Mechanistic Insights into m-Cresol Adsorption on Functional Resins: Surface Chemistry and Adsorption Behavior
Abstract
1. Introduction
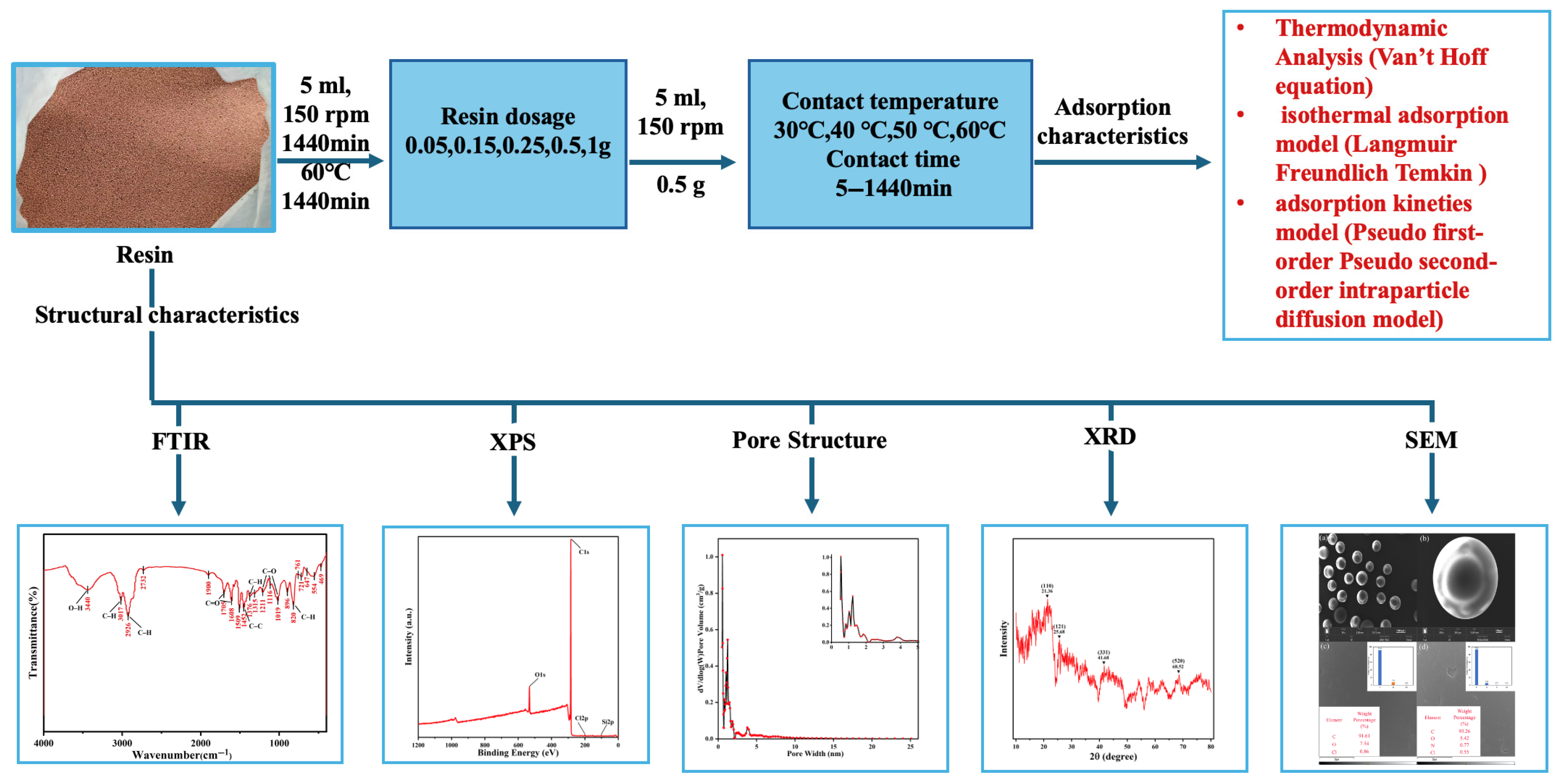
2. Materials and Methods
2.1. Materials
2.2. Batch Adsorption Experiments
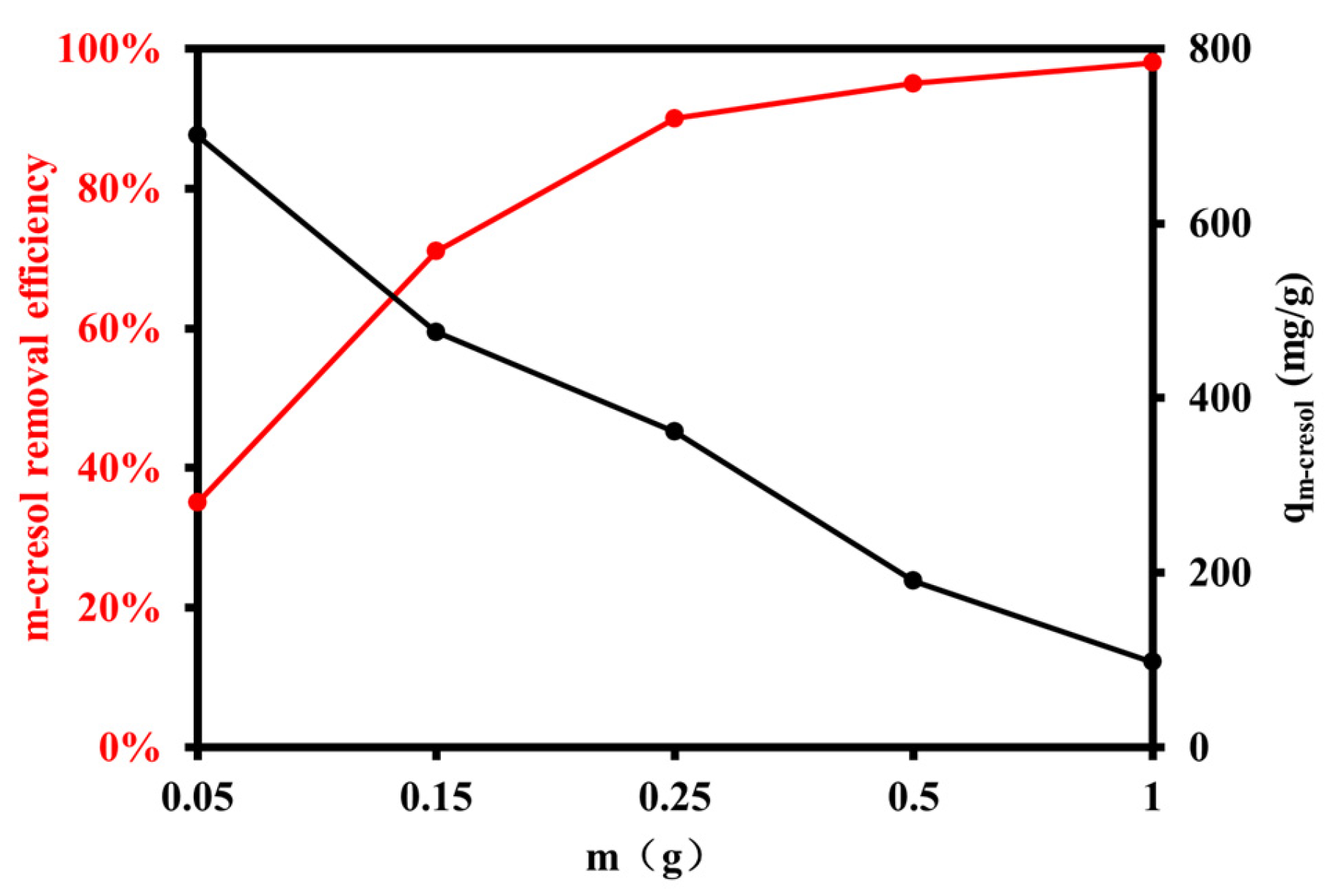
2.2.1. Effect of Resin Dosage
2.2.2. Effect of Contact Time and Temperature
2.2.3. Analytical Methods
3. Results and Discussion
3.1. Characterization of the Materials
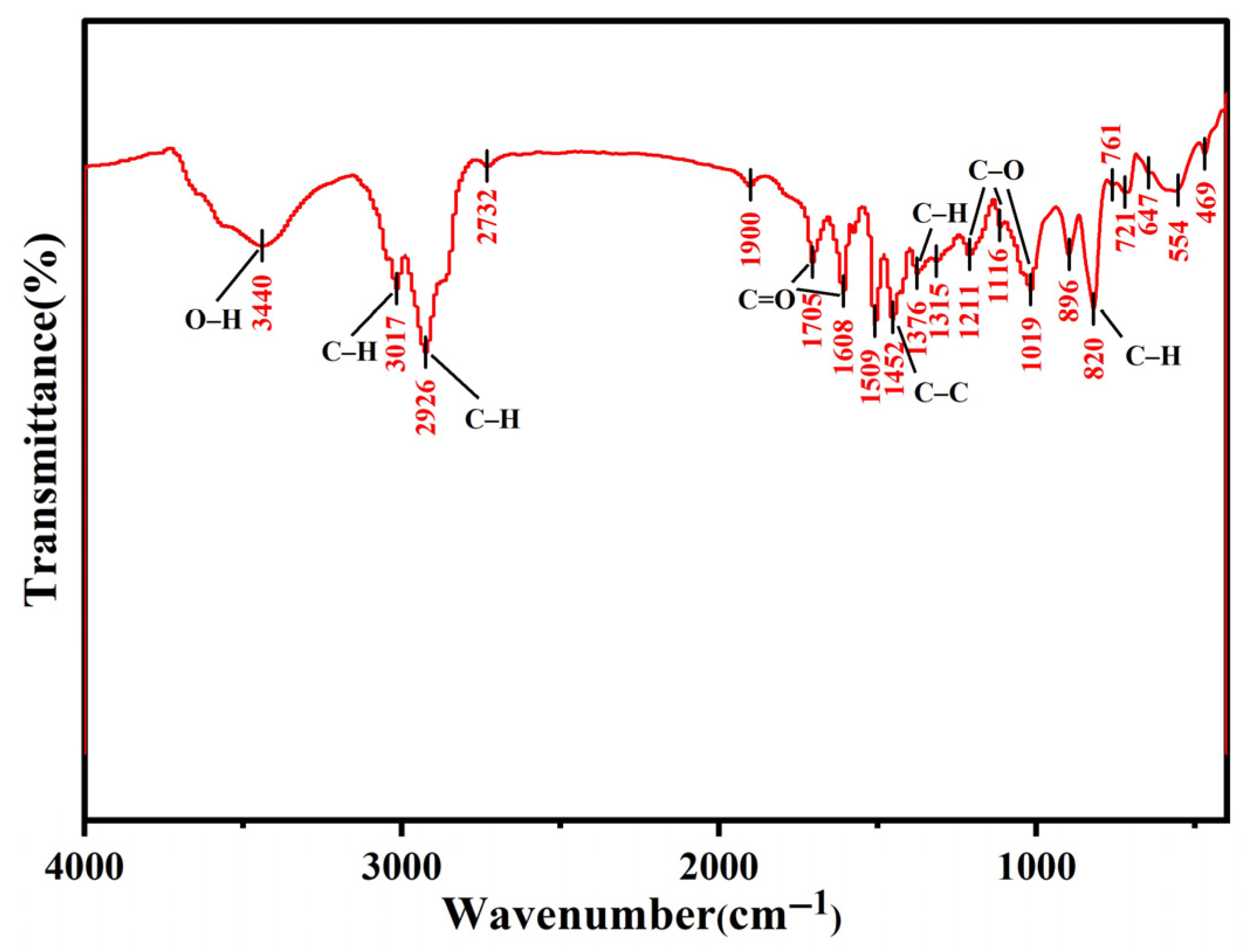
3.1.1. FTIR
3.1.2. XPS
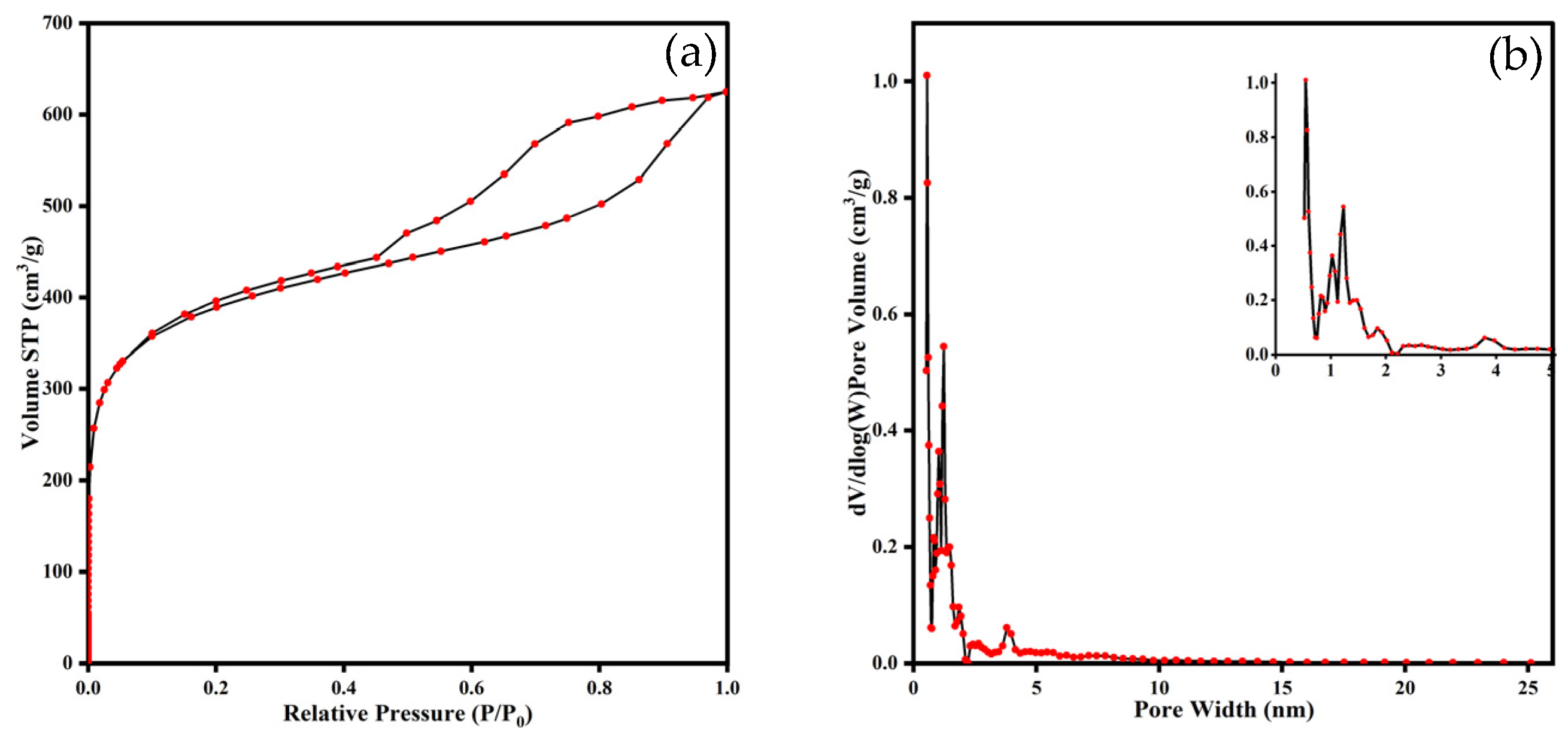
3.1.3. Pore Structure
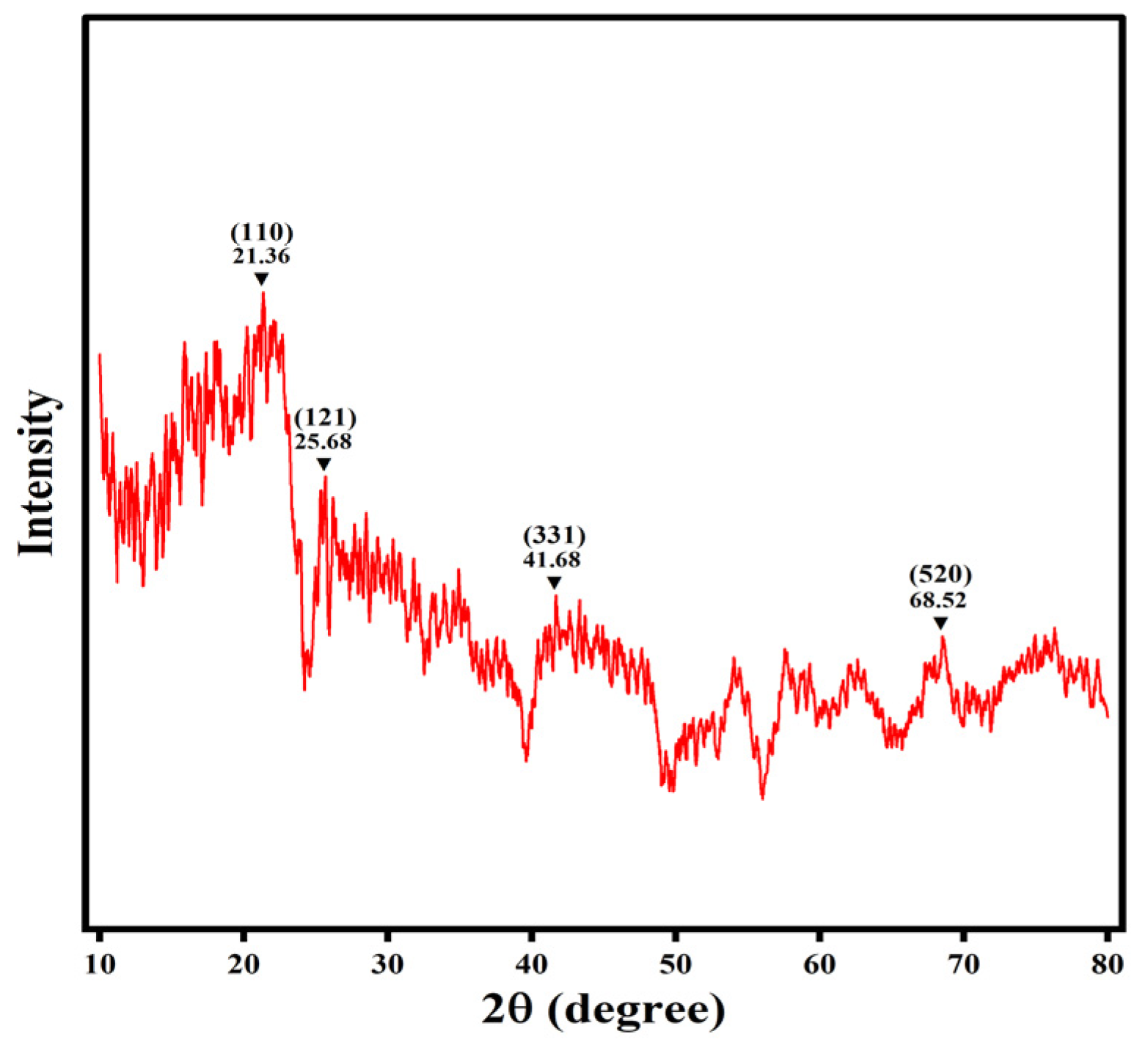
3.1.4. XRD
3.1.5. SEM
3.2. Adsorption Performance
3.2.1. Effect of Resin Dosage
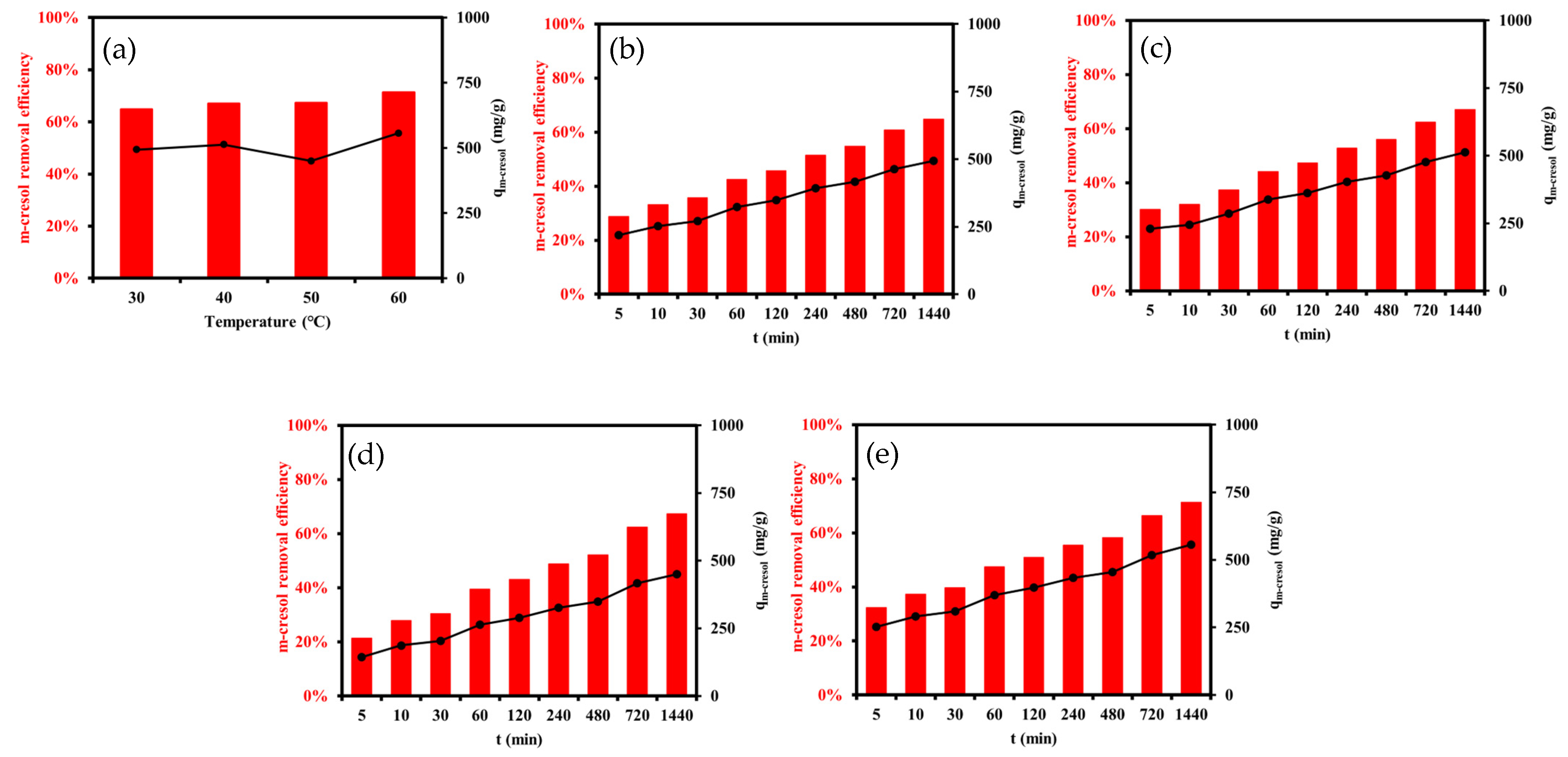
3.2.2. Effects of Time and Temperature
3.2.3. Adsorption Isotherms
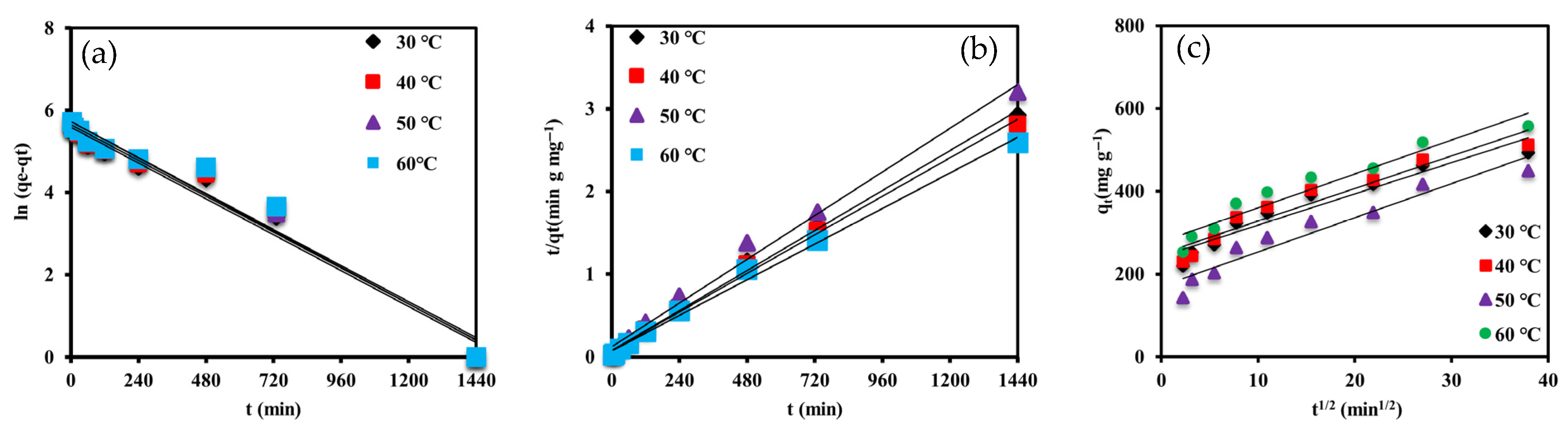
3.2.4. Kinetic Analysis
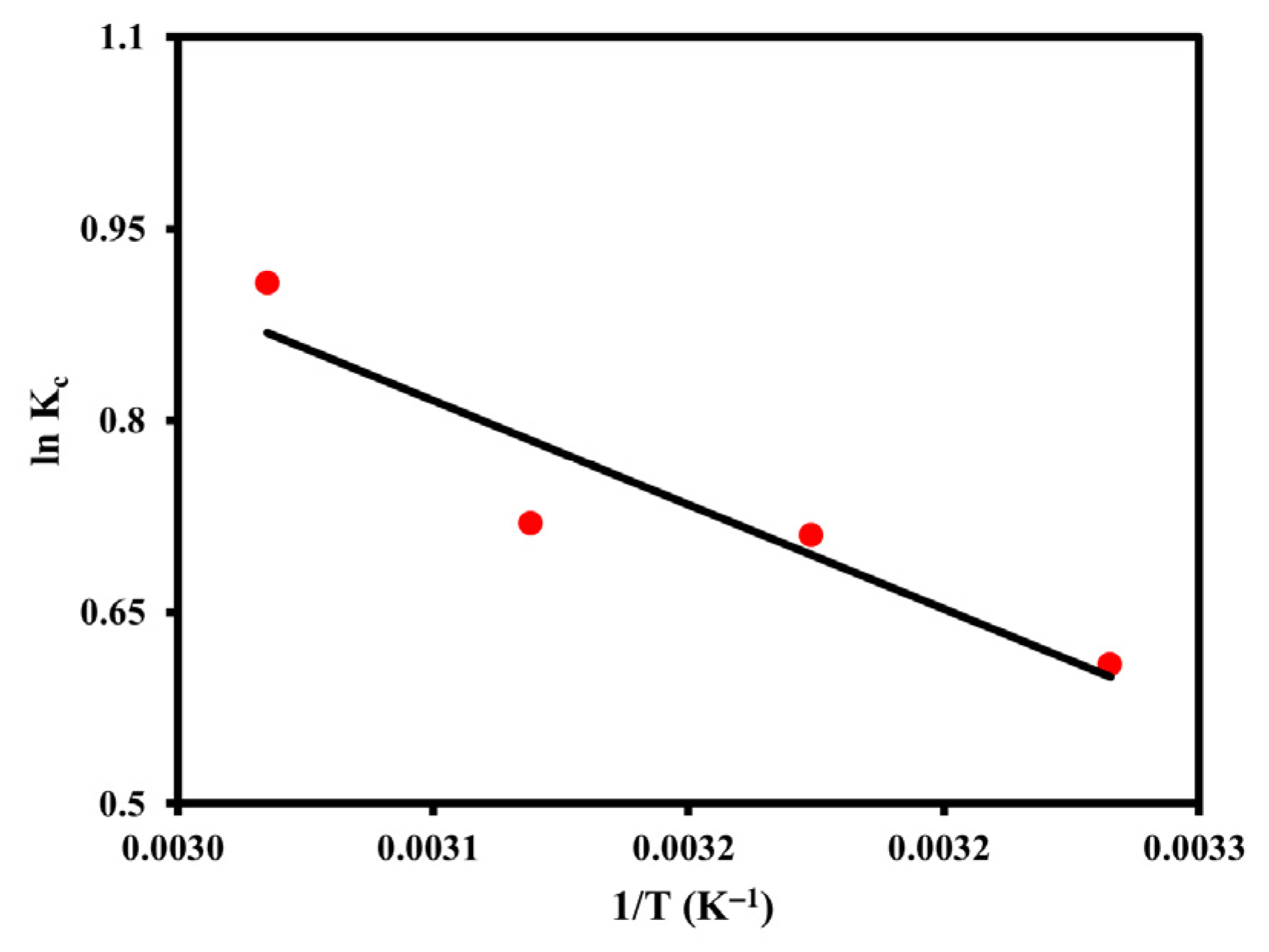
3.2.5. Thermodynamic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Li, X.; Zhu, H.; Xu, X.; Bao, L. Chemical removal of m-cresol: A critical review. Rev. Chem. Eng. 2022, 38, 1023–1044. [Google Scholar] [CrossRef]
- Veeresh, G.S.; Kumar, P.; Mehrotra, I. Treatment of phenol and cresols in upflow anaerobic sludge blanket (UASB) process: A review. Water Res. 2005, 39, 154–170. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Huang, S.; Wu, J.; Xiong, Y.; Liu, F.; Shi, X.; Liao, X.; Xiao, J.; Zhang, S. Analysis of cardiac developmental toxicity induced by m-cresol in early life of zebrafish and its mechanism. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2025, 289, 110123. [Google Scholar] [CrossRef]
