Preparation and Mechanical Properties of Alkali-Treated Wood Flour/Dynamic Polyurethane Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Alkali-Treated Wood Flour
2.3. Preparation of a Low Transition Temperature Mixture
2.4. Preparation of Wood Flour/Dynamic Polyurethane Composites
2.5. Preparation of Reprocessed Wood Flour/Dynamic Polyurethane Composites
2.6. Measurement and Characterization
3. Results and Discussion
3.1. Chemical Structure
3.2. Microstructure and Interface Bonding
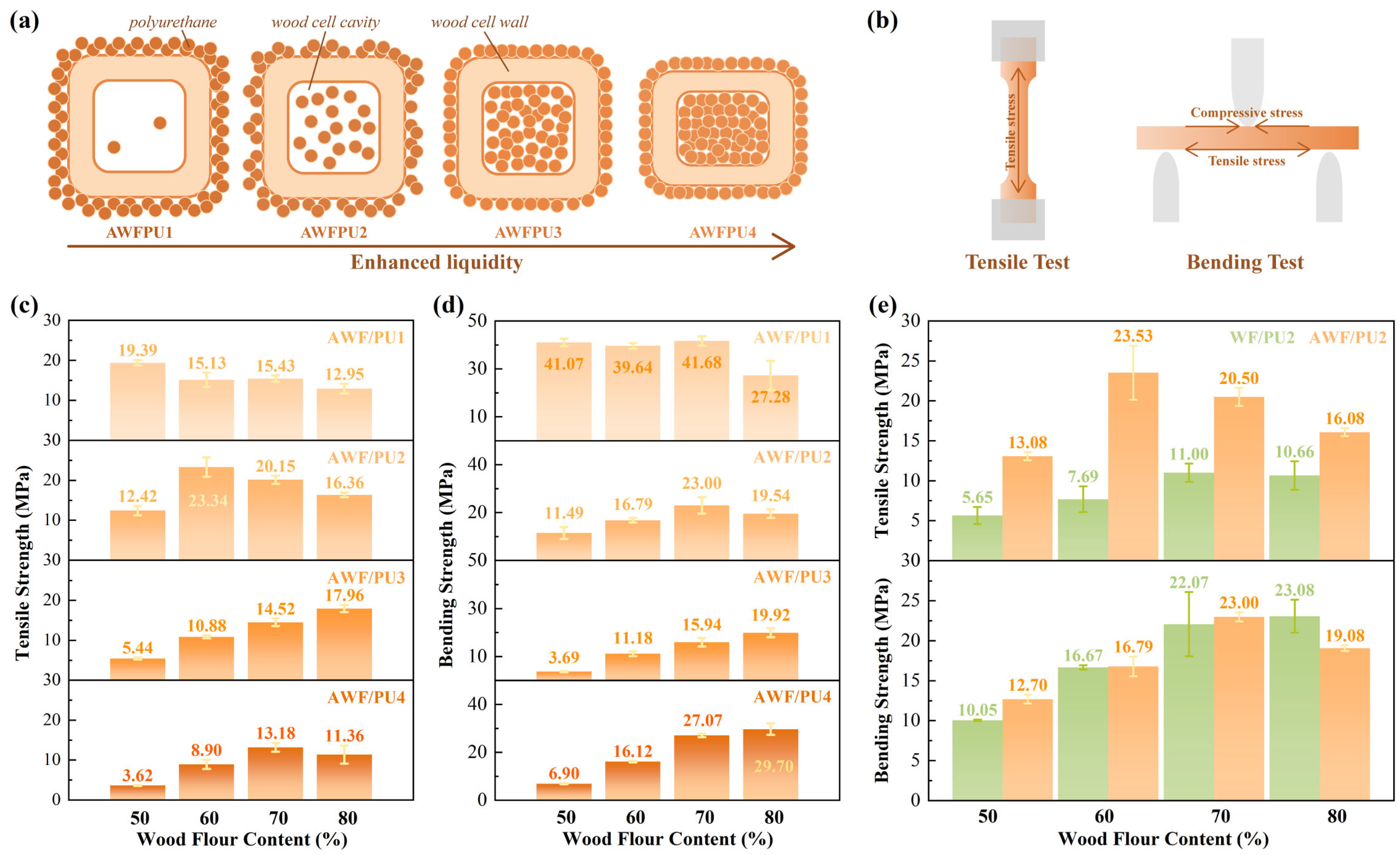
3.3. Mechanical Properties
3.4. Reprocessability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WPC | Wood plastic composite |
| TA | Tannic acid |
| PEG | Polyethylene glycol |
| IPDI | Isophorone diisocyanate |
| WF | Wood flour |
| AWF | Alkali-treated wood flour |
| LTTMs | Low transition temperature mixture |
| WFPU | Wood flour/dynamic polyurethane composites |
| AWFPU | Alkali-treated wood flour/dynamic polyurethane composites |
| Re-WFPU | Reprocessed wood flour/dynamic polyurethane composites |
| Re-AWFPU | Reprocessed alkali-treated wood flour/dynamic polyurethane composites |
References
- Xiao, R.; Yu, Q.; Ye, H.; Shi, Y.; Sheng, Y.; Zhang, M.; Nourani, P.; Ge, S. Visual Design of High-Density Polyethylene into Wood Plastic Composite with Multiple Desirable Features: A Promising Strategy for Plastic Waste Valorization. J. Build. Eng. 2023, 63, 105445. [Google Scholar] [CrossRef]
- Ramli, R.A. A Comprehensive Review on Utilization of Waste Materials in Wood Plastic Composite. Mater. Today Sustain. 2024, 27, 100889. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Guo, S.; Liu, T. A Review of Coextruded Wood–Plastic Composites. Polym. Compos. 2021, 42, 4174–4186. [Google Scholar] [CrossRef]
- Mitaľová, Z.; Mitaľ, D.; Berladir, K. A Concise Review of the Components and Properties of Wood–Plastic Composites. Polymers 2024, 16, 1556. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Luo, Z.; Cai, T.; Cai, D.; Zhang, C.; Qin, P.; Cao, H. The Effect of Corn Varieties on the Production of Fiber-Reinforced High-Density Polyethylene Composites. Biomass Convers. Biorefinery 2018, 8, 953–963. [Google Scholar] [CrossRef]
