Tailoring Microstructure via Rolling to Achieve Concurrent High Strength and Thermal Conductivity in Mg-Zn-Nd-Zr Alloys
Abstract
1. Introduction
2. Experimental Procedure
2.1. Preparation of the Mg Alloys
2.2. Materials Characterization
3. Results and Discussion
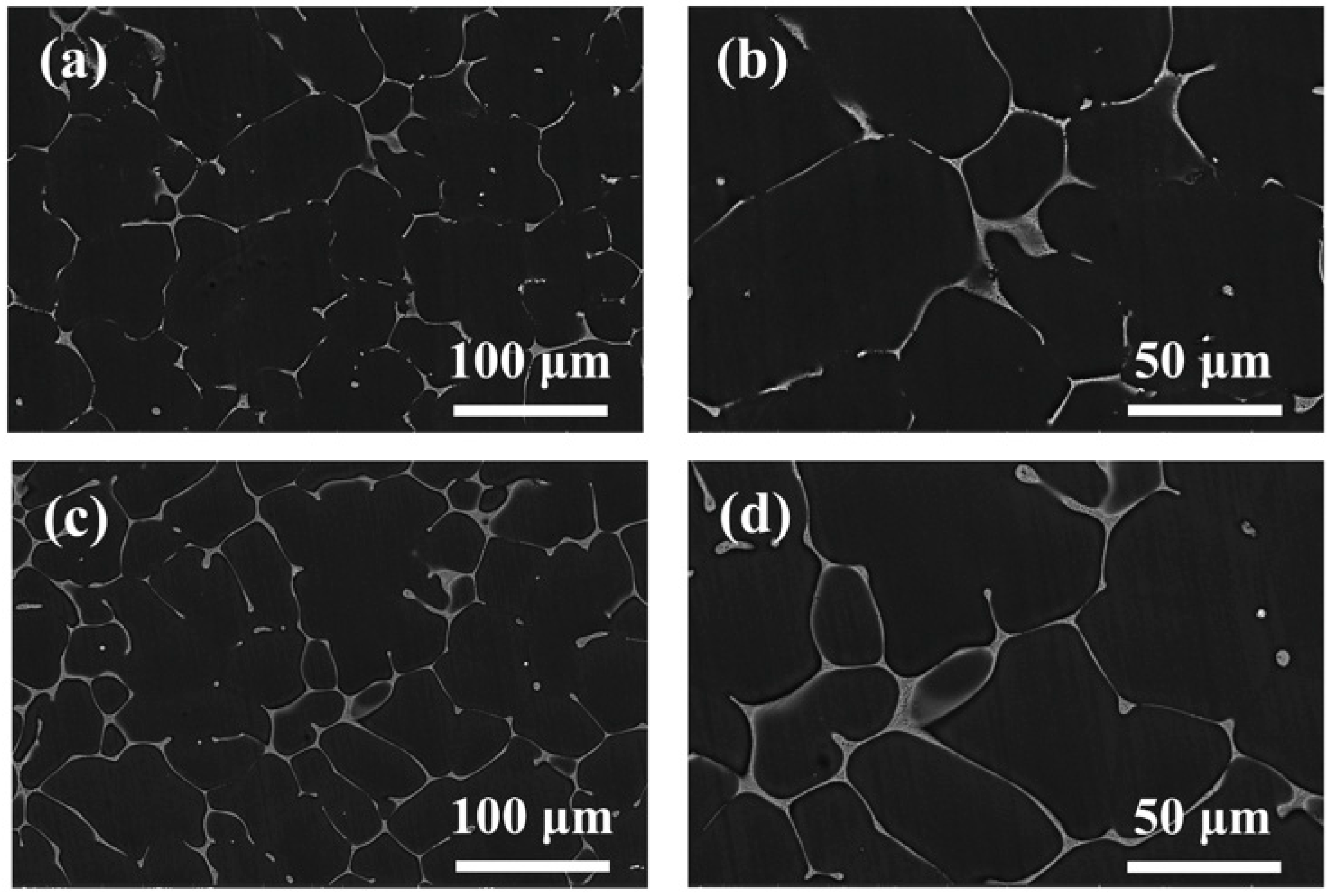
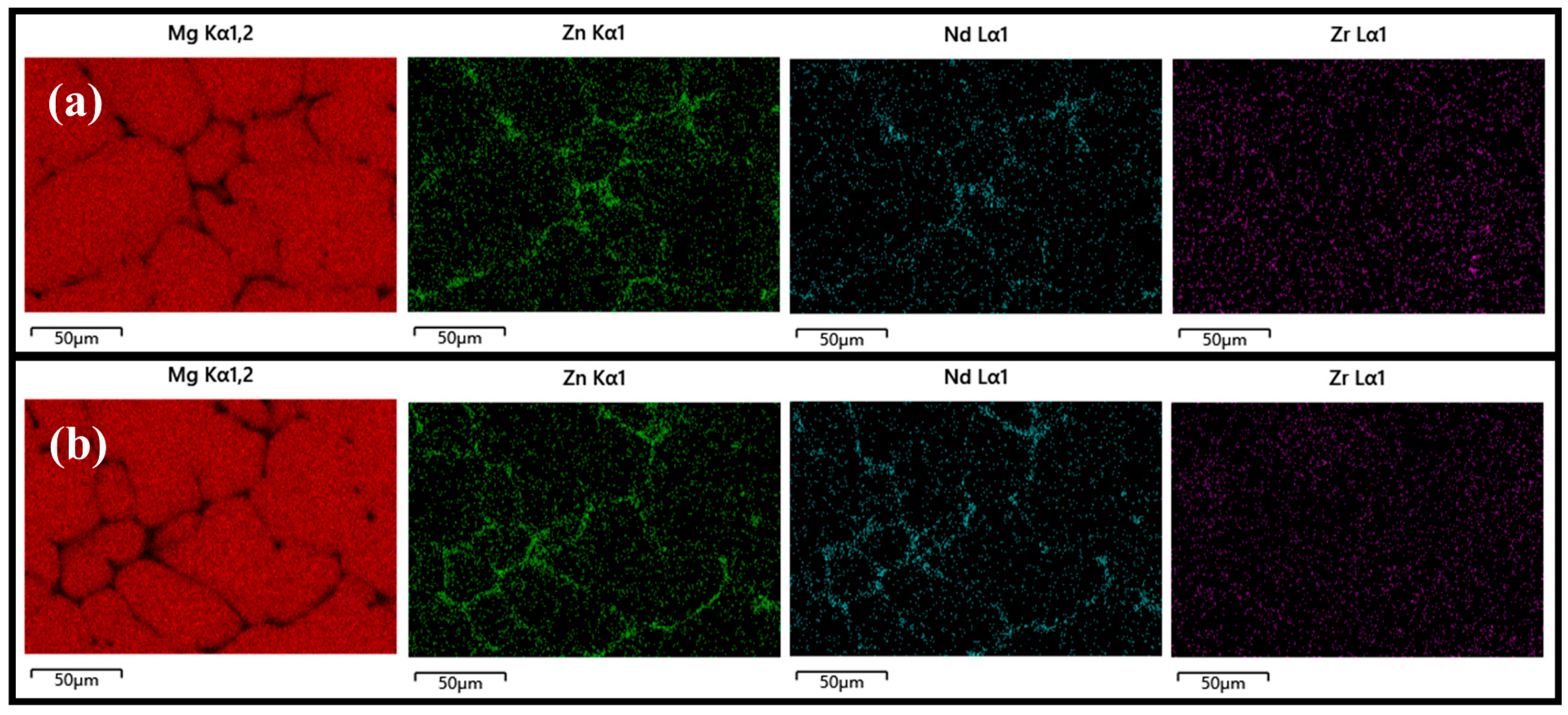
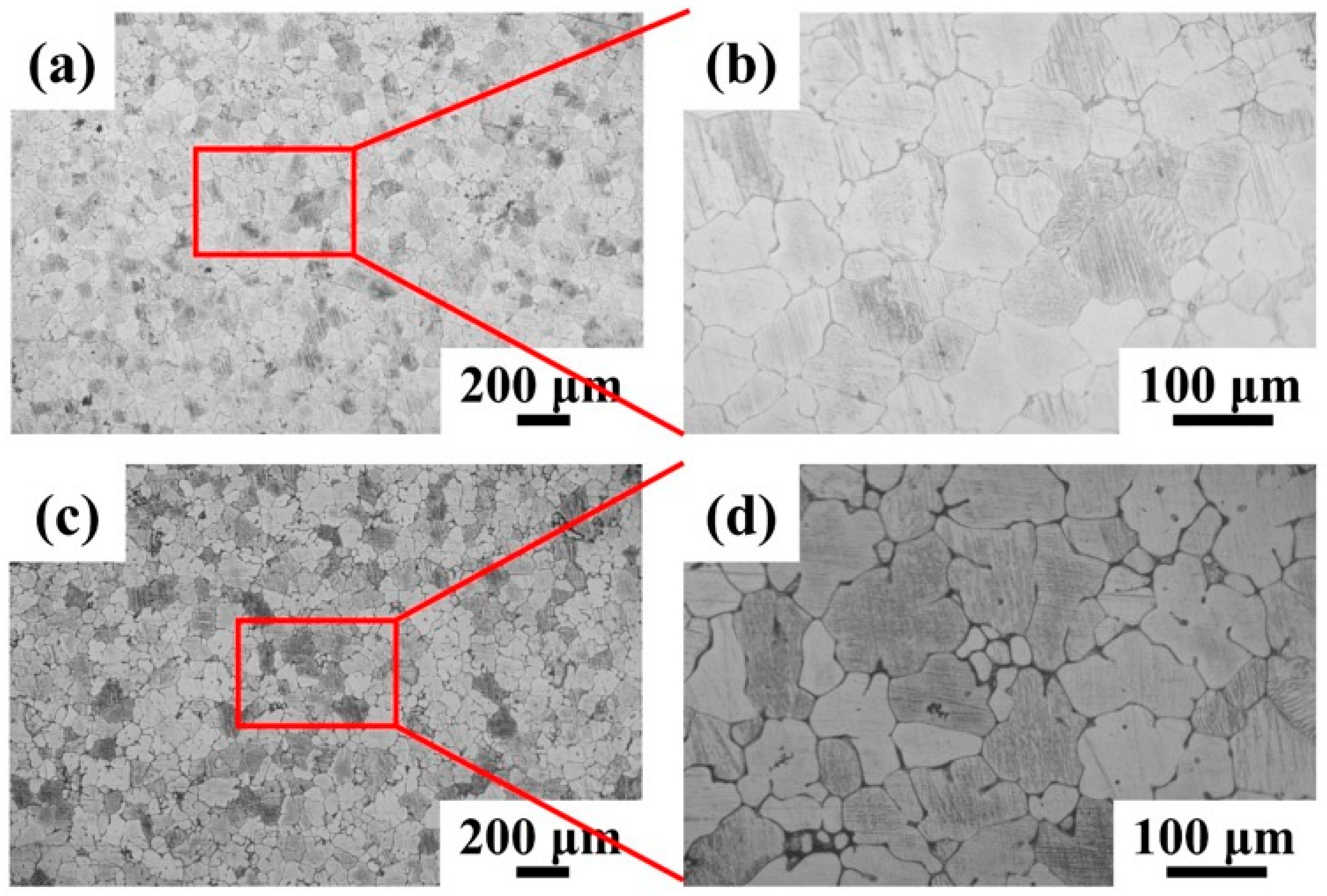
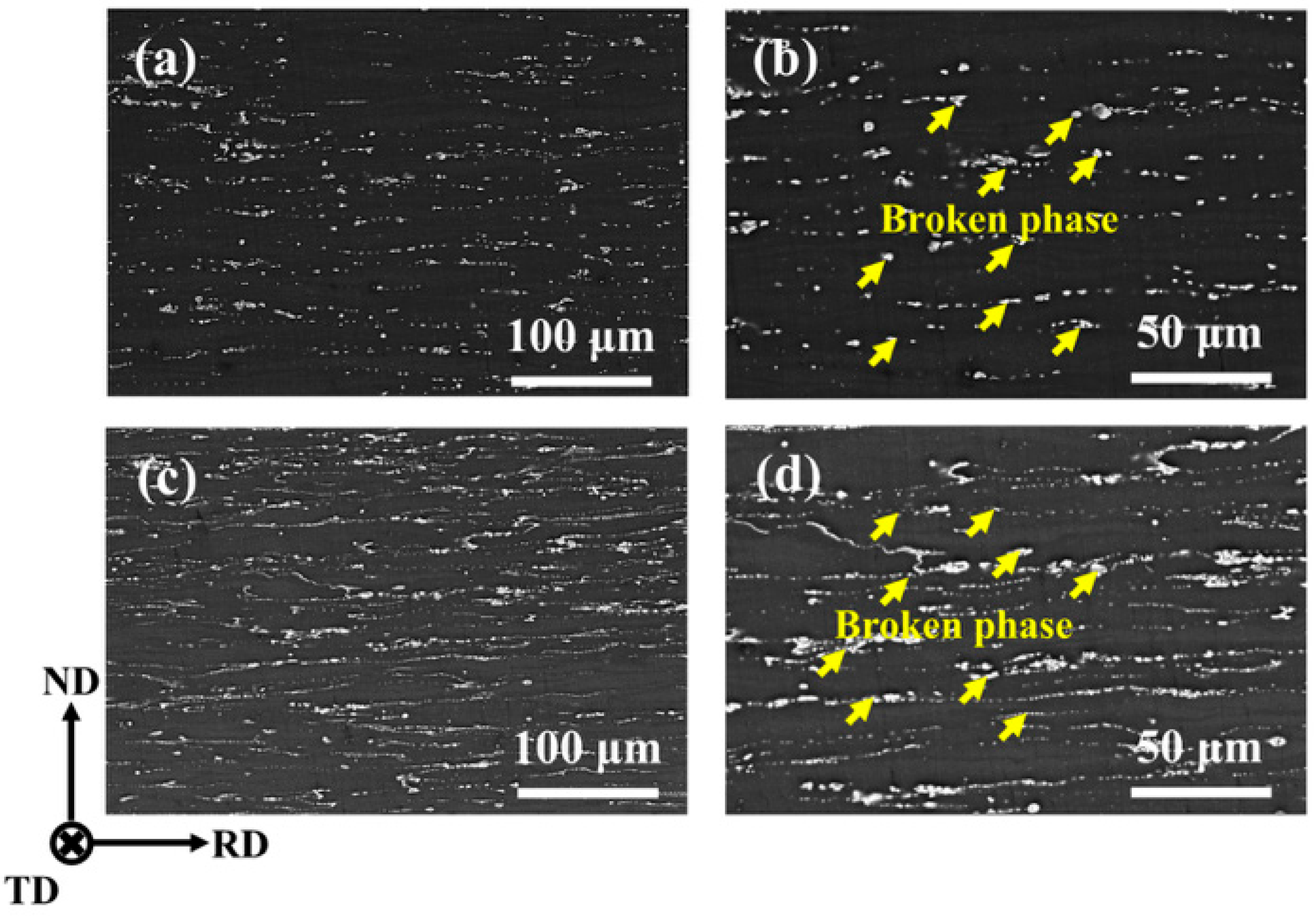
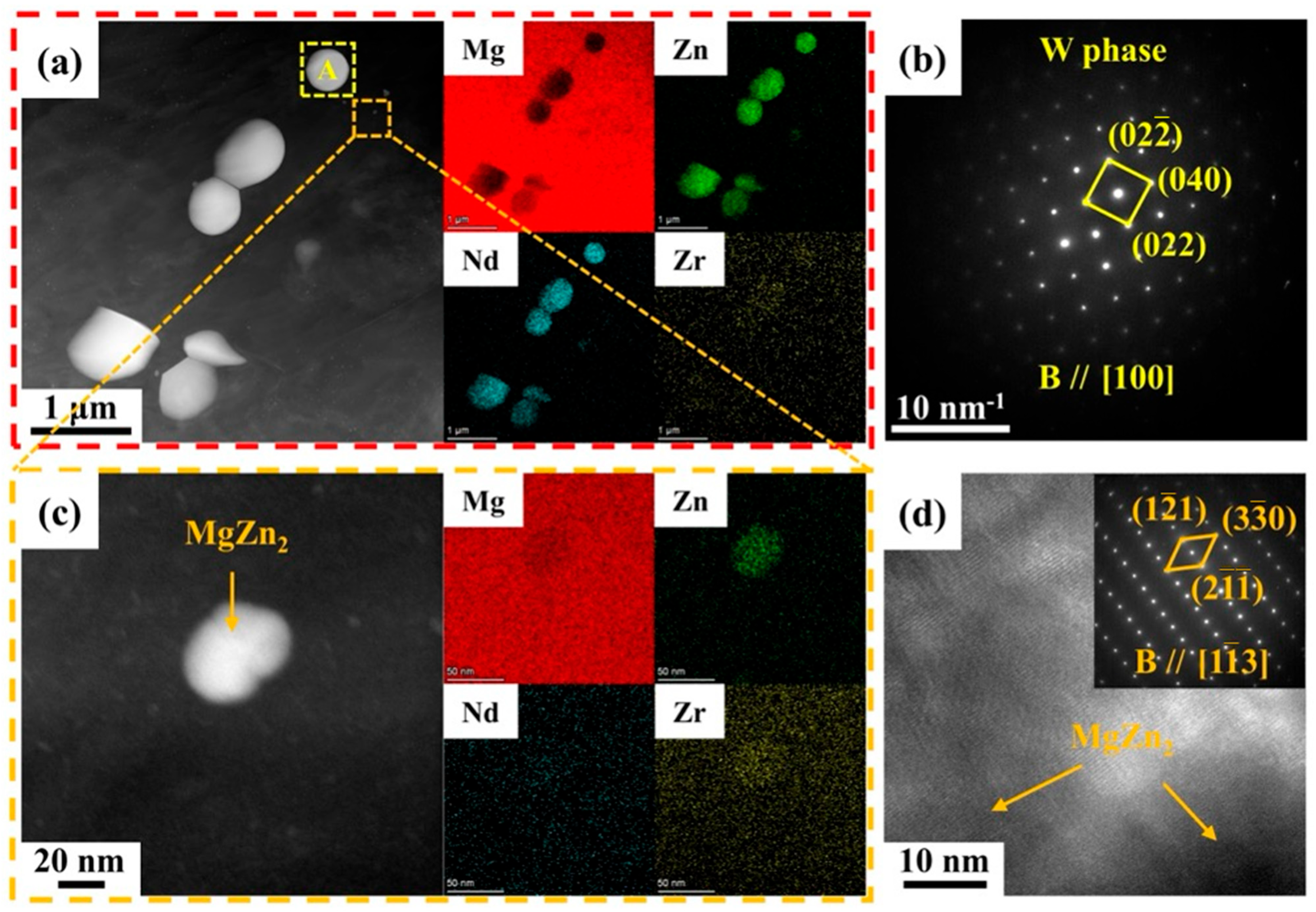
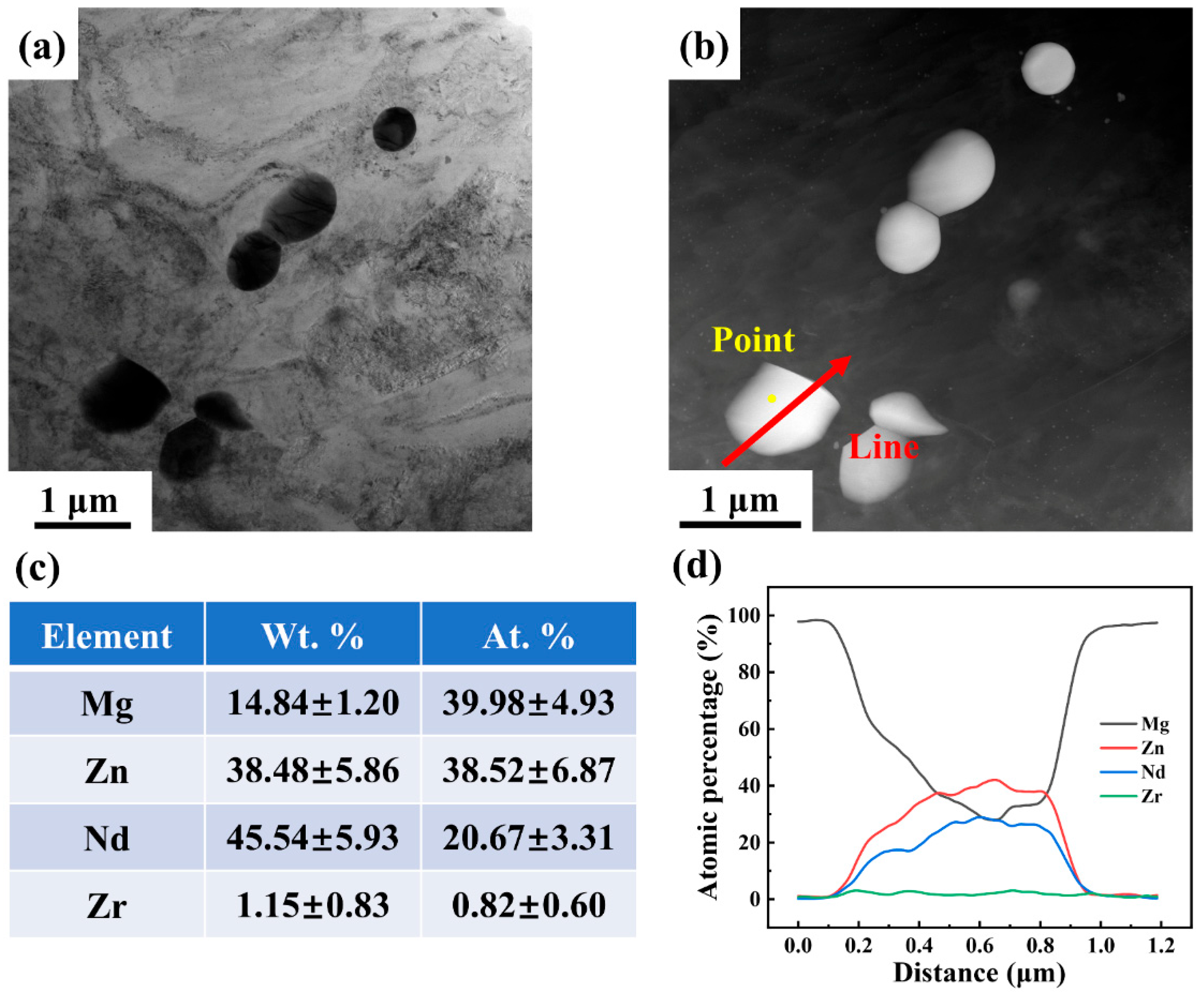
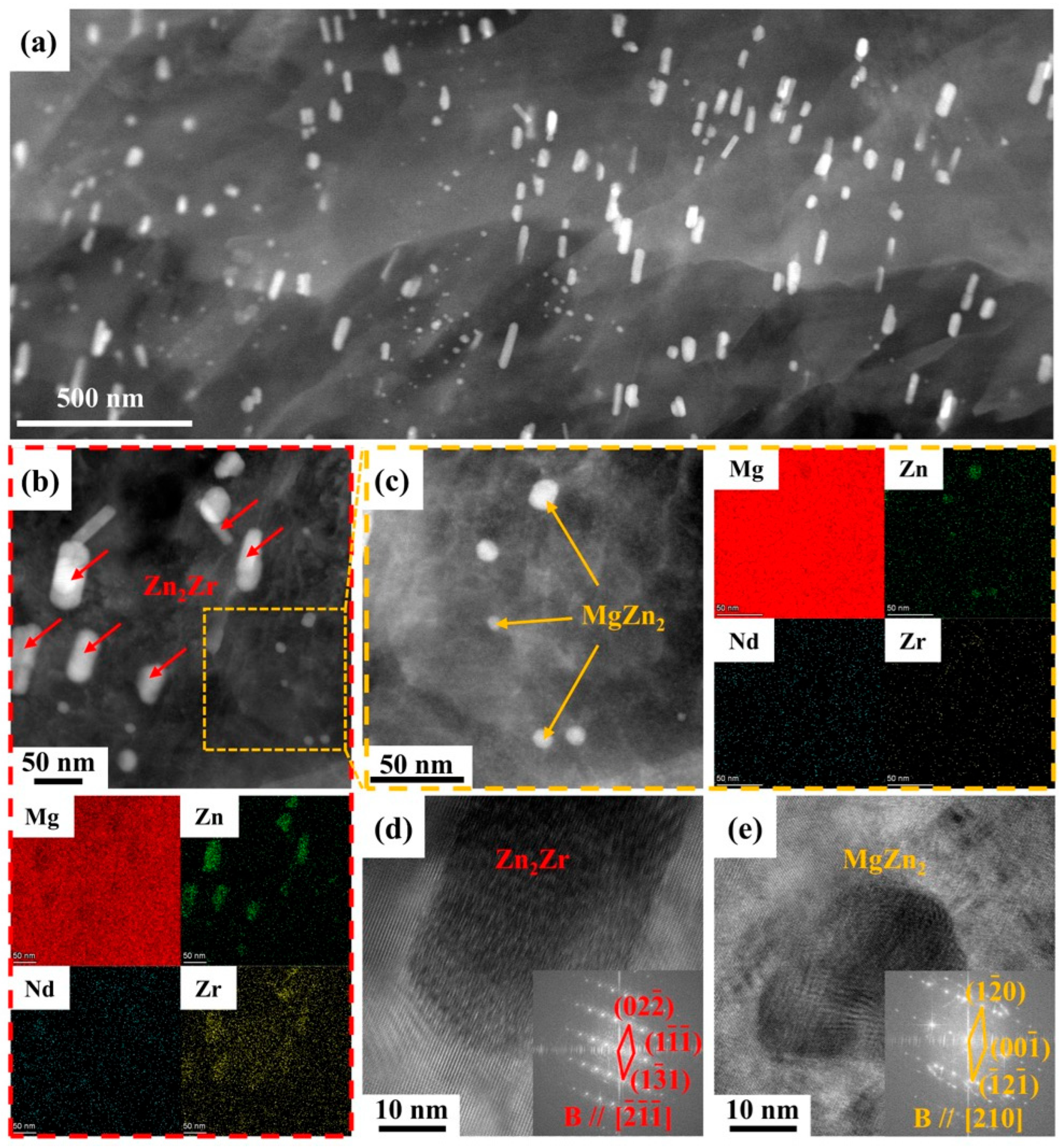
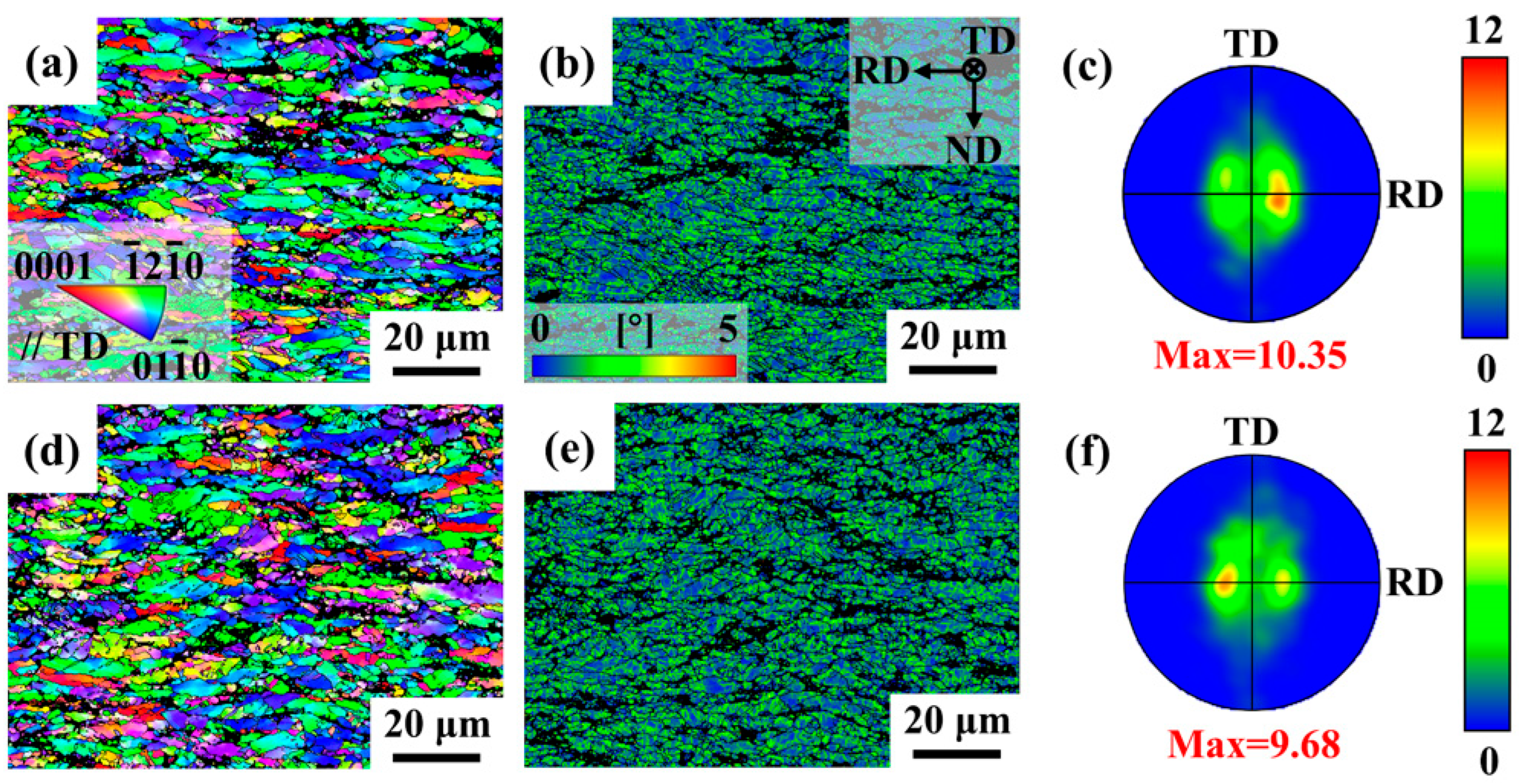
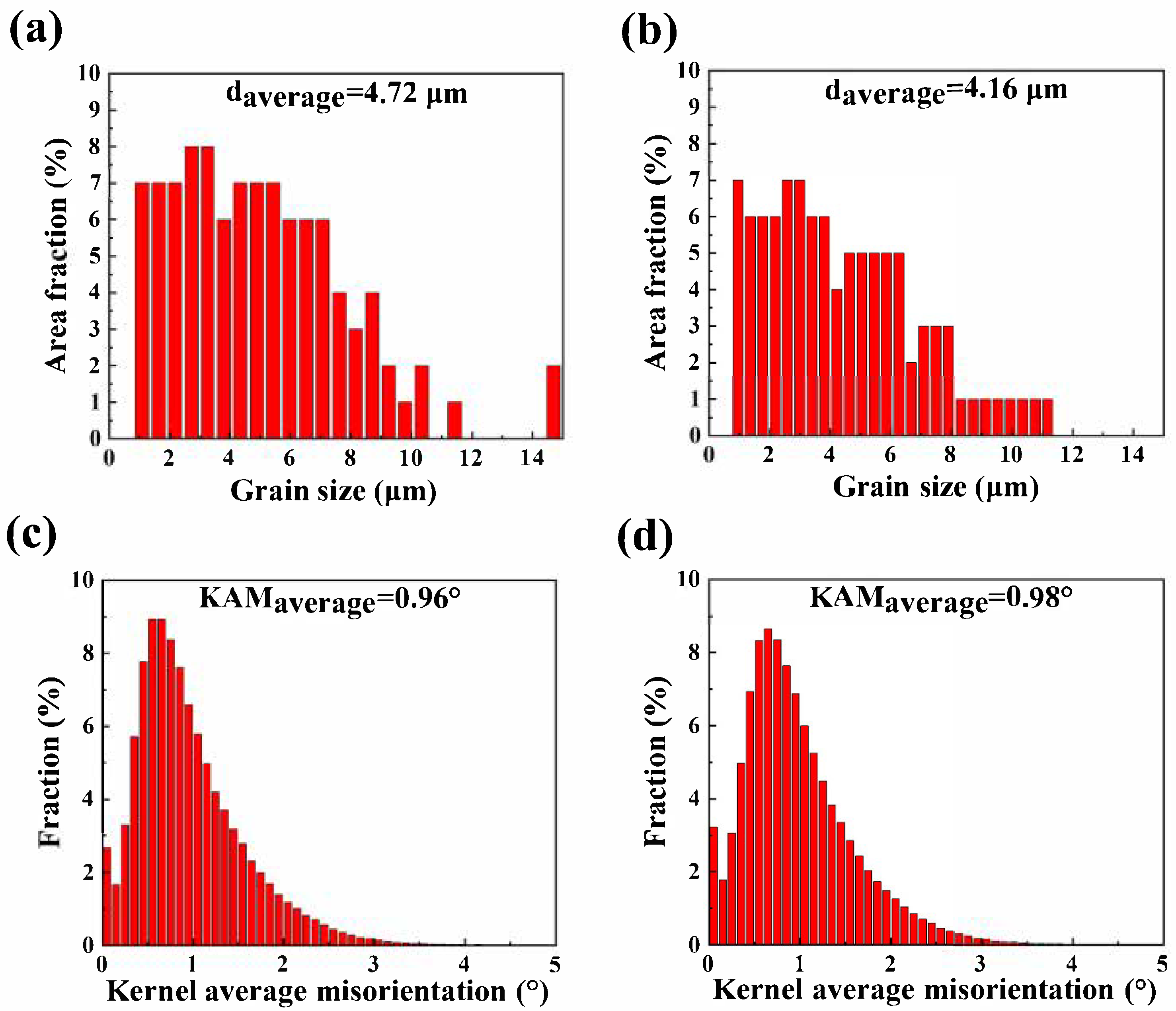
3.1. Microstructure of Mg-5Zn-xNd-0.4Zr Alloys
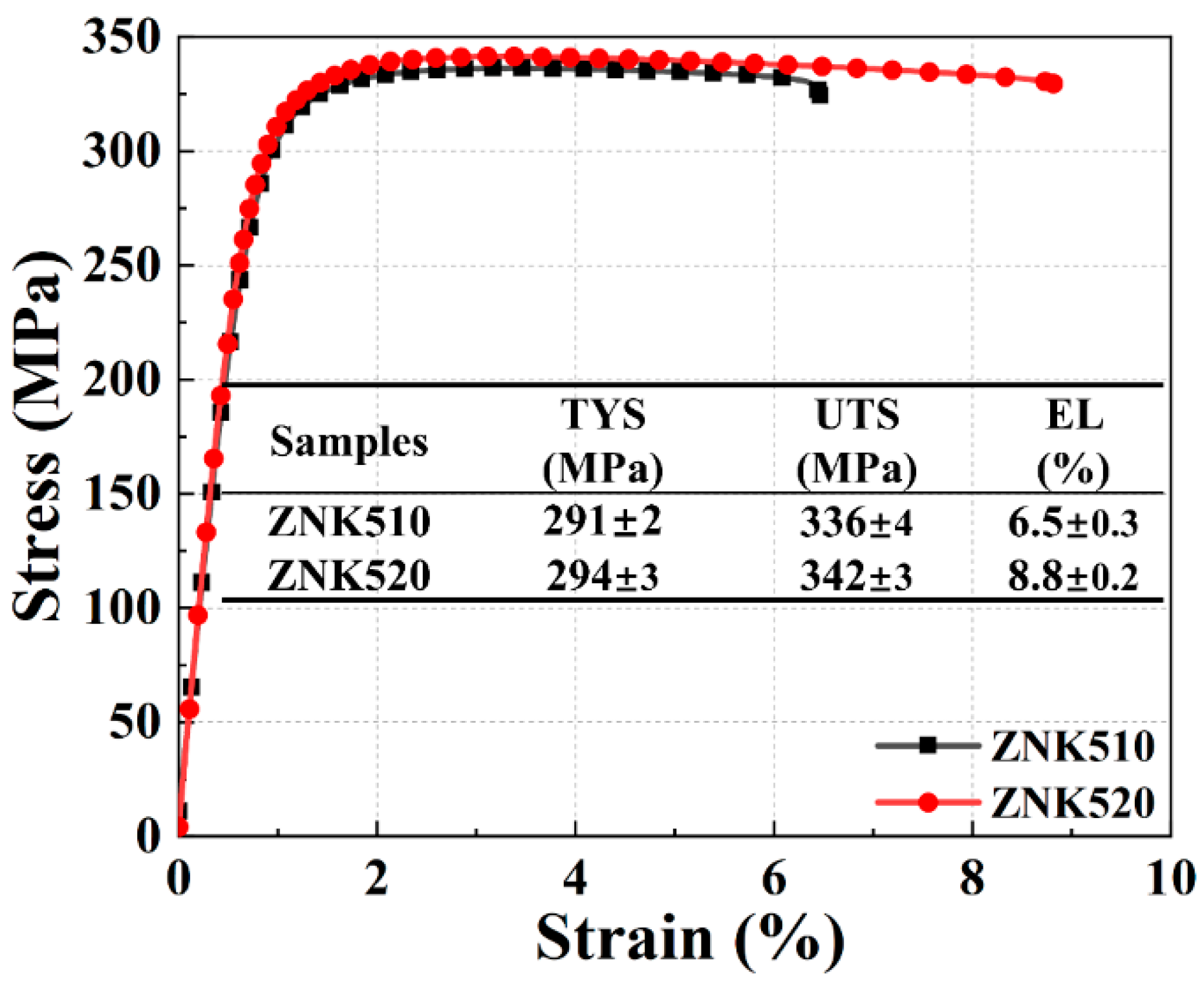
3.2. Mechanical Properties of Mg-5Zn-xNd-0.4Zr Alloys
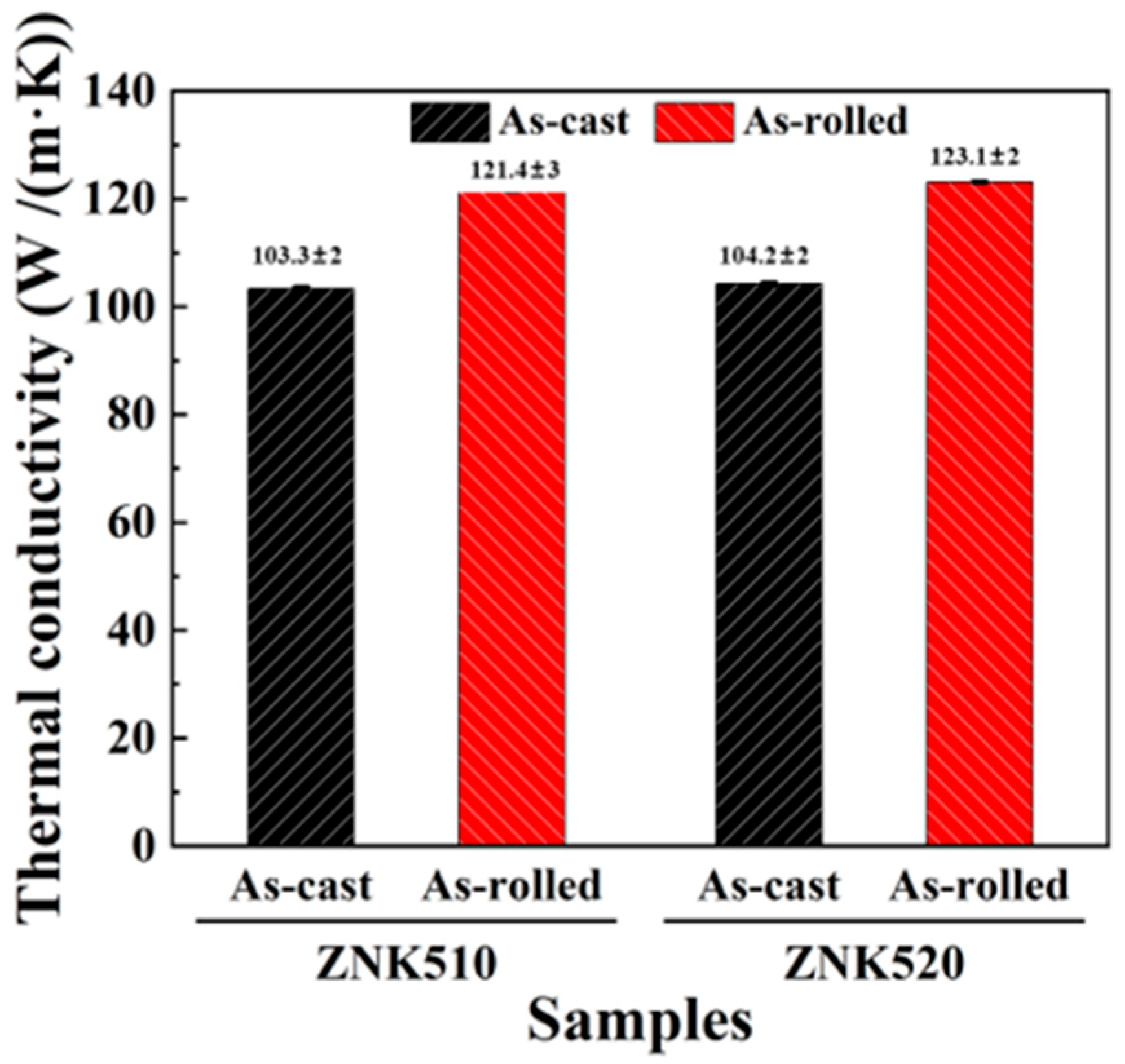
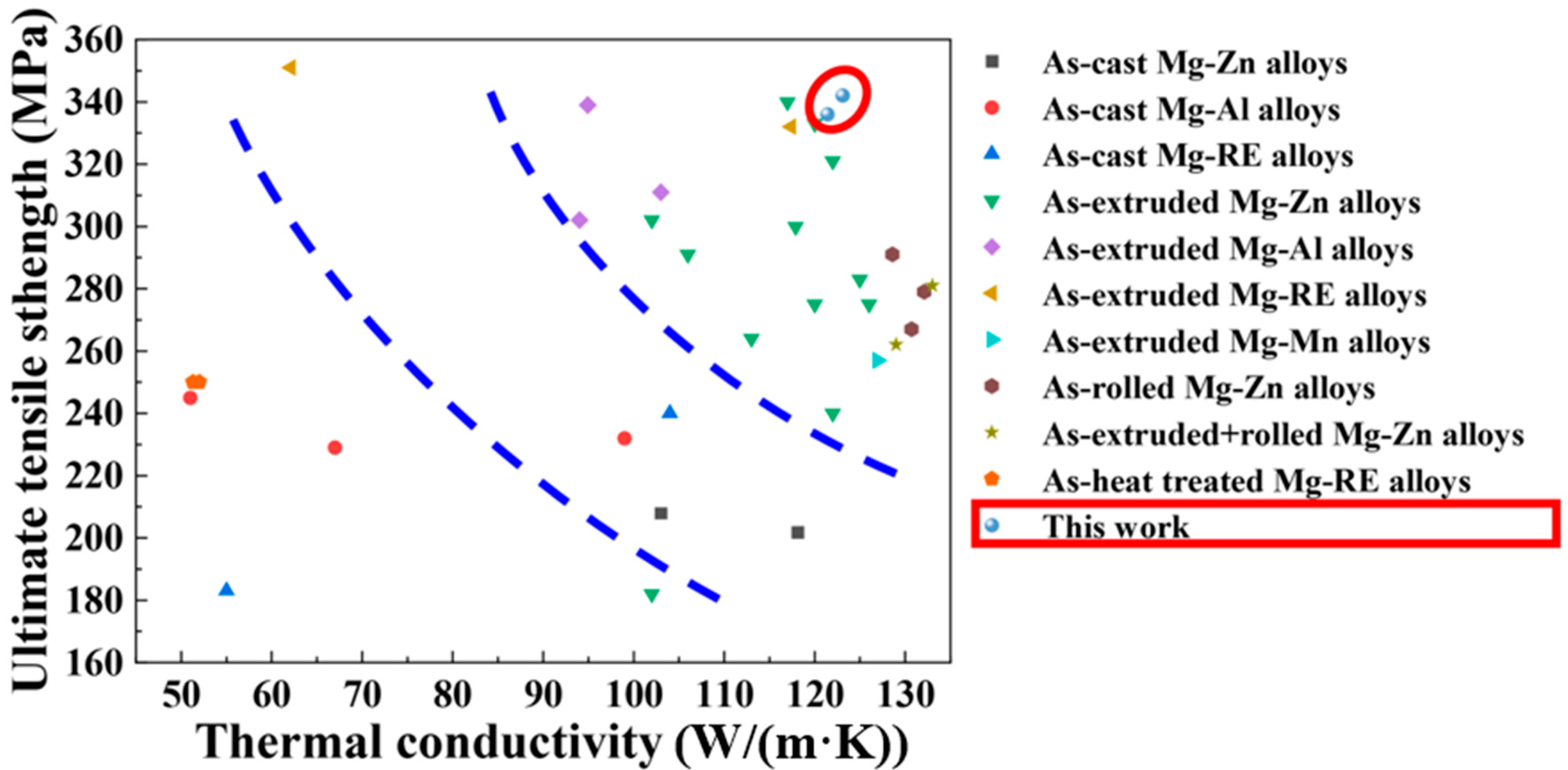
3.3. Thermal Conductivity of Mg-5Zn-xNd-0.4Zr Alloys
4. Conclusions
- The rolled Mg-Zn-Nd-Zr alloys exhibited a combination of elevated strength and thermal conductivity, with ZNK520 showing the best overall performance due to its higher Nd content and increased second-phase formation.
- Grain boundary strengthening, the dynamic precipitation of nanoscale phases during rolling, and microstructure evolution, particularly the formation of basal textures in the ND direction, significantly enhanced both the mechanical properties and thermal conductivity of the alloy.
- Combining composition design with thermal deformation processes, including phase state regulation, is an effective strategy in the pursuit of Mg alloys with simultaneous high strength and thermal conductivity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, J.; She, J.; Chen, D.; Pan, F. Latest Research Advances on Magnesium and Magnesium Alloys Worldwide. J. Magnes. Alloys 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Xu, T.; Yang, Y.; Peng, X.; Song, J.; Pan, F. Overview of Advancement and Development Trend on Magnesium Alloy. J. Magnes. Alloys 2019, 7, 536–544. [Google Scholar] [CrossRef]
