Preparation of Glass-Ceramics Using Zinc-Containing Smelting Slag: Structure, Properties and Solidification of Zinc
Abstract
1. Introduction
2. Experimental Section
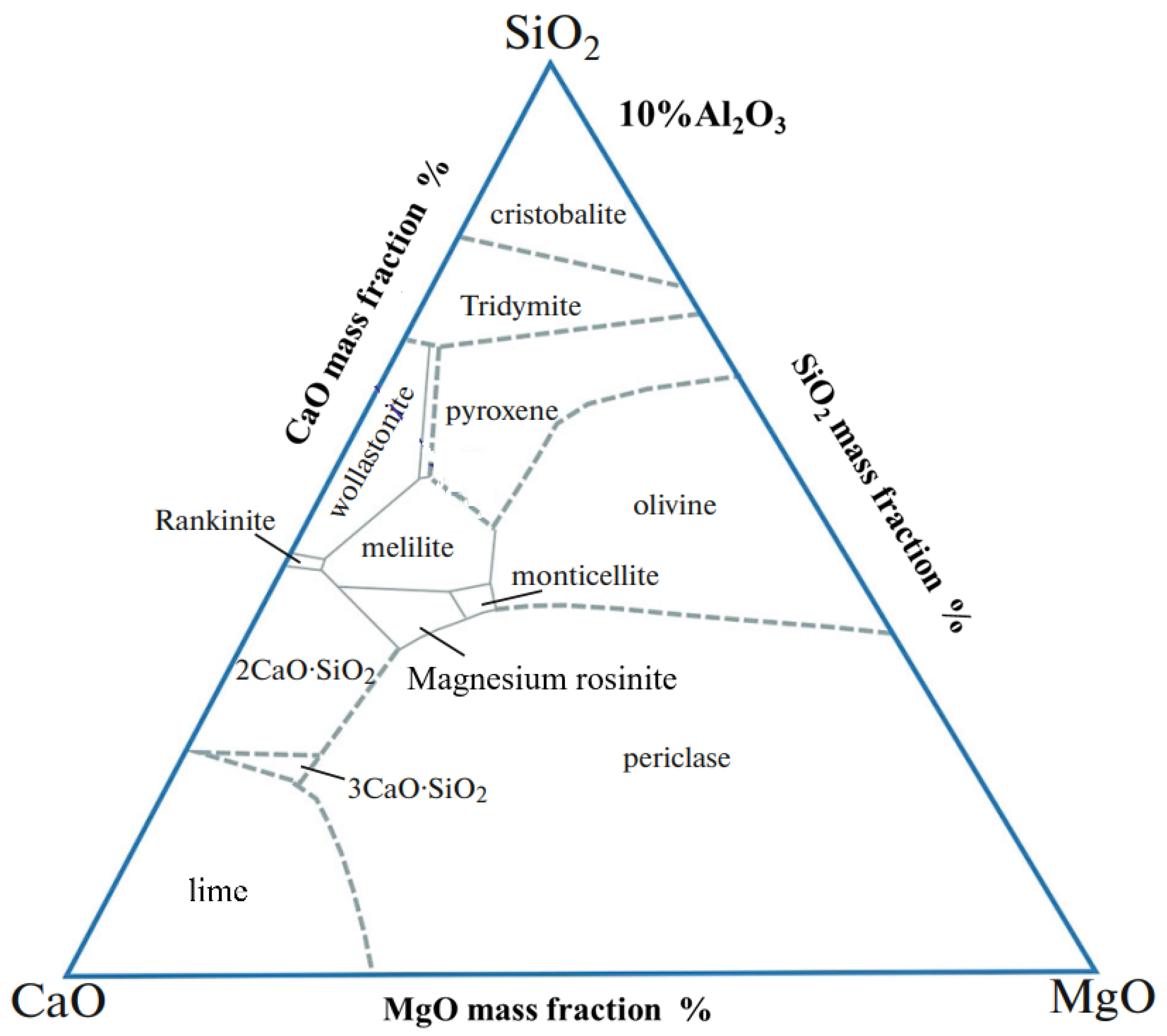
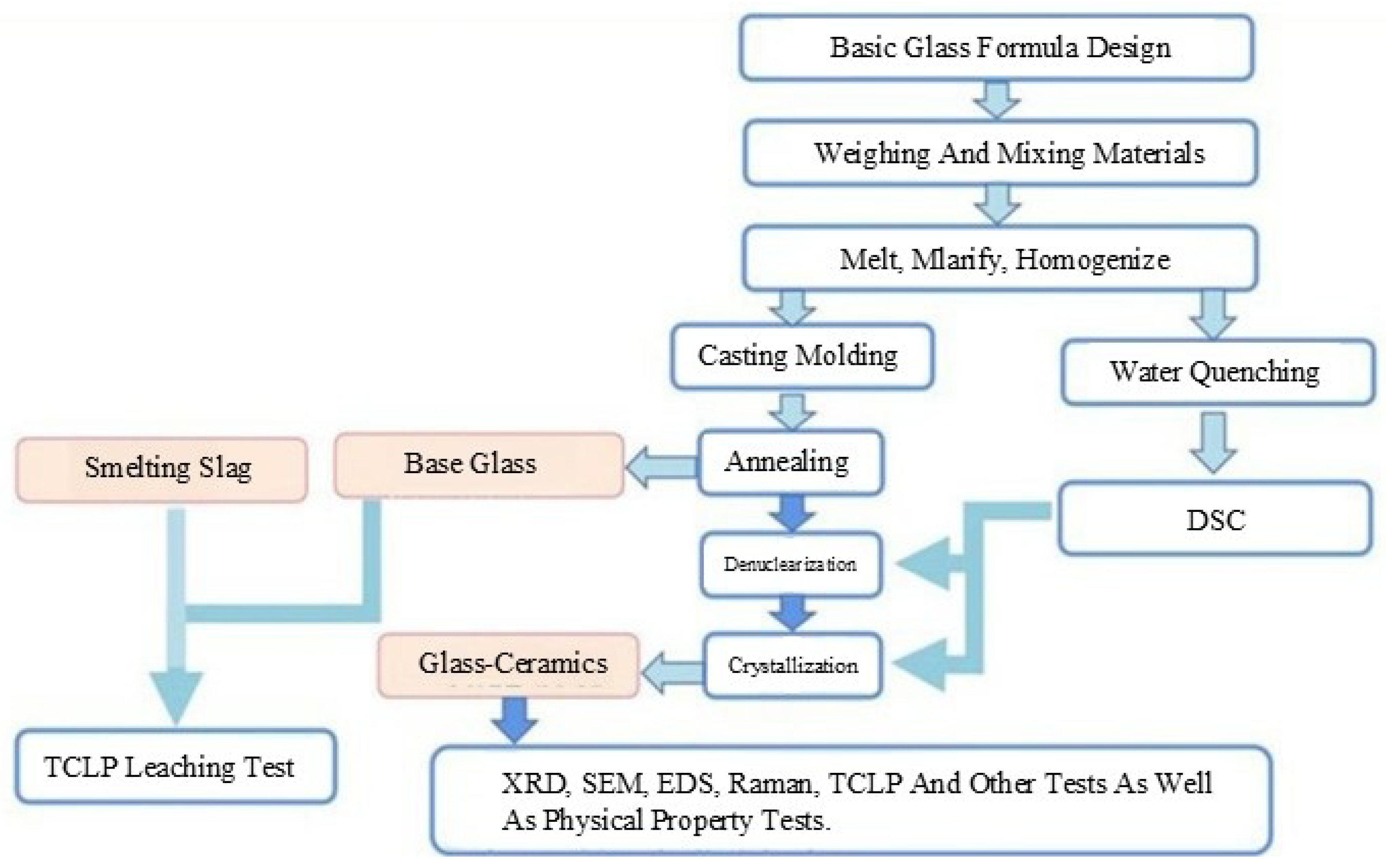
2.1. Sample Preparation Process
- (1)
- Ingredients: Weigh the corresponding raw materials based on the formula.
- (2)
- Mixing: Put the weighed ingredients into the jar mill and run it at a speed of 150 revolutions per minute for 30 min to ensure thorough mixing of the ingredients.
- (3)
- Molten water quenching: Place the well-mixed ingredients in a corundum crucible, place in a high-temperature resistance furnace, and melt at 1550 °C for 3 h, and quickly pour the molten glass liquid into cold water for water quenching to obtain molten water quenching slag. After the molten water quenching slag is crushed and passed through a 200-mesh sieve, DSC testing can be conducted. Correspondingly, the subsequent heat treatment system of the sample can be further determined.
- (4)
- Casting molding: The uniform melt is rapidly poured onto a metal mold to obtain glass, which is transferred to a preheated annealing furnace at 600 °C for annealing for 10 h to reduce residual internal stress. Subsequently, it is cooled to room temperature in the furnace and taken out.
