Surface Treatment, Chemical Characterization, and Debonding Crack Initiation Strength for Veneering Dental Ceramics on Ni-Cr Alloys
Abstract
1. Introduction
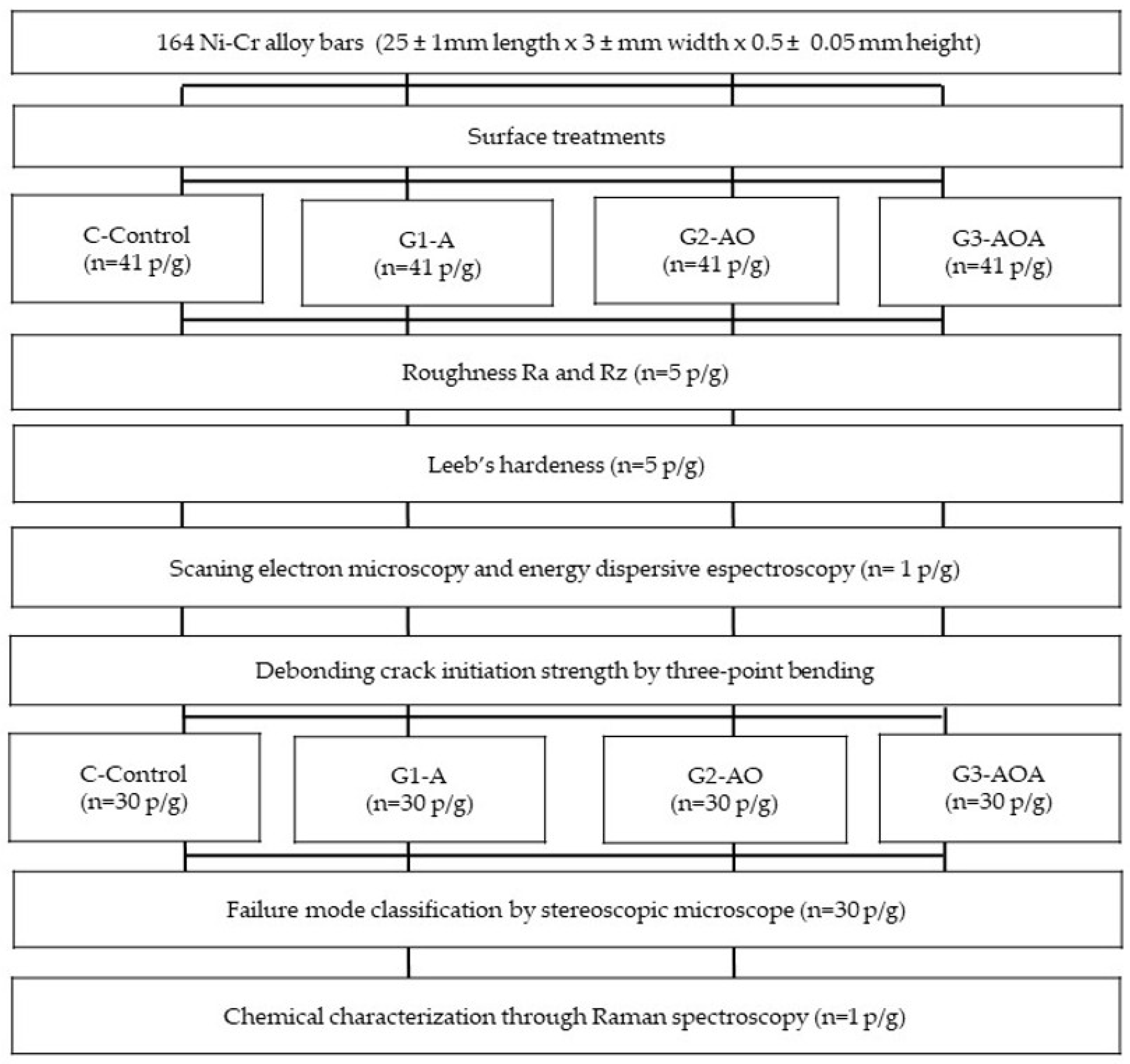
2. Materials and Methods
2.1. Preparation of Specimens
2.2. Surface Treatment and Ceramic Veneering
2.3. Surface Roughness
2.4. Leeb’s Hardness Dynamic Test
2.5. Evaluation Using Scanning Electron Microscopy and Energy-Dispersive Spectrometry
2.6. Evaluation of the Debonding/Cack-Initiation Strength
2.7. Evaluation of the Failure Mode
2.8. Chemical Interface Characterization After Failure Using Raman Spectroscopy
2.9. Statistical Analysis
3. Results
3.1. Surface Roughness and Leeb’s Hardness
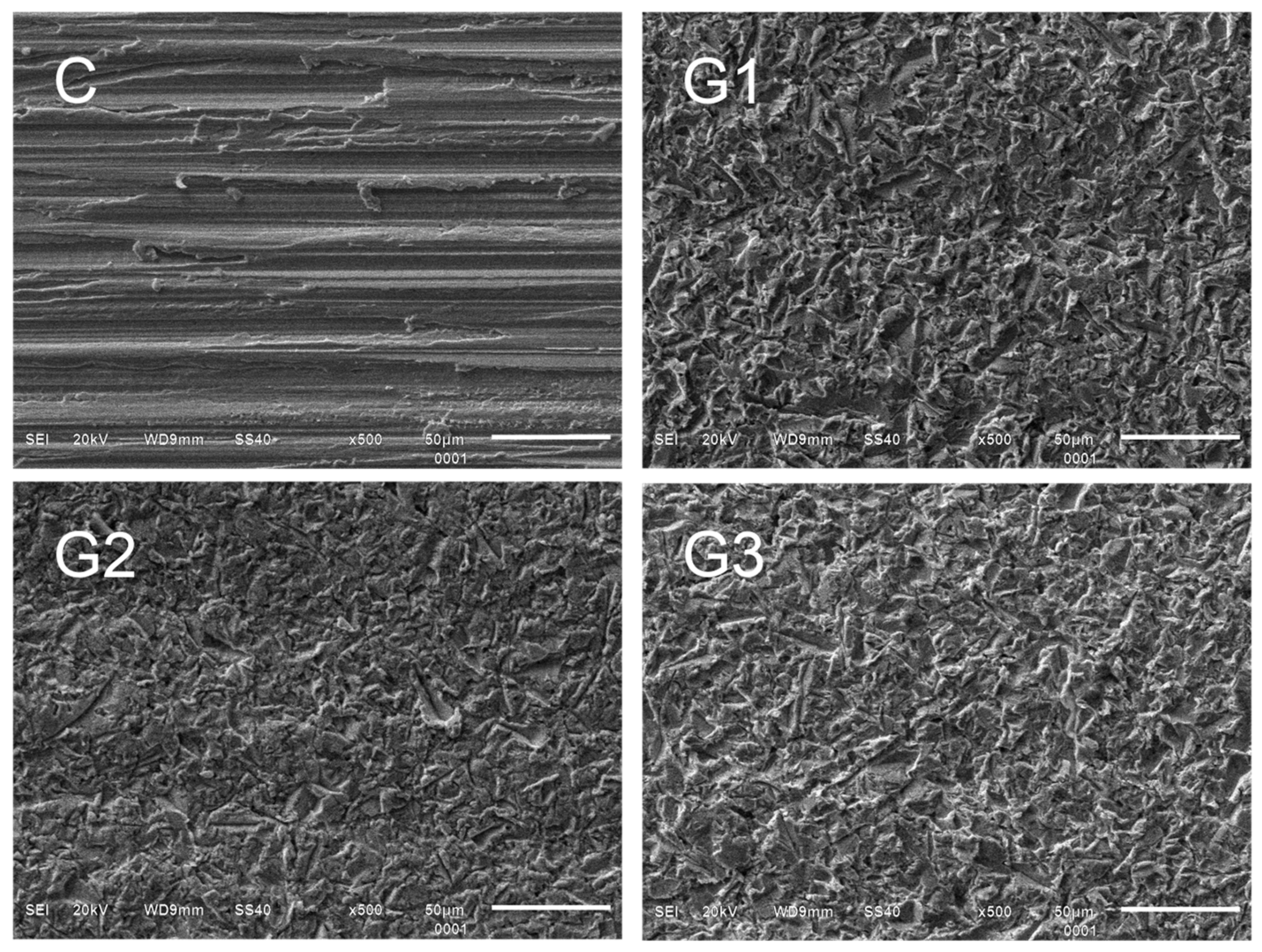
3.2. Surface Morphology According to Scanning Electron Microscopy and Energy-Dispersive Spectroscopy
3.3. Debonding/Crack Initiation Strength
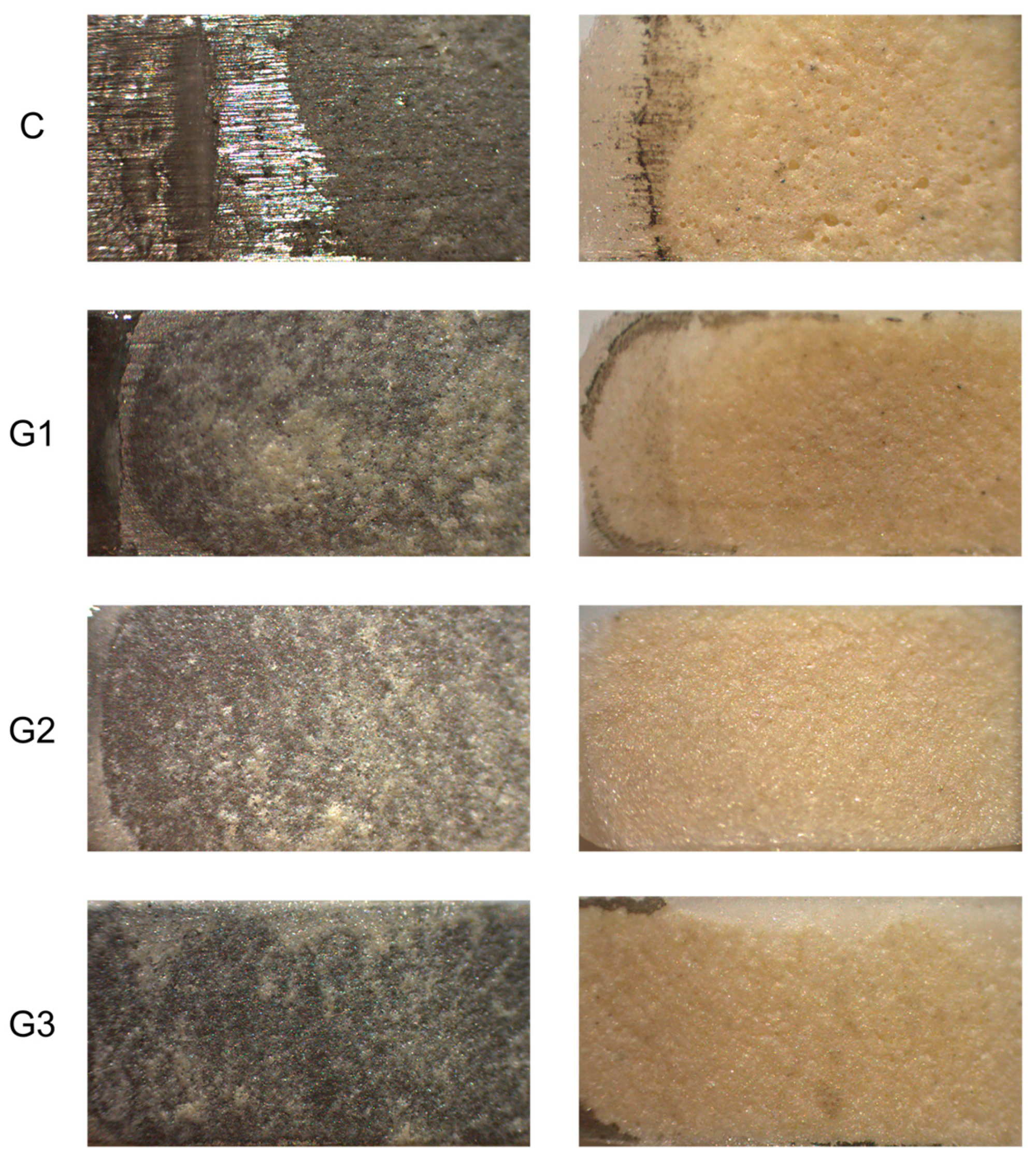
3.4. Failure Mode
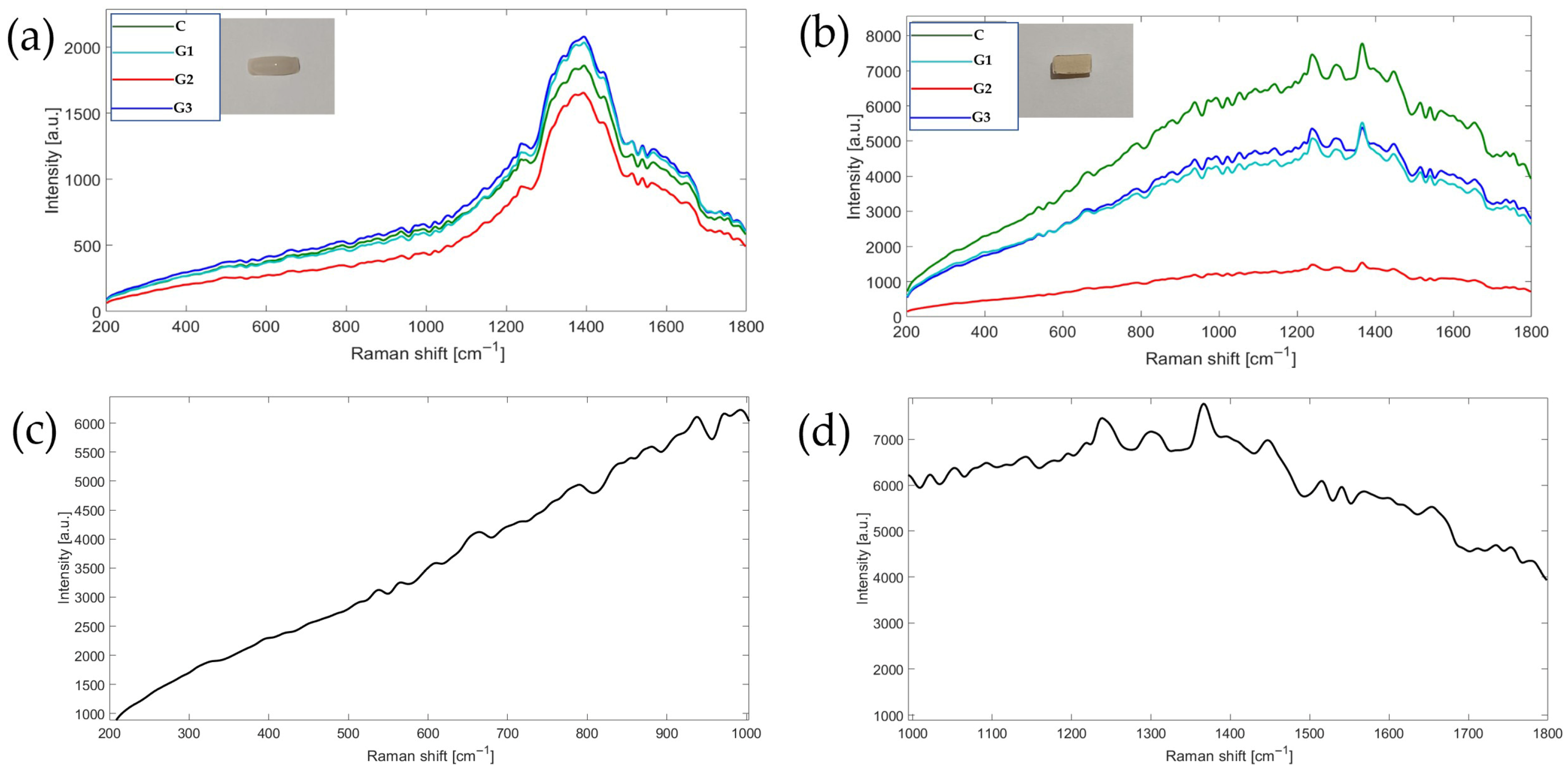
3.5. Chemical Interface Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DCIS | Debonding Crack Initiation Strength |
| APA | Airborne-Particle Abrasion |
| HLD | Leeb’s Hardness |
| SEM | Scanning Electron Microscope |
| EDS | Energy-Dispersive Spectroscopy |
| Al2O3 | Aluminum Oxide |
| MPa | Megapascal |
References
- Porcelain-Fused-to-Metal Crowns Versus All-Ceramic Crowns: A Review of the Clinical and Cost-Effectiveness; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 29 May 2015. Available online: https://www.ncbi.nlm.nih.gov/books/NBK304696/ (accessed on 25 June 2024).