- Prasad, S.V.S.; Prasad, S.B.; Verma, K.; Mishra, R.K.; Kumar, V.; Singh, S. The Role and Significance of Magnesium in Modern Day Research-A Review. J. Magnes. Alloys 2022, 10, 1–61. [Google Scholar] [CrossRef]
- Ji, Z.K.; Qiao, X.G.; Yuan, L.; Cong, F.G.; Wang, G.J.; Zheng, M.Y. Exceptional Fracture Toughness in a High-Strength Mg Alloy with the Synergetic Effects of Bimodal Structure, LPSO, and Nanoprecipitates. Scr. Mater. 2023, 236, 115675. [Google Scholar] [CrossRef]
- Li, L.; Bao, J.; Qiao, M.; Tian, J.; Yang, Y.; Sha, J.; Zhang, Z. Improvement of Strength-Ductility Balance and Corrosion Resistance in as-Extruded Mg-Gd-Zn-Zr Alloys by Zn/Gd Ratio. Mater. Sci. Eng. A 2023, 872, 144979. [Google Scholar] [CrossRef]
- Xiang, C.; Huang, Z.; Wang, Z.; Ding, H.; Xu, S. Effect of Cu Addition on the Microstructure, Mechanical Properties and Thermal Properties of Mg-Al-Ca-Mn Alloy. Mater. Charact. 2023, 202, 113028. [Google Scholar] [CrossRef]
- Feng, L.; Dong, X.; Xia, M.; Zhu, X.; Ji, G.; Yang, H.; Wang, B.; Nyberg, E.A.; Ji, S. Development of High Thermal Conductivity, Enhanced Strength and Cost-Effective Die-Cast Mg Alloy Compared with AE44 Alloy. J. Mater. Res. Technol. 2023, 22, 2955–2966. [Google Scholar] [CrossRef]
- Pan, H.; Pan, F.; Yang, R.; Peng, J.; Zhao, C.; She, J.; Gao, Z.; Tang, A. Thermal and Electrical Conductivity of Binary Magnesium Alloys. J. Mater. Sci. 2014, 49, 3107–3124. [Google Scholar] [CrossRef]
- Ying, T.; Chi, H.; Zheng, M.; Li, Z.; Uher, C. Low-Temperature Electrical Resistivity and Thermal Conductivity of Binary Magnesium Alloys. Acta Mater. 2014, 80, 288–295. [Google Scholar] [CrossRef]
- Li, Z.; Hu, B.; Yao, F.; Jiang, Z.; Zhang, J.; Zhang, Q.; Han, J.; Zhou, L.; Sun, F.; Zeng, X.; et al. Anomalous Increase in Thermal Conductivity of Mg Solid Solutions by Co-Doping with Two Solute Elements. Acta Mater. 2025, 285, 120708. [Google Scholar] [CrossRef]
- Subramaniam, C.; Yasuda, Y.; Takeya, S.; Ata, S.; Nishizawa, A.; Futaba, D.; Yamada, T.; Hata, K. Carbon Nanotube-Copper Exhibiting Metal-like Thermal Conductivity and Silicon-like Thermal Expansion for Efficient Cooling of Electronics. Nanoscale 2014, 6, 2669–2674. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Hou, J.; Du, W. A Review on Thermal Conductivity of Magnesium and Its Alloys. J. Magnes. Alloys 2020, 8, 78–90. [Google Scholar] [CrossRef]
