The Mechanical Reinforcing Mechanism and Self-Healing Properties of Biomimetic Hybrid Cement Composites via In-Situ Polymerization
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.2. Preparation Progress
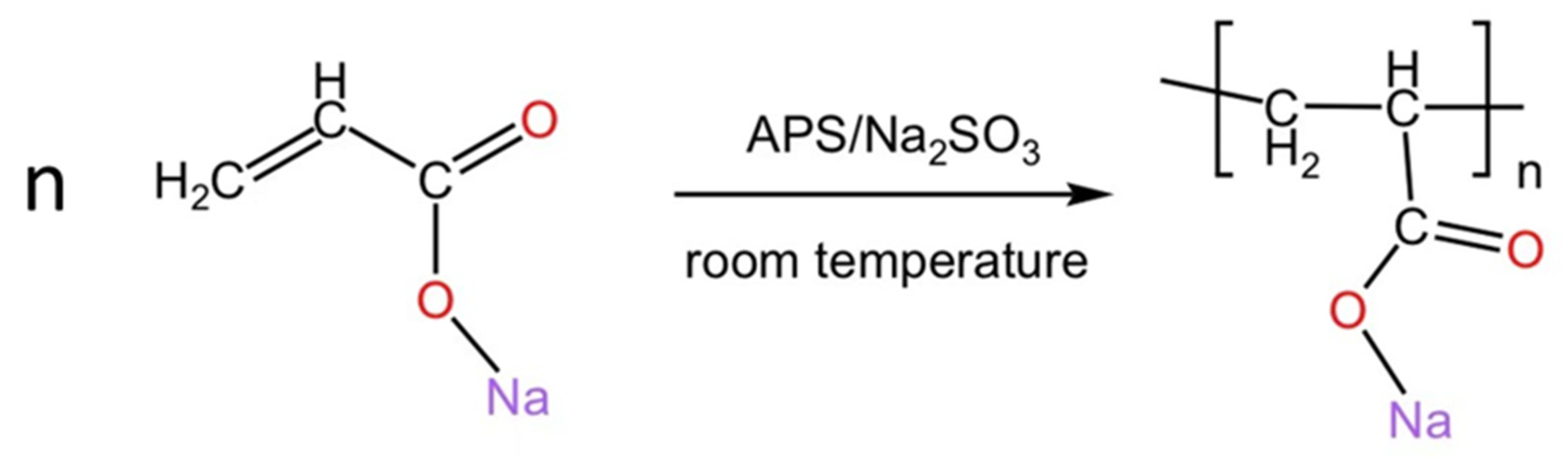
2.2.1. Preparation of PANa
2.2.2. Preparation of CSPA
2.3. Test and Characterization
2.3.1. Structural Characterization of PANa
2.3.2. Effects of ANa Polymerization on Properties of CSPA
2.3.3. Self-Healing Properties of CSPA
3. Results and Discussion
3.1. Structure of PANa
3.1.1. FTIR Analysis
3.1.2. Raman Analysis
3.1.3. Zeta
3.1.4. SEM Analysis
3.2. Effects of ANa Polymerization on the Hydration Process of CSPA
3.2.1. Mechanical Properties of Cement Composites
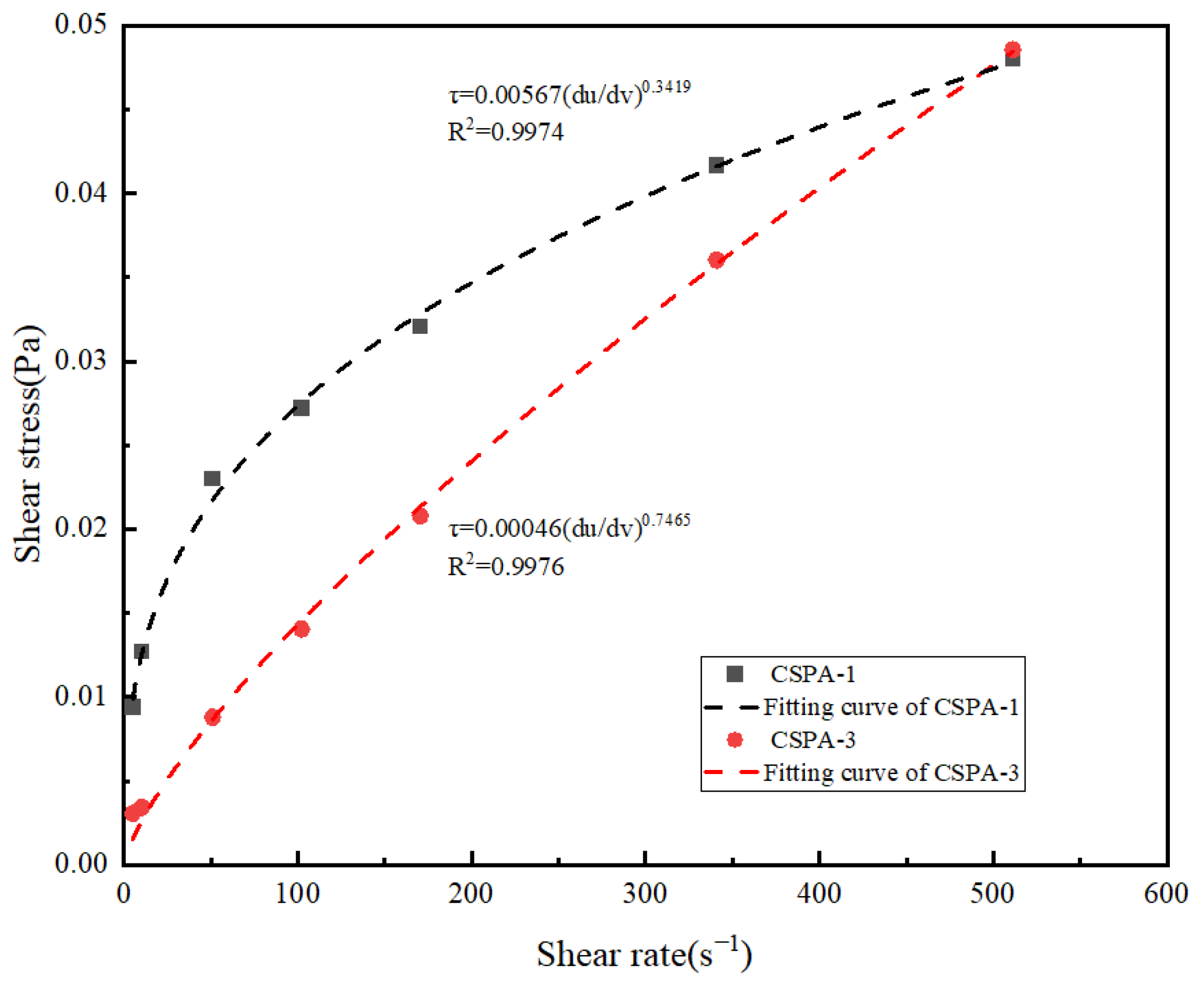
3.2.2. Rheological Properties of CSPA
3.2.3. Hydration Progress
3.2.4. Analysis of Hydration Products of Cement
3.3. Self-Healing Properties of CSPA
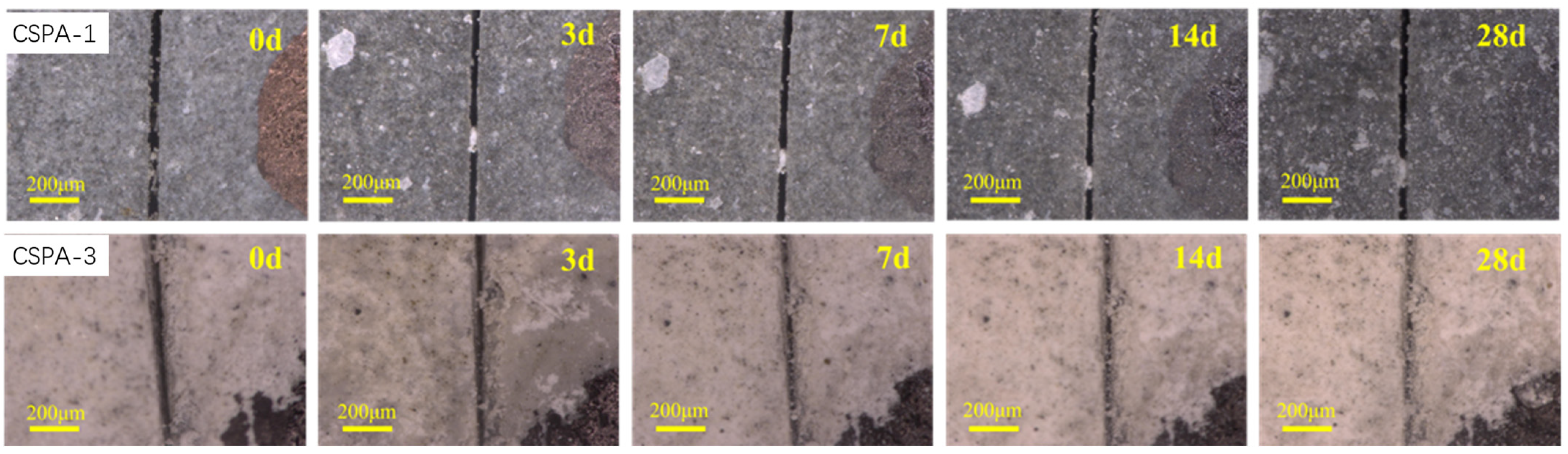
3.3.1. Apparent Self-Healing Performance of CSPA
3.3.2. Apparent Self-Healing Performance of CSPA
4. Conclusions
- (1)
- The polymerization of ANa in the pore solution was confirmed. FTIR spectra show the disappearance of the C=C bond characteristic peak in ANa, while Raman spectra indicate a decrease in the intensity of C=C bond peaks and an increase in C-C bond peaks after polymerization. These results confirm the successful formation of PANa in the pore solution. Additionally, SEM observations of the porous network structure of PANa confirm its binding ability to calcium ions.
- (2)
- The in situ polymerization of the polymer network achieves high-efficiency toughening and late strengthening of the cement. The flexural strength of CSPA-3 surpassed the blank cement sample, achieving 204% of the blank cement sample’s flexural strength after curing for merely 3 days.
- (3)
- The bridge between the polymer network and cement substrate reinforced their bonding performance and made the hydration products form a more dense structure. In addition, the decreased aperture and graded hydration system of CSPA-3 promoted the development of the compressive strength.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adams, K.K.; Mitri, H.S. A Novel Method for the Determination of Direct Tensile Strength of Brittle Materials Using Expansive Cement. Rock Mech. Rock Eng. 2024, 58, 4193–4206. [Google Scholar] [CrossRef]
