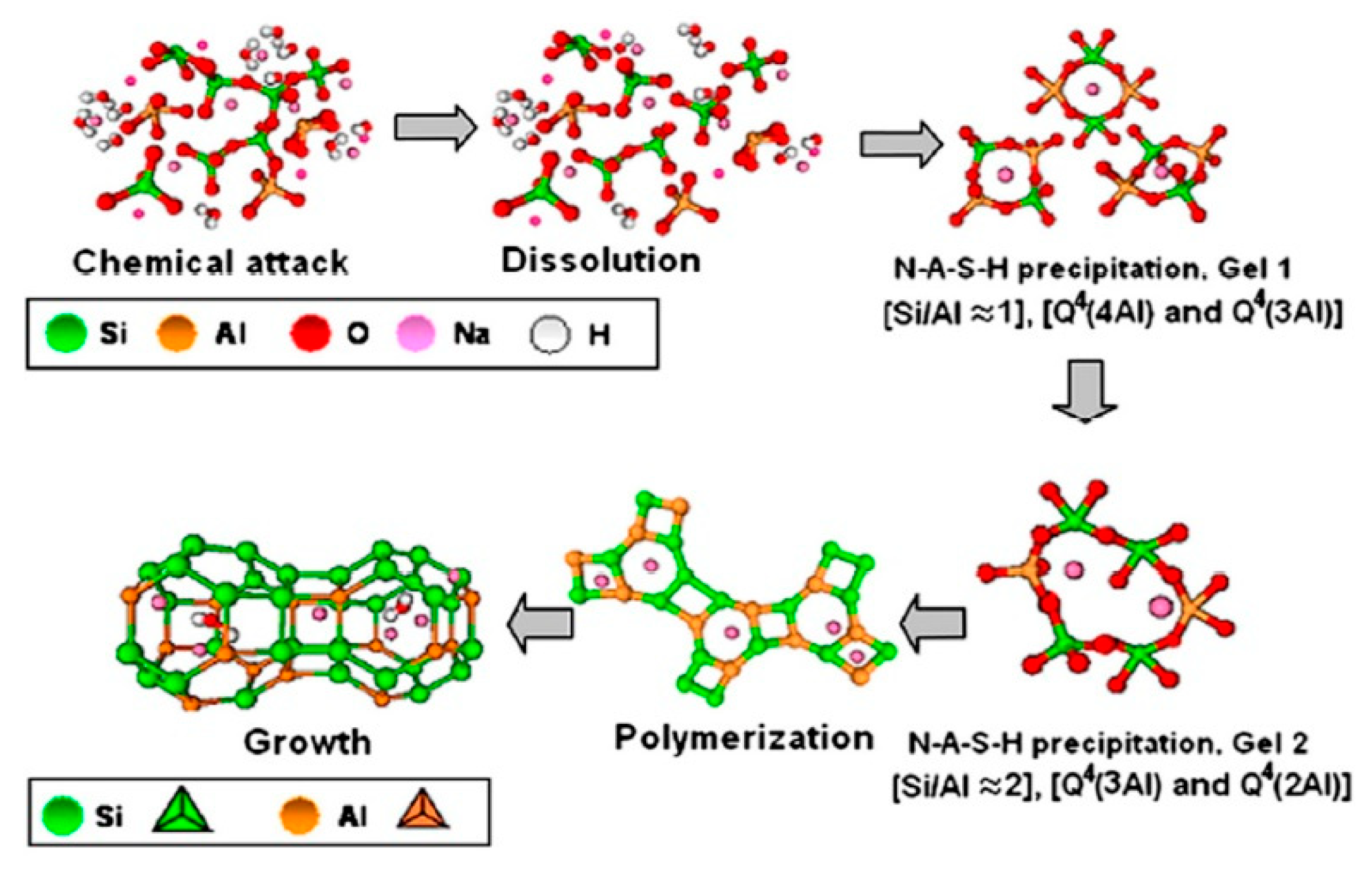
2.4. How Geopolymerization Works and Its Thermal Properties: Phase Chemistry and Gel Structures
- Gel Hardening and Network Growth—progressive cross-linking yields amorphous or semi-crystalline structures with high mechanical strength and durability [30,47]. Nano-silica addition modifies the hydration behavior of reactive aluminosilicates, improving gel densification and early-age strength [102].
2.5. A Sustainable Future with Geopolymers
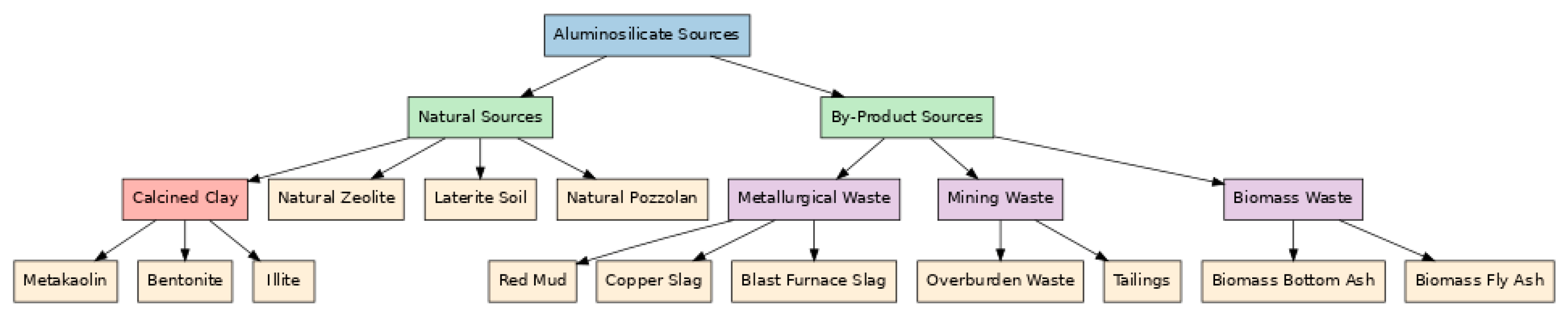
2.6. Aluminosilicate Materials: Classification and Functional Roles in Geopolymer Technology
2.7. Material Constituents of Geopolymeric Systems
2.7.1. Aluminosilicate
2.7.2. Fly Ash (FA)
2.7.3. Ground Granulated Blast Furnace Slag (GGBS)
2.7.4. Metakaolin (MK)
2.7.5. Red Mud (RM)
2.7.6. Waste Glass (WG)
2.7.7. Coal Gasification Fly Ash (CGFA)
2.7.8. Agricultural Ashes (Rice Husk Ash, Bagasse Ash, Bamboo Leaf Ash, Etc.)
2.7.9. Basalt Powder and Other Emerging Precursors
2.8. True Precursors vs. Auxiliary Components
- 1.
- Silica Content (SiO2) and Amorphous Potential
- 2.
- Alumina Content (Al2O3)
- 3.
- Calcium Oxide (CaO) and C–A–S–H Gel Potential
- 4.
- Iron Oxide (Fe2O3) and Chromophoric Effects
- 5.
- Alkali Content (Na2O + K2O)
- 6.
- Sulfur and Titanium Oxides
- 7.
- Emerging and Alternative Precursors
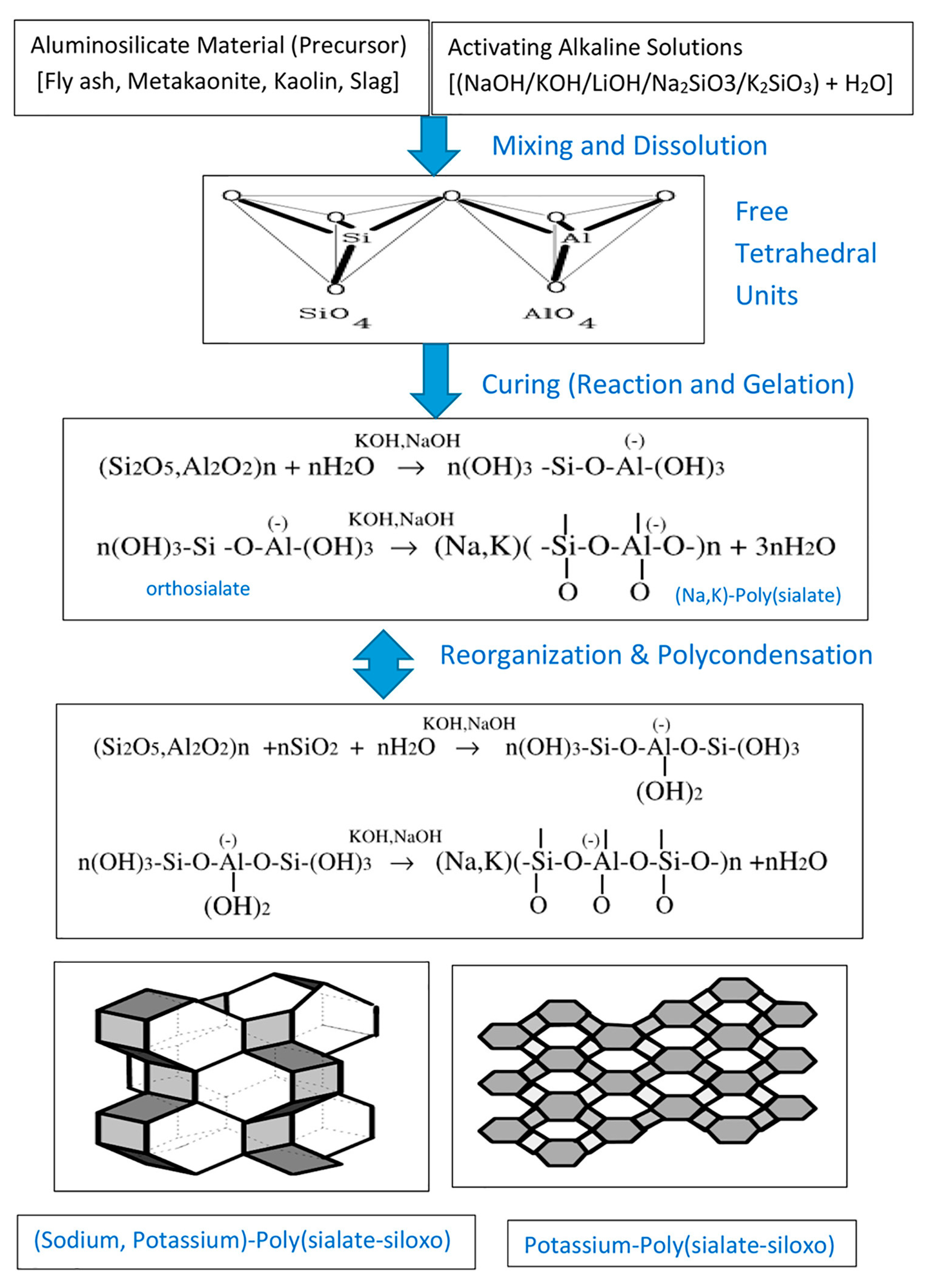
2.9. Chemical Pathways in Geopolymerization: The Role of Hardeners, Alkalination, and Activation
2.10. Optimizing Precursor Reactivity in Geopolymer Synthesis
3. Engineering Geopolymer Properties Through Molar Ratio Design
3.1. Optimizing Molar Ratios
- Viscosity and Workability: As detailed in Section 2.4, higher Si/Al ratios increase viscosity, improving moldability but reducing early workability; optimized ratios in fly ash–slag blends balance rheology and strength [57,89,138].
- Setting and Strength Development: Lower ratios accelerate setting and early strength, whereas higher ratios enhance long-term mechanical stability. Recent results indicate an optimal range of 3.4–3.8 for achieving balanced early and ultimate strength [57,137,139,140]. Zhang et al. [137] reported that alkali-activated geopolymer cement mortar optimized within this range showed superior trench backfilling performance due to its compact microstructure.
