Progress in Surface and Interface Modification Strategies of MXene Materials for Energy Storage Applications
Abstract
1. Introduction
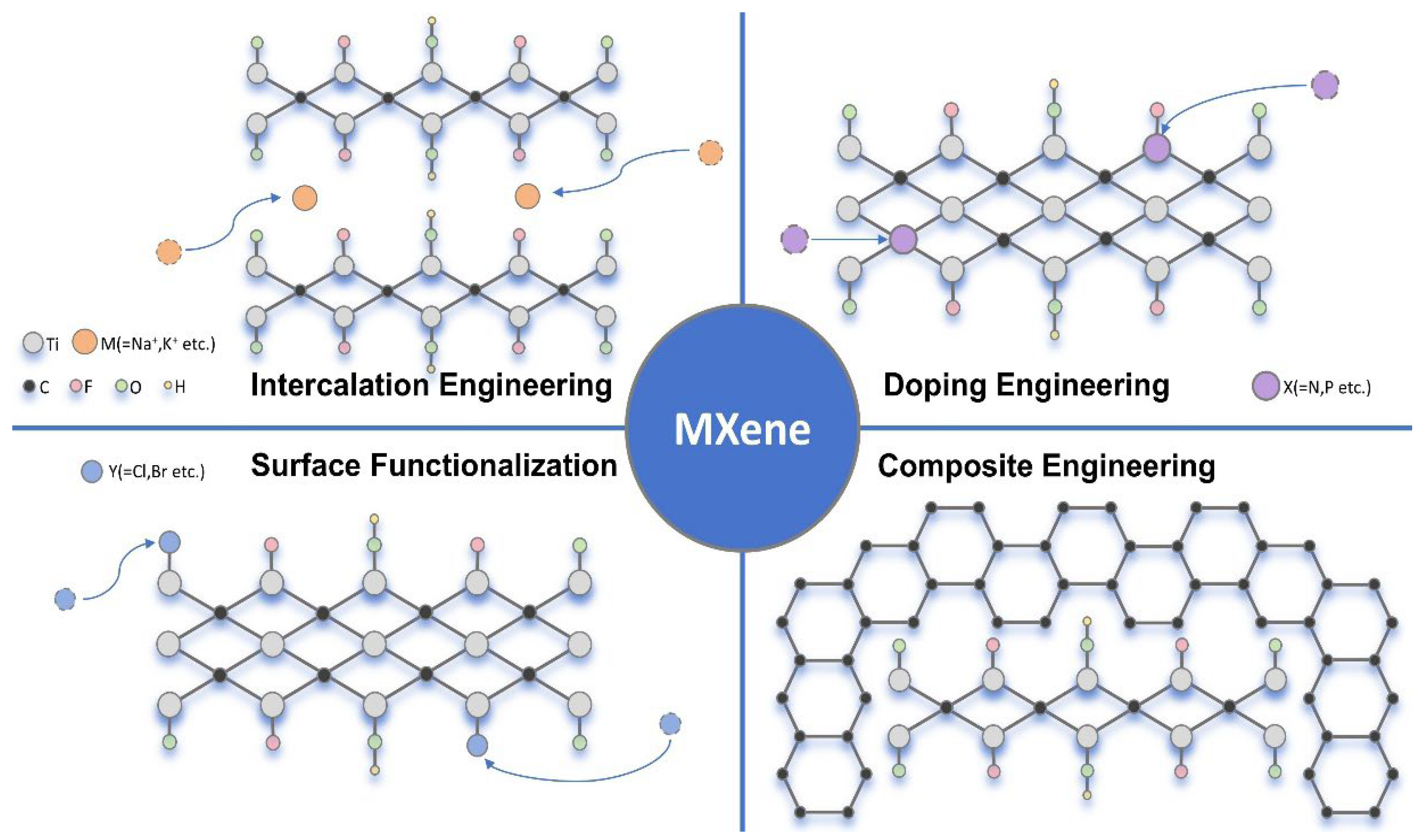
2. Modification Strategies
2.1. Intercalation Engineering of MXene
2.1.1. Metal Ions and Their Oxides’ Intercalation Modification
2.1.2. Non-Metal Ion and Organic Molecule Intercalation Modification
2.2. Surface Functionalization on MXene
2.3. Doping Engineering on MXene
2.4. Composite Engineering on MXene
3. Conclusions and Outlook
Funding
Data Availability Statement
Conflicts of Interest
References
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Sang, X.; Xie, Y.; Lin, M.; Alhabeb, M.; Van Aken, K.L.; Gogotsi, Y.; Kent, P.R.C.; Xiao, K.; Unocic, R.R. Atomic Defects in Monolayer Titanium Carbide (Ti3C2Tx) MXene. ACS Nano 2016, 10, 9193–9200. [Google Scholar] [CrossRef]
- Guan, K.; Dong, L.; Xing, Y.; Li, X.; Luo, J.; Jia, Q.; Zhang, H.; Zhang, S.; Lei, W. Structure and Surface Modification of MXene for Efficient Li/K-Ion Storage. J. Energy Chem. 2022, 75, 330–339. [Google Scholar] [CrossRef]
- Ji, P.; Liu, L.; Liu, Q.; Li, B.; He, S.; Wu, K.; Dong, X.; Liu, Z.; Tai, Y. Oxygen-Rich 3D Hierarchical Porous MXene Prepared by Zn Powder Reduction for Flexible Supercapacitors. Chem. Eng. J. 2024, 497, 154937. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, H.; Fu, T.; Wang, L.; Tang, R.; Tong, Z.; Huang, X. Construction of BiOBr/Ti3C2/Exfoliated Montmorillonite Schottky Junction: New Insights Into Exfoliated Montmorillonite for Inducing MXene Oxygen Functionalization and Enhancing Photocatalytic Activity. Chem. Eng. J. 2022, 438, 135609. [Google Scholar] [CrossRef]
- Baig, M.M.; Saqib, Q.M.; Noman, M.; Sheeraz, M.; Rasheed, A.; Yousuf, M.; Lee, E.; Kim, J.; Ko, Y.; Patil, C.S. Novel Intercalation Approach in MXene Using Modified Silica Nanospheres to Enhance the Surface Charge Density for Superior Triboelectric Performance. Adv. Funct. Mater. 2024, 34, 2408271. [Google Scholar] [CrossRef]
- Chen, B.; Feng, A.; Deng, R.; Liu, K.; Yu, Y.; Song, L. MXene as a Cation-Selective Cathode Material for Asymmetric Capacitive Deionization. ACS Appl. Mater. Interfaces 2020, 12, 13750–13758. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Ng, S.; Lazar, P.; Padinjareveetil, A.K.K.; Michalička, J.; Pumera, M. 2D Germanane-MXene Heterostructures for Cations Intercalation in Energy Storage Applications. Adv. Funct. Mater. 2024, 34, 2308793. [Google Scholar] [CrossRef]
- Huang, M.; Rao, L.; Chen, J.; Wang, X.; Zhou, R.; Yu, F.; Li, X.; Ma, J. Advancements in Electrode Materials: The Role of MXenes in Capacitive Deionization Technology. ACS Mater. Lett. 2025, 7, 1400–1418. [Google Scholar] [CrossRef]
- Li, Z.; Wei, Y.; Liu, Y.; Yan, S.; Wu, M. Dual Strategies of Metal Preintercalation and in Situ Electrochemical Oxidization Operating On MXene for Enhancement of Ion/Electron Transfer and Zinc-Ion Storage Capacity in Aqueous Zinc-Ion Batteries. Adv. Sci. 2023, 10, 2206860. [Google Scholar] [CrossRef]
- Ming, F.; Liang, H.; Zhang, W.; Ming, J.; Lei, Y.; Emwas, A.; Alshareef, H.N. Porous MXenes Enable High Performance Potassium Ion Capacitors. Nano Energy 2019, 62, 853–860. [Google Scholar] [CrossRef]
- Song, S.; Yin, F.; Fu, Y.; Ren, J.; Ma, J.; Liu, Y.; Ma, R.; Ye, W. Simultaneous Regulation of Li-Ion Intercalation and Oxygen Termination Decoration On Ti3C2TX MXene Toward Enhanced Oxygen Electrocatalysis for Li-O2 Batteries. Chem. Eng. J. 2023, 451, 138818. [Google Scholar] [CrossRef]
- Xia, Y.; Que, L.; Yu, F.; Deng, L.; Liu, C.; Sui, X.; Zhao, L.; Wang, Z. Boosting Ion/E− Transfer of Ti3C2 Via Interlayered and Interfacial Co-Modification for High-Performance Li-Ion Capacitors. Chem. Eng. J. 2021, 404, 127116. [Google Scholar] [CrossRef]
