Simulation of Corrosion Cracking in Reinforced Concrete Based on Multi-Phase Multi-Species Electrochemical Phase Field Modeling
Highlights
- Controlling electrochemical reaction kinetics and electric field migration with varied corrosion current density.
- Developing a phase field model that encompasses multiple species and multiple phases.
- Study on the influence of multiple reinforcement configurations on crack patterns.
Abstract
1. Introduction
- (1)
- Linked model of dynamically variable corrosion current density, including electrochemical reactions and mechanical fields, is created based on the phase field approach to facilitate the unified simulation of corrosion and cracking processes.
- (2)
- A multi-phase multi-species coupled phase field model is developed at the fine-scale level, comprehensively integrating multi-species ion transport, electrochemical reactions on the steel surface, and the phase field model.
- (3)
- Simulation of dynamic electrochemical interactions among various rebar configurations for investigating the factors influencing their cracking patterns.
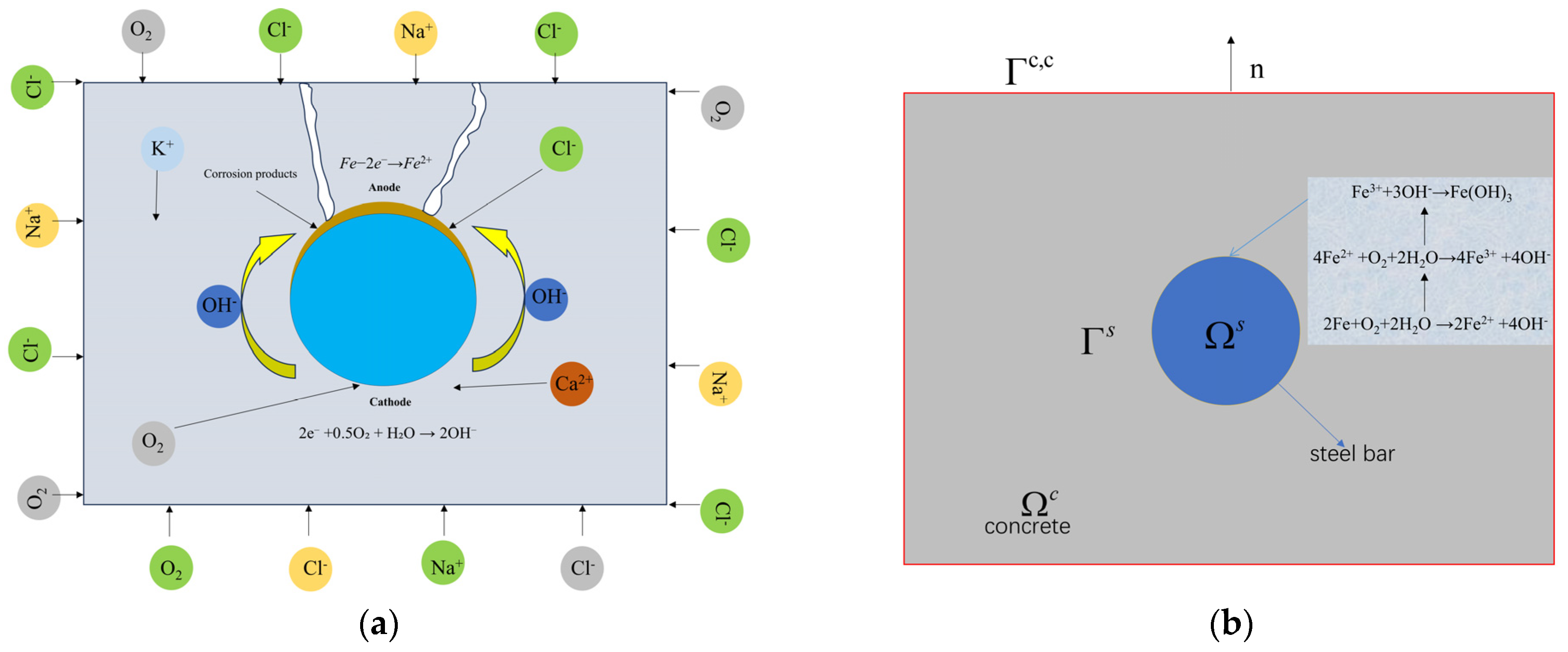
2. Multi-Physics Field Framework
2.1. Electrochemical Processes
2.1.1. Transportation of Substances in Concrete
2.1.2. Electrode Polarization
2.1.3. Electrochemical Corrosion of Reinforcing Steel