- SiO2/Fe2O3: Moderate Fe incorporation enhances nucleation and refines the gel matrix, whereas excess Fe disrupts network cohesion [83].
- Na/Fe and Al/Fe Ratios: Regulate porosity, phase stability, and thermal resistance; higher Al/Fe ratios are particularly advantageous in acidic or sulfate-rich environments [83].
- Strategic Molar Design: By interlinking these ratios, tailored binders can be engineered for chemically aggressive, thermally extreme, or structurally demanding applications. Furthermore, a nonlinear three-component creep model originally proposed for polymer-alloy geocell sheets [146] has been suggested as a transferable predictive framework for modeling time-dependent deformation in geopolymer composites.
3.2. Influence of Activator Chemistry
3.3. Effect of Curing Regimes
3.4. Reaction Kinetics and Gel Phase Evolution
3.5. Microstructural Development and Porosity Control
3.6. Long-Term Durability and Performance Prediction
- 1.
- Microstructure-Driven Service-Life Models
- 2.
- Life Cycle Assessment (LCA) Integration
- 3.
- Performance-Based Predictive Tools
- 4.
- Reliability in Harsh Environments
4. Structural Chemistry and Bonding in Geopolymer Networks
- A.
- Hydroxide ions (OH−) attack Si–O–Si and Al–O–Si bonds, releasing reactive monomeric species. The dissolution rate depends strongly on precursor fineness and activator molarity, as previously established (Section 3.4).
- B.
- Dissolved species align and condense into monomers, which polymerize into stable sialate and sialate-siloxo networks. Their connectivity ranging from simple poly(sialate) to highly cross-linked poly(sialate-disiloxo) directly governs microstructural densification and long-term durability (Section 3.5).
4.1. Classification and Chemistry of Acid-Based Geopolymers
- (1)
- (2)
- Polycondensation among PO43−, Al3+, and silicate components, resulting in the generation of alumino- and silico-phosphate gel networks, with AlPO4 crystals also forming as secondary phases [158];
- (3)
4.2. The Chemistry of Alkali Aluminosilicate (AAS) Geopolymers
5. Geopolymers for Construction and Environmental Applications: A Comparative Study of the Alkali Aluminosilicate (AAS) and Aluminosilicate Phosphate (ASP)
6. Performance and Application-Based Comparison of AAS and ASP Geopolymers
Phase Chemistry and the Structural Role of Gel Phases (N–A–S–H, K-A-S-H, C-S-H, and C–A–S–H) in Geopolymers
7. Conclusions
8. Future Considerations
- Carbon Fiber and Advanced Fiber Reinforcements—The incorporation of fiber reinforcements, particularly chromium-impregnated carbon fibers (CICFs) derived from tannery buffing dust, presents significant potential to enhance the tensile strength, flexural toughness, and crack resistance of alkali aluminosilicate (AAS) and aluminosilicate phosphate (ASP) geopolymers. Although not addressed in the current literature covered in this review, CICF represents a promising future direction due to its dual functionality: improving mechanical properties while immobilizing chromium and other toxic metals. This aligns with the broader sustainability and waste valorization strategies highlighted in Section 1, Section 2 and Section 5. Moreover, hybrid waste stream integration, involving the co-utilization of by-products and hazardous or organic waste sources from nature, industry, or agriculture, could further expand resource availability and improve binder performance.
- Underexplored Industrial and Secondary Raw Materials—Future studies should systematically investigate a broader range of precursors beyond the conventional fly ash and slag. Promising materials include waste glass (high amorphous silica content, enhancing N–A–S–H gel formation), coal gasification fly ash (CGFA) (rich in reactive aluminosilicates, though heavy metal variability requires careful assessment), iron-rich laterites and basalt powders (notable for their dense crystalline phases and excellent performance in ASP systems), and red mud (bauxite residue), which offers potential for hybrid alkali–acid activation. Additionally, phosphorus slag and rice husk ash (RHA) present sustainable options for incorporating calcium, phosphorus, and silica-rich phases, while waste ceramics and demolition waste powders provide low-cost, readily available aluminosilicate sources for large-scale applications. Integrating these diverse secondary materials aligns with circular economy principles and can significantly expand the performance envelope of both AAS and ASP systems.
- Hybrid Waste Stream Integration—Expanding on the principles of circular economy highlighted in Section 1 and Section 5, future work should explore hybrid systems that co-utilize diverse waste streams including agricultural residues, hazardous industrial by-products, and organic wastes. Controlled interaction between such waste streams and aluminosilicate matrices could yield multifunctional binders with improved chemical stability, reduced porosity, and enhanced performance under aggressive environments.
- Development of Multifunctional Geopolymer Matrices—Emerging applications increasingly demand engineered binder systems capable of fulfilling structural, chemical, and environmental functions. Future research should target ASP systems for biomedical and high-temperature applications and AAS systems for structural and environmental remediation contexts. Designing these matrices to perform waste immobilization, heavy metal stabilization, and chemical durability enhancement will require fine-tuned control of gel phases (N–A–S–H, K-A-S-H, and C–A–S–H) and network cross-linking mechanisms (Section 6). The application of thermodynamic modeling and phase assemblage predictions, as demonstrated by [40], could be instrumental in anticipating stable gel phase formation and optimizing precursor combinations for multifunctional matrices.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2θ | Diffraction Angle |
| AAMs | Alkali-Activated Materials |
| AAS | Alkali Aluminosilicate |
| AI | Artificial Intelligence |
| ANNs | Artificial Neural Networks |
| ANFIS | Adaptive Neuro-Fuzzy Inference System |
| ASP | Aluminosilicate Phosphate |
| ASR | Alkali–Silica Reaction |
| Al | Aluminum |
| BLA | Bamboo Leaf Ash |
| BP | Basalt Powder |
| CGFA | Coal Gasification Fly Ash |
| C–A–S–H | Calcium Aluminosilicate Hydrate |
| CH | Calcium Hydroxide |
| CICF | Chromium-Impregnated Carbon Fibers |
| CSH | Calcium Silicate Hydrate |
| CaO | Calcium Oxide |
| DNN | Deep Neural Network |
| DWTS | Desulfurization Waste from Titanium Slag |
| FA | Fly Ash |
| FeO | Ferrous Oxide |
| GEP | Gene Expression Programming |
| GGBFS | Ground Granulated Blast Furnace Slag |
| GPC | Geopolymer Concrete |
| GPS | Granulated Phosphorous Slag |
| IGCC | Integrated Gasification Combined Cycle |
| KASH | Potassium Aluminosilicate Hydrate |
| KOH | Potassium Hydroxide |
| LAC | Lateritic Aluminosilicate Clay |
| LAI | Lateritic Aluminosilicate (Iron-rich) |
| LCA | Life Cycle Assessment |
| MAP | Monoaluminum Phosphate |
| MK-750 | Metakaolin Calcined at 750 °C |
| ML | Machine Learning |
| NASH | Sodium Aluminosilicate Hydrate |
| NOx | Nitrogen Oxides |
| NaOH | Sodium Hydroxide |
| OH | Hydroxide Ion |
| OPC | Ordinary Portland Cement |
| ResNet | Residual Network |
| RF | Random Forest |
| RHA | Rice Husk Ash |
| RM | Red Mud |
| RSA | Rice Straw Ash |
| SBA | Sugarcane Bagasse Ash |
| SCMs | Supplementary Cementitious Materials |
| SDGs | Sustainable Development Goals |
| SEM | Scanning Electron Microscopy |
| Si | Silicon |
| Si/Al | Silicon-to-Aluminum Ratio |
| SVM | Support Vector Machine |
| WG | Waste Glass |
| XRD | X-ray Diffraction |
References
- Prospects, M. Global Construction Trends. 2022. Available online: https://www.market-prospects.com/articles/global-construction-industry-trends (accessed on 17 April 2025).
- Jwaida, Z.; Dulaimi, A.; Mashaan, N.; Othuman Mydin, M.A. Geopolymers: The Green Alternative to Traditional Materials for Engineering Applications. Infrastructures 2023, 8, 98. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Khaiyum, M.Z.; Sarker, S.; Kabir, G. Evaluation of Carbon Emission Factors in the Cement Industry: An Emerging Economy Context. Sustainability 2023, 15, 15407. [Google Scholar] [CrossRef]
- Berry, M.; Cross, D.; Stephens, J. Changing the Environment: An Alternative “Green” Concrete Produced without Portland Cement. In Proceedings of the 2009 World of Coal Ash (WOCA) Conference, Lexington, KY, USA, 4–7 May 2009. [Google Scholar]
- Davidovits, J. Geopolymers: Ceramic-like inorganic polymers. J. Ceram. Sci. Technol. 2017, 8, 335–350. [Google Scholar] [CrossRef]
- Kriven, W.M.; Leonelli, C.; Provis, J.L.; Boccaccini, A.R.; Attwell, C.; Ducman, V.S.; Ferone, C.; Rossignol, S.; Luukkonen, T.; Jannie Emiliano, J.V.; et al. Why geopolymers and alkali-activated materials are key components of a sustainable world: A perspective contribution. J. Am. Ceram. Soc. 2024, 107, 5159–5177. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. (Eds.) Geopolymers: Structure, Processing, Properties and Industrial Applications; Woodhead Publishing: Cambridge, UK, 2009. [Google Scholar] [CrossRef]
- Mushtaq, A.; Ali, S.; Chaudhry, A.H.; Sial, N.; Batool, H. Geopolymers as Supplementary Cementitious Materials to Reduce Carbon Dioxide Emissions. Nat. Environ. Pollut. Technol. 2025, 24 (Suppl. S1), 417–429. [Google Scholar] [CrossRef]
- Adewuyi, Y.G. Recent Advances in Fly-Ash-Based Geopolymers: Potential on the Utilization for Sustainable Environmental Remediation. ACS Omega 2021, 6, 15532–15542. [Google Scholar] [CrossRef]
- Burciaga-Díaz, O.; MDurón-Sifuentes Díaz-Guillén, J.C.; Escalante-Garcia, J.I. Effect of waste glass incorporation on the properties of geopolymers formulated with low purity metakaolin. Cem. Concr. Compos. 2020, 107, 103492. [Google Scholar] [CrossRef]
- Saini, R.; Santosh Deb Barma Rao, D.S.; Basu, S.; Mahajani, S.M. Applied mineralogical investigation on coal gasification ash. Waste Manag. 2023, 167, 1–12. [Google Scholar] [CrossRef]
- Luhar, S.; Nicolaides, D.; Luhar, I. Fire Resistance Behaviour of Geopolymer Concrete: An Overview. Buildings 2021, 11, 82. [Google Scholar] [CrossRef]
- Rihan, M.A.M.; Onchiri, R.O.; Gathimba, N.; Sabuni, B. Assessing the durability performance of geopolymer concrete utilizing fly ash and sugarcane bagasse ash as sustainable binders. Open Ceram. 2024, 20, 100687. [Google Scholar] [CrossRef]
- Amran, M.; Al-Fakih, A.; Chu, S.H.; Fediuk, R.; Haruna, S.; Azevedo, A.; Vatin, N. Long-term durability properties of geopolymer concrete: An in-depth review. Case Stud. Constr. Mater. 2021, 15, e00661. [Google Scholar] [CrossRef]
- Reuters. Comment: Why a Circular Built Environment Makes Economic and Environmental Sense. Available online: https://www.reuters.com/sustainability/climate-energy/comment-why-circular-built-environment-makes-economic-environmental-sense-2024-02-23/ (accessed on 23 January 2025).
- UN Environment Programme. Green and Sustainable Chemistry: Framework Manual; UNEP—UN Environment Programme: Nairobi, Kenya, 2022; Available online: https://www.unep.org/resources/toolkits-manuals-and-guides/green-and-sustainable-chemistry-framework-manual (accessed on 10 February 2025).