- (5)
- After cutting the base glass into regular shapes of specific sizes, the glass samples can undergo further heat treatment and testing.
2.2. Testing Method
3. Results and Discussion
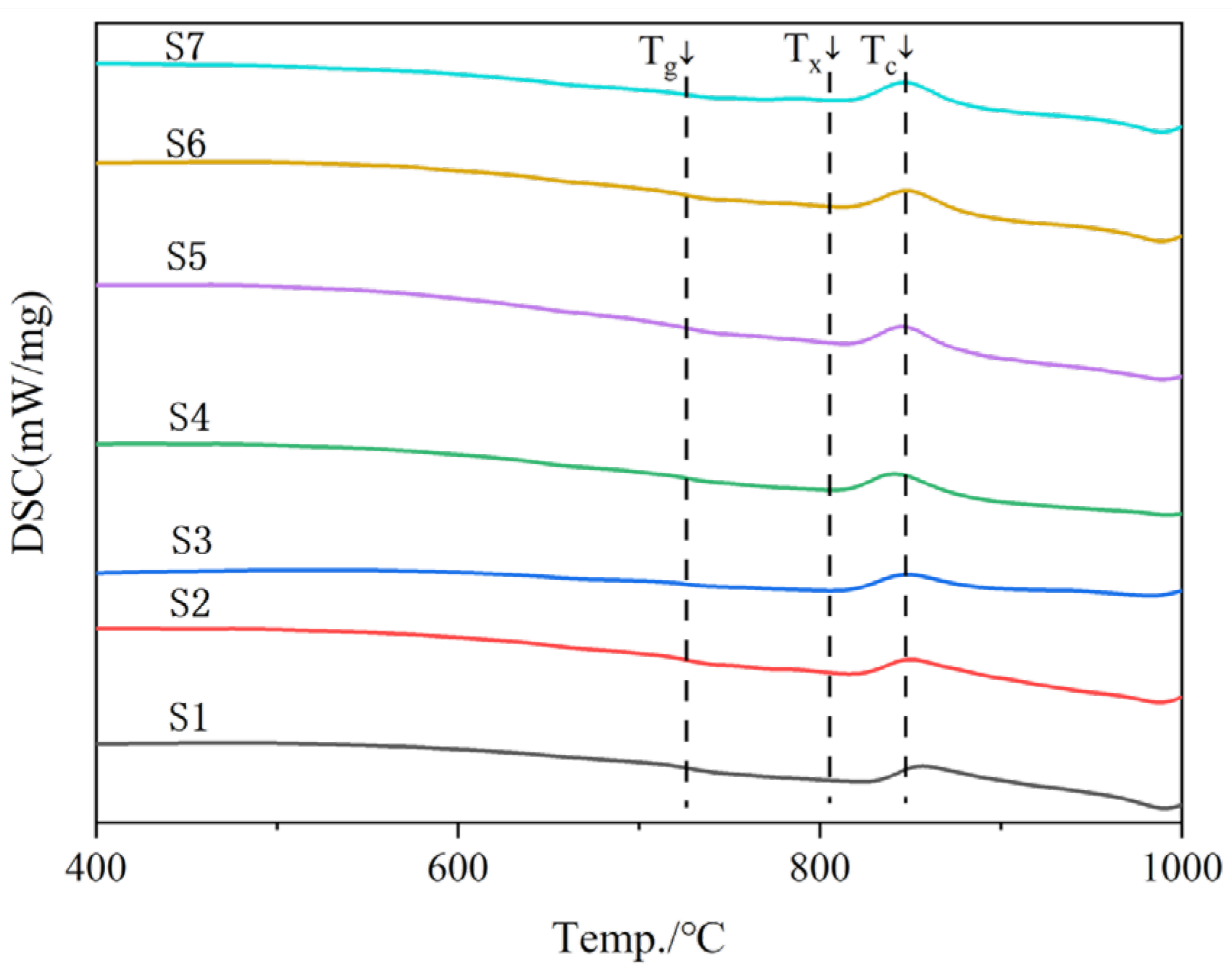
3.1. Differential Scanning Calorimetry
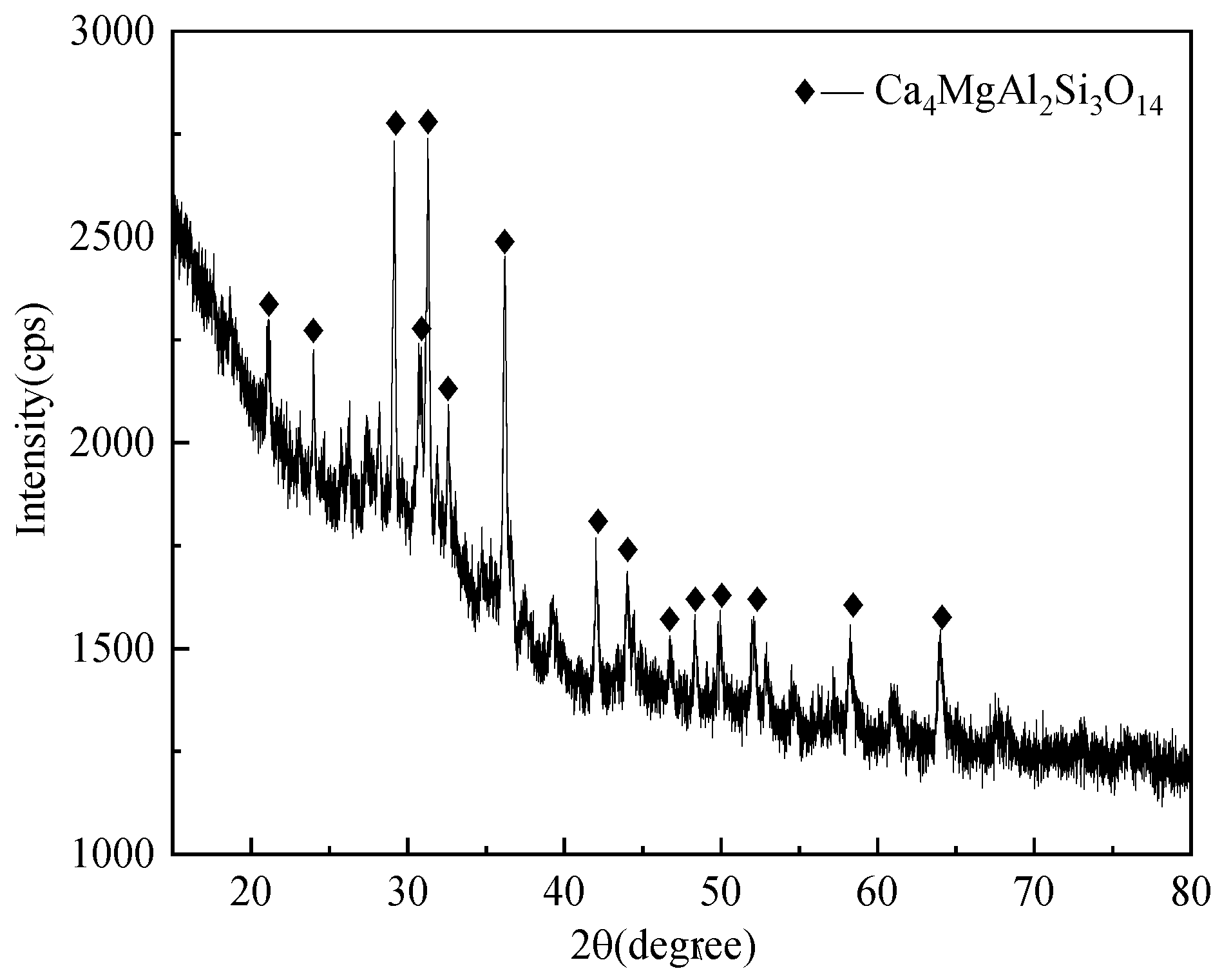
