Biodegradable NR Latex Films with Lignocellulosic and Collagen Hydrolysate Fillers
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kędzia, J.R.; Sitko, A.M.; Haponiuk, J.T.; Kucińska-Lipka, J. Natural Rubber Latex—Origin, Specification and Application. In Application and Characterization of Rubber Materials; Evingür, G.A., Pekcan, Ö., Eds.; IntechOpen: London, UK, 2023. [Google Scholar]
- Rose, K.; Steinbuchel, A. Biodegradation of natural rubber and related compounds: Recent insights into a hardly understood catabolic capability of microorganisms. Appl. Environ. Microbiol. 2005, 71, 2803–2812. [Google Scholar] [CrossRef]
- Subramaniam, A. The chemistry of natural rubberlatex. Immunol. Allergy Clin. N. Am. 1995, 15, 1–20. [Google Scholar] [CrossRef]
- Ali Shah, A.; Hasan, F.; Shah, Z.; Kanwal, N.; Zeb, S. Biodegradation of natural and synthetic rubbers: A review. Int. Biodeter. Biodegr. 2013, 83, 145–157. [Google Scholar] [CrossRef]
- Kurian, T.; Mathew, N.M. Natural Rubber: Production, Properties and Applications. In Biopolymers: Biomedical and Environmental Applications; Kalia, S., Avérous, L., Eds.; Scrivener Publishing LLC.: Beverly, MA, USA, 2011. [Google Scholar]
- Production/Yield Quantities of Rubber, Natural in World from 2000 to 2019; FAO: Rome, Italy, 2021.
- Inkonkoy, F. Sustainability in the Natural Rubber Supply Chain: Getting the Basics Right. SPOTT; Zoological Society of London: London, UK, 2021; Available online: https://www.spott.org/news/sustainability-in-the-natural-rubber-supply-chain (accessed on 14 February 2025).
- Industry Outlook 2022–2024: Natural Rubber. Available online: https://www.krungsri.com/en/research/industry/industry-outlook/agriculture/rubber/io/rubber-2022 (accessed on 11 December 2024).
- Natural Rubber Latex (HS: 400110) Product Trade, Exporters and Importers. Available online: https://oec.world/en/profile/hs/natural-rubber-latex (accessed on 11 December 2024).
- Prata, J.C.; Silva, A.L.P.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. COVID-19 pandemic repercussions on the use and management of plastics. Environ. Sci. Technol. 2020, 54, 7760–7765. [Google Scholar] [CrossRef]
- Bosco, F.; Mollea, C. Biodegradation of Natural Rubber: Microcosm Study. Water Air Soil Pollut. 2021, 232, 227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Maria, F.; Beccaloni, E.; Bonadonna, L.; Cini, C.; Confalonieri, E.; La Rosa, G.; Milana, M.R.; Testai, E.; Scaini, F. Minimization of spreading of SARS-CoV-2 via household waste produced by subjects affected by COVID-19 or in quarantine. Sci. Total Environ. 2020, 743, 140803. [Google Scholar] [CrossRef]
- Klemeš, J.J.; Fan, Y.V.; Tan, R.R.; Jiang, P. Minimising the present and future plastic waste, energy and environmental footprints related to COVID-19. Renew. Sustain. Energy Rev. 2020, 127, 109883. [Google Scholar] [CrossRef]
- Soares, F.A.; Steinbuchel, A. Natural rubber degradation products: Fine chemicals and reuse of rubber waste. Eur. Polym. J. 2022, 165, 111001. [Google Scholar] [CrossRef]
- Kędzia, J.; Haponiuk, J.; Formela, K. Natural Rubber Latex Wastes from Balloon Production as Valuable Source of Raw Material: Processing, Physico-Mechanical Properties, and Structure. J. Compos. Sci. 2024, 8, 365. [Google Scholar] [CrossRef]
- Patipnun, S.; Bunchuai, C.; Lertsarawut, P.; Saenboonruang, K. Enhancement of degradation resistance and biodegradability in natural rubber films through addition of gamma-irradiated mangosteen powder as biofiller. React. Funct. Polym. 2025, 210, 106198. [Google Scholar] [CrossRef]
- Altenhoff, A.L.; de Witt, J.; Andler, R.; Steinbüchel, A. Impact of additives of commercial rubber compounds on the microbial and enzymatic degradation of poly(cis-1,4-isoprene). Biodegradation 2019, 30, 13–26. [Google Scholar] [CrossRef]
- Cui, C.; Jiang, M.; Zhang, C.; Zhang, N.; Jin, F.J.; Li, T.; Lee, H.G.; Jin, L. Assembly strategies for rubber-degrading microbial consortia based on omics tools. Front. Bioenerg. Biotechnol. 2023, 11, 1326395. [Google Scholar] [CrossRef]
- Guerra, N.B.; Pegorin, G.S.A.; Boratto, M.H.; de Barros, N.R.; de Oliveira Graeff, C.F.; Herculano, R.D. Biomedical applications of natural rubber latex from the rubber tree Hevea brasiliensis. Mat. Sci. Eng. 2021, 126, 112126. [Google Scholar] [CrossRef]
- Amnuaypornsri, S.; Sakdapipanich, J.; Tanaka, Y. Highly purified natural rubber by saponification of latex: Analysis of green and cured properties. J. Appl. Polym. Sci. 2010, 118, 3524–3531. [Google Scholar] [CrossRef]
- Burfield, D.R. Storage hardening of natural rubber: An examination of current mechanistic proposals. J. Nat. Rubber Res. 1986, 1, 202–208. [Google Scholar]
- Berekaa, M.M.; Linos, A.; Reichelt, R.; Keller, U.; Steinbüchel, A. Effect of pretreatment of rubber material on its biodegradability by various rubber degrading bacteria. FEMS Microbiol. Lett. 2000, 184, 199–206. [Google Scholar] [CrossRef]
- Cherian, E.; Jayachandran, K. Microbial Degradation of Natural Rubber Latex by a novel species of Bacillus sp. SBS25 isolated from Soil. Int. J. Environ. Res. 2009, 3, 599–604. [Google Scholar]
- Rolling Car Tires into the Global Circular Economy. Available online: https://news.mongabay.com/2023/08/rolling-car-tires-into-the-global-circular-economy/ (accessed on 11 December 2024).