- Khoshnazar, R.; Alizadeh, R.; Beaudoin, J.J.; Raki, L. The physico-mechanical stability of C–S–H/polyaniline nanocomposites. Mater. Struct. 2015, 48, 67–75. [Google Scholar] [CrossRef]
- Farhan, A.H.; Dawson, A.R.; Thom, N.H. Effect of cementation level on performance of rubberized cement-stabilized aggregate mixtures. Mater. Des. 2016, 97, 98–107. [Google Scholar] [CrossRef]
- Musso, S.; Robisson, A.; Maheshwar, S.; Ulm, F.-J. Stimuli-Responsive Cement-Reinforced Rubber. ACS Appl. Mater. Interfaces 2014, 6, 6962–6968. [Google Scholar] [CrossRef]
- Childers, M.I.; Nguyen, M.-T.; Rod, K.A.; Koech, P.K.; Um, W.; Chun, J.; Glezakou, V.-A.; Linn, D.; Roosendaal, T.J.; Wietsma, T.W.; et al. Polymer-Cement Composites with Self-Healing Ability for Geothermal and Fossil Energy Applications. Chem. Mater. 2017, 29, 4708–4718. [Google Scholar] [CrossRef]
- Du, J.; Bu, Y.; Shen, Z. Interfacial properties and nanostructural characteristics of epoxy resin in cement matrix. Constr. Build. Mater. 2018, 164, 103–112. [Google Scholar] [CrossRef]
- Pang, B.; Zhang, Y.; Liu, G. Study on the effect of waterborne epoxy resins on the performance and microstructure of cement paste. Constr. Build. Mater. 2018, 167, 831–845. [Google Scholar] [CrossRef]
- Myszka, B.; Hurle, K.; Zheng, K.; Wolf, S.E.; Boccaccini, A.R. Mechanical improvement of calcium carbonate cements by in situ HEMA polymerization during hardening. J. Mater. Chem. B 2019, 7, 3403–3411. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Y.; Ming, X.; Chen, B.; Li, Z. Enhancing anti-washout behavior of cement paste by polyacrylamide gelation: From floc properties to mechanism. Cem. Concr. Compos. 2023, 136, 104887. [Google Scholar] [CrossRef]
- Robisson, A.; Maheshwari, S.; Musso, S.; Thomas, J.J.; Auzerais, F.M.; Han, D.; Qu, M.; Ulm, F.-J. Reactive elastomeric composites: When rubber meets cement. Compos. Sci. Technol. 2013, 75, 77–83. [Google Scholar] [CrossRef]
- Fonseca, C.S.; Silva, M.F.; Mendes, R.F.; Hein, P.R.G.; Zangiacomo, A.L.; Savastano, H.; Tonoli, G.H.D. Jute fibers and micro/nanofibrils as reinforcement in extruded fiber-cement composites. Constr. Build. Mater. 2019, 211, 517–527. [Google Scholar] [CrossRef]
- Rod, K.A.; Fernandez, C.A.; Nguyen, M.-T.; Gardiner, J.B.; Huerta, N.J.; Glezakou, V.-A.; Varga, T.; Rousseau, R.; Koech, P.K. Polymer-cement composites with adhesion and re-adhesion (healing) to casing capability for geothermal wellbore applications. Cem. Concr. Compos. 2020, 107, 103490. [Google Scholar] [CrossRef]