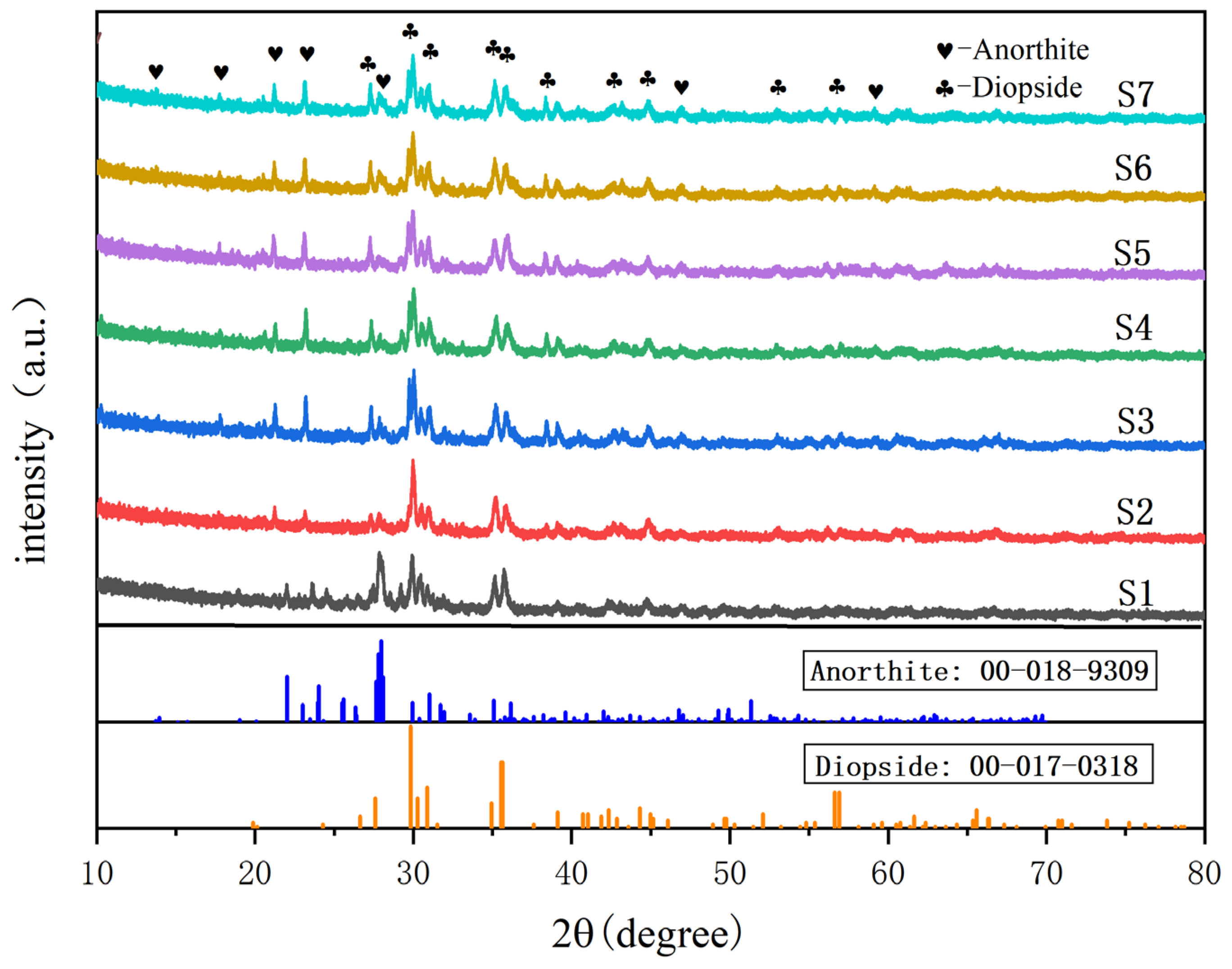
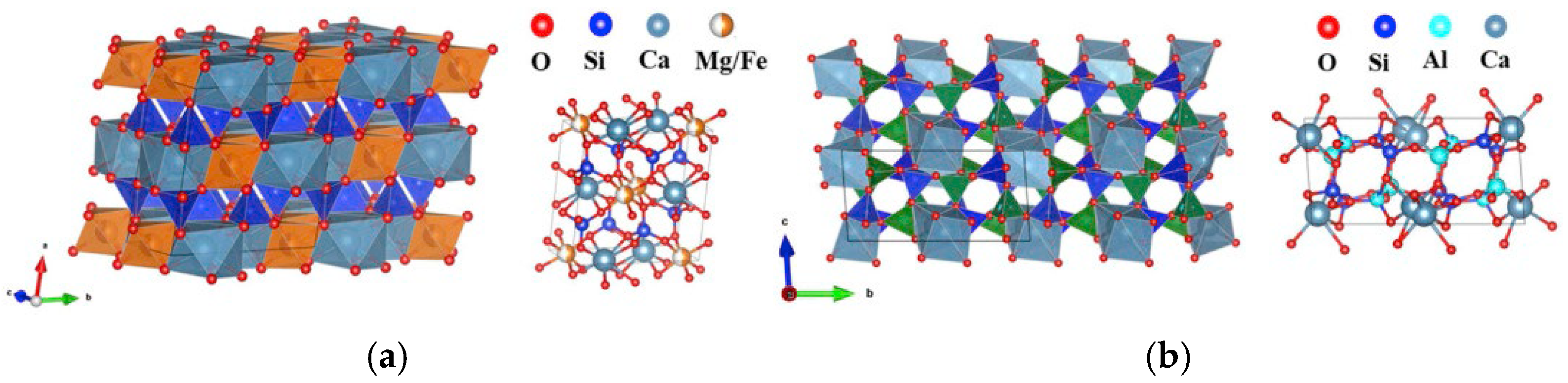
3.2. Phase Composition Analysis of Slag Glass-Ceramics
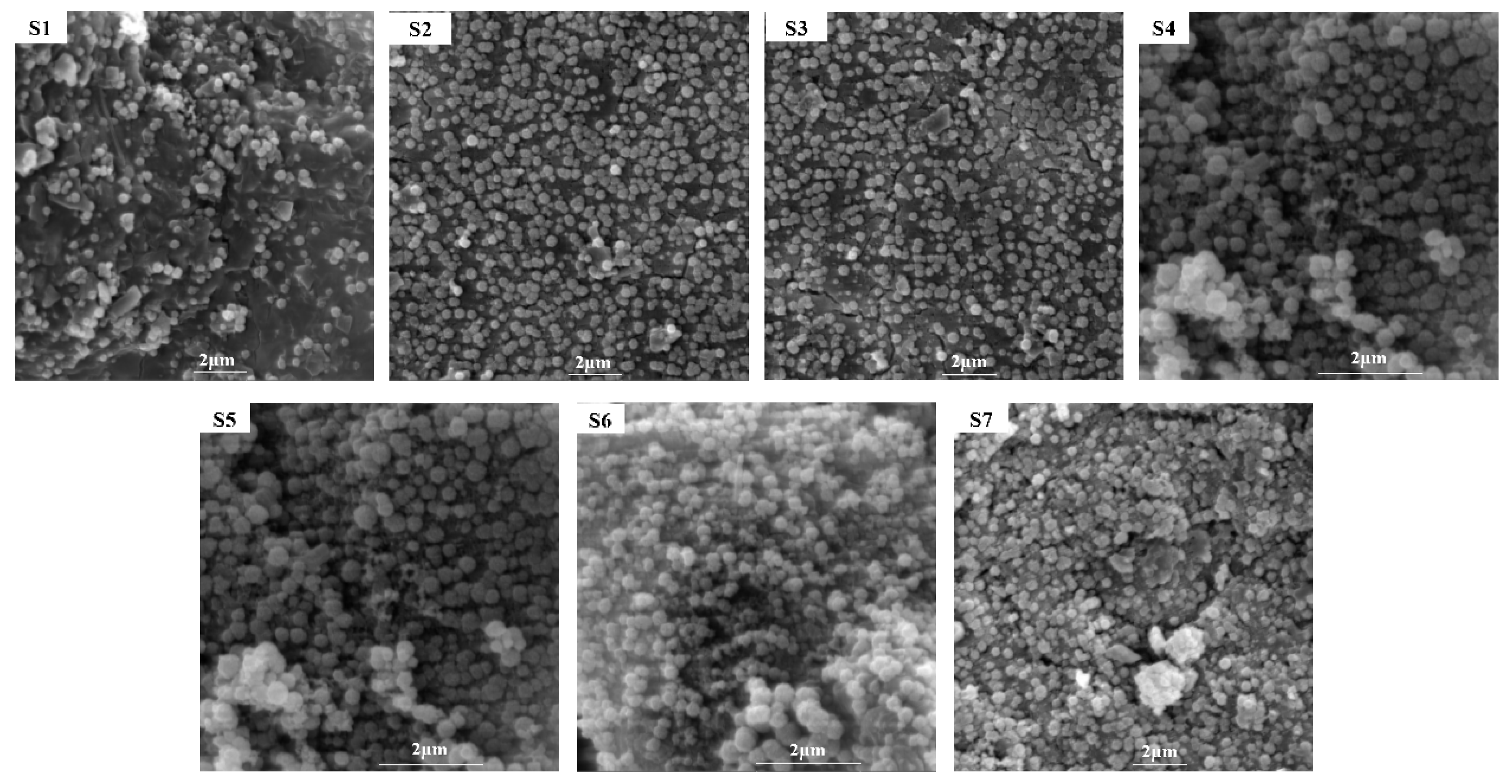
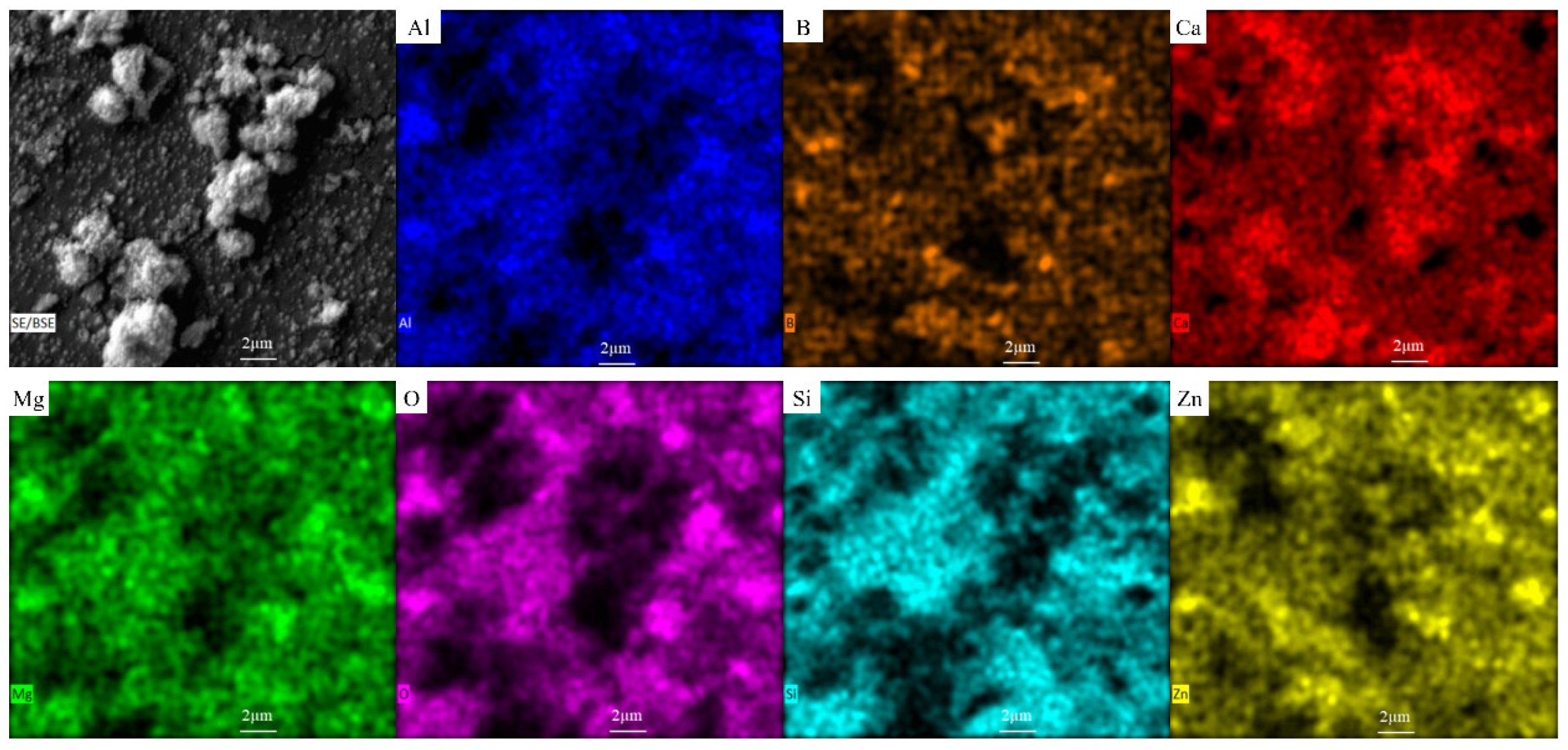
3.3. Microstructure Analysis of Glass-Ceramics
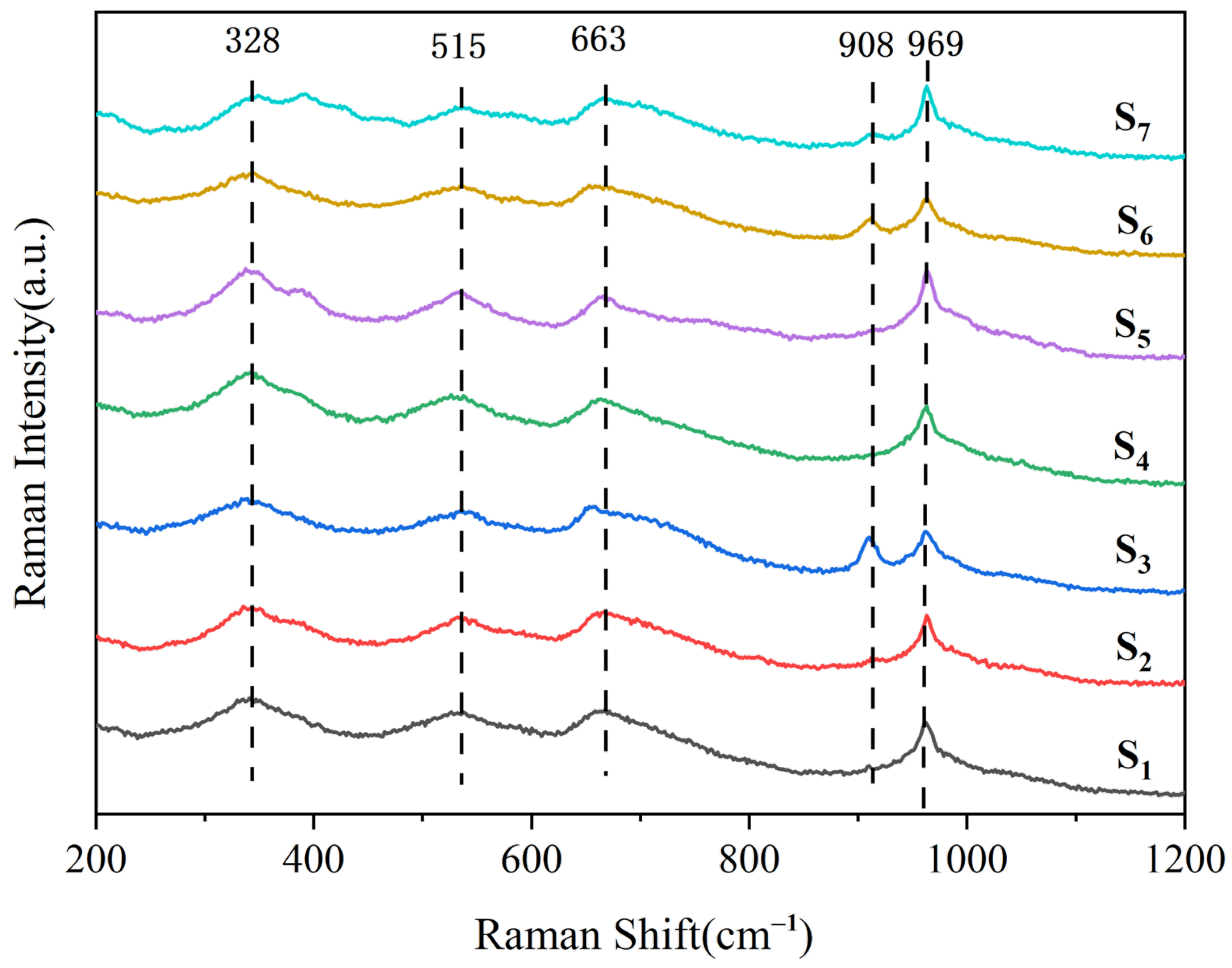
3.4. Raman Spectroscopy Analysis
