Determining the Sulfate Content in Phosphogypsum and Cement-Based Materials Based on Conductivity Titration
Abstract
1. Introduction
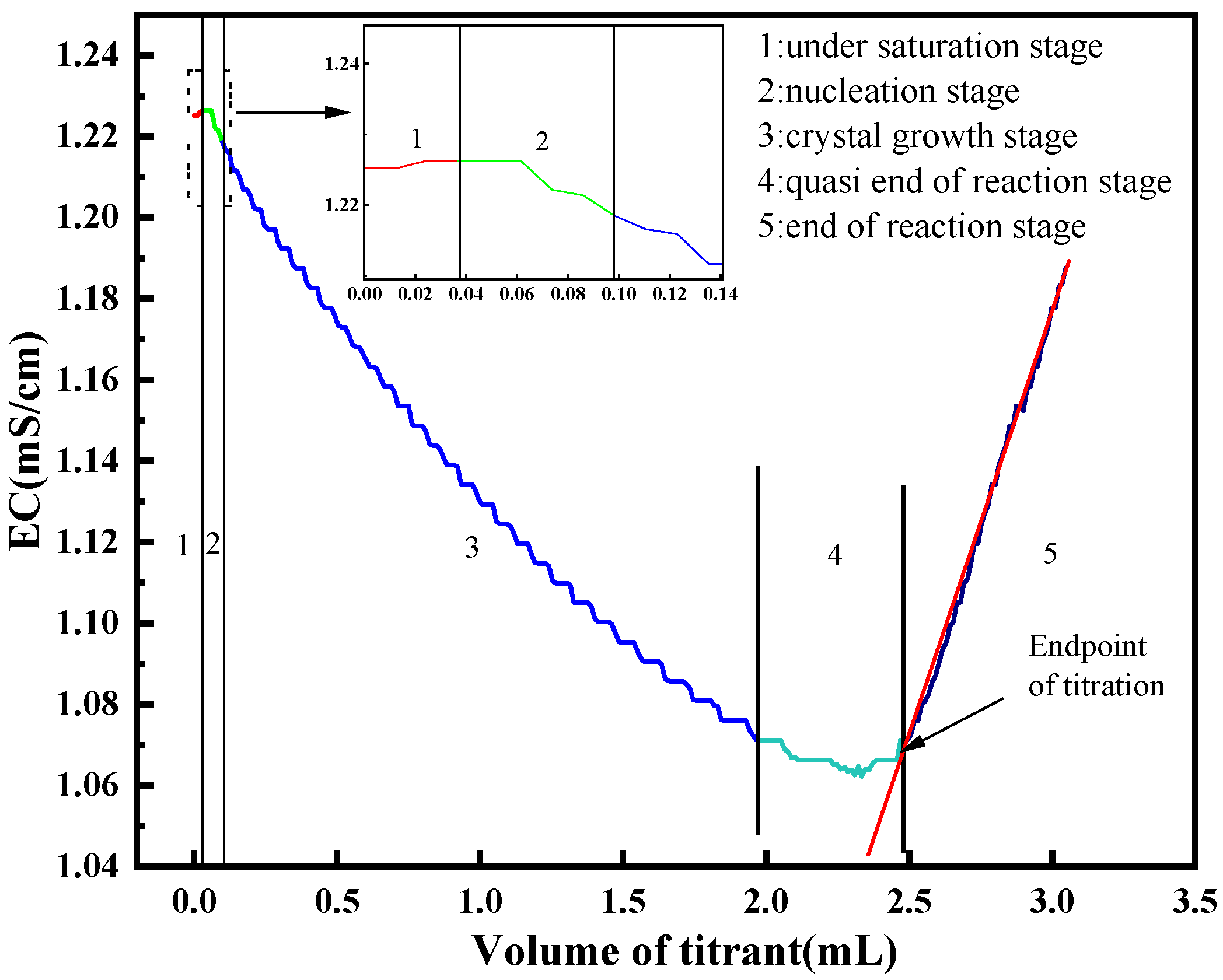
2. Conductivity Titration Theory
3. Materials and Methods
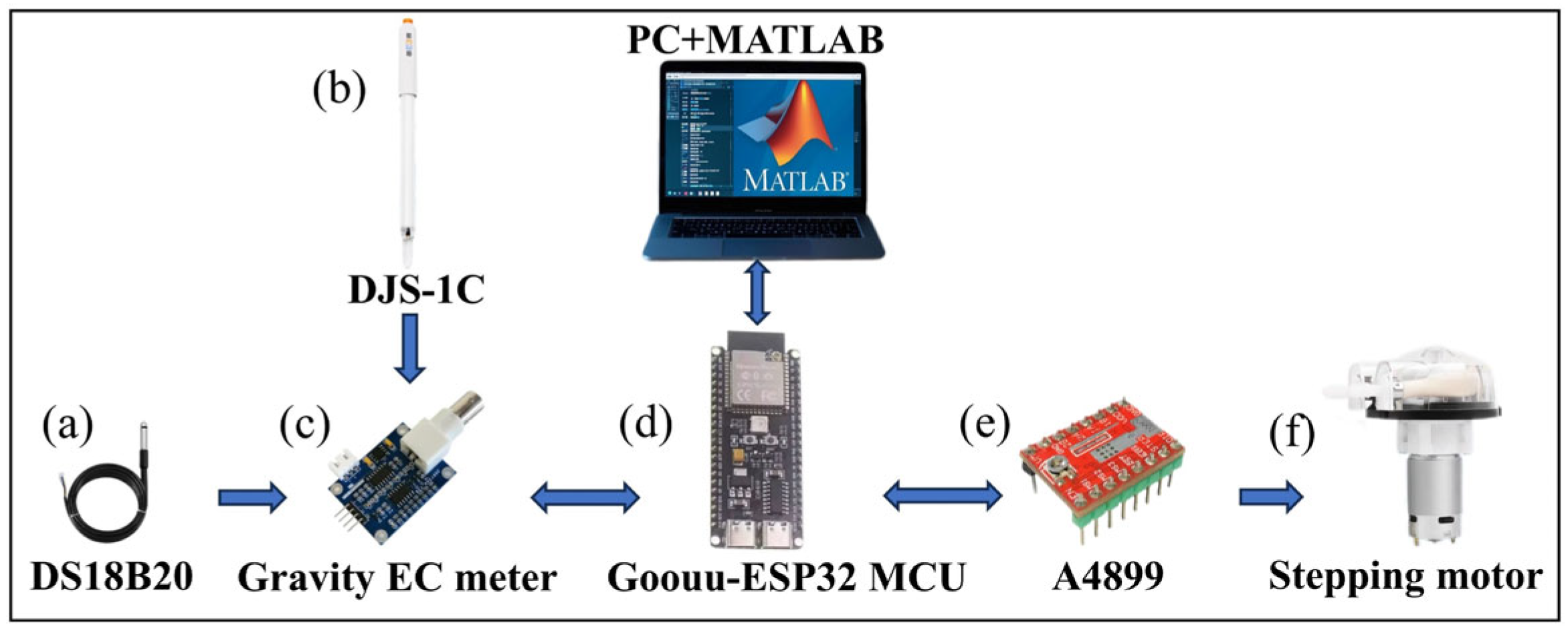
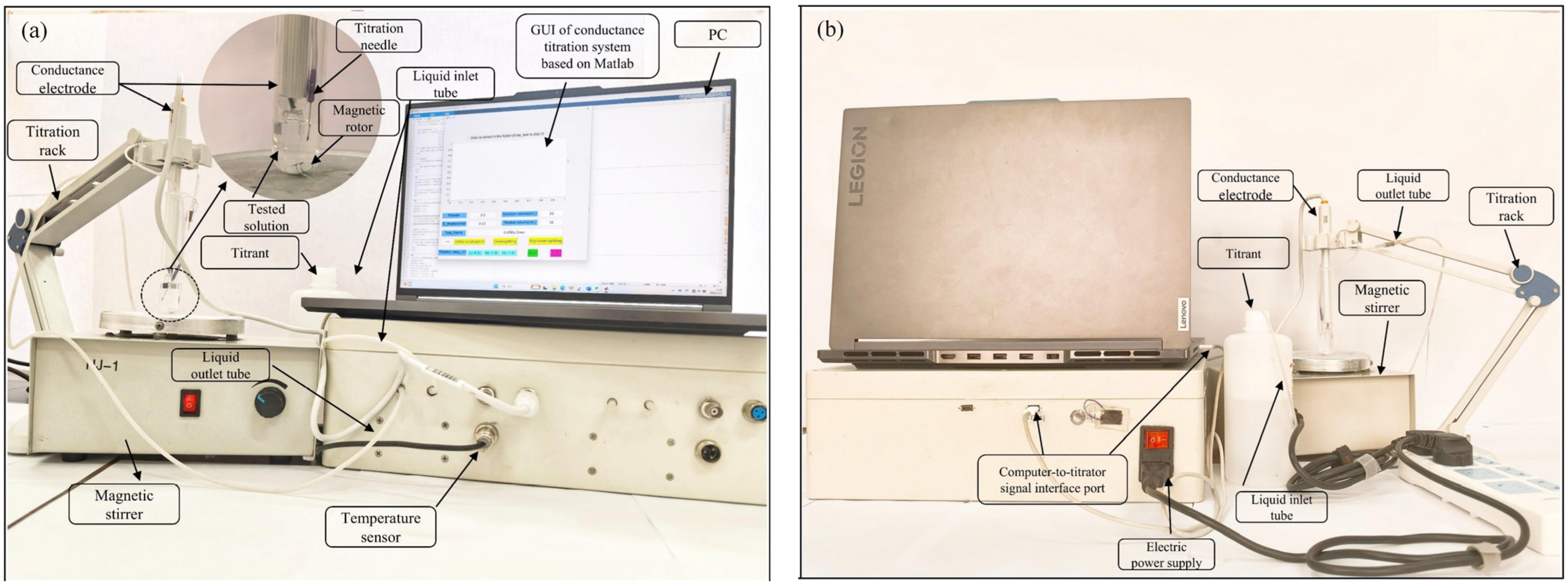
3.1. Conductivity Titrators
3.1.1. Goouuu-ESP32 MCU
3.1.2. Conductivity Sensor
3.1.3. A 42-Step Motor
3.1.4. Temperature Sensor