- Zheng, T.; Su, Y.; Zhang, X.; Zhou, H.; Qian, C. Effect and Mechanism of Encapsulation-Based Spores on Self-Healing Concrete at Different Curing Ages. ACS Appl. Mater. Interfaces 2020, 12, 52415–52432. [Google Scholar] [CrossRef]
- Ying, Y.; Hu, M.; Han, J.; Liu, W.; Qi, B.; Guo, J. Self-healing in cementitious system using interface enhanced capsules prepared at room temperature. J. Clean. Prod. 2023, 395, 136465. [Google Scholar] [CrossRef]
- Zheng, Q.; Xie, Z.; Li, J.; Li, W.; Jiang, Z. Autogenously self-healable cementitious composite incorporating autolytic mineral microspheres: Hydration regulation and structural alteration. Compos. Part B Eng. 2023, 259, 110724. [Google Scholar] [CrossRef]
- Finnemore, A.; Cunha, P.; Shean, T.; Vignolini, S.; Guldin, S.; Oyen, M.; Steiner, U. Biomimetic layer-by-layer assembly of artificial nacre. Nat. Commun. 2012, 3, 966. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; She, W.; Zuo, W.; Zhou, Y.; Huang, J.; Zhang, Z.; Geng, Z.; Yao, Y.; Zhang, W.; Zheng, L.; et al. Hierarchical Toughening of a Biomimetic Bulk Cement Composite. ACS Appl. Mater. Interfaces 2020, 12, 53297–53309. [Google Scholar] [CrossRef]
- Yuan, C.; Yangzezhi, Z.; Yang, Z.; Wei, Z.; Weihuan, L.; Wei, S.; Jiaping, L.; Changwen, M. Multi-layered cement-hydrogel composite with high toughness, low thermal conductivity, and self-healing capability. Nat. Commun. 2023, 14, 3438. [Google Scholar]
- Liu, M.; Hu, M.; Zou, S.; Lu, H.; Yu, J.; Guo, J. Biomimetic anisotropic hydrogel as a smart self-healing agent of sustainable cement-based infrastructure. Cem. Concr. Compos. 2024, 154, 105763. [Google Scholar] [CrossRef]
- Yin, B.; Qi, D.; Hua, X.; Fan, F.; Han, K.; Hou, Y.; Hou, D.; Chen, B. Mechanical properties and micro-mechanism of cement-based materials strengthened by in-situ organic-inorganic polymerization. Cem. Concr. Compos. 2023, 142, 105202. [Google Scholar] [CrossRef]
- Narupai, B.; Willenbacher, J.; Bates, M.W.; Barbon, S.M.; Zerdan, R.B.; McGrath, A.J.; Lee, I.H.; Anastasaki, A.; Discekici, E.H.; Laitar, D.S.; et al. Low-Temperature, Rapid Copolymerization of Acrylic Acid and Sodium Acrylate in Water. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1414–1419. [Google Scholar] [CrossRef]
- Chen, B.; Qiao, G.; Hou, D.; Wang, M.; Li, Z. Cement-based material modified by in-situ polymerization: From experiments to molecular dynamics investigation. Compos. Part B Eng. 2020, 194, 108036. [Google Scholar] [CrossRef]
