Green Low-Temperature Activation and Curing for High-Toughness Geopolymer Binders from Diabase Tailings
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Thermal Pretreatment
2.2. Analytical Methods
3. Structural Characterization of Activated Diabase Tailings
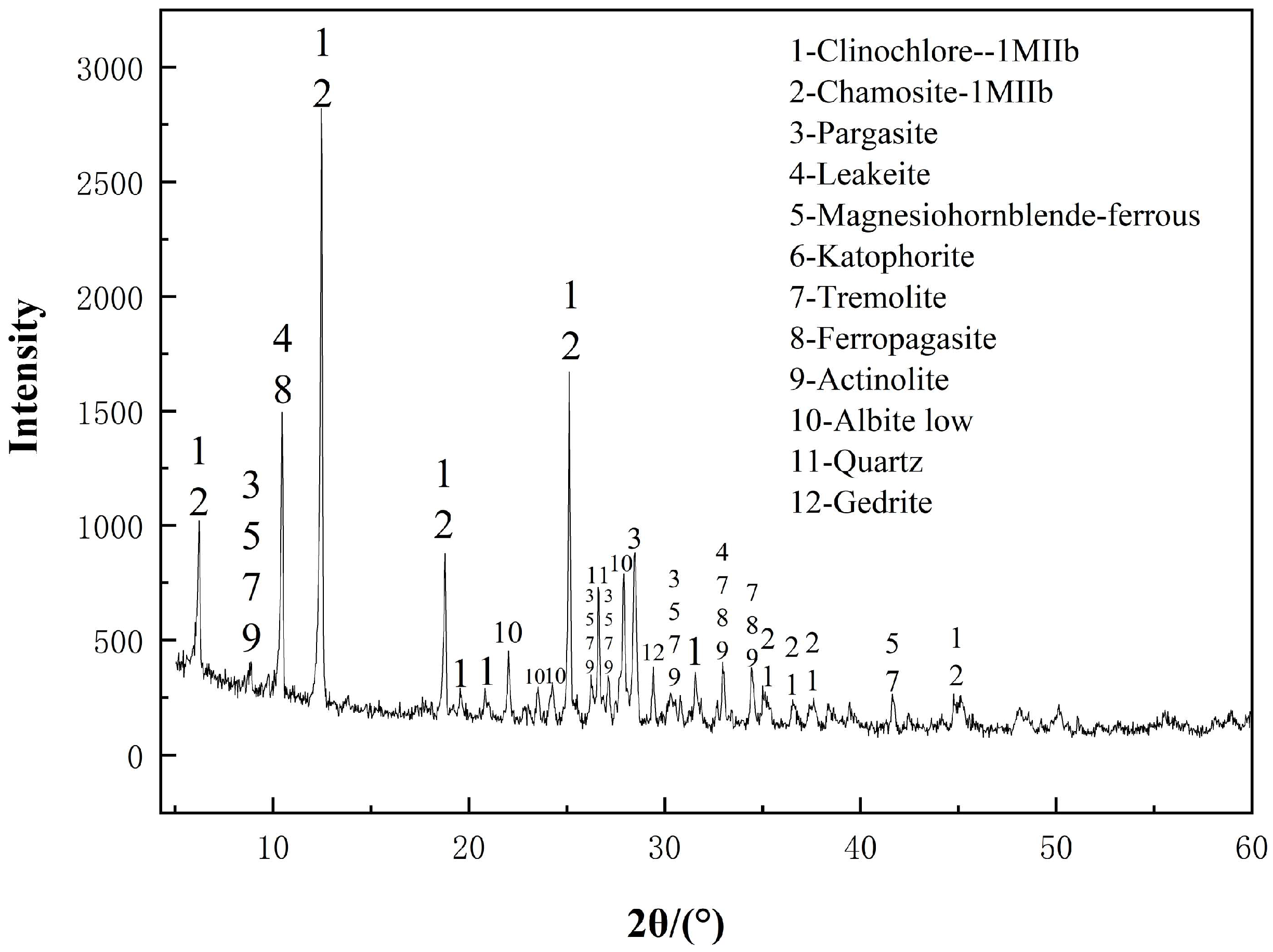
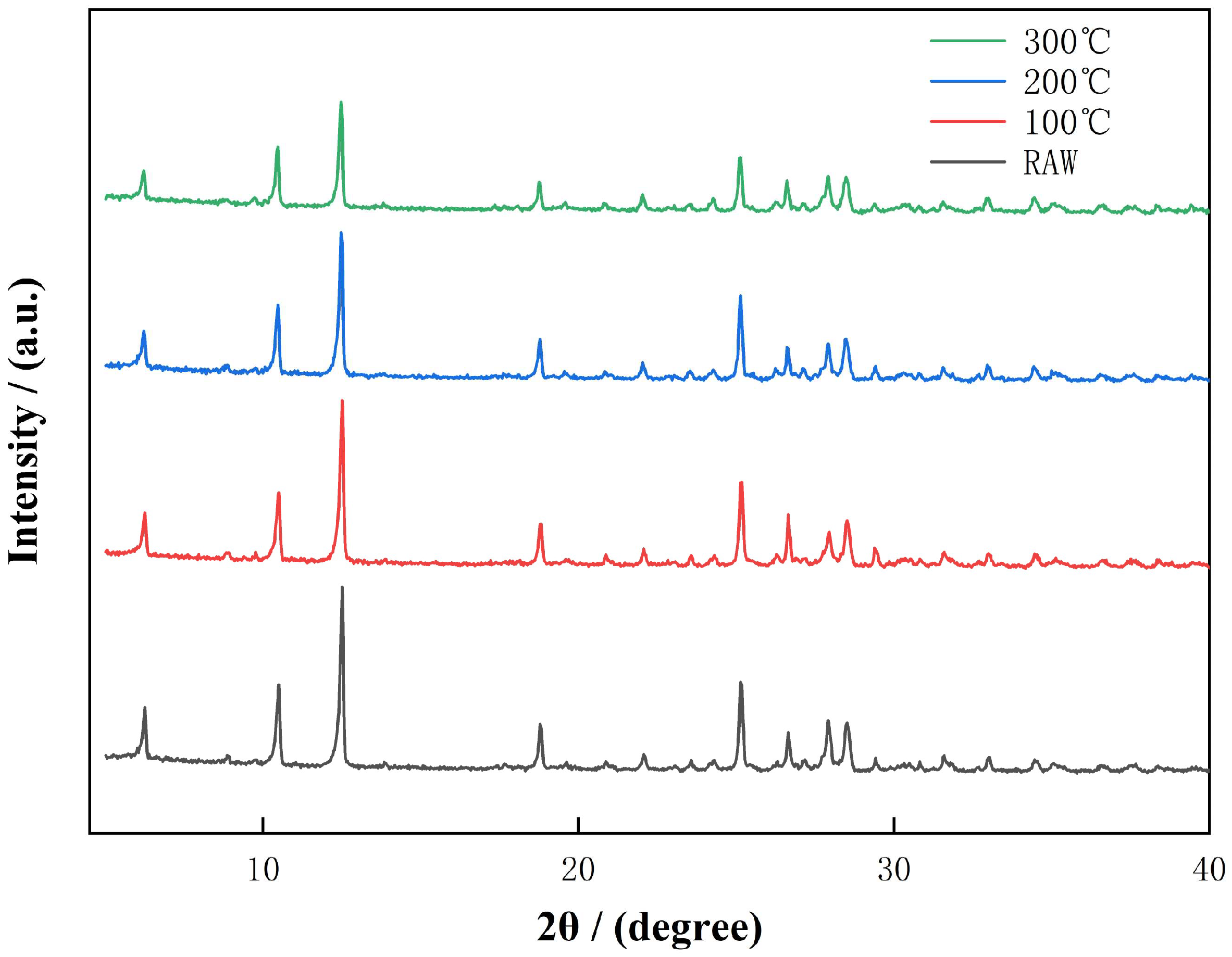
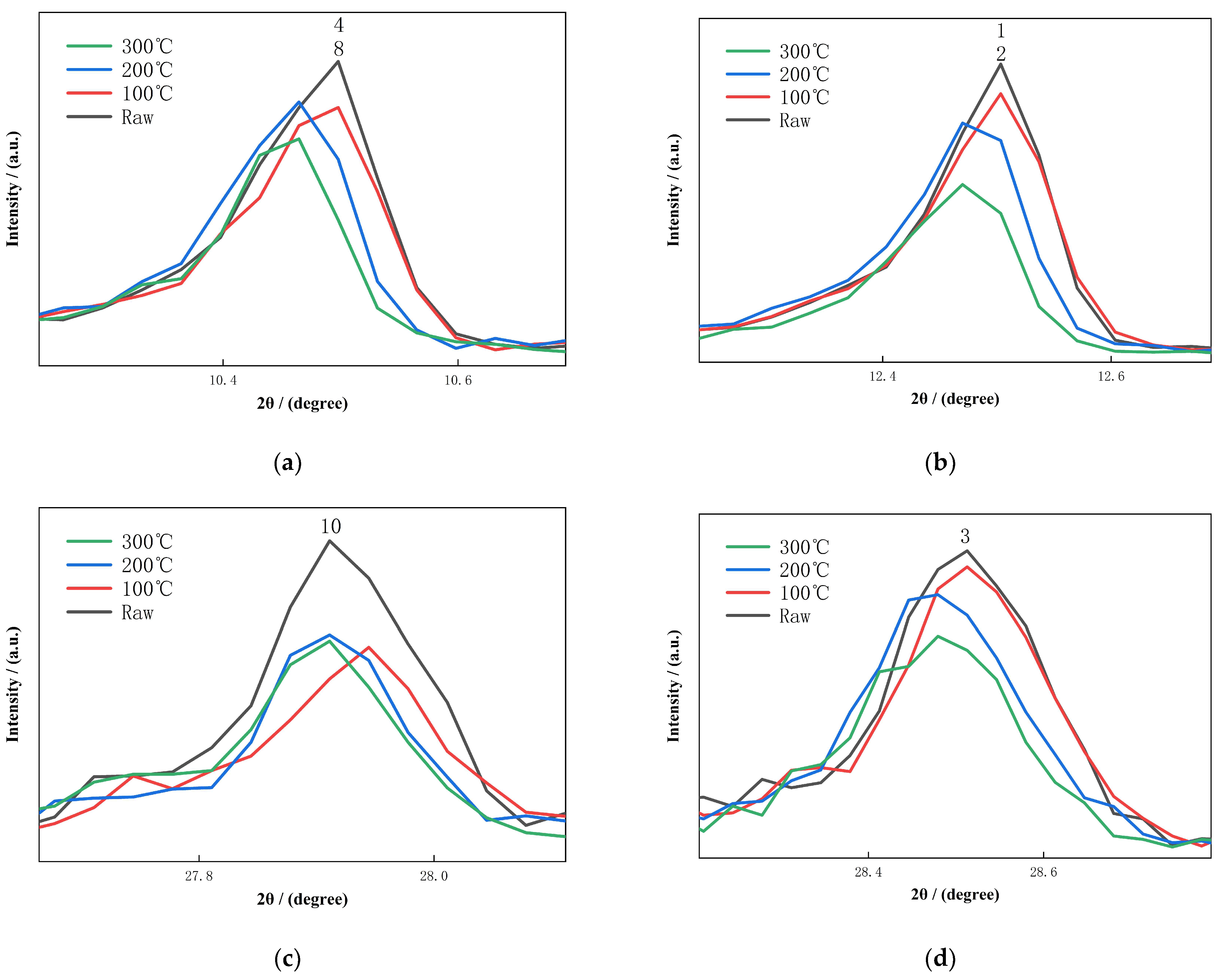
3.1. XRD Analysis of Activated Diabase Tailings
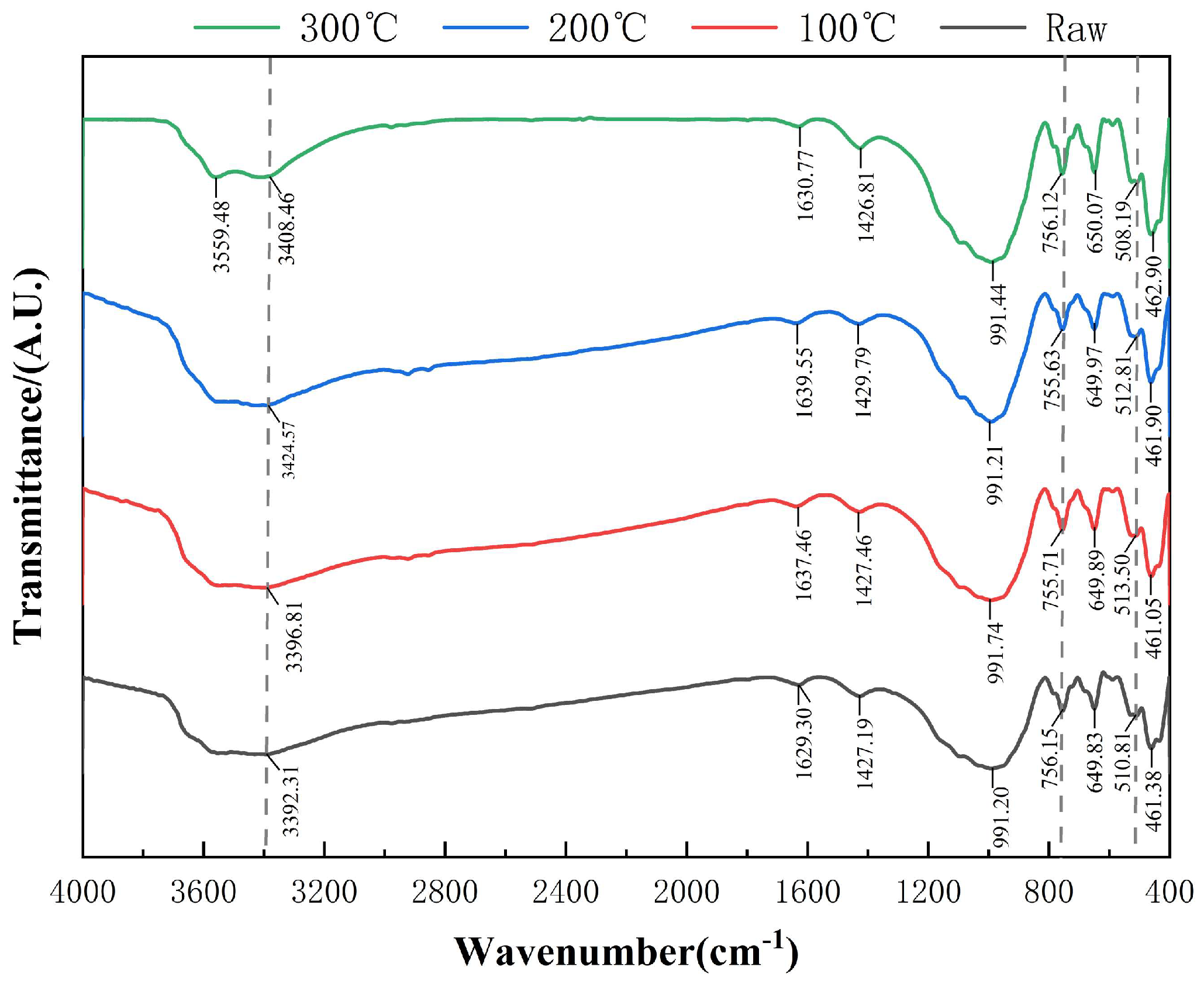
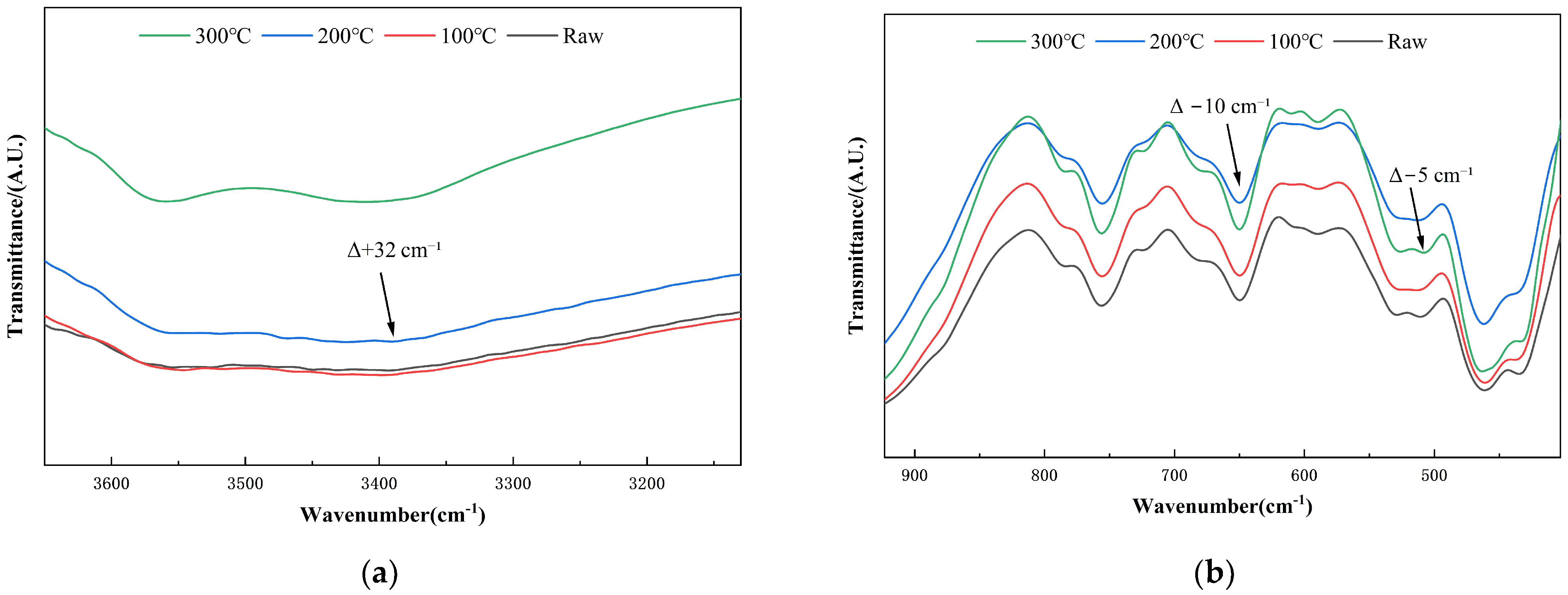
3.2. FTIR Analysis of Activated Diabase Tailings
4. Mechanical Behavior of Diabase Tailings-Based Geopolymers (DTG)
4.1. Analysis of Strength Development
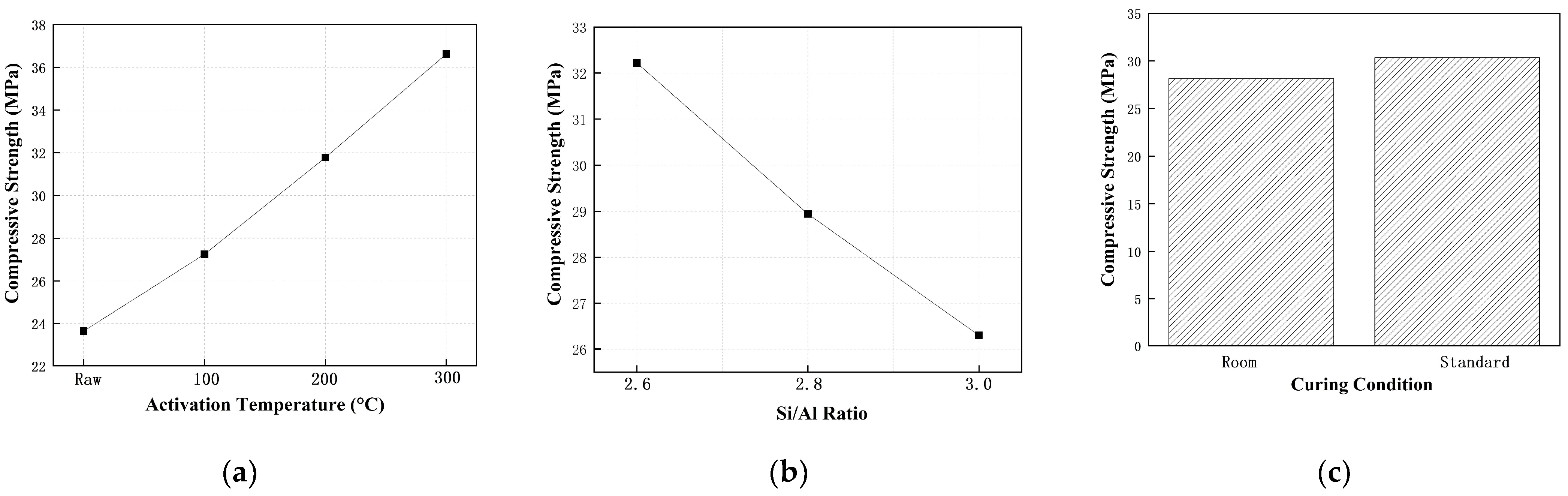
4.1.1. Effect of Thermal Activation Temperature