- Jayathilaka, L.P.I.; Ariyadasa, T.U.; Egodage, S.M. Development of biodegradable natural rubber latex composites by employing corn derivative bio-fillers. J. Appl. Polym. Sci. 2020, 137, e49205. [Google Scholar] [CrossRef]
- Humwong, A.; Poltabtim, W.; Kerdsang, P.; Saenboonruang, K. Roles of Chitosan as Bio-Fillers in Radiation-Vulcanized Natural Rubber Latex and Hybrid Radiation and Peroxide-Vulcanized Natural Rubber Latex: Physical/Mechanical Properties under Thermal Aging and Biodegradability. Polymers 2021, 13, 3940. [Google Scholar] [CrossRef]
- ISO 287:2017; Paper and Board—Determination of Moisture Content of a Lot—Oven-Drying Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- TAPPI T280 om-99. (1999); Acetone Extractives of Wood and Pulp, Test Method T280 pm-99. TAPPI Press: Atlanta, GA, USA, 1999.
- TAPPI T212 om-12 (2012); One Percent Sodium Hydroxide Solubility. TAPPI Press: Atlanta, GA, USA, 2012.
- Seifert, K. Zur Frage der cellulose-Schnellbestimmung nach der Acetylacetone method. Das Papier 1956, 20, 301. [Google Scholar]
- TAPPI T222 om-15 (2015); Acid-Insoluble Lignin in Wood and Pulp. TAPPI Press: Atlanta, GA, USA, 2015.
- Przepiórkowska, A.; Tadeusiak, A.; Prochoń, M. Sposób Otrzymywania Napełniacza Mieszanki Kauczukowej Karboksylowanego Kauczuku Butadienowo-Akrylonitrylowego Oraz Mieszanka Kauczukowa Zawierająca ten Napełniacz. Patent of the Patent Office of the Republic of Poland UP RP PL214972, 14 March 2013. [Google Scholar]
