The Influence of the Annealing Process on the Mechanical Properties of Chromium Nitride Thin Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Thin Films Deposition
2.2. Characterization
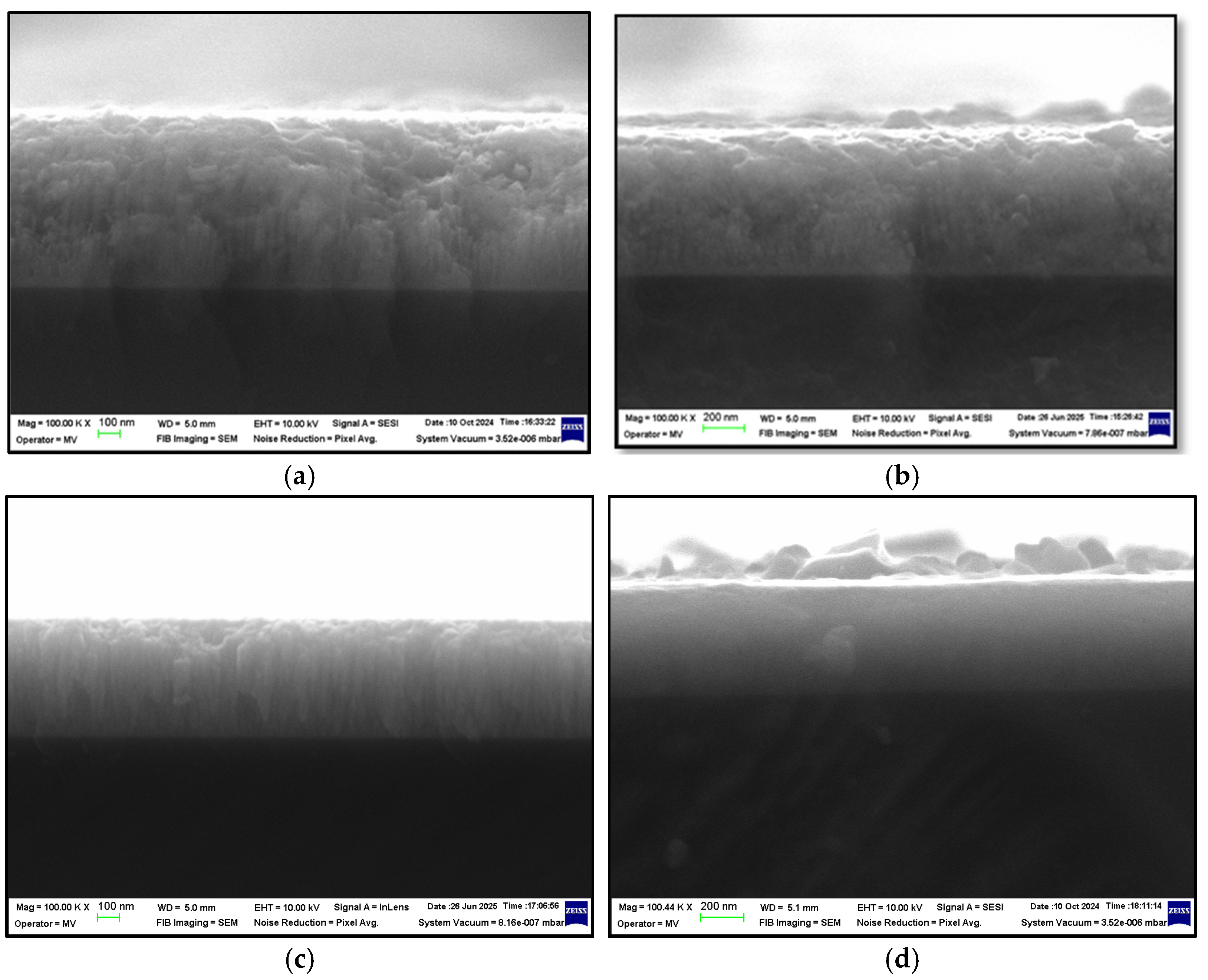
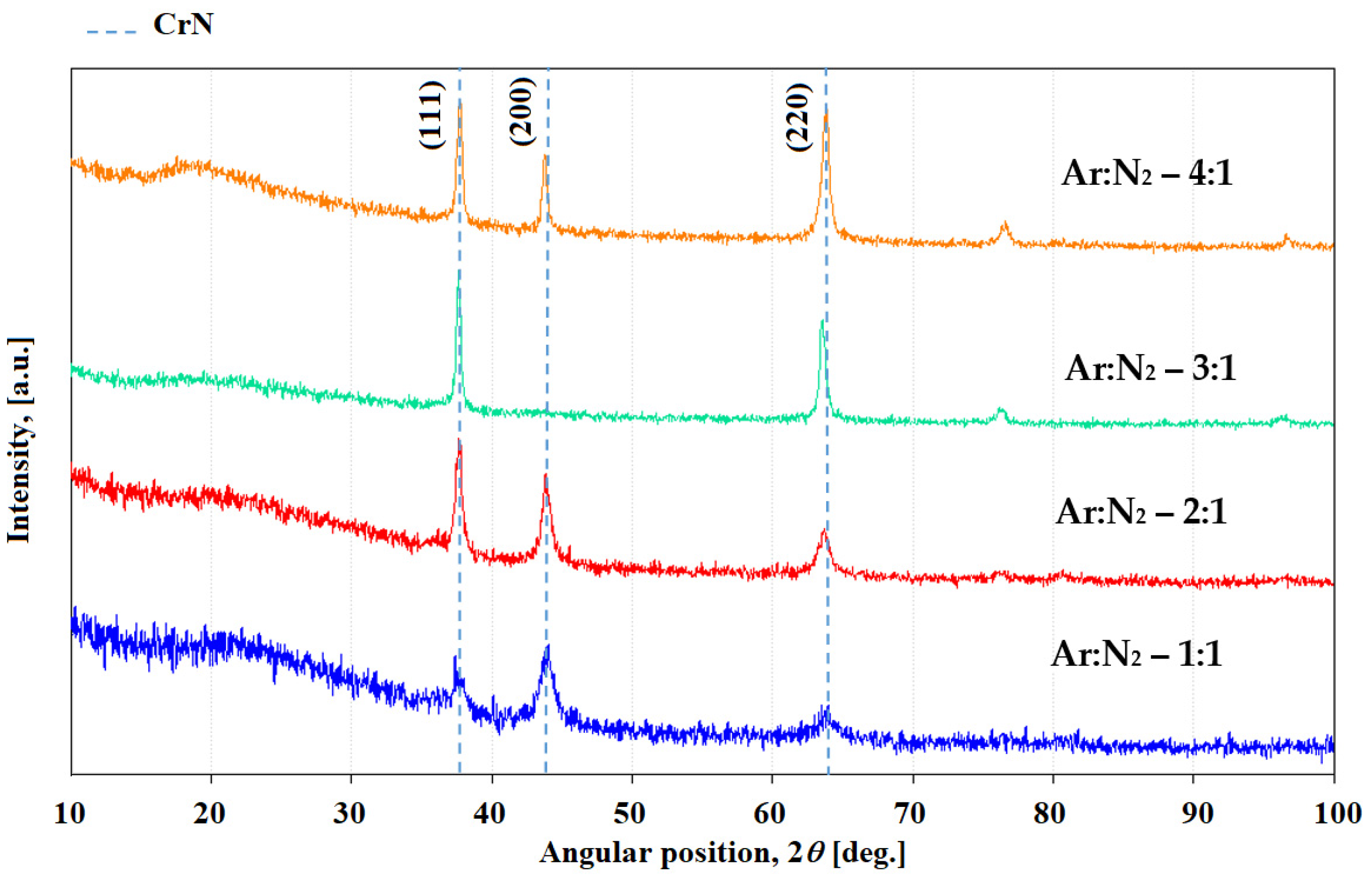
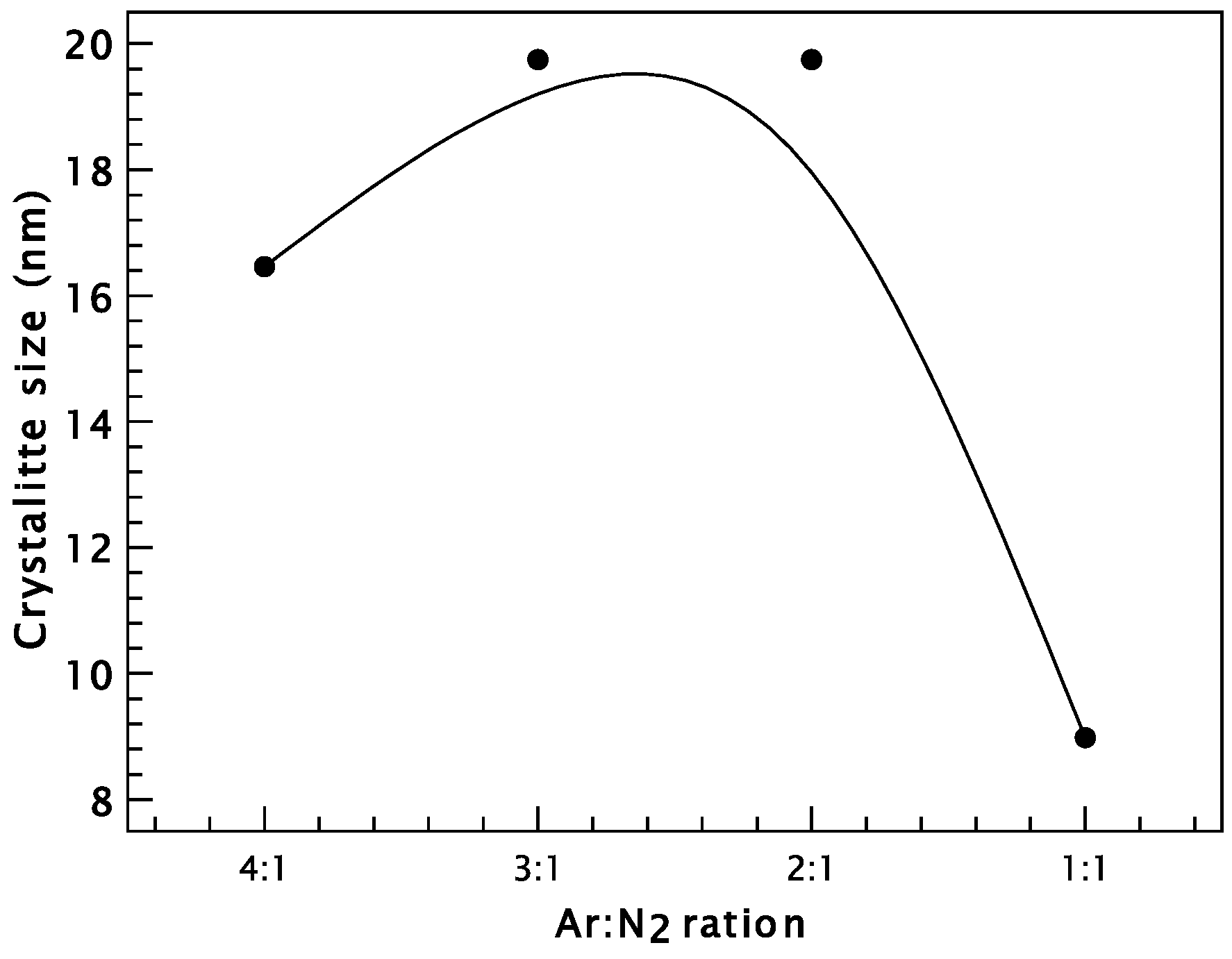
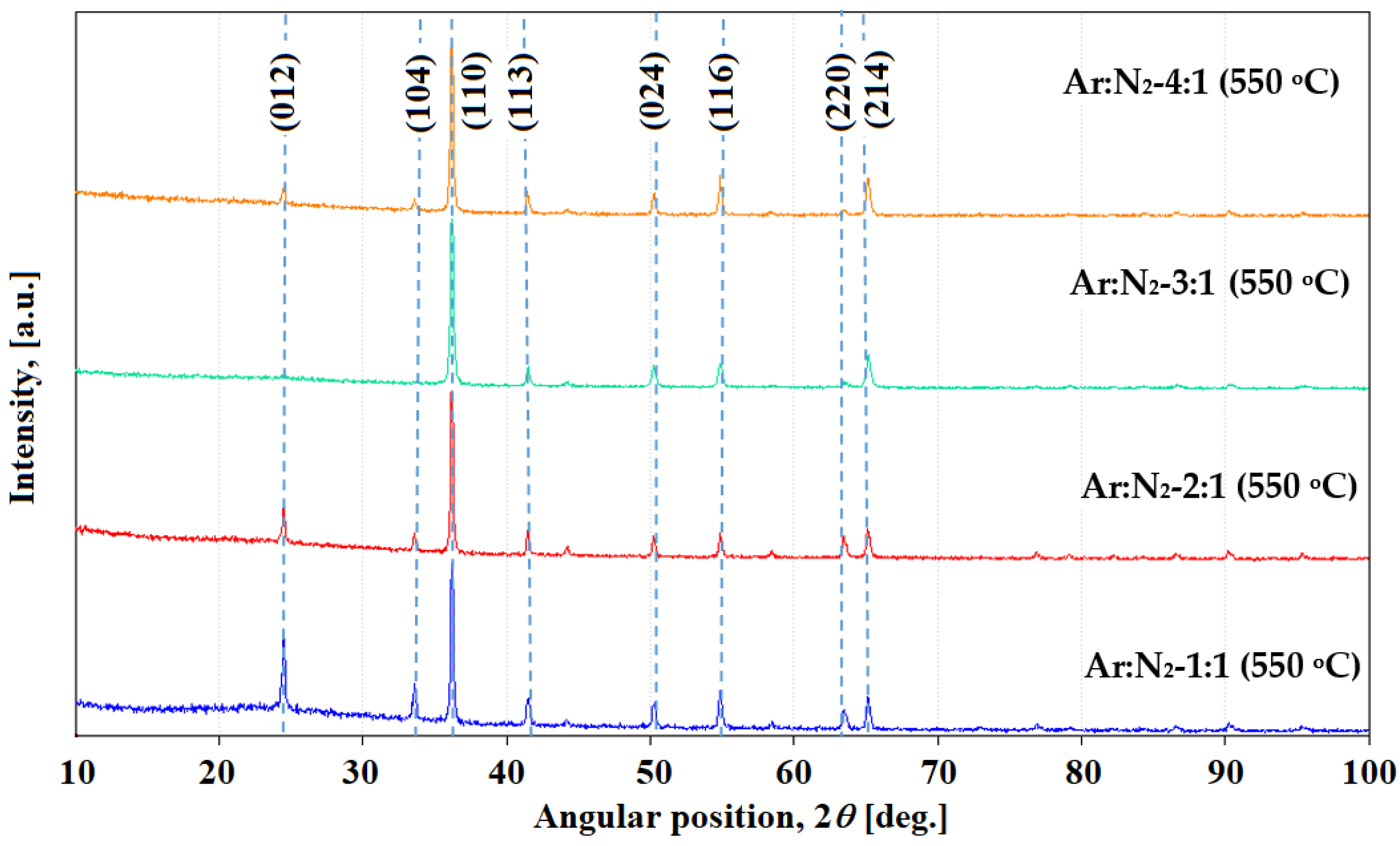
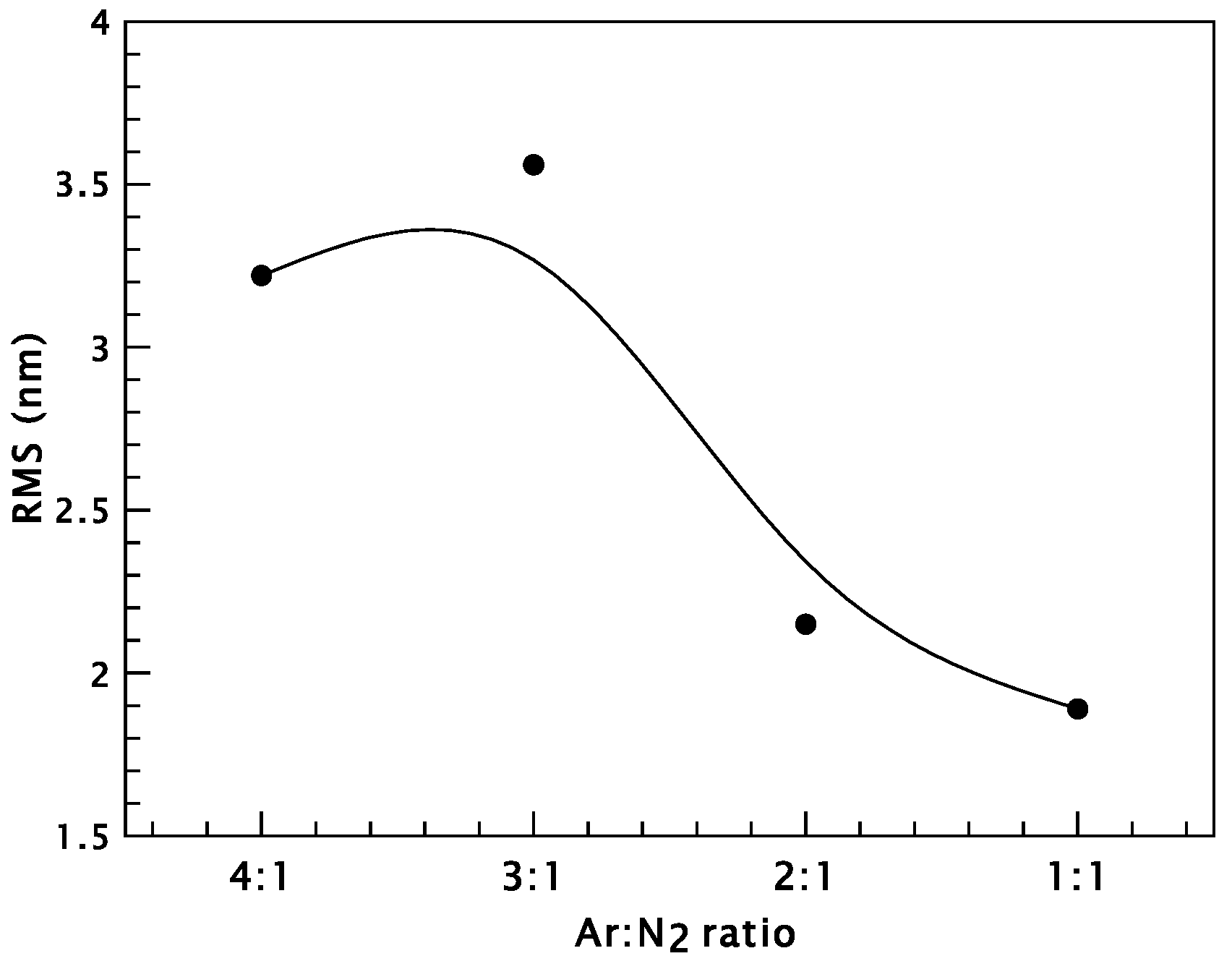
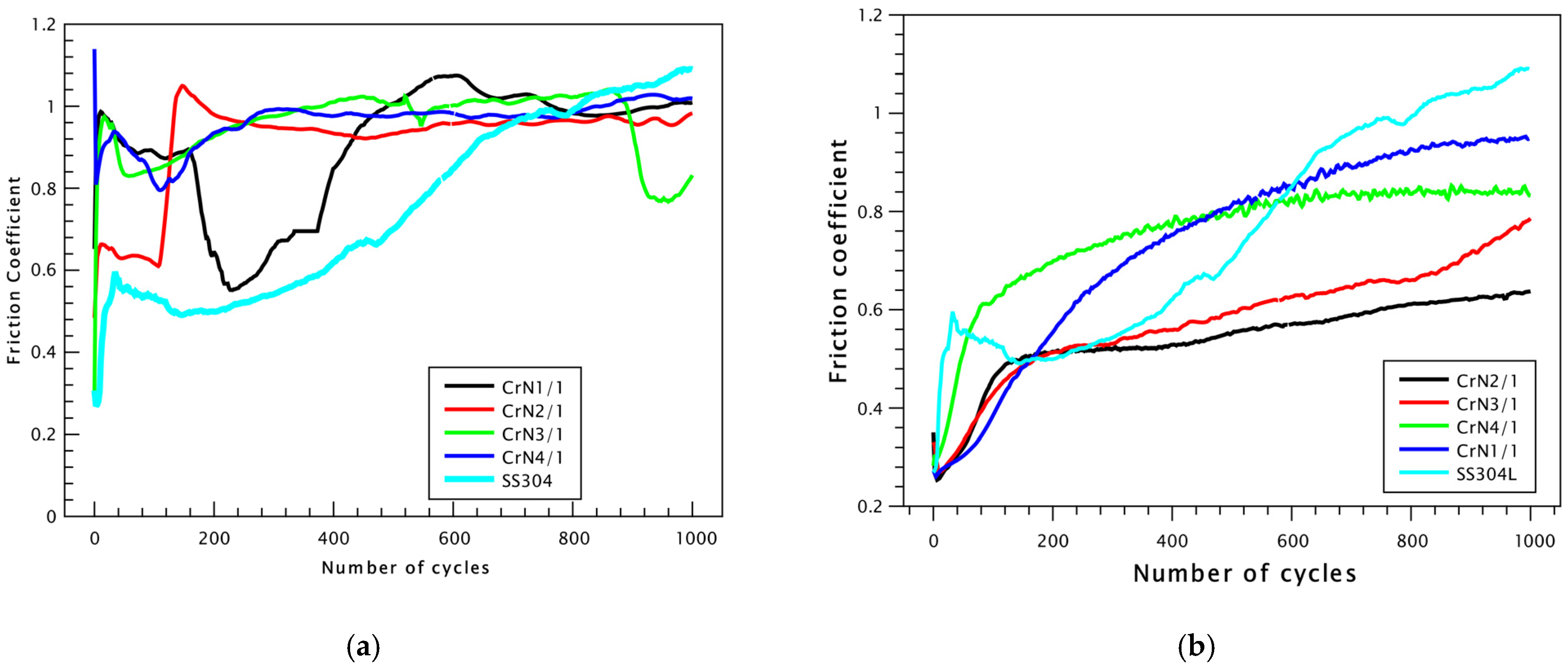
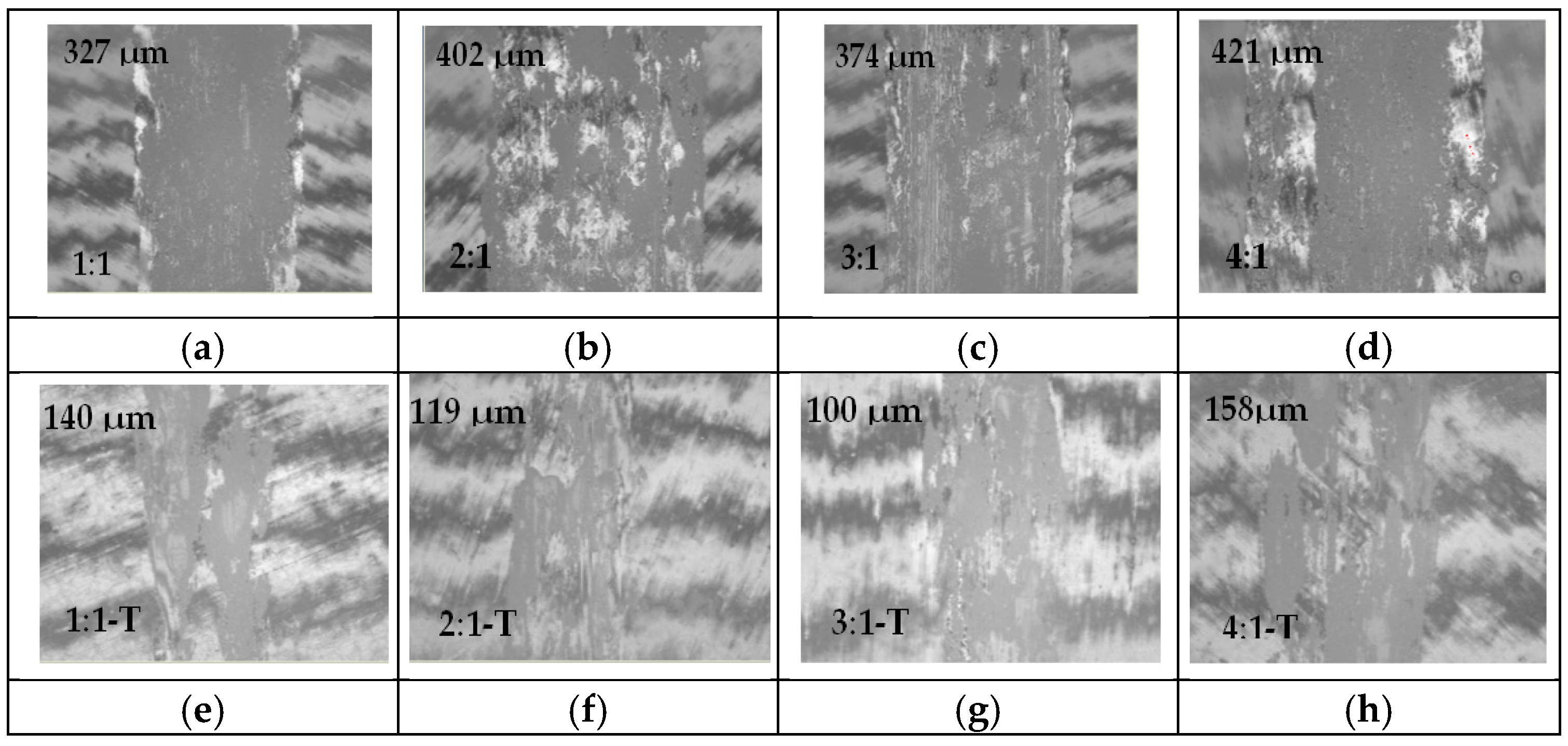
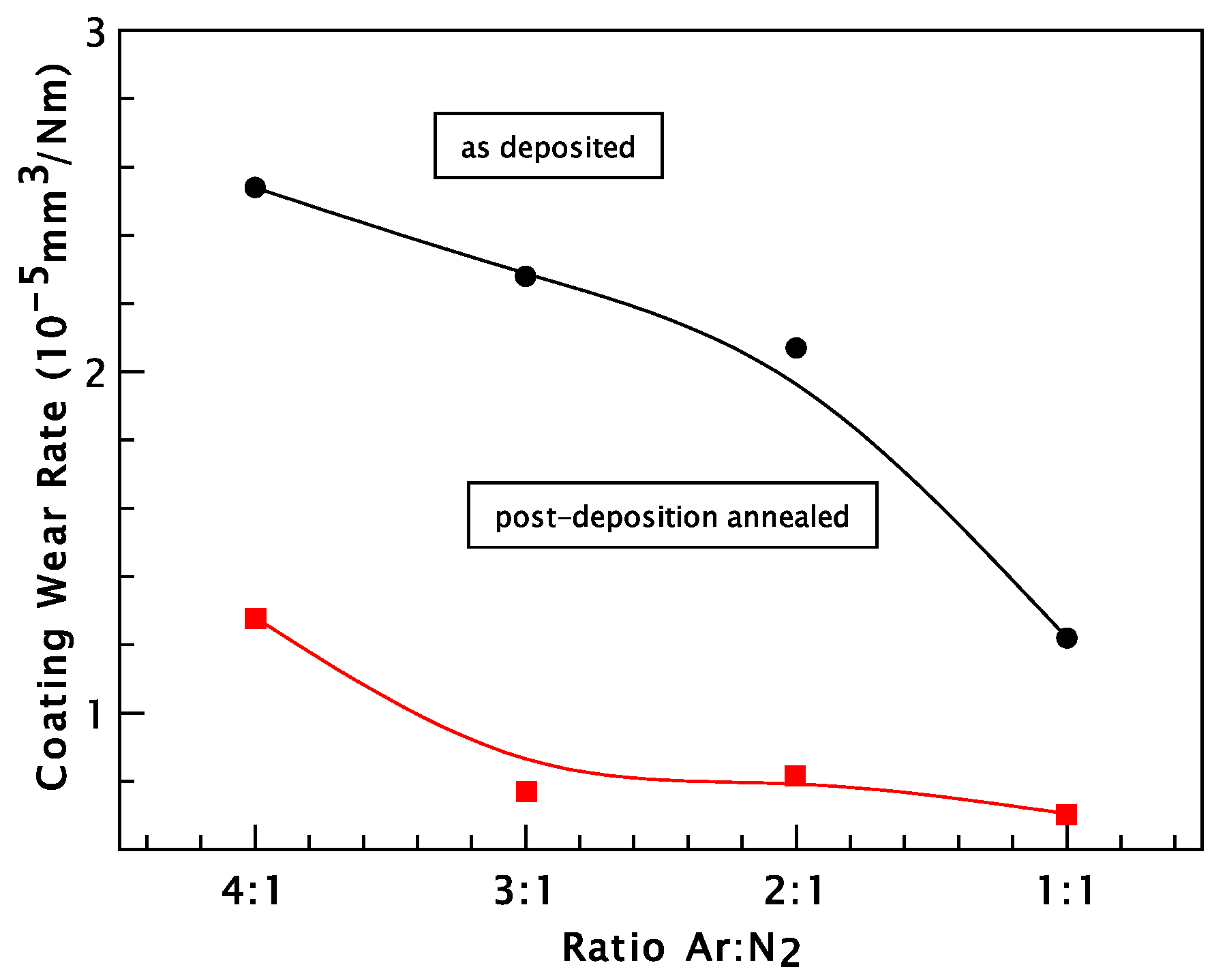
3. Results and Discussion
Chromium Nitride Thin Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veprek, S. The search for novel, superhard materials. J. Vac. Sci. Technol. A 1999, 17, 2401–2420. [Google Scholar] [CrossRef]
- Holmberg, K.; Matthews, A. Coatings Tribology: Properties, Mechanisms, Techniques and Applications in Surface Engineering; Elsevier: Amsterdam, The Netherlands, 2009; Volume 56. [Google Scholar]
- Greene, J.E. Tracing the Recorded History of Thin-Film Sputter Deposition: From the 1800s to 2017. J. Vac. Sci. Technol. A 2017, 35, 05C204. [Google Scholar] [CrossRef]
- Meneve, J. Advanced thin film technologies for industrial applications. Thin Solid Films 2020, 709, 138272. [Google Scholar]
- Vilhena, L.; Ferreira, F.; Oliveira, J.C.; Ramalho, A. Rapid and Easy Assessment of Friction and Load-Bearing Capacity in Thin Coatings. Electronics 2022, 11, 296. [Google Scholar] [CrossRef]
- Stoiber, M.; Badisch, E.; Lugmair, C.; Mitterer, C. Low-friction TiN coatings deposited by PACVD. Surf. Coat. Technol. 2003, 163–164, 451–456. [Google Scholar] [CrossRef]
