Preparation and Physical Properties of Red Mud Based Artificial Lightweight Aggregates
Abstract
1. Introduction
2. Materials and Method
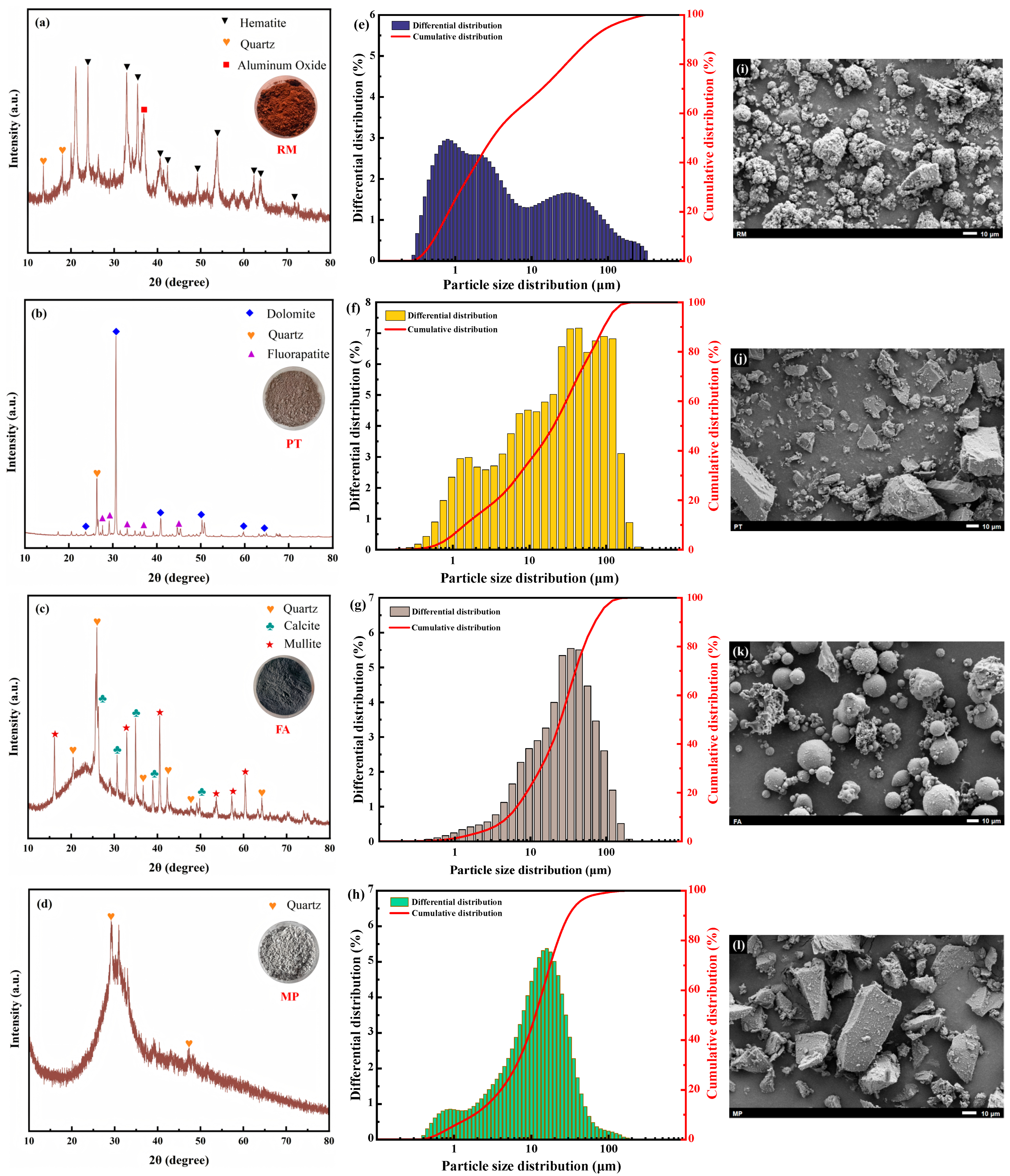
2.1. Raw Materials
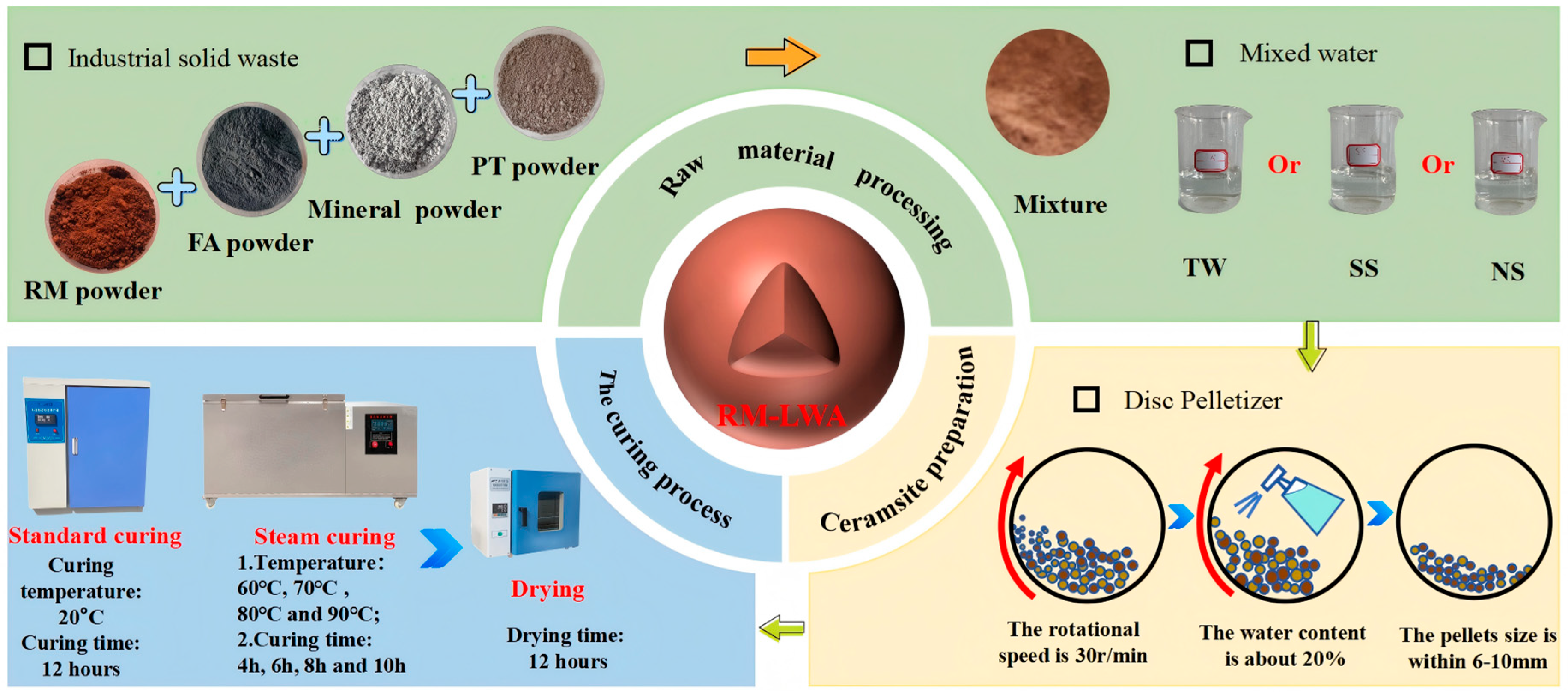
2.2. Preparation of RM-LWA
2.3. Curing Conditions
2.4. Testing Techniques
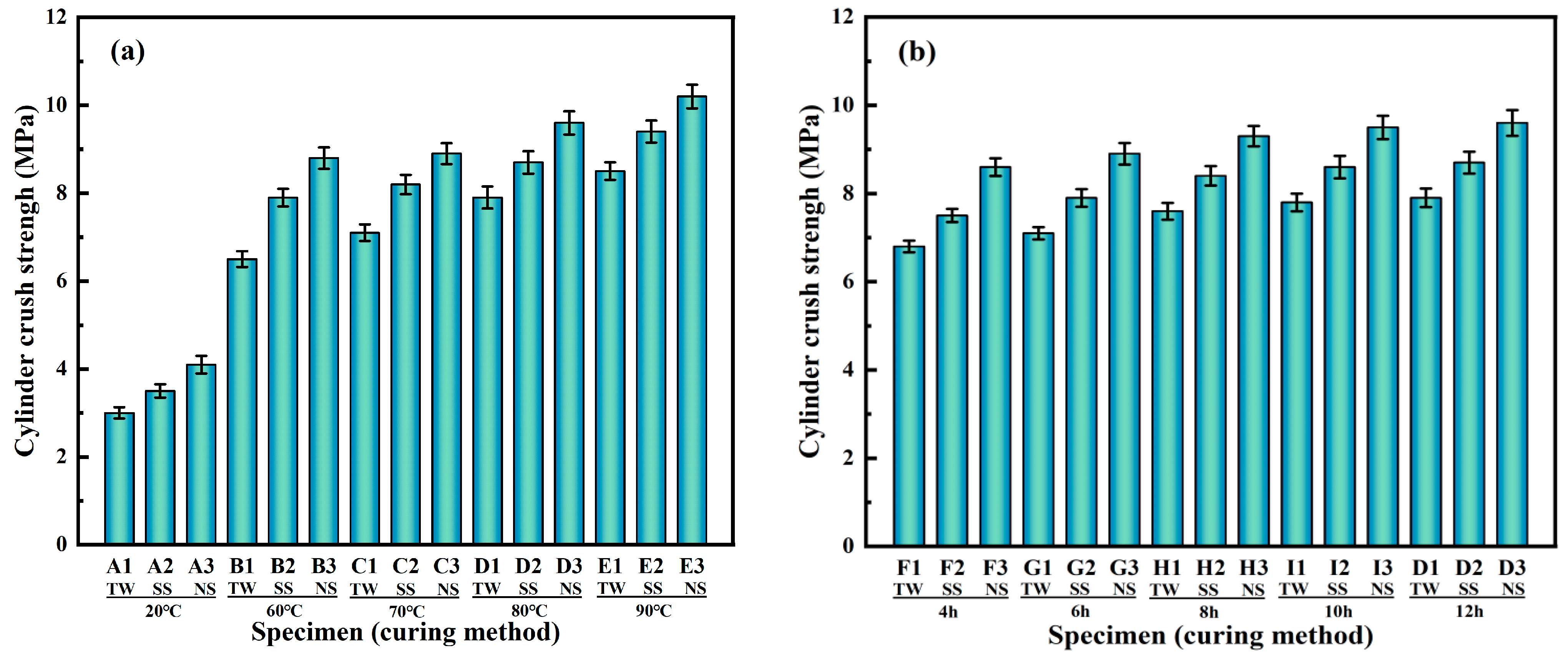
2.4.1. Determination of Cylinder Crush Strength
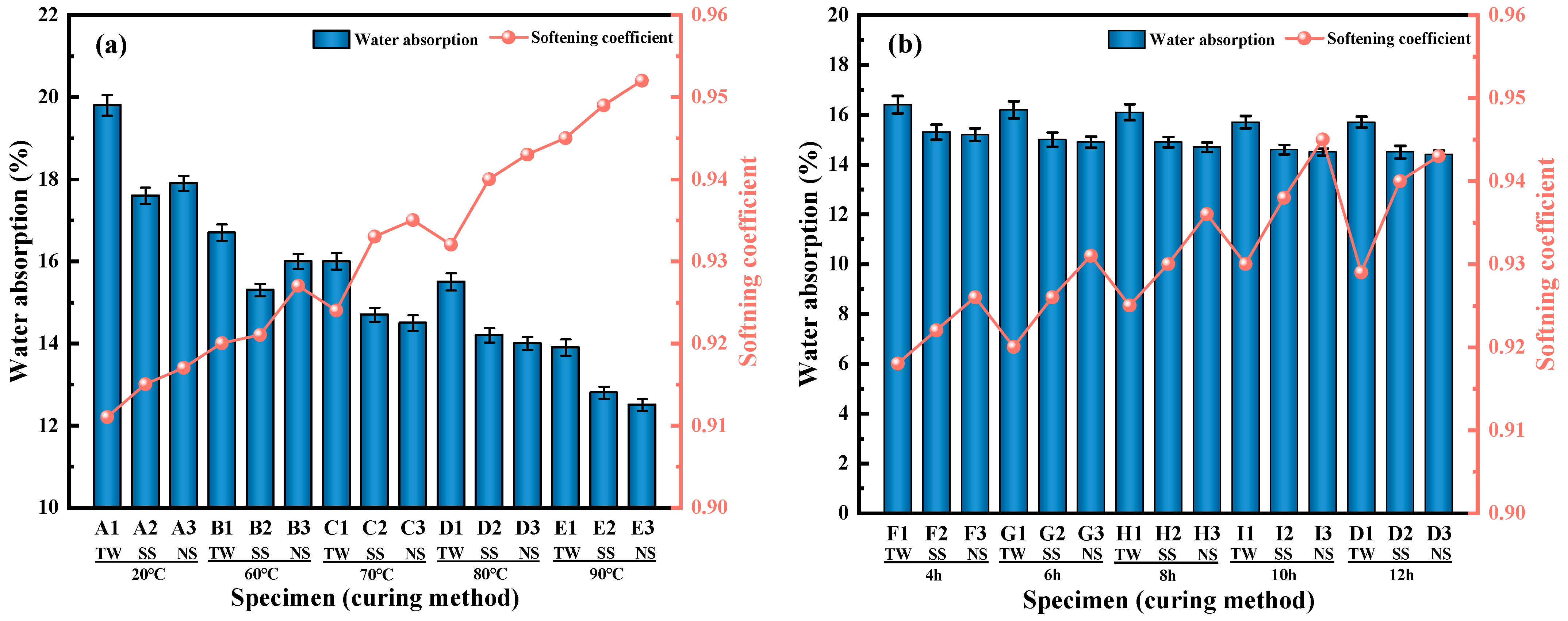
2.4.2. Determination of 1 h Water Absorption
2.4.3. Determination of Softening Coefficient
2.4.4. Determination of Bulk and Apparent Density
- (1)
- Apparent density
- (2)
- Bulk density
2.4.5. Determination of Void Ratio