- Zielińska, M.; Seyger, R.; Dierkes, W.K.; Bieliński, D.; Noordermeer, J.W.M. Swelling of EPDM rubber for oil-well applications as influenced by medium composition and temperature. Part I. Literature and background. Elastomery 2016, 2, 6–17. [Google Scholar]
- ISO 37:1998; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. International Organization for Standardization: Geneva, Switzerland, 1998.
- PN-71/C-04238; Hardness Determination with Shore Method. Polish Committee for Standardization: Warsaw, Poland, 1971.
- Horcas, I.; Fern’andez, R.; G’omez-Rodríguez, J.M.; Colchero, J.; G’omez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 7. [Google Scholar] [CrossRef]
- Chang, Y.P.; Cheah, P.B.; Seow, C.C. Plasticizing—Antiplasticizing Effects of Water on Physical Properties of Tapioca Starch Films in the Glassy State. J. Food Sci. 2000, 65, 445–451. [Google Scholar] [CrossRef]
- Debarshi, N.; Rodriguez-Uribe, A.; Tao, W.; Manjusri, M.; Mohanty, A.K. Green composites from plasticized poly(3-hydroxybutyrate-co-3-hydroxyvalerate) bioplastic and cellulosic fiber: Effect of compatibilizer and grafting percentage on interfacial adhesion and biocomposite performance. Int. J. Biol. Macromol. 