- Phillip, E.; Choo, T.F.; Khairuddin, N.W.A.; Abdel Rahman, R.O. On the Sustainable Utilization of Geopolymers for Safe Management of Radioactive Waste: A Review. Sustainability 2023, 15, 1117. [Google Scholar] [CrossRef]
- Ramesh, V.; Muthramu, B.; Rebekhal, D. A review of sustainability assessment of geopolymer concrete through AI-based life cycle analysis. AI Civ. Eng. 2025, 4, 3. [Google Scholar] [CrossRef]
- Neupane, K. Evaluation of environmental sustainability of one-part geopolymer binder concrete. Clean. Mater. 2022, 6, 100138. [Google Scholar] [CrossRef]
- Zhu, L. Study on the Performance and Carbon Emission of Geopolymers Concrete. Trans. Eng. Technol. Res. 2024, 4, 231–238. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- Snellings, R.; Mertens, G.; Elsen, J. Supplementary Cementitious Materials. Rev. Miner. Geochem. 2012, 74, 211–278. [Google Scholar] [CrossRef]
- Tulashie, S.K.; Ebo, P.; Ansah, J.K.; Mensah, D. Production of Portland pozzolana cement from rice husk ash. Materialia 2021, 16, 101048. [Google Scholar] [CrossRef]
- Assi, L.N.; Carter, K.; Deaver, E.; Ziehl, P. Review of availability of source materials for geopolymer/sustainable concrete. J. Clean. Prod. 2020, 263, 121477. [Google Scholar] [CrossRef]
- Dai, T.; Fang, C.; Liu, T.; Zheng, S.; Lei, G.; Jiang, G. Waste glass powder as a high temperature stabilizer in blended oil well cement pastes: Hydration, microstructure and mechanical properties. Constr. Build. Mater. 2024, 439, 137359. [Google Scholar] [CrossRef]
- Singh, A.; Bhadauria, S.S.; Thakare, A.A.; Kumar, A.; Mudgal, M.; Chaudhary, S. Durability assessment of mechanochemically activated geopolymer concrete with a low molarity alkali solution. Case Stud. Constr. Mater. 2024, 20, e02715. [Google Scholar] [CrossRef]
- Nukah, P.D.; Abbey, S.J.; Booth, C.A.; Oti, J. Development of low carbon concrete and prospective of geopolymer concrete using lightweight coarse aggregate and cement replacement materials. Constr. Build. Mater. 2024, 428, 136295. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2006, 42, 2917–2933. [Google Scholar] [CrossRef]
- Nawaz, M.; Heitor, A.; Sivakumar, M. Geopolymers in construction—Recent developments. Constr. Build. Mater. 2020, 260, 120472. [Google Scholar] [CrossRef]
- Lee, S.; van Riessen, A. A Review on Geopolymer Technology for Lunar Base Construction. Materials 2022, 15, 4516. [Google Scholar] [CrossRef]
- Wang, K.-T.; Tang, Q.; Cui, X.-M.; He, Y.; Liu, L.-P. Development of near-zero water consumption cement materials via the geopolymerization of tektites and its implication for lunar construction. Sci. Rep. 2016, 6, 29659. [Google Scholar] [CrossRef]
- Nasa.gov. NASA TecPort. Available online: https://techport.nasa.gov/projects/13755 (accessed on 13 February 2025).
- Chen, Y.; Sha, A.; Jiang, W.; Lu, Q.; Du, P.; Hu, K.; Li, C. Eco-friendly bismuth vanadate/iron oxide yellow composite heat-reflective coating for sustainable pavement: Urban heat island mitigation. Constr. Build. Mater. 2025, 470, 140645. [Google Scholar] [CrossRef]
- Almutairi, A.L.; Tayeh, B.A.; Adesina, A.; Isleem, H.F.; Zeyad, A.M. Potential applications of geopolymer concrete in construction: A review. Case Stud. Constr. Mater. 2021, 15, e00733. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Maged, A.; Eloffy, M.G.; Zahran, M.; Kharbish, S.; Elwakeel, K.Z.; Bhatnagar, A. Geopolymers as sustainable eco-friendly materials: Classification, synthesis routes, and applications in wastewater treatment. Sep. Purif. Technol. 2023, 324, 124631. [Google Scholar] [CrossRef]
- Richardson, I.G. The calcium silicate hydrates. Cem. Concr. Res. 2008, 38, 137–158. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D.E. Compatibility studies between N–A–S–H and C–A–S–H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Xiao, R.; Jiang, X.; Zhang, M.; Polaczyk, P.; Huang, B. Analytical investigation of phase assemblages of alkali-activated materials in CaO-SiO2-Al2O3 systems: The management of reaction products and designing of precursors. Mater. Des. 2020, 194, 108975. [Google Scholar] [CrossRef]
- Rathnayaka, M.; Karunasinghe, D.; Gunasekara, C.; Wijesundara, K.; Lokuge, W.; Law, D.W. Machine learning approaches to predict compressive strength of fly ash-based geopolymer concrete: A comprehensive review. Constr. Build. Mater. 2024, 419, 135519. [Google Scholar] [CrossRef]
- Ünal, M.T.; Gökçe, H.S.; Ayough, P.; Alnahhal, A.M.; Şimşek, O.; Nehdi, M.L. Nanomaterial and fiber-reinforced sustainable geopolymers: A systematic critical review. Constr. Build. Mater. 2023, 404, 133325. [Google Scholar] [CrossRef]
- Moujoud, Z.; Sair, S.; Ousaleh, H.A.; Ayouch, I.; El Bouari, A.; Tanane, O. Geopolymer composites reinforced with natural Fibers: A review of recent advances in processing and properties. Constr. Build. Mater. 2023, 388, 131666. [Google Scholar] [CrossRef]
- Pilehvar, S.; Baltazar, L.G. Advances in Geopolymer Composites: From Synthesis to Sustainable Applications. Crystals 2024, 14, 738. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, Z.; Gedela, R.; Tang, C.; Feng, Y.; Liu, J.; Yao, H. Performance and geocell-soil interaction of sand subgrade reinforced with high-density polyethylene, polyester, and polymer-blend geocells: 3D numerical studies. Comput. Geotech. 2025, 178, 106949. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Kaze, R.C.; Naghizadeh, A.; Tchadjie, L.; Adesina, A.; Djobo, J.N.Y.; Nemaleu, J.G.D.; Kamseu, E.; Melo, U.C.; Tayeh, B.A. Lateritic soils based geopolymer materials: A review. Constr. Build. Mater. 2022, 344, 128157. [Google Scholar] [CrossRef]
- He, J.; Zhang, J.; Yu, Y.; Zhang, G. The strength and microstructure of two geopolymers derived from metakaolin and red mud-fly ash admixture: A comparative study. Constr. Build. Mater. 2012, 30, 80–91. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Li, Y.; Ren, Y.; Wang, Y.; Zhang, W. Effects of sulfate on the mechanical performances and hydration characteristics of red mud based non-burnt brick. Constr. Build. Mater. 2020, 262, 120722. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Alrefaei, Y.; Dai, J.-G. Silico-Aluminophosphate and Alkali-Aluminosilicate Geopolymers: A Comparative Review. Front. Mater. 2019, 6, 106. [Google Scholar] [CrossRef]
- Arora, S.; Jangra, P.; Lim, Y.Y.; Pham, T.M. Strength, durability, and microstructure of self-compacting geopolymer concrete produced with copper slag aggregates. Environ. Sci. Pollut. Res. 2022, 30, 666–684. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, H.; Mungule, M.; KRIyer, K. Issues and challenges for development of geopolymer concrete. Mater. Today Proc. 2022, 65, 1567–1574. [Google Scholar] [CrossRef]
- Martínez, A.; Miller, S.A. A review of drivers for implementing geopolymers in construction: Codes and constructability. Resour. Conserv. Recycl. 2023, 199, 107238. [Google Scholar] [CrossRef]
- Hamsashree; Pandit, P.; Prashanth, S.; Katpady, D.N. Durability of alkali-activated fly ash-slag concrete- state of art. Innovative Infrastruct. Solut. 2024, 9, 222. [Google Scholar] [CrossRef]
- Madirisha, M.M.; Dada, O.R.; Ikotun, B.D. Chemical Fundamentals of Geopolymers in Sustainable Construction. Mater. Today Sustain. 2024, 27, 100842. [Google Scholar] [CrossRef]
- Martínez, A.; Miller, S.A. Life cycle assessment and production cost of geopolymer concrete: A meta-analysis. Resour. Conserv. Recycl. 2024, 215, 108018. [Google Scholar] [CrossRef]
- Houhou, M.; Leklou, N.; Ranaivomanana, H.; Penot, J.D.; de Barros, S. Geopolymers in nuclear waste storage and immobilization: Mechanisms, applications, and challenges. Discov. Appl. Sci. 2025, 7, 126. [Google Scholar] [CrossRef]
- Mikulčić, H.; Cabezas, H.; Vujanović, M.; Duić, N. Environmental assessment of different cement manufacturing processes based on Emergy and Ecological Footprint analysis. J. Clean. Prod. 2016, 130, 213–221. [Google Scholar] [CrossRef]
- Esparham, A. A review of the features of geopolymer cementitious composites for use in green construction and sustainable urban development. Cent. Asian J. Environ. Sci. Technol. Innov. 2022, 3, 64–74. [Google Scholar] [CrossRef]
- Horan, C.; Genedy, M.; Juenger, M.; van Oort, E. Fly Ash-Based Geopolymers as Lower Carbon Footprint Alternatives to Portland Cement for Well Cementing Applications. Energies 2022, 15, 8819. [Google Scholar] [CrossRef]
- Kudłacik-Kramarczyk, S.; Drabczyk, A.; Figiela, B.; Korniejenko, K. Geopolymers: Advanced Materials in Medicine, Energy, Anticorrosion and Environmental Protection. Materials 2023, 16, 7416. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Dai, F.; Chen, H.; He, Y.; Wang, Z.; Wang, R. Fabrication of stalk fiber/geopolymers-based slow-release fertilizer with agricultural waste and loess for promoting plant growth. J. Environ. Chem. Eng. 2023, 11, 109481. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Z.; Wen, Y.; Song, X.; Tan, W.K.; Ong, C.N.; Li, J. Recent Advances in Superabsorbent Hydrogels Derived from Agro Waste Materials for Sustainable Agriculture: A Review. J. Agric. Food Chem. 2024, 72, 22399–22419. [Google Scholar] [CrossRef] [PubMed]
- Fahimzadeh, M.; Sukiato, F.; Chew, K.L.; Amri, A.Y.; Pasbakhsh, P.; Tan, J.B.L.; Singh, R.; Yuan, P. Sustainable Artificial Coral Reef Restoration Using Nanoclays and Composite Hydrogel Microcapsules. RSC Sustain. 2024, 2, 616–620. [Google Scholar] [CrossRef]
- Sha, F.; Dong, Y.; Gu, S.; Fan, X.; Xiao, W. Study on novel alkali-activated cementitious grout for scour control of offshore foundation. Geomech. Energy Environ. 2025, 42, 100663. [Google Scholar] [CrossRef]
- Ikotun, J.O.; Aderinto, G.E.; Madirisha, M.M.; Katte, V.Y. Geopolymer Cement in Pavement Applications: Bridging Sustainability and Performance. Sustainability 2024, 16, 5417. [Google Scholar] [CrossRef]
- Luetkenhorst, W.; Altenburg, T.; Pegels, A.; Vidican, G. Green Industrial Policy: Managing Transformation Under Uncertainty; German Development Institute/Deutsches Institut für Entwicklungspolitik: Bonn, Germany, 2014. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Joseph, D. Geopolymers: Inorganic Polymeric New Materials; Geopolymer Institute: Saint-Quentin, France, 2006; Available online: https://www.geopolymer.org/library/technical-papers/12-geopolymers-inorganic-polymeric-new-materials/ (accessed on 20 February 2025).