- Ghasemi, A.; Fereiduni, E.; Balbaa, M.; Elbestawi, M.; Habibi, S. Unraveling the Low Thermal Conductivity of the LPBF Fabricated Pure Al, AlSi12, and AlSi10Mg Alloys through Substrate Preheating. Addit. Manuf. 2022, 59, 103148. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, Y.; Chen, T.; Wu, L.; Chen, X.; Jiang, B.; Wang, J.; Pan, F. Multi-Solute Solid Solution Behavior and Its Effect on the Properties of Magnesium Alloys. J. Magnes. Alloys 2022, 10, 1786–1820. [Google Scholar] [CrossRef]
- Li, N.; Wang, C.; Monclús, M.A.; Yang, L.; Molina-Aldareguia, J.M. Solid Solution and Precipitation Strengthening Effects in Basal Slip, Extension Twinning and Pyramidal Slip in Mg-Zn Alloys. Acta Mater. 2021, 221, 117374. [Google Scholar] [CrossRef]
- Bazhenov, V.E.; Koltygin, A.V.; Sung, M.C.; Park, S.H.; Tselovalnik, Y.V.; Stepashkin, A.A.; Rizhsky, A.A.; Belov, M.V.; Belov, V.D.; Malyutin, K.V. Development of Mg–Zn–Y–Zr Casting Magnesium Alloy with High Thermal Conductivity. J. Magnes. Alloys 2021, 9, 1567–1577. [Google Scholar] [CrossRef]
- Chen, L.; Lü, S.; Guo, W.; Li, J.; Wu, S. High Thermal Conductivity of Highly Alloyed Mg-Zn-Cu Alloy and Its Mechanism. J. Alloys Compd. 2022, 918, 165614. [Google Scholar] [CrossRef]
- Su, C.; Li, D.; Luo, A.A.; Ying, T.; Zeng, X. Effect of Solute Atoms and Second Phases on the Thermal Conductivity of Mg-RE Alloys: A Quantitative Study. J. Alloys Compd. 2018, 747, 431–437. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, X.; Du, J. Microstructure, Thermal Conductivity and Mechanical Properties of the Mg–Zn–Sb Ternary Alloys. Met. Mater. Int. 2021, 27, 4477–4486. [Google Scholar] [CrossRef]
- Ying, T.; Zheng, M.Y.; Li, Z.T.; Qiao, X.G.; Xu, S.W. Thermal Conductivity of As-Cast and as-Extruded Binary Mg–Zn Alloys. J. Alloys Compd. 2015, 621, 250–255. [Google Scholar] [CrossRef]
- Buha, J. The Effect of Ba on the Microstructure and Age Hardening of an Mg–Zn Alloy. Mater. Sci. Eng. A 2008, 491, 70–79. [Google Scholar] [CrossRef]