- Yoshio, Y.; Shibata, T.; Itabashi, M. Formation of chromium nitride films by nitridation of electroplated chromium. Surf. Coat. Technol. 1995, 76–77, 474–479. [Google Scholar]
- Rie, K.T. Chromium coatings: Electroplating. Surf. Coat. Technol. 2000, 125, 1–10. [Google Scholar]
- European Parliament and Council. Directive 2012/19/EU on Waste Electrical and Electronic Equipment (WEEE). Off. J. Eur. Union 2012, L 197, 38–71. [Google Scholar]
- European Parliament and Council. Directive 2011/65/EU on the Restriction of Hazardous Substances (RoHS) in Electrical and Electronic Equipment. Off. J. Eur. Union 2011, L 174, 88–110. [Google Scholar]
- U.S. Environmental Protection Agency (EPA). Integrated Risk Information System (IRIS) on Hexavalent Chromium; EPA: Washington, DC, USA, 1998. [Google Scholar]
- Carlsson, J.-O.; Martin, P.M. Chemical Vapor Deposition. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Martin, P.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2010; pp. 314–363. [Google Scholar]
- Ghazal, H.; Sohail, N. Sputtering Deposition. In Thin Films—Deposition Methods and Applications; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Ohring, M. Materials Science of Thin Films, 2nd ed.; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Paulitsch, J.; Mayrhofer, P.H.; Münz, W.-D.; Schenkel, M. Structure and Mechanical Properties of CrN/TiN Multilayer Coatings Prepared by a Combined HIPIMS/UBMS Deposition Technique. Thin Solid Films 2008, 517, 1239–1244. [Google Scholar] [CrossRef]