- Nayak, S.; Khuntia, S.K.; Mohanty, S.D.; Mohapatra, J. Investigation and Fabrication of Thermo-Mechanical Properties of Ceiba Pentandra Bark Fiber/Poly (Vinyl) Alcohol Composites for Automobile Dash Board and Door Panel Applications. J. Nat. Fibers 2020, 19, 450–462. [Google Scholar] [CrossRef]
- Zheng, S.; Liu, G. Polymeric Emissive Materials Based on Dynamic Covalent Bonds. Molecules 2022, 27, 6635. [Google Scholar] [CrossRef]
- Chakma, P.; Konkolewicz, D. Dynamic Covalent Bonds in Polymeric Materials. Angew. Chem. Int. Ed. 2019, 58, 9682–9695. [Google Scholar] [CrossRef]
- Das, M.; Parathodika, A.R.; Maji, P.; Naskar, K. Dynamic Chemistry: The Next Generation Platform for Various Elastomers and Their Mechanical Properties with Self-Healing Performance. Eur. Polym. J. 2023, 186, 111844. [Google Scholar] [CrossRef]
- Cai, Y.; Zou, H.; Zhou, S.; Chen, Y.; Liang, M. Room-Temperature Self-Healing Ablative Composites Via Dynamic Covalent Bonds for High-Performance Applications. ACS Appl. Polym. Mater. 2020, 2, 3977–3987. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Phan, T.T.T.; Nam, Y.J.; Lee, J.S. Dynamic Covalent Bond Network-Based Carbon Nanocomposite for a Self-Healing Tactile Sensor. ACS Appl. Electron. Mater. 2023, 5, 4417–4425. [Google Scholar] [CrossRef]
- Mo, J.; Wang, H.; Yan, M.; Huang, J.; Li, R.; Sun, D.; Lei, J.; Qiu, X.; Liu, W. Construction of Interfacial Dynamic Bonds for High Performance Lignin/Polymer Biocomposites. Front. Chem. Sci. Eng. 2023, 17, 1372–1388. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and Stabilization of Polyurethane Elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Duan, X.; Cao, W.; He, X.; Wang, M.; Cong, R.; Zhang, Z.; Ning, C.; Wang, C.; Zhao, S.; Li, Z.; et al. Realization of Dual Crosslinked Network Robust, High Toughness Self-Healing Polyurethane Elastomers for Electronics Applications. Chem. Eng. J. 2023, 476, 146536. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, M.; Gao, M.; Zhang, Y.; Ye, Q.; Zhu, X. Research Progress on Wood Delignification and Its Application. Chem. Ind. For. Prod. 2024, 44, 52–64. [Google Scholar] [CrossRef]
- Wang, J.; Fishwild, S.J.; Begel, M.; Zhu, J.Y. Properties of Densified Poplar Wood through Partial Delignification with Alkali and Acid Pretreatment. J. Mater. Sci. 2020, 55, 14664–14676. [Google Scholar] [CrossRef]
- Shakeel, U.; Zhang, Y.; Topakas, E.; Wang, W.; Liang, C.; Qi, W. Unraveling Interplay between Lignocellulosic Structures Caused by Chemical Pretreatments in Enhancing Enzymatic Hydrolysis. Carbohydr. Polym. 2024, 334, 122037. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, U.; Zhang, Y.; Liang, C.; Wang, W.; Qi, W. Unrevealing the Influence of Reagent Properties on Disruption and Digestibility of Lignocellulosic Biomass During Alkaline Pretreatment. Int. J. Biol. Macromol. 2024, 266, 131193. [Google Scholar] [CrossRef] [PubMed]
- Ishikura, Y.; Abe, K.; Yano, H. Bending Properties and Cell Wall Structure of Alkali-Treated Wood. Cellulose 2009, 17, 47–55. [Google Scholar] [CrossRef]
- Oka, D.; Kobayashi, K.; Isobe, N.; Ogawa, Y.; Yokoyama, T.; Kimura, S.; Kim, U.-J.; Tokuyasu, K.; Wada, M. Enzymatic Hydrolysis of Wood with Alkaline Treatment. J. Wood Sci. 2013, 59, 484–488. [Google Scholar] [CrossRef]
- Chang, W.-P.; Kim, K.-J.; Gupta, R.K. Moisture Absorption Behavior of Wood/Plastic Composites Made with Ultrasound-Assisted Alkali-Treated Wood Particulates. Compos. Interfaces 2012, 16, 937–951. [Google Scholar] [CrossRef]
- Wang, H. Study on Poplar Powder/Dynamic Polyurethane Composites Based on Low Transition Temperature Mixture. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2023. Available online: https://link.cnki.net/doi/10.27009/d.cnki.gdblu.2023.000058 (accessed on 30 July 2025).