- Yin, X.; Zheng, W.; Tang, H.; Yang, L.; Lu, C.; Pan, L.; Zhang, P.; Sun, Z. Unraveling Cation Intercalation Mechanism in MXene for Enhanced Supercapacitor Performance. Energy Storage Mater. 2024, 72, 103688. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Z.; Xie, G.; Guo, X.; Guo, Z.; Song, F.; Li, G.; Chen, C.; Xie, X.; Zhang, N.; et al. Achieving High-Performance 3D K+-Pre-Intercalated Ti3C2Tx MXene for Potassium-Ion Hybrid Capacitors Via Regulating Electrolyte Solvation Structure. Angew. Chem. Int. Ed. 2021, 60, 26246–26253. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, T.; Li, X.L.; Zhou, W.; Ding, M. A “Two-Birds-One-Stone” Strategy to Enhance Capacitive Deionization Performance of Flexible Ti3 C2TX MXene Film Electrodes by Surface Modification. J. Mater. Chem. A 2024, 12, 8734–8746. [Google Scholar] [CrossRef]
- Lu, D.; Lu, Y.; Liang, Y.; Li, J.; Xu, J.; Wu, J.; Huang, H.; Xu, S.; Liang, X.; Zhou, W. The Phosphorus Doping Modification of Ti3C2TX MXene Films Assisted by Tripolyphosphate-Crosslinking for Flexible Supercapacitors. J. Energy Storage 2024, 100, 113524. [Google Scholar] [CrossRef]
- Sun, Y.; Li, T.; Liu, X.; Liu, Y.; Zada, A.; Han, Y.; Han, Y.; Chen, J.; Dang, A. Exceptional Suppression of the Self-Discharge Behavior of Supercapacitors by Precisely Tuning the Surface Assets of MXene by a Spontaneous Single-Atom Doping Strategy. Nano Lett. 2025, 25, 3875–3882. [Google Scholar] [CrossRef]
- Kumar, S.; Mehdi, S.M.Z.; Taunk, M.; Kumar, S.; Aherwar, A.; Singh, S.; Singh, T. Synergistic Effects of Polymer Integration On the Properties, Stability, and Applications of MXenes. J. Mater. Chem. A 2025, 3, 11050–11113. [Google Scholar] [CrossRef]
- Luo, Y.; Que, W.; Tang, Y.; Kang, Y.; Bin, X.; Wu, Z.; Yuliarto, B.; Gao, B.; Henzie, J.; Yamauchi, Y. Regulating Functional Groups Enhances the Performance of Flexible Microporous MXene/Bacterial Cellulose Electrodes in Supercapacitors. ACS Nano 2024, 18, 11675–11687. [Google Scholar] [CrossRef]
- Xue, Y.; Chao, S.; Xu, M.; Wu, Q.; Zhang, Q.; Liu, Y.; Wu, F.; Liu, L.; Javed, M.S.; Zhang, W. Multi-Layers Hexagonal Hole MXene Trap Constructed by Carbon Vacancy Defect Regulation Strategy Enables High Energy Density Potassium-Ions Storage. Energy Storage Mater. 2024, 71, 103558. [Google Scholar] [CrossRef]
- Zhang, T.; Chang, L.; Zhang, X.; Wan, H.; Liu, N.; Zhou, L.; Xiao, X. Simultaneously Tuning Interlayer Spacing and Termination of MXenes by Lewis-Basic Halides. Nat. Commun. 2022, 13, 6731. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yao, X.; Wang, G.; Xie, Y.; Wu, T.; Zhou, N.; Wei, Y.; Qu, G. Interlayer Spacing Optimization Combined with Zinc-Philic Engineering Fostering Efficient Zn2+ Storage of V2CTX MXenes for Aqueous Zinc-Ion Batteries. Small 2025, 21, 2408930. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, X.; Wang, H.; Wang, P.; Wu, K.; Cheng, Y.; Xiao, B. Achieving High Electrical Conductivity, Energy Storage Capacity and Cycling Stability in Ammoniated Mo2TiC2TX MXenes as an Anode for Lithium-Ion Batteries. J. Mater. Chem. A 2024, 12, 26962–26979. [Google Scholar] [CrossRef]
- Mullani, S.; Kim, C.; Lokhande, V.; Ji, T. MXene Structural and Surface Modifications for Enhanced Li-Ion Diffusion in Lithium-Ion Capacitors: A Critical Mini Review of Recent Advances. Chem. Eng. J. 2025, 510, 161565. [Google Scholar] [CrossRef]
- Qiu, X.; Dai, L.; Li, H.; Qu, K.; Li, R. Pillaring Behavior of Organic Molecules On MXene: Insights From Molecular Dynamics Simulations. Langmuir 2023, 39, 14912–14921. [Google Scholar] [CrossRef]
- Li, J.; Lu, M.; Zheng, W.; Zhang, W. Ion-Intercalation Architecture for Robust Functionalization of Two-Dimensional MXenes. Energy Storage Mater. 2024, 64, 103068. [Google Scholar] [CrossRef]
- Zou, J.; Wu, J.; Wang, Y.; Deng, F.; Jiang, J.; Zhang, Y.; Liu, S.; Li, N.; Zhang, H.; Yu, J. Additive-Mediated Intercalation and Surface Modification of MXenes. Chem. Soc. Rev. 2022, 51, 2972–2990. [Google Scholar] [CrossRef]
- Arole, K.; Pas, S.E.; Thakur, R.M.; Amiouny, L.A.; Kabir, M.H.; Dujovic, M.; Radovic, M.; Lutkenhaus, J.L.; Green, M.J.; Liang, H. Effects of Intercalation On ML-Ti3C2TZ MXene Properties and Friction Performance. ACS Appl. Mater. Interfaces 2024, 16, 64156–64165. [Google Scholar] [CrossRef]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and Delamination of Layered Carbides and Carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, X.; Ai, W.; He, Z.; Lou, S.; Tang, Z.; Hang, F.; Liu, Z.; Ou, Y.; Hu, X. Intercalation-Deintercalation Engineering of Van Der Waals Stacked MXene Films for Wearable Thermoelectrics and Sensing. Chem. Eng. J. 2025, 512, 162603. [Google Scholar] [CrossRef]