- Resan, S.F.; Zemam, S.K.; Abed, M.S. Developing Self-Curing Cement Sand Mortar Using Sodium Polyacrylate. J. Eng. Sustain. Dev. 2019, 23, 95–107. [Google Scholar] [CrossRef]
- Chen, B.; Xu, B.; Sun, Z. Rheological properties of cement paste with in-situ polymerization of sodium acrylate: Roles of polymerization and hydration. Constr. Build. Mater. 2024, 457, 139381. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, Z.; Hu, X.; Chen, B.; Li, Z.; Liang, R.; Sun, G. A mechanical strong polymer-cement composite fabricated by in situ polymerization within the cement matrix. J. Build. Eng. 2021, 42, 103048. [Google Scholar] [CrossRef]
- Han, J.; Hu, M.; Ying, Y.; Liu, M.; Yan, X.; Guo, J. Efficient healing of existed cracks in cement via synergistic effects of cement matrix activation and monomer polymerization. Constr. Build. Mater. 2023, 406, 133394. [Google Scholar] [CrossRef]
- Gb/T 1346-2024; National Technical Committee for Cement Standardization (SAC/TC.184) “Test Methods for Water Requirement of Standard Consistency, Setting Time and Soundness of the Portland Cement”. Standardization Administration of the People’s Republic of China: Beijing, China, 2024.
- GBT 19139-2012; National Technical Committee 355 on Petroleum of Standardization Administration of China (SAC/TC.355) “Testing of Well Cements”. Standardization Administration of the People’s Republic of China: Beijing, China, 2012.
- Rougelot, T.; Burlion, N.; Bernard, D.; Skoczylas, F. About microcracking due to leaching in cementitious composites: X-ray microtomography description and numerical approach. Cem. Concr. Res. 2010, 40, 271–283. [Google Scholar] [CrossRef]
- Pouyanne, A.; Boudache, S.; Hilloulin, B.; Loukili, A.; Roziere, E. Experimental Investigation on the Effects of Mineral Water Composition on the Leaching of Cement-Based Materials. Materials 2024, 17, 1548. [Google Scholar] [CrossRef] [PubMed]
- Li, I.C.; Chen, Y.-H.; Chen, Y.-C. Adsorption properties of ammonium nitrogen from aqueous solutions using sodium humate/poly(sodium acrylate) hydrogel. J. Taiwan Inst. Chem. Eng. 2024, 161, 105516. [Google Scholar] [CrossRef]
- Yu, C.; Liao, R.; Cai, X.; Yu, X. Sodium polyacrylate modification method to improve the permeant performance of bentonite in chemical resistance. J. Clean. Prod. 2019, 213, 242–250. [Google Scholar] [CrossRef]