- Kelly, P.J.; Arnell, R.D. Magnetron sputtering: A review of recent developments and applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Musil, J. Flexible design of nanocomposite coatings. Surf. Coat. Technol. 2012, 207, 50–65. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M. Review of growth defects in thin films prepared by PVD techniques. Coatings 2020, 10, 447. [Google Scholar] [CrossRef]
- Chowdhury, S.; Hjort, V.; Shu, R.; Greczynski, G.; le Febvrier, A.; Eklund, P.; Magnuson, M. Thermoelectric Properties and Electronic Structure of Cr(Mo,V)Nx Thin Films Studied by Synchrotron and Lab-Based X-ray Spectroscopy. Phys. Rev. B 2023, 108, 205134. [Google Scholar] [CrossRef]
- Tang, P.H.; Song, R.G.; Chai, G.Z.; Mao, J. Microstructure and nanoindentation hardness of TiN/AlN multilayer films prepared by pulsed laser deposition. Surf. Eng. 2012, 28, 165–170. [Google Scholar] [CrossRef]
- Lackner, J.M.; Waldhauser, W.; Major, L.; Kot, M. Tribology and micromechanics of chromium nitride based multilayer coatings on soft and hard substrates. Coatings 2014, 4, 121–138. [Google Scholar] [CrossRef]
- Ul-Hami, A. The effect of deposition conditions on the properties of Zr-carbide, Zr-nitride and Zr-carbonitride coatings—A review. Mater. Adv. 2020, 1, 988–1011. [Google Scholar] [CrossRef]
- Liao, Y.-H.; Wu, F.-B. Microstructure evolution and mechanical properties of refractory molybdenum-tungsten nitride coatings. Surf. Coat. Technol. 2024, 476, 130154. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, S.; Zheng, J.; Dong, Y.; Zhang, C.; Li, J.; Chen, Z.; Zhang, J.; Sun, D. Excellent anti-corrosion and conductivity of NbN coated on Ti bipolar plate by controlling N2 flow rates. J. Alloys Compd. 2024, 976, 173033. [Google Scholar] [CrossRef]