2.2. Fracture Phase Field Model
2.2.1. Unified Phase Field Theory
2.2.2. Constitutive Relationship Based on Characteristic Strain
2.2.3. Crack-Induced Diffusion Coefficient Variation
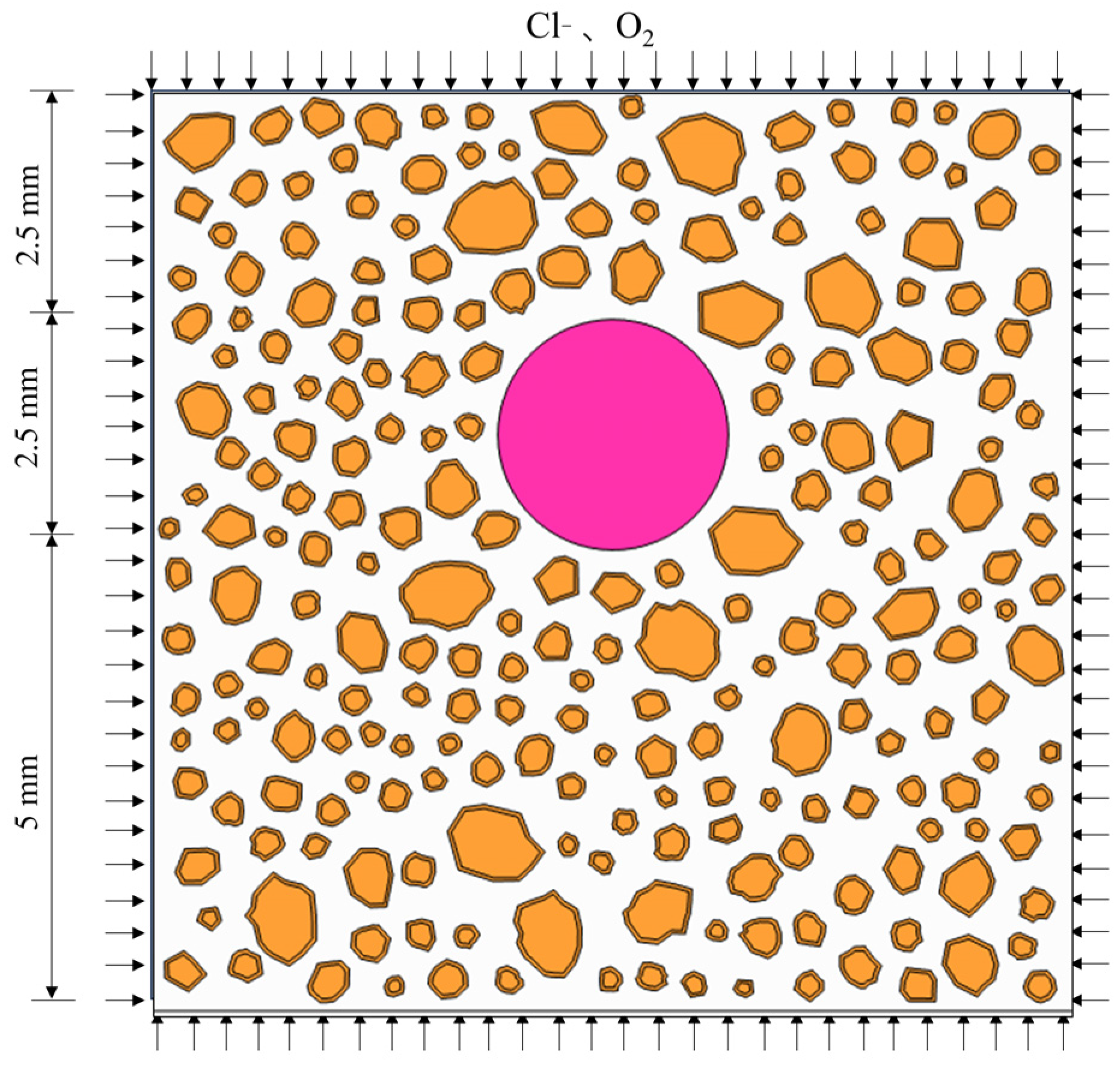
3. Numerical Validation
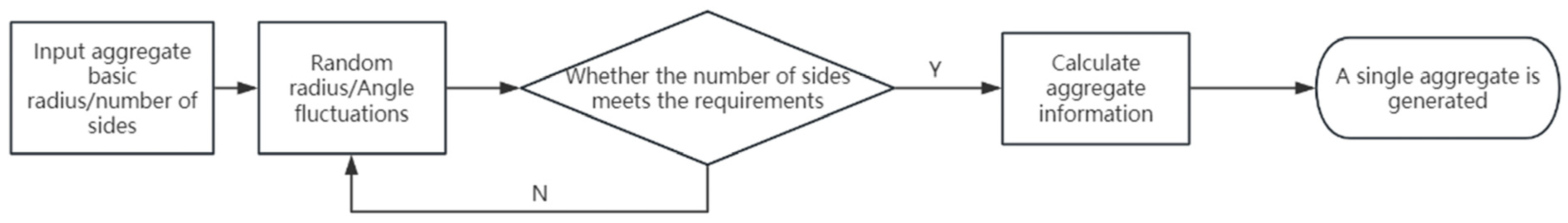
3.1. Numerical Implementation
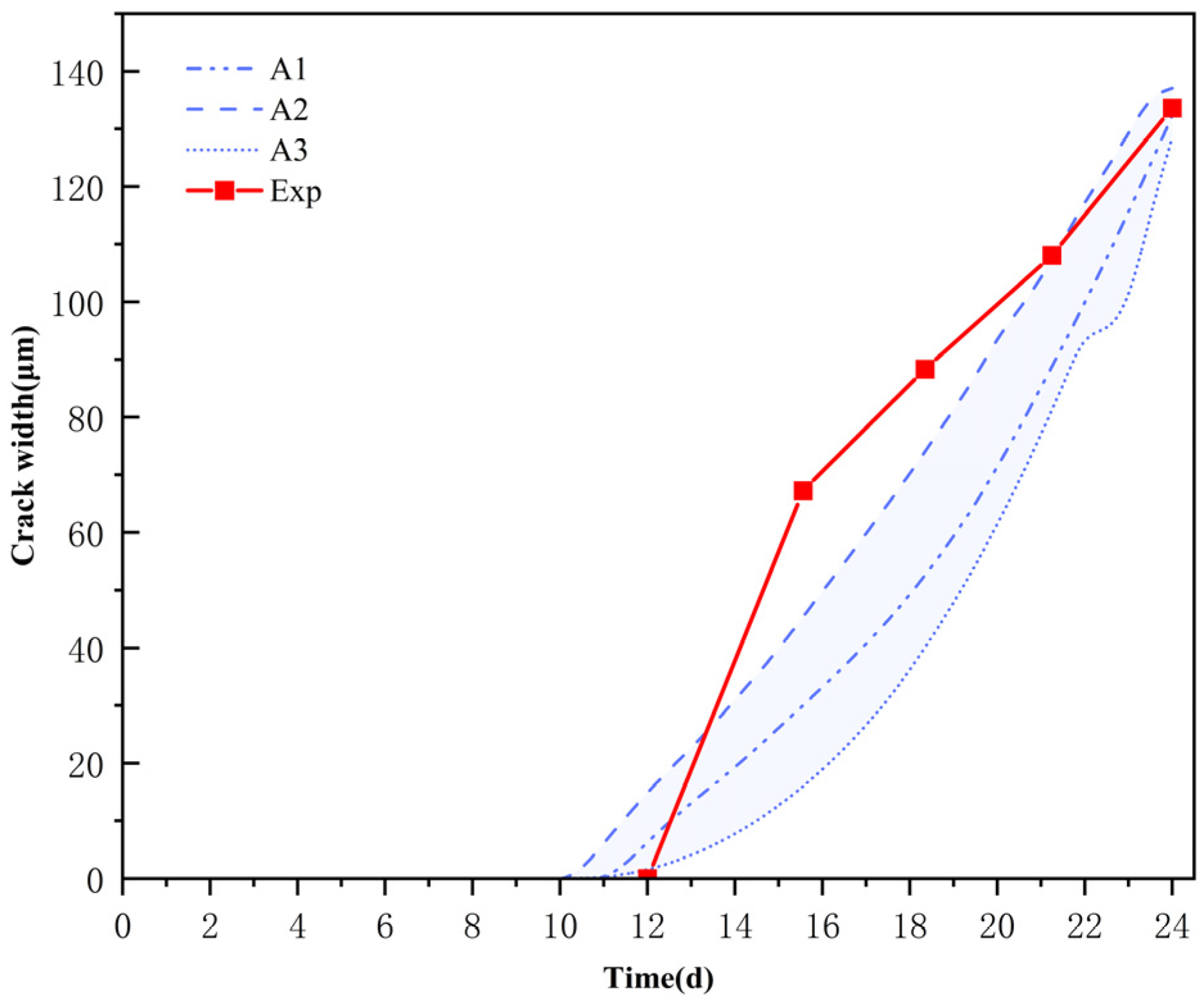
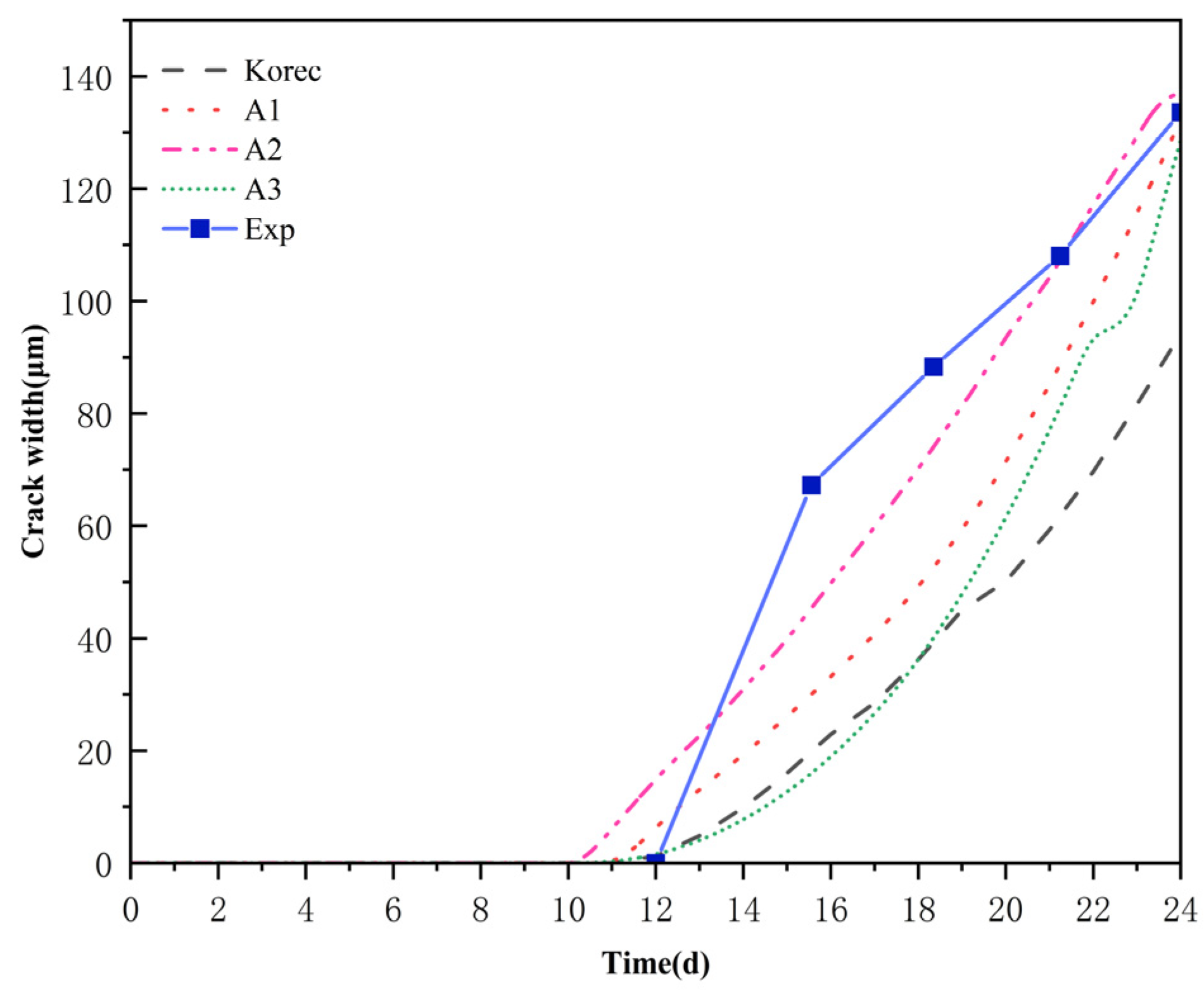
3.2. Verification of Corrosion-Induced Cracking of the Protective Layer of Concrete
| Parameters | Value |
|---|---|
| Anodic Tafel slop, | 0.09 |
| Cathodic Tafel slope, | −0.14 |
| Anodic equilibrium potential, | −0.78 |
| Cathodic equilibrium potential, | 0.16 |