- Murli, C.; Song, Y. Pressure-Induced Polymerization of Acrylic Acid: A Raman Spectroscopic Study. J. Phys. Chem. B 2010, 114, 9744–9750. [Google Scholar] [CrossRef]
- Johan, R.P.; Martin, A.; Jan, L.; Christer, E. Differences in Binding of a Cationic Surfactant to Cross-Linked Sodium Poly(Acrylate) and Sodium Poly(Styrene Sulfonate) Studied by Raman Spectroscopy. Langmuir 2005, 21, 2761–2765. [Google Scholar]
- Nap, R.J.; Szleifer, I. Effect of calcium ions on the interactions between surfaces end-grafted with weak polyelectrolytes. J. Chem. Phys. 2018, 149, 163309. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Perez, P.M.; da Silva, R.M.P.; Strehin, I.; Kouwer, P.H.J.; Leeuwenburgh, S.C.G.; Messersmith, P.B. Self-Healing Hydrogels Formed by Complexation between Calcium Ions and Bisphosphonate-Functionalized Star-Shaped Polymers. Macromolecules 2017, 50, 8698–8706. [Google Scholar] [CrossRef]
- Heinze, M.; Horn, C.; Pospiech, D.; Boldt, R.; Kobsch, O.; Eckstein, K.; Jehnichen, D.; Voit, B.; Baudis, S.; Liska, R.; et al. Polymer Networks for Enrichment of Calcium Ions. Polymers 2021, 13, 3506. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Wang, S.; Zheng, Y.; Chi, B.; Guo, J.; Xu, Y.; Jiang, M.; Wang, Z.; Cui, S. Formation of tannic acid-calcium polymeric network in pore solution: Characterization and kinetics. J. Build. Eng. 2023, 80, 108121. [Google Scholar] [CrossRef]
- Yang, K.; Tang, Z.; Li, W.; Long, Z.; He, J.; Ma, G.; Li, Y.; Xiang, Y.; Xie, Y.; Long, G. A comprehensive review on the toughening technologies of cement-based materials: From multiscale materials to advanced processes. Constr. Build. Mater. 2024, 456, 139274. [Google Scholar] [CrossRef]
- Liu, M.; Hu, M.; Li, P.; Zhang, H.; Zhao, J.; Guo, J. A new application of fluid loss agent in enhancing autogenous healing ability and improving mechanical properties of oil well cement. Cem. Concr. Compos. 2022, 128, 104419. [Google Scholar] [CrossRef]
- Mohammed, A.; Mahmood, W.; Ghafor, K. Shear stress limit, rheological properties and compressive strength of cement-based grout modified with polymers. J. Build. Pathol. Rehabil. 2019, 5, 3. [Google Scholar] [CrossRef]
- Kai, C.; Jun, C.; Sabri, S.M.M.; Jiandong, H. Influence of Graphene Nanoplates on Dispersion, Hydration Behavior of Sulfoaluminate Cement Composites. Materials 2022, 15, 5357. [Google Scholar] [CrossRef]