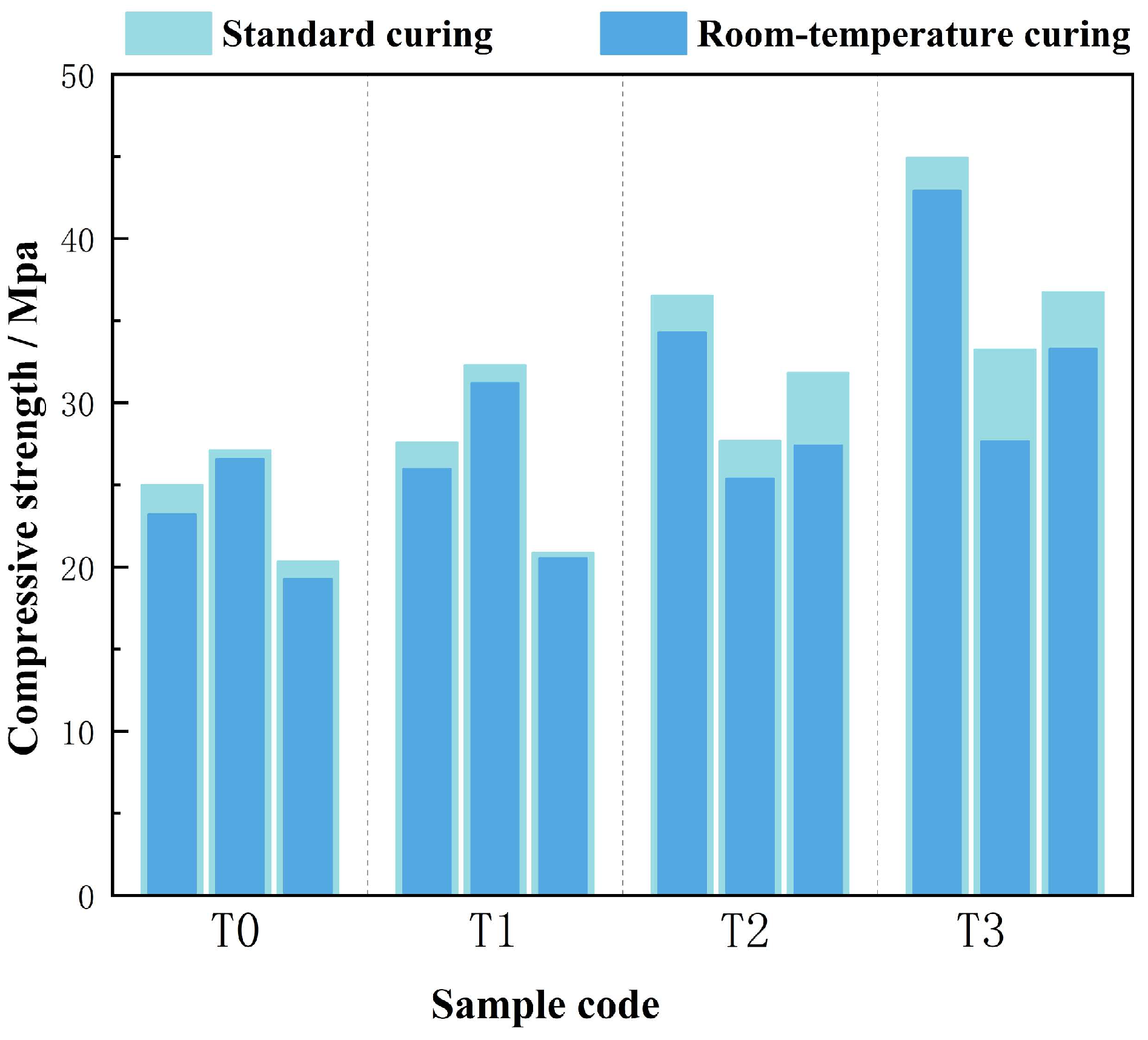
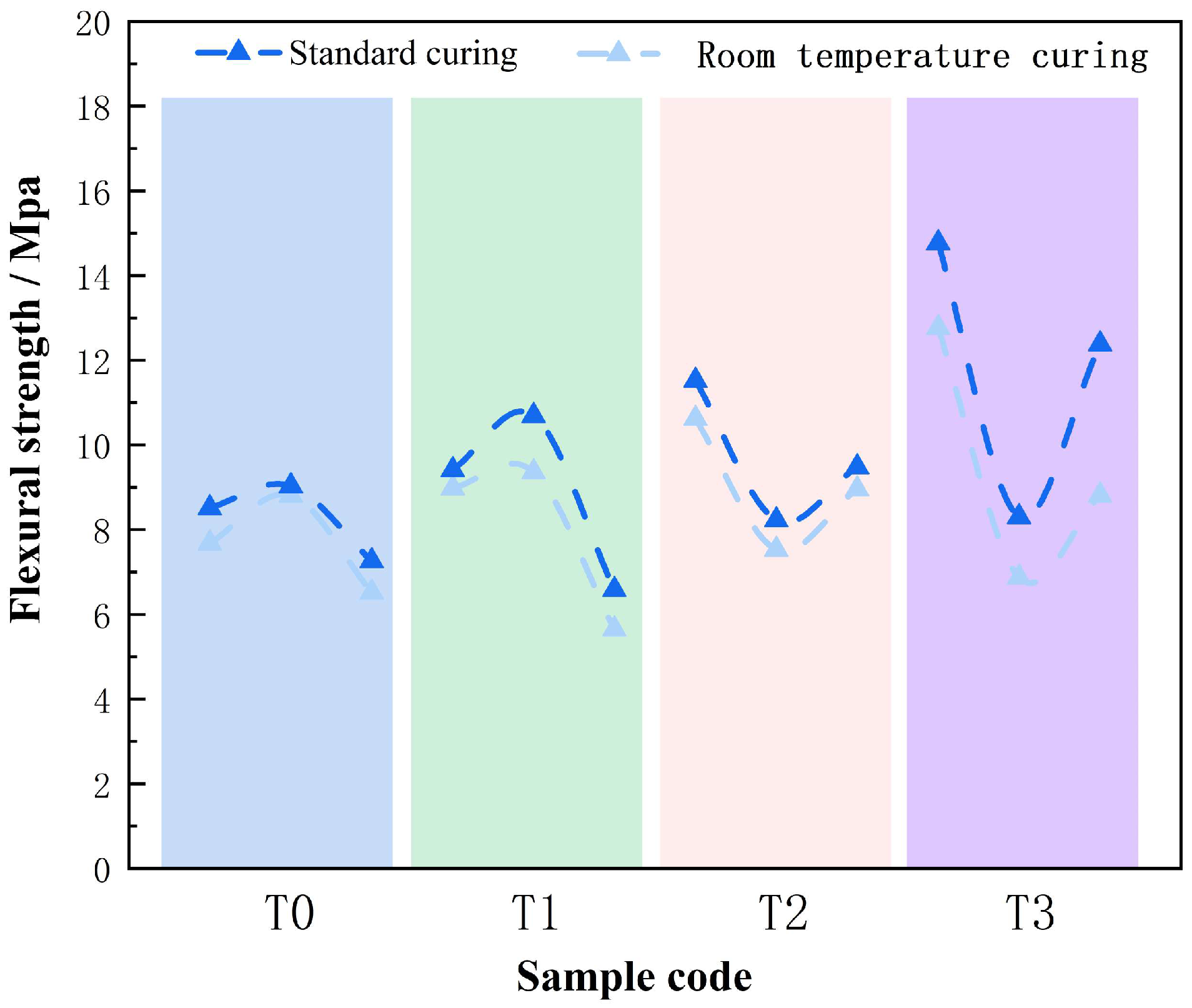
4.1.2. Effect of Curing Conditions
4.1.3. Statistical Analysis of Orthogonal Design
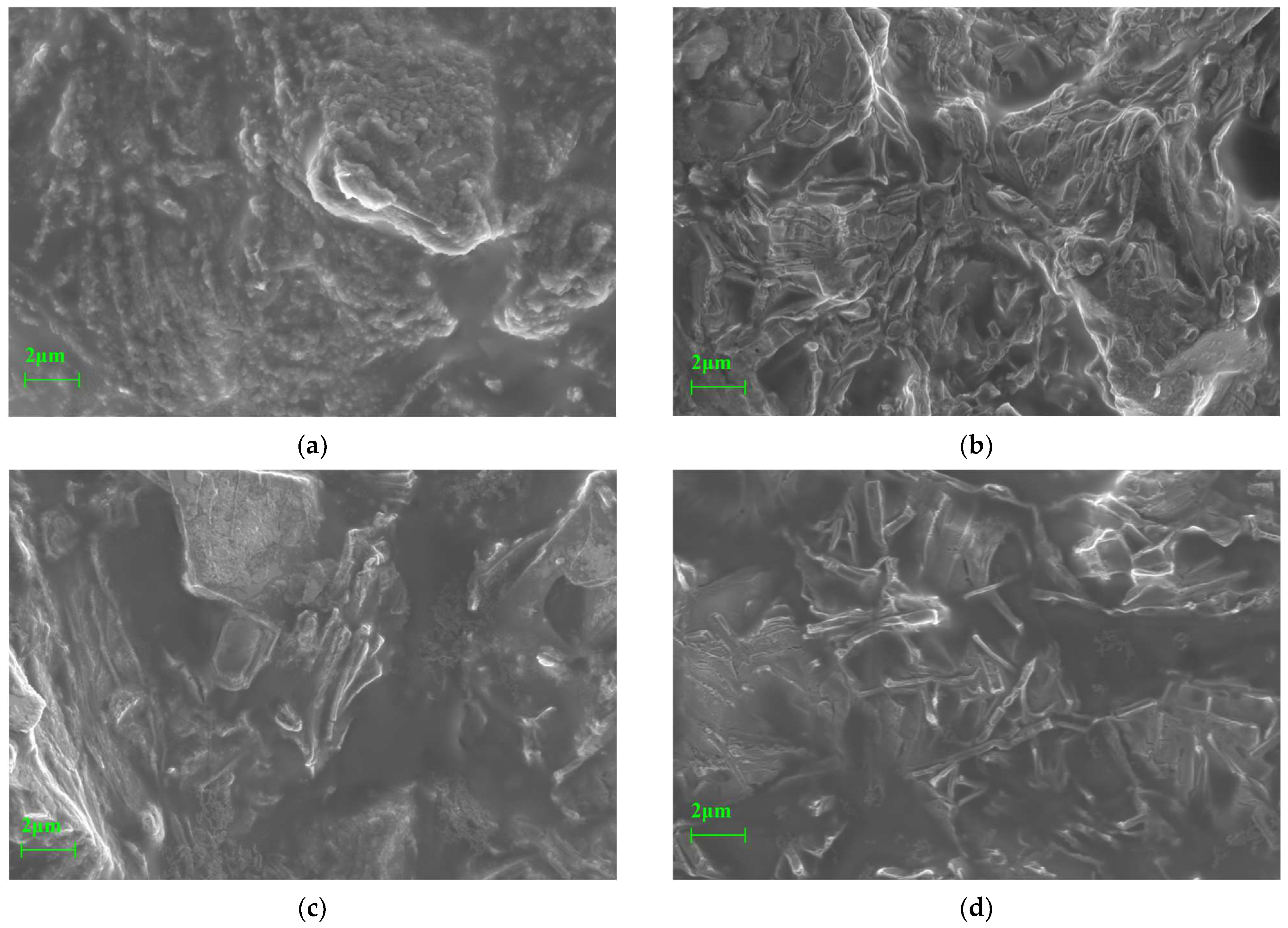
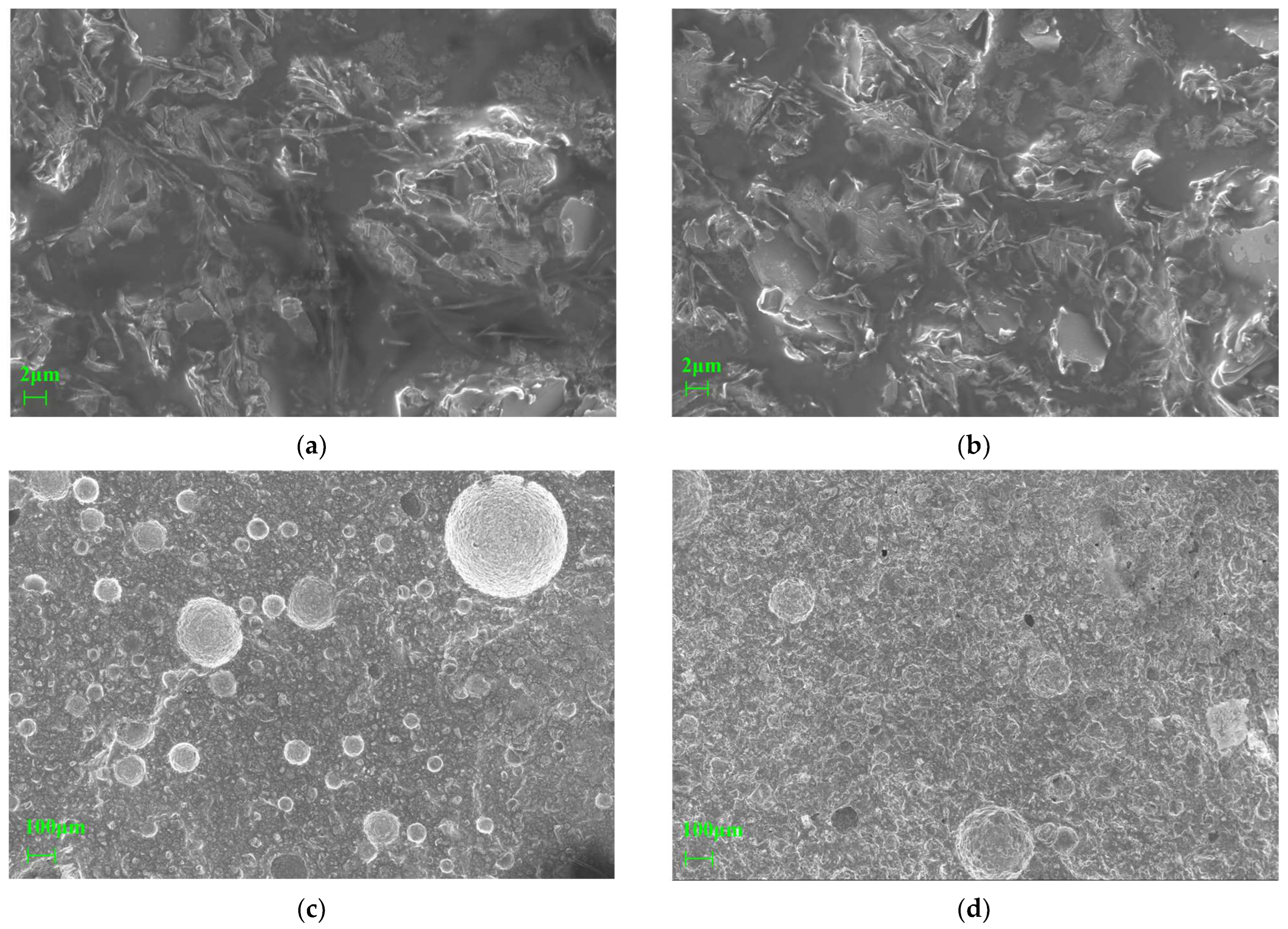
4.2. Microstructural Characterization by SEM
4.2.1. Microstructural Features at Different Activation Temperatures
4.2.2. Microstructural Features Under Different Curing Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kossoff, D.; Dubbin, W.E.; Alfredsson, M.; Edwards, S.J.; Macklin, M.G.; Hudson-Edwards, K.A. Mine tailings dams: Characteristics, failure, environmental impacts, and remediation. Appl. Geochem. 2014, 51, 229–245. [Google Scholar] [CrossRef]