- Chen, H.; Xie, T.; Liu, Q.; Huang, Y.; Liu, B.; Luo, Q.; Li, Q. Mechanism and Prediction of Aging Time Related Thermal Conductivity Evolution of Mg-Zn Alloys. J. Alloys Compd. 2023, 930, 167392. [Google Scholar] [CrossRef]
- Li, B.; Hou, L.; Wu, R.; Zhang, J.; Li, X.; Zhang, M.; Dong, A.; Sun, B. Microstructure and Thermal Conductivity of Mg-2Zn-Zr Alloy. J. Alloys Compd. 2017, 722, 772–777. [Google Scholar] [CrossRef]
- Li, X.; Du, W.; Lou, F.; Ding, N.; Du, X.; Li, S. Dual-Phase Synergistically Enhancing Mechanical Properties and Thermal Conductivity of Hot-Extruded Mg-8Gd-1Er-8Zn-1Mn Alloy. J. Magnes. Alloys 2024, 13, 1176–1186. [Google Scholar] [CrossRef]
- Liu, H.; Zuo, J.; Nakata, T.; Xu, C.; Wang, G.; Shi, H.; Tang, G.; Wang, X.; Kamado, S.; Geng, L. Effects of La Addition on the Microstructure, Thermal Conductivity and Mechanical Properties of Mg-3Al-0.3Mn Alloys. Materials 2022, 15, 1078. [Google Scholar] [CrossRef]
- Liu, H.; Nakata, T.; Xu, C.; Tang, G.; Li, D.; Wang, X.; Wang, G.; Kamado, S.; Geng, L. Effects of La and Ce on the Microstructure, Thermal Conductivity and Strength Synergy of the as-Extruded Mg-Mn-RE Alloys. J. Magnes. Alloys 2024, 13, 654–667. [Google Scholar] [CrossRef]
- Zhang, Z.; Kim, J.; Li, M.; Gao, Y.; Hu, Y.; Jiang, B.; Pan, F. Effects of Nd Content on the Microstructures and Mechanical Properties of ZK60 Mg Alloy and Corresponding Strengthening Mechanisms. Mater. Sci. Eng. A 2024, 901, 146504. [Google Scholar] [CrossRef]
- Lv, S.; Meng, F.; Lu, X.; Yang, Q.; Qiu, X.; Duan, Q.; Meng, J. Influence of Nd Addition on Microstructures and Mechanical Properties of a Hot-Extruded Mg−6.0Zn−0.5Zr (Wt.%) Alloy. J. Alloys Compd. 2019, 806, 1166–1179. [Google Scholar] [CrossRef]
- Zhang, Z.; Kim, J.; Pu, H.; Zhou, S.; Hu, Y.; Huang, G.; Jiang, B.; Pan, F. Improving mechanical properties of Mg–Zn–Nd–Zr alloy by low alloying with Yb and corresponding strengthening mechanisms. J. Mater. Res. Technol. 2024, 33, 2023–2034. [Google Scholar] [CrossRef]