- Xu, M.; Lei, S.; Qi, J.; Dou, Q.; Liu, L.; Lu, Y.; Huang, Q.; Shi, S.; Yan, X. Opening Magnesium Storage Capability of Two-Dimensional MXene by Intercalation of Cationic Surfactant. ACS Nano 2018, 12, 3733–3740. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Matsumoto, R.A.; Zhao, W.; Osti, N.C.; Popov, I.; Thapaliya, B.P.; Fleischmann, S.; Misra, S.; Prenger, K.; Tyagi, M. Engineering the Interlayer Spacing by Pre-Intercalation for High Performance Supercapacitor MXene Electrodes in Room Temperature Ionic Liquid. Adv. Funct. Mater. 2021, 31, 2104007. [Google Scholar] [CrossRef]
- Osti, N.C.; Liang, K.; Prenger, K.; Thapaliya, B.P.; Tyagi, M.; Dai, S.; Naguib, M.; Mamontov, E. Optimizing Protic Ionic Liquid Electrolyte for Pre-Intercalated Ti3C2TX MXene Supercapacitor Electrodes. APL Energy 2024, 2, 4. [Google Scholar] [CrossRef]
- Valurouthu, G.; Panigrahi, R.; Saraf, M.; Shuck, C.E.; Mallik, B.S.; Kurra, N.; Gogotsi, Y. Ambipolar Electrochemistry of Pre-Intercalated Ti3C2Tx MXene in Ionic Liquid Electrolyte. Batter. Supercaps 2023, 6, e202300009. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, C.J.; Zhou, F.; Dong, Y.; Shi, X.; Nicolosi, V.; Wu, Z.; Bao, X. Ionic Liquid Pre-Intercalated MXene Films for Ionogel-Based Flexible Micro-Supercapacitors with High Volumetric Energy Density. J. Mater. Chem. A 2019, 7, 9478–9485. [Google Scholar] [CrossRef]
- Campanella, B.; Simoncini, M.; Passaglia, E.; Cicogna, F.; Ciancaleoni, G.; González-Rivera, J.; Bernazzani, L.; Bramanti, E. Ecofriendly Preparation of Rosmarinic Acid-Poly(Vinyl Alcohol) Biofilms Using NADES/DES, Ultrasounds and Optimization Via a Mixture-Process Design Strategy. Materials 2024, 17, 377. [Google Scholar] [CrossRef]
- Długosz, O. Natural Deep Eutectic Solvents in the Synthesis of Inorganic Nanoparticles. Materials 2023, 16, 627. [Google Scholar] [CrossRef]
- Pang, J.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.; Rummeli, M.H. Applications of 2D MXenes in Energy Conversion and Storage Systems. Chem. Soc. Rev. 2019, 48, 72–133. [Google Scholar] [CrossRef]
- Bashir, T.; Ismail, S.A.; Wang, J.; Zhu, W.; Zhao, J.; Gao, L. MXene Terminating Groups =O,–F Or–OH,–F Or =O,–OH,–F, Or =O,–OH,–Cl? J. Energy Chem. 2023, 76, 90–104. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Y.; Zhang, M.; Sui, J.; Peng, W.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. N-Butyllithium-Treated Ti3C2TX MXene with Excellent Pseudocapacitor Performance. ACS Nano 2019, 13, 9449–9456. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Lin, C.; Yang, Y.; Xu, L.; Du, X.; Xie, J.; Lin, J.; Sun, J. Achieving High Pseudocapacitance of 2D Titanium Carbide (MXene) by Cation Intercalation and Surface Modification. Adv. Energy Mater. 2017, 7, 1602725. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Wu, Y.; Huang, H.; Jiang, Q. Chemically Functionalized Two-Dimensional Titanium Carbide MXene by in Situ Grafting-Intercalating with Diazonium Ions to Enhance Supercapacitive Performance. J. Phys. Chem. Solids 2018, 115, 172–179. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Luo, K.; Li, Y.; Chang, K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P. Element Replacement Approach by Reaction with Lewis Acidic Molten Salts to Synthesize Nanolaminated MAX Phases and MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Qin, G.; Luo, K.; Lu, J.; Li, Y.; Liang, G.; Huang, Z.; Zhou, J.; Hultman, L. Halogenated Ti3C2 MXenes with Electrochemically Active Terminals for High-Performance Zinc Ion Batteries. ACS Nano 2021, 15, 1077–1085. [Google Scholar] [CrossRef]
- Kamysbayev, V.; Filatov, A.S.; Hu, H.; Rui, X.; Lagunas, F.; Wang, D.; Klie, R.F.; Talapin, D.V. Covalent Surface Modifications and Superconductivity of Two-Dimensional Metal Carbide MXenes. Science 2020, 369, 979–983. [Google Scholar] [CrossRef]