3.5. Physical Properties of Samples with Different Content of Smelting Slag
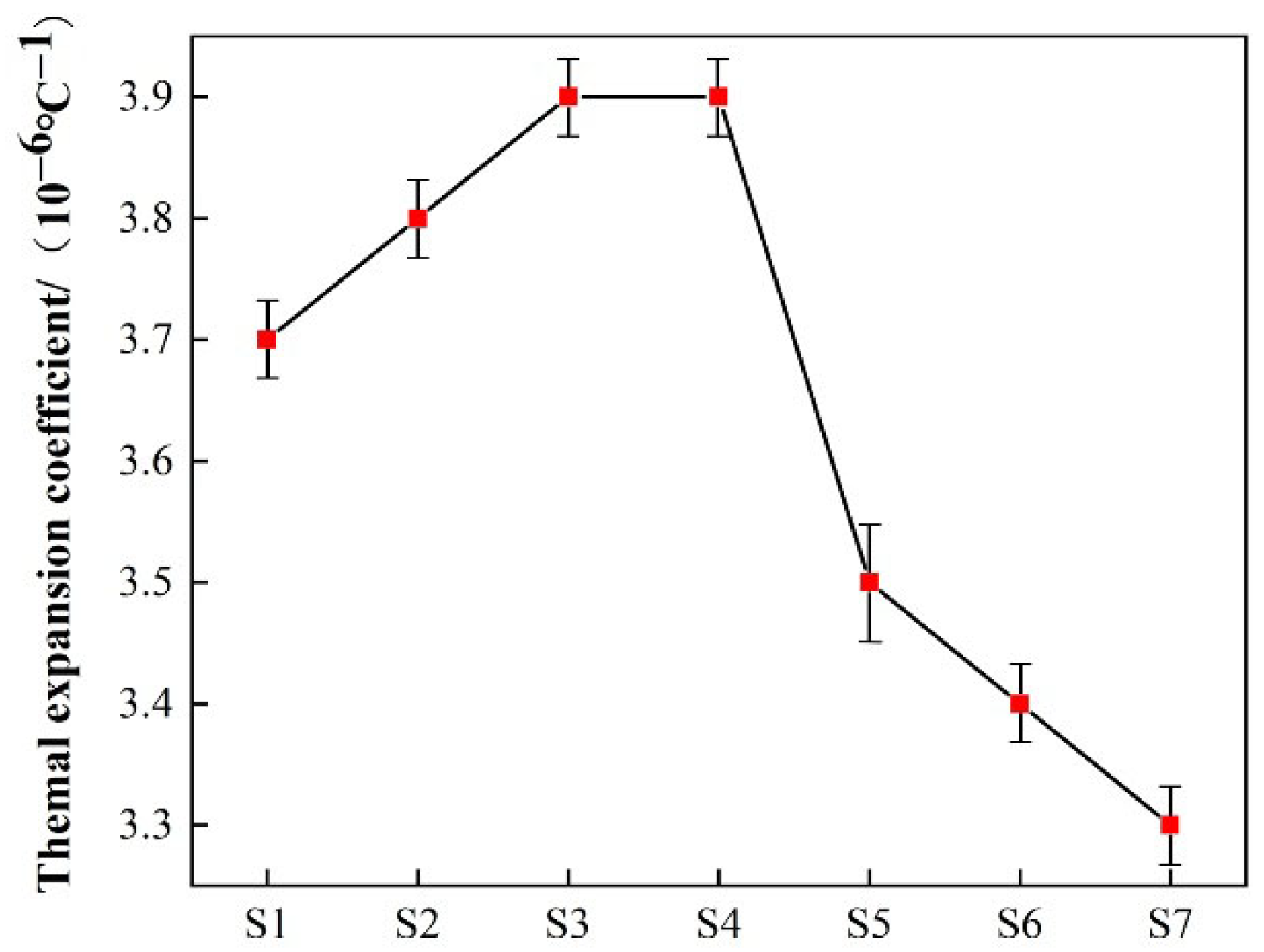
3.6. Coefficient of Thermal Expansion
3.7. Study on Acid and Alkali Corrosion Resistance of Slag Glass Ceramics
3.8. Toxicity Leaching Test
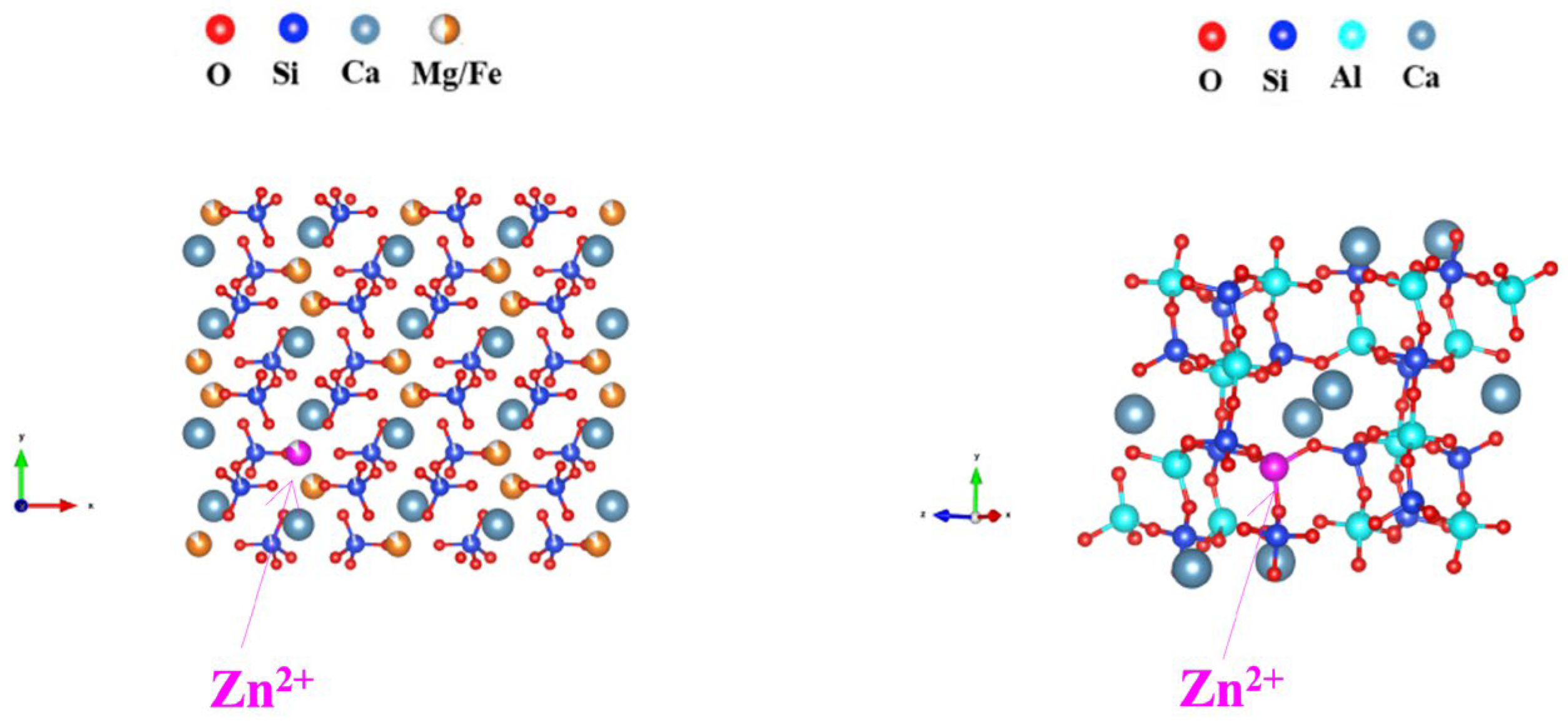
3.9. Heavy Metal Curing Mechanism of Slag Glass-Ceramics
4. Conclusions
- (1)
- Zinc as a network modifier can effectively enter the glass structure. As the doping amount of zinc-containing smelting slag continuously increases, the glass transition temperature and crystallization temperature decrease, which benefits the crystallization of the glass.