- Zengin, H.; Turen, Y. Effect of Y addition on microstructure and corrosion behavior of extruded Mg–Zn–Nd–Zr alloy. J. Magnes. Alloys 2020, 8, 640–653. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, K.; Li, T.; Li, X.; Li, Y.; Ma, M.; Luo, P.; Luo, G.; Hao, Y. Anisotropy of Thermal Conductivity and Mechanical Properties in Mg–5Zn–1Mn Alloy. Mater. Des. 2012, 40, 257–261. [Google Scholar] [CrossRef]
- Tong, X.; You, G.; Ding, Y.; Xue, H.; Wang, Y.; Guo, W. Effect of Grain Size on Low-Temperature Electrical Resistivity and Thermal Conductivity of Pure Magnesium. Mater. Lett. 2018, 229, 261–264. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Turakhodjaevr, N.; Li, X.; Shi, H.; Zhang, Y.; Hu, X.; Xu, C. Overcoming Oxidation and Enhancing Dispersion of Nanoparticles via Molten Salt: Configurational Distribution of TiCnp in Pure Mg. J. Magnes. Alloys 2024, in press. [CrossRef]
- Zhang, X.; Wang, X.; Shi, H.; Turakhodjaevr, N.; Li, X.; Zhang, Y.; Hu, X.; Xu, C. Molten Salt-Assisted Dispersion-Wetting Synergy: A Breakthrough Strategy for Fabrication of Nanoparticle-Reinforced Mg Composites via Melt Processing. Adv. Compos. Hybrid Mater. 2025, 8, 295. [Google Scholar] [CrossRef]
- Park, S.J.; Heogh, W.; Yang, J.; Kang, S.; Jeong, W.; Lee, H.; Jang, T.-S.; Jung, H.-D.; Jahazi, M.; Han, S.C.; et al. Meta-structure of amorphous-inspired 65.1Co28.2Cr5.3Mo lattices augmented by artificial intelligence. Adv. Compos. Hybrid Mater. 2024, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Meng, F.; Lv, S.; Guan, K.; Yang, Q.; Qin, P.; Qiu, X.; Tian, Z.; Zhang, D.; Meng, J. Influence of Various Yb Additions on Microstructures of a Casting Mg−8Gd−1.2Zn−0.5Zr Alloy. J. Alloys Compd. 2019, 789, 720–729. [Google Scholar] [CrossRef]
- Feng, X.; Wang, X.; Xu, C.; Hu, X.; Shi, H.; Li, X.; Lu, Z. High-Modulus Magnesium Alloy: Control of Microstructure and Mechanical Properties via In-Situ Synthesis of the Al2RE Phase. J. Magnes. Alloys 2024, in press. [CrossRef]
- Gao, M.; Yang, K.; Tan, L.; Ma, Z. Role of Bimodal-Grained Structure with Random Texture on Mechanical and Corrosion Properties of a Mg-Zn-Nd Alloy. J. Magnes. Alloys 2022, 10, 2147–2157. [Google Scholar] [CrossRef]