- Tian, F.; Wang, F.; Nie, W.; Zhang, X.; Xia, X.; Chang, L.; Pang, Z.; Yu, X.; Li, G.; Hu, S. Tailoring Oxygen-Depleted and Unitary Ti3C2TX Surface Terminals by Molten Salt Electrochemical Etching Enables Dendrite-Free Stable Zn Metal Anode. Angew. Chem. Int. Ed. 2024, 63, e202408996. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Que, L.; Yu, F.; Deng, L.; Liang, Z.; Jiang, Y.; Sun, M.; Zhao, L.; Wang, Z. Tailoring Nitrogen Terminals On MXene Enables Fast Charging and Stable Cycling Na-Ion Batteries at Low Temperature. Nano-Micro Lett. 2022, 14, 143. [Google Scholar] [CrossRef]
- Li, H.; Chen, S.; Boukhvalov, D.W.; Yu, Z.; Humphrey, M.G.; Huang, Z.; Zhang, C. Switching the Nonlinear Optical Absorption of Titanium Carbide MXene by Modulation of the Surface Terminations. ACS Nano 2022, 16, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Mei, S.; Ye, X.; Yuan, H.; Li, X.; Tan, J.; Zhao, X.; Wu, T.; Chen, X.; Wu, F.; et al. Enhancing Lithium–Sulfur Battery Performance with MXene: Specialized Structures and Innovative Designs. Adv. Sci. 2024, 11, 2404328. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Su, D.; Xie, X.; Guo, X.; Bao, W.; Shao, G.; Wang, G. Immobilizing Polysulfides with MXene-Functionalized Separators for Stable Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2016, 8, 29427–29433. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, Q.; Liu, Y.; Xu, B. Status and Prospects of MXene-Based Lithium–Sulfur Batteries. Adv. Funct. Mater. 2021, 31, 2100457. [Google Scholar] [CrossRef]
- Zhang, X.; Ni, Z.; Bai, X.; Shen, H.; Wang, Z.; Wei, C.; Tian, K.; Xi, B.; Xiong, S.; Feng, J. Hierarchical Porous N-Doped Carbon Encapsulated Fluorine-Free MXene with Tunable Coordination Chemistry by One-Pot Etching Strategy for Lithium–Sulfur Batteries. Adv. Energy Mater. 2023, 13, 2301349. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, S.; Cui, Z.; Li, Z.; Wu, S.; Xu, W.; Ba, T.; Liang, Y.; Jiang, H. Solvent-Free One-Step Green Synthesis of MXenes by "Gas-Phase Selective Etching". Energy Storage Mater. 2024, 70, 103503. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, S.; Cui, Z.; Li, Z.; Wu, S.; Xu, W.; Gao, Z.; Ba, T.; Liang, C.; Liang, Y. Dual Redox Reaction Sites for Pseudocapacitance Based On Ti and− P Functional Groups of Ti3C2PBrX MXene. Angew. Chem. Int. Ed. 2024, 63, e202403508. [Google Scholar]
- Ma, W.; Li, T.; Tang, H.; Qiao, Z. MXenes Etching Method and Dispersion in Organic Solvents. Chin. J. Appl. Chem. 2023, 40, 1044–1053. [Google Scholar]
- Wang, D.; Li, F.; Lian, R.; Xu, J.; Kan, D.; Liu, Y.; Chen, G.; Gogotsi, Y.; Wei, Y. A General Atomic Surface Modification Strategy for Improving Anchoring and Electrocatalysis Behavior of Ti3C2T2 MXene in Lithium–Sulfur Batteries. ACS Nano 2019, 13, 11078–11086. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, S.; Hu, R.; Gu, J.; Cui, Y.; Li, B.; Chen, W.; Liu, C.; Shang, J.; Yang, S. Catalytic Conversion of Polysulfides On Single Atom Zinc Implanted MXene Toward High-Rate Lithium–Sulfur Batteries. Adv. Funct. Mater. 2020, 30, 2002471. [Google Scholar] [CrossRef]
- Sahoo, S.; Kumar, R.; Hussain, I.; Zhang, K. Heteroatom Doping in 2D MXenes for Energy Storage/Conversion Applications. Adv. Powder Mater. 2024, 3, 100246. [Google Scholar] [CrossRef]
- Guan, Y.; Jiang, S.; Ding, Y.; Xiao, B.; Pi, Y.; Wang, Z.; Cong, Y. Insight Into the Mechanism of Nitrogen Doping in MXenes with Controllable Surface Chemistry. Mater. Today Energy 2024, 44, 101642. [Google Scholar] [CrossRef]
- Li, D.; Zheng, X.; Boutinaud, P.; Hu, Y.; Xiao, S.; Xu, J.; Wang, C.; Hou, Y.; He, Z.; Huang, W. An Overview of Nitrogen-Doped MXenes and their Recent Developments. Responsive Mater. 2024, 2, e20240015. [Google Scholar] [CrossRef]
- Bao, W.; Liu, L.; Wang, C.; Choi, S.; Wang, D.; Wang, G. Facile Synthesis of Crumpled Nitrogen-Doped Mxene Nanosheets as a New Sulfur Host for Lithium–Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1702485. [Google Scholar] [CrossRef]
- Chen, X.; Zhai, X.; Hou, J.; Cao, H.; Yue, X.; Li, M.; Chen, L.; Liu, Z.; Ge, G.; Guo, X. Tunable Nitrogen-Doped Delaminated 2D MXene Obtained by NH3/Ar Plasma Treatment as Highly Efficient Hydrogen and Oxygen Evolution Reaction Electrocatalyst. Chem. Eng. J. 2021, 420, 129832. [Google Scholar] [CrossRef]