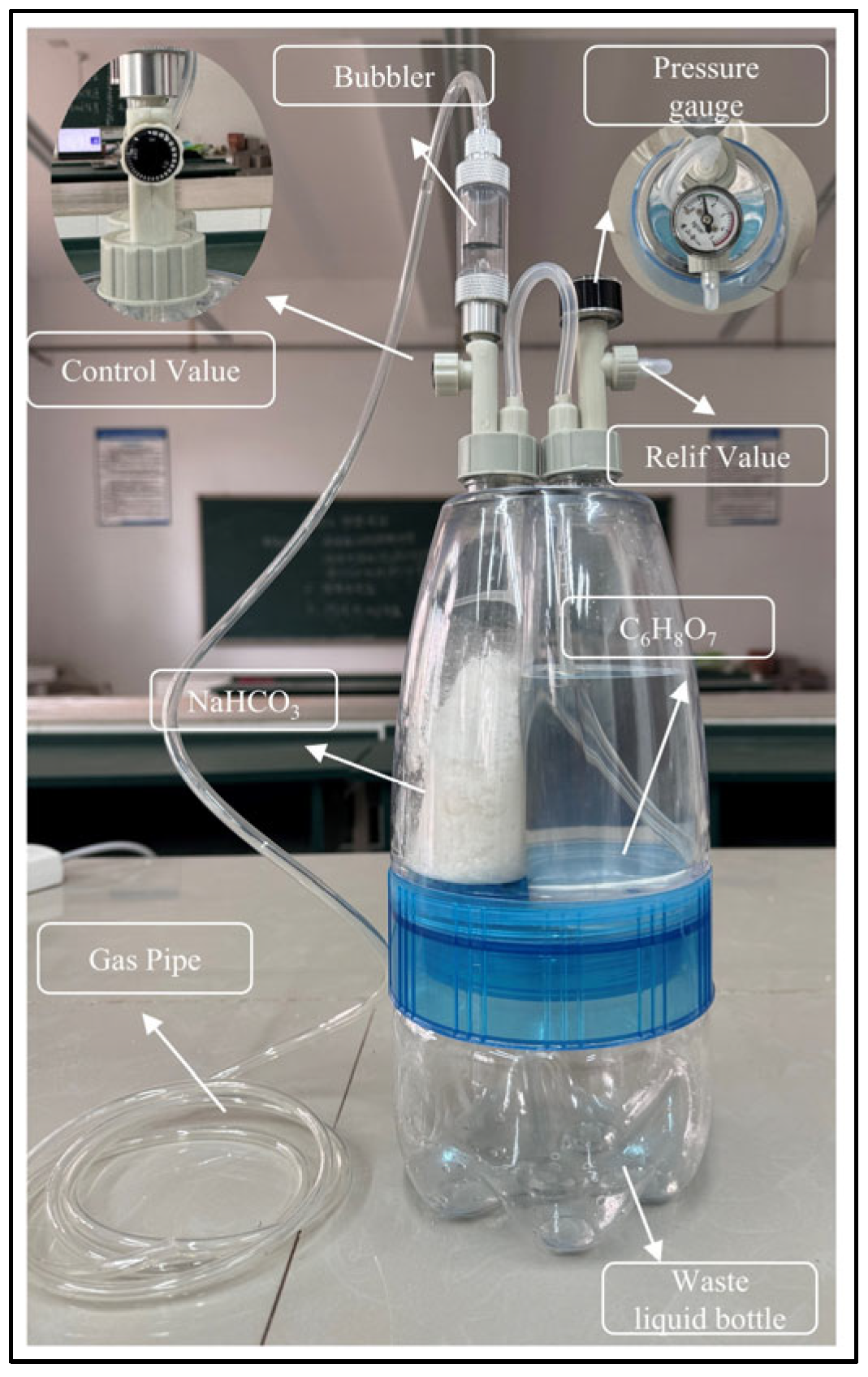
3.1.5. CO2 Generator
3.1.6. Other Tools
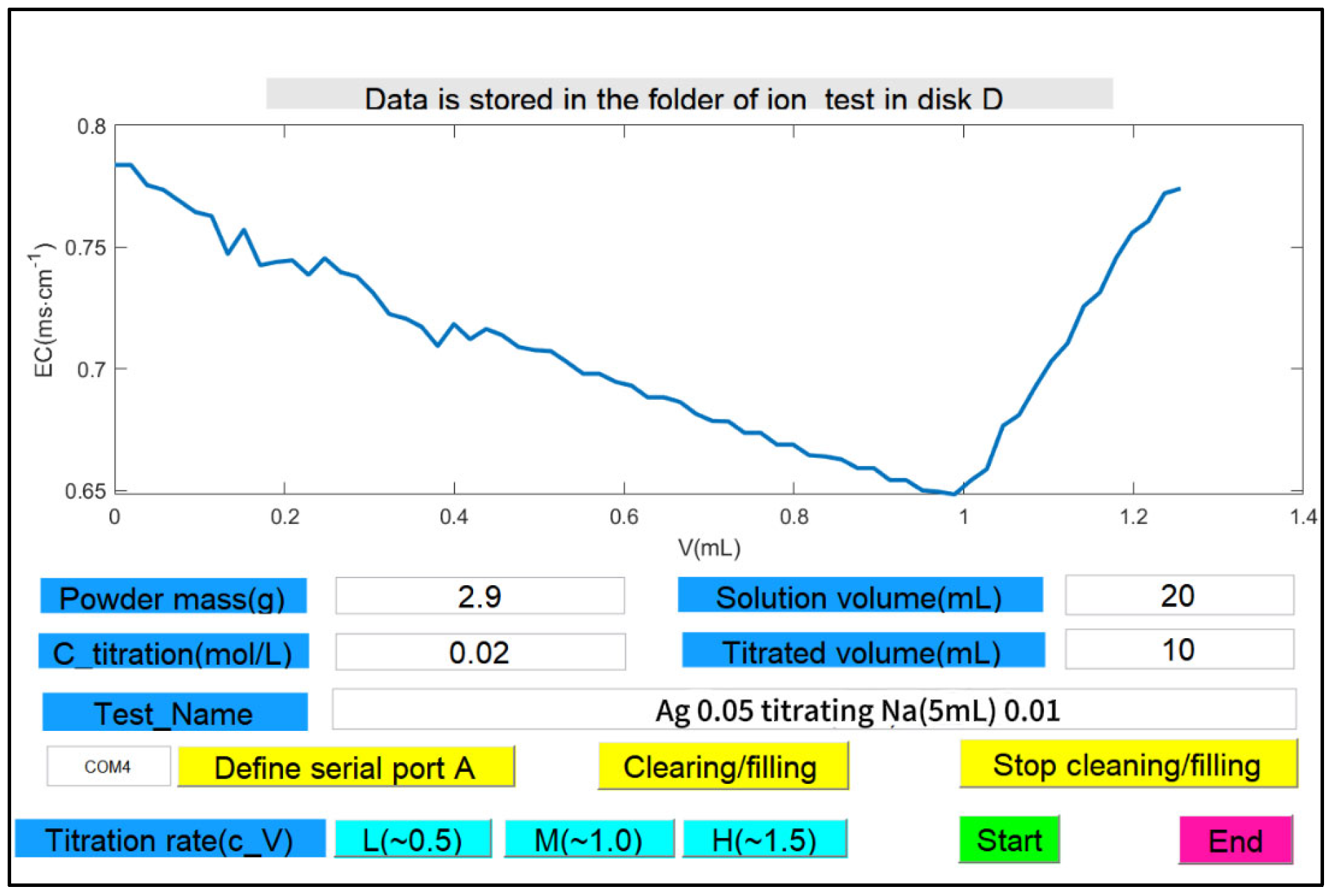
3.2. Software for Conductivity Titration
3.3. Preparation of Solutions
3.4. Conductivity Titration Procedure
3.4.1. Conditions for Conductivity Titration
3.4.2. Conductivity Electrode Calibration
3.4.3. Titration Volume Correction
3.4.4. Calculation of Sulfate Ion Test Results
4. Results and Discussion