- Salas, D.A.; Ramirez, A.D.; Ulloa, N.; Baykara, H.; Boero, A.J. Life cycle assessment of geopolymer concrete. Constr. Build. Mater. 2018, 190, 170–177. [Google Scholar] [CrossRef]
- Ji, X.; Huang, X.; Zhong, S.; Zhou, J. Advancing toward a low-carbon infrastructure: Emission reduction potential of geopolymer road maintenance. Mater. Today Sustain. 2025, 30, 101121. [Google Scholar] [CrossRef]
- Celerier, H.; Jouin, J.; Gharzouni, A.; Mathivet, V.; Sobrados, I.; Tessier-Doyen, N.; Rossignol, S. Relation between working properties and structural properties from 27Al, 29Si and 31P NMR and XRD of acid-based geopolymers from 25 to 1000 °C. Mater. Chem. Phys. 2019, 228, 293–302. [Google Scholar] [CrossRef]
- Zribi, M.; Baklouti, S. Investigation of Phosphate based geopolymers formation mechanism. J. Non-Cryst. Solids 2021, 562, 120777. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, Z.; Liu, X. Comprehensive Understanding of Aluminosilicate Phosphate Geopolymers: A Critical Review. Materials 2022, 15, 5961. [Google Scholar] [CrossRef]
- Garg, N.; Özçelik, V.O.; Skibsted, J.; White, C.E. Nanoscale Ordering and Depolymerization of Calcium Silicate Hydrates in the Presence of Alkalis. J. Phys. Chem. C 2019, 123, 24873–24883. [Google Scholar] [CrossRef]
- Xu, X.; Bao, S.; Zhang, Y.; Luo, Y.; Qin, L.; Li, S. Role of particle fineness and reactive silicon-aluminum ratio in mechanical properties and microstructure of geopolymers. Constr. Build. Mater. 2021, 313, 125483. [Google Scholar] [CrossRef]
- Liu, J.; Doh, J.-H.; Dinh, H.L.; Ong, D.E.L.; Zi, G.; You, I. Effect of Si/Al molar ratio on the strength behavior of geopolymer derived from various industrial waste: A current state of the art review. Constr. Build. Mater. 2022, 329, 127134. [Google Scholar] [CrossRef]
- Sellami, M.; Barre, M.; Toumi, M. Synthesis, thermal properties and electrical conductivity of phosphoric acid-based geopolymer with metakaolin. Appl. Clay Sci. 2019, 180, 105192. [Google Scholar] [CrossRef]
- Goryunova, K.; Gahramanli, Y.; Muradkhanli, V.; Nadirov, P. Phosphate-activated geopolymers: Advantages and application. RSC Adv. 2023, 13, 30329–30345. [Google Scholar] [CrossRef] [PubMed]
- Davidovits, J.; Davidovits, R. Ferro-sialate geopolymers. In Technical Papers # 27; Geopolymer Institute: Saint-Quentin, France, 2020; Available online: www.geopolymer.org (accessed on 15 February 2025).
- Kaze, R.C.; Moungam, L.M.B.à.; Cannio, M.; Rosa, R.; Kamseu, E.; Melo, U.C.; Leonelli, C. Microstructure and engineering properties of Fe2O3(FeO)-Al2O3-SiO2 based geopolymer composites. J. Clean. Prod. 2018, 199, 849–859. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A. An overview of the chemistry of alkali-activated cement-based binders. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Woodhead Publishing: Cambridge, UK, 2015; pp. 19–47. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, Z.; Tian, X.; Wu, J.; Wang, L. Review on the impact of metakaolin-based geopolymer’s reaction chemistry, nanostructure and factors on its properties. Constr. Build. Mater. 2024, 412, 134760. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers Based on Natural and Synthetic Metakaolin a Critical Review. In Proceedings of the 41st International Conference on Advanced Ceramics and Composites: Ceramic Engineering and Science Proceedings, Daytona Beach, FL, USA, 22–27 January 2017; Wiley-American Ceramic Society: Westerville, OH, USA, 2018; pp. 201–214. [Google Scholar] [CrossRef]
- Bai, C.; Zheng, K.; Sun, F.; Wang, X.; Zhang, L.; Zheng, T.; Colombo, P.; Wang, B. A review on metakaolin-based porous geopolymers. Appl. Clay Sci. 2024, 258, 107490. [Google Scholar] [CrossRef]
- Long, Q.; Zhao, Y.; Zhang, B.; Yang, H.; Luo, Z.; Li, Z.; Zhang, G.; Liu, K. Interfacial Behavior of Slag, Fly Ash, and Red Mud-Based Geopolymer Mortar with Concrete Substrate: Mechanical Properties and Microstructure. Buildings 2024, 14, 652. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, Z.; Wang, J.; Guo, J.; Hu, S.; Ling, Y. Properties of fresh and hardened fly ash/slag based geopolymer concrete: A review. J. Clean. Prod. 2020, 270, 122389. [Google Scholar] [CrossRef]
- Kolawole, J.T.; Olusola, K.O.; Babafemi, A.J.; Olalusi, O.B.; Fanijo, E. Blended cement binders containing bamboo leaf ash and ground clay brick waste for sustainable concrete. Materialia 2021, 15, 101045. [Google Scholar] [CrossRef]
- Morsy, M.I.; Alakeel, K.A.; Ahmed, A.E.; Abbas, A.M.; Omara, A.I.; Abdelsalam, N.R.; Emaish, H.H. Recycling rice straw ash to produce low thermal conductivity and moisture-resistant geopolymer adobe bricks. Saudi J. Biol. Sci. 2022, 29, 3759–3771. [Google Scholar] [CrossRef]
- Zribi, M.; Baklouti, S. Phosphate-based geopolymers: A critical review. Polym. Bull. 2021, 79, 6827–6855. [Google Scholar] [CrossRef]
- Al-Alwan, A.A.K.; Al-Bazoon, M.; IMussa, F.; Alalwan, H.A.; Shadhar, M.H.; Mohammed, M.M.; Mohammed, M.F. The Impact of Using Rice Husk Ash as a Replacement Material in concrete: An Experimental Study. J. King Saud Univ.-Eng. Sci. 2022, 36, 249–255. [Google Scholar] [CrossRef]
- Adeleke, B.O.; Kinuthia, J.M.; Oti, J.; Pirrie, D.; Power, M. Mechanical and Microstructural Investigation of Geopolymer Concrete Incorporating Recycled Waste Plastic Aggregate. Materials 2024, 17, 1340. [Google Scholar] [CrossRef]
- Lao, J.-C.; Xu, L.-Y.; Huang, B.-T.; Dai, J.-G.; Shah, S.P. Strain-hardening Ultra-High-Performance Geopolymer Concrete (UHPGC): Matrix design and effect of steel fibers. Compos. Commun. 2022, 30, 101081. [Google Scholar] [CrossRef]
- Oti, J.; Adeleke, B.O.; Casabuena, L.R.; Kinuthia, J.M.; Sule, S. Utilization of a PFA-GGBS-Based Precursor in Geopolymer Concrete Production as a Sustainable Substitute for Conventional Concrete. Materials 2025, 18, 1309. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.; Zhang, W.; Ye, J.; Wang, R. Solidification performance and mechanism of typical radioactive nuclear waste by geopolymers and geopolymer ceramics: A review. Prog. Nucl. Energy 2024, 169, 105106. [Google Scholar] [CrossRef]
- Ricciotti, L.; Apicella, A.; Perrotta, V.; Aversa, R. Geopolymer Materials for Bone Tissue Applications: Recent Advances and Future Perspectives. Polymers 2023, 15, 1087. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, A.; Al Rashid, A.; Koç, M. 3D printing of alkali-activated geopolymers for sustainable and circular economy advancements. Circ. Econ. 2024, 3, 100101. [Google Scholar] [CrossRef]
- Kondepudi, K.; Subramaniam, K.V.L. Formulation of alkali-activated fly ash-slag binders for 3D concrete printing. Cem. Concr. Compos. 2021, 119, 103983. [Google Scholar] [CrossRef]
- Jin, Q.; Zhang, P.; Wu, J.; Sha, D. Mechanical Properties of Nano-SiO2 Reinforced Geopolymer Concrete under the Coupling Effect of a Wet–Thermal and Chloride Salt Environment. Polymers 2022, 14, 2298. [Google Scholar] [CrossRef]
- Zheng, D.; Monasterio, M.; Feng, W.; Tang, W.; Cui, H.; Dong, Z. Hydration Characteristics of Tricalcium Aluminate in the Presence of Nano-Silica. Nanomaterials 2021, 11, 199. [Google Scholar] [CrossRef]
- Dai, J.; Wang, Q.; Xie, C.; Xue, Y.; Duan, Y.; Cui, X. The Effect of Fineness on the Hydration Activity Index of Ground Granulated Blast Furnace Slag. Materials 2019, 12, 2984. [Google Scholar] [CrossRef]
- Oti, J.; Adeleke, B.O.; Mudiyanselage, P.R.; Kinuthia, J. A Comprehensive Performance Evaluation of GGBS-Based Geopolymer Concrete Activated by a Rice Husk Ash-Synthesised Sodium Silicate Solution and Sodium Hydroxide. Recycling 2024, 9, 23. [Google Scholar] [CrossRef]
- Tahwia, A.M.; Aldulaimi, D.S.; Abdellatief, M.; Youssf, O. Physical, Mechanical and Durability Properties of Eco-Friendly Engineered Geopolymer Composites. Infrastructures 2024, 9, 191. [Google Scholar] [CrossRef]
- Pereira, M.A.; Vasconcelos, D.C.L.; Vasconcelos, W.L. Synthetic Aluminosilicates for Geopolymer Production. Mater. Res. 2019, 22, e20180508. [Google Scholar] [CrossRef]
- Farooq, F.; Jin, X.; Faisal Javed, M.; Akbar, A.; Izhar Shah, M.; Aslam, F.; Alyousef, R. Geopolymer concrete as sustainable material: A state of the art review. Constr. Build. Mater. 2021, 306, 124762. [Google Scholar] [CrossRef]
- van Riessen, A.; Jamieson, E.; Gildenhuys, H.; Skane, R.; Allery, J. Using XRD to Assess the Strength of Fly-Ash- and Metakaolin-Based Geopolymers. Materials 2025, 18, 2093. [Google Scholar] [CrossRef]
- Adeleke, B.; Kinuthia, J.; Oti, J.; Ebailila, M. Physico-mechanical evaluation of geopolymer concrete activated by sodium hydroxide and silica fume-synthesised sodium silicate solution. Materials 2023, 16, 2400. [Google Scholar] [CrossRef]
- Christiansen, M.U. An Investigation of Waste Glass-Based Geopolymers Supplemented with Alumina. Ph.D. Dissertation, Michigan Technological University, Houghton, MI, USA, 2013. [Google Scholar] [CrossRef]
- Paiva, H.; Velosa, A.; Cachim, P.; Ferreira, V.M. Effect of pozzolans with different physical and chemical characteristics on concrete properties. Mater. Construcción 2016, 66, e083. [Google Scholar] [CrossRef]
- Mrozek, D.; Mrozek, M.; Krzywoń, R. Mechanical properties of geopolymers synthesized using basalt powder as a partial substitute for metakaolin. Sci. Rep. 2025, 15, 13156. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Zhuge, Y.; Chow, C.W.; Keegan, A.; Liu, Y.; Siddique, R. Mechanical performance and phase analysis of an eco-friendly alkali-activated binder made with sludge waste and blast-furnace slag. J. Clean. Prod. 2022, 374, 134024. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; van Deventer, J.S.J.; Lukey, G.C. The effect of composition and temperature on the properties of fly ash- and kaolinite-based geopolymers. Chem. Eng. J. 2002, 89, 63–73. [Google Scholar] [CrossRef]
- Safari, Z.; Kurda, R.; Al-Hadad, B.; Mahmood, F.; Tapan, M. Mechanical characteristics of pumice-based geopolymer paste. Resour. Conserv. Recycl. 2020, 162, 105055. [Google Scholar] [CrossRef]