- Xu, C.; Jiang, W.; Guo, L.; Shen, M.; Li, B.; Wang, J. High Supercapacitance Performance of Nitrogen-Doped Ti3C2Tx Prepared by Molten Salt Thermal Treatment. Electrochim. Acta 2022, 403, 139528. [Google Scholar] [CrossRef]
- Yu, L.; Fan, Z.; Shao, Y.; Tian, Z.; Sun, J.; Liu, Z. Versatile N-Doped MXene Ink for Printed Electrochemical Energy Storage Application. Adv. Energy Mater. 2019, 9, 1901839. [Google Scholar] [CrossRef]
- Liu, K.; Xia, Q.; Si, L.; Kong, Y.; Shinde, N.; Wang, L.; Wang, J.; Hu, Q.; Zhou, A. Defect Engineered Ti3C2TX MXene Electrodes by Phosphorus Doping with Enhanced Kinetics for Supercapacitors. Electrochim. Acta 2022, 435, 141372. [Google Scholar] [CrossRef]
- Huang, H.; Yan, L.; Liang, Y.; Li, C.; Li, J.; Liang, X.; Xu, S.; Zhou, W.; Guo, J. Enhanced Pseudocapacitance of Ti3C2TX MXene by UV Photochemical Doping. Appl. Phys. Lett. 2023, 123, 13. [Google Scholar] [CrossRef]
- Panda, A.; Kim, H. Phosphorus-Decorated Mo-MXene/CQD Hybrid: A 2D/0D Architecture for Bifunctional Electrochemical Water Splitting. Nanoscale 2021, 13, 14795–14806. [Google Scholar] [CrossRef]
- Li, J.; Yan, D.; Hou, S.; Li, Y.; Lu, T.; Yao, Y.; Pan, L. Improved Sodium-Ion Storage Performance of Ti3C2TX MXenes by Sulfur Doping. J. Mater. Chem. A 2018, 6, 1234–1243. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zhou, J.; Sun, D.; Li, H. Preparation of High Conductive Medium and Establishment of Laege Capacity Conductive Channel. Adv. Mater. 2023, 35, 2307363. [Google Scholar] [CrossRef]
- Wu, Y.; Bo, T.; Tu, H.; Wang, L.; Shi, W. U and Co Dual Single-Atom Doped MXene for Accelerating Electrocatalytic Hydrogen Evolution Activity. Small 2024, 20, 2402847. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yan, W.; Liu, Z.; Liu, X.; Tian, Y.; Cui, X. Optimizing Electronic Structure of Mo2TiC2Tx MXene through Nb Doping for Enhanced Electrochemical Performance. Nano Res. 2024, 17, 7174–7181. [Google Scholar] [CrossRef]
- Jaberi, S.S.; Asen, P.; Esfandiar, A.; Tolstoy, V.P. MXene/Carbon Hybrid Nanostructures and Heteroatom-Doped Derivatives for Enhanced Electrochemical Energy Storage. J. Energy Storage 2024, 90, 111751. [Google Scholar] [CrossRef]
- Yu, L.P.; Zhou, X.H.; Lu, L.; Xu, L.; Wang, F.J. MXene/Carbon Nanotube Hybrids: Synthesis, Structures, Properties, and Applications. ChemSusChem 2021, 14, 5079–5111. [Google Scholar] [CrossRef]
- Yuan, X.; Li, S.; Qin, R.; Zhang, M.; Zhu, B.; Cao, W. Advances in Constructing Carbon Fiber-MXene Multilevel Structures to Reinforce Interfacial Properties of Carbon Fiber Reinforced Polymer Composites. Polym. Compos. 2025, 46, 5041–5063. [Google Scholar] [CrossRef]
- Muthukutty, B.; Kumar, P.S.; Vivekanandan, A.K.; Sivakumar, M.; Lee, S.; Lee, D. Progress and Perspective in Harnessing MXene–Carbon-Based Composites (0–3D): Synthesis, Performance, and Applications. Chemosphere 2024, 355, 141838. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Liu, S.C.; Wu, Y.X.; Wu, Y.Y.; Peng, L.D.; Wang, Y.J.; Nie, F.; Zhao, L.; Lv, P.Y.; Cao, C.F. Chemical-Physical Synergistic Assembly of MXene/CNT Nanocoatings in Silicone Foams for Reliable Piezoresistive Sensing in Harsh Environments. Small 2024, 20, 2406102. [Google Scholar] [CrossRef]
- Yi, G.; Du, L.; Wei, G.; Zhang, H.; Yu, H.; Quan, X.; Chen, S. Selective Molecular Separation with Conductive MXene/CNT Nanofiltration Membranes Under Electrochemical Assistance. J. Membr. Sci. 2022, 658, 120719. [Google Scholar] [CrossRef]
- Zhao, X.; Zha, X.; Tang, L.; Pu, J.; Ke, K.; Bao, R.; Liu, Z.; Yang, M.; Yang, W. Self-Assembled Core-Shell Polydopamine@MXene with Synergistic Solar Absorption Capability for Highly Efficient Solar-to-Vapor Generation. Nano Res. 2020, 13, 255–264. [Google Scholar] [CrossRef]
- Qin, J.; Lu, H.; Yang, S.; Tian, T.; Su, W. Investigation of Dielectric and Energy Storage Properties of PDA@MXene/Poly(Vinylidene Fluoride) Nanodielectrics. Polym. Bull. 2025, 82, 5037–5059. [Google Scholar] [CrossRef]