2.4.6. Microstructural Analysis
2.4.7. Freeze-Thaw Cycling Analysis
2.4.8. Leaching Properties of Heavy Metals
3. Results and Discussion
3.1. Physical Properties of RM-LWA
3.1.1. Cylinder Crush Strength
3.1.2. Softening Coefficient and 1 h Water Absorption
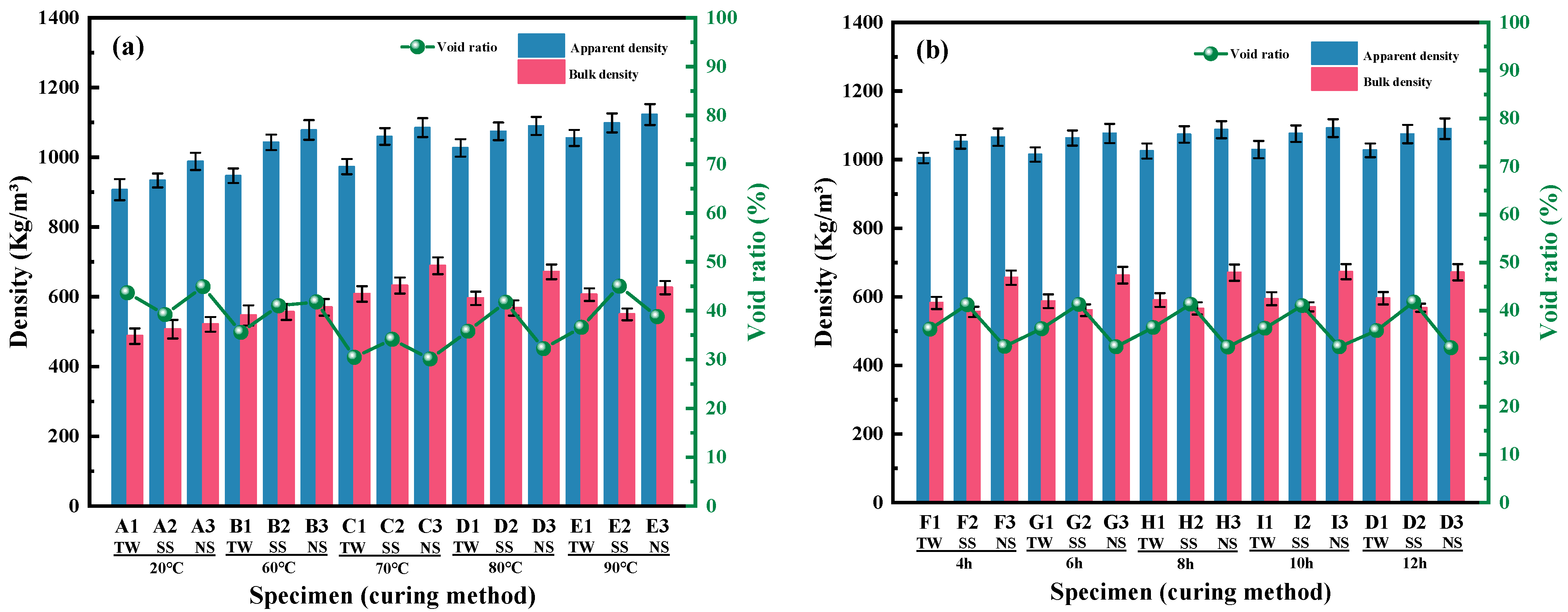
3.1.3. Apparent Density, Bulk Density, and Void Ratio
3.2. Microstructure Analysis
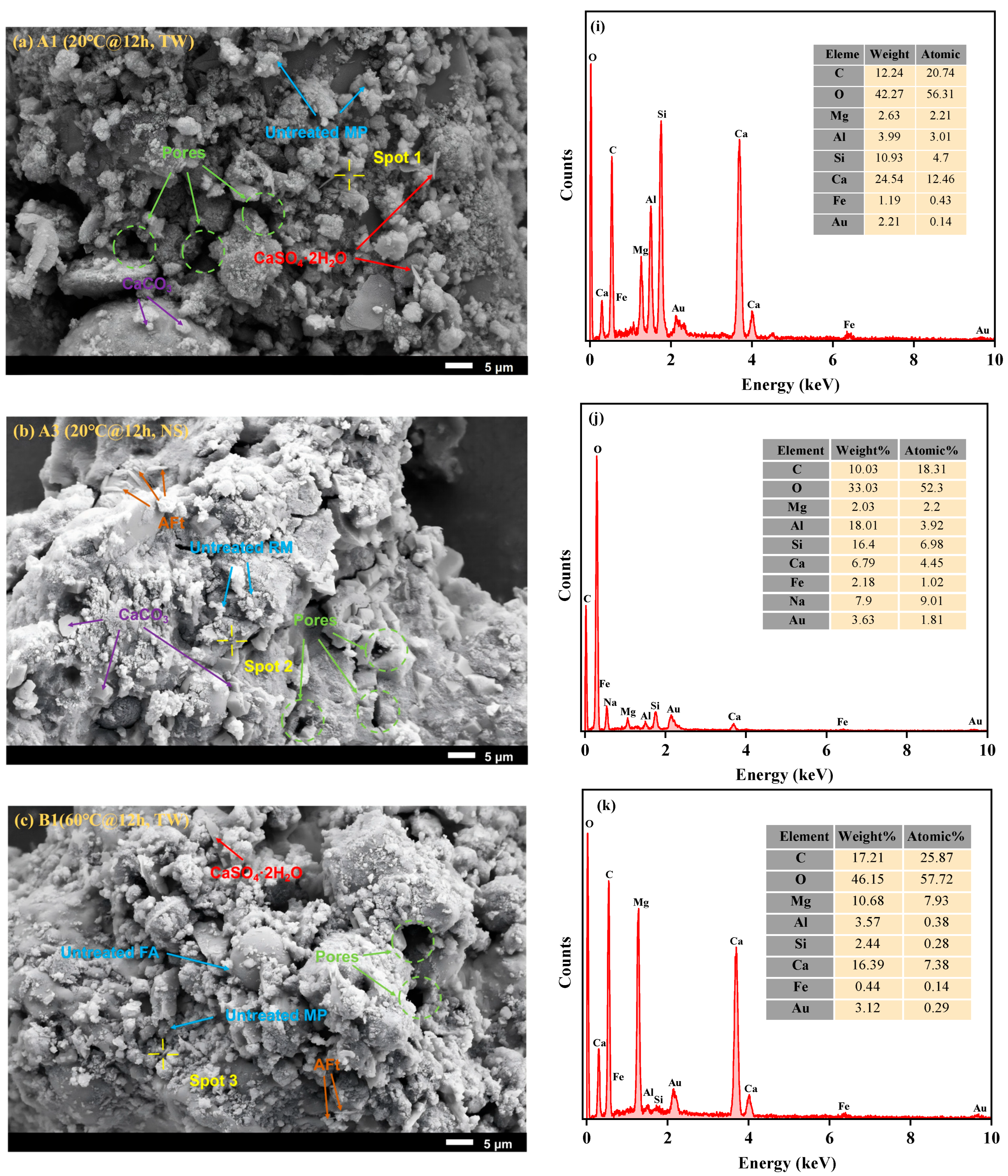
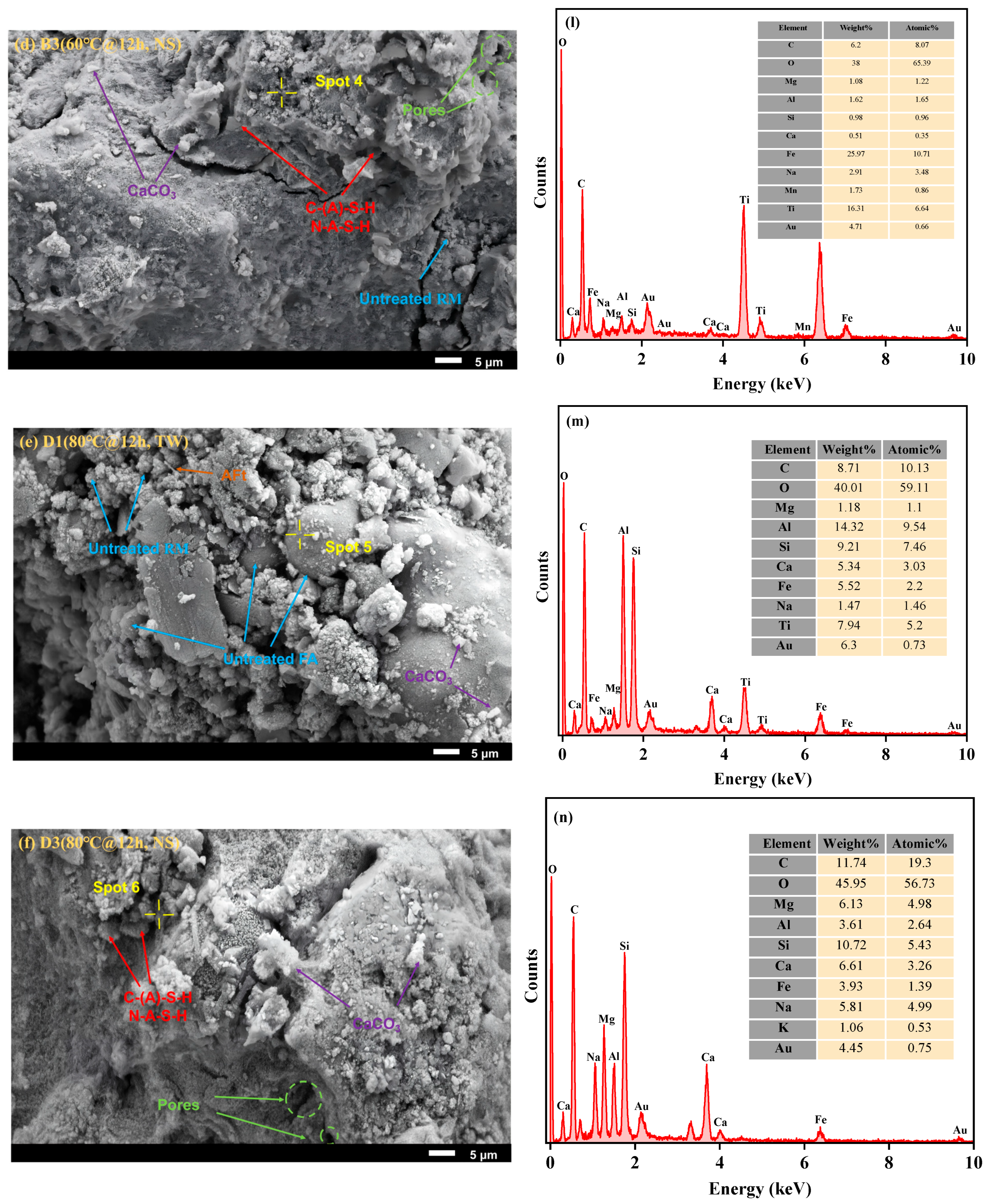
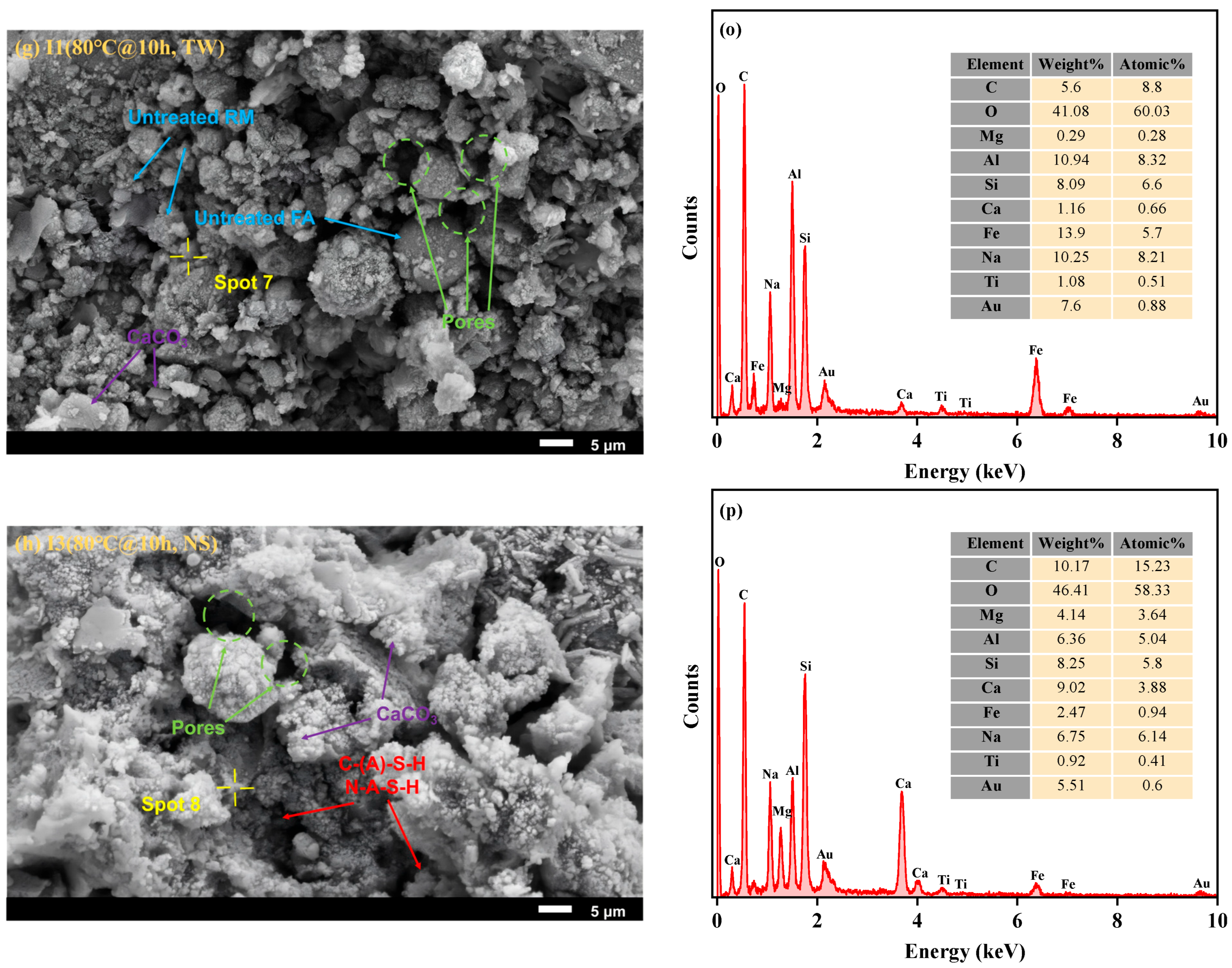
3.2.1. SEM-EDS
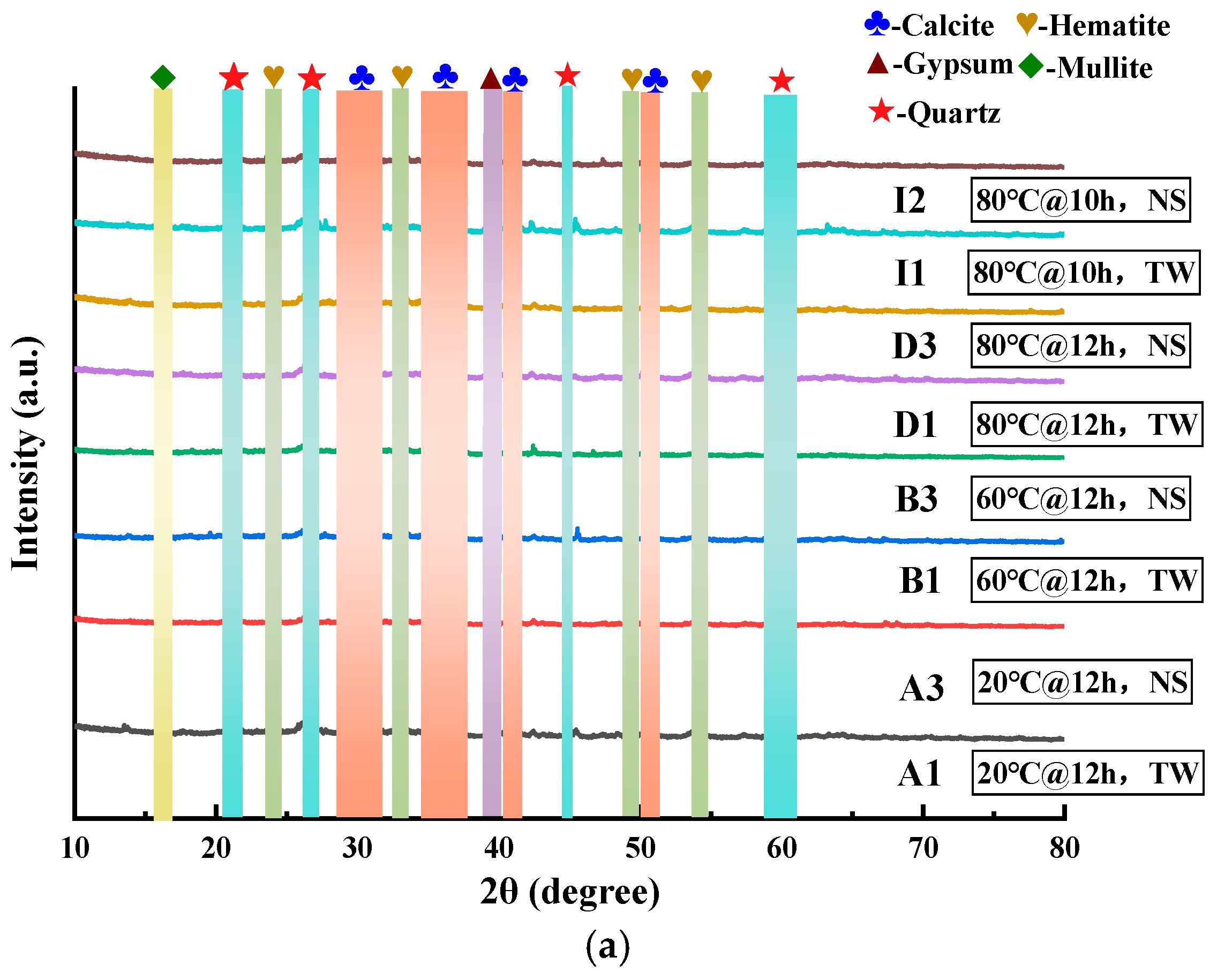
3.2.2. XRD
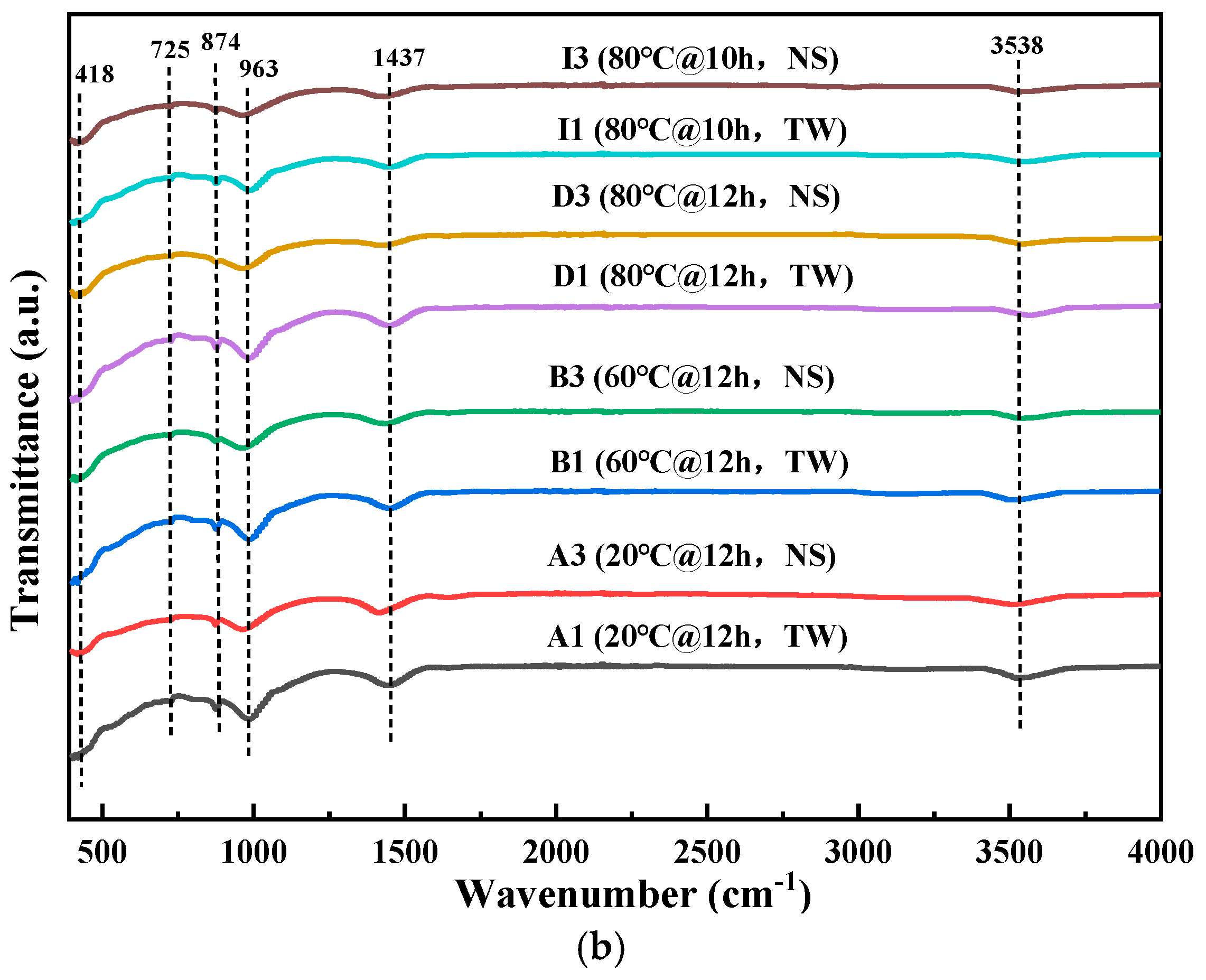
3.2.3. FTIR
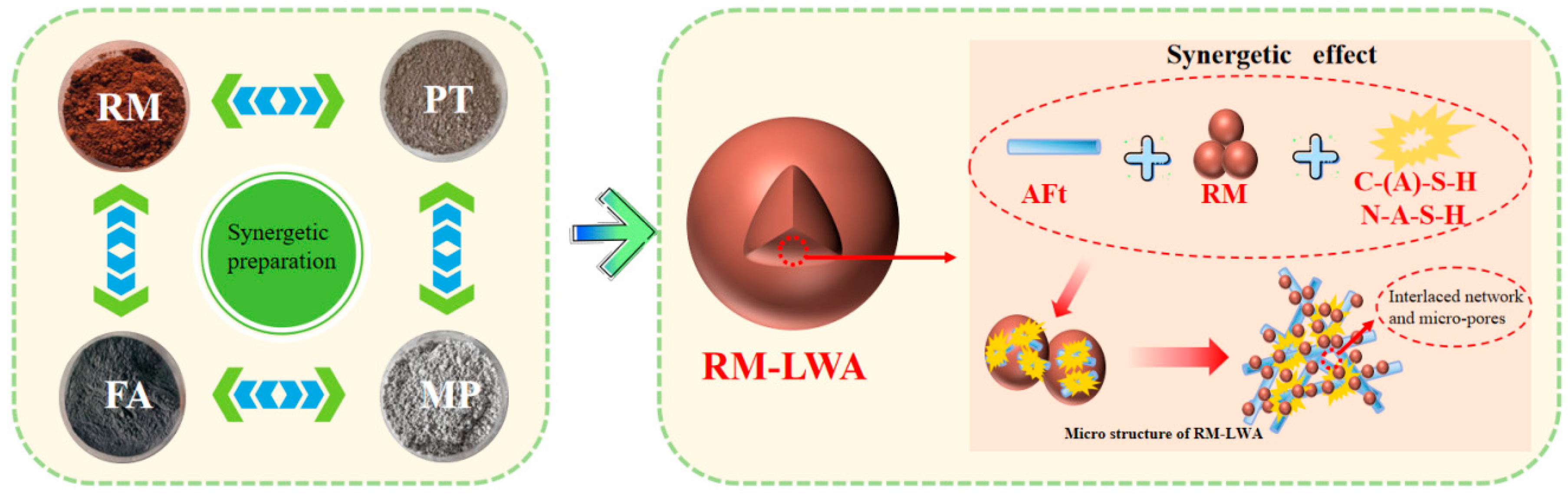