- Zin, V.; Montagner, F.; Deambrosis, S.M.; Mortalò, C.; Litti, L.; Meneghetti, M.; Miorin, E. Mechanical and tribological properties of Ta-N and Ta-Al-N coatings deposited by reactive high power impulse magnetron sputtering. Materials 2022, 15, 3354. [Google Scholar] [CrossRef]
- Smith, J.; Brown, A.; Wang, L. Mechanical and thermal properties of transition metal nitrides. J. Mater. Sci. 2020, 55, 4893–4902. [Google Scholar]
- Lee, M.; Kim, K. Dry deposition techniques for chromium nitride thin films. Thin Solid Films 2019, 680, 45–52. [Google Scholar]
- Johnson, P. Environmental impacts of hexavalent chromium in electrochemical processes. Environ. Sci. Technol. 2018, 53, 3456–3465. [Google Scholar]
- Aubert, A.; Danroc, J.; Gaucher, A.; Terrat, J.P. Hard chrome and molybdenum coatings produced by physical vapour deposition. Thin Solid Films 1985, 126, 61–67. [Google Scholar] [CrossRef]
- ASTM G99-17; Test Method for Wear Testing with a Pin-on-Disk Apparatus. ASTM: West Conshohocken, PA, USA, 2023.
- Silcotek. Wear and Friction Analysis of Thin Coatings. Available online: https://www.silcotek.com/hs-fs/hub/22765/file-341679011-pdf/docs/t-09-113_silcotek_tribology_testing_final_report.pdf (accessed on 4 July 2024).
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic Modulus Using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- ISO 14577-1:2015; Metallic Materials—Instrumented Indentation Test for Hardness and Materials Parameters, Part 1: Test Method. ISO: Geneva, Switzerland, 2015.