| Anodic exchange current density, | 3 × 10−4 |
| Cathodic exchange current density, | 1 × 10−5 |
| Concrete resistivity, | 200 |
| Variables | Cl− | Na+ | Ca2+ | OH− | K+ | O2 |
|---|---|---|---|---|---|---|
| Charge number | −1 | 1 | 2 | −1 | 1 | - |
| Diffusion coefficient (×10−11 m2/s) | 1.2 | 1.33 | 0.79 | 5.27 | 1.96 | 600 |
| Initial concentration (mol/m3) | 0 | 40 | 15 | 140 | 70 | 0.156 |
| Boundary concentration (mol/m3) | - | - | 0 | 0 | 0 | 0.268 |
| Phases | Young’s Modulus (MPa) | Poisson’s Ratio | Failure Strength (MPa) | Fracture Energy (N/m) | Porosity |
|---|---|---|---|---|---|
| Aggregate | 70,000 | 0.2 | - | - | - |
| Matrix | 25,000 | 0.2 | 3 | 40 | 0.26 |
| ITZ | 15,000 | 0.2 | 1.5 | 20 | 0.26 |
| Precipitate | 440 | 0.4 | - | - | 0.16 |
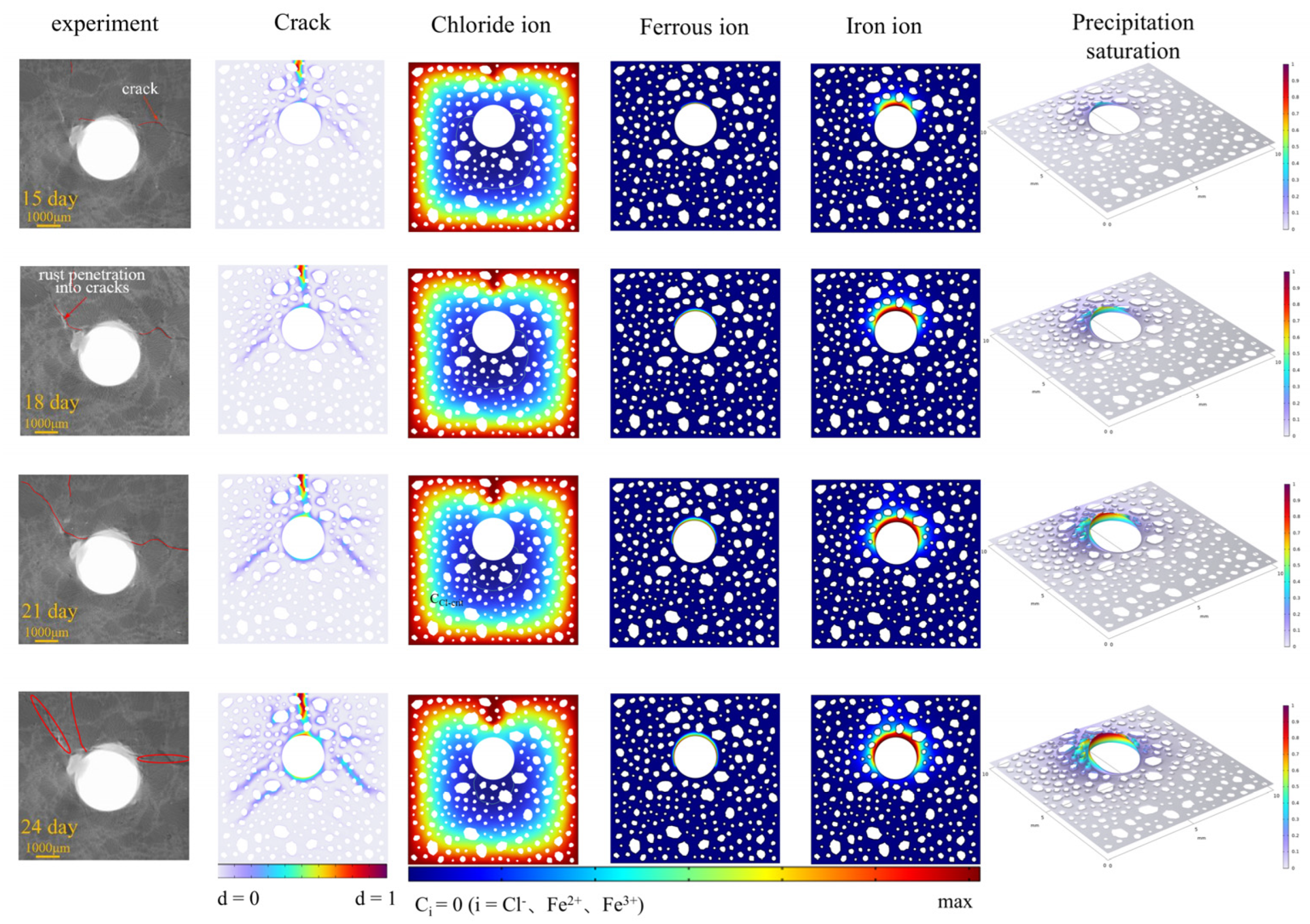
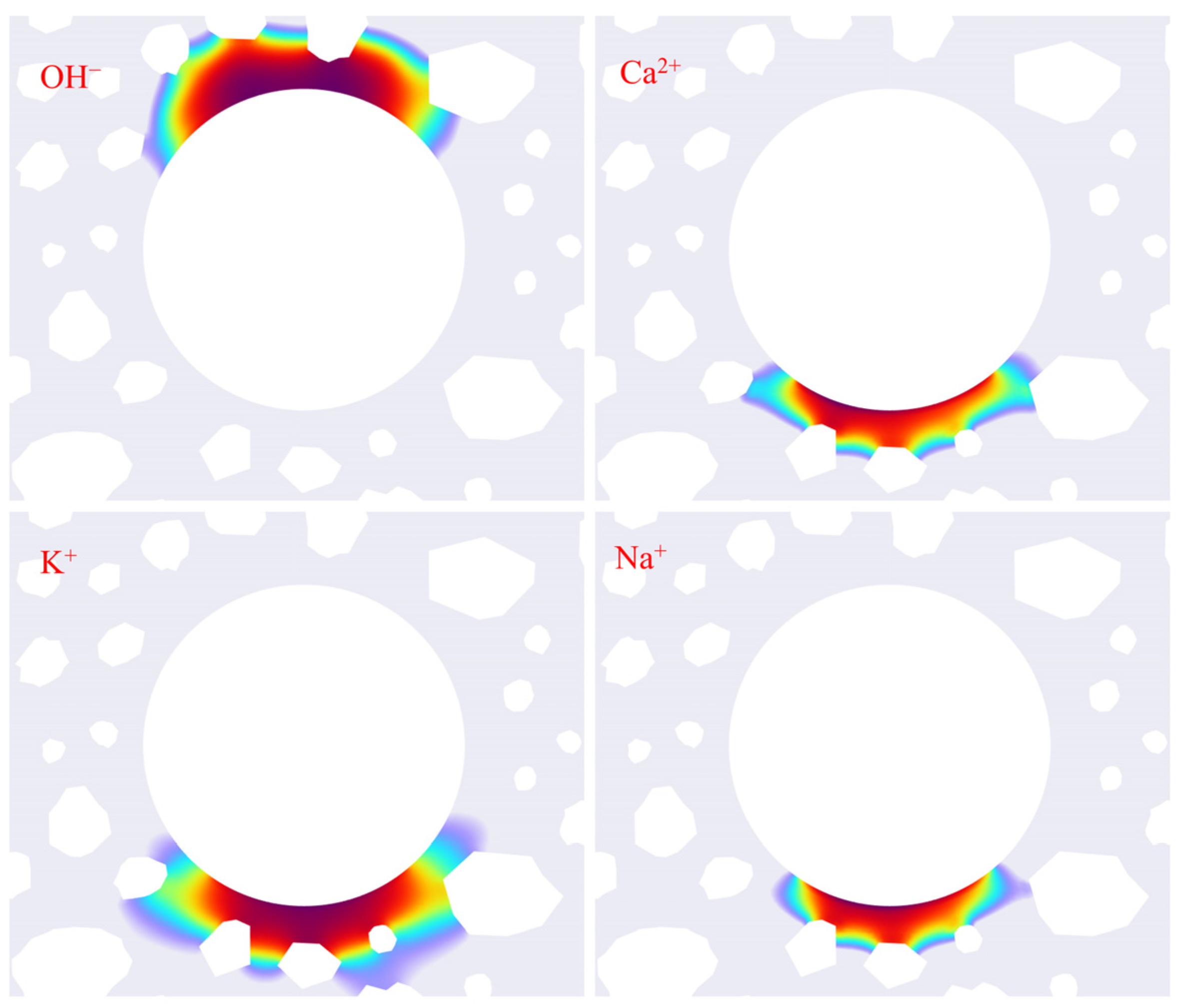
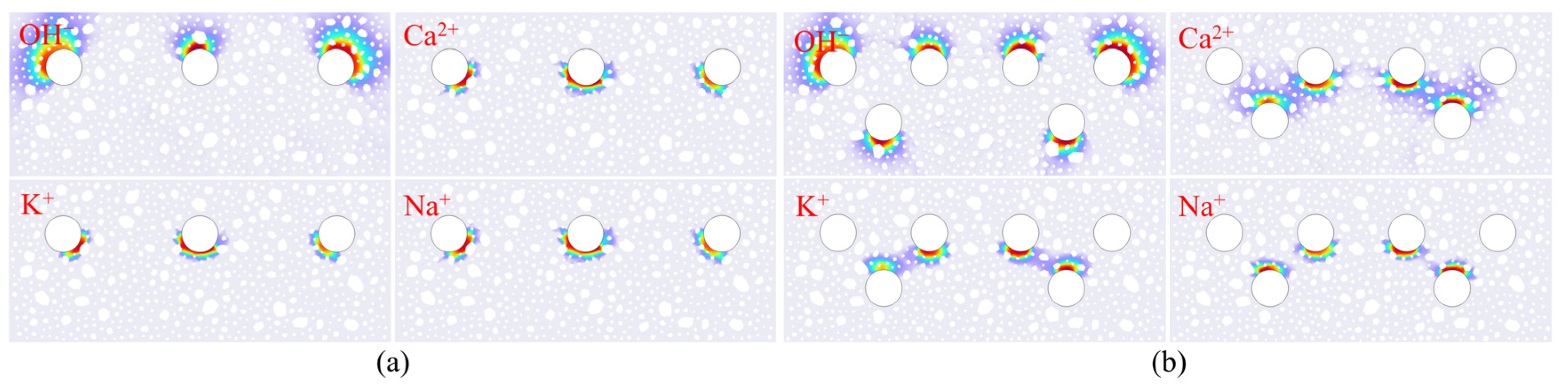
3.3. Ion Concentration Distribution Verification
4. Results and Discussion
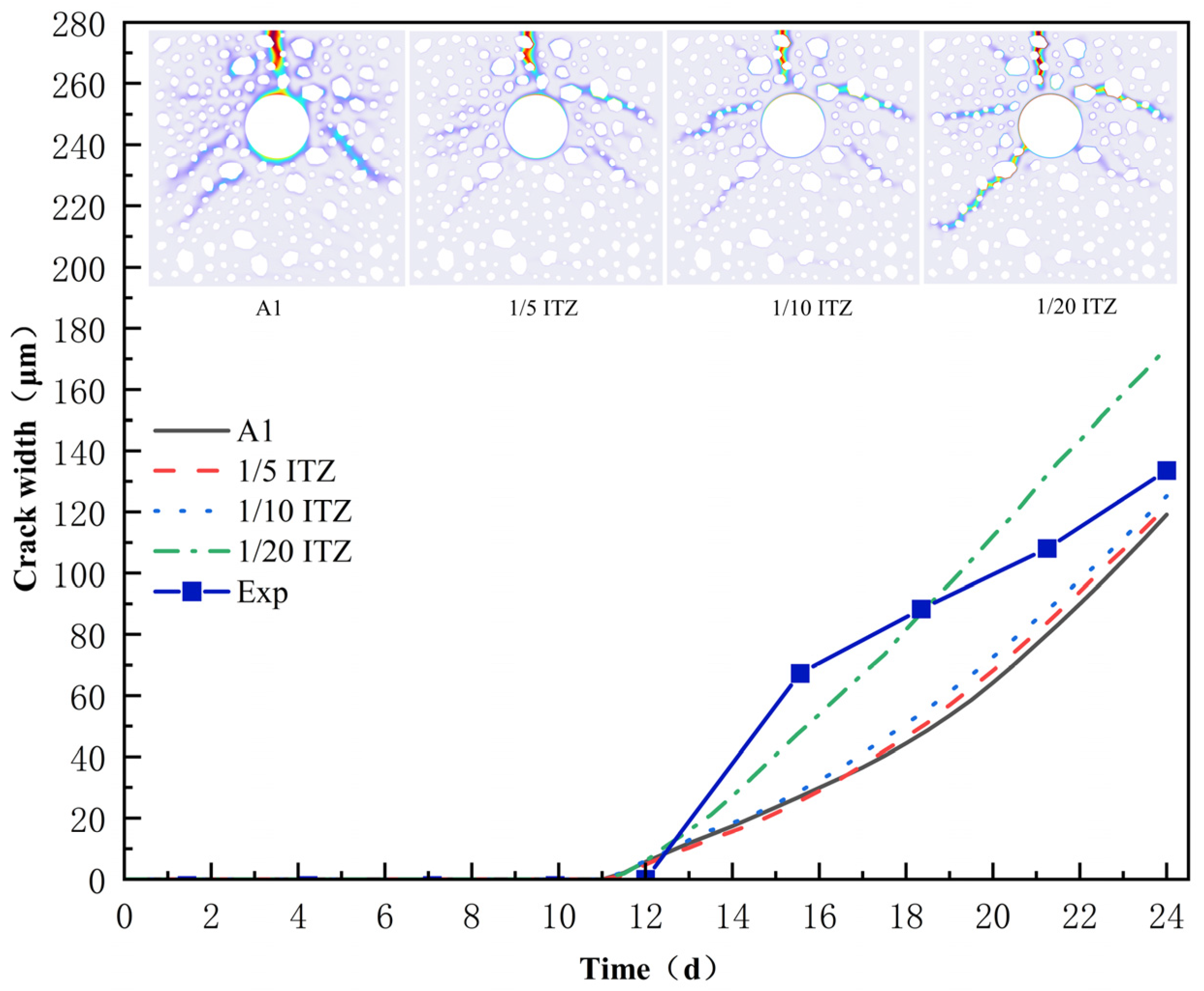
4.1. Mechanism of ITZ Strength on Crack Expansion Mode of Reinforced Concrete
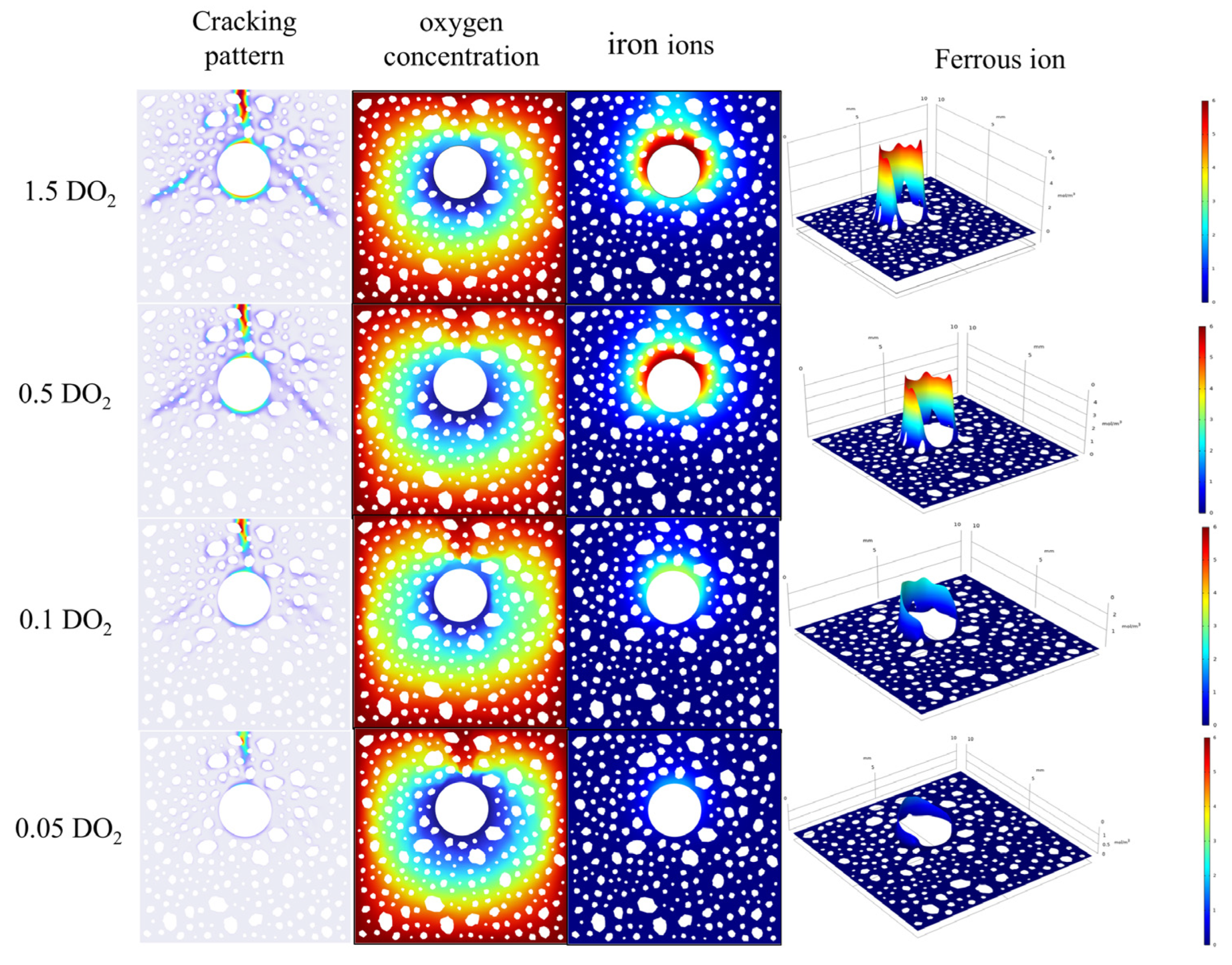
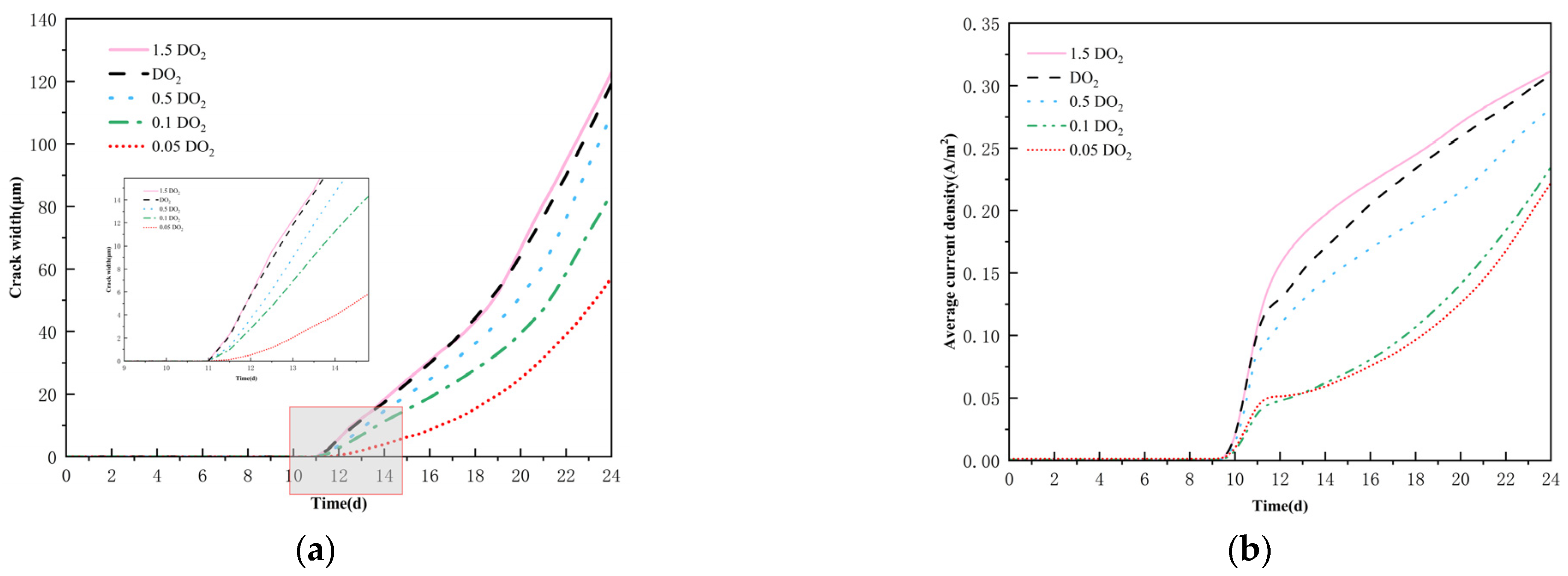
4.2. Effect of Oxygen Diffusion Coefficient on Corrosion Cracking
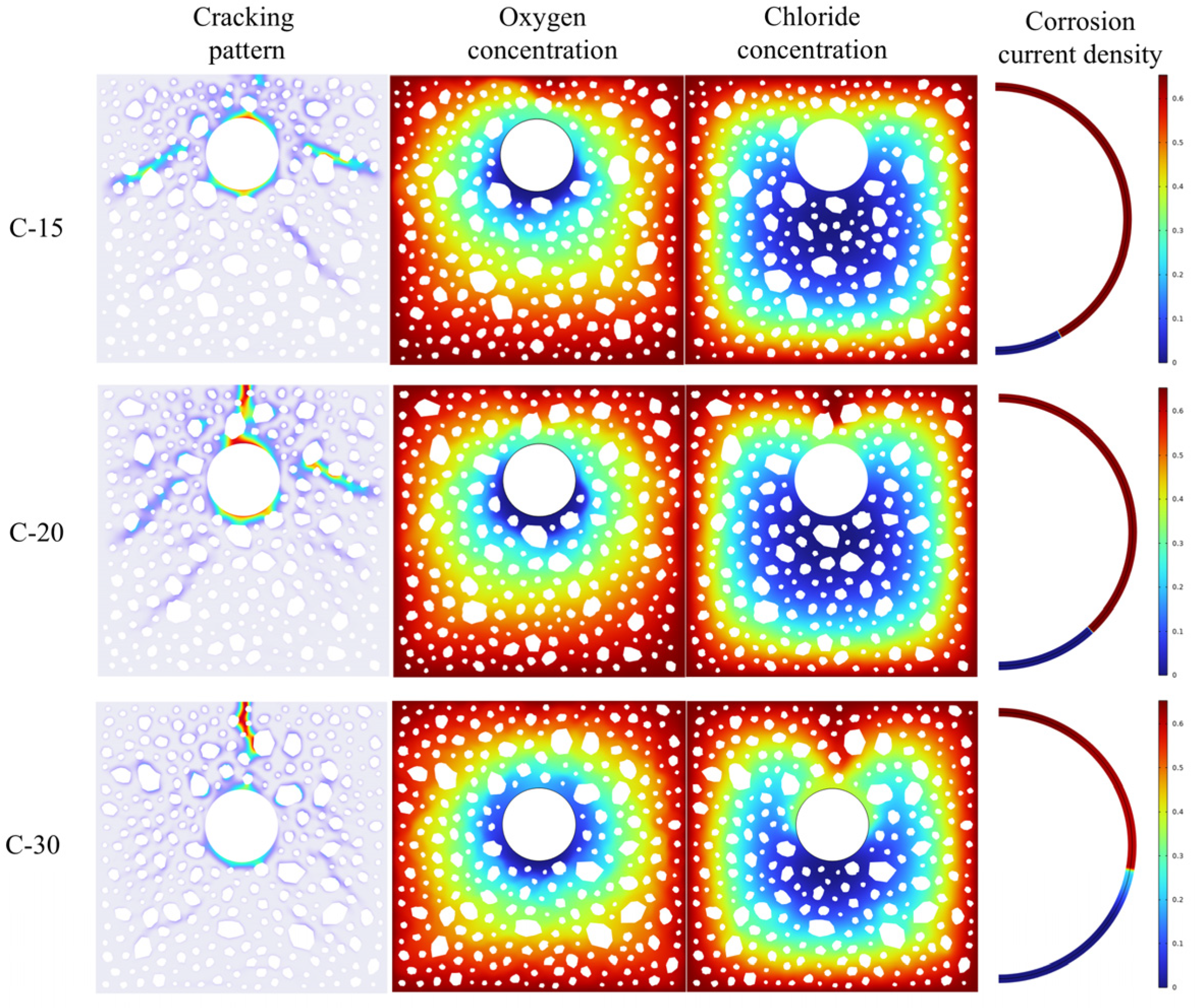
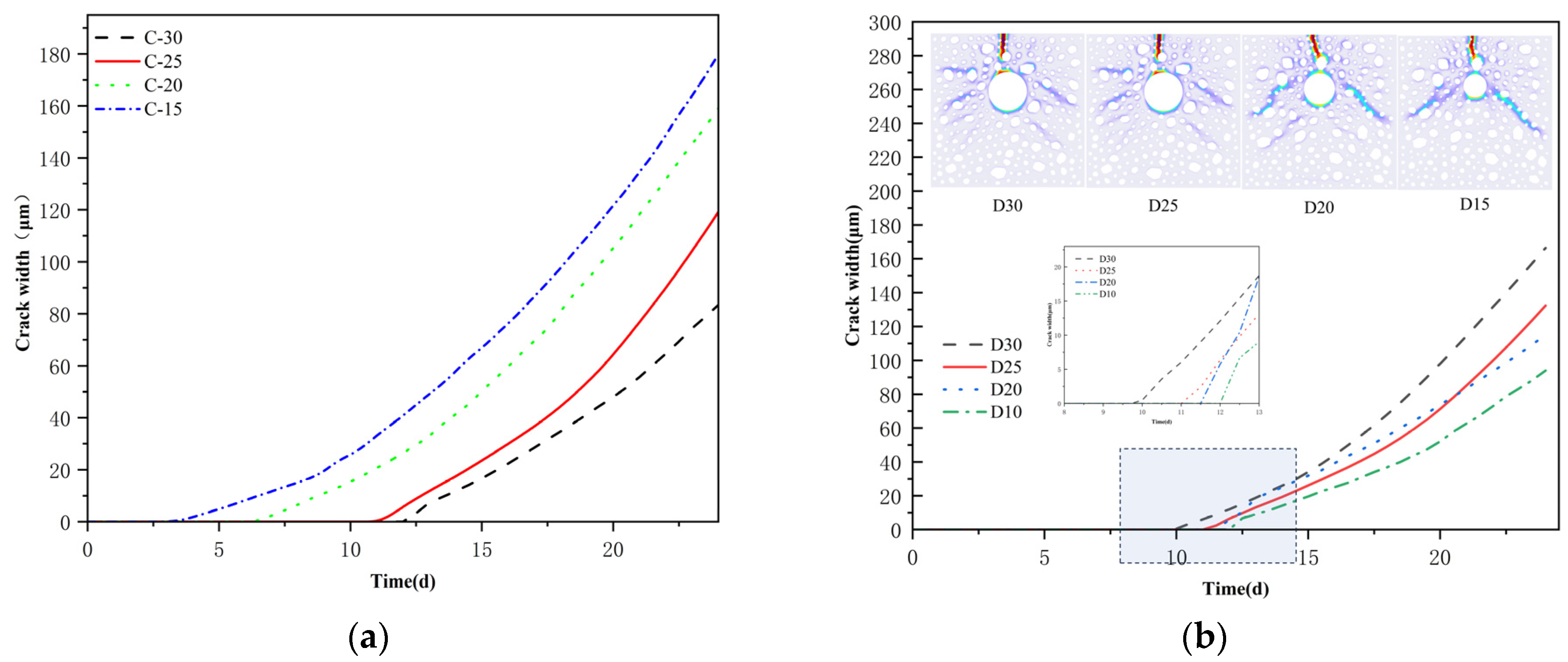
4.3. Effect of Protective Layer Thickness and Reinforcement Diameter on Corrosion Cracking