- Gröbner, J.; Kozlov, A.; Fang, X.Y.; Geng, J.; Nie, J.F.; Schmid-Fetzer, R. Phase Equilibria and Transformations in Ternary Mg-Rich Mg–Y–Zn Alloys. Acta Mater. 2012, 60, 5948–5962. [Google Scholar] [CrossRef]
- Huang, K.; Logé, R.E. A Review of Dynamic Recrystallization Phenomena in Metallic Materials. Mater. Des. 2016, 111, 548–574. [Google Scholar] [CrossRef]
- Hradilová, M.; Montheillet, F.; Fraczkiewicz, A.; Desrayaud, C.; Lejček, P. Effect of Ca-Addition on Dynamic Recrystallization of Mg–Zn Alloy during Hot Deformation. Mater. Sci. Eng. A 2013, 580, 217–226. [Google Scholar] [CrossRef]
- Li, X.; Zhao, J.; Zhao, Z.; Shi, H.; Li, X.; Xu, C.; Hu, X.; Wang, X. Fabrication of High-Strength and High-Thermal-Conductivity Mg-Zn-Gd-Y Alloy Rolled Sheets via Synergistic Modulation of Dislocation and Recrystallization. J. Mater. Eng. Perform. 2025. [Google Scholar] [CrossRef]
- Yuan, W.; Panigrahi, S.K.; Su, J.-Q.; Mishra, R.S. Influence of Grain Size and Texture on Hall–Petch Relationship for a Magnesium Alloy. Scr. Mater. 2011, 65, 994–997. [Google Scholar] [CrossRef]
- Liu, C.; Chen, X.; Chen, J.; Atrens, A.; Pan, F. The Effects of Ca and Mn on the Microstructure, Texture and Mechanical Properties of Mg-4 Zn Alloy. J. Magnes. Alloys 2021, 9, 1084–1097. [Google Scholar] [CrossRef]
- Wan, Y.; Tang, B.; Gao, Y.; Tang, L.; Sha, G.; Zhang, B.; Liang, N.; Liu, C.; Jiang, S.; Chen, Z.; et al. Bulk Nanocrystalline High-Strength Magnesium Alloys Prepared via Rotary Swaging. Acta Mater. 2020, 200, 274–286. [Google Scholar] [CrossRef]
- Jain, J.; Cizek, P.; Poole, W.J.; Barnett, M.R. Precipitate Characteristics and Their Effect on the Prismatic-Slip-Dominated Deformation Behaviour of an Mg–6 Zn Alloy. Acta Mater. 2013, 61, 4091–4102. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, Z.H.; Ma, H.; Zhang, H.; Zhang, Z.M.; Xu, C.J.; Zhou, N.; Kuang, M.; Huang, J.C. High Strength Mg-1.4Gd-1.2Y-0.4Zn Sheet and Its Strengthening Mechanisms. Mater. Sci. Eng. A 2019, 747, 17–26. [Google Scholar] [CrossRef]
- Nagaraj Nandihalli, Takao Mori, and Holger Kleinke, Effect of addition of SiC and Al2O3 refractories on Kapitza resistance of antimonide-telluride. AIP Adv. 2018, 8, 095009. [CrossRef]