- Yadav, K.P.; Samanta, A.K. Characterization and development of alccofine based sustainable concrete—A review. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Falayi, T. A comparison between fly ash- and basic oxygen furnace slag-modified gold mine tailings geopolymers. Int. J. Energy Environ. Eng. 2019, 11, 207–217. [Google Scholar] [CrossRef]
- Hu, M.; Zhu, X.; Long, F. Alkali-activated fly ash-based geopolymers with zeolite or bentonite as additives. Cem. Concr. Compos. 2009, 31, 762–768. [Google Scholar] [CrossRef]
- Yong, C.L.; Mo, K.H.; Koting, S. Phosphorus slag in supplementary cementitious and alkali activated materials: A review on activation methods. Constr. Build. Mater. 2022, 352, 129028. [Google Scholar] [CrossRef]
- Dulaimi, A.; Shanbara, H.K.; Al-Rifaie, A. The mechanical evaluation of cold asphalt emulsion mixtures using a new cementitious material comprising ground-granulated blast-furnace slag and a calcium carbide residue. Constr. Build. Mater. 2020, 250, 118808. [Google Scholar] [CrossRef]
- Matsimbe, J.; Dinka, M.; Olukanni, D.; Musonda, I. Geopolymer: A Systematic Review of Methodologies. Materials 2022, 15, 6852. [Google Scholar] [CrossRef]
- Van Deventer, J.S.J. Technical and commercial progress in the adoption of geopolymer cement. Miner. Eng. 2012, 29, 89–104. [Google Scholar] [CrossRef]
- Castillo, H.; Collado, H.; Droguett, T.; Sánchez, S.; Vesely, M.; Garrido, P.; Palma, S. Factors Affecting the Compressive Strength of Geopolymers: A Review. Minerals 2021, 11, 1317. [Google Scholar] [CrossRef]
- Oti, J.; Adeleke, B.O.; Anowie, F.X.; Kinuthia, J.M.; Ekwulo, E. Mechanical Properties of a Sustainable Low-Carbon Geopolymer Concrete Using a Pumice-Derived Sodium Silicate Solution. Materials 2024, 17, 1792. [Google Scholar] [CrossRef]
- Soriano, L.; Font, A.; Borrachero, M.V.; Monzó, J.M.; Payá, J.; Tashima, M.M. Biomass ashes to produce an alternative alkaline activator for alkali-activated cements. Mater. Lett. 2022, 308, 131198. [Google Scholar] [CrossRef]
- Silvestro, L.; Scolaro, T.P.; Ruviaro, A.S.; Lima, G.T.d.S.; Gleize, P.J.P.; Pelisser, F. Use of biomass wood ash to produce sustainable geopolymeric pastes. Constr. Build. Mater. 2023, 370, 130641. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Rose, V.; de Gutierrez, R.M. Evolution of binder structure in sodium silicate-activated slag-metakaolin blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Derouiche, R.; Baklouti, S. Phosphoric acid based geopolymerization: Effect of the mechanochemical and the thermal activation of the kaolin. Ceram. Int. 2021, 47, 13446–13456. [Google Scholar] [CrossRef]
- Segura, I.P.; Ranjbar, N.; Damø, A.J.; Jensen, L.S.; Moreira, M.; Jensen, P.A. A review: Alkali-activated cement and concrete production technologies available in the industry. Heliyon 2023, 9, e15718. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Geopolymer foam concrete: An emerging material for sustainable construction. Constr. Build. Mater. 2014, 56, 113–127. [Google Scholar] [CrossRef]
- Wei, M.; Chen, L.; Lei, N.; Li, H.; Huang, L. Mechanical properties and microstructures of thermally activated ultrafine recycled fine powder cementitious materials. Constr. Build. Mater. 2025, 475, 141195. [Google Scholar] [CrossRef]
- De Gutiérrez, R.M.; Torres, J.; Vizcayno, C.; Castello, R. Influence of the calcination temperature of kaolin on the mechanical properties of mortars and concretes containing metakaolin. Clay Miner. 2008, 43, 177–183. [Google Scholar] [CrossRef]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Swathi, R.B.; Vidjeapriya, R. Influence of precursor materials and molar ratios on normal, high, and ultra-high performance geopolymer concrete—A state of art review. Constr. Build. Mater. 2023, 392, 132006. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, T.; Guo, H.; Chen, J.; Liu, Y.; Yuan, P. Effect of the SiO2/Al2O3 Molar Ratio on the Microstructure and Properties of Clay-based Geopolymers: A Comparative Study of Kaolinite-based and Halloysite-based Geopolymers. Clays Clay Miner. 2022, 70, 882–902. [Google Scholar] [CrossRef]
- Xu, W.; Tang, Z.; Xie, Y.; Long, G.; Kai, M.; Zhang, Z.; Bu, M.; Zaland, S. Understanding the impact of synthesis parameters on the pore structure properties of fly ash-based geopolymers. Constr. Build. Mater. 2024, 411, 134640. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Guo, H.; Li, Y.; Wei, L. Mechanical properties of alkali activated geopolymer cement mortar for non vibratory compacted trench backfilling. Sci. Rep. 2025, 15, 12347. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mohapatra, S.; Gaur, A.; Dwivedi, G.; Soni, A. Study of various properties of geopolymer concrete—A review. Mater. Today Proc. 2021, 46, 5687–5695. [Google Scholar] [CrossRef]
- Faradilla, F.S.; Nugroho, D.T.; Hidayati, R.E.; Nurlina; Bayuaji, R.; Hartanto, D.; Fansuri, H. Optimization of SiO2/Al2O3 Ratio in the Preparation of Geopolymer from High Calcium Fly Ash. IOP Conf. Ser. Earth Environ. Sci. 2020, 616, 012051. [Google Scholar] [CrossRef]
- Dehghani, A.; Aslani, F.; Panah, N.G. Effects of initial SiO2/Al2O3 molar ratio and slag on fly ash-based ambient cured geopolymer properties. Constr. Build. Mater. 2021, 293, 123527. [Google Scholar] [CrossRef]
- Aziz, I.H.A.; Abdullah, M.M.A.B.; Razak, R.A.; Yahya, Z.; Salleh, M.A.A.M.; Chaiprapa, J.; Rojviriya, C.; Vizureanu, P.; Sandu, A.V.; Tahir, M.F.; et al. Mechanical Performance, Microstructure, and Porosity Evolution of Fly Ash Geopolymer after Ten Years of Curing Age. Materials 2023, 16, 1096. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Lin, K.-L.; Wang, D.; Hwang, C.-L.; Shiu, H.-S.; Chang, Y.-M.; Cheng, T.-W. Effects SiO2/Na2O molar ratio on mechanical properties and the microstructure of nano-SiO2 metakaolin-based geopolymers. Constr. Build. Mater. 2014, 53, 503–510. [Google Scholar] [CrossRef]
- Thang, N.H. Geopolymerization: A Review on Physico-chemical Factors Influence to the Reaction Process. J. Polym. Compos. 2020, 8, 01–09p. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, D.; Wang, Y.; Sun, R.; Rui, Y. Effects of the n(H2O: Na2Oeq) ratio on the geopolymerization process and microstructures of fly ash-based geopolymers. J. Non-Cryst. Solids 2019, 511, 19–28. [Google Scholar] [CrossRef]
- Altameemi, S.; Adeleke, B.O.; Kinuthia, J.M.; Oti, J. Understanding the Effect of Waiting for the Dissolution of Sodium Hydroxide in Geopolymer Concrete Mixes. Materials 2025, 18, 849. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xiao, H.; Chen, L.; Chen, P.; Lu, Z.; Tang, C.; Yao, H. Application of the non-linear three-component model for simulating accelerated creep behavior of polymer-alloy geocell sheets. Geotext. Geomembr. 2025, 53, 70–80. [Google Scholar] [CrossRef]
- Siyal, A.A.; Saphira, M.; Shamsuddin, R.; Ridzuan, M.B. A comprehensive review of synthesis kinetics and formation mechanism of geopolymers. RSC Adv. 2024, 14, 446–462. [Google Scholar] [CrossRef]
- Kubba, Z.; Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Asaad, M.A.; Ismail, M.; Tahir, M.M.d.; Mirza, J. Impact of curing temperatures and alkaline activators on compressive strength and porosity of ternary blended geopolymer mortars. Case Stud. Constr. Mater. 2018, 9, e00205. [Google Scholar] [CrossRef]
- Bao, W.; Yin, Y.; Mi, W.; Chen, R.; Lin, X. Durability and microstructural evolution of high-performance ecological geopolymer concrete under low-pressure–salt-erosion–freeze–thaw cycling conditions. Constr. Build. Mater. 2024, 426, 136197. [Google Scholar] [CrossRef]
- Wu, R.; Gu, Q.; Gao, X.; Huang, J.; Guo, Y.; Zhang, H. Effect of curing conditions on the alkali-activated blends: Microstructure, performance and economic assessment. J. Clean. Prod. 2024, 445, 141344. [Google Scholar] [CrossRef]
- Negahban, E.; Bagheri, A.; Sanjayan, J. Pore structure profile of ambient temperature-cured geopolymer concrete and its effect on engineering properties. Constr. Build. Mater. 2023, 406, 133311. [Google Scholar] [CrossRef]
- Geopolymer.org. About Geopolymerization; Geopolymer Institute: Saint-Quentin, France, 2006; Available online: https://www.geopolymer.org/science/about-geopolymerization/?u (accessed on 18 March 2025).
- Chanh, N.V.; Trung, B.D.; Tuan, D.V. Recent Research Geopolymer Concrete. In Proceedings of the 3rd ACF International Conference-ACF/VCA, Ho Chi Minh City, Vietnam, 11–13 November 2008. Available online: https://www.researchgate.net/profile/Prem-Baboo/post/Is-Seawater-suitable-for-Geopolymer-concrete/attachment/59d636d679197b80779943c7/AS%3A390575271497729%401470131809520/download/A18.pdf (accessed on 21 July 2025).
- Liguori, B.; Capasso, I.; De Pertis, M.; Ferone, C.; Cioffi, R. Geopolymerization Ability of Natural and Secondary Raw Materials by Solubility Test in Alkaline Media. Environments 2017, 4, 56. [Google Scholar] [CrossRef]
- Davidovits, J. 30 Years of Successes and Failures in Geopolymer Applications. In Market Trends and Potential Breakthroughs, Proceedings of the Geopolymer 2002 International Conference, Melbourne, Australia, 28–29 October 2002; Geopolymer Institute: Saint-Quentin, France, 2002; Available online: https://www.geopolymer.org/wp-content/uploads/30YearsGEOP.pdf (accessed on 21 July 2025).
- Peng, Y.; Zhao, T.; Miao, J.; Kong, L.; Li, Z.; Liu, M.; Jiang, X.; Zhang, Z.; Wang, W. Evaluation framework for bitumen-aggregate interfacial adhesion incorporating pull-off test and fluorescence tracing method. Constr. Build. Mater. 2024, 451, 138773. [Google Scholar] [CrossRef]
- Zribi, M.; Samet, B.; Baklouti, S. Investigation of Dealumination in Phosphate-Based Geopolymer Formation Process: Factor Screening and Optimization. Minerals 2022, 12, 1104. [Google Scholar] [CrossRef]
- Louati, S.; Baklouti, S.; Samet, B. Acid based geopolymerization kinetics: Effect of clay particle size. Appl. Clay Sci. 2016, 132–133, 571–578. [Google Scholar] [CrossRef]
- Dahal, S. Synthesis of Alkali Activated Geopolymer Cement from Clay. J. Civ. Environ. Eng. 2022, 12, 455. [Google Scholar]
- Joseph, D. Geopolymer Chemistry and Applications, 5th ed; Geopolymer Institute: Saint-Quentin, France, 2008; Available online: https://www.geopolymer.org/learning/book-geopolymer-chemistry-and-applications/ (accessed on 14 February 2025).