3.2.4. Formation Mechanism of RM-LWA Pore Structure
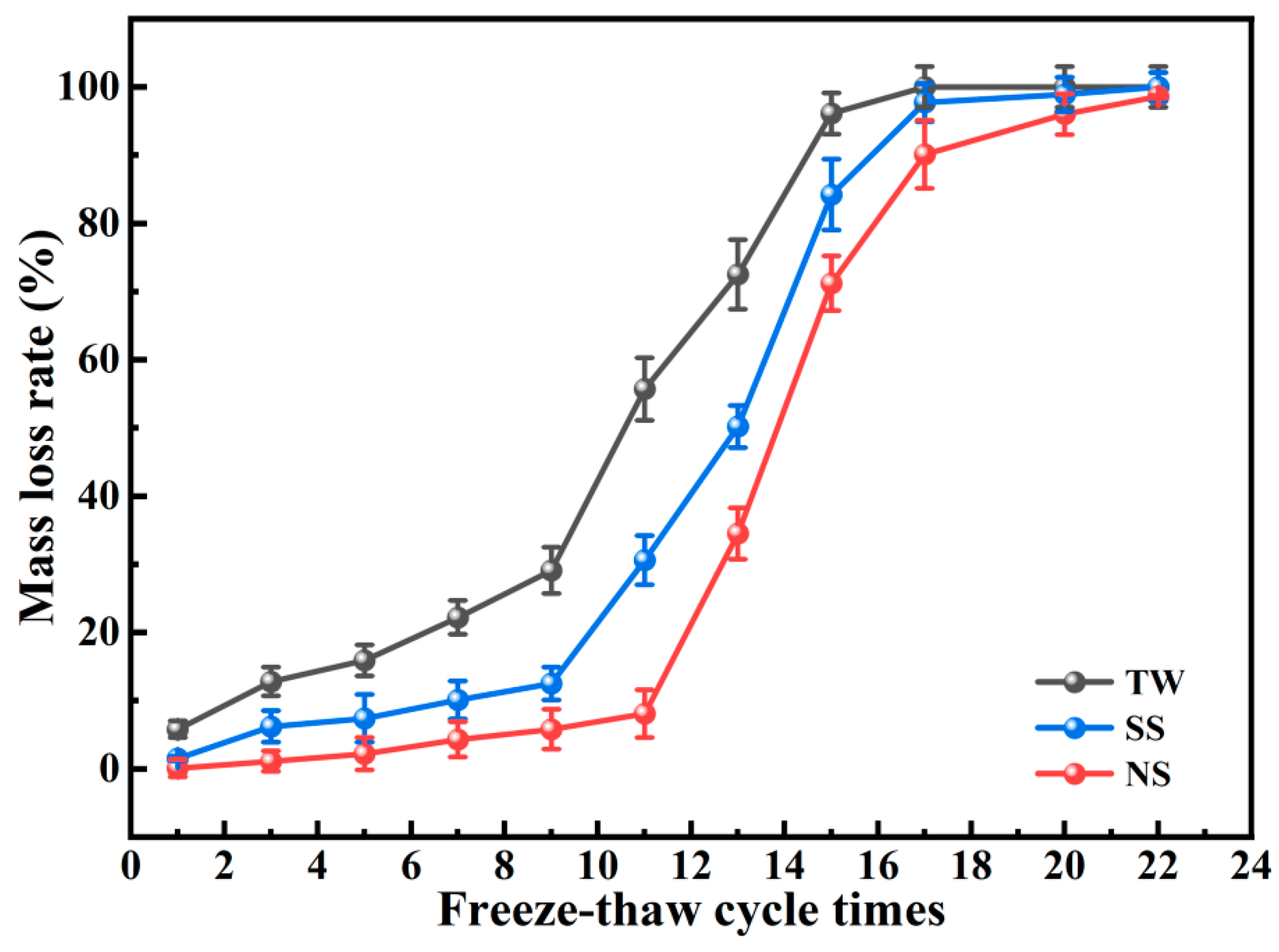
3.3. Freeze–Thaw Resistance
3.4. Leaching Properties of Heavy Metals
4. Risks, Mitigation Strategies, and Application FIELDS of RM-LWA
4.1. Risks, Mitigation Strategies
4.2. Application Fields
4.2.1. Non-Structural Fill Materials
4.2.2. Insulation Blocks and Wall Panels
4.2.3. Eco-Friendly Backfill in Landscaping and Civil Engineering
5. Conclusions
- (1)
- As opposed to TW and SS, when the curing conditions were standard, the cylinder crush strength value for RM-LWA samples made by adding NS increased by 33% and 14%, respectively. In addition, when steam-cured at 80 °C, the cylinder crush strength of lightweight aggregates obtained by incorporating NS exceeded 90% of the value at a 90 °C curing temperature, indicating that further increasing the temperature has a limited impact on the strength. At this time, the softening coefficient is 0.94, and the 1 h water absorption rate reaches 14%.
- (2)
- Microscopic tests showed that the maintenance method did not change the type of mineral phases produced but changed their quantity. The hydrating substances identified in RM-LWA primarily comprise N-A-S-H and C-(A)-S-H. During the hydration process, a robust colloidal framework is generated and uniformly distributed across the particle surfaces, thereby improving the strength to a certain degree.
- (3)
- With standard curing conditions, RM-LWA containing NS exhibited superior freeze–thaw resistance, showing a complete 100% mass loss following 22 freeze–thaw cycles. Heavy metal leachability assays reveal that the elution concentrations of heavy metals (Cr, Pb, As, Cu, and Ni) in RM-LWA specimens all conform to the regulatory thresholds for non-hazardous waste disposal into the environment.
6. Future Perspectives
- (1)
- Building on the existing findings of this study, future research will focus on the neglected alkali metals (such as K, Na) in RM, conducting in-depth investigations into their impacts on the environment.