- Krastanov, A.; Alexieva, Z.; Yemendzhiev, H. Microbial degradation of phenol and phenolic derivatives. Eng. Life Sci. 2013, 13, 76–87. [Google Scholar] [CrossRef]
- Zou, L.; Wang, Y.; Huang, C.; Li, B.; Lyu, J.; Wang, S.; Lu, H.; Li, J. Meta-cresol degradation by persulfate through UV/O3 synergistic activation: Contribution of free radicals and degradation pathway. Sci. Total Environ. 2021, 754, 142219. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Chen, J.; Zeng, Z.; Huang, Z.; Niu, Y. Periodate activation with polyaniline-derived carbon for bisphenol A degradation: Insight into the roles of nitrogen dopants and non-radical species formation. Chem. Eng. J. 2024, 499, 156077. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X. Systematic method of screening deep eutectic solvents as extractive solvents for m-cresol/cumene separation. Sep. Purif. Technol. 2022, 291, 120853. [Google Scholar] [CrossRef]
- Said, K.A.M.; Ismail, A.F.; Karim, Z.A.; Abdullah, M.S.; Hafeez, A. A review of technologies for the phenolic compounds recovery and phenol removal from wastewater. Process Saf. Environ. Prot. 2021, 151, 257–289. [Google Scholar] [CrossRef]
- Dąbrowski, A.; Podkościelny, P.; Hubicki, Z.; Barczak, M. Adsorption of phenolic compounds by activated carbon—A critical review. Chemosphere 2005, 58, 1049–1070. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Adsorption of phenolic compounds on low-cost adsorbents: A review. Adv. Colloid Interface Sci. 2008, 143, 48–67. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Kolya, H.; Kang, C.-W. Bio-based polymeric flocculants and adsorbents for wastewater treatment. Sustainability 2023, 15, 9844. [Google Scholar] [CrossRef]