- Yao, H.; Fan, M.; Huang, T.; Yuan, Q.; Xie, Z.; Chen, Z.; Li, Y.; Wang, J. Retardation and bridging effect of anionic polyacrylamide in cement paste and its relationship with early properties. Constr. Build. Mater. 2021, 306, 124822. [Google Scholar] [CrossRef]
- Zhang, M.; Shen, J.; Yang, R.; Ji, H.; Ding, J. Effect of Curing Age on the Microstructure and Hydration Behavior of Oil Well Cement Paste Cured at High Temperature. J. Mater. Civ. Eng. 2021, 33, 1–12. [Google Scholar] [CrossRef]
- Vafaei, B.; Ghahremaninezhad, A. Self-healing effect of hydrogels in cement slag and fly ash pastes. Constr. Build. Mater. 2024, 438, 137036. [Google Scholar] [CrossRef]
- Pai, S.; Kini, M.S.; Rangasamy, G.; Selvaraj, R. Mesoporous calcium hydroxide nanoparticle synthesis from waste bivalve clamshells and evaluation of its adsorptive potential for the removal of Acid Blue 113 dye. Chemosphere 2023, 313, 137476. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Wang, F.; Zhang, J.; Guo, L.; Zhang, Y.; Li, Y.; Lin, J.; Lu, Z.; Jiang, J. Deciphering the influence of superabsorbent polymers on cement hydration and portlandite formation. Constr. Build. Mater. 2024, 418, 135455. [Google Scholar] [CrossRef]
- Kontoyannis, C.G.; Vagenas, N.V. Calcium carbonate phase analysis using XRD and FT-Raman spectroscopy. Analyst 2000, 125, 251–255. [Google Scholar] [CrossRef]
- Fordham, C.J.; Smalley, I.J. A simple thermogravimetric study of hydrated cement. Cem. Concr. Res. 1985, 15, 141–144. [Google Scholar] [CrossRef]
- Im, S.; Jee, H.; Suh, H.; Kanematsu, M.; Morooka, S.; Taku, K.; Yuhei, N.; Machida, A.; Kim, J.; Bae, S. Temperature effects on local structure, phase transformation, and mechanical properties of calcium silicate hydrates. J. Am. Ceram. Soc. 2021, 104, 4803–4818. [Google Scholar] [CrossRef]
- Chomyen, P.; Sinthupinyo, S.; Hanpongpun, W.; Wianglor, K.; Aodkeng, S.; Chaipanich, A. Thermogravimetric analysis and compressive strength of cement mixes using Thai Lopburi calcined clay as supplementary cementitious material. J. Therm. Anal. Calorim. 2024, 149, 7197–7203. [Google Scholar] [CrossRef]
- Galan, I.; Glasser, F.P.; Andrade, C. Calcium carbonate decomposition. J. Therm. Anal. Calorim. 2012, 111, 1197–1202. [Google Scholar] [CrossRef]














| Methods | Compressive Strength (28 d) | Flexural Strength (28 d) | Self-Healing Efficiency | Ref. |
|---|---|---|---|---|