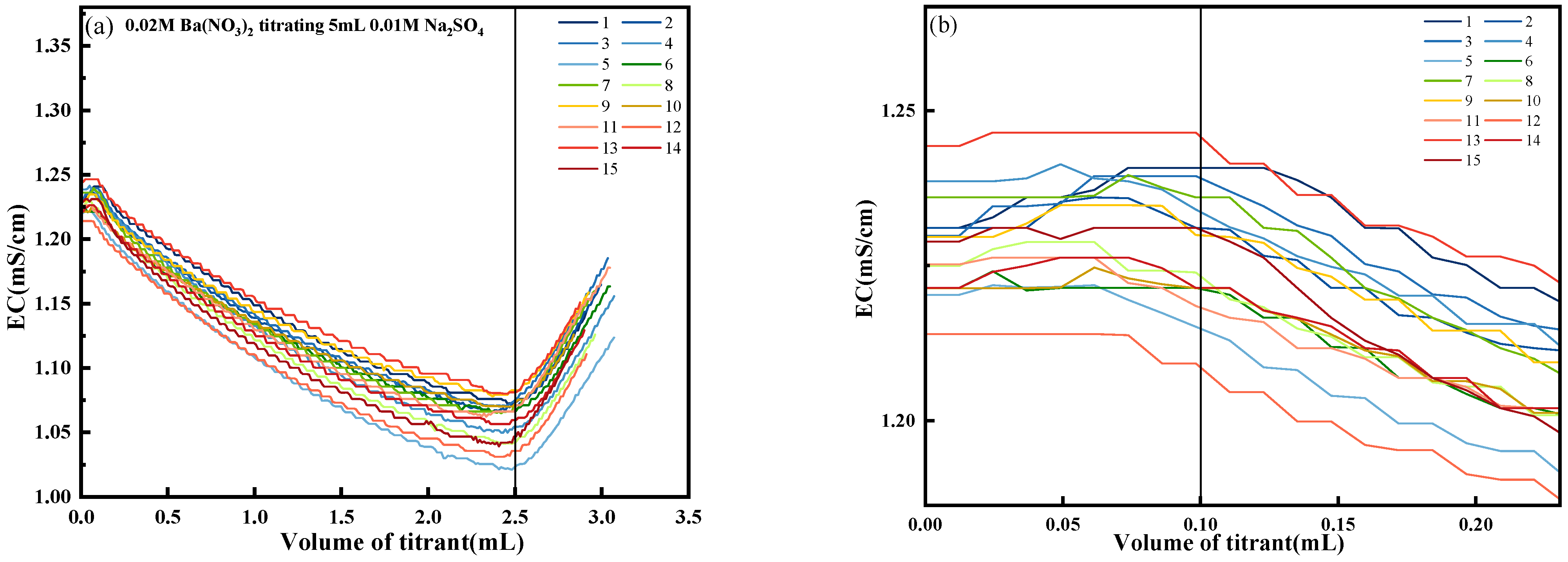
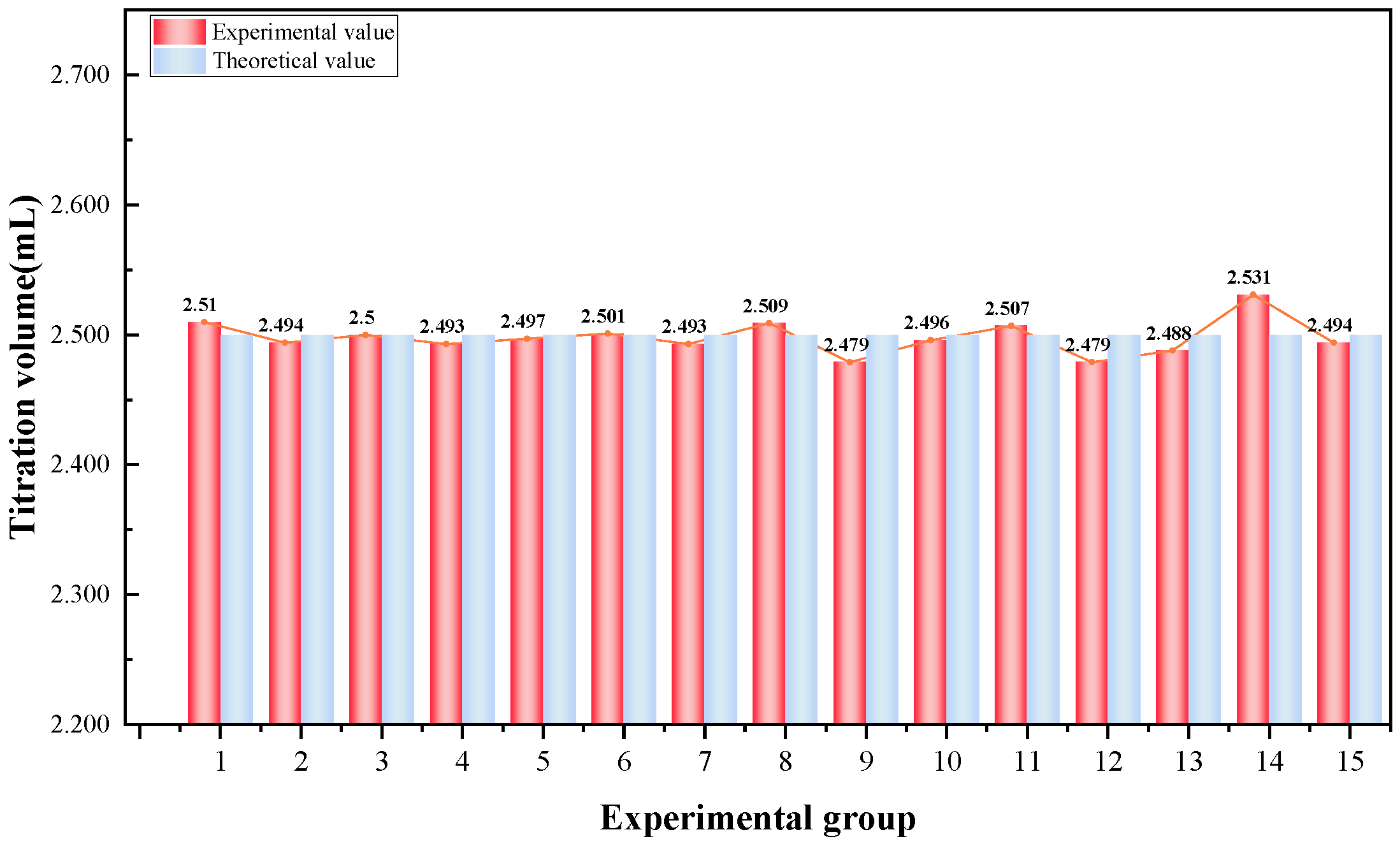
4.1. Precision and Stability of Conductivity Titration
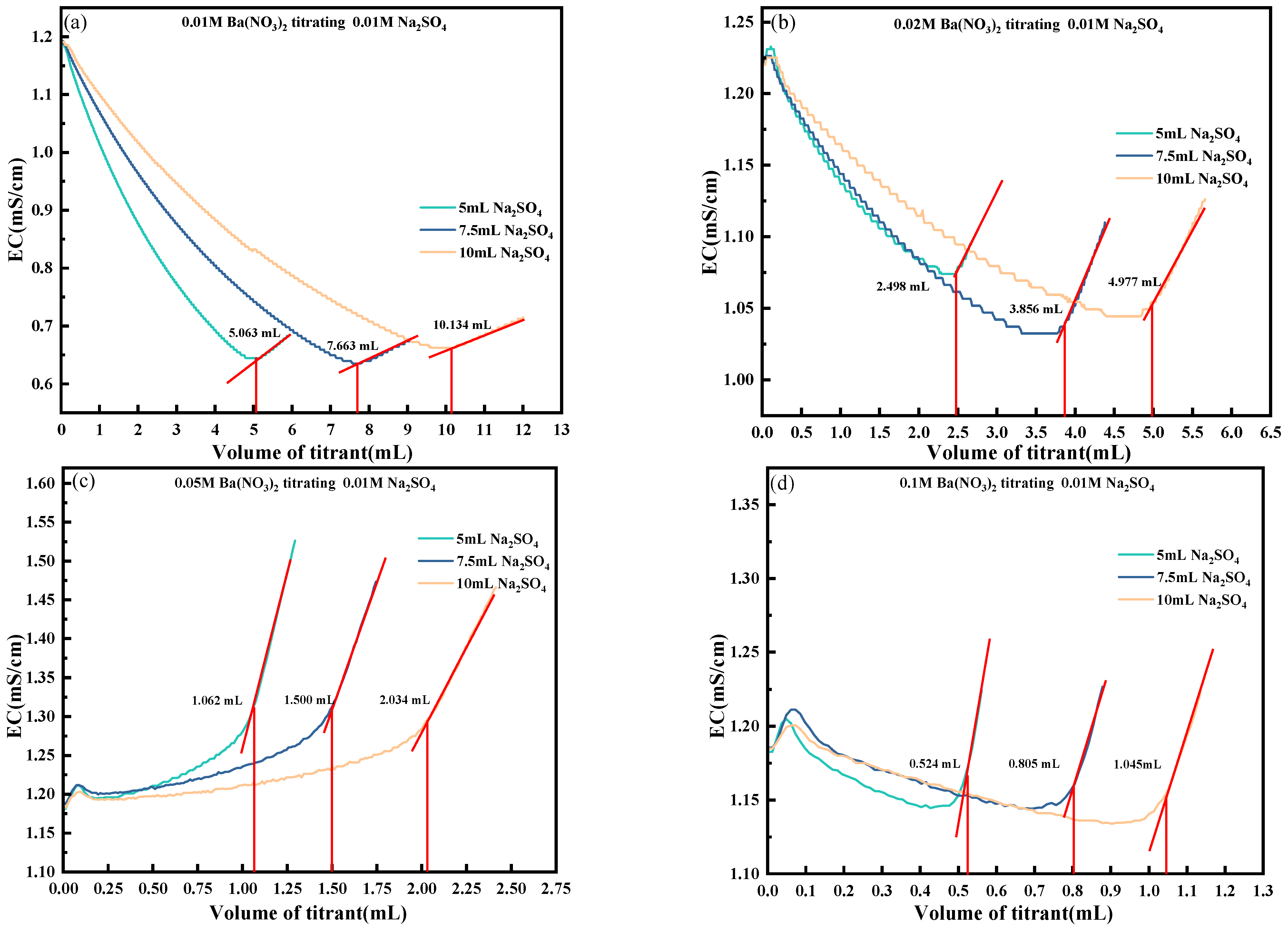
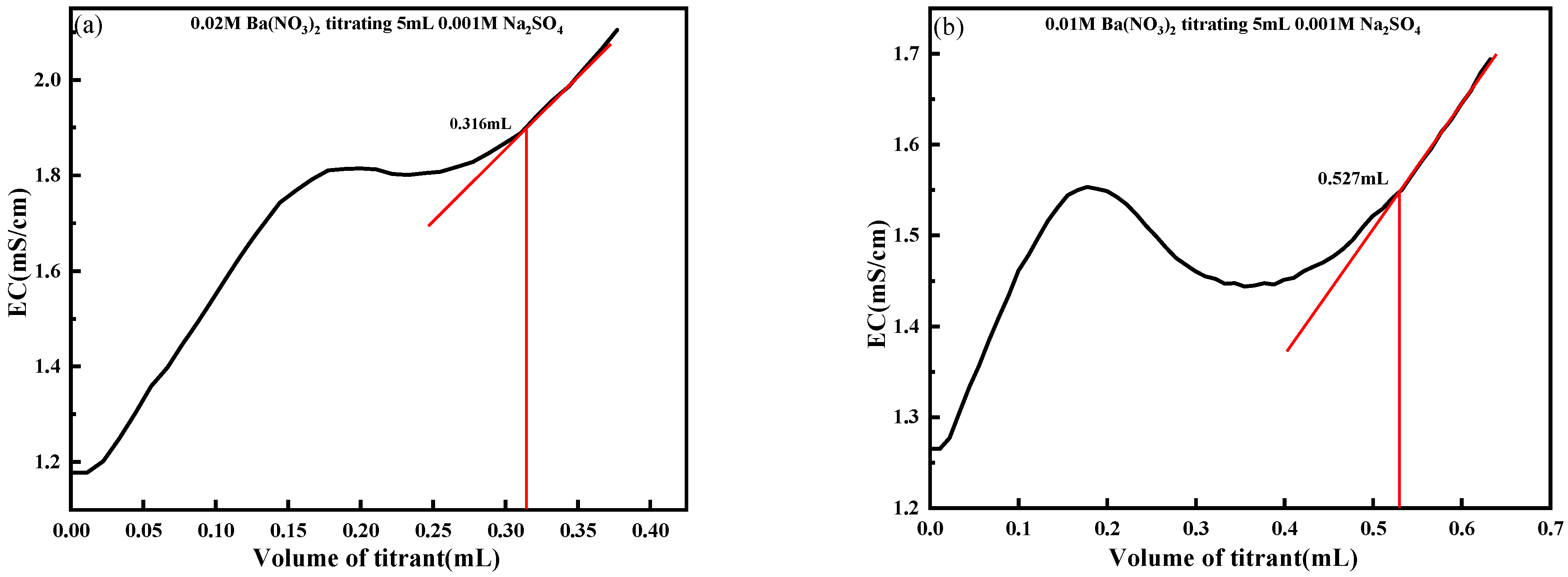
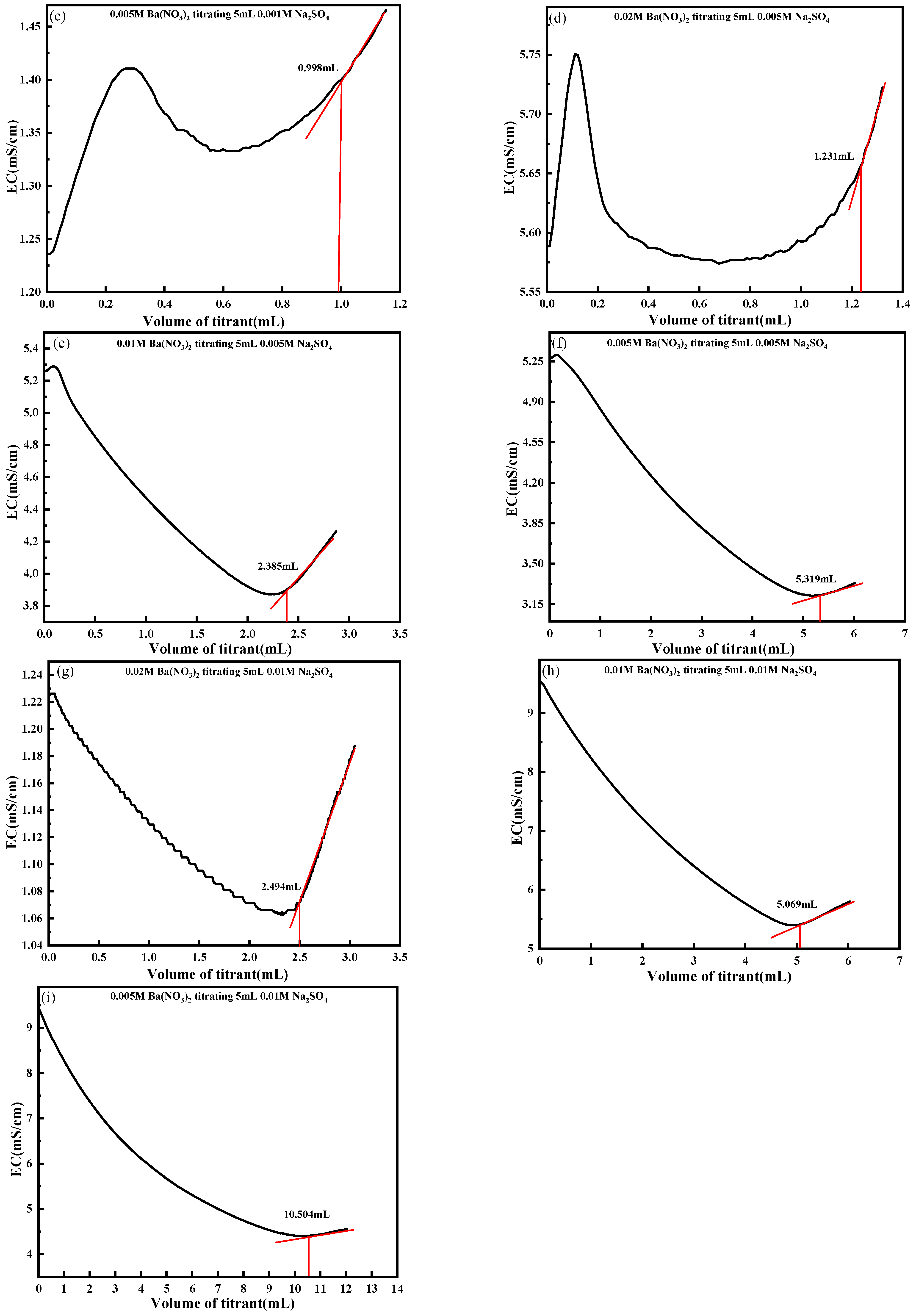
4.2. Effect of the Solution Volume to Be Measured
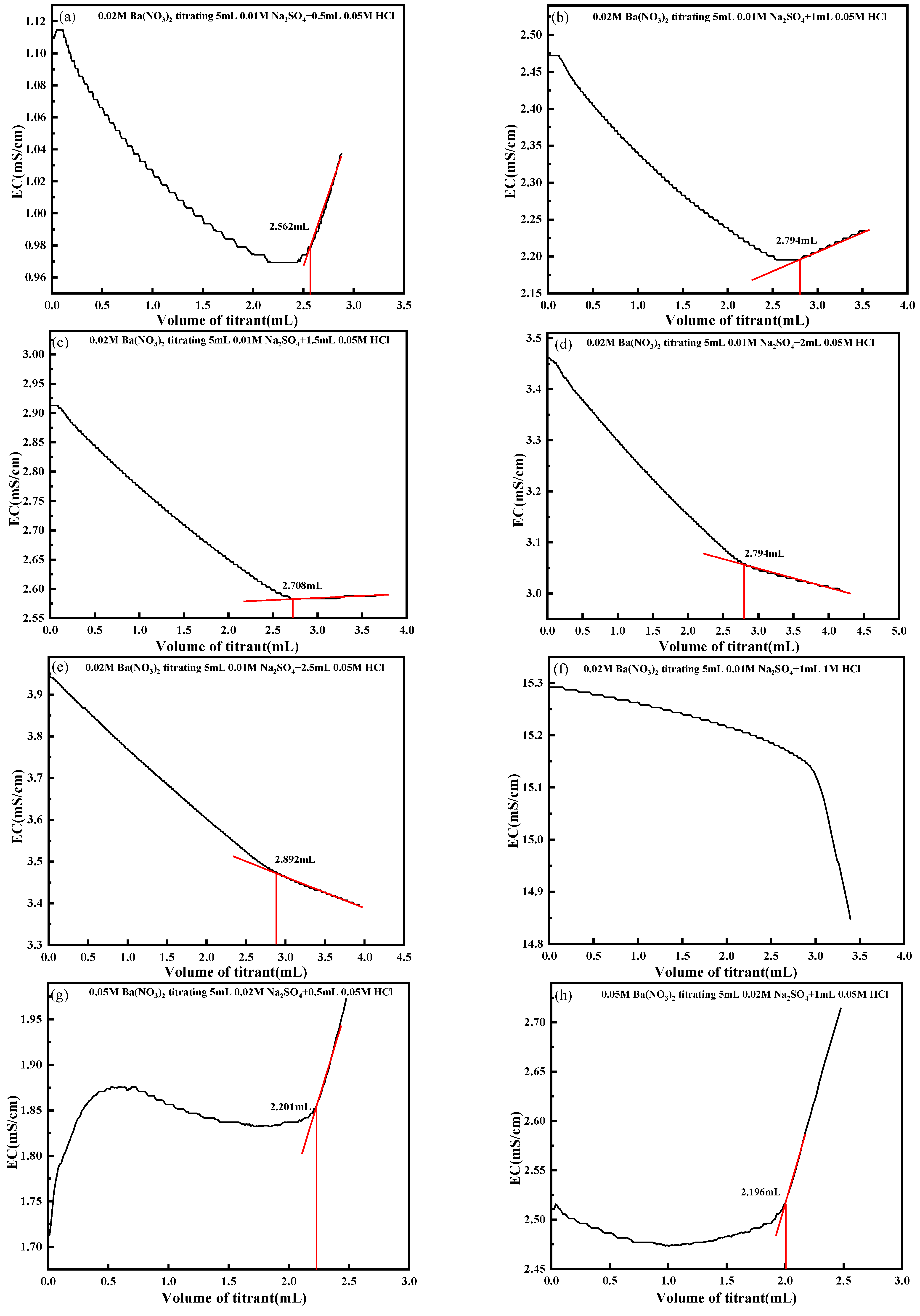
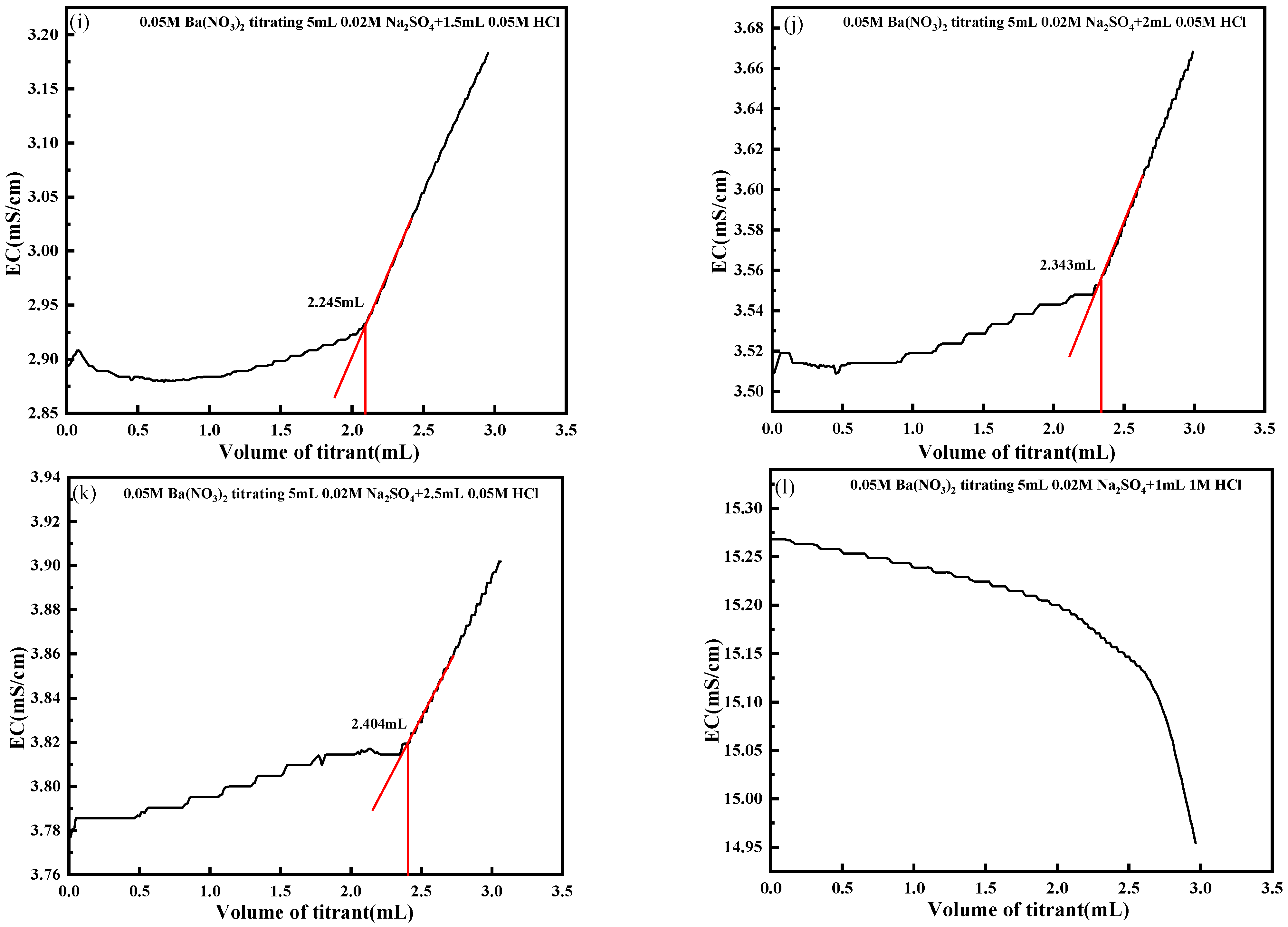
4.3. Effect of Titrant Concentration
4.4. Effect of H+ on Conductivity