- Walton, T.R. A 10-year longitudinal study of fixed prosthodontics: Clinical characteristics and outcome of single-unit metal-ceramic crowns. Int. J. Prosthodont. 1999, 12, 519–526. [Google Scholar] [PubMed]
- Pjetursson, B.E.; Tan, K.; Lang, N.P.; Brägger, U.; Egger, M.; Zwahlen, M. A systematic review of the survival and complication rates of fixed partial dentures (FPDs) after an observation period of at least 5 years. Clin. Oral Implants Res. 2004, 15, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Pretti, M.; Hilgert, E.; Bottino, M.A.; Avelar, R.P. Evaluation of the shear bond strength of the union between two CoCr-alloys and a dental ceramic. J. Appl. Oral Sci. 2004, 12, 280–284. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pjetursson, B.E.; Valente, N.A.; Strasding, M.; Zwahlen, M.; Liu, S.; Sailer, I. A systematic review of the survival and complication rates of zirconia-ceramic and metal-ceramic single crowns. Clin. Oral Implants Res. 2018, 29, 199–214. [Google Scholar] [CrossRef]
- Ozcan, M.; Niedermeier, W. Clinical study on the reasons for and location of failures of metal-ceramic restorations and survival of repairs. Int. J. Prosthodont. 2002, 15, 299–302. [Google Scholar] [PubMed]
- Sailer, I.; Strasding, M.; Valente, N.A.; Zwahlen, M.; Liu, S.; Pjetursson, B.E. A systematic review of the survival and complication rates of zirconia-ceramic and metal-ceramic multiple-unit fixed dental prostheses. Clin. Oral Implants Res. 2018, 29, 184–198. [Google Scholar] [CrossRef]