- (2)
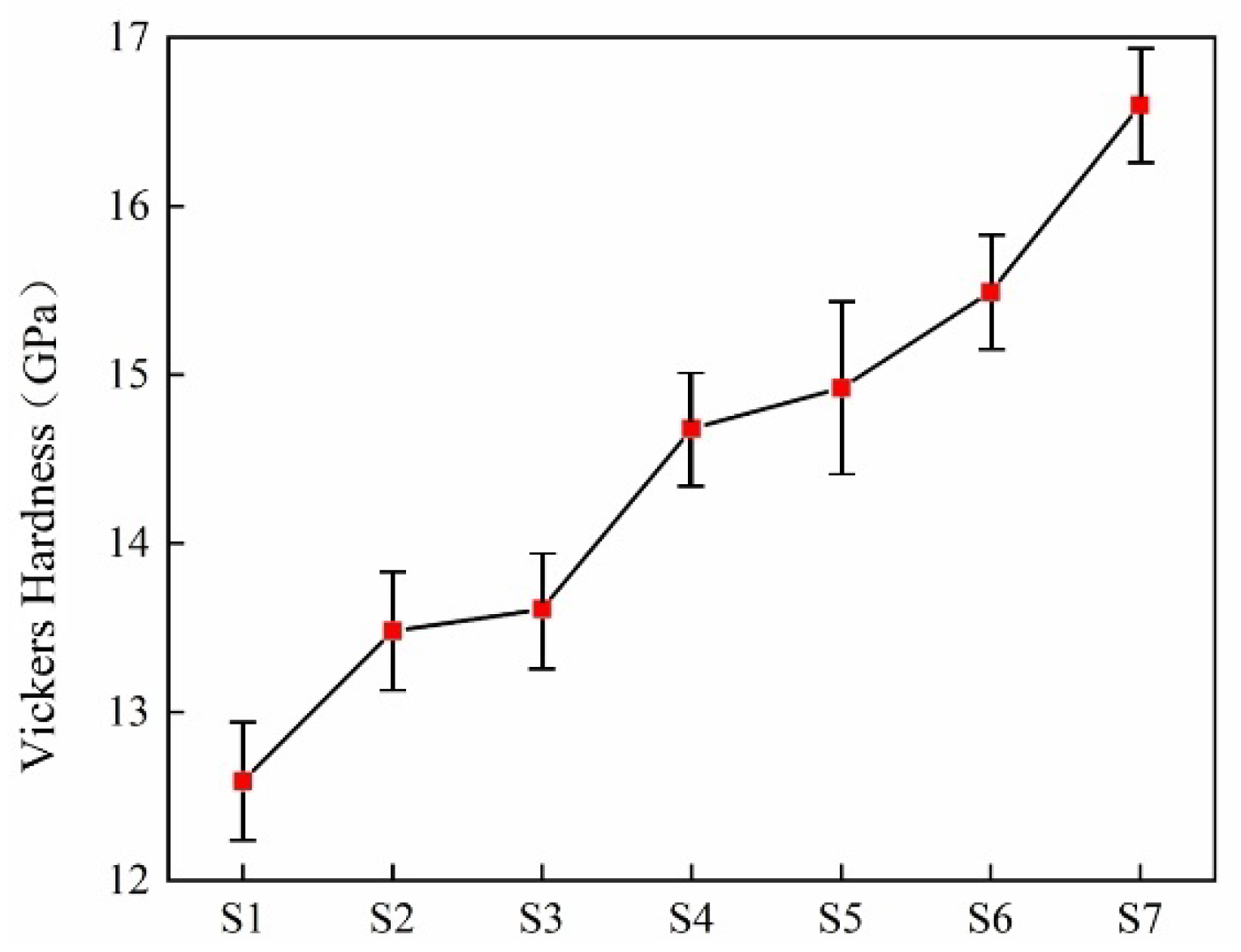
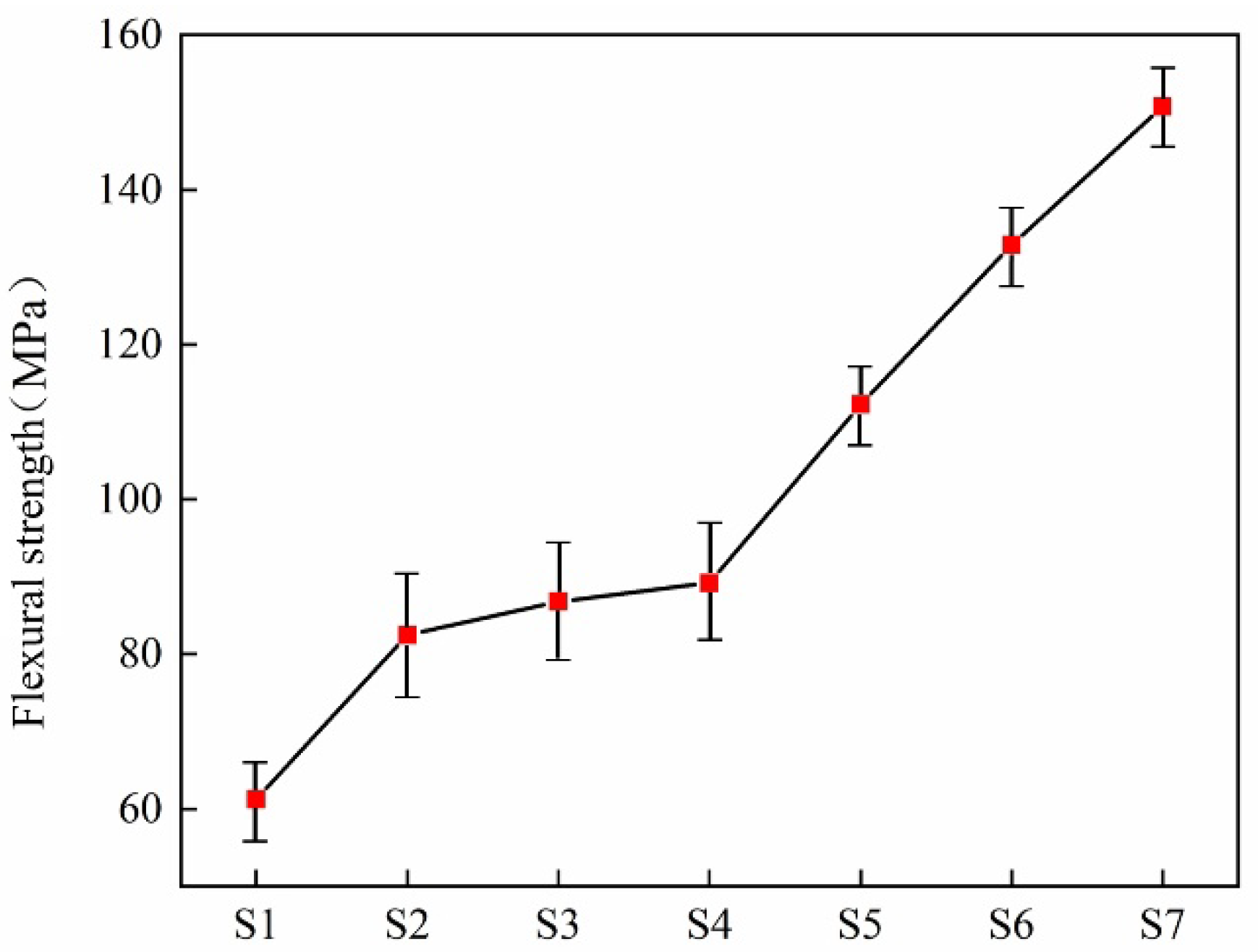
- The main crystalline phases in the glass-ceramics are diopside and anorthite. With the increase in the doping amount of smelting slag, the glass ceramics form a denser microstructure; at the same time, the density and acid and alkali resistance of the sample increase. The introduction of zinc significantly enhances the mechanical properties of the glass-ceramics. The maximum Vickers hardness reached 19.60 GPa, and the maximum flexural strength achieved was 160.75 MPa. The glass-ceramics exhibit excellent physical and chemical properties.
- (3)
- The heavy metal zinc, whose content is high in the smelting slag, is cured in the amorphous and crystalline phases of the slag glass-ceramics as a solid solution, where Zn2+ substitutes for Al3+ and Fe/Mg2+ in the crystal. The slag glass-ceramics effectively cure heavy metal zinc, and the leaching concentration of zinc meets the requirements of the toxicity leaching standard, and the leaching rate tends to stabilize. This approach reduces environmental pollution and health risks while lowering disposal costs. The zinc-containing smelting slag is transformed into high-performance, non-toxic, and harmless glass-ceramic materials, achieving the resource utilization of solid waste.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Zhang, X.; Xing, Z.; Cen, R.; Du, C. Assessment and Source Apportionment of Heavy Metal Pollution in Soil of a Gold Ore Slag Muck Historical Remains Point. Shandong Chem. Ind. 2024, 53, 218–222. [Google Scholar]
- Guo, Y.; Du, Y.; Wei, Y.; Wei, Y.; Zhao, D.; Zhang, H.; Deng, L.; Chen, H.; Zhao, M. Crystallization, microstructural evolution, heavy metals migration, and solidification mechanism of blast furnace slag glass-ceramics. Ceram. Int. 2024, 50, 18462–18472. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhang, Y.; Chai, Y.; Zhao, F.; Luo, G. Effect of Cr2O3 on the viscosity and structure of slag (or glass) of CaO-MgO-Al2O3-SiO2 system. Korean J. Chem. Eng. 2023, 40, 1783–1791. [Google Scholar] [CrossRef]
- Ghosh, S. Ceramic and glass-ceramic fillers in dental composites: A review. J. Met. Mater. Miner. 2020, 30, 22–30. [Google Scholar] [CrossRef]
- Reddy, A.A.; Tulyaganov, U.D.; Kharton, V.V.; Ferreira, J.M.F. Development of bilayer glass-ceramic SOFC sealants via optimizing the chemical composition of glasses—A review. J. Solid State Electrochem. 2015, 19, 2899–2916. [Google Scholar] [CrossRef]
- Ma, M.; Yu, Y.; Huang, Y. Effect of heating rate on crystallisation and properties of R2O–CaO–MgO–CuO–Al2O3–SiO2 glass-ceramics. Ceram. Int. 2024, 50, 12326–12332. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Xu, W.; Zhang, Y.; Shi, Y.; Ma, J.; Ouyang, S.L.; Du, Y. The mechanism of the crystalline characteristics of spinel-induced epitaxial growth of diopside in CMAS glass-ceramics. J. Eur. Ceram. Soc. 2020, 41, 1603–1612. [Google Scholar] [CrossRef]
- Pi, Y.; Zhang, W.; Zhang, Y.; Zou, W.; Zhang, Z.; Wu, F. Migration and transformation of heavy metals in glass-ceramics and the mechanism of stabilization. Ceram. Int. 2021, 47, 24663–24674. [Google Scholar] [CrossRef]
- Li, B.; Jing, K.; Bian, H. Effect of Zn/Zr ratio on mechanical, dielectric, and thermal properties of MgO-Al2O3-SiO2 glass-ceramics. J. Non-Cryst. Solids 2018, 500, 487–492. [Google Scholar] [CrossRef]
- Pei, P.Y.; Wang, J.; Wang, Y.; Hu, W.; Wang, B.; Zhou, J.N. Waste control by waste: Extraction of valuable metals from mixed metallurgical dust by boiling furnace roasting. Sep. Purif. Technol. 2024, 346, 127452. [Google Scholar] [CrossRef]
- Yao, R.; Zhang, R.; Lang, T.; Zhou, Y.; Shi, Y.; Ma, C.; He, Z. Transparent zinc silicate/ zinc oxide crystallized glass-ceramics for water remediation application under visible light. Ceram. Int. 2023, 49, 10420–10427. [Google Scholar] [CrossRef]
- Yu, H.; Liu, J.; Zeng, M.; He, L. Structure and dielectric properties of zinc borate glass–ceramics modified by magnesium. J. Mater. Sci. Mater. Electron. 2016, 27, 7109–7114. [Google Scholar] [CrossRef]
- Hao, Y.; Pu, Y.; Zhang, J.; Peng, X.; Shang, Y.; Xie, H.; Zhang, L.; Wang, B.; Zhang, X. Energy-storage performance of NaNbO3-based ceramic capacitor derived from a high DOP glass network structure. Ceram. Int. 2024, 5, 19355–19364. [Google Scholar] [CrossRef]