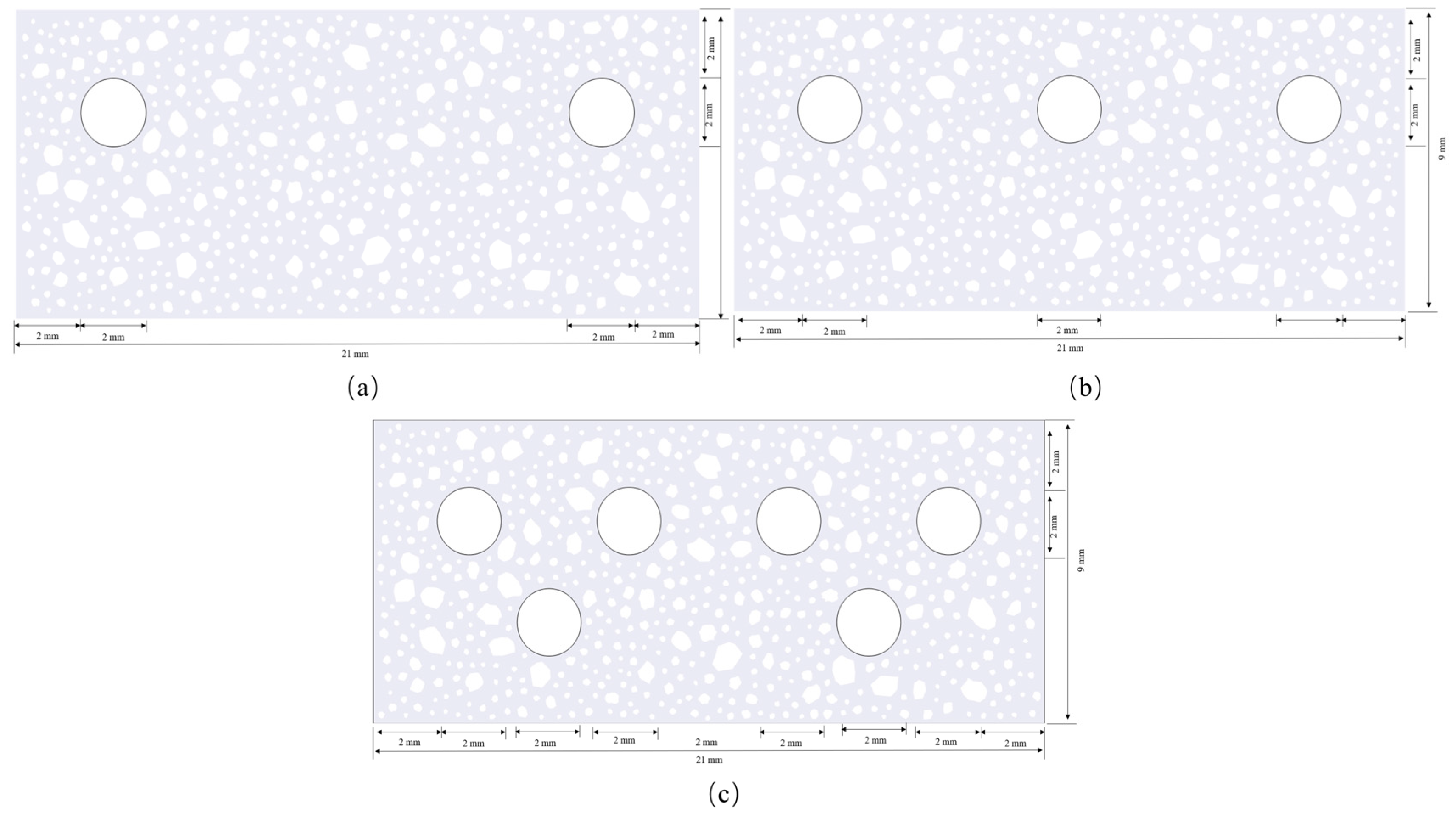
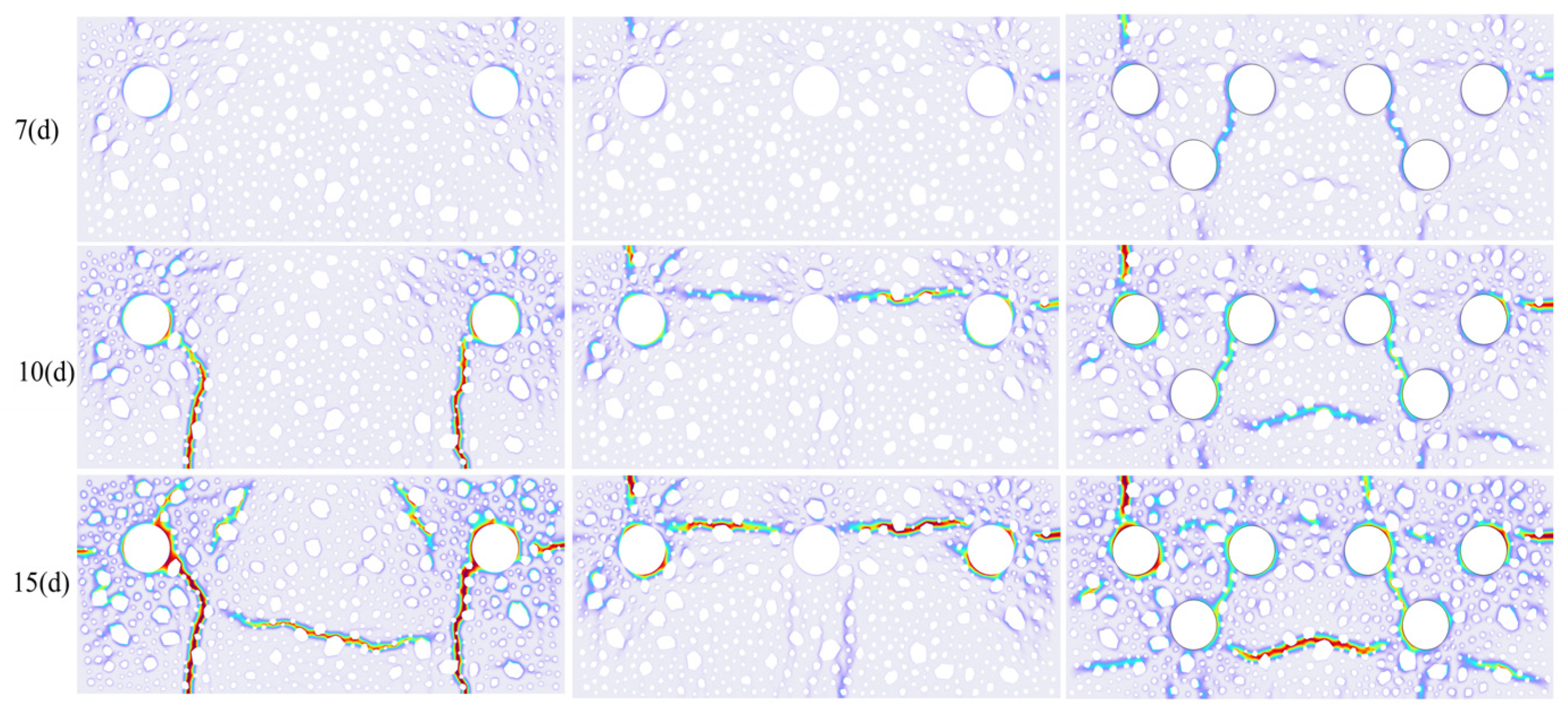
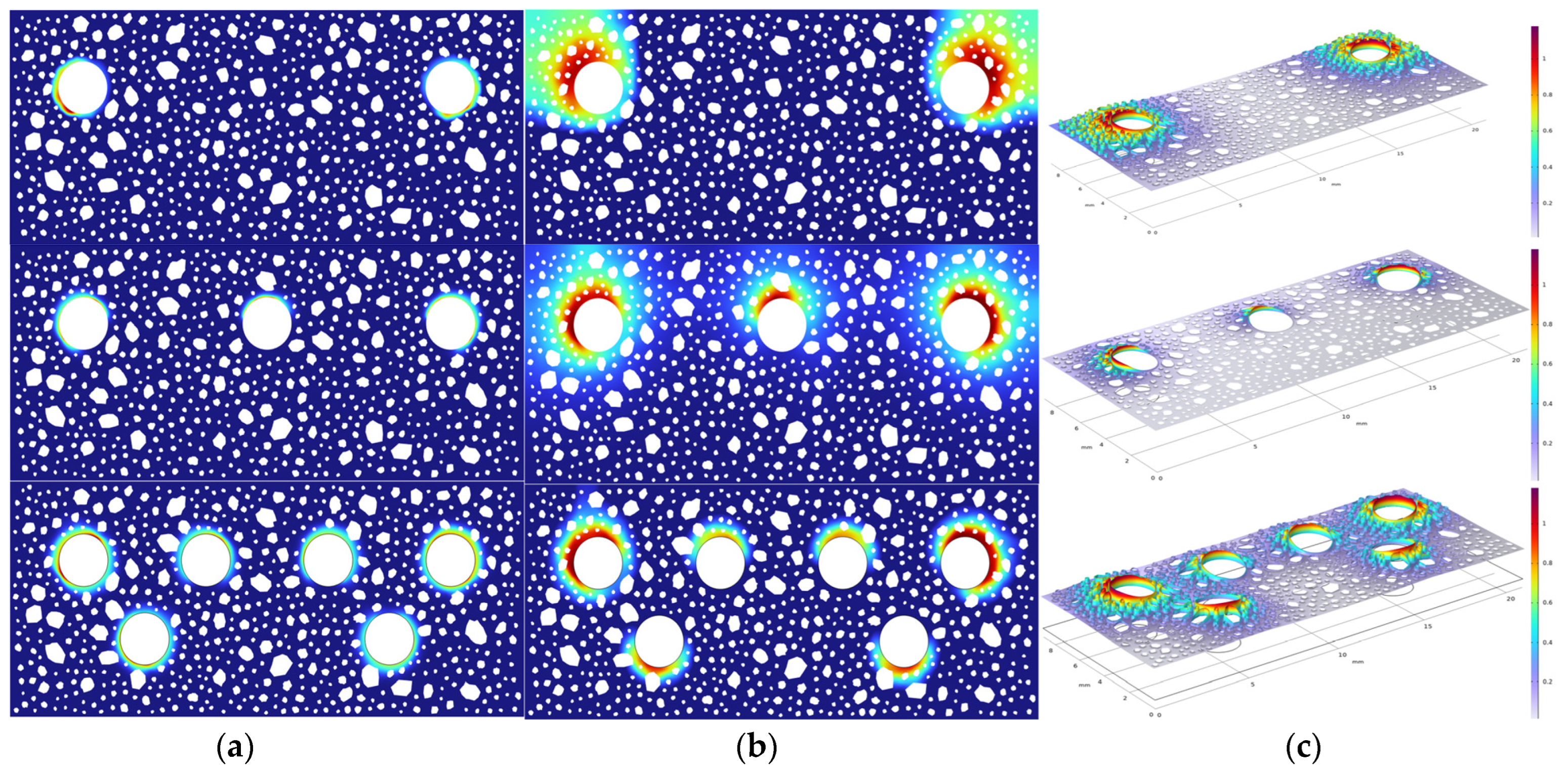
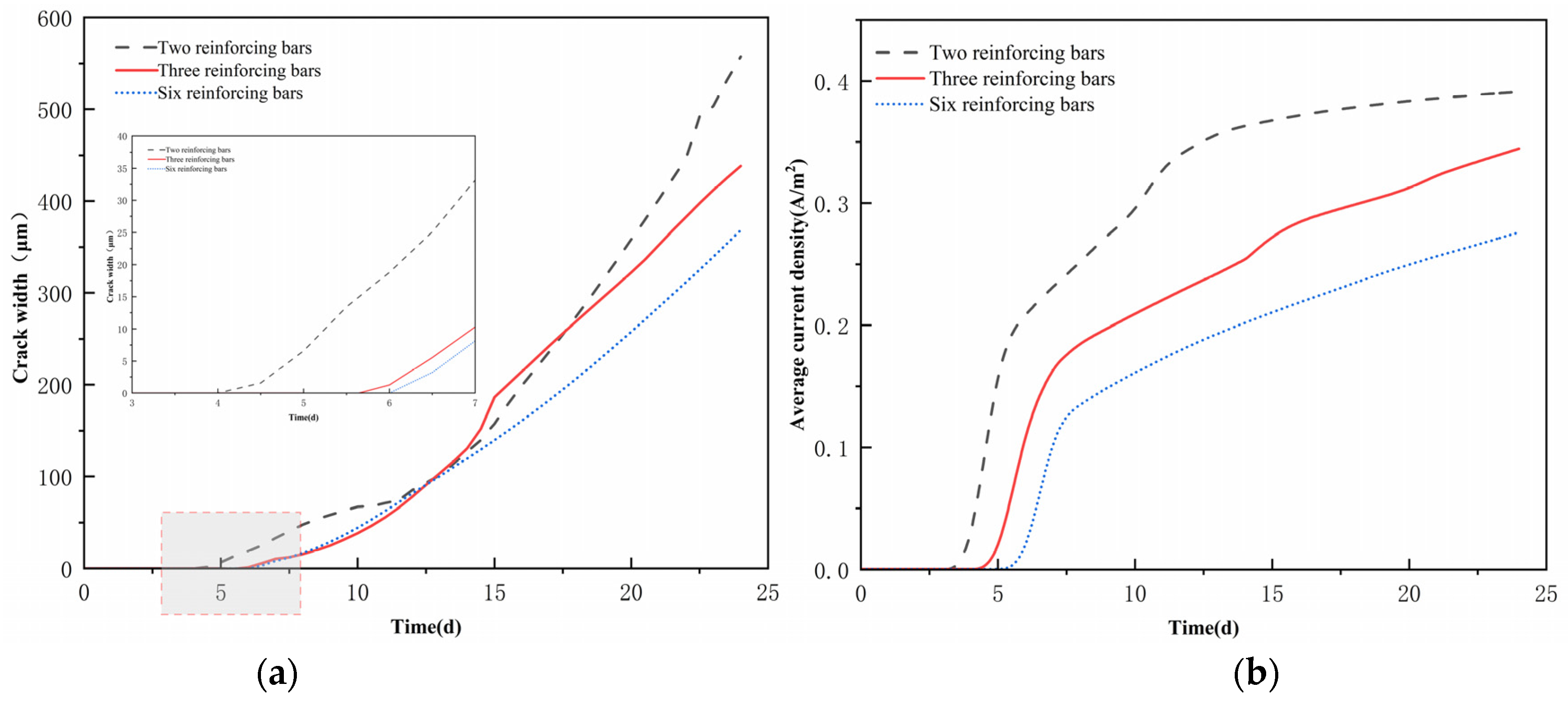
4.4. Cracking Patterns for Multiple Rebar Configurations
4.5. Discussion
5. Potential Applications and Developments
6. Conclusions
- (1)
- Through integrating a unified phase field model with continuous electrochemical reactions and multi-species transport, the localized steel dissolution process can be accurately emulated, and corrosion regions effectively differentiated. The localized corrosion current density functions as a precise gauge of reaction rate and extent.