2025, 315, 144107. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, L.; Wang, R.; Yu, H.; Zheng, T.; Lian, Y.; Luo, M.; Liao, S.; Liu, H.; Peng, Z. Research of strain induced crystallization and tensile properties of vulcanized natural rubber based on crosslink densities. Ind. Crops Prod. 2023, 202, 117070. [Google Scholar] [CrossRef]
- Huneau, B. Strain-Induced Crystallization of Natural Rubber: A Review of X-ray Diffraction Investigations. Rubber Chem. Technol. 2011, 84, 425–452. [Google Scholar] [CrossRef]
- Ho, C.C.; Khew, M.C. Surface Free Energy Analysis of Natural and Modified Natural Rubber Latex Films by Contact Angle Method. Am. Chem. Soc. 2000, 16, 1407–1414. [Google Scholar] [CrossRef]
- Qazanfarzadeh, Z.; Kumaravel, V. Hydrophobisation approaches of protein-based bioplastics. Trends Food Technol. 2023, 138, 27–43. [Google Scholar] [CrossRef]
- Matveev, Y.I.; Grinberg, V.Y.; Tolstoquzov, V.B. The plasticizing effect of water on proteins, polysaccharides and their mixtures. Glassy state of biopolymers, foods and seeds. Food Hydrocoll. 2000, 14, 425–437. [Google Scholar] [CrossRef]
- Kumar Gupta, P.; Sai Raghunath, S.; Venkatesh Prasanna, D.; Venkat, P.; Shree, V.; Chithananthan, C.; Choudhary, S.; Surender, K.; Geetha, K. An Update on Overview of Cellulose, Its Structure and Applications. In