- Joias, R.M.; Tango, R.N.; Junho de Araujo, J.E.; Junho de Araujo, M.A.; Ferreira Anzaloni Saavedra Gde, S.; Paes-Junior, T.J.; Kimpara, E.T. Shear bond strength of a ceramic to Co-Cr alloys. J. Prosthet. Dent. 2008, 99, 54–59. [Google Scholar] [CrossRef]
- Bagby, M.; Marshall, S.J.; Marshall, G.W., Jr. Metal ceramic compatibility: A review of the literature. J. Prosthet. Dent. 1990, 63, 21–25. [Google Scholar] [CrossRef]
- O’Brien, W.J.; Ryge, G. Contact angles of drops of enamels on metals. J. Prosthet. Dent. 1965, 15, 1094–1100. [Google Scholar] [CrossRef]
- Czepułkowska-Pawlak, W.; Klimek, L.; Makówka, M.; Wołowiec-Korecka, E. Effect of Ni-Cr Alloy Surface Abrasive Blasting on Its Wettability by Liquid Ceramics. Materials 2021, 14, 2007. [Google Scholar] [CrossRef]
- Rosentiel, S.F.; Land, M.F.; Fujimoto, J. Restauraciones de Metal Cerámica. In Prótesis Fija Contemporánea, 4th ed.; Rosentiel, S.F., Land, M.F., Fujimoto, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 740–774. [Google Scholar]
- Borom, M.P.; Pask, J.A. Role of adherence oxides in the development of chemical bonding at glass metal interfaces. J. Am. Ceram. Soc. 1966, 49, 1–6. [Google Scholar] [CrossRef]
- Wataha, J.C. Alloys for prosthodontic restorations. J. Prosthet. Dent. 2002, 87, 351–363. [Google Scholar] [CrossRef]
- Wagner, W.C.; Asgar, K.; Bigelow, W.C.; Flinn, R.A. Effect of interfacial variables on metal-porcelain bonding. J. Biomed. Mater. Res. 1993, 27, 531–537. [Google Scholar] [CrossRef] [PubMed]