- (2)
- ITZ strength exerts a notable influence on the cracking mode of the concrete cover: under high ITZ strength, cracks tend to propagate along interfaces, giving rise to “aggregate boundary-type” main cracks; under low ITZ strength, cracks breach interface constraints and directly penetrate the concrete. At extremely low ITZ strength, cracks penetrating aggregates aggravate structural damage, accompanied by “multi-directional dispersed” secondary cracks and a marked increase in the maximum crack width.
- (3)
- The oxygen diffusion coefficient modulates the corrosion reaction rates within the concrete; low oxygen concentrations impede crack width development. An increase in concrete cover thickness lessens the crack width and defers cracking initiation. A reduction in the reinforcement diameter advances the cracking onset, with the ultimate crack width exhibiting a decreasing tendency.
- (4)
- In multi-bar systems, dense reinforcement intensifies competition in cathodic oxygen reduction reactions, engendering local oxygen-deficient zones. This shifts electrochemical reactions from activation control to mass transfer control, with the initial corrosion time extending logarithmically as the number of bars rises. The spatial superposition of rust expansion stresses from adjacent bars diminishes the stress concentration factor in the concrete matrix, resulting in a decreasing trend in the surface crack width with an increasing reinforcement ratio.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanz, B.; Planas, J.; Sancho, J.M. A method to determine the constitutive parameters of oxide in accelerated corrosion tests of reinforced concrete specimens. Cem. Concr. Res. 2017, 101, 68–81. [Google Scholar] [CrossRef]
- Angst, U.; Elsener, B.; Larsen, C.K.; Vennesland, Ø. Critical chloride content in reinforced concrete—A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Glasser, F.P.; Marchand, J.; Samson, E. Durability of concrete—Degradation phenomena involving detrimental chemical reactions. Cem. Concr. Res. 2008, 38, 226–246. [Google Scholar] [CrossRef]
- Šauman, Z. Carbonization of porous concrete and its main binding components. Cem. Concr. Res. 1971, 1, 645–662. [Google Scholar] [CrossRef]
- Ye, H.; Jin, N.; Fu, C.; Jin, X. Rust distribution and corrosion-induced cracking patterns of corner-located rebar in concrete cover. Constr. Build. Mater. 2017, 156, 684–691. [Google Scholar] [CrossRef]
- Pedrosa, F.; Andrade, C. Corrosion induced cracking: Effect of different corrosion rates on crack width evolution. Constr. Build. Mater. 2017, 133, 525–533. [Google Scholar] [CrossRef]
- Jang, B.S.; Oh, B.H. Effects of non-uniform corrosion on the cracking and service life of reinforced concrete structures. Cem. Concr. Res. 2010, 40, 1441–1450. [Google Scholar] [CrossRef]
- Chen, A.; Pan, Z.; Ma, R. Mesoscopic Simulation of Steel Rebar Corrosion Process in Concrete and Its Damage to Concrete Cover. In Bridge Design, Assessment and Monitoring; Routledge: London, UK, 2018; pp. 62–77. [Google Scholar]
- Du, X.; Jin, L.; Zhang, R. Modeling the cracking of cover concrete due to non-uniform corrosion of reinforcement. Corros. Sci. 2014, 89, 189–202. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, R.; Du, X.; Li, Y. Investigation on the cracking behavior of concrete cover induced by corner located rebar corrosion. Eng. Fail. Anal. 2015, 52, 129–143. [Google Scholar] [CrossRef]
- Jin, L.; Liu, M.; Zhang, R.; Du, X. Cracking of cover concrete due to non-uniform corrosion of corner rebar: A 3D meso-scale study. Constr. Build. Mater. 2020, 245, 118449. [Google Scholar] [CrossRef]
- Zhao, Y.; Karimi, A.R.; Wong, H.S.; Hu, B.; Buenfeld, N.R.; Jin, W. Comparison of uniform and non-uniform corrosion induced damage in reinforced concrete based on a Gaussian description of the corrosion layer. Corros. Sci. 2011, 53, 2803–2814. [Google Scholar] [CrossRef]
- Xi, X.; Yang, S.; Li, C.Q. A non-uniform corrosion model and meso-scale fracture modelling of concrete. Cem. Concr. Res. 2018, 108, 87–102. [Google Scholar] [CrossRef]
- Bažant, Z.P.; Oh, B.H. Crack band theory for fracture of concrete. Matériaux Et Constr. 1983, 16, 155–177. [Google Scholar] [CrossRef]
- Cao, C.; Cheung, M.M.S.; Chan, B.Y.B. Modelling of interaction between corrosion-induced concrete cover crack and steel corrosion rate. Corros. Sci. 2013, 69, 97–109. [Google Scholar] [CrossRef]
- Bazant, Z.P.; Pijaudier-Cabot, G. Nonlocal continuum damage, localization instability and convergence. J. Appl. Mech. 1988, 55, 287–293. [Google Scholar] [CrossRef]
- Moës, N.; Dolbow, J.; Belytschko, T. A finite element method for crack growth without remeshing. Int. J. Numer. Methods Eng. 1999, 46, 131–150. [Google Scholar] [CrossRef]
- Ambati, M.; Gerasimov, T.; De Lorenzis, L. A review on phase-field models of brittle fracture and a new fast hybrid formulation. Comput. Mech. 2015, 55, 383–405. [Google Scholar] [CrossRef]
- Javili, A.; Morasata, R.; Oterkus, E.; Oterkus, S. Peridynamics review. Math. Mech. Solids 2019, 24, 3714–3739. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W.; Guan, J.; Zhou, X.; Guo, L. Fully coupled peridynamic model for analyzing the chemo-diffusion-mechanical behavior of sulfate attack in concrete. Constr. Build. Mater. 2023, 409, 133874. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Zhou, W.; Chen, Z.; Bobaru, F. Peridynamic modeling of early-age cracking behaviour in continuously reinforced concrete pavement. Int. J. Pavement Eng. 2023, 24, 2111422. [Google Scholar] [CrossRef]
- Hermann, A.; Shojaei, A.; Höche, D.; Jafarzadeh, S.; Bobaru, F.; Cyron, C.J. Nonlocal Nernst-Planck-Poisson System for Modeling Electrochemical Corrosion in Biodegradable Magnesium Implants. J. Peridynamics Nonlocal Model. 2025, 7, 1–32. [Google Scholar] [CrossRef]
- Wu, J.Y. A unified phase-field theory for the mechanics of damage and quasi-brittle failure. J. Mech. Phys. Solids 2017, 103, 72–99. [Google Scholar] [CrossRef]
- Wu, J.Y.; Nguyen, V.P. A length scale insensitive phase-field damage model for brittle fracture. J. Mech. Phys. Solids 2018, 119, 20–42. [Google Scholar] [CrossRef]
- Wu, J.Y. A geometrically regularized gradient-damage model with energetic equivalence. Comput. Methods Appl. Mech. Eng. 2018, 328, 612–637. [Google Scholar] [CrossRef]
- Wu, J.Y. Unified phase field theory, algorithms and applications for damage destruction of solid structures. J. Mech. 2021, 53, 301–329. [Google Scholar]
- Wei, C.; Wojnar, C.S.; Wu, C. Hydro-chemo-mechanical phase field formulation for corrosion induced cracking in reinforced concrete. Cem. Concr. Res. 2021, 144, 106404. [Google Scholar] [CrossRef]
- Fang, X.; Pan, Z.; Chen, A.; Tian, H.; Ma, R. Phase-field method for modeling non-uniform corrosion-induced cracking in concrete. Eng. Fract. Mech. 2023, 281, 109131. [Google Scholar] [CrossRef]
- Fang, X.; Pan, Z.; Ma, R. A multi-phase-field framework for non-uniform corrosion and corrosion-induced concrete cracking. Comput. Methods Appl. Mech. Eng. 2023, 414, 116196. [Google Scholar] [CrossRef]
- Korec, E.; Jirásek, M.; Wong, H.S.; Martínez-Pañeda, E. A phase-field chemo-mechanical model for corrosion-induced cracking in reinforced concrete. Constr. Build. Mater. 2023, 393, 131964. [Google Scholar] [CrossRef]