- Ralli, Z.G.; Pantazopoulou, S.J. State of the art on geopolymer concrete. Int. J. Struct. Integr. 2020; ahead-of-print. [Google Scholar] [CrossRef]
- Rajini, B.; Rao, A.V.N.; Sashidhar, C. Cost Analysis of Geopolymer Concrete Over Conventional Concrete. Int. J. Civ. Eng. Technol. 2020, 11, 23–30. [Google Scholar] [CrossRef]
- Ozcelikci, E.; Kul, A.; Gunal, M.F.; Ozel, B.F.; Yildirim, G.; Ashour, A.; Sahmaran, M. A comprehensive study on the compressive strength, durability-related parameters and microstructure of geopolymer mortars based on mixed construction and demolition waste. J. Clean. Prod. 2023, 396, 136522. [Google Scholar] [CrossRef]
- Zulkifly, K.; Cheng-Yong, H.; Yun-Ming, L.; Abdullah, M.M.A.B.; Shee-Ween, O.; Khalid, M.S.B. Effect of phosphate addition on room-temperature-cured fly ash-metakaolin blend geopolymers. Constr. Build. Mater. 2021, 270, 121486. [Google Scholar] [CrossRef]
- Mathivet, V.; Jouin, J.; Parlier, M.; Rossignol, S. Control of the alumino-silico-phosphate geopolymers properties and structures by the phosphorus concentration. Mater. Chem. Phys. 2021, 258, 123867. [Google Scholar] [CrossRef]
- Priya, E.; Sharma, J.; Sarkar, S.; Maji, P.K. Phosphate Recovery from Wastewater by Cellulose-based Adsorbent Derived from Agricultural Waste and its Reuse as a Slow-Release Fertilizer. J. Environ. Chem. Eng. 2025, 13, 116716. [Google Scholar] [CrossRef]
- Nasir, M.; Mahmood, A.H.; Bahraq, A.A. History, recent progress, and future challenges of alkali-activated binders—An overview. Constr. Build. Mater. 2024, 426, 136141. [Google Scholar] [CrossRef]
- Amer, I.; Kohail, M.; El-Feky, M.; Rashad, A.; Khalaf, M.A. A review on alkali-activated slag concrete. Ain Shams Eng. J. 2021, 12, 1475–1499. [Google Scholar] [CrossRef]
- Albidah, A.; Alghannam, M.; Abbas, H.; Almusallam, T.; Al-Salloum, Y. Characteristics of metakaolin-based geopolymer concrete for different mix design parameters. J. Mater. Res. Technol. 2021, 10, 84–98. [Google Scholar] [CrossRef]
- Barbosa, V.F.F.; MacKenzie, K.J.D.; Thaumaturgo, C. Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: Sodium polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Liu, J.; Fan, D.; Qu, H.; Zhou, L.; Zheng, S. Effects of Different Calcium Sources on Mechanical Properties of Metakaolin Geopolymers. Materials 2024, 17, 2087. [Google Scholar] [CrossRef] [PubMed]
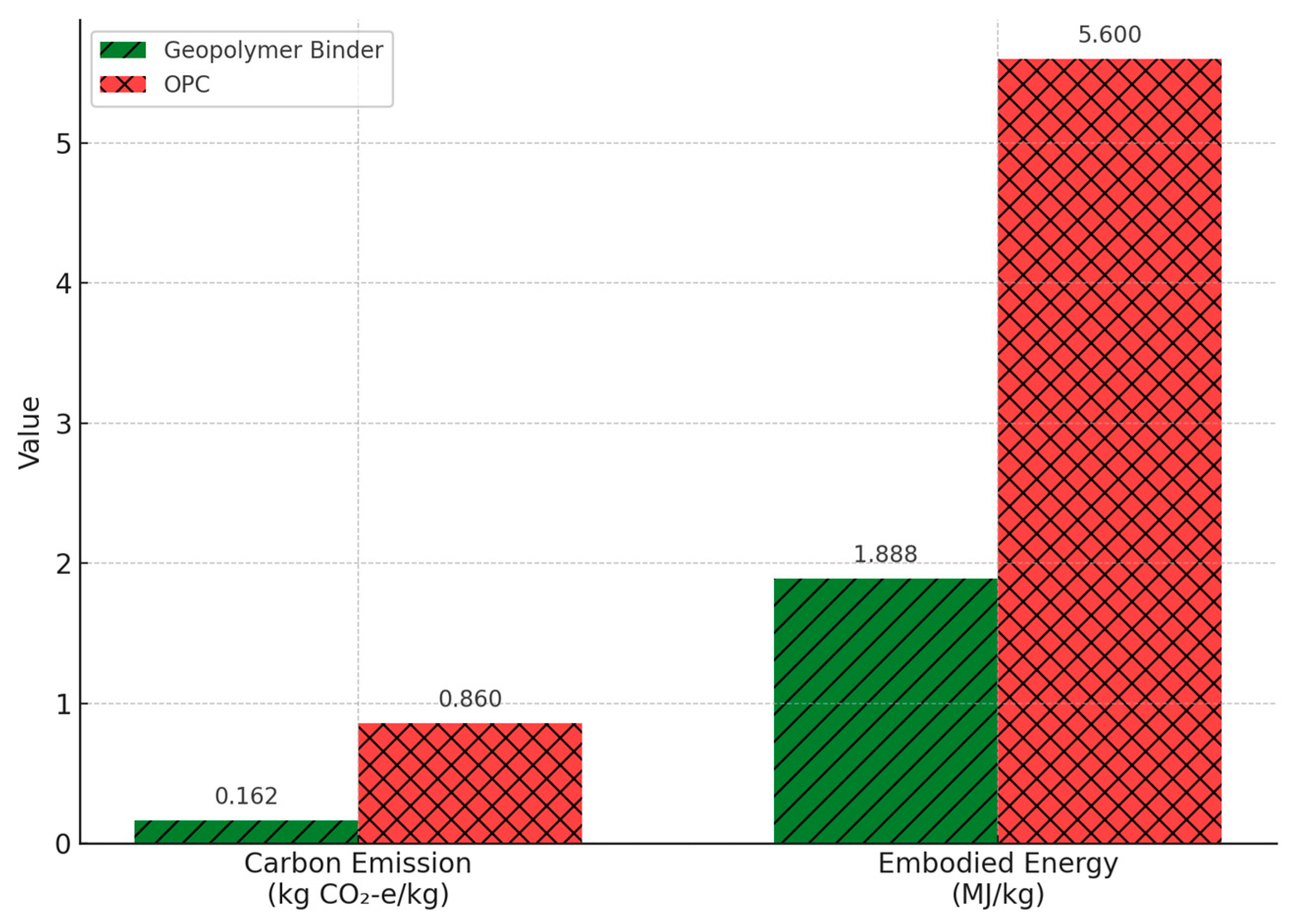
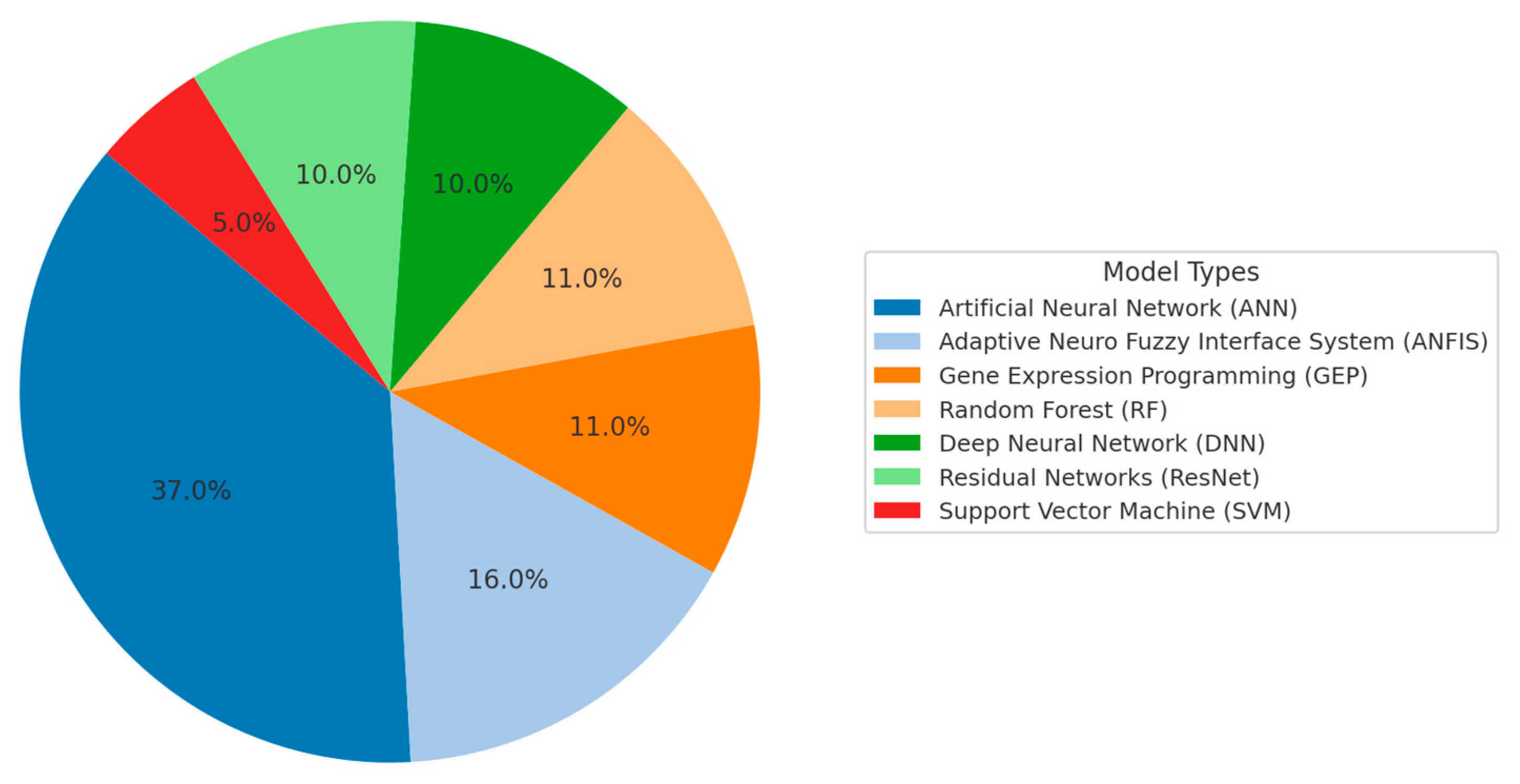
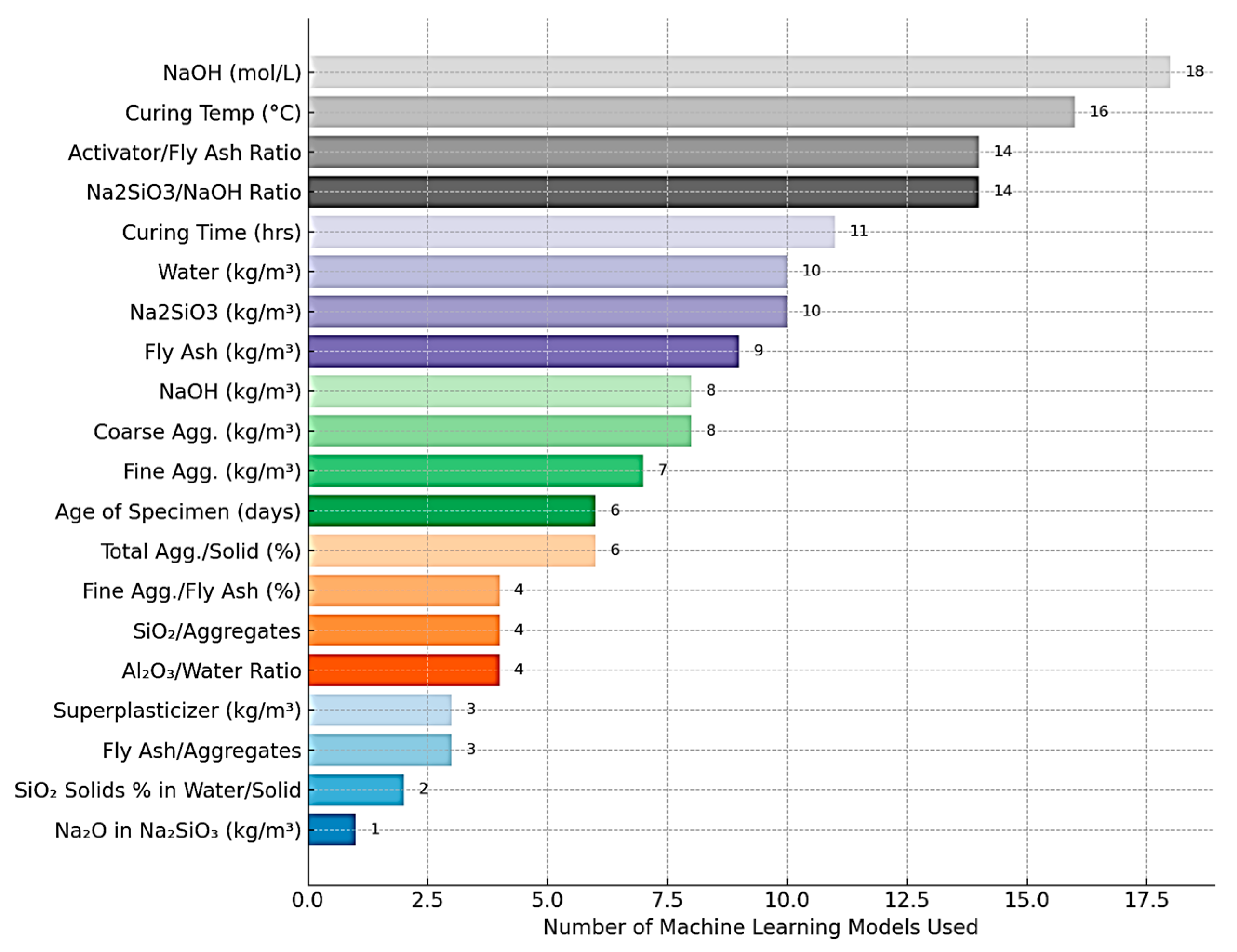
| Geopolymer | OPC | |||||
|---|---|---|---|---|---|---|
| Ingredients kg (per kg of binder) | Fly Ash | GGBS | Sodium Silicate | Sodium Carbonate | Total | |
| 0.5 | 0.32 | 0.09 | 0.09 | 1 | ||
| Carbon emission (kg CO2-e/kg) | 0.0135 | 0.0457 | 0.0803 | 0.0225 | 0.162 | 0.86 |
| Embodied energy (MJ/kg) | 0.05 | 0.1056 | 1.611 | 0.1215 | 1.888 | 5.6 |
| Binder Type | Main Reaction Gel | Ca Content | Typical Precursors | Primary Durability Focus |
|---|---|---|---|---|
| Geopolymers—Low-Ca AAM | N–A–S–H (sodium aluminosilicate hydrate) | <5–10 wt% Ca | Fly ash, metakaolin, waste glass, red mud 1 | ASR risk, drying shrinkage |
| High-Ca AAM (e.g., Slag-Based) | C–A–S–H (calcium aluminosilicate hydrate) | >10 wt% Ca | GGBS, slag-rich blends, limestone-rich by-products | Carbonation, sulfate and acid attack |
| SDG | Geopolymer Contribution | Key Research Gaps | Citation |
|---|---|---|---|
| SDG 9—Industry, Innovation, and Infrastructure | Development of resilient precast infrastructure, pavements, and advanced Alkali-Activated Slag—Alkali-Activated Silicophosphate systems for structural applications. | Long-term structural performance under varied climatic and loading conditions. | [15,52,53] |