Cellulose; Pascual, A.R., Martin, M.A.E., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Hu, L.; Fang, X.; Du, M.; Luo, F.; Guo, S. Hemicellulose-Based Polymers Processing and Application. Am. J. Plant Sci. 2020, 11, 2066–2079. [Google Scholar] [CrossRef]
- Ribeiro, M.; Santos, A.I.; Veiga, F.; Figueiras, A. Lignin nanoparticle-based nanocomposite hydrogels for biomedical applications. In Functional Nanocomposite Hydrogels. Synthesis, Characterization, and Biomedical Applications. Nanotechnology in Biomedicine; Elsevier: Amsterdam, The Netherlands, 2023; pp. 69–90. [Google Scholar]
- Akbas, A.; Yuhana, A.Y. Recycling of rubber waste as fuel and its additives. Recycling 2021, 6, 78. [Google Scholar] [CrossRef]
- Mbese, Z.; Alven, S.; Aderibigbe, B.A. Collagen-Based Nanofibers for Skin Regeneration and Wound Dressing Applications. Polymers 2021, 13, 4368. [Google Scholar] [CrossRef]
- Rahmah, M.; Khusyairi, A.R.A.; Khairunnisya, H.N.; Nurbalgis, M.S. Oven ageing versus UV ageing properties of natural rubber cup lump/EPDM rubber blend with mangosteen powder (MPP) as natural antioxidant. IOP Conf. Ser. Mater. Sci. Eng. 2019, 548, 012014. [Google Scholar] [CrossRef]
- Nair, A.R.; Sambhudevan, S.S.; Shankar, B. Synthesis, characterization and dye removal properties of cellulose nanocrystals embedded natural rubber latex composites. Cellul. Chem. Technol. 2019, 53, 263–270. [Google Scholar] [CrossRef]