- Korec, E.; Jirásek, M.; Wong, H.S.; Martínez-Pañeda, E. Phase-field chemo-mechanical modelling of corrosion-induced cracking in reinforced concrete subjected to non-uniform chloride-induced corrosion. Theor. Appl. Fract. Mech. 2024, 129, 104233. [Google Scholar] [CrossRef]
- Guo, W.; Yan, S.; Gao, G. Electro-Chemo-Mechanical coupled model for corrosion and crack propagation in reinforced concrete. J. Build. Eng. 2025, 108, 112988. [Google Scholar] [CrossRef]
- Zhao, P.; Si, Z.; Huang, L.; He, Y.; Liu, X.; Zhang, Y. A review of the repair measures for reinforced concrete affected by chloride ion corrosion. J. Build. Eng. 2025, 263, 112028. [Google Scholar] [CrossRef]
- Du, F.; Jin, Z.; She, W.; Xiong, C.; Feng, G.; Fan, J. Chloride ions migration and induced reinforcement corrosion in concrete with cracks: A comparative study of current acceleration and natural marine exposure. Constr. Build. Mater. 2020, 263, 120099. [Google Scholar] [CrossRef]
- Ožbolt, J.; Balabanić, G.; Periškić, G.; Kušter, M. Modelling the effect of damage on transport processes in concrete. Constr. Build. Mater. 2010, 24, 1638–1648. [Google Scholar] [CrossRef]
- Liu, Q.F.; Su, R.K.L.; Xu, F. Quantification of the actual expansion and deposition of rust in reinforced concrete. Constr. Build. Mater. 2021, 297, 123760. [Google Scholar] [CrossRef]
- Galan, I.; Glasser, F.P. Chloride in cement. Adv. Cem. Res. 2015, 27, 63–97. [Google Scholar] [CrossRef]
- Xia, J.; Li, T.; Fang, J.X.; Jin, W.L. Numerical simulation of steel corrosion in chloride contaminated concrete. Constr. Build. Mater. 2019, 228, 116745. [Google Scholar] [CrossRef]
- Hu, X.; Xu, H.; Xi, X.; Zhang, P.; Yang, S. Meso-scale phase field modelling of reinforced concrete structures subjected to corrosion of multiple reinforcements. Constr. Build. Mater. 2022, 321, 126376. [Google Scholar] [CrossRef]
- Xiao, J.; Ying, J.; Shen, L. FEM simulation of chloride diffusion in modeled recycled aggregate concrete. Constr. Build. Mater. 2012, 29, 12–23. [Google Scholar] [CrossRef]
- Li, L.J.; Liu, Q.F.; Tang, L.; Hu, Z.; Wen, Y.; Zhang, P. Chloride penetration in freeze–thaw induced cracking concrete: A numerical study. Constr. Build. Mater. 2021, 302, 124291. [Google Scholar] [CrossRef]
- Liu, Q.F.; Hu, Z.; Wang, X.E.; Zhao, H.; Qian, K.; Li, L.J.; Meng, Z. Numerical study on cracking and its effect on chloride transport in concrete subjected to external load. Constr. Build. Mater. 2022, 325, 126797. [Google Scholar] [CrossRef]
- Fang, X.; Pan, Z.; Ma, R.; Chen, A. A multi-phase multi-species phase field model for non-uniform corrosion and chloride removal in concrete. J. Build. Eng. 2024, 82, 108214. [Google Scholar] [CrossRef]
- Walraven, J.C.; Reinhardt, H.W. Concrete mechanics. Part a: Theory and experiments on the mechanical behavior of cracks in plain and reinforced concrete subjected to shear loading. Nasa Sti/Recon Tech. Rep. N 1981, 82, 25417. [Google Scholar]
- Hong, S.; Qin, S.; Dong, P.; Li, G.; Zhang, Y.; Xing, F.; Dong, B. Quantification of rust penetration profile in reinforced concrete deduced by inverse modeling. Cem. Concr. Compos. 2020, 111, 103622. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.; Tang, Z.; Huang, Q. Experimental and numerical investigation of chloride-induced reinforcement corrosion and mortar cover cracking. Cem. Concr. Compos. 2020, 111, 103620. [Google Scholar] [CrossRef]
- Cheng, X.; Xia, J.; Wang, W.L.; Jin, S.J.; Huang, N.; Jin, W.L. Numerical modeling of the effect of concrete porosity evolution on electrochemical chloride removal from concrete structures. Constr. Build. Mater. 2021, 267, 120929. [Google Scholar] [CrossRef]
- Yang, Z.J.; Li, B.B.; Wu, J.Y. X-ray computed tomography images based phase-field modeling of mesoscopic failure in concrete. Eng. Fract. Mech. 2019, 208, 151–170. [Google Scholar] [CrossRef]
- Qiu, J.R.; Feng, D.C.; Wu, G. A multi-physics dual-phase field model for chloride-induced localized corrosion process and cracking in reinforced concrete. Comput. Methods Appl. Mech. Eng. 2025, 434, 117578. [Google Scholar] [CrossRef]
- Liu, Q. Multiphase numerical simulation of concrete at the micro-scale based on multi-ion transport. J. Silic. Sci. 2018, 46, 1074–1080. [Google Scholar] [CrossRef]
- Rao, G.A.; Raghu Prasad, B.K. Influence of type of aggregate and surface roughness on the interface fracture properties. Mater. Struct. 2004, 37, 328–334. [Google Scholar] [CrossRef]
- Gu, X.; Hong, L.; Wang, Z.; Lin, F. Experimental study and application of mechanical properties for the interface between cobblestone aggregate and mortar in concrete. Constr. Build. Mater. 2013, 46, 156–166. [Google Scholar] [CrossRef]
- Gu, X.; Jia, J.; Wang, Z.; Hong, L.; Lin, F. Determination of mechanical parameters for elements in meso-mechanical models of concrete. Front. Struct. Civ. Eng. 2013, 7, 391–401. [Google Scholar] [CrossRef]
- Trawiński, W.; Bobiński, J.; Tejchman, J. Two-dimensional simulations of concrete fracture at aggregate level with cohesive elements based on X-ray μCT images. Eng. Fract. Mech. 2016, 168, 204–226. [Google Scholar] [CrossRef]
- Chen, S.; Zhuang, H.; Zhou, Y.; Li, S.; Li, C. Numerical simulation of chloride-induced reinforcement corrosion in cracked concrete based on mesoscopic model. Constr. Build. Mater. 2024, 441, 137408. [Google Scholar] [CrossRef]
- Millard, S.G.; Law, D.; Bungey, J.H.; Cairns, J. Environmental influences on linear polarisation corrosion rate measurement in reinforced concrete. Ndt E Int. 2001, 34, 409–417. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C. Corrosion rate monitoring in the laboratory and on-site. Constr. Build. Mater. 1996, 10, 315–328. [Google Scholar] [CrossRef]
- Hussain, Z.; Lin, Z.; Pan, H.; Huang, Y.; Tang, F.; Jiang, L. Synergizing empirical and AI methods to examine nano-silica’s microscale contribution to epoxy coating corrosion resistance. Ceram. Int. 2024, 50, 47172–47191. [Google Scholar] [CrossRef]
- Xie, M.; Wei, Z.; Zhao, J.; Chen, Y. Failure analysis of corroded hydrogen-blended natural gas pipelines based on finite element analysis and genetic algorithm-back propagation neural network. Reliab. Eng. Syst. Saf. 2025, 262, 111174. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, T.; Li, H.; Wu, K.; Chen, J.; Zhou, Z.; Wu, Y. Simulation of Corrosion Cracking in Reinforced Concrete Based on Multi-Phase Multi-Species Electrochemical Phase Field Modeling. Materials 2025, 18, 3742. https://doi.org/10.3390/ma18163742
Yao T, Li H, Wu K, Chen J, Zhou Z, Wu Y. Simulation of Corrosion Cracking in Reinforced Concrete Based on Multi-Phase Multi-Species Electrochemical Phase Field Modeling. Materials. 2025; 18(16):3742. https://doi.org/10.3390/ma18163742
Chicago/Turabian StyleYao, Tianhao, Houmin Li, Keyang Wu, Jie Chen, Zhengpeng Zhou, and Yunlong Wu. 2025. "Simulation of Corrosion Cracking in Reinforced Concrete Based on Multi-Phase Multi-Species Electrochemical Phase Field Modeling" Materials 18, no. 16: 3742. https://doi.org/10.3390/ma18163742
APA StyleYao, T., Li, H., Wu, K., Chen, J., Zhou, Z., & Wu, Y. (2025). Simulation of Corrosion Cracking in Reinforced Concrete Based on Multi-Phase Multi-Species Electrochemical Phase Field Modeling. Materials, 18(16), 3742. https://doi.org/10.3390/ma18163742