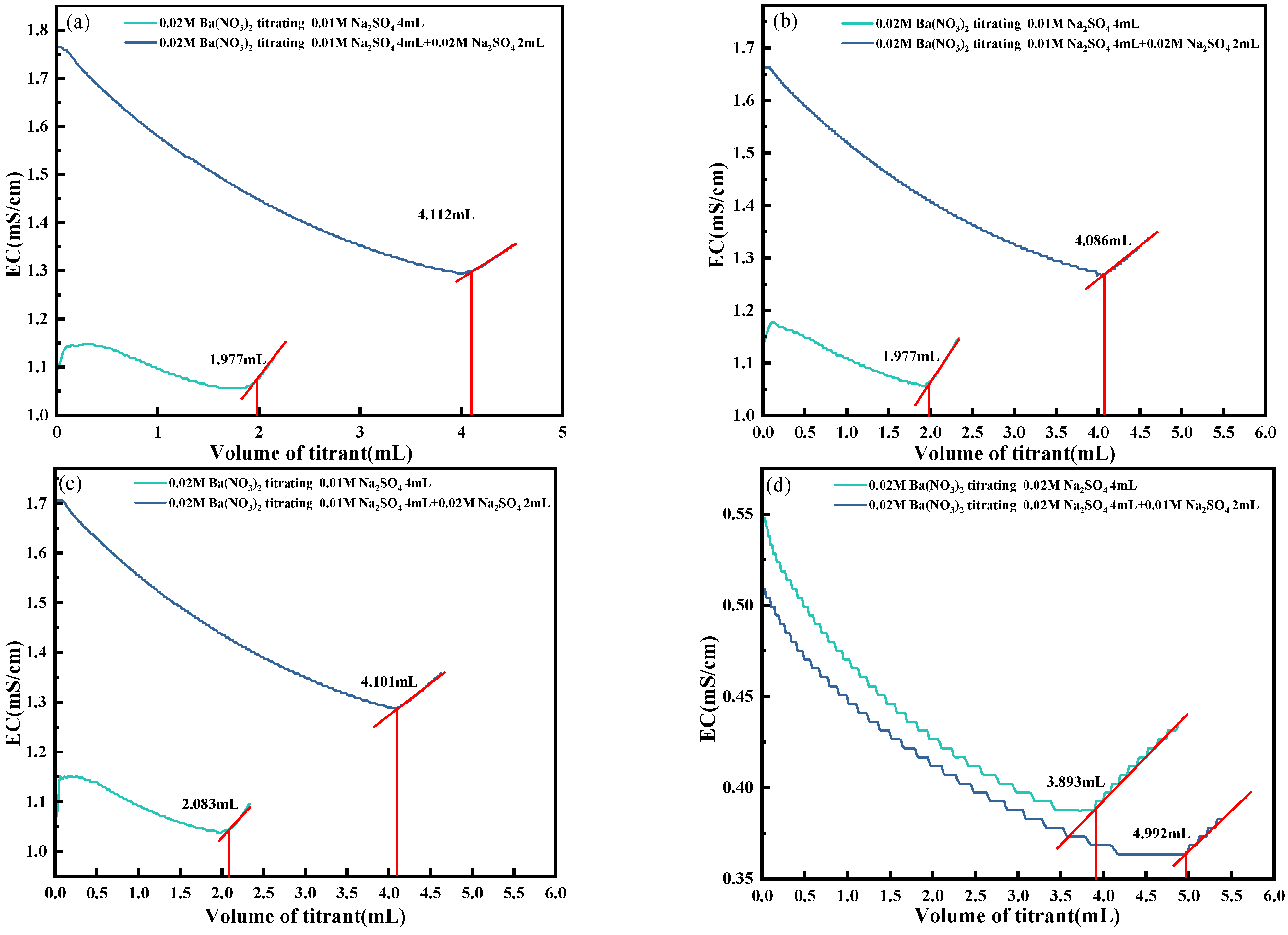
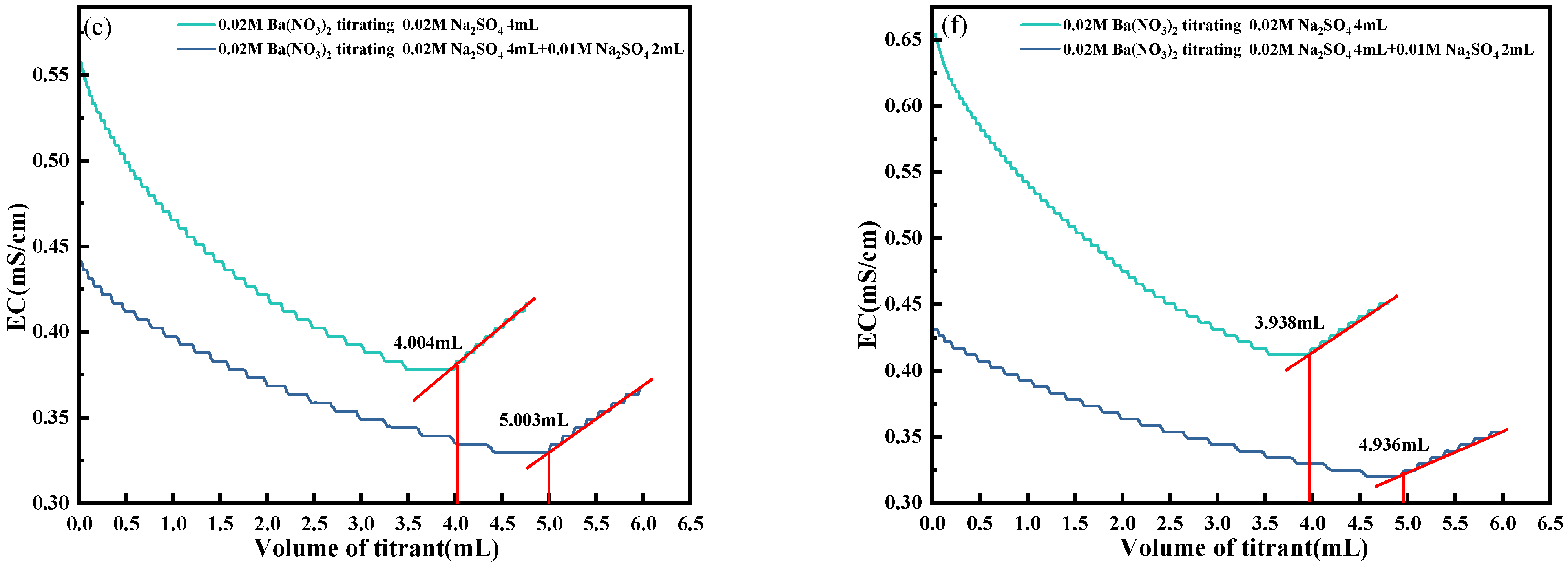
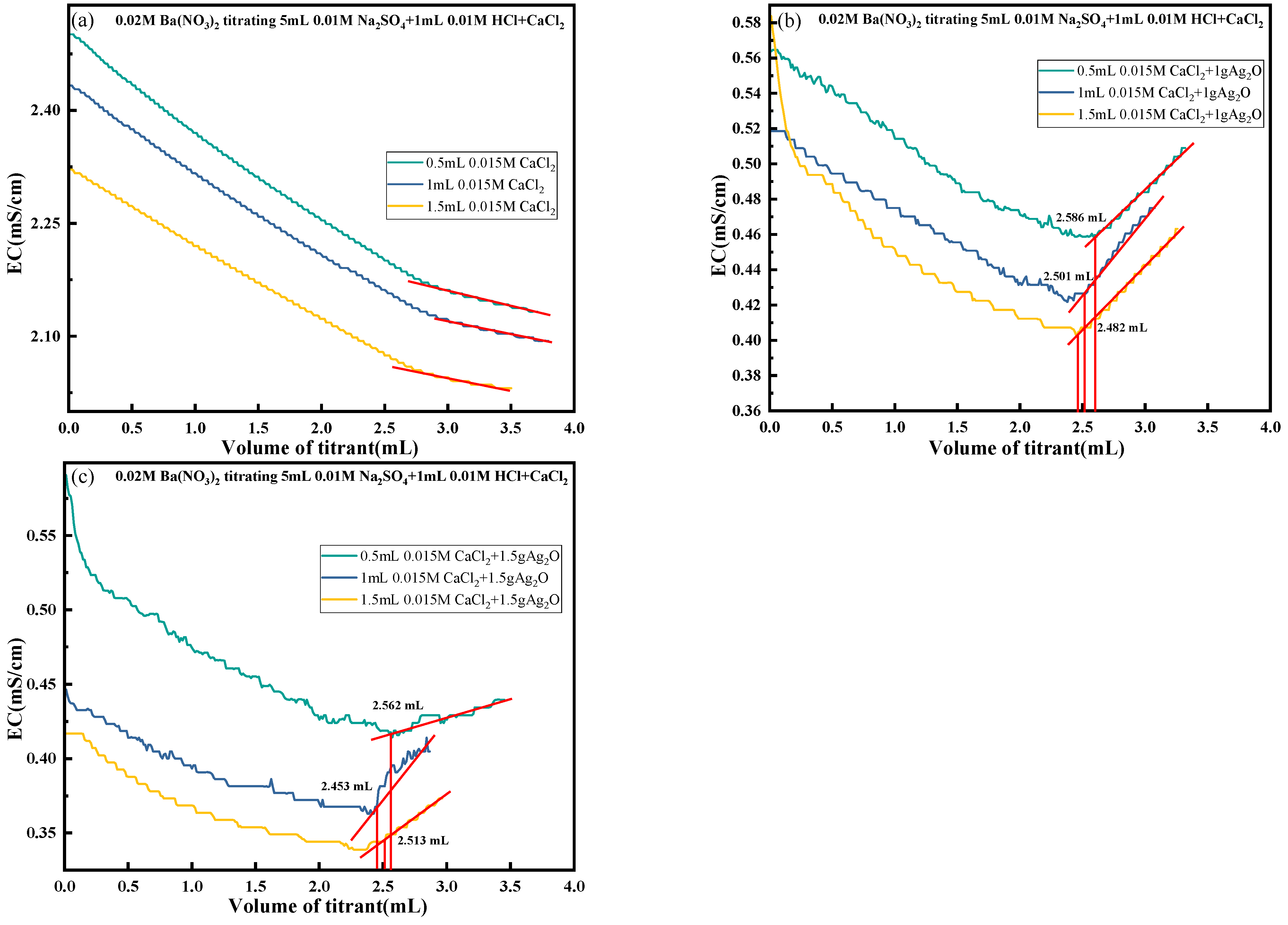
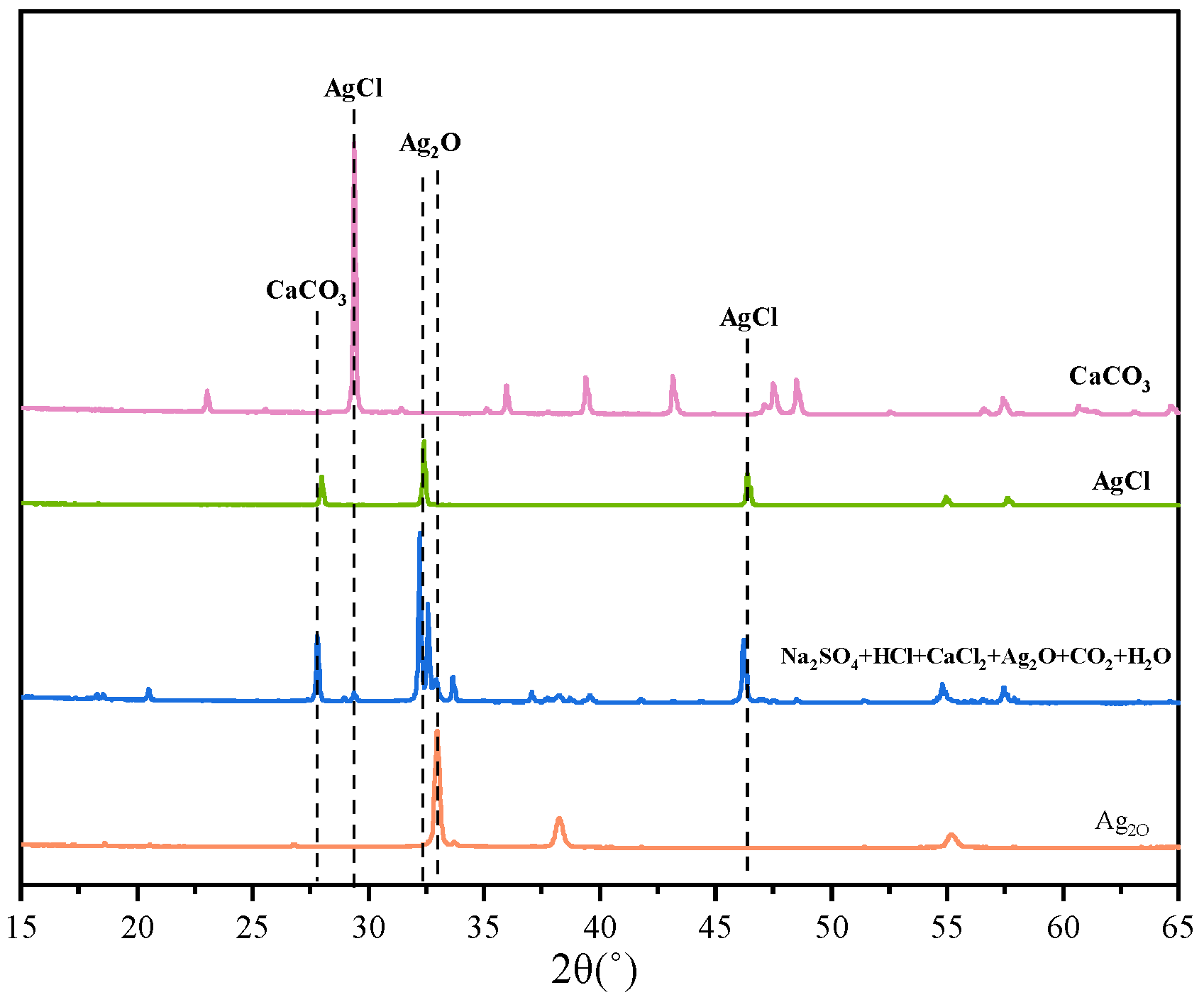
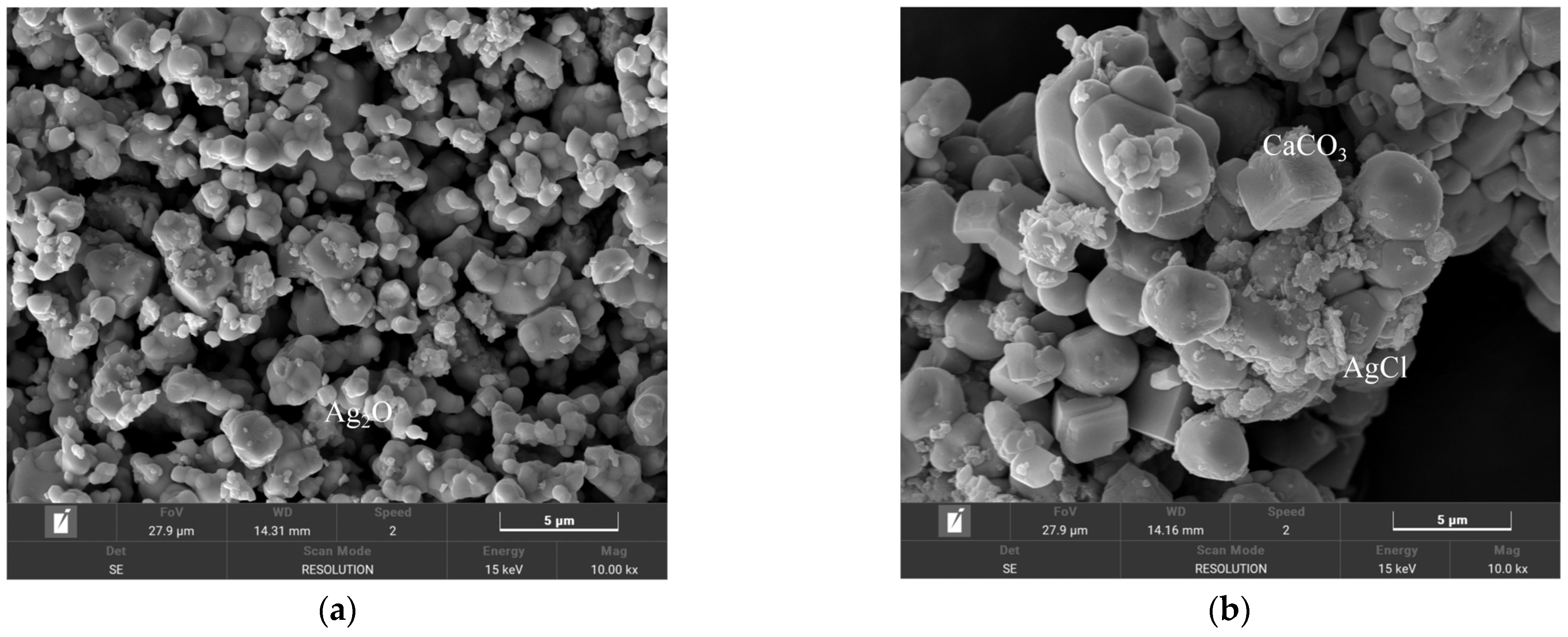
4.5. Elimination of H+, Cl− and Ca2+
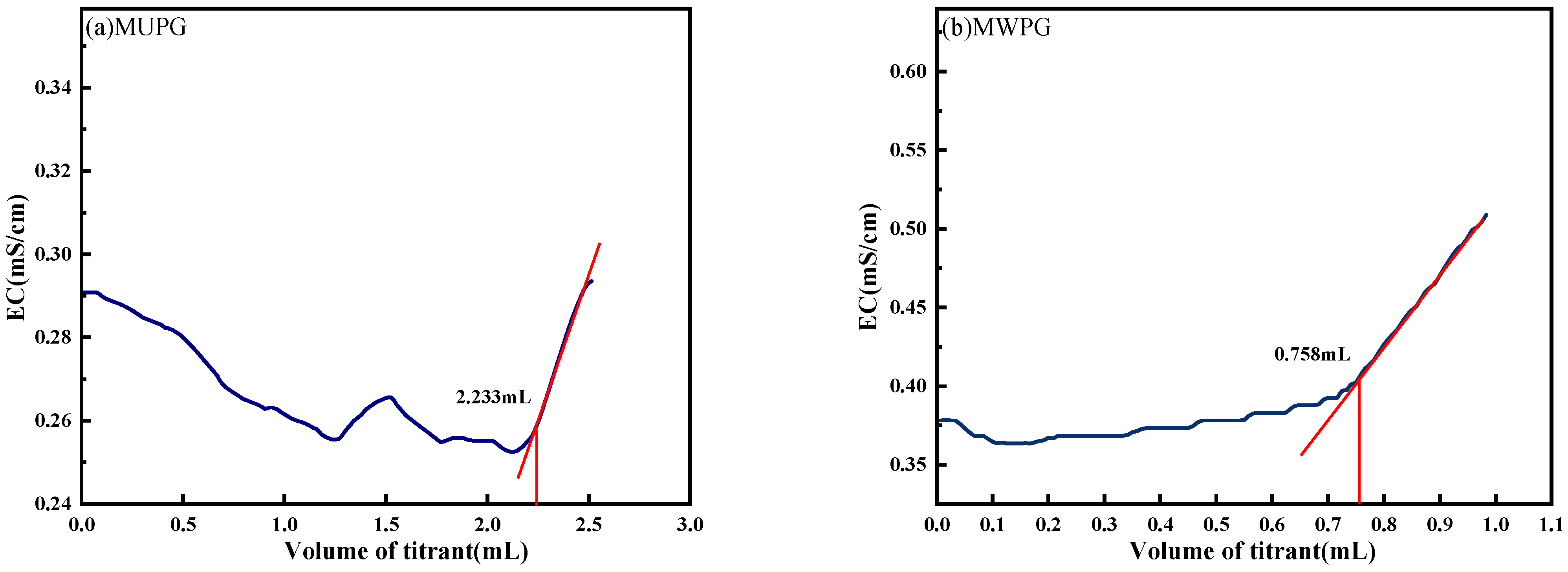
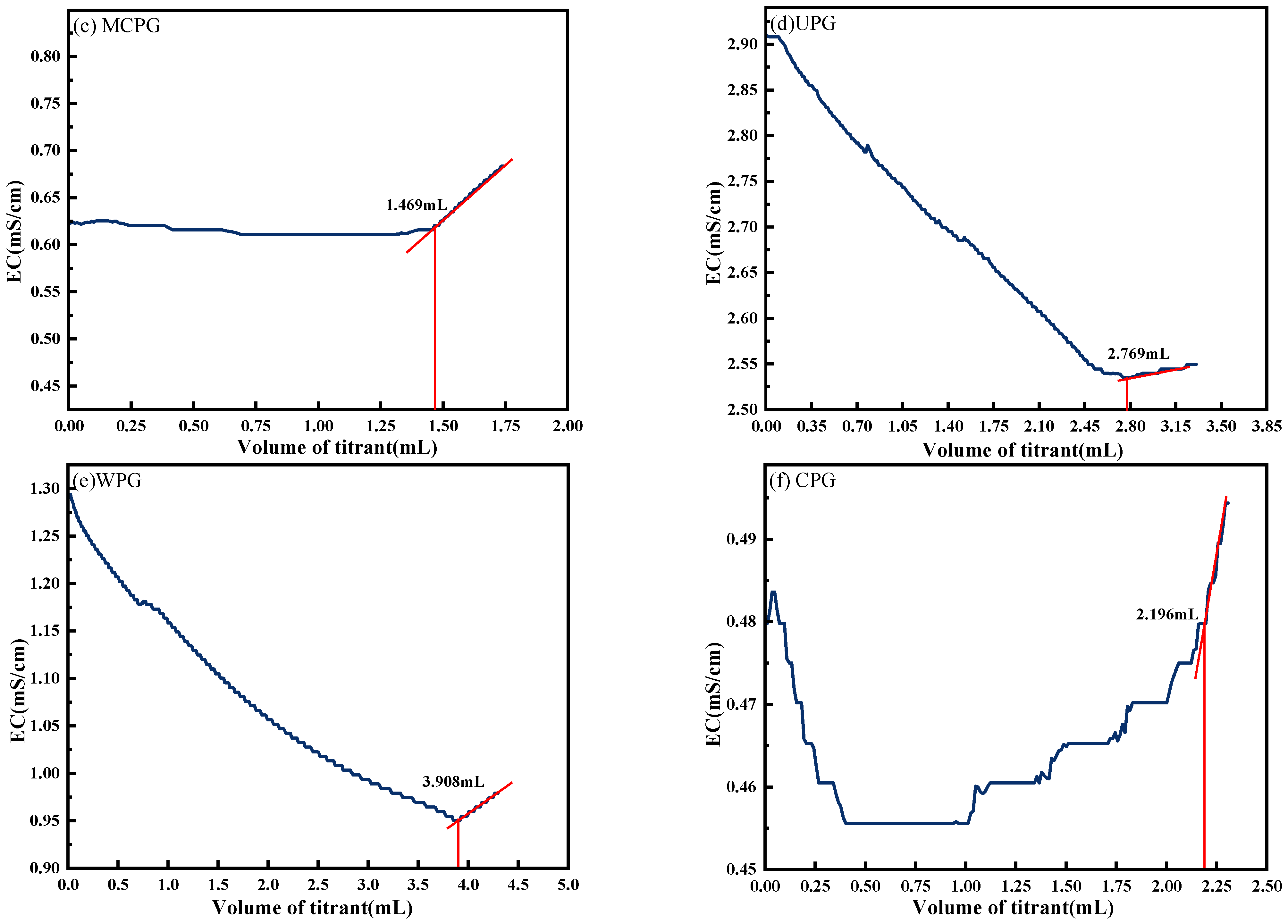
4.6. Determination of SO42− Concentration in PG and Cement-Based Materials by Conductivity Titration
5. Conclusions
- A conductivity titration system based on the Goouuu-ESP32 microcontroller was developed for the quantitative detection of sulfate ions. Experimental validation yielded an average Ba(NO3)2 titration volume of 2.498 mL, with a standard deviation of 0.013 mL, a coefficient of variation of 0.52%, an average relative error of 0.32%, and recovery rates ranging from 103.2% to 103.9%, indicating excellent accuracy and reproducibility of the system.
- The influence of key titration parameters, including the solution volume and titrant concentration, was comprehensively analyzed. The results suggest that a test solution volume of 5 mL and a Ba(NO3)2 concentration approximately twice that of the SO42− content yielded optimal endpoint clarity. These optimized conditions enhance conductivity responses and mitigate endpoint ambiguity caused by excess or insufficient titrant addition.
- To address the interference of high-conductivity ions such as H+, Cl−, and Ca2+, a synergistic elimination method involving Ag2O and CO2 was developed. This approach effectively reduced ionic interference by promoting the formation of weakly conductive precipitates, including AgCl and CaCO3, thereby restoring the dominant contribution of SO42− to solution conductivity and ensuring accurate endpoint detection.