- Cai, Y.; Fang, Y.; Cao, W.; He, P.; Cao, M. MXene-CNT/PANI Ternary Material with Excellent Supercapacitive Performance Driven by Synergy. J. Alloy. Compd. 2021, 868, 159159. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, X.; Jin, X.; Zhang, C.; Zhang, X.; Liu, X.; Li, Y.; Li, Y.; Lin, J.; Gao, H. Multifunction Integration within Magnetic CNT-Bridged MXene/CoNi Based Phase Change Materials. eScience 2024, 4, 100292. [Google Scholar] [CrossRef]
- He, S.; Sun, X.; Zhang, H.; Yuan, C.; Wei, Y.; Li, J. Preparation Strategies and Applications of MXene-Polymer Composites: A Review. Macromol. Rapid Commun. 2021, 42, 2100324. [Google Scholar] [CrossRef] [PubMed]
- Saharudin, M.S.; Ayub, A.; Hasbi, S.; Muhammad-Sukki, F.; Shyha, I.; Inam, F. Recent Advances in MXene Composites Research, Applications and Opportunities. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Munir, K.S.; Zheng, Y.; Zhang, D.; Lin, J.; Li, Y.; Wen, C. Microstructure and Mechanical Properties of Carbon Nanotubes Reinforced Titanium Matrix Composites Fabricated Via Spark Plasma Sintering. Mater. Sci. Eng. A 2017, 688, 505–523. [Google Scholar] [CrossRef]
- Thirumal, V.; Yuvakkumar, R.; Kumar, P.S.; Ravi, G.; Velauthapillai, D. Si@ MXene/Graphene Crumbled Spherical Nanocomposites. Int. J. Energy Res. 2022, 46, 21548–21557. [Google Scholar] [CrossRef]
- Shao, R.; Wang, G.; Chai, J.; Lin, J.; Zhao, G.; Zeng, Z.; Wang, G. Multifunctional Janus-Structured Polytetrafluoroethylene-Carbon Nanotube-Fe3O4/MXene Membranes for Enhanced EMI Shielding and Thermal Management. Nano-Micro Lett. 2025, 17, 1–22. [Google Scholar] [CrossRef]
- Wang, J.; He, J.; Kan, D.; Chen, K.; Song, M.; Huo, W. MXene Film Prepared by Vacuum-Assisted Filtration: Properties and Applications. Crystals 2022, 12, 1034. [Google Scholar] [CrossRef]
- Liu, C.; Ma, Y.; Xie, Y.; Zou, J.; Wu, H.; Peng, S.; Qian, W.; He, D.; Zhang, X.; Li, B. Enhanced Electromagnetic Shielding and Thermal Management Properties in MXene/Aramid Nanofiber Films Fabricated by Intermittent Filtration. ACS Appl. Mater. Interfaces 2023, 15, 4516–4526. [Google Scholar] [CrossRef]
- Yang, B.; Wang, L.; Zhao, J.; Pang, R.; Yuan, B.; Tan, J.; Song, S.; Nie, J.; Zhang, M. A Robust, Flexible, Hydrophobic, and Multifunctional Pressure Sensor Based On an MXene/Aramid Nanofiber (ANF) Aerogel Film. ACS Appl. Mater. Interfaces 2022, 14, 47075–47088. [Google Scholar] [CrossRef]
- Liang, L.; Yao, C.; Yan, X.; Feng, Y.; Hao, X.; Zhou, B.; Wang, Y.; Ma, J.; Liu, C.; Shen, C. High-Efficiency Electromagnetic Interference Shielding Capability of Magnetic Ti3C2TX MXene/CNT Composite Film. J. Mater. Chem. A 2021, 9, 24560–24570. [Google Scholar] [CrossRef]
- Peng, T.; Wang, S.; Xu, Z.; Tang, T.; Zhao, Y. Multifunctional MXene/Aramid Nanofiber Composite Films for Efficient Electromagnetic Interference Shielding and Repeatable Early Fire Detection. ACS Omega 2022, 7, 29161–29170. [Google Scholar] [CrossRef] [PubMed]
- Allah, A.E.; Wang, J.; Kaneti, Y.V.; Li, T.; Farghali, A.A.; Khedr, M.H.; Nanjundan, A.K.; Ding, B.; Dou, H.; Zhang, X. Auto-Programmed Heteroarchitecturing: Self-Assembling Ordered Mesoporous Carbon Between Two-Dimensional Ti3C2TX MXene Layers. Nano Energy 2019, 65, 103991. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, E.; Kim, D.; Ahn, C.W.; Kim, B.S.; Ahn, K.H.; Lee, Y.; Park, J.D. Tuning the Microstructure and Rheological Properties of MXene-Polymer Composite Ink by Interaction Control. Korea-Aust. Rheol. J. 2023, 35, 117–125. [Google Scholar] [CrossRef]
- Liu, Z.; Xiong, H.; Luo, Y.; Zhang, L.; Hu, K.; Zhang, L.; Gao, Y.; Qiao, Z.A. Interface-Induced Self-Assembly Strategy Toward 2D Ordered Mesoporous Carbon/MXene Heterostructures for High-Performance Supercapacitors. ChemSusChem 2021, 14, 4422–4430. [Google Scholar] [CrossRef]
- Solangi, N.H.; Abbas, A.; Mubarak, N.M.; Karri, R.R.; Aleithan, S.H.; Kazmi, J.; Ahmad, W.; Khan, K. Insight Mechanism of MXene for the Future Generation of Highly Efficient Energy Storage Device. Mater. Today Sustain. 2024, 27, 100896. [Google Scholar] [CrossRef]
