Ultrasound-Activated BiOI/Ti3C2 Heterojunctions in 3D-Printed Piezocatalytic Antibacterial Scaffolds for Infected Bone Defects
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of BiOI/Ti3C2
2.3. Physicochemical Characterizations of BT
2.4. Electrochemical Measurement
2.5. Preparation of Composite Scaffolds
2.6. Measurement of Extracellular ROS
2.7. In Vitro Bacterial Inhibition Testing
2.8. Evaluation of Bacterial Activity in Vitro
2.8.1. ROS Staining
2.8.2. LIVE/DEAD Staining
2.9. Cell Cytotoxicity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Fabrication and Characterization of BT
3.2. Characterization of Piezoelectric Properties of BT
3.3. Sonodynamic Performance of the Scaffolds
3.4. In Vitro Antibacterial Properties of the Scaffolds
3.5. Extracellular Bacterial ROS Detection
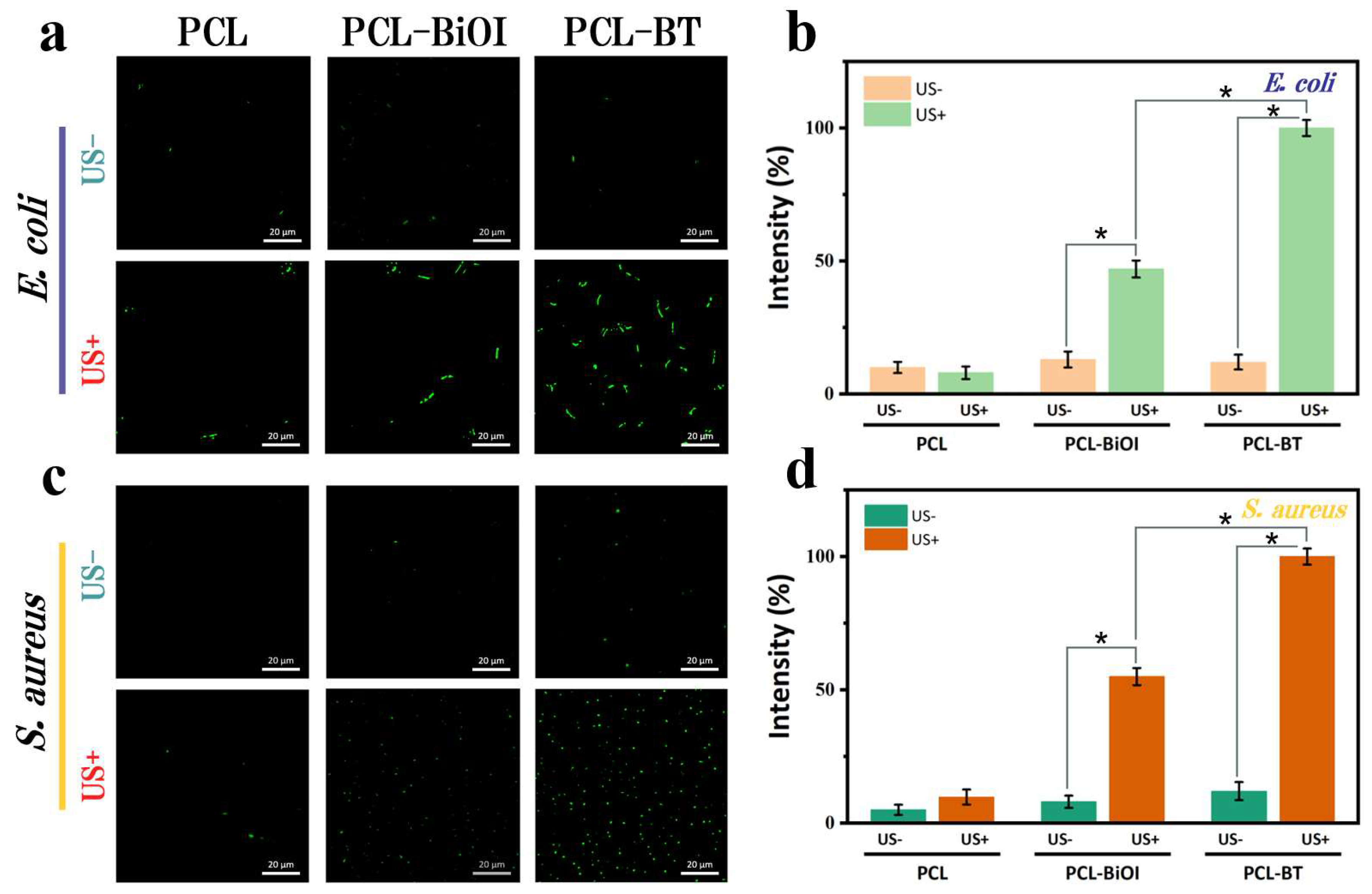
3.6. In Vitro Antibacterial Activity Testing
3.7. In Vitro Cytocompatibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Sang, L.; Liu, Y.; Bai, Z. Sono-Piezo Dynamic Therapy: Utilizing Piezoelectric Materials as Sonosensitizer for Sonodynamic Therapy. Adv. Sci. 2025, 12, e2417439. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, H.; Lu, Q.; Sun, J.; Zhang, P.; Zeng, L.; Vasilev, K.; Zhao, Y.; Chen, Y.; Liu, P. Spontaneous formation of MXene-oxidized sono/chemo-dynamic sonosensitizer/nanocatalyst for antibacteria and bone-tissue regeneration. J. Nanobiotechnol. 2023, 21, 193. [Google Scholar] [CrossRef]
- Shuai, C.; Liu, G.; Yang, Y.; Qi, F.; Peng, S.; Yang, W.; He, C.; Wang, G.; Qian, G. A strawberry-like Ag-decorated barium titanate enhances piezoelectric and antibacterial activities of polymer scaffold. Nano Energy 2020, 74, 104825. [Google Scholar] [CrossRef]
- He, C.; Feng, P.; Hao, M.; Tang, Y.; Wu, X.; Cui, W.; Ma, J.; Ke, C. Nanomaterials in Antibacterial Photodynamic Therapy and Antibacterial Sonodynamic Therapy. Adv. Funct. Mater. 2024, 34, 2402588. [Google Scholar] [CrossRef]
- Gao, Z.; Song, Z.; Guo, R.; Zhang, M.; Wu, J.; Pan, M.; Du, Q.; He, Y.; Wang, X.; Gao, L.; et al. Mn Single-Atom Nanozyme Functionalized 3D-Printed Bioceramic Scaffolds for Enhanced Antibacterial Activity and Bone Regeneration. Adv. Healthc. Mater. 2024, 13, e2303182. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, Q.; Cao, W. Multifunctional Porphyrin-Based Sonosensitizers for Sonodynamic Therapy. Adv. Funct. Mater. 2024, 34, 2405844. [Google Scholar] [CrossRef]