- The leaching–filtering–treatment of the filtration–titration process accurately detected SO42− in the sulfate determination of cement-based and PG-based materials, which verified the applicability of the method for industrial solid waste resource utilization and concrete durability assessment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qu, F.; Zhang, Y.; Li, M.; Dong, W.; Li, W.; Tsang, D.C.W. Resource recycling of industrial waste phosphogypsum in cementitious materials: Pretreatment, properties, and applications. J. Environ. Manag. 2025, 376, 124291. [Google Scholar] [CrossRef]
- Ren, H.; Mao, R.; Wu, H.; Liang, X.; Zhou, J.; Zhang, Z. Preparation and properties of phosphogypsum-based calcined coal gangue composite cementitious materials. Case Stud. Constr. Mater. 2024, 21, e03963. [Google Scholar] [CrossRef]
- Deng, Q.; Bai, J.; Luo, C.; Liao, X.; He, Q.; Tan, H.; Dong, F.; Chen, W.; Jiang, J. Preparation of calcium-based cementitious material by decomposing phosphogypsum as the sole calcium source utilizing biomass synergized with iron accelerators. Process Saf. Environ. Prot. 2025, 195, 106775. [Google Scholar] [CrossRef]
- Ji, Z.R.; Chen, K.S.; Chen, J.X. Research on strength characteristics and mechanism of cement stabilized phosphogypsum materials under dry and wet cycles. Sci. Rep. 2025, 15, 20. [Google Scholar] [CrossRef]
- Tang, L.; Yu, Z.; He, Z.; Pei, S. Evaluation of the workability, mechanical strength, leaching toxicity and durability of sulfate solid waste composite cementitious materials. Sustain. Chem. Pharm. 2024, 42, 101847. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, L.; Bindiganavile, V.; Yi, C. Coupled models to describe the combined diffusion-reaction behaviour of chloride and sulphate ions in cement-based systems. Constr. Build. Mater. 2020, 243, 118232. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Liu, C.; Liu, Z. A mechanistic model of the degradation of cement-based materials subjected to sulfate attack. Constr. Build. Mater. 2024, 421, 135654. [Google Scholar] [CrossRef]
- Wu, L.; Yi, C.; Feng, Q.; Huang, X.; Mao, Z. Numerical simulation of sulfate attack in cement based materials: Considering dynamic boundary calcium concentration. Case Stud. Constr. Mater. 2024, 21, e04091. [Google Scholar] [CrossRef]
- Chen, D.; Guo, W.; Chen, D.; Guo, L.; Cai, B.; Ye, T. Mechanistic modeling for coupled chloride-sulfate attack in cement-based materials. Constr. Build. Mater. 2024, 455, 139231. [Google Scholar] [CrossRef]
- Gao, R.D.; Li, Q.B.; Zhao, S.B. Concrete Deterioration Mechanisms under Combined Sulfate Attack and Flexural Loading. J. Mater. Civ. Eng. 2013, 25, 39–44. [Google Scholar] [CrossRef]
- Gao, R.D.; Li, Q.B.; Zhao, S.B.; Yang, X.M. Deterioration Mechanisms of Sulfate Attack on Concrete under Alternate Action. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2010, 25, 355–359. [Google Scholar] [CrossRef]
- Zhang, F.H.; Zhang, L.; Yang, L.G.; Feng, T.G.; Chen, L.; Zhong, X.C. The Cement Content Measurement of Cement Mixing Piles with EDTA Titration Method. Ksce J. Civ. Eng. 2018, 22, 4306–4315. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, T.; Zou, D.; Zhou, A. Compressive strength assessment of sulfate-attacked concrete by using sulfate ions distributions. Constr. Build. Mater. 2021, 293, 123550. [Google Scholar] [CrossRef]
- Zou, D.; Qin, S.; Liu, T.; Jivkov, A. Experimental and numerical study of the effects of solution concentration and temperature on concrete under external sulfate attack. Cem. Concr. Res. 2021, 139, 106284. [Google Scholar] [CrossRef]
- Cheewasedtham, A.W.; Kovuttikulrangsie, S. Determining Proportion of Soap Constituent in Latex Sample Involves Processing Latex Sample to Prevent Coagulation and Subsequent Conductometric Titration for Eventually Determining Proportion of Soap Constituent in the Latex Sample. MY142005-A, 16 August 2010. [Google Scholar]
- Liu, X.-M.; He, D.-Q.; Fang, K.-J. Adsorption of cationic copolymer nanoparticles onto bamboo fiber surfaces measured by conductometric titration. Chin. Chem. Lett. 2015, 26, 1174–1178. [Google Scholar] [CrossRef]
- Xu, F.; Ma, G. Determination of Sulfur Trioxide in Fly Ash by Conductometric Titration and Analysis of Influence Factors. Bull. Chin. Ceram. Soc. 2003, 22, 43. [Google Scholar]
- Azmat, R.; Saleem, A.; Ahmed, T.; Ahmed, W. A new, innovative, simple method to determine the concentration of phosphate and sulphate ions in an aqueous extract of plants using conductometric titration. Pure Appl. Chem. 2025, 97, 427–438. [Google Scholar] [CrossRef]
- Farris, S.; Mora, L.; Capretti, G.; Piergiovanni, L. Charge Density Quantification of Polyelectrolyte Polysaccharides by Conductometric Titration: An Analytical Chemistry Experiment. J. Chem. Educ. 2012, 89, 121–124. [Google Scholar] [CrossRef]
- Volmer, D.A.; Curbani, L.; Parker, T.A.; Garcia, J.; Schultz, L.D.; Borges, E.M. Determination of Titratable Acidity in Wine Using Potentiometric, Conductometric, and Photometric Methods. J. Chem. Educ. 2017, 94, 1296–1302. [Google Scholar] [CrossRef]
- Raymond, L.; Morin, F.G.; Marchessault, R.H. Degree of deacetylation of chitosan using conductometric titration and solid-state NMR. Carbohydr. Res. 1993, 246, 331–336. [Google Scholar] [CrossRef]
- Katz, D.A.; Willis, C. Two Safe Student Conductivity Apparatus. J. Chem. Educ. 1994, 71, 330. [Google Scholar] [CrossRef]
- Smith, K.C.; Edionwe, E.; Michel, B. Conductimetric Titrations: A Predict−Observe−Explain Activity for General Chemistry. J. Chem. Educ. 2010, 87, 1217–1221. [Google Scholar] [CrossRef]
- Colvin, D.W.; Propst, R.C. A recording conductometric titrator. Anal. Chem. 1960, 32, 1858–1861. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Pang, B.; Liu, C.; Liu, Z. Study on the optimal conductivity titration parameters for SO42− in cement-based materials. Measurement 2024, 237, 115277. [Google Scholar] [CrossRef]
- T/CECS 10433-2024; Determination of Sulfate Ion Content in Concrete—Conductivity Titration Method. China Engineering Construction Standardization Association: Beijing, China, 2024.
- Garcia, J.; Schultz, L.D. Determination of Sulfate by Conductometric Titration: An Undergraduate Laboratory Experiment. J. Chem. Educ. 2016, 93, 910–914. [Google Scholar] [CrossRef]
- Anechiţei, L.; Cojocaru, T.; Munteanu, G.; Bulgariu, L. Simple methods for quantitative determination of sulphate ions from aqueous media with industrial applications. Bull. Polytech. Inst. Jassy Constructions. Archit. Sect. 2019, 65, 27–37. [Google Scholar]
- Dong, E.L.; Fu, S.Y.; Wu, C.Q.; Lv, W.; Liu, X.; Zhang, L.Y.; Feng, Y.; Shui, Z.H.; Yu, R. Value-added utilization of phosphogypsum industrial by-products in producing green Ultra-High performance Concrete: Detailed reaction kinetics and microstructure evolution mechanism. Constr. Build. Mater. 2023, 389, 16. [Google Scholar] [CrossRef]
- Ren, J.; Yang, H.H.; Du, S.M.; Wang, D.F.; Shao, J.H.; Dong, Z.Q.; Xiao, M.L.; Cao, Y.B.; Mao, J.H.; Zhang, Z.H. Fabrication of bulk hydrophobic building gypsum using modified phosphogypsum additives: Preparation, performance, and mechanism. Constr. Build. Mater. 2025, 463, 16. [Google Scholar] [CrossRef]
- Degirmenci, N. Utilization of phosphogypsum as raw and calcined material in manufacturing of building products. Constr. Build. Mater. 2008, 22, 1857–1862. [Google Scholar] [CrossRef]


| Group | Initial Concentration (mol/L) | Spiked Concentration (mol/L) | Theoretical Volume Increment (mL) | Actual Volume Increment (mL) | Recovery Rate (%) |
|---|---|---|---|---|---|
| A | 0.1 | 0.02 | 2 | 2.079 | 103.9 |
| B | 0.2 | 0.01 | 1 | 1.032 | 103.2 |
| Group | Titrant Concentration (mol/L) | Concentration of the Test Solution (mol/L) |
|---|---|---|
| A | 0.02/0.01/0.005 | 0.001 |
| B | 0.02/0.01/0.005 | 0.005 |
| C | 0.02/0.01/0.005 | 0.010 |
| Group | HCl Concentration (mol/L) | Volume of HCl (mL) | Theoretical Titration Volume (mL) | Actual Titration Volume (mL) | Impact Rate (%) |
|---|---|---|---|---|---|
| A | 0.05 | 0.5 | 2.500 | 2.562 | 2.48 |
| 0.05 | 1.0 | 2.500 | 2.794 | 11.76 | |
| 0.05 | 1.5 | 2.500 | 2.708 | 8.32 | |
| 0.05 | 2.0 | 2.500 | 2.794 | 11.76 | |
| 0.05 | 2.5 | 2.500 | 2.892 | 15.68 | |
| 1.00 | 1.0 | 2.500 | - | - | |
| B | 0.05 | 0.5 | 2.000 | 2.201 | 10.05 |
| 0.05 | 1.0 | 2.000 | 2.196 | 9.80 | |
| 0.05 | 1.5 | 2.000 | 2.245 | 12.25 | |
| 0.05 | 2.0 | 2.000 | 2.343 | 17.15 | |
| 0.05 | 2.5 | 2.000 | 2.404 | 20.20 | |
| 1.00 | 1.0 | 2.000 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Zhang, J.; Zhou, J.; Sun, Y.; Ren, J.; Li, X.; Liu, Z. Determining the Sulfate Content in Phosphogypsum and Cement-Based Materials Based on Conductivity Titration. Materials 2025, 18, 3758. https://doi.org/10.3390/ma18163758
Wang D, Zhang J, Zhou J, Sun Y, Ren J, Li X, Liu Z. Determining the Sulfate Content in Phosphogypsum and Cement-Based Materials Based on Conductivity Titration. Materials. 2025; 18(16):3758. https://doi.org/10.3390/ma18163758
Chicago/Turabian StyleWang, Dafu, Jieming Zhang, Jingting Zhou, Yudong Sun, Jun Ren, Xincheng Li, and Zhiyong Liu. 2025. "Determining the Sulfate Content in Phosphogypsum and Cement-Based Materials Based on Conductivity Titration" Materials 18, no. 16: 3758. https://doi.org/10.3390/ma18163758
APA StyleWang, D., Zhang, J., Zhou, J., Sun, Y., Ren, J., Li, X., & Liu, Z. (2025). Determining the Sulfate Content in Phosphogypsum and Cement-Based Materials Based on Conductivity Titration. Materials, 18(16), 3758. https://doi.org/10.3390/ma18163758