- Cacciuttolo, C.; Atencio, E. An alternative technology to obtain dewatered mine tailings: Safe and control environmental management of filtered and thickened copper mine tailings in Chile. Minerals 2022, 12, 1334. [Google Scholar] [CrossRef]
- Gitari, M.W.; Akinyemi, S.A.; Thobakgale, R.; Petrik, L.F. Physicochemical and mineralogical characterization of Musina mine copper and New Union gold mine tailings. J. Afr. Earth Sci. 2018, 137, 218–228. [Google Scholar] [CrossRef]
- GRID-Arendal. Global Tailings Dam Portal; GRID-Arendal: Arendal, Norway, 2020. [Google Scholar]
- Vizureanu, P.; Nergis, D.D.B.; Sandu, A.V.; Nergis, D.D. The physical and mechanical characteristics of geopolymers using mine tailings as precursors. In Advances in Geopolymer-Zeolite Composites; IntechOpen: Rijeka, Croatia, 2021; pp. 1–20. [Google Scholar]
- Zhang, J.; Fu, Y.; Wang, A.; Chen, W. Research on the mechanical properties and microstructure of fly ash-based geopolymers modified by molybdenum tailings. Constr. Build. Mater. 2023, 385, 131530. [Google Scholar] [CrossRef]
- Qing, L.; Chuanming, L.; Huili, S.; Jingxuan, L. Use of activated quartz powder as an alkaline source for producing one-part Ca-rich slag-based cementitious materials. J. Build. Eng. 2023, 64, 105586. [Google Scholar] [CrossRef]
- Opiso, E.M.; Tabelin, C.B.; Maestre, C.V.; Yu, S.K. Utilization of palm oil fuel ash (POFA) as an admixture for the synthesis of a gold mine tailings-based geopolymer composite. Minerals 2023, 13, 232. [Google Scholar] [CrossRef]