- Gu, C.; Yan, K.; Bo, L.; Zhou, X.; He, Y.; Feng, J.; Qin, J. Alkaline Prehydrolysis Prolongs Resin Life and Enhances the Adsorption of Phenolic Compounds. Water 2023, 15, 2566. [Google Scholar] [CrossRef]
- Yu, Y.; Zhuang, Y.-Y.; Wang, Z.-H. Adsorption of water-soluble dye onto functionalized resin. J. Colloid Interface Sci. 2001, 242, 288–293. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Vavouraki, A.I.; Kornaros, M.E.; Paraskeva, C.A. Purification of olive mill wastewater phenols through membrane filtration and resin adsorption/desorption. J. Hazard. Mater. 2015, 285, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Yue, J.; Shan, L.; Tao, G.; Wang, X.; Qiu, A. Process research of macroporous resin chromotography for separation of N-(p-coumaroyl) serotonin and N-feruloylserotonin from Chinese safflower seed extracts. Sep. Purif. Technol. 2008, 62, 370–375. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Z.; Xi, H. Influence of the microporosity and surface chemistry of polymeric resins on adsorptive properties toward phenol. J. Hazard. Mater. 2004, 113, 131–135. [Google Scholar] [CrossRef]
- Liu, F.-Q.; Xia, M.-F.; Yao, S.-L.; Li, A.-M.; Wu, H.-S.; Chen, J.-L. Adsorption equilibria and kinetics for phenol and cresol onto polymeric adsorbents: Effects of adsorbents/adsorbates structure and interface. J. Hazard. Mater. 2008, 152, 715–720. [Google Scholar] [CrossRef]