- .Sripriya, R.; Kumar, R. A Novel Enzymatic Method for Preparation and Characterization of Collagen Film from Swim Bladder of Fish Rohu (Labeo rohita). Food Nutr. Sci. 2015, 6, 1468–1478. [Google Scholar] [CrossRef]
- Tran, N.T.T.; Wang, T.-H.; Lin, C.-H.; Tsai, Y.-C.; Lai, C.-H.; Tai, Y.; Yung, B.Y.M. Direct Synthesis of Rev Peptide-Conjugated Gold Nanoparticles and Their Application in Cancer Therapeutics. Bioconjugate Chem. 2011, 22, 1394–1401. [Google Scholar] [CrossRef] [PubMed]

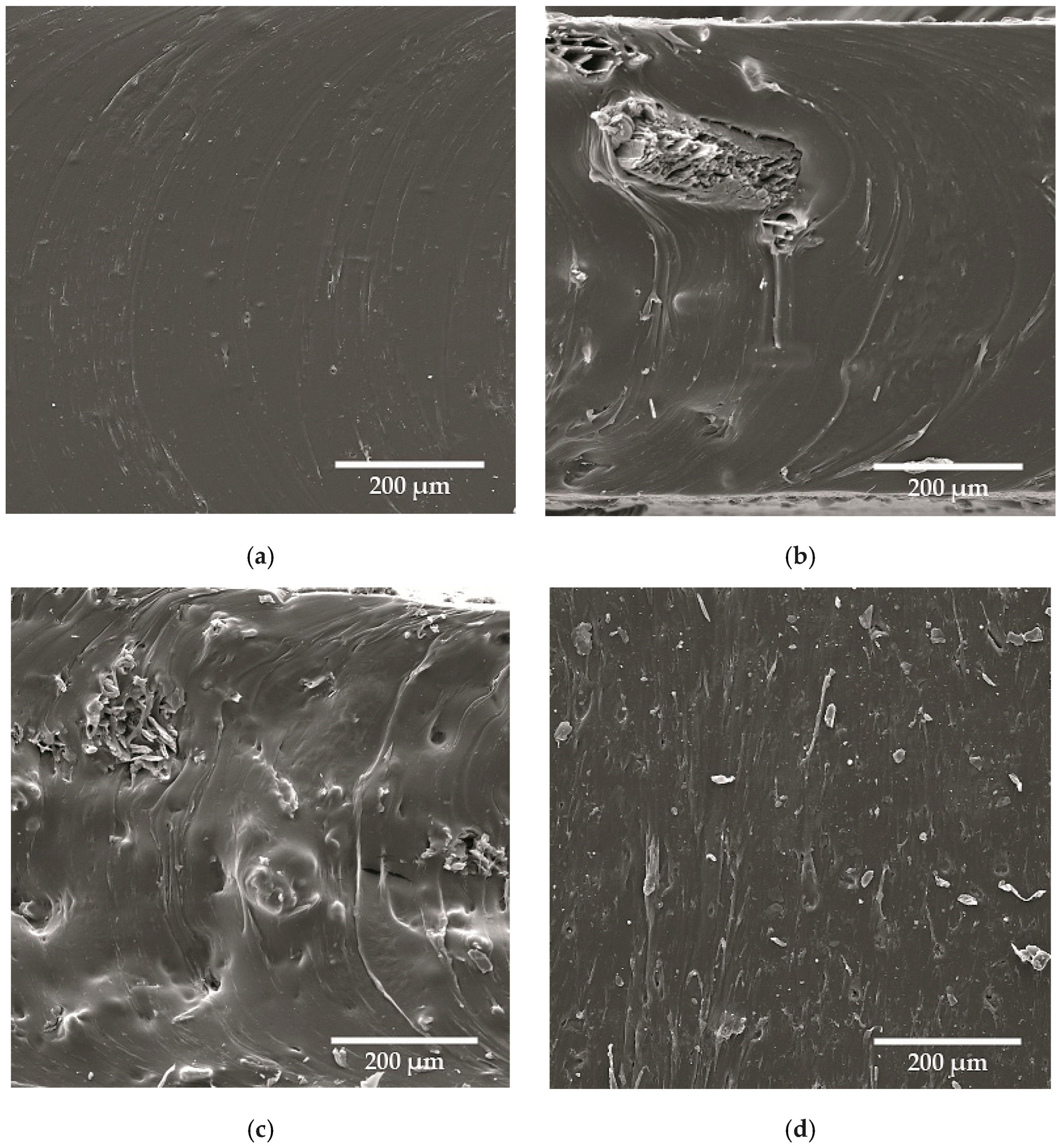
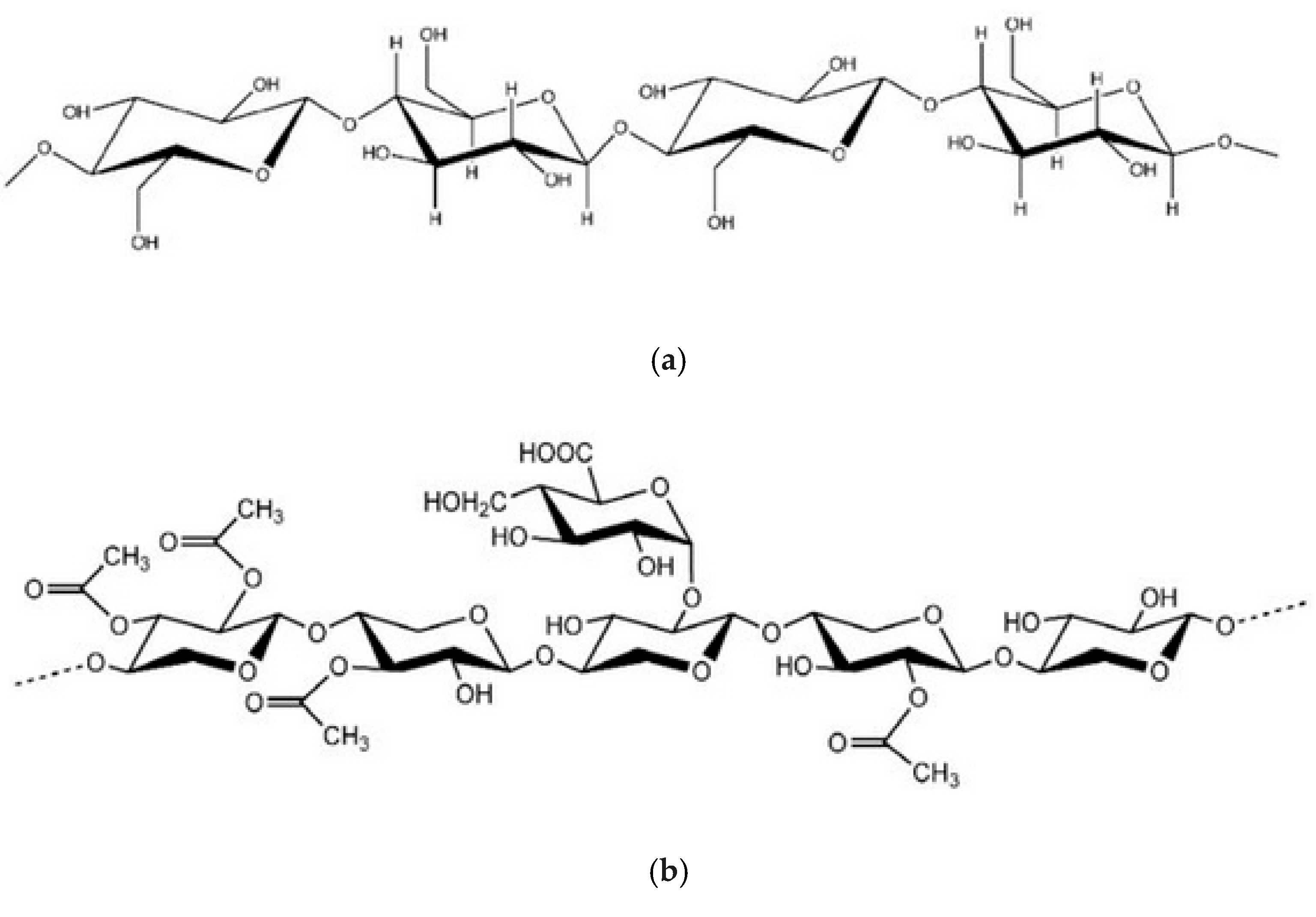
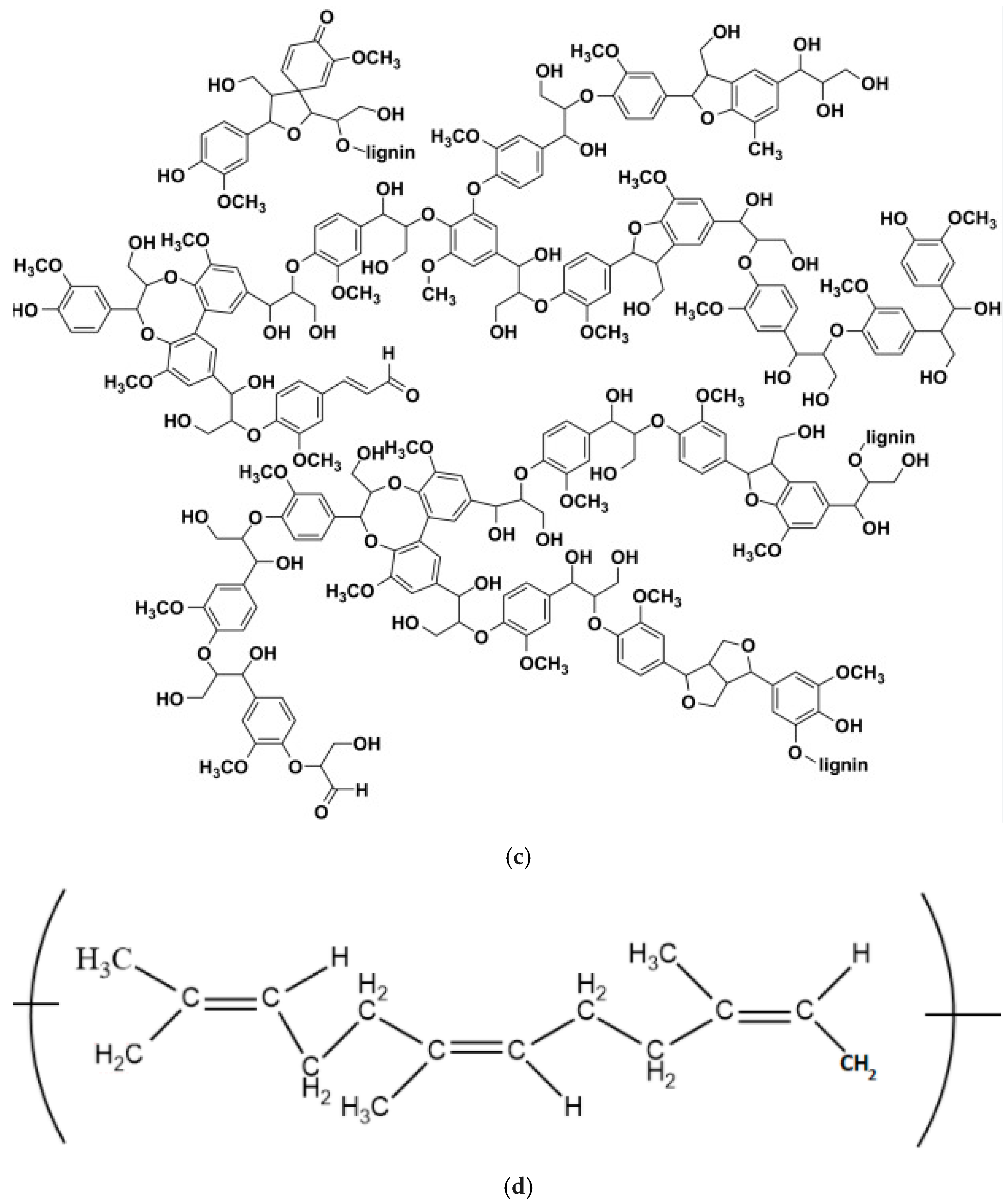
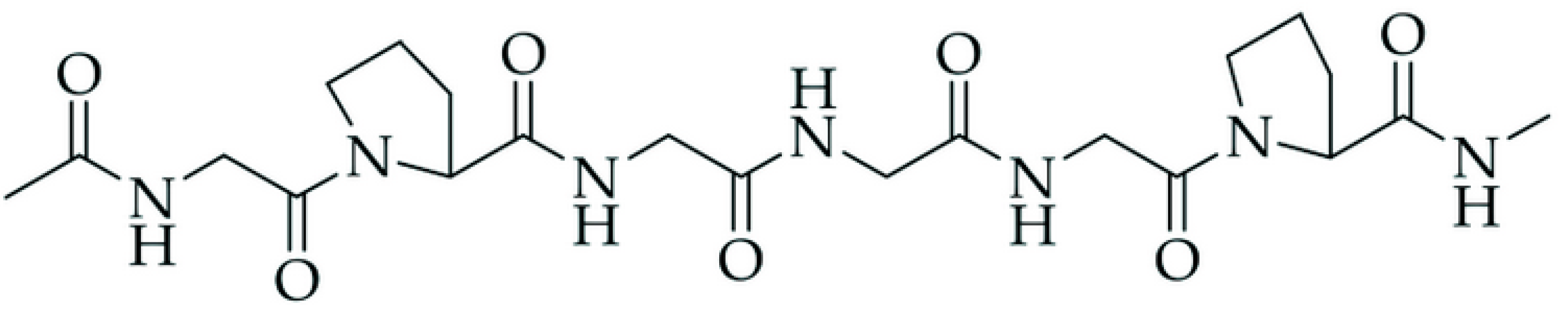

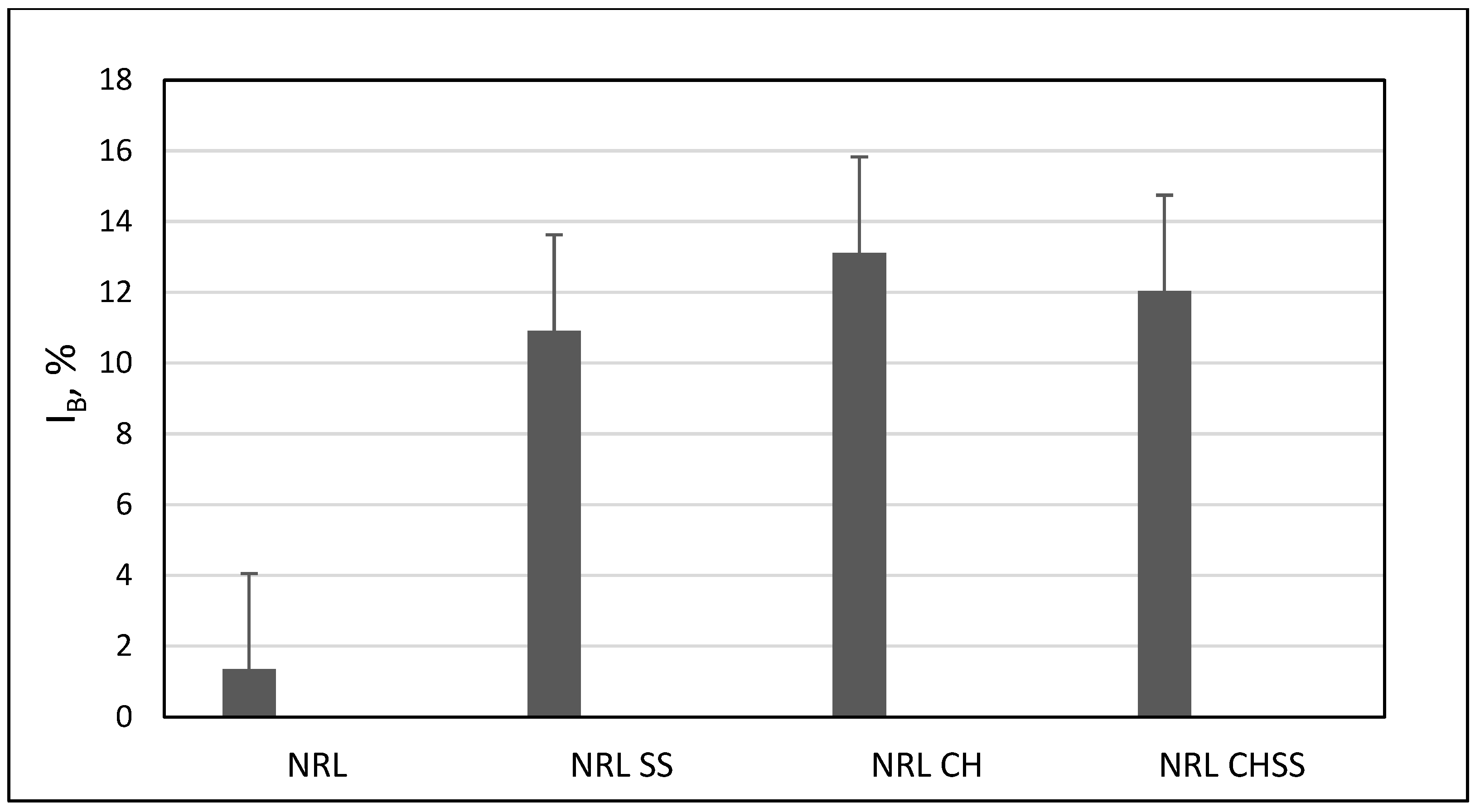
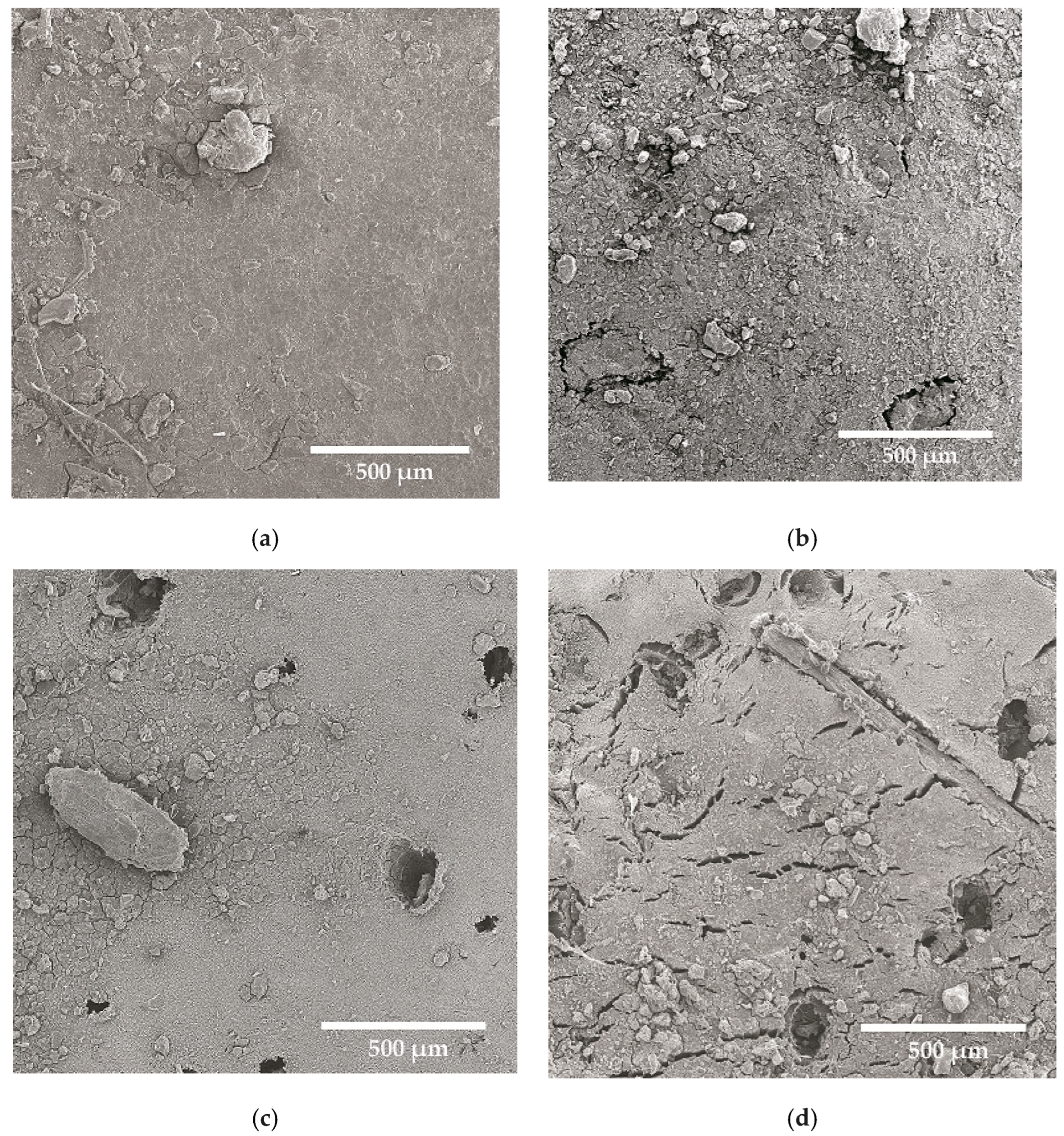
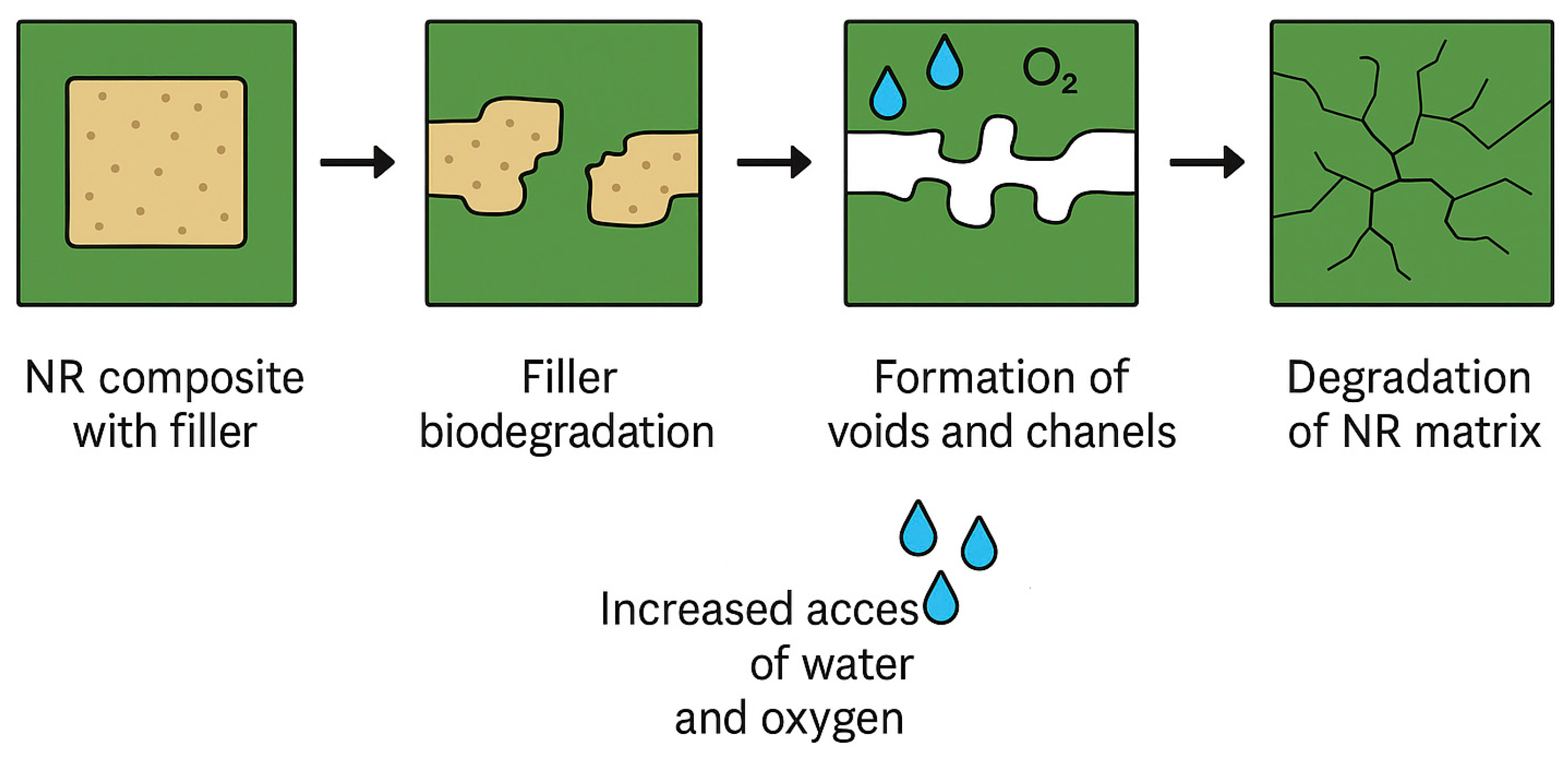
| NRL | NRL F | NRL CH | NRL CHF | |
|---|---|---|---|---|
| NR latex | 100 | 100 | 100 | 100 |
| Wood/non-wood flour | - | 4 | - | 2 |
| Collagen hydrolysate | - | - | 4 | 2 |
| TSb, MPa | Eb, % | °Shore A Hardness | Vr |
Water
Content, % | |
|---|---|---|---|---|---|
| NRL | 9.832 ± 1.185 | 614 ± 2 | 42.42 | 0.172 | 0.69 |
| NRL PS | 11.180 ± 1.548 | 704 ± 55 | 50.14 | 0.149 | 1.54 |
| NRL SS | 11.521 ± 0.598 | 1260 ± 99 | 53.06 | 0.152 | 0.78 |
| NRL BS | 11.430 ± 0.851 | 1620 ± 54 | 47.78 | 0.149 | 1.22 |