- Castillo, H.; Droguett, T.; Vesely, M.; Collado, H. Simple compressive strength results of sodium-hydroxide- and sodium-silicate-activated copper flotation tailing geopolymers. Appl. Sci. 2022, 12, 5876. [Google Scholar] [CrossRef]
- Ilieva, D.; Surleva, A.; Murariu, M.; Kirilova, E. Evaluation of ICP-OES method for heavy metal and metalloids determination in sterile dump material. Solid State Phenom. 2018, 273, 159–166. [Google Scholar]
- Ke, Y.; Liang, S.; Hou, H.; Zhou, Y. A zero-waste strategy to synthesize geopolymer from iron-recovered Bayer red mud combined with fly ash. Constr. Build. Mater. 2022, 322, 126176. [Google Scholar] [CrossRef]
- Kumar, S.; Djobo, J.N.Y.; Kumar, A.; Kumar, R. Geopolymerization behavior of fine iron-rich fraction of brown fly ash. J. Build. Eng. 2016, 8, 172–178. [Google Scholar] [CrossRef]
- Castillo, H.; Collado, H.; Droguett, T.; Vesely, M. Factors affecting the compressive strength of geopolymers: A review. Minerals 2021, 11, 1317. [Google Scholar] [CrossRef]
- Mitov, I.; Stoilova, A.; Yordanov, B.; Savov, D. Technological research on converting iron ore tailings into a marketable product. J. S. Afr. Inst. Min. Metall. 2021, 121, 181–186. [Google Scholar] [CrossRef]
- Burduhos-Nergis, D.D.; Vizureanu, P.; Sandu, A.V.; Ciobanu, G. XRD and TG-DTA study of new phosphate-based geopolymers with coal ash or metakaolin as aluminosilicate source and mine tailings addition. Materials 2022, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- EN 12457-2:2002; Characterisation of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges. European Committee for Standardization: Brussels, Belgium, 2002.