- Zhang, N.; He, L.; Yang, X.; Chen, C.; Liu, Y.; Lu, Q.; Huang, Y.; Ding, S.; Liu, R. Preparation; Characterization; Evaluation of “Double Resistance” Activity of Gallnut Tannic Acid Microcapsules. Food Ferment. Ind. 2024, 50, 121–127. [Google Scholar] [CrossRef]
- Xv, Z.; Ma, X.; Zhang, D.; Huang, W.; Dai, X.; Song, W.; Cheng, C. Preparation of Polyethylene Glycol Modified Lyocell Fiber and Study on Its Antigen Fibrillation. J. Cellul. Sci. Technol. 2021, 29, 19–25. [Google Scholar] [CrossRef]
- Xia, M. The Synthesis Process of Mixed Acid Peg Ester Was Studied by Infrared Spectroscopy. J. Qiqihar Univ. 2003, 19, 32–34. [Google Scholar]
- Wang, H.; Fu, Y.; Liu, Y.; Li, J.; Sun, X.; Liu, T. Synthesis of Dynamic Polyurethanes by Utilizing Low-Transition-Temperature Mixtures of Pyrogallol and Poly(Ethylene Glycol). ACS Appl. Polym. Mater. 2024, 6, 8786–8797. [Google Scholar] [CrossRef]
- Yen, F.-S.; Hong, J.-L. Hydrogen-Bond Interactions between Ester and Urethane Linkages in Small Model Compounds and Polyurethanes. Macromolecules 1997, 30, 7927–7938. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.; Zhang, L.; Huang, H.; Peng, R.; Jin, B. Synthesis and Characterization of Random Block Hydroxyl-Terminated Polyfluoroether-Based Polyurethane Elastomers with Fluorine-Containing Side Chains. Polymers 2023, 15, 288. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Liu, Y.; Fu, Y.; Zhang, X.; Qi, B.; Liu, T. Preparation and Closed-Loop Recycling of Ultra-High-Filled Wood Flour/Dynamic Polyurethane Composites. Polymers 2023, 15, 1418. [Google Scholar] [CrossRef]
- Liu, S.; Yue, K.; Qian, J.; Lu, D.; Wu, P.; Li, Q.; Zhang, Z. Integrated Approach for Improving Mechanical and High-Temperature Properties of Fast-Growing Poplar Wood Using Lignin-Controlled Treatment Combined with Densification. Int. J. Biol. Macromol. 2024, 280, 135949. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhou, Y.; Wen, S.; Zhou, C. A Review: Additive Manufacturing of Wood-Plastic Composites. Cellulose 2024, 31, 5315–5341. [Google Scholar] [CrossRef]
- Dikobe, D.G.; Luyt, A.S. Thermal and Mechanical Properties of Pp/Hdpe/Wood Powder and Mapp/Hdpe/Wood Powder Polymer Blend Composites. Thermochim. Acta 2017, 654, 40–50. [Google Scholar] [CrossRef]
- Rao, J.; Zhou, Y.; Fan, M. Revealing the Interface Structure and Bonding Mechanism of Coupling Agent Treated Wpc. Polymers 2018, 10, 266. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Nie, M.; Chen, N.; Han, D.; Liu, B.; Cheng, L. Amide Wax-Assisted Interfacial Compatibilization of Maleic Anhydride-Grafted Polyethylene for High-Performance Wood-Plastic Composites. Wood Mater. Sci. Eng. 2024, 19, 1–9. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diao, Y.; Li, M.; Yu, C.; Han, Z.; Wang, S.; Liu, Y.; Wu, J.; Liu, T. Preparation and Mechanical Properties of Alkali-Treated Wood Flour/Dynamic Polyurethane Composites. Materials 2025, 18, 3817. https://doi.org/10.3390/ma18163817
Diao Y, Li M, Yu C, Han Z, Wang S, Liu Y, Wu J, Liu T. Preparation and Mechanical Properties of Alkali-Treated Wood Flour/Dynamic Polyurethane Composites. Materials. 2025; 18(16):3817. https://doi.org/10.3390/ma18163817
Chicago/Turabian StyleDiao, Yifan, Manyu Li, Chenglei Yu, Zhenqi Han, Shuyuan Wang, Yue Liu, Jianguo Wu, and Tian Liu. 2025. "Preparation and Mechanical Properties of Alkali-Treated Wood Flour/Dynamic Polyurethane Composites" Materials 18, no. 16: 3817. https://doi.org/10.3390/ma18163817
APA StyleDiao, Y., Li, M., Yu, C., Han, Z., Wang, S., Liu, Y., Wu, J., & Liu, T. (2025). Preparation and Mechanical Properties of Alkali-Treated Wood Flour/Dynamic Polyurethane Composites. Materials, 18(16), 3817. https://doi.org/10.3390/ma18163817