- DeHoff, P.H.; Anusavice, K.J.; Hojjatie, B. Thermal incompatibility analysis of metal-ceramic systems based on flexural displacement data. J. Biomed. Mater. Res. 1998, 41, 614–623. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Q.; Li, N.; Xu, S. Evaluation of metal-ceramic bond characteristics of three dental Co-Cr alloys prepared with different fabrication techniques. J. Prosthet. Dent. 2016, 116, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Al Jabbari, Y.S.; Koutsoukis, T.; Barmpagadaki, X.; Zinelis, S. Metallurgical and interfacial characterization of PFM Co-Cr dental alloys fabricated via casting, milling or selective laser melting. Dent. Mater. 2014, 30, e79–e88. [Google Scholar] [CrossRef]
- Wu, L.; Zhu, H.; Gai, X.; Wang, Y. Evaluation of the mechanical properties and porcelain bond strength of cobalt-chromium dental alloy fabricated by selective laser melting. J. Prosthet. Dent. 2014, 111, 51–55. [Google Scholar] [CrossRef]
- Revilla-León, M.; Al-Haj Husain, N.; Methani, M.M.; Özcan, M. Chemical composition, surface roughness, and ceramic bond strength of additively manufactured cobalt-chromium dental alloys. J. Prosthet. Dent. 2021, 125, 825–831. [Google Scholar] [CrossRef]
- Elkallaf, E.T.; Abdel-Aziz, S.A.; El-Sharkawy, Z.R. Effect of Metal Surface Treatment on Bonding of Porcelain to Recycled Cobalt Chromium and Nickel Chromium. Al-Azhar J. Dent. Sci 2024, 11, 76–84. [Google Scholar] [CrossRef]