- (2)
- RM, PT, FA, and MP have been studied for RM-LWA, but exploring more solid waste types can broaden sources and improve resource efficiency. Further research on material proportions, characteristics, and interactions is needed for higher-performance RM-LWA.
- (3)
- Steam-cured artificial lightweight aggregates have high early strength (shortening construction periods) but limited late-stage strength. Future research should explore modified coatings to improve performance.
- (4)
- Future research will conduct 6–8-month RM-LWA field exposure tests under simulated diverse climates to monitor heavy metal leaching under natural stressors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lima, M.S.; Thives, L.P. Evaluation of red mud as filler in Brazilian dense graded asphalt mixtures. Constr. Build. Mater. 2020, 260, 119894. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Uthayakumar, M.; Arumugaprabu, V. Development and sustainability of industrial waste-based red mud hybrid composites. J. Clean. Prod. 2019, 230, 862–868. [Google Scholar] [CrossRef]
- Kim, C.-J.; Chung, C.-S.; Jung, J.-M.; Kim, Y.-R.; Kang, D.-W.; Kim, H.-E.; Shin, K.-H.; Choi, K.-Y. Long-term effects of chromium from red mud (bauxite residue) ocean dumping on the benthic environment in South Korea. Mar. Pollut. Bull. 2023, 196, 115584. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Guo, W.; Jia, Y.; Xue, C.; Qiu, Y.; Zhao, Q.; Wang, D. Preparation of non-sintered lightweight aggregate ceramsite based on red mud-carbide slag-fly ash: Strength and curing method optimization. J. Clean. Prod. 2022, 372, 133788. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, N. Utilization of red mud in cement production: A review. Waste Manag. Res. 2011, 29, 1053–1063. [Google Scholar] [CrossRef]
- Jia, K.; Zhou, Z.; Singh, S.V.; Wang, C. A review of the engineered treatment of red mud: Construction materials, metal recovery, and soilization revegetation. Results Eng. 2024, 24, 102927. [Google Scholar] [CrossRef]
- Pan, X.; Wu, H.; Lv, Z.; Yu, H.; Tu, G. Recovery of valuable metals from red mud: A comprehensive review. Sci. Total Environ. 2023, 904, 166686. [Google Scholar] [CrossRef]
- Padhan, A.; Paul, B. Unlocking the potential of red mud: Advanced strategies for economic optimization and sustainable recovery of critical minerals. J. Environ. Manag. 2025, 389, 126040. [Google Scholar] [CrossRef] [PubMed]
- Swain, B.; Akcil, A.; Lee, J.-C. Red mud valorization an industrial waste circular economy challenge; review over processes and their chemistry. Crit. Rev. Environ. Sci. Technol. 2022, 52, 520–570. [Google Scholar] [CrossRef]
- Bose, B.P. Comprehensive Utilizations of Red Mud with Emphasis on Circular Economy: An Approach towards Achieving the United Nations Sustainable Development Goals. Int. J. Earth Sci. Knowl. Appl. 2024, 6, 253–261. [Google Scholar]
- Swain, B. Red mud: An environmental challenge but overlooked treasure for critical rare earth metals. MRS Bull. 2022, 47, 289–302. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Luo, H.; Wang, H.; Chen, L.; Liu, M.; Tang, M.; He, B.-J. Preparation and properties of alkali-activated red mud-based artificial lightweight aggregates. Constr. Build. Mater. 2024, 449, 138304. [Google Scholar] [CrossRef]
- Ma, M.; Tam, V.W.; Le, K.N.; Li, W. Challenges in current construction and demolition waste recycling: A China study. Waste Manag. 2020, 118, 610–625. [Google Scholar] [CrossRef]
- Aslam, M.S.; Huang, B.; Cui, L. Review of construction and demolition waste management in China and USA. J. Environ. Manag. 2020, 264, 110445. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Z.; Wang, K.; Wang, F.; Jiang, H.; Liang, M.; Wei, J.; Airey, G. Sustainable utilization of bauxite residue (Red Mud) as a road material in pavements: A critical review. Constr. Build. Mater. 2021, 270, 121419. [Google Scholar] [CrossRef]
- Xie, W.; Zhou, F.; Liu, J.; Bi, X.; Huang, Z.; Li, Y.; Chen, D.; Zou, H.; Sun, S. Synergistic reutilization of red mud and spent pot lining for recovering valuable components and stabilizing harmful element. J. Clean. Prod. 2020, 243, 118624. [Google Scholar] [CrossRef]
- Khairul, M.; Zanganeh, J.; Moghtaderi, B.M. The composition, recycling and utilisation of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Wang, L.; Sun, N.; Tang, H.; Sun, W. A review on comprehensive utilization of red mud and prospect analysis. Minerals 2019, 9, 362. [Google Scholar] [CrossRef]
- Molineux, C.J.; Newport, D.J.; Ayati, B.; Wang, C.; Connop, S.P.; Green, J.E. Bauxite residue (red mud) as a pulverised fuel ash substitute in the manufacture of lightweight aggregate. J. Clean. Prod. 2016, 112, 401–408. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.-S.; Chen, Z.; Xue, Q.; Sun, Q.; Zhou, Y.; Chen, X.; Liu, L.; Poon, C.S. Production of lightweight aggregate ceramsite from red mud and municipal solid waste incineration bottom ash: Mechanism and optimization. Constr. Build. Mater. 2021, 287, 122993. [Google Scholar] [CrossRef]
- Liu, S.; Yang, C.; Liu, W.; Yi, L.; Qin, W. A novel approach to preparing ultra-lightweight ceramsite with a large amount of fly ash. Front. Environ. Sci. Eng. 2020, 14, 62. [Google Scholar] [CrossRef]
- Zhao, Q.; Shi, Y.; Xue, C.; Jia, Y.; Guo, W.; Wang, D.; Wang, S.; Gao, Y. Investigation of various curing methods on the properties of red mud-calcium carbide slag-based artificial lightweight aggregate ceramsite fabricated through alkali-activated cold-bonded pelletization technology. Constr. Build. Mater. 2023, 401, 132956. [Google Scholar] [CrossRef]
- Gesoğlu, M.; Güneyisi, E.; Mahmood, S.F.; Öz, H.Ö.; Mermerdaş, K. Recycling ground granulated blast furnace slag as cold bonded artificial aggregate partially used in self-compacting concrete. J. Hazard. Mater. 2012, 235, 352–358. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Hu, Z.; Wang, Z.; Cao, Z.; Wang, W. Production of a low-carbon and economical high-strength artificial aggregate from gold tailings for the preparation of lightweight aggregate concrete. Cem. Concr. Compos. 2025, 161, 106100. [Google Scholar] [CrossRef]
- Deng, X.; Li, J.; Du, D.; Wang, T. Manufacturing non-sintered ceramsite from dredged sediment, steel slag, and fly ash for lightweight aggregate: Production and characterization. Environ. Sci. Pollut. Res. Int. 2024, 31, 15078–15090. [Google Scholar] [CrossRef]
- Shenwei, Z.; Linying, Y.; Haibin, H.; Yiping, Z.; Lei, H.; Yujia, Z.; Yajing, Y.; Jianli, J. Preparation and environmental toxicity of non-sintered ceramsite using coal gasification coarse slag. Arch. Environ. Prot. 2019, 45, 84–90. [Google Scholar]
- Małgorzata, F.; Danuta, B.-H.; Magdalena, W. Utilization of sewage sludge in the manufacture of lightweight aggregate. Environ. Monit. Assess. 2016, 188, 10. [Google Scholar]
- Luisa, B.; Alessandro, B.; Fernanda, A.; Isabella, L.; Grazia, G.; Maria, T.C.P.; Carmen, M.G. Environmental impact estimation of ceramic lightweight aggregates production starting from residues. Int. J. Appl. Ceram. Technol. 2020, 18, 353–368. [Google Scholar] [CrossRef]
- Kalle, K.; Abhijit, M.; Mirja, I.; Priyadharshini, P. Utilization of fine concrete waste as a lightweight aggregate via granulation: Technical and environmental assessment. J. Clean. Prod. 2024, 434, 139938. [Google Scholar]
- Tesovnik, A.; Ottosen, L.M.; Ducman, V. Carbonation of lightweight alkali-activated aggregates based on biomass fly ash: Effect on microstructure and leaching behavior. Case Stud. Constr. Mater. 2025, 23, e05014. [Google Scholar] [CrossRef]
- GB/T 17431.1-2010; Lightweight Aggregates and Its Test Methods—Part 1: Lightweight Aggregates. China Building Materials Federation: Beijing, China, 2010.
- GB/T 17431.2-2010; National Standards of the People’s Republic of China, Lightweight Aggregates and Its Test Methods—Part 2. Test Methods for Lightweight Aggregate. Standards Press of China: Beijing, China, 2010.
- GB/T 50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. National Standard of the People’s Republic of China: Beijing, China, 2009.
- HJ 557-2010; Solid Waste-Extraction Procedure for Leaching Toxicity-Horizontal Vibration Method. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2010.