| NRL WS | 8.941 ± 0.365 | 1152 ± 151 | 47.92 | 0.145 | 1.90 |
| NRL RSS | 7.540 ± 0.871 | 1209 ± 31 | 37.88 | 0.149 | 1.52 |
| NRL MS | 4.453 ± 0.723 | 607 ± 32 | 43.00 | 0.148 | 1.12 |
| NRL CH | 2.459 ± 0.323 | 1209 ± 32 | 37.88 | 0.148 | 1.55 |
| NRL CH SS | 7.540 ± 0.841 | 570 ± 46 | 39.30 | 0.145 | 1.54 |
| SFE, mJ/m2 | γd, mJ/m2 | γp, mJ/m2 | |
|---|---|---|---|
| NR latex | 32.2 | 25.8 | 6.5 |
| PS | 32.8 | 32.6 | 0.3 |
| SS | 32.0 | 28.7 | 3.2 |
| BS | 34.1 | 32.7 | 1.4 |
| WS | 16.1 | 16.0 | 0.1 |
| RSS | 37.2 | 32.6 | 0.1 |
| MS | 37.2 | 33.0 | 4.2 |
| CH | 21.9 | 14.2 | 7.7 |
| Cellulose | Lignin | Hemicellulose | Extractives | Mineral Substances | |
|---|---|---|---|---|---|
| PS | 40.02 | 30.59 | 17.77 | 2.98 | 0.26 |
| SS | 38.44 | 29.53 | 17.01 | 0.63 | 0.18 |
| BS | 40.48 | 19.88 | 18.92 | 1.66 | 0.07 |
| WS | 28.31 | 32.25 | 47.69 | 6.95 | 2.36 |
| RSS | 34.71 | 24.33 | 22.36 | 3.14 | 1.68 |
| MS | 40.91 | 22.47 | 22.40 | 1.12 | 0.99 |
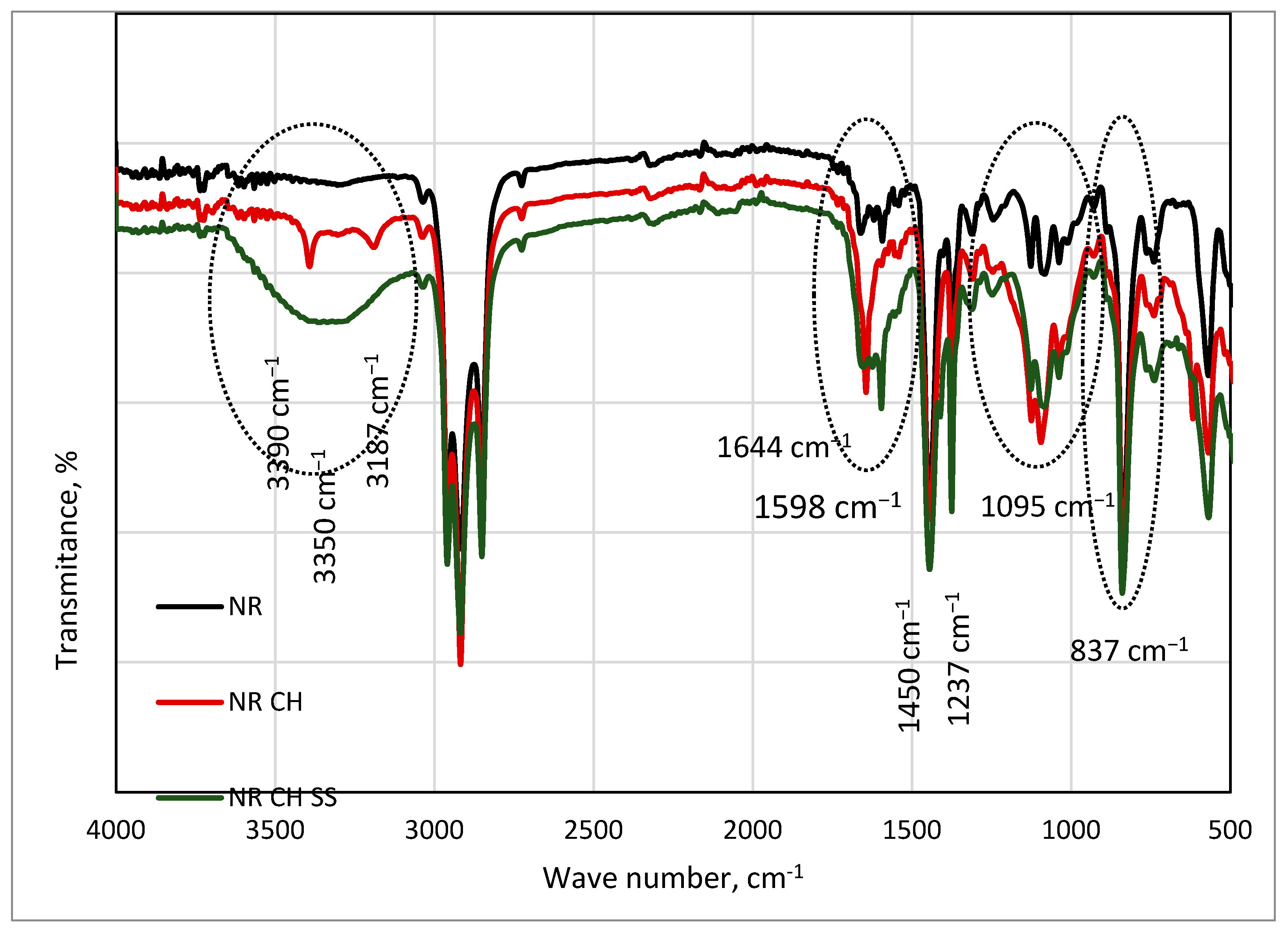
| Band Intensity | Wave Number, cm−1 | Absorption Group | Assignment |
|---|---|---|---|
| Strong | 2960 | CH | Stretching vibration |
| Weak | 1658 | C=C | Stretching vibration |
| Strong | 1444, 1375 | CH2 | Deformation |
| Strong | 842 | CH3 | Out of plane |
| Band Intensity | Wave Number, cm−1 | Absorption Group | Assignment |