- Al-Mashhadani, M.M.S.; Raja Awan, R.A.; Ismail, N.H. Metal surface treatments and their effect on metal-ceramic bond strength: A review. MJM 2025, 21, 338–349. [Google Scholar]
- Asproudi, G.; Galiatsatos, P.; Galiatsatos, A. Research on the Role of Surface Treatment of the Metal Surface on the Strength of the Metal-Ceramic Bond. J. Contemp. Dent. Pract. 2023, 24, 188–194. [Google Scholar]
- ISO 9693:2019(E); Dentistry-Compatibility Testing for Metal-Ceramic and Ceramic-Ceramic Systems. International Organizazion for Standardization: Geneva, Switzerland, 2019.
- Ho, B.J.; Tsoi, J.K.; Liu, D.; Lung, C.Y.; Wong, H.M.; Matinlinna, J.P. Effects of sandblasting distance and angles on resin cement bonding to zirconia and titanium. Int. J. Adhes. Adhes. 2015, 62, 25–31. [Google Scholar] [CrossRef]
- Flores-Ferreyra, B.I.; Scougall-Vilchis, R.J.; Velazquez-Enriquez, U.; Garcia-Contreras, R.; Aguillon-Sol, L.; Olea-Mejia, O.F. Effect of airborne-particle abrasion and, acid and alkaline treatments on shear bond strength of dental zirconia. Dent. Mater. J. 2019, 38, 182–188. [Google Scholar] [CrossRef] [PubMed]
- ISO 4287:1998; Geometrical Product Specifications (GPS)—Surface Texture: Profile Method–Terms, Definitions and Surface Texture Parameters. International Organization for Standardization: Geneva, Switzerland, 1998.
- ASTM A956-06:2006; Standard Test Method for Leeb Hardness Testing of Steel Products. American Society for Testing and Materials: West Conshohocken, PA, USA, 2006.