- Zhang, P.; Ru, X.; Li, H.; Liang, H.; Wang, H.; Yang, C.; Zhang, X.; Liu, Z.; Zhang, Q.; Chen, Y. Efficient MXene/CNT Electromagnetic Shielding Composite Films with Self-Assembly Multilayer Structure. J. Mater. Sci.-Mater. Electron. 2023, 34, 39. [Google Scholar] [CrossRef]
- Li, S.; Peng, Z.; Huang, Y.; Tan, L.; Chen, Y. Electrostatic Self-Assembly of MXene and Carbon Nanotube@MnO2 Multilevel Hybrids for Achieving Fast Charge Storage Kinetics in Aqueous Asymmetric Supercapacitors. J. Mater. Chem. A 2022, 10, 23886–23895. [Google Scholar] [CrossRef]
- Thirumal, V.; Yuvakkumar, R.; Kumar, P.S.; Ravi, G.; Keerthana, S.P.; Velauthapillai, D. Facile Single-Step Synthesis of MXene@ CNTs Hybrid Nanocomposite by CVD Method to Remove Hazardous Pollutants. Chemosphere 2022, 286, 131733. [Google Scholar] [CrossRef]
- Wan, Y.; Xiong, P.; Liu, J.; Feng, F.; Xun, X.; Gama, F.M.; Zhang, Q.; Yao, F.; Yang, Z.; Luo, H. Ultrathin, Strong, and Highly Flexible Ti3C2TX MXene/Bacterial Cellulose Composite Films for High-Performance Electromagnetic Interference Shielding. ACS Nano 2021, 15, 8439–8449. [Google Scholar] [CrossRef]
- Wen, C.; Liu, H.; Luo, L.; Cui, Z.; Li, X.; Jin, J.; Yan, S.; Lin, P.; Sa, B. Ultrathin and Flexible PDA Modified MXene/Bacterial Cellulose Composite Film with a Dense Lamellar Structure for Enhanced Electromagnetic Interference Shielding Performance. J. Mater. Chem. C 2024, 12, 17037–17049. [Google Scholar] [CrossRef]
- Li, X.; You, W.; Xu, C.; Wang, L.; Yang, L.; Li, Y.; Che, R. 3D Seed-Germination-Like MXene with in Situ Growing CNTs/Ni Heterojunction for Enhanced Microwave Absorption Via Polarization and Magnetization. Nano-Micro Lett. 2021, 13, 1–14. [Google Scholar] [CrossRef]
- Kulkarni, M.; Lalic, A.; Balu, R.; Zhang, H.; Dutta, N.K.; Roy Choudhury, N. Hydrothermal Synthesis of MXenes in Alkali Environment and Development of MXene/PEDOT:PSS Composite Electrodes for Supercapacitor Applications. MRS Adv. 2024, 9, 1310–1317. [Google Scholar] [CrossRef]
- Yang, B.; Liu, B.; Chen, J.; Ding, Y.; Sun, Y.; Tang, Y.; Yan, X. Realizing High-Performance Lithium Ion Hybrid Capacitor with a 3D MXene-Carbon Nanotube Composite Anode. Chem. Eng. J. 2022, 429, 132392. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.; Gu, H.; Cui, Y.; Gao, R.; Dai, W. In Situ Growth of Cd0.5Zn0.5S Nanorods On Ti3C2 MXene Nanosheet for Efficient Visible-Light-Driven Photocatalytic Hydrogen Evolution. Acta Phys.-Chim. Sin. 2024, 41, 100031. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Lin, Y.; Zhang, Y. Hydrothermal Synthesis of MoS2/MXene Composites for High-Performance Supercapacitors. Mater. Today Commun. 2025, 44, 111864. [Google Scholar] [CrossRef]
- Guo, T.; Lei, Y.; Hu, X.; Yang, G.; Liang, J.; Huang, Q.; Li, X.; Liu, M.; Zhang, X.; Wei, Y. Hydrothermal Synthesis of MXene-MoS2 Composites for Highly Efficient Removal of Pesticides. Appl. Surf. Sci. 2022, 588, 152597. [Google Scholar] [CrossRef]
- Dangbegnon, J.; Garino, N.; Angelozzi, M.; Laurenti, M.; Seller, F.; Serrapede, M.; Zaccagnini, P.; Moras, P.; Cocuzza, M.; Ouisse, T. High-Performance Novel Asymmetric MXene@ CNT//N-Doped CNT Flexible Hybrid Device with Large Working Voltage for Energy Storage. J. Energy Storage 2023, 63, 106975. [Google Scholar] [CrossRef]
- Sikkema, R.; Baker, K.; Zhitomirsky, I. Electrophoretic Deposition of Polymers and Proteins for Biomedical Applications. Adv. Colloid. Interface. Sci. 2020, 284, 102272. [Google Scholar] [CrossRef]
- Yang, R.; Gui, X.; Yao, L.; Hu, Q.; Yang, L.; Zhang, H.; Yao, Y.; Mei, H.; Tang, Z. Ultrathin, Lightweight, and Flexible CNT Buckypaper Enhanced Using MXenes for Electromagnetic Interference Shielding. Nano-Micro Lett. 2021, 13, 66. [Google Scholar] [CrossRef]
- Namvari, M.; Chakrabarti, B.K. Electrophoretic Deposition of MXenes and their Composites: Toward a Scalable Approach. Adv. Colloid. Interface. Sci. 2024, 331, 103208. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Huang, L.; Liu, Z.; Tian, Y.; Zhang, X. Ti3C2Tx MXene-Modified Domestic High-Modulus High-Strength Carbon Fibers Based On Electrophoretic Deposition Method. Acta Mater. Compos. Sin. 2024, 41, 2968–2979. [Google Scholar]