| Through an innovative biomimetic layered structure design, a multi-layered cement-hydrogel composite material was constructed. | / | Increased by 107% | / | [18] |
| A biomimetic anisotropic hydrogel, namely a alginate/polyacrylamide/halloysite nanotube hybrid hydrogel (SA/AM/HNTs-RDC), was prepared as a self-healing agent. | Decreased by 42% | / | The recovery rate of compressive strength was approximately 92.8%. | [19] |
| A plug-in network structure was developed through the in situ polymerization of zirconium phosphate and acrylamide. | Decreased | Increased by 105% | / | [20] |
| In the ordinary Portland cement system, an organic network of the cementitious matrix was prepared through the in situ polymerization of sodium acrylate monomers. | Increased by 15% | Increased by 200% | / | [22] |
| The in situ polymerization-modified cement paste with different amounts of ANa monomers was used to regulate the rheological behavior. | / | / | / | [24] |
| By using sodium silicate, sodium silicate/sodium acrylate (ANa), and sodium silicate/sodium polyacrylate as healing agents, efficient healing of existing cracks was achieved through the synergistic reaction between the activation of the cement matrix and the organic polymerization of sodium acrylate. | / | / | The recovery rate of compressive strength was approximately 119.99% | [26] |
| Components | CaO | SiO2 | Fe2O3 | SO3 | Others |
|---|---|---|---|---|---|
| Content/% | 61.39 | 21.36 | 4.56 | 3.96 | 4.77 |
| Cement/(g) | M/(g) | I/(mg) | I′/(mg) | Water/(g) | |
|---|---|---|---|---|---|
| CSPA-1 (Blank) | 800 | 0 | 0 | 0 | 352 |
| CSPA-2 (2%M) | 800 | 16 | 288 | 240 | 352 |
| CSPA-3 (4%M) | 800 | 32 | 576 | 480 | 352 |
| CSPA-4 (8%M) | 800 | 64 | 1152 | 960 | 352 |
| Test | Mold Size/mm3 | Total Curing Time/d |
|---|---|---|
| Flexural strength | 40 × 40 × 100 | 3 |
| Compressive strength | 50.8 × 50.8 × 50.8 | 28 |
| TG/BET (hydration products) | 50.8 × 50.8 × 50.8 | 28 |
| SEM (hydration products) | 50.8 × 50.8 × 50.8 | 28 |
| Self-healing efficiency | 18 × 16 × 18 | 28 |
| SEM/TG/XRD/FTIR (healing product) | 18 × 16 × 18 | 28 |
| 0 h | 1.5 h | 3 h | ||||||
|---|---|---|---|---|---|---|---|---|
| Peak Weighting Center/(cm−1) | Peak Area Percentage/(%) | Standard Error | Peak Weighting Center/(cm−1) | Peak Area Percentage/(%) | Standard Error | Peak Weighting Center/(cm−1) | Peak Area Percentage/(%) | Standard Error |
| 1642.31 | 31.67 | 0.06028 | 1642.90 | 15.27 | 0.03512 | 1642.69 | 23.13 | 0.07638 |