- Artun, J.; Bergland, S. Clinical trials with crystal growth conditioning as an alternative to acid-etch enamel pretreatment. Am. J. Orthod. 1984, 85, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Eilers, P.H. A perfect smoother. Anal. Chem. 2003, 75, 3631–3636. [Google Scholar] [CrossRef]
- Colomban, P.; Tournie, A.; Bellot-Gurlet, L. Raman identification of glassy silicates used in ceramics, glass and jewellery: A tentative differentiation guide. J. Raman Spectrosc. 2006, 37, 841–852. [Google Scholar] [CrossRef]
- Brawer, S.A.; White, W.B. Raman spectroscopic investigation of the structure of silicate glasses. I. The binary alkali silicates. J. Chem. Phys. 1975, 63, 2421–2432. [Google Scholar] [CrossRef]
- Bancroft, G.M.; Nesbitt, H.W.; Henderson, G.S.; O’Shaughnessy, C.; Withers, A.C.; Neuville, D.R. Lorentzian dominated lineshapes and linewidths for Raman symmetric stretch peaks (800–1200 cm−1) in Qn (n = 1–3) species of alkali silicate glasses/melts. J. Non-Cryst. Solids 2018, 484, 72–83. [Google Scholar] [CrossRef]
- Neves, A.; Friedel, R.; Callapez, M.E.; Swank, S.D. Safeguarding our dentistry heritage: A study of the history and con-servation of nineteenth–twentieth century dentures. Herit. Sci. 2023, 11, 142. [Google Scholar] [CrossRef]
- Wachs, I.E. Raman and IR studies of surface metal oxide species on oxide supports: Supported metal oxide catalysts. Catal. Today 1996, 27, 437–455. [Google Scholar] [CrossRef]
- van Noort, R.; Barbour, M.E. Atomic building blocks. In Introduction to Dental Materials E-Book, 5th ed.; van Noort, R., Barbour, M.E., Eds.; Elsevier Health Sciences: New York, NY, USA, 2023; pp. 56–59. [Google Scholar]
- Li, J.; Ye, X.; Li, B.; Liao, J.; Zhuang, P.; Ye, J. Effect of oxidation heat treatment on the bond strength between a ceramic and cast and milled cobalt-chromium alloys. Eur. J. Oral Sci. 2015, 123, 297–304. [Google Scholar] [CrossRef]
- Gowri, N.S.; Reddy, K.M.; Shastry, Y.M.; Aditya, S.V.; Dubey, D. The effect of ceramic bonder on shear bond strength at the metal-ceramic interface in casted and direct metal laser sintering cobalt-chromium alloy—An in vitro study. J. Indian Prosthodont. Soc. 2024, 24, 159–164. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, X.; Li, N.; Yan, J. Effects of posttreatment on the metal-ceramic bond properties of selective-laser-melted Co-Cr dental alloy-Part 2: Heat treatment after porcelain firing. J. Prosthet. Dent. 2025, 133, 292–300. [Google Scholar] [CrossRef]
- Deepak, K.; Ahila, S.C.; Muthukumar, B.; Vasanthkumar, M. Comparative evaluation of effect of laser on shear bond strength of ceramic bonded with two base metal alloys: An in-vitro study. Indian J. Dent. Res. 2013, 24, 610–615. [Google Scholar] [CrossRef]
- Meenakshi, T.; Bharathi, M.; Komala, J. Evaluation of the Effect of recasting Nickel-chromium Base Metal Alloy on the Metal-ceramic Bond Strength: An in vitro Study. J. Contemp. Dent. Pract. 2017, 18, 837–841. [Google Scholar] [CrossRef]
- Abdullah Alsadon, O. Adhesion concepts and techniques for laboratory-processed indirect dental restorations. Saudi Dent. J. 2022, 34, 661–668. [Google Scholar] [CrossRef] [PubMed]
- von Fraunhofer, J.A. Adhesion and cohesion. Int. J. Dent. 2012, 2012, 951324. [Google Scholar] [CrossRef] [PubMed]
- Toduran, S.; Ciocan, L.T.; Spinu, T.; Galbinasu, B.M.; Patrascu, I.; Vasilescu, V.G. Adhesion evaluation of dental ceramics sintered on novel titanium alloys. Rom. J. Stomatol. 2023, 69, 2013–2022. [Google Scholar] [CrossRef]
- Kotian, R.; Mariam, S.P.; Naik, S.; Prashanthi, M. Effect of heat treatment on the microstructure and hardness of Ni-Cr base metal alloys. Indian Prosthodont. Soc. 2008, 8, 17–21. [Google Scholar] [CrossRef]