- Hu, N.; Fu, F.; Luo, B.; Ye, Y.; Chen, D.; Ou, Z.; Li, J. Preparation, characterization and self-foaming mechanism of total-tailings-based foamed glass-ceramics. Ceram. Int. 2023, 49, 31881–31890. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, P.; Zeng, L.; Jiao, B.; Shiau, Y.; Li, D. Preparation of Glass-Ceramics via Cosintering and Solidification of Hazardous Waste Incineration Residue and Chromium-Containing Sludge. ACS Omega 2021, 6, 23723–23730. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, H.; Wu, C.; Sun, Y.; Chen, R.; Guo, X. Properties of Glass-Ceramics Prepared from Industrial Multi-Wastes. Separations 2023, 10, 498. [Google Scholar] [CrossRef]
- Erol, M.; Demirler, U.; Kbayraksk, C.U. Characterization investigations of glass-ceramics developed from Seyitomer thermal power plant fly ash. J. Eur. Ceram. Soc. 2003, 23, 757–763. [Google Scholar] [CrossRef]
- Xu, W.C.; Cao, Z.; Ma, R.; Zhang, Y.X.; Wu, N.N.; Ouyang, S.L. Migration Mechanism and Magnetic Properties of Fe Ionsin Glass–Ceramics of an Iron-Rich CMAS System. Glass Phys. Chem. 2023, 49, 463–477. [Google Scholar]
- JC/T 258-1993; Test Methods for Performance of Cast Stone Products-Acid and Alkali Resistance Tests. State Bureau of Building Materials Industry: Beijing, China, 1993.
- HJ/T 299-2007; Solid Waste Leaching Toxicity Leaching Method Sulfuric Acid-Nitric Acid Method. Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 2007.
- Jia, R. Study on the Structure and Properties of Glass-Ceramics Prepared from Chromium-Containing Steel Slag and the Solidification of Heavy Metals. Master’s Thesis, Inner Mongolia University of Science & Technology, Baotou, China, 2022. [Google Scholar]
- Van, P.; Papenfuss, C.; Muschik, W. Griffith cracks in the mesoscopic microcrack theory. J. Phys. A Math. Gen. 2004, 37, 5315–5328. [Google Scholar] [CrossRef]
- Huang, D. Physical Geology; Higher Education Press: Beijing, China, 2004; pp. 1–339. [Google Scholar]
- Yang, Q.; Jiang, Z. The study of criterion for glass crystallization kinetics. J. Chin. Ceram. Soc. 1994, 22, 419–426. [Google Scholar]
- Xu, X.; Chu, Y.; Sun, S. ZnO, Effect of the Crystal Nucleus of ZnO and ZnO-Fe2O3 on Glass-Ceramics Microstructure. Bull. Chin. Silic. Soc. 2004, 3, 24–27. [Google Scholar]
- Sun, Q.; Kang, X. Experiment of Preparing Feldspar Glass-ceramics and Luminescence Properties. Multipurp. Util. Miner. Resour. 2020, 4, 151–155. [Google Scholar]
- Ouyang, S.L.; Zhang, Y.X.; Chen, Y.X.; Zhao, Z.; Wen, M.; Li, B.; Shi, Y.; Zhang, M.; Liu, S. Preparation of glass-ceramics using chromium-containing stainless steel slag: Crystal structure and solidification of heavy metal chromium. Sci. Rep. 2019, 9, 1964. [Google Scholar] [CrossRef]
- Ramteke, R.; Kumari, K.; Bhattacharya, S.; Rahman, M.R. Synthesis and characterization of zinc oxide incorporated iron borate glass-ceramic. J. Alloys Compd. 2019, 811, 151876. [Google Scholar] [CrossRef]
- Li, C.; Zhang, G.; Zheng, H.; Zhang, F.; Liu, K. Characterization of glass-ceramics developed from zinc leaching residue by sintering method. Ceram. Int. 2024, 50, 8302–8317. [Google Scholar] [CrossRef]
- Mu, N.O.; Chen, D.M.; Li, B. Influence of Zn/Ba ratio on crystallization, sintering behavior and properties of CaO-Al2O3-SiO2 glass-ceramics. J. Non-Cryst. Solids 2023, 622, 122662. [Google Scholar] [CrossRef]
- Margha, H.F.; Abdel-Hameed, M.A.S.; Kato, S.; Shigeo, S.; Kojima, T. Effect of ZrO2 addition on Vickers Hardness of modified basalt glass-ceramics. J. Ceram. Soc. Jpn. 2007, 115, 429–433. [Google Scholar] [CrossRef]
- Seiichi, T.; Koji, M.; Kouichi, N. Antimicrobial and mechanical properties of silver ion-exchanged transparent lithium-mica glass-ceramics. Ceram. Int. 2023, 49, 15959–15968. [Google Scholar]
- Soares, O.V.; Rodrigues, M.A. Improvements on sintering and thermal expansion of lithium aluminum silicate glass-ceramics. Ceram. Int. 2020, 46, 17430–17436. [Google Scholar] [CrossRef]
- Chen, W.P.; Jia, R.Z.; Zhang, Y.X.; Ouyang, S.L.; Wu, N.N. The excellent properties and solidifying effect of Cr providing by micro-crystal grain in slag glass-ceramics. J. Ceram. Soc. Jpn. 2022, 130, 376–383. [Google Scholar] [CrossRef]
- Lara, C.; Pascual, M.; Durán, A. Glass-forming ability, sinterability and thermal properties in the systems RO-BaO-SiO2 (R = Mg, Zn). J. Non-Cryst. Solids 2004, 348, 149–155. [Google Scholar] [CrossRef]
- Charitidis, C.A.; Karakasidis, T.E.; Kavouras, P.; Karakostas, T. The size effect of crystalline inclusions on the fracture modes in glass-ceramic materials. J. Phys. Condens. Matter. 2007, 19, 266209. [Google Scholar] [CrossRef]