- Li, X.; Fu, X.; Zhang, Y.; Zhang, Q.; Wei, Z.; Wang, K.; Li, R.K.; Shi, D.; Jin, J. Electrospun Sodium Titanate-MXene/Carbon Nanofibers as Binder-Free Electrode for Enhanced Hybrid Capacitive Deionization. Chem. Eng. J. 2025, 511, 162040. [Google Scholar] [CrossRef]
- He, M.; Qian, W.; Li, H.; Li, Z.; Chen, H.; Zhou, Y.; Bu, X.; Wang, Y. Ultrathin and Flexible Carbonized MXene@ PAN/Ni Films with Alternating Multilayered Structure for Superior EMI Shielding, Joule Heating and Mechanical Performance. Chem. Eng. J. 2025, 506, 159777. [Google Scholar] [CrossRef]
- You, M.; Xin, B. MXene Nanosheets and Carbon Nanofiber Hybrid Membranes for Electrochemical Energy Storage Materials. Fiber. Polym. 2024, 25, 3323–3330. [Google Scholar] [CrossRef]
- Ding, X.; Xun, H.; Cao, Q.; Zhang, X.; Zhou, H.; Niu, H. High-Comfort, Ultrathin Air-Layer Nanofiber Composite Membrane for Thermal Insulation in Complex Environments. Sustain. Mater. Technol. 2025, 44, e01382. [Google Scholar] [CrossRef]
- Ji, Y.; Li, W.; You, Y.; Xu, G. In Situ Synthesis of M (Fe, Cu, Co and Ni)-MOF@ MXene Composites for Enhanced Specific Capacitance and Cyclic Stability in Supercapacitor Electrodes. Chem. Eng. J. 2024, 496, 154009. [Google Scholar] [CrossRef]
- Hu, M.; Wang, L.; Li, S.; Yan, W. Research Progress of MXene as Anodes for Sodium-Ion Batteries. Acta Mater. Compos. Sin. 2025, 42, 1158–1177. [Google Scholar]
- Niu, S.; Xu, Z.; Kang, X.; Zhao, L.; Zou, R.; Liu, W.; Ran, F. Customizing MXene@ CoSe2 for Cathode Host of Lithium–Sulfur Batteries to Promote the Redox Kinetics of Lithium Polysulfides. J. Mater. Sci. Mater. Electron. 2024, 35, 693. [Google Scholar] [CrossRef]
- Wang, L.; Guo, J.; Qi, Q.; Li, X.; Ge, Y.; Li, H.; Chao, Y.; Du, J.; Cui, X. Revisiting Dipole-Induced Fluorinated-Anion Decomposition Reaction for Promoting a LiF-Rich Interphase in Lithium-Metal Batteries. Nano-Micro Lett. 2025, 17, 111. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, Y.; Wang, Z.; Wang, Z.; Dong, Z.; Zeng, Q.; Hui, K.N.; Liu, Z.; Peng, Z. A High-Rate and Ultrastable Re2Te5/MXene Anode for Potassium Storage Enabled by Amorphous/Crystalline Heterointerface Engineering. Adv. Mater. 2024, 36, 2407134. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Q.; Meng, X.; Zhen, M.; Hu, Z.; Shen, B. Research Status and Perspectives of MXene-Based Materials for Aqueous Zinc-Ion Batteries. Rare Metals 2024, 43, 1867–1885. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Liu, X.; Yang, R.; Qiu, J.; Xu, J.; Lu, B.; Rosen, J.; Qin, L.; Jiang, J. MXene-Stabilized VS2 Nanostructures for High-Performance Aqueous Zinc Ion Storage. Adv. Sci. 2024, 11, 2401252. [Google Scholar] [CrossRef]





















| Modification Strategy | Key Advantages | Key Drawbacks | Typical Applications |
|---|---|---|---|
| Intercalation |
|
| Li+/Na+/K+ batteries; high-rate supercapacitors; ion sieving |
| Surface Functionalization |
|
| High-power electrodes; Li–S battery separators; electrocatalysis |
| Doping Engineering |
|
| Metal-ion batteries; advanced catalysts; low-temperature storage |
| Composite Engineering |
|
| Flexible/stable electrodes; EMI shielding; photothermal films |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Hu, J.; Liu, X.; Liu, F. Progress in Surface and Interface Modification Strategies of MXene Materials for Energy Storage Applications. Materials 2025, 18, 3576. https://doi.org/10.3390/ma18153576
Han Y, Hu J, Liu X, Liu F. Progress in Surface and Interface Modification Strategies of MXene Materials for Energy Storage Applications. Materials. 2025; 18(15):3576. https://doi.org/10.3390/ma18153576
Chicago/Turabian StyleHan, Yizhao, Junhua Hu, Xinhong Liu, and Fanfan Liu. 2025. "Progress in Surface and Interface Modification Strategies of MXene Materials for Energy Storage Applications" Materials 18, no. 15: 3576. https://doi.org/10.3390/ma18153576
APA StyleHan, Y., Hu, J., Liu, X., & Liu, F. (2025). Progress in Surface and Interface Modification Strategies of MXene Materials for Energy Storage Applications. Materials, 18(15), 3576. https://doi.org/10.3390/ma18153576