- Yamamoto, M.; Tanaka, M.; Furukimi, O. Hardness-Deformation Energy Relationship in Metals and Alloys: A Comparative Evaluation Based on Nanoindentation Testing and Thermodynamic Consideration. Materials 2021, 14, 7217. [Google Scholar] [CrossRef]
- Niem, T.; Gonschorek, S.; Wöstmann, B. Evaluation of the damping capacity of common CAD/CAM restorative materials. J. Mech. Behav. Biomed. Mater. 2022, 126, 104987. [Google Scholar] [CrossRef]
- Nieva, N.; Arreguez, C.; Carrizo, R.N.; Molé, C.S.; Lagarrigue, G.M. Bonding Strength Evaluation on Metal/Ceramic Interfaces in Dental Materials. Procedia Mater. Sci. 2012, 1, 475–482. [Google Scholar] [CrossRef]
- do Nascimento, C.; Miani, P.K.; Bezzon, O.L.; Gonçalves, M.; de Albuquerque, R.F., Jr. Shear bond strength between Ni-Cr alloy bonded to a ceramic substrate. Gerodontology 2012, 29, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Park, W.U.; Jung, S.H.; Zhao, J.; Hwang, K.H.; Lee, J.K.; Mitchell, J.C. Effects of Oxide Layer on the Bonding Strength of Ni-Cr Alloys with Porcelain Ceramics. J. Nanosci. Nanotechnol. 2015, 15, 5901–5904. [Google Scholar] [CrossRef] [PubMed]
- Pagnano, V.O.; Leal, M.B.; Catirse, A.B.C.E.B.; Curylofo, P.A.; Silva, R.F.; Macedo, A.P. Effect of oxidation heat treatment with airborne-particle abrasion on the shear bond strength of ceramic to base metal alloys. J. Prosth. Dent. 2021, 126, 804.e1–804.e9. [Google Scholar] [CrossRef]
- Pisch, I.V.; Radion, E.V. The effect of oxides on stabilization of zirconium dioxide. Glass Ceram 1999, 56, 397–400. [Google Scholar] [CrossRef]
- Galhenage, R.P.; Yan, H.; Tenney, S.A.; Park, N.; Henkelman, G.; Albrecht, P.; Mullins, D.R.; Chen, D.A. Understanding the Nucleation and Growth of Metals on TiO2: Co Compared to Au, Ni, and Pt. J. Phys. Chem. 2013, 117, 7191–7201. [Google Scholar] [CrossRef]
- Cetiner, D.; Paksoy, A.; Tazegul, O.; Baydogan, M.; Güleryüz, H.; Cimenoglu, H.; Atar, E. Thermal Oxidation of Cold Sprayed Titanium-Based Coating Deposited on Co-Cr Alloy. J. Therm. Spray Technol. 2018, 27, 1414–1427. [Google Scholar] [CrossRef]
- Li, H.; Chen, L.; Yuan, X.; Zhang, W.; Smith, J.R.; Evans, A.G. Interfacial Stoichiometry and Adhesion at Metal/α-Al2O3 Interfaces. J. Am. Ceram. Soc. 2011, 94, s154–s159. [Google Scholar] [CrossRef]
- Nagao, K.; Neaton, J.B.; Ashcroft, N.W. First-principles study of adhesion at Cu/SiO2 interfaces. Phys. Rev. B 2003, 68, 125403. [Google Scholar] [CrossRef]
- Scherrer, S.S.; Quinn, G.D.; Quinn, J.B. Fractographic failure analysis of a Procera AllCeram crown using stereo and scanning electron microscopy. Dent. Mater. 2008, 24, 1107–1113. [Google Scholar] [CrossRef]
- Davis, M.J.; McGregor, A. Assessing adhesive bond failures: Mixed-mode bond failures explained. In Proceedings of the ISASI, Australian Safety Seminar, Canberra, Australia, 4 June 2010; pp. 4–6. [Google Scholar]



| Material | Composition | Batch No. | Manufacturer |
|---|---|---|---|
| VeraBond Type 5 | Ni 77.9% Cr 12.6% Mo 5% Al 2.9%, Be 1.9%, Co | 210712 | AalbaDent, Fairfield, CA, USA |
| Ceramco® 3 Opaque A2 | Powder porcelain, Sodium Potassium aluminosilicate 60–70% WT, Tin Oxide 0–20% WT | 23003177 | Dentslpy Sirona, York, PA, USA |
| Ceramco® 3 Dentin A2 | Powder porcelain, Sodium Potassium Aluminosilicate 60–70% WT, Tin Oxide 0–20% WT | 23003701 | Denstply Sirona, York, PA, USA |
| Ceramco® 3 Enamel Clear | Powder porcelain, Sodium Potassium Aluminosilicate 60–70% WT, Tin Oxide 0–20% WT | 23003671 | Dentsply Sirona, York, PA, USA |
| DU | Modeling liquid, Non-hazardous ingredients Mixture | 18003537 | Dentsply Sirona, York, PA, USA |
| Smart Vest | Phosphate bonded investment | F271123 | Smart Family, Guadalajara, Jalisco, Mexico |
| Kemdent Wax | Calibrated modeling wax #28 | 43662 | Dental Products Ltd., Wiltshire, UK |