- Pan, H.; Pan, F.; Peng, J.; Gou, J.; Tang, A.; Wu, L.; Dong, H. High-Conductivity Binary Mg–Zn Sheet Processed by Cold Rolling and Subsequent Aging. J. Alloys Compd. 2013, 578, 493–500. [Google Scholar] [CrossRef]
- Wu, M.; Jiang, X.; Chen, J.; Yan, H.; Xia, W.; Su, B.; Deng, Y. High Strength-Thermal Conductivity Mg–Ga–Ca–Ce Sheet by Hot-Extrusion and Rolling. Met. Mater. Int. 2024, 30, 13–24. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, H.; Nagaumi, H.; Yang, X. Tracing the Microstructures, Mechanical Properties and Thermal Conductivity of Low-Temperature Extruded MgMn Alloys with Various Cerium Additions. Mater. Charact. 2023, 196, 112658. [Google Scholar] [CrossRef]
- Liu, Y.F.; Jia, X.J.; Qiao, X.G.; Xu, S.W.; Zheng, M.Y. Effect of La Content on Microstructure, Thermal Conductivity and Mechanical Properties of Mg–4Al Magnesium Alloys. J. Alloys Compd. 2019, 806, 71–78. [Google Scholar] [CrossRef]
- Rong, J.; Xiao, W.; Fu, Y.; Zhao, X.; Yan, P.; Ma, C.; Chen, M.; Huang, C. A High Performance Mg–Al–Ca Alloy Processed by High Pressure Die Casting: Microstructure, Mechanical Properties and Thermal Conductivity. Mater. Sci. Eng. A 2022, 849, 143500. [Google Scholar] [CrossRef]
- Li, G.; Zhang, J.; Wu, R.; Feng, Y.; Liu, S.; Wang, X.; Jiao, Y.; Yang, Q.; Meng, J. Development of High Mechanical Properties and Moderate Thermal Conductivity Cast Mg Alloy with Multiple RE via Heat Treatment. J. Mater. Sci. Technol. 2018, 34, 1076–1084. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, J.; Qin, P.; Liu, S.; Yang, Q.; Meng, J.; Wu, R.; Xie, J. Characterization of Elevated-Temperature High Strength and Decent Thermal Conductivity Extruded Mg-Er-Y-Zn Alloy Containing Nano-Spaced Stacking Faults. Mater. Charact. 2019, 155, 109823. [Google Scholar] [CrossRef]
- Li, Z.H.; Sasaki, T.T.; Shiroyama, T.; Miura, A.; Uchida, K.; Hono, K. Simultaneous Achievement of High Thermal Conductivity, High Strength and Formability in Mg-Zn-Ca-Zr Sheet Alloy. Mater. Res. Lett. 2020, 8, 335–340. [Google Scholar] [CrossRef]