- Deng, C.; Liu, Y.; Yang, D.; Cao, X.; Gai, H.; Xiao, M.; Huang, T.; Zhu, Q.; Song, H. Synthesis of poly (ionic liquid) s with high specific surface areas for m-cresol adsorption. Microporous Mesoporous Mater. 2023, 362, 112778. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Yang, L.; Zhao, Y.; Zhang, X.; Shen, R.; Sun, D.; Qian, J. Film-like bacterial cellulose based molecularly imprinted materials for highly efficient recognition and adsorption of cresol isomers. Chem. Eng. J. 2020, 382, 123007. [Google Scholar] [CrossRef]
- Chen, D.; Liu, F.; Zong, L.; Sun, X.; Zhang, X.; Zhu, C.; Tao, X.; Li, A. Integrated adsorptive technique for efficient recovery of m-cresol and m-toluidine from actual acidic and salty wastewater. J. Hazard. Mater. 2016, 312, 192–199. [Google Scholar] [CrossRef]
- Zorba, T.; Papadopoulou, E.; Hatjiissaak, A.; Paraskevopoulos, K.; Chrissafis, K. Urea-formaldehyde resins characterized by thermal analysis and FTIR method. J. Therm. Anal. Calorim. 2008, 92, 29–33. [Google Scholar] [CrossRef]
- Kapadiya, P.; Adhikari, J. Prediction of thermodynamic properties of m-cresol: Comparison of TraPPE-UA and OPLS-AA force fields. J. Mol. Liq. 2025, 425, 127211. [Google Scholar] [CrossRef]
- González, M.G.; Cabanelas, J.C.; Baselga, J. Applications of FTIR on epoxy resins-identification, monitoring the curing process, phase separation and water uptake. Infrared Spectrosc. -Mater. Sci. Eng. Technol. 2012, 2, 261–284. [Google Scholar]
- Delgado, A.H.; Young, A.M. Modelling ATR-FTIR spectra of dental bonding systems to investigate composition and polymerisation kinetics. Materials 2021, 14, 760. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, S.; Kumar, A.; Lai, C.W.; Naushad, M.; Shehnaz; Iqbal, J.; Stadler, F.J. Activated carbon as superadsorbent and sustainable material for diverse applications. Adsorpt. Sci. Technol. 2022, 2022, 4184809. [Google Scholar] [CrossRef]