- Zang, Z.; Wang, J.; Liu, L.; Shen, B. Preparation and characterization of glass-ceramic via co-sintering of coal fly ash and oil shale ash-derived amorphous slag. Ceram. Int. 2019, 45, 20058–20065. [Google Scholar] [CrossRef]
- Xu, D.; Lin, H.; Yang, W.; Li, C.; Liu, L.; Li, S.; Zeng, F. From structure to luminescence investigation of B2O3-GeO2-BaO-ZnO glass doped with Dy3+. J. Non-Cryst. Solids 2024, 634, 122965. [Google Scholar] [CrossRef]
- Liu, L.; Yu, H.; Li, Y.; Zhang, Z. Stabilization behavior and mechanism of heavy metals in eco-friendly glass-ceramics derived from wastes. J. Clean. Prod. 2020, 269, 122417. [Google Scholar] [CrossRef]
- Lin, X.Q.; Chen, J.; Xu, S.X.; Mao, T.; Liu, W.; Wu, J.; Li, X.; Yan, J. Solidification of heavy metals and PCDD/Fs from municipal solid waste incineration fly ash by the polymerization of calcium carbonate oligomers. Chemosphere 2022, 288, 132420. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, F.Y.; Zhou, F.; Lu, M.; Hou, H.; Li, J.; Liu, D.; Wang, T. Early solidification/stabilization mechanism of heavy metals (Pb, Cr and Zn) in Shell coal gasification fly ash based geopolymer. Sci. Total Environ. 2022, 802, 149905. [Google Scholar] [CrossRef]
| Chemical Composition | SiO2 | Al2O3 | Na2O | MgO | K2O | CaO | TiO2 | BaO | Cu | Cr | Zn | TFe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | 26.65 | 14.29 | 5.32 | 3.97 | 0.40 | 29.78 | 0.25 | 0.51 | 1.04 | 0.71 | 2.36 | 14.72 |
| Specimen | Mass Fraction/% | ||||||
|---|---|---|---|---|---|---|---|
| Fly Ash | Smelting Slag | Quartz Sand | CaO | Na2CO3 | Borax | CaF2 | |
| S1 | 45 | 30.0 | 6.0 | 8.0 | 2.5 | 8.2 | 5 |
| S2 | 40 | 35.0 | 2.0 | 7.7 | 2.2 | 8.2 | 5 |
| S3 | 35 | 40.0 | 0 | 7.5 | 2.1 | 8.2 | 5 |
| S4 | 30 | 45.0 | 0 | 7.2 | 2.0 | 8.2 | 5 |
| S5 | 25 | 50.0 | 0 | 6.9 | 1.8 | 8.2 | 5 |
| S6 | 20 | 55.0 | 0 | 6.6 | 1.6 | 8.2 | 5 |
| S7 | 20 | 60.0 | 0 | 6.3 | 1.4 | 8.2 | 5 |
| Sample | Tg/°C | Tc/°C | ΔT/°C |
|---|---|---|---|
| S1 | 731 | 868 | 137 |
| S2 | 726 | 863 | 142 |
| S3 | 720 | 862 | 142 |
| S4 | 710 | 847 | 137 |
| S5 | 708 | 865 | 157 |
| S6 | 698 | 867 | 169 |
| S7 | 686 | 841 | 155 |
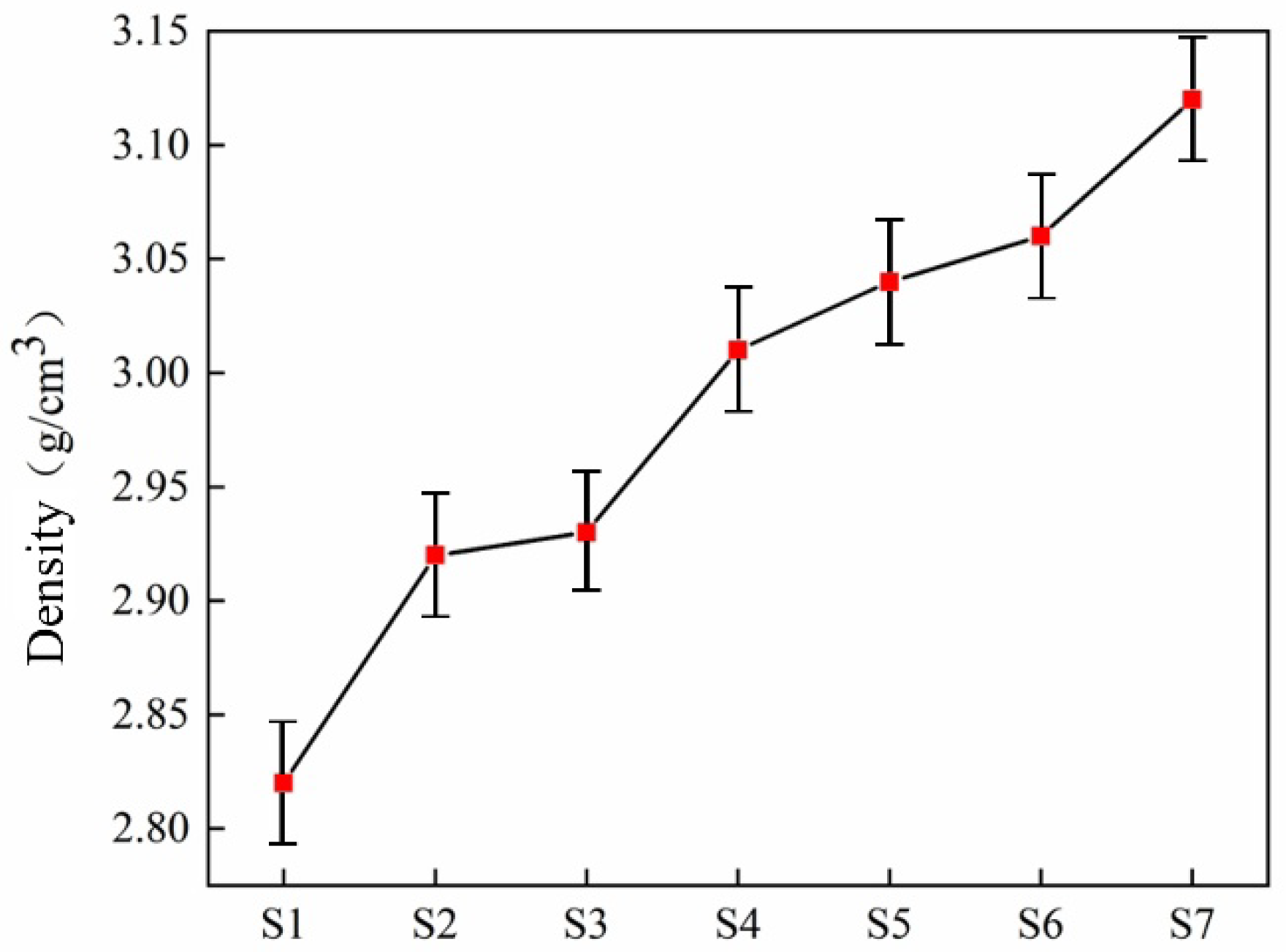
| Sample | Density (g·cm−3) | Vickers Hardness (GPa) | Flexural Strength (MPa) |
|---|---|---|---|
| S1 | 2.82 0.025 | 12.59 0.4 | 61.25 5.00 |
| S2 | 2.92 0.025 | 13.48 0.4 | 82.45 7.50 |
| S3 | 2.93 0.025 | 13.61 0.4 | 86.8 7.50 |
| S4 | 3.01 0.025 | 14.68 0.4 | 89.2 7.50 |
| S5 | 3.04 0.025 | 14.92 0.6 | 112.25 5.00 |
| S6 | 3.06 0.025 | 15.49 0.4 | 132.86 5.00 |
| S7 | 3.12 0.025 | 16.60 0.4 | 150.75 5.00 |
| Sample | Acid-Resisting (20% H2SO4)/% | Alkaline-Resisting (20% NaOH)/% |
|---|---|---|
| S1 | 73.24 0.37 | 96.29 0.48 |
| S2 | 74.56 0.37 | 97.35 0.49 |
| S3 | 76.91 0.38 | 97.41 0.49 |
| S4 | 77.42 0.39 | 97.66 0.49 |
| S5 | 79.36 0.40 | 97.72 0.49 |
| S6 | 79.83 0.40 | 97.76 0.49 |
| S7 | 80.96 0.40 | 97.84 0.49 |
| Samples | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|
| Zn concentration (mg·L−1) | 4.42 0.02 | 3.45 0.17 | 3.82 0.2 | 16.70 0.08 | 32.00 0.16 | 27.91 0.14 | 24.15 0.12 |
| Metals | Mg | Al | Ca | Fe | Fe | Zn |
|---|---|---|---|---|---|---|
| Ionic radius (pm) | 65 | 50 | 99 | 76 | 64 | 74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, N.; Huang, J.; Qiu, J.; Li, Z.; Li, X.; Li, B.; Li, N.; Zhang, Y.; Ouyang, S. Preparation of Glass-Ceramics Using Zinc-Containing Smelting Slag: Structure, Properties and Solidification of Zinc. Materials 2025, 18, 3555. https://doi.org/10.3390/ma18153555
Wu N, Huang J, Qiu J, Li Z, Li X, Li B, Li N, Zhang Y, Ouyang S. Preparation of Glass-Ceramics Using Zinc-Containing Smelting Slag: Structure, Properties and Solidification of Zinc. Materials. 2025; 18(15):3555. https://doi.org/10.3390/ma18153555
Chicago/Turabian StyleWu, Nannan, Junhui Huang, Junxi Qiu, Zonghang Li, Xiaofan Li, Bohan Li, Nianzhe Li, Yuxuan Zhang, and Shunli Ouyang. 2025. "Preparation of Glass-Ceramics Using Zinc-Containing Smelting Slag: Structure, Properties and Solidification of Zinc" Materials 18, no. 15: 3555. https://doi.org/10.3390/ma18153555
APA StyleWu, N., Huang, J., Qiu, J., Li, Z., Li, X., Li, B., Li, N., Zhang, Y., & Ouyang, S. (2025). Preparation of Glass-Ceramics Using Zinc-Containing Smelting Slag: Structure, Properties and Solidification of Zinc. Materials, 18(15), 3555. https://doi.org/10.3390/ma18153555