- ISO 14577-4:2016; Metallic Materials—Instrumented Indentation Test for Hardness and Materials Parameters, Part 4: Test Method for Metallic and Non-Metallic Coatings. ISO: Geneva, Switzerland, 2016.
- Lungu, M.V.; Tălpeanu, D.; Ciobanu, R.C.; Cojocaru, A.; Pătroi, D.; Marinescu, V.; Caramitu, A.R. Evaluation of Magnetron Sputtered TiAlSiN-Based Thin Films as Protective Coatings for Tool Steel Surfaces. Coatings 2024, 14, 1184. [Google Scholar] [CrossRef]
- Shah, H.N.; Jayaganthan, R.; Kaur, D.; Chandra, R. Influence of sputtering parameters and nitrogen on the microstructure of chromium nitride thin films deposited on steel substrate by direct-current reactive magnetron sputtering. Thin Solid Films 2010, 518, 5762–5768. [Google Scholar] [CrossRef]
- Smith, J.; Lee, K. Influence of strain energy on CrN thin film texture during reactive sputtering. Coatings 2020, 10, 123. [Google Scholar]
- Zhang, L.; Wang, Y.; Chen, H. Effect of nitrogen flow rate on the microstructure and properties of CrN thin films. Materials 2019, 12, 456. [Google Scholar]
- Baker, M.A.; Gilmore, R.; Lemoine, P.; Roberts, K.G.; Teer, D.G. Structural and mechanical properties of CrN coatings deposited by dc reactive magnetron sputtering. Surf. Coat. Technol. 2000, 123, 242–249. [Google Scholar]
- Smith, J.; Brown, A.; Lee, C. Thermal stability and oxidation behavior of CrN coatings. Surf. Coat. Technol. 2014, 250, 100–106. [Google Scholar]
- Patterson, A.L. The Scherrer Formula for X-ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Shukla, G.; Khare, A. Dependence of N2 pressure on the crystal structure and surface quality of AlN thin films deposited via pulsed laser deposition technique at room temperature. Appl. Surf. Sci. 2008, 255, 2057–2062. [Google Scholar] [CrossRef]