- Raymundo-Pinero, E.; Cazorla-Amorós, D.; Linares-Solano, A.; Find, J.; Wild, U.; Schlögl, R. Structural characterization of N-containing activated carbon fibers prepared from a low softening point petroleum pitch and a melamine resin. Carbon 2002, 40, 597–608. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Z.; Qian, M.; Zhang, H.; Xie, H.; Chen, C. Effect of 10-methacryloxydecyl dihydrogen phosphate concentration on chemical coupling of methacrylate resin to yttria-stabilized zirconia. J. Adhes. Dent. 2017, 19, 349–355. [Google Scholar]
- Winer, K.; Colmenares, C.; Smith, R.; Wooten, F. Interaction of water vapor with clean and oxygen-covered uranium surfaces. Surf. Sci. 1987, 183, 67–99. [Google Scholar] [CrossRef]
- Horikawa, T.; Do, D.; Nicholson, D. Capillary condensation of adsorbates in porous materials. Adv. Colloid Interface Sci. 2011, 169, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Groen, J.C.; Peffer, L.A.; Pérez-Ramírez, J. Pore size determination in modified micro-and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Walton, K.S.; Snurr, R.Q. Applicability of the BET method for determining surface areas of microporous metal–organic frameworks. J. Am. Chem. Soc. 2007, 129, 8552–8556. [Google Scholar] [CrossRef] [PubMed]
- Galarneau, A.; Villemot, F.; Rodriguez, J.; Fajula, F.; Coasne, B. Validity of the t-plot method to assess microporosity in hierarchical micro/mesoporous materials. Langmuir 2014, 30, 13266–13274. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Ko, T.H.; Kuo, W.S.; Chang, Y.H. Microstructural changes of phenolic resin during pyrolysis. J. Appl. Polym. Sci. 2001, 81, 1084–1089. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Zong, J.; Shu, Z.; Xiao, Q.; Wang, X.; Zhou, F.; Huang, J. Acetamido-functionalized hyper-crosslinked polymers for efficient removal of phenol in aqueous solution. Sep. Purif. Technol. 2022, 287, 120566. [Google Scholar] [CrossRef]
- Pasquet, P.; Bertagnolli, C.; Villain-Gambier, M.; Trébouet, D. Investigation of phenolic compounds recovery from brewery wastewater with coupled membrane and adsorption process. J. Environ. Chem. Eng. 2024, 12, 112478. [Google Scholar] [CrossRef]
- Jones, I.; Zhu, M.; Zhang, J.; Zhang, Z.; Preciado-Hernandez, J.; Gao, J.; Zhang, D. The application of spent tyre activated carbons as low-cost environmental pollution adsorbents: A technical review. J. Clean. Prod. 2021, 312, 127566. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Tellinghuisen, J. Van’t Hoff analysis of K°(T): How good… or bad? Biophys. Chem. 2006, 120, 114–120. [Google Scholar] [CrossRef]
- Liu, Y. Some consideration on the Langmuir isotherm equation. Colloids Surf. A: Physicochem. Eng. Asp. 2006, 274, 34–36. [Google Scholar] [CrossRef]
- Ghosal, P.S.; Gupta, A.K. Determination of thermodynamic parameters from Langmuir isotherm constant-revisited. J. Mol. Liq. 2017, 225, 137–146. [Google Scholar] [CrossRef]
- Chu, K.H. Revisiting the Temkin isotherm: Dimensional inconsistency and approximate forms. Ind. Eng. Chem. Res. 2021, 60, 13140–13147. [Google Scholar] [CrossRef]