| 1556.75 | 1.46 | 0.03000 | 1559.36 | 3.20 | 0.09165 | 1560.06 | 3.67 | 0.08145 |
| 1432.60 | 29.63 | 0.03000 | 1432.46 | 21.71 | 0.02082 | 1432.97 | 19.81 | 0.05508 |
| 1365.43 | 5.17 | 0.08622 | 1364.86 | 3.12 | 0.04000 | 1356.41 | 0.69 | 0.02082 |
| 1283.27 | 21.18 | 0.03215 | 1284.72 | 12.18 | 0.04041 | 1283.17 | 13.68 | 0.04041 |
| 1067.42 | 3.45 | 0.01528 | 1069.85 | 4.23 | 0.06110 | 1071.87 | 6.15 | 0.03786 |
| 900.39 | 7.44 | 0.04509 | 872.00 | 40.29 | 0.05508 | 876.33 | 32.88 | 0.12858 |
| Sample | Curing Time/d | Surface Area (m2/g) | Total Pore Volume of Pores (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|
| CSPA-1 | 3 | 15.4411 | 0.07953 | 20.6022 |
| 14 | 28.1092 | 0.08849 | 12.5924 | |
| 28 | 31.0427 | 0.08939 | 11.5183 | |
| CSPA-3 | 3 | 5.5207 | 0.04133 | 29.9455 |
| 14 | 9.2676 | 0.05787 | 24.9773 | |
| 28 | 19.7404 | 0.1221 | 24.7411 |
| Sample | Curing Time/d | Percentage of Mass Loss/% | ||
|---|---|---|---|---|
| 100–200 °C | 400–440 °C | 550–650 °C | ||
| CSPA-1 | 3 | 3.25 | 3.37 | 1.15 |
| 14 | 3.63 | 3.61 | 1.46 | |
| 28 | 3.64 | 4.67 | 1.51 | |
| CSPA-3 | 3 | 1.12 | 0.3 | 2.38 |
| 14 | 1.31 | 0.31 | 2.50 | |
| 28 | 3.28 | 3.55 | 3.25 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, W.; Zhao, J.; Guo, B.; Li, S.; Shen, J.; Liu, M.; Han, J.; Xing, S.; Hu, M.; Guo, J. The Mechanical Reinforcing Mechanism and Self-Healing Properties of Biomimetic Hybrid Cement Composites via In-Situ Polymerization. Materials 2025, 18, 3763. https://doi.org/10.3390/ma18163763
Bao W, Zhao J, Guo B, Li S, Shen J, Liu M, Han J, Xing S, Hu M, Guo J. The Mechanical Reinforcing Mechanism and Self-Healing Properties of Biomimetic Hybrid Cement Composites via In-Situ Polymerization. Materials. 2025; 18(16):3763. https://doi.org/10.3390/ma18163763
Chicago/Turabian StyleBao, Wenhui, Jian Zhao, Bumin Guo, Shuan Li, Jinwei Shen, Mengyuan Liu, Jingmin Han, Susu Xing, Miaomiao Hu, and Jintang Guo. 2025. "The Mechanical Reinforcing Mechanism and Self-Healing Properties of Biomimetic Hybrid Cement Composites via In-Situ Polymerization" Materials 18, no. 16: 3763. https://doi.org/10.3390/ma18163763
APA StyleBao, W., Zhao, J., Guo, B., Li, S., Shen, J., Liu, M., Han, J., Xing, S., Hu, M., & Guo, J. (2025). The Mechanical Reinforcing Mechanism and Self-Healing Properties of Biomimetic Hybrid Cement Composites via In-Situ Polymerization. Materials, 18(16), 3763. https://doi.org/10.3390/ma18163763