| SDG 12—Responsible Consumption and Production | Valorization of industrial by-products (fly ash, GGBS, rice husk ash, waste glass, red mud, coal gasification fly ash). | Precursor variability, consistent quality control, and supply chain sustainability. | [9,11,12,25] |
| SDG 13—Climate Action | Up to 80% CO2 reduction compared to OPC; Life Cycle Assessment studies confirm significant environmental benefits. | Comprehensive life-cycle assessment for emerging precursors (waste glass, CGFA, red mud). | [16,17,18,19,60] |
| Precursor | SEM Observations (Morphology) | XRD Observations (Phase Characteristics) | Microstructural Implications | Citation |
|---|---|---|---|---|
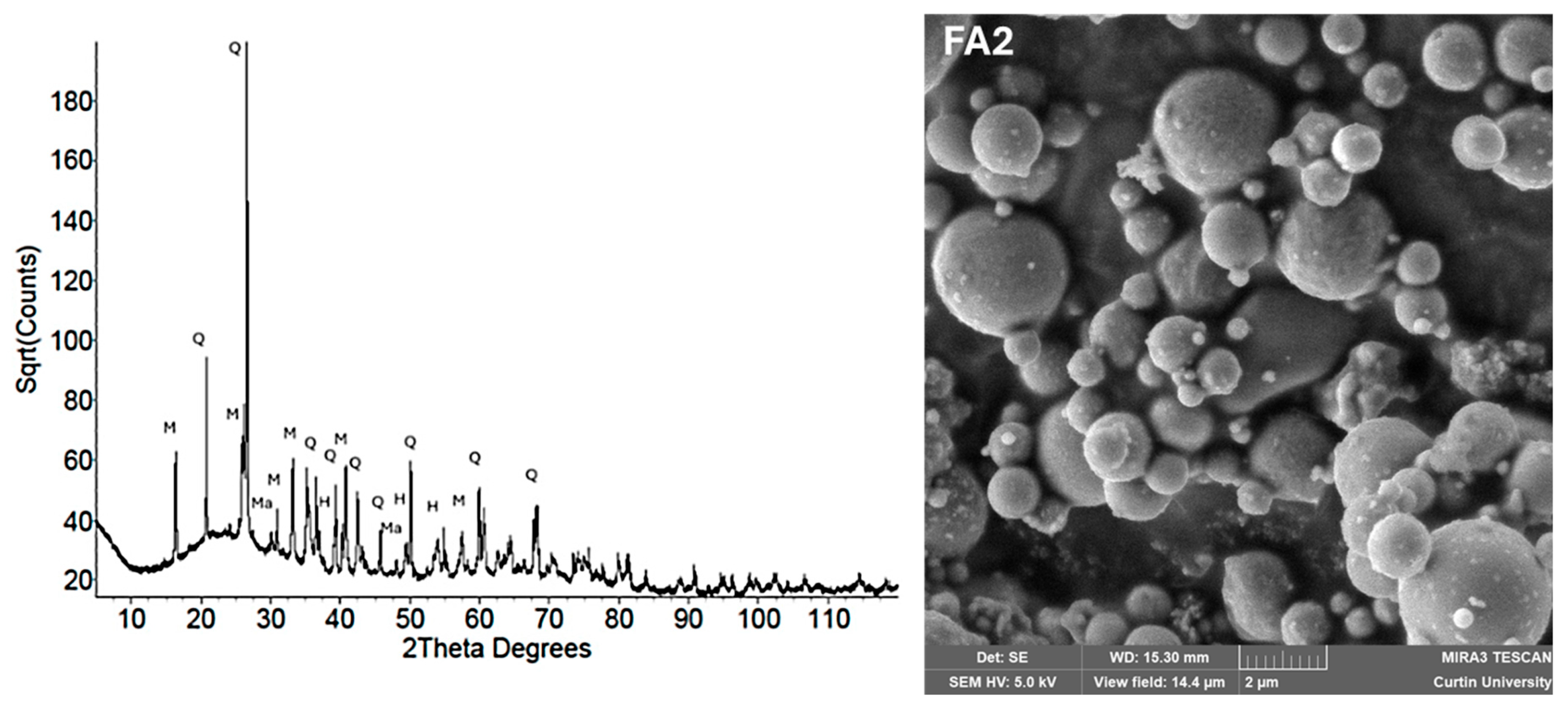
| Fly Ash (FA) | Predominantly spherical, smooth glassy microspheres; some porous cenospheres | Dominant amorphous hump (20–35° 2θ); crystalline quartz, mullite, hematite peaks | Spherical morphology enhances workability; high amorphous phase improves dissolution and geopolymerization | [108] |
| GGBS | Irregular, angular, rough particles; glassy texture visible | Broad amorphous hump (25–35° 2θ); minor crystalline merwinite and akermanite | High Ca amorphous phase accelerates C–A–S–H gel formation; irregular morphology slightly lowers flowability | [103,104,109] |
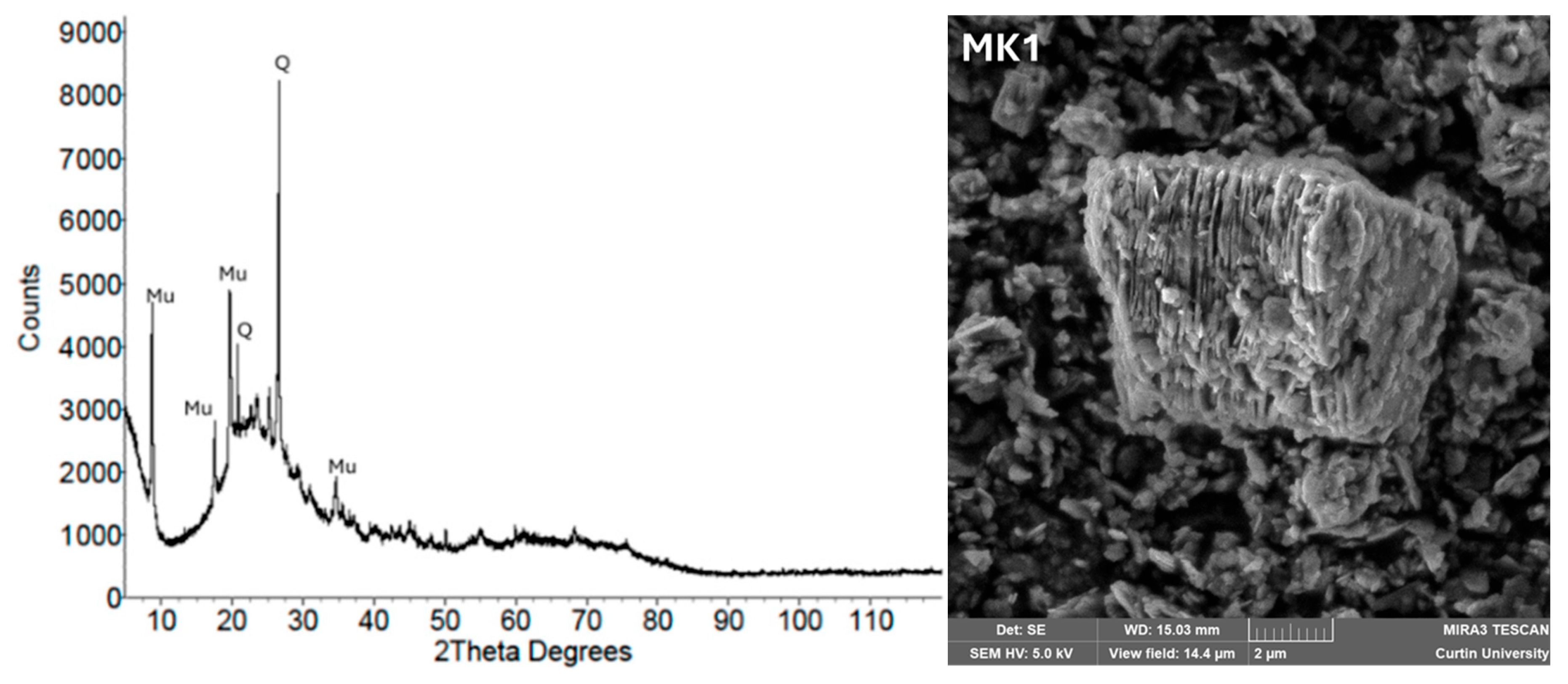
| Metakaolin (MK) | Plate-like, flaky particles with high surface area | Semi-crystalline; quartz and muscovite peaks; amorphous aluminosilicate derived from dehydroxylated kaolinite | High surface area and amorphous content enhance reactivity; improves strength and durability | [108] |
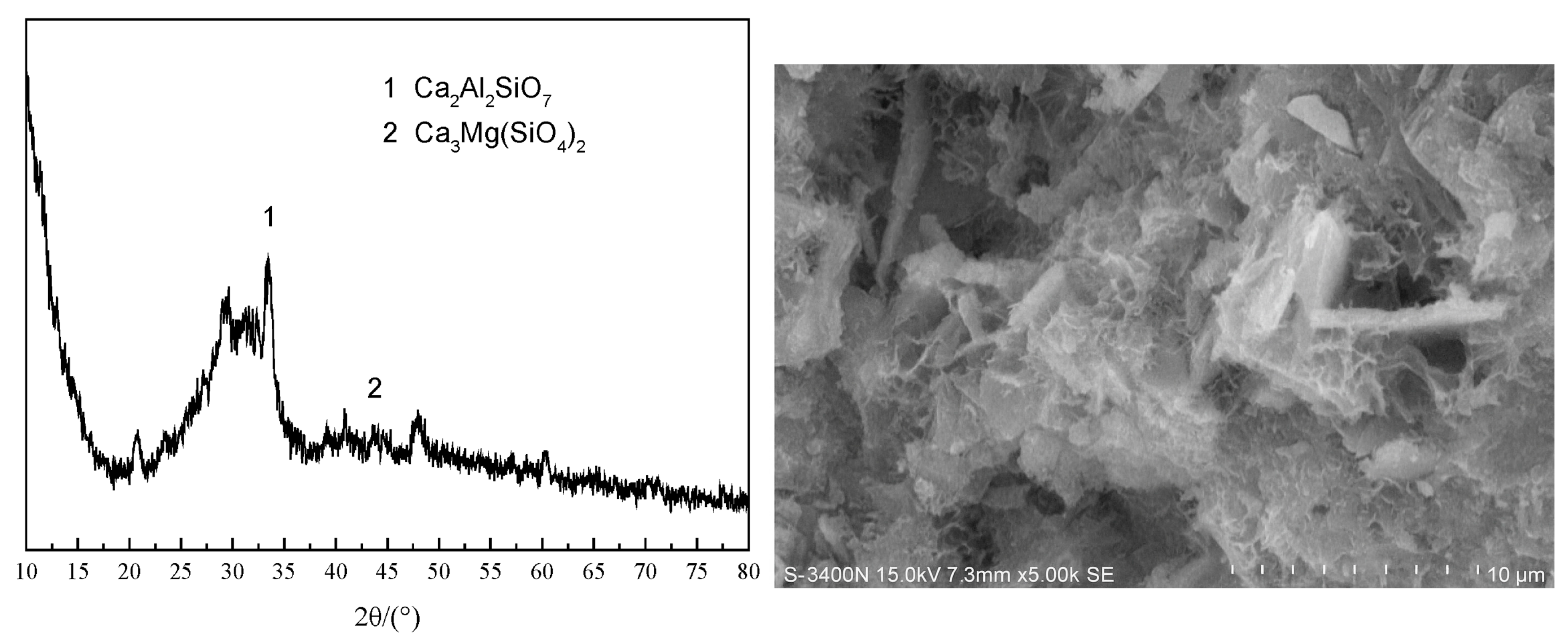
| Red Mud (RM) | Agglomerated, irregular plate-like particles; dense clusters | Crystalline hematite, goethite, perovskite, gibbsite; low amorphous content | High Fe2O3 & CaO contribute to (Fe)-A-S-H or C–A–S–H gel formation; excessive Fe reduces workability | [49,51] |
| Waste Glass (WG) | Angular, sharp-edged particles; smooth fractured surfaces | Broad amorphous hump (20–30° 2θ); minor crystalline quartz and wollastonite | High reactive silica improves polymerization; angular morphology may need alumina-rich blends for balance | [11,26] |
| Coal Gasification Fly Ash (CGFA) | Finer than FA; mixed cenospheres and angular grains | High amorphous content; crystalline quartz, mullite, magnetite | High reactivity and fine size accelerate gel formation; durable performance still under research | [12] |
| Bamboo Leaf Ash (BLA) | Irregular porous particles; fibrous ash texture | High amorphous silica (~73%); minor crystalline quartz | High silica improves N–A–S–H gel formation; porous morphology increases water demand | [90] |
| Rice Husk Ash (RHA) | Fine, highly porous particles; honeycomb-like structure | Broad amorphous silica hump; crystalline cristobalite if over-burnt | High reactive silica boosts polymerization; porous structure may influence workability | [93,104] |
| Rice Straw Ash (RSA) | Flaky, irregular porous ash particles | High amorphous SiO2 (~69%); some quartz peaks | Similar to RHA; promising as low-cost precursor; moderate CaO enables hybrid C–A–S–H gel potential | [91] |
| Basalt Powder (BP) | Angular, dense, rough particles | Moderate amorphous content; crystalline plagioclase and pyroxene | Moderate CaO (7–8%) supports hybrid binder systems; dense morphology may reduce flowability | [112] |
| Precursors | Chemical Composition (%) | Citation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 | SO3 | ||
| Fly ash | 52.4 | 25.8 | 8.4 | 6.42 | 2.27 | - | 1.47 | 1.31 | 0.86 | [95] |