- Manjarrez, L.; Nikvar-Hassani, R.; Zhang, L. Experimental study of geopolymer binder synthesized with copper mine tailings and low-calcium copper slag. J. Mater. Civ. Eng. 2019, 31, 04019156. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.; Zhang, Q.; Chen, Y. Application of geopolymers for treatment of industrial solid waste containing heavy metals: State-of-the-art review. J. Clean. Prod. 2023, 390, 136053. [Google Scholar] [CrossRef]
- He, X.; Zhang, Y.; Qaidi, S.M.A.; Awoyera, P.O. Mine tailings-based geopolymers: A comprehensive review. Ceram. Int. 2022, 48, 24192–24212. [Google Scholar] [CrossRef]
- Cristelo, N.; Coelho, J.; Oliveira, M.; Silva, R.V. Recycling and application of mine tailings in alkali-activated cements and mortars—Strength development and environmental assessment. Appl. Sci. 2020, 10, 2084. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, X.; Hu, Z.; Liu, M. Synthesis of one-part geopolymers from alkaline-activated molybdenum tailings. J. Clean. Prod. 2024, 443, 142989. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Cao, C.; Yu, Q. Experimental assessment of utilizing copper tailings as alkali-activated materials. Constr. Build. Mater. 2023, 408, 134039. [Google Scholar] [CrossRef]
- Demeusy, B.; Madanski, D.; Bouzahzah, H.; Tasev, G. Mineralogical study of electrom grain size, shape and mineral chemistry in process streams from the Krumovgrad mine, Bulgaria. Miner. Eng. 2023, 198, 108080. [Google Scholar] [CrossRef]
- Ilieva, D.; Argirova, M.; Angelova, L.; Surleva, A. Application of chemical and biological tests for estimation of current state of a tailing dump and surrounding soil. Environ. Sci. Pollut. Res. 2020, 27, 1386–1396. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 5th ed.; Institut Géopolymère: Saint-Quentin, France, 2008. [Google Scholar]
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). Standardization Administration of China: Beijing, China, 2021.
- Liu, H.; Jiang, S.; Zhang, X.; Zhang, J. Low-temperature activation of basalt tailings for geopolymer production. Minerals 2022, 12, 955. [Google Scholar]
- Chen, Y.; Xu, L.; Zhang, J.; Wang, Y. Effect of stepwise activation on the reactivity of silicate-rich mine tailings. Constr. Build. Mater. 2023, 366, 130215. [Google Scholar]
- Zhao, X.; Li, Q.; Wang, J.; Shi, C. Environmental benefits and performance of geopolymers from industrial solid waste: A review. J. Clean. Prod. 2023, 382, 135347. [Google Scholar]
- GB/T 1345-2005; Test Method for Fineness of Cement—Sieve Analysis Method. Standardization Administration of China: Beijing, China, 2005.
- GB/T 8074-2008; Testing Method for Specific Surface of Cement—Blaine Method. Standardization Administration of China: Beijing, China, 2008.
- GB/T 208-2014; Test Method for Determining Cement Density. Standardization Administration of China: Beijing, China, 2014.