- Huang, W.; Wang, H. Geopolymer pervious concrete modified with granulated blast furnace slag: Microscale characterization and mechanical strength. J. Clean. Prod. 2021, 328, 129469. [Google Scholar] [CrossRef]
- Rehman, M.U.; Rashid, K.; Haq, E.U.; Hussain, M.; Shehzad, N. Physico-mechanical performance and durability of artificial lightweight aggregates synthesized by cementing and geopolymerization. Constr. Build. Mater. 2020, 232, 117290. [Google Scholar] [CrossRef]
- Chuan, H.D.L.; Abd, R.R.; Marwan, K.; Zarina, Y.; Bakri, A.M.M.A.; Doru, B.N.D.; Hamzah, F.; Ratna, E.; Rosnita, M.; Alida, A. Artificial Lightweight Aggregates Made from Pozzolanic Material: A Review on the Method, Physical and Mechanical Properties, Thermal and Microstructure. Materials 2022, 15, 3929. [Google Scholar] [CrossRef]
- Abbas, W.; Khalil, W.; Nasser, I. Production of lightweight Geopolymer concrete using artificial local lightweight aggregate. MATEC Web Conf. 2018, 162, 02024. [Google Scholar] [CrossRef]
- Risdanareni, P.; Schollbach, K.; Wang, J.; Belie, N.D. The effect of NaOH concentration on the mechanical and physical properties of alkali activated fly ash-based artificial lightweight aggregate. Constr. Build. Mater. 2020, 259, 119832. [Google Scholar] [CrossRef]
- Bekkeri, G.B.; Shetty, K.K.; Nayak, G. Synthesis of artificial aggregates and their impact on performance of concrete: A review. J. Mater. Cycles Waste Manag. 2023, 25, 1988–2011. [Google Scholar] [CrossRef]
- Hu, S.; Gong, X.; Li, Q.; Fan, Z. Preparation and Characterization of Non-Sintered Ceramsites From Alkali-Activated Foundry Dust. Int. J. Met. 2024, 18, 706–716. [Google Scholar] [CrossRef]
- Liu, J.; Niu, R.; Hu, J.; Ren, Y.; Zhang, W.; Liu, G.; Li, Z.; Xing, F.; Ren, J. The performance and microstructure of alkali-activated artificial aggregates prepared from municipal solid waste incineration bottom ash. Constr. Build. Mater. 2023, 403, 133012. [Google Scholar] [CrossRef]
- Ying, K.S.; Hanizam, A. Utilisation of Recycled Silt from Water Treatment and Palm Oil Fuel Ash as Geopolymer Artificial Lightweight Aggregate. Sustainability 2021, 13, 6091. [Google Scholar] [CrossRef]
- Qin, J.; Cui, C.; Cui, X.; Hussain, A.; Yang, C. Preparation and characterization of ceramsite from lime mud and coal fly ash. Constr. Build. Mater. 2015, 95, 10–17. [Google Scholar] [CrossRef]
- Jin, J.; Ying, W.; Jiawei, L.; Ming, W.; Chuanlin, W.; Wei, B. Characterization and mechanism of sintered light aggregate ceramsite with engineering excavated soil. Structures 2024, 70, 107699. [Google Scholar] [CrossRef]
- Zhuo, L.; Rongxin, G.; Xiao-Yong, W.; Chaoshu, F.; Run-Sheng, L. Construction ceramsite from low-silicon red mud: Design, preparation, and sintering mechanism analysis. Process Saf. Environ. Prot. 2023, 176, 166–179. [Google Scholar]
- Pei, J.; Ye, P.; Lu, B.; Lu, W.; Jiang, F.; Lu, Z.; Pan, X.; Xu, Z. Ultra-lightweight ceramsites from waste glass and red mud: Performance and microstructural analysis. J. Environ. Chem. Eng. 2025, 13, 115876. [Google Scholar] [CrossRef]
- Jun, J.; Song, C.; Chao, J.; Gui, W.; Tie, L.; Tianyuan, X.; Luo, L.; Wenjing, D.; Xu, Y.; Tao, D.; et al. Preparation and properties of high-strength lightweight aggregate ceramsite from nepheline tailings. Constr. Build. Mater. 2023, 368, 130458. [Google Scholar] [CrossRef]
- Fan, C.Y.; Xian, H.W.; Hao, Z.Y.; Ci, W.Y.; Jun, P.; Li, A.S. Process and property optimization of ceramsite preparation by Bayan Obo tailings and blast furnace slag. J. Iron Steel Res. Int. 2023, 30, 1381–1389. [Google Scholar] [CrossRef]
- Jiannan, P.; Xiaolin, P.; Yibo, W.; Zhongyang, L.; Haiyan, Y.; Ganfeng, T. Effects of alkali and alkaline-earth oxides on preparation of red mud based ultra-lightweight ceramsite. Ceram. Int. 2023, 49, 18379–18387. [Google Scholar]
- Liu, B.; Zhang, Q.; Feng, Y.; Chen, Q.; Guo, L. Mechanical and microstructural analysis of cemented tailings backfill by copper slag through alkaline activation emphasizing red mud. Constr. Build. Mater. 2024, 428, 136341. [Google Scholar] [CrossRef]
- Xu, D.; Ni, W.; Wang, Q.; Xu, C.; Li, K. Ammonia-soda residue and metallurgical slags from iron and steel industries as cementitious materials for clinker-free concretes. J. Clean. Prod. 2021, 307, 127262. [Google Scholar] [CrossRef]
- Walkley, B.; Nicolas, R.S.; Sani, M.-A.; Rees, G.J.; Hanna, J.V.; van Deventer, J.S.; Provis, J.L. Phase evolution of C-(N)-ASH/NASH gel blends investigated via alkali-activation of synthetic calcium aluminosilicate precursors. Cem. Concr. Res. 2016, 89, 120–135. [Google Scholar] [CrossRef]
- Guo, W.; Wang, S.; Xu, Z.; Zhang, Z.; Zhang, C.; Bai, Y.; Zhao, Q. Mechanical performance and microstructure improvement of soda residue–carbide slag–ground granulated blast furnace slag binder by optimizing its preparation process and curing method. Constr. Build. Mater. 2021, 302, 124403. [Google Scholar] [CrossRef]
- Abdelfattah, M.M.; Géber, R.; Abdel-Kader, N.A.; Kocserha, I. Assessment of the mineral phase and properties of clay-Ca bentonite lightweight aggregates. Arab. J. Geosci. 2022, 15, 205. [Google Scholar] [CrossRef]
- Nie, Q.; Hu, W.; Ai, T.; Huang, B.; Shu, X.; He, Q. Strength properties of geopolymers derived from original and desulfurized red mud cured at ambient temperature. Constr. Build. Mater. 2016, 125, 905–911. [Google Scholar] [CrossRef]
- Sglavo, V.M.; Campostrini, R.; Maurina, S.; Carturan, G.; Monagheddu, M.; Budroni, G.; Cocco, G. Bauxite ‘red mud’in the ceramic industry. Part 1: Thermal behaviour. J. Eur. Ceram. Soc. 2000, 20, 235–244. [Google Scholar] [CrossRef]
- Wang, J.; Liu, E.; Li, L. Multiscale investigations on hydration mechanisms in seawater OPC paste. Constr. Build. Mater. 2018, 191, 891–903. [Google Scholar] [CrossRef]
- Zhai, Q.; Kurumisawa, K. Effect of accelerators on Ca(OH)2 activated ground granulated blast-furnace slag at low curing temperature. Cem. Concr. Compos. 2021, 124, 104272. [Google Scholar] [CrossRef]
- Choudhary, H.K.; Anupama, A.; Kumar, R.; Panzi, M.; Matteppanavar, S.; Sherikar, B.N.; Sahoo, B. Observation of phase transformations in cement during hydration. Constr. Build. Mater. 2015, 101, 122–129. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, K.-S.; Jung, S.S.; Hwang, J.I.; Choi, J.-S.; Sohn, I. Valorization of electric arc furnace primary steelmaking slags for cement applications. Waste Manag. 2015, 41, 85–93. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Z.; Bai, Y.; Zhao, G.; Sang, Z.; Zhao, Q. Development and characterization of a new multi-strength level binder system using soda residue-carbide slag as composite activator. Constr. Build. Mater. 2021, 291, 123367. [Google Scholar] [CrossRef]
- Zhong, M.; Meng, J.; Ning, B.; Na, F.; Cui, T.; Shi, X.; Cui, T. Preparation and alkali excitation mechanism of coal gangue-iron ore tailings non-sintering ceramsite. Constr. Build. Mater. 2024, 426, 136209. [Google Scholar] [CrossRef]
- Zhang, N.; Li, H.; Liu, X. Hydration mechanism and leaching behavior of bauxite-calcination-method red mud-coal gangue based cementitious materials. J. Hazard. Mater. 2016, 314, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Qin, Z.; Lin, Z.; Shen, P.; Chen, L.; Chen, G.; Zhang, L.; Gao, J.; Liu, S.; Yang, N. Study on the physical mechanical properties and freeze-thaw resistance of energy storage concrete with artificial phase change aggregate. J. Build. Eng. 2024, 99, 111506. [Google Scholar] [CrossRef]
- Tian, Y.; Lai, Y.; Pei, W.; Qin, Z.; Li, H. Study on the physical mechanical properties and freeze-thaw resistance of artificial phase change aggregates. Constr. Build. Mater. 2022, 329, 127225. [Google Scholar] [CrossRef]
- Yeon, J.H.; Kim, K.-K. Potential applications of phase change materials to mitigate freeze-thaw deteriorations in concrete pavement. Constr. Build. Mater. 2018, 177, 202–209. [Google Scholar] [CrossRef]
- GB 5085.3-2007; Identification Standards for Hazardous Wastes-Identification for Extraction Toxicity. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2007.