| Zeta Sand | 99.80% Al2O3 | U112682/A | Zhermack Technical, Roma, Italy |
| Material | Pre-Heating Tem. (°C) | Drying Time (min) | Heating Rate (°C/min) | Final Temp °C | Holding Time (s) | Vacuum Start/Stop |
|---|---|---|---|---|---|---|
| Oxidation | 600 | 1 | 70 | 980 | 0 | No vacuum |
| First opaque | 650 | 5 | 70 | 970 | 0 | 650/970 |
| Second opaque | 650 | 5 | 70 | 970 | 0 | 650/970 |
| Dentine | 650 | 3 | 45 | 930 | 60 | 650/930 |
| Enamel | 650 | 3 | 45 | 930 | 60 | 650/930 |
| Group | Ra µm | Rz µm | HLD |
|---|---|---|---|
| C | 0.91 ± 0.26 | 5.09 ± 1.20 | 575.06 ± 81.33 |
| G1-APA | 1.15 ± 0.14 * | 8.14 ± 0.65 * | 624.73 ± 104.0 * |
| G2-APA-O | 0.76 ± 0.05 | 5.57 ± 0.52 | 537.73 ± 45.12 |
| G3-APA-O-APA | 1.03 ± 0.04 | 7.55 ± 0.74 | 492.73 ± 35.11 |
| Spectrum | O | Al | Si | S | Ti | Cr | Co | Ni | Total |
|---|---|---|---|---|---|---|---|---|---|
| Control | 0.87 | 3.33 | 0.29 | 1.87 | 0.46 | 13.19 | 0.56 | 79.43 | 100.00 |
| G1-APA | 8.23 | 10.13 | - | 1.52 | 0.27 | 11.18 | 0.40 | 68.28 | 100.00 |
| G2-APA-O | 30.40 | 7.66 | - | 1.07 | 0.26 | 9.51 | 0.35 | 50.74 | 100.00 |
| G2-APA-O-APA | 11.80 | 11.76 | 0.27 | 1.33 | 0.39 | 10.49 | 0.38 | 63.57 | 100.00 |
| Group | DCIS MPa |
|---|---|
| C | 37.51 ± 30.81 |
| G1-APA | 33.35 ± 37.30 |
| G2-APA-O | 63.97 ± 44.40 * |
| G3-APA-O-APA | 41.19 ± 33.88 |
| Mixed Failure | Mixed Failure | Cohesive Failure | ||
|---|---|---|---|---|
| Surface Treatment | Score 1 n (%) | Score 2 n (%) | Score 3 n (%) | Total |
| C | 5 (16.6) | 25 (83.3) | 0 | 30 |
| G1-APA | 0 | 30 (100) | 0 | 30 |
| G2-APA-O | 0 | 24 (80) | 6 (20) | 30 |
| G3-APA-O-APA | 11 (36.6) | 19 (63.3) | 0 | 30 |
| Total | 16 (13.3) | 98 (81.7) | 6 (5) | 120 |
| Surface Treatment | Adhesive | Cohesive | Mixed |
|---|---|---|---|
| C | 0 | 0 | 30 |
| G1-APA | 0 | 0 | 30 |
| G2-APA-O | 0 | 6 | 24 |
| G3-APA-O-APA | 0 | 0 | 30 |
| Total | 0 | 6 | 114 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Ferreyra, B.I.; Moyaho-Bernal, M.d.l.A.; Chavarría-Lizárraga, H.N.; Castro-Ramos, J.; Franco-Romero, G.; Velázquez-Enríquez, U.; Flores-Ledesma, A.; Reyes-Cervantes, E.; Ley-García, A.K.; Velasco-León, E.d.C.; et al. Surface Treatment, Chemical Characterization, and Debonding Crack Initiation Strength for Veneering Dental Ceramics on Ni-Cr Alloys. Materials 2025, 18, 3822. https://doi.org/10.3390/ma18163822
Flores-Ferreyra BI, Moyaho-Bernal MdlA, Chavarría-Lizárraga HN, Castro-Ramos J, Franco-Romero G, Velázquez-Enríquez U, Flores-Ledesma A, Reyes-Cervantes E, Ley-García AK, Velasco-León EdC, et al. Surface Treatment, Chemical Characterization, and Debonding Crack Initiation Strength for Veneering Dental Ceramics on Ni-Cr Alloys. Materials. 2025; 18(16):3822. https://doi.org/10.3390/ma18163822
Chicago/Turabian StyleFlores-Ferreyra, Blanca Irma, María de los Angeles Moyaho-Bernal, Héctor Nahum Chavarría-Lizárraga, Jorge Castro-Ramos, Guillermo Franco-Romero, Ulises Velázquez-Enríquez, Abigailt Flores-Ledesma, Eric Reyes-Cervantes, Ana Karina Ley-García, Estela del Carmen Velasco-León, and et al. 2025. "Surface Treatment, Chemical Characterization, and Debonding Crack Initiation Strength for Veneering Dental Ceramics on Ni-Cr Alloys" Materials 18, no. 16: 3822. https://doi.org/10.3390/ma18163822
APA StyleFlores-Ferreyra, B. I., Moyaho-Bernal, M. d. l. A., Chavarría-Lizárraga, H. N., Castro-Ramos, J., Franco-Romero, G., Velázquez-Enríquez, U., Flores-Ledesma, A., Reyes-Cervantes, E., Ley-García, A. K., Velasco-León, E. d. C., & Carrasco-Gutiérrez, R. G. (2025). Surface Treatment, Chemical Characterization, and Debonding Crack Initiation Strength for Veneering Dental Ceramics on Ni-Cr Alloys. Materials, 18(16), 3822. https://doi.org/10.3390/ma18163822