| Ground Granulated Blast Furnace Slag | 32.19 | 13.89 | 0.35 | 40.45 | 6.67 | 0.28 | 0.32 | 0.74 | 4.74 | [95,113] |
| Desulfurization Waste from Titanium Slag | 33.07 | 48.21 | 6.74 | 2.94 | 1.48 | 0.28 | 1.21 | 0.51 | 1.70 | [113] |
| Kaolinite | 51.30 | 32.60 | 1.10 | 0.10 | 0.30 | 0.20 | 0.30 | 1.10 | 0.00 | [114] |
| Rice Husk Ash | 83.10 | 2.15 | 1.10 | 4.70 | 1.50 | 0.10 | 2.96 | - | 1.20 | [93] |
| Pumice | 75.23 | 14.04 | 1.95 | 0.52 | 0.22 | 2.09 | 5.05 | 0.11 | 0.29 | [115] |
| Silica Fume | 96.9 | 0.15 | 0.06 | 0.53 | 1.1 | - | 0.78 | - | 0.12 | [95] |
| Sugarcane Bagasse Ash | 76.00 | 9.00 | 4.20 | 3.10 | 2.70 | - | 3.83 | 0.46 | - | [14] |
| Tektite | 69.84 | 12.16 | 8.40 | 2.54 | 2.03 | 1.07 | 2.28 | 0.78 | - | [33] |
| Alccofine | 37.53 | 24.57 | 0.92 | 29.46 | 5.23 | 0.03 | 0.61 | - | 0.18 | [116] |
| Red Mud | 29.18 | 30.01 | 8.71 | 15.96 | 0.89 | 8.22 | 0.80 | 2.70 | 2.73 | [51] |
| Waste Glass | 69.65 | 0.85 | 0.50 | 14.45 | 2.89 | 9.69 | 0.39 | 0.10 | 0.55 | [11] |
| Coal gasification fly ash | 61.30 | 24.94 | 3.86 | 1.11 | 0.67 | 0.09 | 1.48 | 2.01 | 0.021 | [12] |
| Gold Mine Tailings | 74.50 | 6.98 | 7.03 | 0.53 | 5.16 | 0.27 | 1.26 | 0.44 | 3.05 | [117] |
| Zeolite | 70.92 | 11.71 | 0.92 | 2.10 | 0.33 | 2.06 | 3.09 | - | - | [118] |
| Bentonite | 68.10 | 15.44 | 0.34 | 0.77 | 3.79 | 2.58 | 0.93 | 0.12 | - | [118] |
| Basalt Powder | 45.00 | 15.00 | 15.00 | 7.50 | 5.20 | 3.00 | 1.00 | 2.00 | - | [112] |
| Bamboo Leaf Ash | 72.97 | 2.85 | 2.31 | 4.98 | 1.23 | - | 6.07 | 0.41 | 0.55 | [90] |
| Rice straw ash | 69.20 | 5.30 | 0.90 | 3.46 | 2.81 | 3.43 | 6.40 | - | - | [91] |
| Granulated Phosphorus Slag | 39.11 | 2.38 | 0.74 | 47.47 | 4.60 | 0.03 | 0.15 | - | 0.22 | [119] |
| Framework Type | General Formula | Polymeric Description | Citations |
|---|---|---|---|
| Siloxo | –Si–O–Si–O– | Poly(siloxo) | [6,70] |
| Sialate | –Si–O–Al–O– | Poly(sialate) | [6,70] |
| Sialate-siloxo | –Si–O–Al–O–Si–O– | Poly(sialate–siloxo) | [6,70] |
| Sialate-disiloxo | –Si–O–Al–O–Si–O–Si–O– | Poly(sialate–disiloxo) | [6,70] |
| Phosphate | –P–O–P–O– | Poly(phosphate) | [6] |
| Phospho-siloxo | –P–O–Si–O–P–O– | Poly(phosphor–siloxo) | [6] |
| Phospho-sialate | –P–O–Si–O–Al–O–P–O– | Poly(phosphor–sialate) | [6] |
| Alumino-phospho | –Al–O–P–O– | Poly(alumino–phospho) | [6] |
| Ferro-sialate | –Fe–O–Si–O–Al–O–Si–O– | Poly(ferro–sialate) | [6] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohan Kumar, S.G.K.; Kinuthia, J.M.; Oti, J.; Adeleke, B.O. Geopolymer Chemistry and Composition: A Comprehensive Review of Synthesis, Reaction Mechanisms, and Material Properties—Oriented with Sustainable Construction. Materials 2025, 18, 3823. https://doi.org/10.3390/ma18163823
Mohan Kumar SGK, Kinuthia JM, Oti J, Adeleke BO. Geopolymer Chemistry and Composition: A Comprehensive Review of Synthesis, Reaction Mechanisms, and Material Properties—Oriented with Sustainable Construction. Materials. 2025; 18(16):3823. https://doi.org/10.3390/ma18163823
Chicago/Turabian StyleMohan Kumar, Sri Ganesh Kumar, John M. Kinuthia, Jonathan Oti, and Blessing O. Adeleke. 2025. "Geopolymer Chemistry and Composition: A Comprehensive Review of Synthesis, Reaction Mechanisms, and Material Properties—Oriented with Sustainable Construction" Materials 18, no. 16: 3823. https://doi.org/10.3390/ma18163823
APA StyleMohan Kumar, S. G. K., Kinuthia, J. M., Oti, J., & Adeleke, B. O. (2025). Geopolymer Chemistry and Composition: A Comprehensive Review of Synthesis, Reaction Mechanisms, and Material Properties—Oriented with Sustainable Construction. Materials, 18(16), 3823. https://doi.org/10.3390/ma18163823