- Araújo, C.S.; Almeida, I.L.; Rezende, H.C.; Marcionilio, S.M.; Léon, J.J.; de Matos, T.N. Elucidation of mechanism involved in adsorption of Pb (II) onto lobeira fruit (Solanum lycocarpum) using Langmuir, Freundlich and Temkin isotherms. Microchem. J. 2018, 137, 348–354. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Singh, S.K.; Townsend, T.G.; Mazyck, D.; Boyer, T.H. Equilibrium and intra-particle diffusion of stabilized landfill leachate onto micro-and meso-porous activated carbon. Water Res. 2012, 46, 491–499. [Google Scholar] [CrossRef]
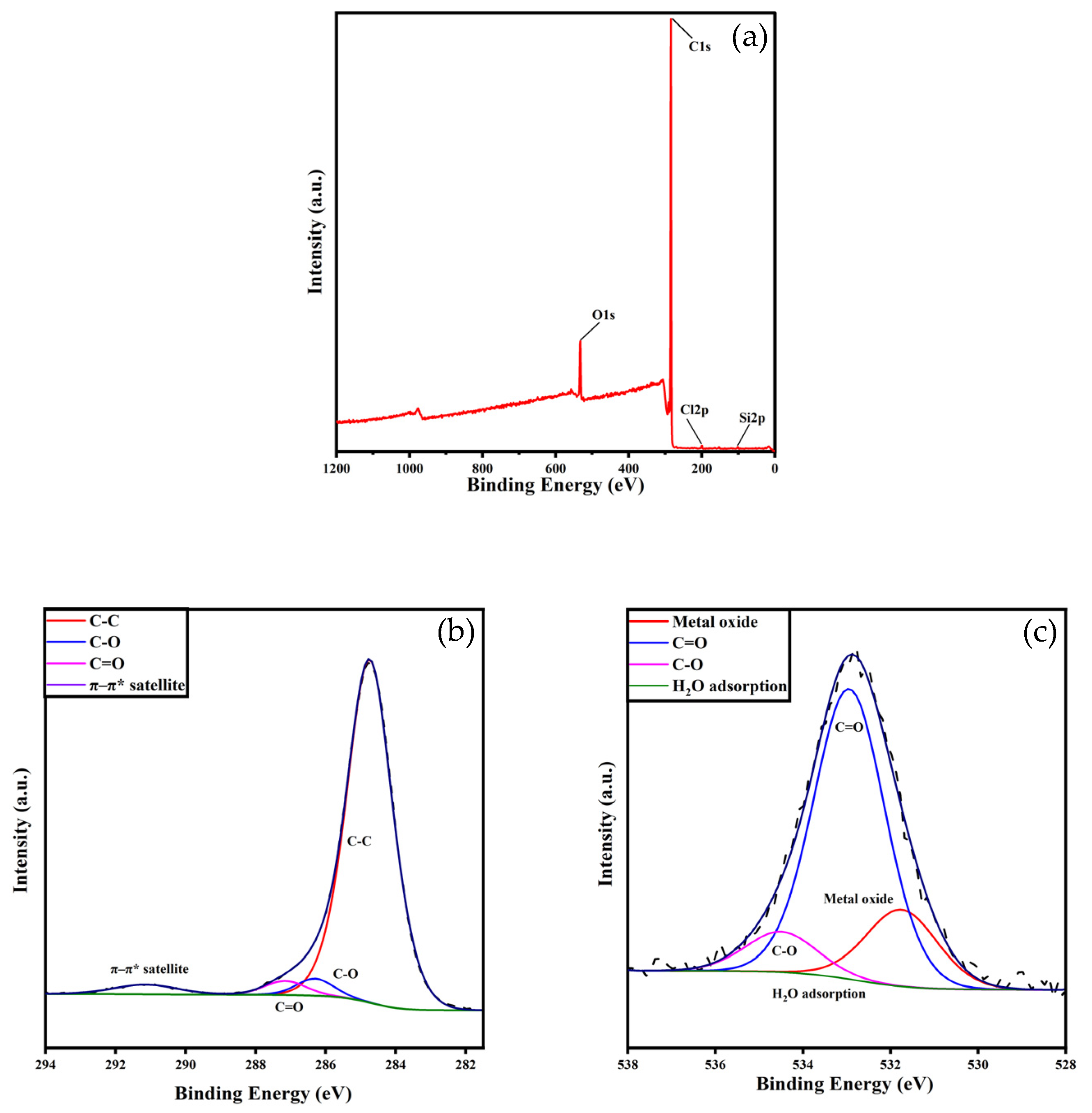
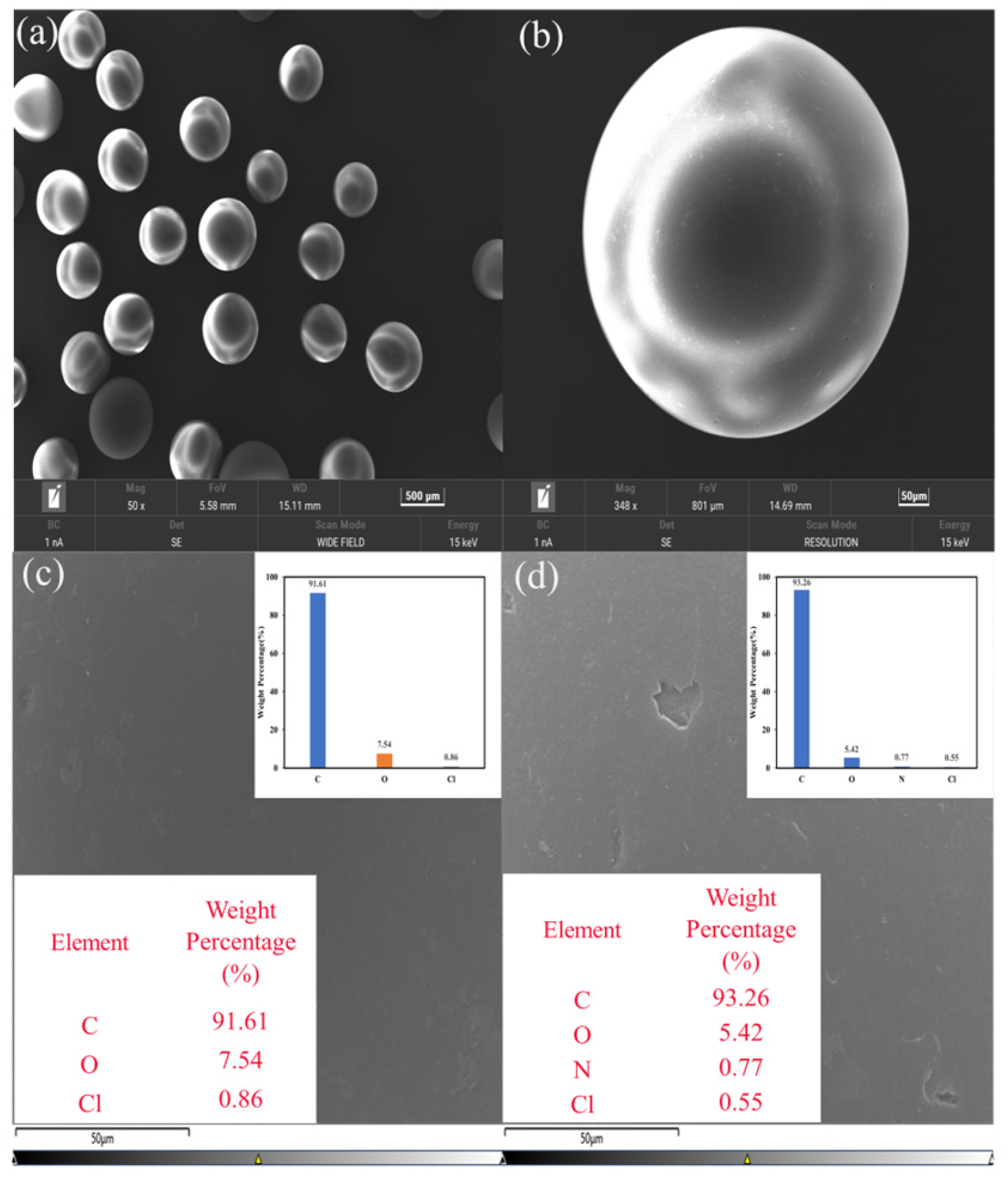
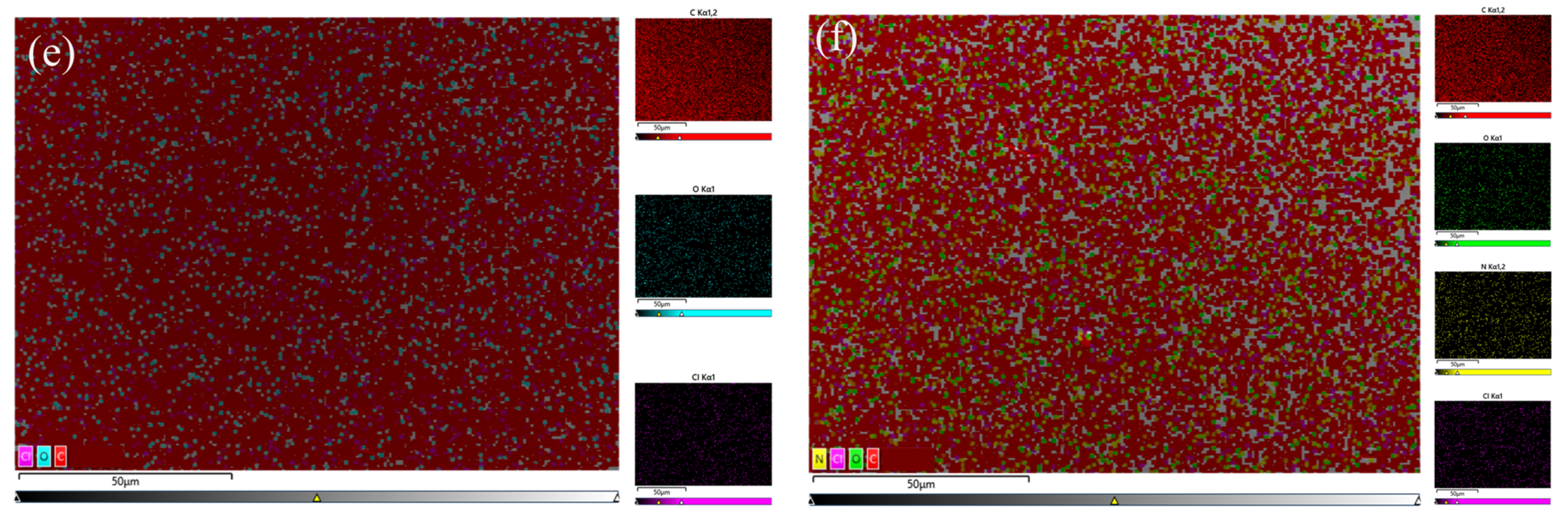

| Parameters | Binding Energy (eV) | Relative Content (%) |
|---|---|---|
| Surface concentration (at %) | ||
| C | 91.71 | |
| O | 7.12 | |
| Cl | 0.48 | |
| Si | 0.69 | |
| C surface concentration (at %) | ||
| C-C | 284.8 | 89.84 |
| C-O | 286.3 | 1.96 |
| C=O | 286.9 | 4.79 |
| π-π* satellite | 291.2 | 3.41 |
| O surface concentration (at %) | ||
| C=O | 531.8 | 18.82 |
| C-O | 532.9 | 71.23 |
| H2O adsorption | 534.5 | 9.95 |
| BET Surface Areas (m2 g−1) | t-Plot Micropore Surface Areas (m2 g−1) | BJH Adsorption Surface Areas (m2 g−1) | BJH Desorption Surface Areas (m2 g−1) | DFT Pore Volume (cm3 g−1) | DFT Surface Areas (m2 g−1) |
|---|---|---|---|---|---|
| 1439 | 611.0 | 177.6 | 106.5 | 0.404 | 1093 |
| Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|
| qmax (mg g−1) | b (L mg −1) | R2 | KF (mg g−1) (L mg−1)−1/n | 1/n | R2 | k1 (mg g −1) | k2 (L mg−1) | R2 |
| 833.3 | 0.3077 | 0.9953 | 9.636 | 0.5607 | 0.9501 | 173.8 | 3.523 | 0.9753 |
| Category | Parameter | Unit | 30 °C | 40 °C | 50 °C | 60 °C |
|---|---|---|---|---|---|---|
| PFO | k1 | min−1 | 0.0036 | 0.0036 | 0.0037 | 0.0037 |