- Siline, M.; Mehsas, B. Effect of Increasing the Blaine Fineness of Metakaolin on Its Chemical Reactivity. J. Build. Eng. 2022, 56, 104778. [Google Scholar] [CrossRef]
- Ilieva, D.; Atanasova, V.; Mihailova, I.; Nikolov, A.; Kostova, Y. Characterization of Bulgarian Copper Mine Tailings as a Potential Geopolymer Raw Material: Fineness Below 100 µm Enhances Reactivity. Materials 2024, 17, 542. [Google Scholar] [CrossRef]
- Morales-Aranibar, C.G.; Silva, G.; Martinez, J.; Torres, R.; Fernandez, J. Reuse of Mine Tailings Through Geopolymerization: Influence of Particle Size on Reactivity, Workability, and Mechanical Performance. Sustainability 2025, 17, 2617. [Google Scholar]
- He, J.; Jie, Y.; Zhang, J.; Yu, Y.; Zhang, G. Synthesis and characterization of red mud and rice husk ash-based geopolymer composites. Cem. Concr. Compos. 2013, 37, 108–118. [Google Scholar] [CrossRef]
- GB/T 33568-2017; Test Method for Strength of Cement Mortar (ISO Method). Standards Press of China: Beijing, China, 2017. (In Chinese)
- Provis, J.L.; van Deventer, J.S.J. Geopolymers: Structure, Processing, Properties and Industrial Applications; Woodhead Publishing: Cambridge, UK, 2009. [Google Scholar]
- Huang, X.; Tian, Y.; Jiang, J.; Wang, Q.; Liu, Y. Enhancement mechanism of iron ore tailings in geopolymer concrete. Constr. Build. Mater. 2024, 439, 137362. [Google Scholar] [CrossRef]
- Zhang, L.; Ahmari, S.; Zhang, J. Synthesis and characterization of fly ash modified mine tailings-based geopolymers. Constr. Build. Mater. 2011, 25, 3773–3781. [Google Scholar] [CrossRef]
- Liu, Q.; Li, W.; Yu, L.; Zhang, Y.; Li, G. Alkali-hydrothermal activation of mine tailings. Ceram. Int. 2022, 48, 28945–28955. [Google Scholar] [CrossRef]
- Krishna, R.S.; Shaikh, F.U.A.; Mishra, J.; Reddy, G.P. Mine tailings-based geopolymers: Properties, applications and industrial prospects. Ceram. Int. 2021, 47, 17826–17843. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, Y.; Zhang, J.; Xu, Q. Preparation of geopolymers from thermally activated lithium slag. J. Build. Eng. 2024, 88, 107234. [Google Scholar] [CrossRef]
- Palma, G.; Bolaños, H.; Huamani, R.; Clements, C.; Hedayat, A. Optimization of geopolymers for sustainable management of mine tailings: Impact on mechanical, microstructural and toxicological properties. Minerals 2024, 14, 997. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vibr. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Solouki, A.; Viscomi, G.; Lamperti, R.; Tataranni, P. Quarry waste as precursors in geopolymers for civil engineering applications: A decade in review. Materials 2020, 13, 3146. [Google Scholar] [CrossRef]
- Maruthupandian, S.; Chaliasou, N.-A.; Akram, S.; Chrysanthou, A. Characterisation and activation of graphite mine tailings for use in cementitious binders. Constr. Build. Mater. 2025, preprint, in press. [Google Scholar] [CrossRef]
- Li, F.; Cui, X.; Hu, Z.; Liu, X.; Wang, Z.; Zhou, C.; Fan, X. Study on the Preparation of Cemented Filling Materials from Molybdenum Tailings. Non-Met. Mines 2021, 44, 49–52. (In Chinese) [Google Scholar]
- GB 175-2023; Common Portland Cement. Standards Press of China: Beijing, China, 2023. (In Chinese)
- Heraiz, H.; Mu, X.; Li, J.; Lei, B.; Zhang, S.; Li, Y.; Zhu, S.; Ni, W.; Hitch, M. Optimization of Ultra-High-Performance Concrete Using a Clinker-Free Binder and Iron Mine Tailings Aggregate. Minerals 2024, 15, 28. [Google Scholar] [CrossRef]

| Chemical Composition | SiO2 | Fe2O3 | Al2O3 | CaO | MgO | TiO2 | Na2O | K2O | MnO |
|---|---|---|---|---|---|---|---|---|---|
| Tailings/(wt%) | 49.05 | 16.99 | 15.6 | 10.05 | 5.05 | 0.91 | 0.78 | 0.73 | 0.36 |
| Chemical Composition | P2O5 | SO3 | CuO | PdO | Rh | SrO | ZrO2 | Rb2O | / |
| Tailings/(wt%) | 0.14 | 0.14 | 0.05 | 0.05 | 0.04 | 0.02 | 0.01 | 0.01 | / |
| NO. | Activation Temperature (°C) | Silicate Modulus | Si/Al | Curing Condition |
|---|---|---|---|---|
| T01 | Raw | 1.2 | 2.6 | Room-temperature curing |
| T02 | Raw | 1.2 | 2.8 | Room-temperature curing |
| T03 | Raw | 1.2 | 3.0 | Room-temperature curing |
| BT01 | Raw | 1.2 | 2.6 | Standard curing |
| BT02 | Raw | 1.2 | 2.8 | Standard curing |
| BT03 | Raw | 1.2 | 3.0 | Standard curing |
| T11 | 100 | 1.2 | 2.6 | Room-temperature curing |
| T12 | 100 | 1.2 | 2.6 | Room-temperature curing |
| T13 | 100 | 1.2 | 2.8 | Room-temperature curing |
| BT11 | 100 | 1.2 | 3.0 | Standard curing |
| BT12 | 100 | 1.2 | 2.6 | Standard curing |
| BT13 | 100 | 1.2 | 2.8 | Standard curing |
| T21 | 200 | 1.2 | 3.0 | Room-temperature curing |
| T22 | 200 | 1.2 | 2.6 | Room-temperature curing |
| T23 | 200 | 1.2 | 2.8 | Room-temperature curing |
| BT21 | 200 | 1.2 | 3.0 | Standard curing |
| BT22 | 200 | 1.2 | 2.6 | Standard curing |
| BT23 | 200 | 1.2 | 2.8 | Standard curing |
| T31 | 300 | 1.2 | 3.0 | Room-temperature curing |
| T32 | 300 | 1.2 | 2.6 | Room-temperature curing |
| T33 | 300 | 1.2 | 2.8 | Room-temperature curing |
| BT31 | 300 | 1.2 | 3.0 | Standard curing |
| BT32 | 300 | 1.2 | 2.6 | Standard curing |
| BT33 | 300 | 1.2 | 2.8 | Standard curing |
| Factor | Sum of Squares (SS) | Degrees of Freedom (df) | F-Value | p-Value |
|---|---|---|---|---|
| Activation Temp | 562.276 | 3 | 10.737 | 0.0003 |
| Si/Al Ratio | 158.325 | 2 | 4.535 | 0.0264 |
| Curing | 29.04 | 1 | 1.664 | 0.2144 |
| Residual | 296.764 | 17 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Yao, Y.; Zhang, L.; Hu, X.; Yang, X. Green Low-Temperature Activation and Curing for High-Toughness Geopolymer Binders from Diabase Tailings. Materials 2025, 18, 3815. https://doi.org/10.3390/ma18163815
Hu Y, Yao Y, Zhang L, Hu X, Yang X. Green Low-Temperature Activation and Curing for High-Toughness Geopolymer Binders from Diabase Tailings. Materials. 2025; 18(16):3815. https://doi.org/10.3390/ma18163815
Chicago/Turabian StyleHu, Yanan, Yong Yao, Lingling Zhang, Xianming Hu, and Xinchun Yang. 2025. "Green Low-Temperature Activation and Curing for High-Toughness Geopolymer Binders from Diabase Tailings" Materials 18, no. 16: 3815. https://doi.org/10.3390/ma18163815
APA StyleHu, Y., Yao, Y., Zhang, L., Hu, X., & Yang, X. (2025). Green Low-Temperature Activation and Curing for High-Toughness Geopolymer Binders from Diabase Tailings. Materials, 18(16), 3815. https://doi.org/10.3390/ma18163815