- Huang, J.-H.; Ho, C.-H.; Yu, G.-P. Effect of nitrogen flow rate on the structure and mechanical properties of ZrN thin films on Si(100) and stainless steel substrates. Mater. Chem. Phys. 2007, 102, 31–38. [Google Scholar] [CrossRef]
- Paul, H. Mayrhofer, Florian Rovere, Martin Moser, Christian Strondl, Roel Tietema, hermally induced transitions of CrN thin films. Scr. Mater. 2007, 57, 249–252. [Google Scholar]
- Lin, J.; Moore, J.J.; Wang, J.; Sproul, W.D. High temperature oxidation behavior of CrN/AlN superlattice films. Thin Solid Film. 2011, 519, 2402–2408. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lu, F.H. Oxidation behavior of chromium nitride films. Thin Solid Films 2006, 515, 2179–2184. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Li, J.; Chen, Z. Effect of Annealing on the Microstructure and Mechanical Properties of CrN Coatings Prepared by Reactive Magnetron Sputtering. Coatings 2021, 11, 1234. [Google Scholar]
- Saladukhin, I.; Abadias, G.; Uglov, V.; Zlotski, S.; Janse van Vuuren, A.; O’Connell, J.H. Structural Properties and Oxidation Resistance of ZrN/SiNx, CrN/SiNx and AlN/SiNx Multilayered Films Deposited by Magnetron Sputtering Technique. Coatings 2020, 10, 149. [Google Scholar] [CrossRef]
- Hirth, J.P.; Lothe, J. Theory of Dislocations, 2nd ed.; Wiley: New York, NY, USA, 1982. [Google Scholar]
- Hull, D.; Bacon, D.J. Introduction to Dislocations, 5th ed.; Butterworth-Heinemann: Oxford, UK, 2011. [Google Scholar]