- Ji, H.; Zhang, Y.; Ren, R.; Guo, Y.; Du, Y.; Zhao, X.; Feng, G. Preparation and performance influencing factors of multi-component solid waste based non-sintered ceramsites. Constr. Build. Mater. 2025, 489, 142159. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Wang, Y.; Zhao, J. Non-sintered ceramsite from alkali-activated gasification slag for adsorption through the regulation of physical properties and pore structure. J. Clean. Prod. 2024, 477, 143820. [Google Scholar] [CrossRef]
- Feng, C.; Wang, J.; Chen, M.; Zhao, X.; Zhang, W.; Zhu, J.; Du, M. Study on the modification effect of recycled fine aggregates treated by synergistic alkali activation using volcanic ash and cement powder wrapping. J. Mater. Res. Technol. 2025, 36, 2874–2887. [Google Scholar] [CrossRef]
- Elias, L.; Bijimol, B.I.; Geethanjali, C.V.; Anil, A.; Bhagya, T.C.; Shibli, S.M.A. Fine-recycled concrete aggregate based cementitious polymer composite coating for corrosion and biocorrosion protection of concrete reinforcing steel bar. Constr. Build. Mater. 2025, 472, 140877. [Google Scholar] [CrossRef]
- Colangelo, F.; Messina, F.; Cioffi, R. Recycling of MSWI fly ash by means of cementitious double step cold bonding pelletization: Technological assessment for the production of lightweight artificial aggregates. J. Hazard. Mater. 2015, 299, 181–191. [Google Scholar] [CrossRef]
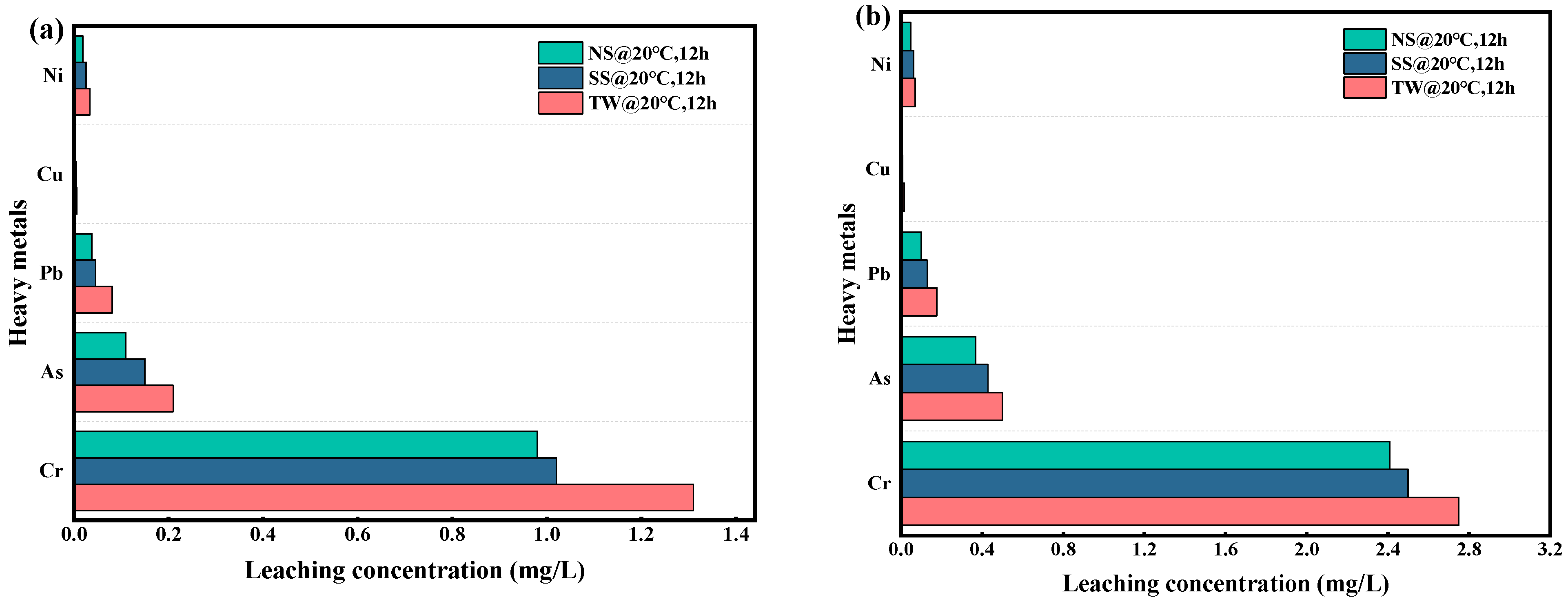
| Material/wt% | CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | TiO2 | K2O | Na2O | LOIa |
|---|---|---|---|---|---|---|---|---|---|---|
| RM | 0.48 | 14.11 | 16.53 | 50.10 | 0.58 | 0.13 | 4.84 | 0.11 | 4.46 | 9.16 |
| PT | 30.35 | 35.12 | 13.32 | 1.42 | 1.36 | 7.94 | 1.58 | 1.84 | 0.34 | 7.26 |
| FA | 20.88 | 37.16 | 21.10 | 3.58 | 3.36 | 3.87 | 2.75 | 4.08 | 2.15 | 1.43 |
| MP | 44.05 | 21.95 | 16.68 | 0.15 | 3.51 | 9.30 | 1.05 | 0.28 | 0.79 | - |
| RM-LWA | Type of Additive | Curing Condition |
|---|---|---|
| A1 | TW | Standard curing at 20 °C over a 12 h period |
| A2 | SS | |
| A3 | NS | |
| B1 | TW | Curing with steam at 60 °C over a 12 h period |
| B2 | SS | |
| B3 | NS | |
| C1 | TW | Curing with steam at 70 °C over a 12 h period |
| C2 | SS | |
| C3 | NS | |
| D1 | TW | Curing with steam at 80 °C over a 12 h period |
| D2 | SS | |
| D3 | NS | |
| E1 | TW | Curing with steam at 90 °C over a 12 h period |
| E2 | SS | |
| E3 | NS | |
| F1 | TW | Curing with steam at 80 °C over a 4 h period |
| F2 | SS | |
| F3 | NS | |
| G1 | TW | Curing with steam at 80 °C over a 6 h period |
| G2 | SS | |
| G3 | NS | |
| H1 | TW | Curing with steam at 80 °C over an 8 h period |
| H2 | SS | |
| H3 | NS | |
| I1 | TW | Curing with steam at 80 °C over a 10 h period |
| I2 | SS | |
| I3 | NS |
| Raw Materials | Sintering Temperature (°C) | The ρap Value (kg/m3) | 1 h Water Absorption (%) | Crush Strength (MPa) | References |
|---|---|---|---|---|---|
| Silica fume | 1120 | 691 | 1.33 | 5.54 | [46] |
| Lime mud | 1050 | 740 | 39.03 | 4.73 | [44] |
| Waste glass | 1125 | 482 | 7.2 | 1.43 | [47] |
| Nepheline tailings | 1150 | 410 | 3.5 | 1.8 | [48] |
| Bayan Obo tailings | 1130 | 1940 | 31.42 | 1.89 | [49] |
| Red mud | 1150 | 450 | 9.0 | 1.12 | [50] |
| Engineering excavated soil | 1175 | 967.1 | 11.39 | 5.02 | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, R.; Zhao, Y.; Luo, H.; Leng, H.; Wu, W.; Song, B.; He, B.-J. Preparation and Physical Properties of Red Mud Based Artificial Lightweight Aggregates. Materials 2025, 18, 3741. https://doi.org/10.3390/ma18163741
Han R, Zhao Y, Luo H, Leng H, Wu W, Song B, He B-J. Preparation and Physical Properties of Red Mud Based Artificial Lightweight Aggregates. Materials. 2025; 18(16):3741. https://doi.org/10.3390/ma18163741
Chicago/Turabian StyleHan, Rubin, Yunrui Zhao, Hui Luo, Hongxiu Leng, Wenbo Wu, Bukai Song, and Bao-Jie He. 2025. "Preparation and Physical Properties of Red Mud Based Artificial Lightweight Aggregates" Materials 18, no. 16: 3741. https://doi.org/10.3390/ma18163741
APA StyleHan, R., Zhao, Y., Luo, H., Leng, H., Wu, W., Song, B., & He, B.-J. (2025). Preparation and Physical Properties of Red Mud Based Artificial Lightweight Aggregates. Materials, 18(16), 3741. https://doi.org/10.3390/ma18163741