| qe | mg g−1 | 267.1 | 284.9 | 306.5 | 307.6 | |
| R2 | 0.9718 | 0.9633 | 0.9627 | 0.9558 | ||
| PSO | k2 | g mg−1 min−1 | 5.270 × 10−5 | 4.769 × 10−5 | 4.027 × 10−5 | 4.583 × 10−5 |
| qe | mg g−1 | 500 | 526.3 | 454.5 | 555.6 | |
| R2 | 0.9963 | 0.9957 | 0.9915 | 0.9948 | ||
| IPD | kp | g mg−1 min−1/2 | 7.551 | 7.883 | 8.255 | 8.187 |
| C | mg g−1 | 242.5 | 248.6 | 170.6 | 277.9 | |
| R2 | 0.9223 | 0.9216 | 0.9257 | 0.9274 |
| T (K) | Kc | ΔGv (kJ mol−1) | ΔH (kJ mol−1) | ΔS (J K−1 mol−1) |
|---|---|---|---|---|
| 303.15 | 1.839 | −1.542 | 9.090 | 35.09 |
| 313.15 | 2.035 | −1.893 | ||
| 323.15 | 2.441 | −2.244 | ||
| 333.15 | 2.479 | −2.595 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Z.; Liu, Z.; He, X.; Zeng, Z. Mechanistic Insights into m-Cresol Adsorption on Functional Resins: Surface Chemistry and Adsorption Behavior. Materials 2025, 18, 3628. https://doi.org/10.3390/ma18153628
Wang Y, Wang Z, Liu Z, He X, Zeng Z. Mechanistic Insights into m-Cresol Adsorption on Functional Resins: Surface Chemistry and Adsorption Behavior. Materials. 2025; 18(15):3628. https://doi.org/10.3390/ma18153628
Chicago/Turabian StyleWang, Yali, Zhenrui Wang, Zile Liu, Xiyue He, and Zequan Zeng. 2025. "Mechanistic Insights into m-Cresol Adsorption on Functional Resins: Surface Chemistry and Adsorption Behavior" Materials 18, no. 15: 3628. https://doi.org/10.3390/ma18153628
APA StyleWang, Y., Wang, Z., Liu, Z., He, X., & Zeng, Z. (2025). Mechanistic Insights into m-Cresol Adsorption on Functional Resins: Surface Chemistry and Adsorption Behavior. Materials, 18(15), 3628. https://doi.org/10.3390/ma18153628