- Estrin, Y.; Vinogradov, A. Extreme grain refinement by severe plastic deformation: A wealth of challenging science. Acta Mater. 2013, 61, 782–817. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Langdon, T.G. Principles of equal-channel angular pressing as a processing tool for grain refinement. Prog. Mater. Sci. 2006, 51, 881–981. [Google Scholar] [CrossRef]
- Polcar, T.; Martinez, R.; Vítů, T.; Kopecký, L.; Rodriguez, R.; Cavaleiro, A. High temperature tribology of CrN and multilayered Cr/CrN coatings. Surf. Coat. Technol. 2009, 203, 3254–3259. [Google Scholar] [CrossRef]


| Nanoindentation Testing-Measurement Conditions | CrN Coatings | SS304L Steel Substrate |
|---|---|---|
| Loading type | linear | linear |
| Indenter approach speed to the sample (nm/min) | 1000 | 2000 |
| Loading/unloading rate (nm/min) | 300 | 600 |
| Pause at Fmax (s) | 10 | 10 |
| Data acquisition frequency (Hz) | 10 | 10 |
| Poisson’s ratio (ν) | 0.20 | 0.30 |
| Sample | N2 Flux (sccm) | Ar Flow Rate (sccm) | Film Thickness (nm) | Deposition Rate (nm/min) |
|---|---|---|---|---|
| CrN4/1 | 10 | 40 | 805 | 17.88 |
| CrN3/1 | 12 | 36 | 800 | 17.77 |
| CrN2/1 | 16 | 32 | 680 | 15.11 |
| CrN1/1 | 25 | 25 | 545 | 11.11 |
| Sample | 2θ (Degree) | (hkl) | d (Å) | FWHM (Degree) | D (nm) | δ (Lines/m2) |
|---|---|---|---|---|---|---|
| CrN4/1 | 37.74 | (111) | 2.3816 | 0.48 | 16.46 | 3.69 × 1015 |
| CrN3/1 | 37.66 | (111) | 2.3866 | 0.40 | 19.75 | 2.56 × 1015 |
| CrN2/1 | 37.69 | (111) | 2.3847 | 0.40 | 19.75 | 2.56 × 1015 |
| CrN1/1 | 37.69 | (111) | 2.3850 | 0.88 | 8.98 | 1.26 × 1016 |
| Sample | Cr (at. %) | N (at. %) | O (at. %) | Si (at. %) |
|---|---|---|---|---|