|---|---|---|---|
| Weak | 3390 | CH2 NH | Stretching vibr. Tension amide A |
| Weak | 3187 | CH2 NH | Stretching vibr. Tension amide B |
| Medium | 1644 | C=O | Stretching vib. amide I |
| Medium | 1598 | CN | Stretching vib. amide II |
| Strong | 1450 | C=O | Stretching vibrations |
| Strong | 1237 | CN | Stretching vib. amide III |
| Weak | 1095 | OH | Stretching vib. |
| Strong | 837 | CH3 | Out of plane |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kmiotek, M.; Prochoń, M.; Sąsiadek-Andrzejczak, E. Biodegradable NR Latex Films with Lignocellulosic and Collagen Hydrolysate Fillers. Materials 2025, 18, 3711. https://doi.org/10.3390/ma18153711
Kmiotek M, Prochoń M, Sąsiadek-Andrzejczak E. Biodegradable NR Latex Films with Lignocellulosic and Collagen Hydrolysate Fillers. Materials. 2025; 18(15):3711. https://doi.org/10.3390/ma18153711
Chicago/Turabian StyleKmiotek, Magdalena, Mirosława Prochoń, and Elżbieta Sąsiadek-Andrzejczak. 2025. "Biodegradable NR Latex Films with Lignocellulosic and Collagen Hydrolysate Fillers" Materials 18, no. 15: 3711. https://doi.org/10.3390/ma18153711
APA StyleKmiotek, M., Prochoń, M., & Sąsiadek-Andrzejczak, E. (2025). Biodegradable NR Latex Films with Lignocellulosic and Collagen Hydrolysate Fillers. Materials, 18(15), 3711. https://doi.org/10.3390/ma18153711