- Huang, X.; Bian, M.; Nakatsugawa, I.; Chino, Y.; Sato, M.; Yamazaki, K.; Kido, F.; Ueda, H.; Inoue, M. Simultaneously Achieving Excellent Mechanical Properties and High Thermal Conductivity in a High Mn-Containing Mg-Zn-Ca-Al-Mn Sheet Alloy. J. Alloys Compd. 2021, 887, 161394. [Google Scholar] [CrossRef]
- Zhong, L.; Peng, J.; Li, M.; Wang, Y.; Lu, Y.; Pan, F. Effect of Ce Addition on the Microstructure, Thermal Conductivity and Mechanical Properties of Mg–0.5Mn Alloys. J. Alloys Compd. 2016, 661, 402–410. [Google Scholar] [CrossRef]
| Alloys | Nominal Composition | Actual Composition | |||
|---|---|---|---|---|---|
| Mg | Zn | Nd | Zr | ||
| ZNK510 | Mg-5Zn-1Nd-0.4Zr | 92.88 ± 2.34 | 5.56 ± 0.97 | 1.02 ± 0.03 | 0.54 ± 0.0 |
| ZNK520 | Mg-5Zn-2Nd-0.4Zr | 91.55 ± 3.12 | 5.88 ± 0.88 | 2.18 ± 0.06 | 0.39 ± 0.1 |
| Samples | Area Fraction (%) | Average Size (μm) |
|---|---|---|
| As-rolled ZNK510 | 4.50 | 2.36 |
| As-rolled ZNK520 | 8.03 | 2.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, H.; Zhang, X.; Li, X.; Zhang, Y.; Li, S.; Wang, Y.; Wang, X.; Hu, X.; Li, X.; Xu, C.; et al. Tailoring Microstructure via Rolling to Achieve Concurrent High Strength and Thermal Conductivity in Mg-Zn-Nd-Zr Alloys. Materials 2025, 18, 3578. https://doi.org/10.3390/ma18153578
Shi H, Zhang X, Li X, Zhang Y, Li S, Wang Y, Wang X, Hu X, Li X, Xu C, et al. Tailoring Microstructure via Rolling to Achieve Concurrent High Strength and Thermal Conductivity in Mg-Zn-Nd-Zr Alloys. Materials. 2025; 18(15):3578. https://doi.org/10.3390/ma18153578
Chicago/Turabian StyleShi, Hailong, Xiaohuan Zhang, Xin Li, Yining Zhang, Siqi Li, You Wang, Xiaojun Wang, Xiaoshi Hu, Xuejian Li, Chao Xu, and et al. 2025. "Tailoring Microstructure via Rolling to Achieve Concurrent High Strength and Thermal Conductivity in Mg-Zn-Nd-Zr Alloys" Materials 18, no. 15: 3578. https://doi.org/10.3390/ma18153578
APA StyleShi, H., Zhang, X., Li, X., Zhang, Y., Li, S., Wang, Y., Wang, X., Hu, X., Li, X., Xu, C., Gan, W., & Ding, C. (2025). Tailoring Microstructure via Rolling to Achieve Concurrent High Strength and Thermal Conductivity in Mg-Zn-Nd-Zr Alloys. Materials, 18(15), 3578. https://doi.org/10.3390/ma18153578