| CrN4/1 | 49.4 | 29.1 | 20.2 | 1.3 |
| CrN3/1 | 48.4 | 31.3 | 19.3 | 1.1 |
| CrN2/1 | 47.8 | 31.4 | 20.0 | 0.8 |
| CrN1/1 | 38.6 | 38.0 | 18.2 | 5.1 |
| Sample | Cr (at. %) | N (at. %) | O (at. %) | Si (at. %) |
|---|---|---|---|---|
| CrN4/1 | 40.3 | 3.3 | 56.2 | 0.2 |
| CrN3/1 | 41.2 | 10.1 | 48.3 | 0.4 |
| CrN2/1 | 38.2 | 18.8 | 41.6 | 1.5 |
| CrN1/1 | 37.6 | 18.5 | 38.6 | 5.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chițanu, E.; Iordache, I.; Codescu, M.M.; Marinescu, V.E.; Sbârcea, G.B.; Pătroi, D.; Zevri, L.; Nadolu, A.C. The Influence of the Annealing Process on the Mechanical Properties of Chromium Nitride Thin Films. Materials 2025, 18, 3605. https://doi.org/10.3390/ma18153605
Chițanu E, Iordache I, Codescu MM, Marinescu VE, Sbârcea GB, Pătroi D, Zevri L, Nadolu AC. The Influence of the Annealing Process on the Mechanical Properties of Chromium Nitride Thin Films. Materials. 2025; 18(15):3605. https://doi.org/10.3390/ma18153605
Chicago/Turabian StyleChițanu, Elena, Iulian Iordache, Mirela Maria Codescu, Virgil Emanuel Marinescu, Gabriela Beatrice Sbârcea, Delia Pătroi, Leila Zevri, and Alexandra Cristiana Nadolu. 2025. "The Influence of the Annealing Process on the Mechanical Properties of Chromium Nitride Thin Films" Materials 18, no. 15: 3605. https://doi.org/10.3390/ma18153605
APA StyleChițanu, E., Iordache, I., Codescu, M. M., Marinescu, V. E., Sbârcea, G. B., Pătroi, D., Zevri, L., & Nadolu, A. C. (2025). The Influence of the Annealing Process on the Mechanical Properties of Chromium Nitride Thin Films. Materials, 18(15), 3605. https://doi.org/10.3390/ma18153605