- Jia, W.; Wang, T.; Chen, F.; Liu, Z.; Hou, X.; Cao, W.; Zhao, X.; Lu, B.; Hu, Y.; Dong, Y.; et al. Low-Intensity Pulsed Ultrasound Responsive Scaffold Promotes Intramembranous and Endochondral Ossification via Ultrasonic, Thermal, and Electrical Stimulation. ACS Nano 2025, 19, 4422–4439. [Google Scholar] [CrossRef]
- He, Z.; Du, J.; Miao, Y.; Li, Y. Recent Developments of Inorganic Nanosensitizers for Sonodynamic Therapy. Adv. Healthc. Mater. 2023, 12, e2300234. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Jiao, Y.; Li, J.; Li, Y.; Hao, Y.L.; Zuo, Y. In Vitro Study on the Piezodynamic Therapy with a BaTiO3-Coating Titanium Scaffold under Low-Intensity Pulsed Ultrasound Stimulation. ACS Appl. Mater. Interfaces 2021, 13, 49542–49555. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, X.; Yue, Y.; He, S.; Cao, J.; He, W.; Toctocan Tai, T.; Wang, D.; Zhou, Z.; Deng, Y. Low-Intensity Ultrasound-Activated Cavitation Effect Triggers Piezoelectric Catalysis Coordinating Respiratory Chain Interference Tactics Against Bacterial Infection. Adv. Funct. Mater. 2024, 35, 2419426. [Google Scholar] [CrossRef]
- Wang, C.; Lei, J.; Mao, C.; Wu, S.; Zheng, Y.; Liang, C.; Yang, L.; Zhu, S.; Li, Z.; Jiang, H.; et al. Defective S-TiO2−x/CeO2 Heterojunction for Mutual Reinforcing Chemodynamic/Sonocatalytic Antibacterial Therapy and Sonoelectric/Ion-Activated Bone Regeneration. Adv. Funct. Mater. 2023, 33, 2306493. [Google Scholar] [CrossRef]
- Wang, C.; Sun, W.; Xiang, Y.; Wu, S.; Zheng, Y.; Zhang, Y.; Shen, J.; Yang, L.; Liang, C.; Liu, X. Ultrasound-Activated Piezoelectric MoS2 Enhances Sonodynamic for Bacterial Killing. Small Sci. 2023, 3, 2300022. [Google Scholar] [CrossRef]
- Shuai, C.; Zhang, Z.; Chen, M.; Sun, B.; Long, X.; Wang, G.; Peng, S. 3D-Printed Scaffold Achieves Synergistic Chemo-Sonodynamic Therapy for Tumorous Bone Defect. ACS Appl. Bio Mater. 2025, 8, 3920–3931. [Google Scholar] [CrossRef]
- Shuai, C.; Long, X.; Yang, Y.; Sun, B.; Zhang, Z.; Wang, G.; Peng, S. Poly(L-lactic acid)-BiFeO3/Ti3C2 Scaffolds for Antibacterial Sonodynamic Therapy. ACS Appl. Nano Mater. 2024, 7, 25132–25142. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, P.; Shah, N.H.; Cui, Y.; Wang, Y. A Comprehensive Review of Inorganic Sonosensitizers for Sonodynamic Therapy. Int. J. Mol. Sci. 2023, 24, 12001. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Zhu, D.; Chen, Y.; Zhang, C.; Li, G.; Fang, Q.; Chang, M.; Chen, Y.; Gao, Y. Ultrasound-Responsive 4D Bioscaffold for Synergistic Sonopiezoelectric-Gaseous Osteosarcoma Therapy and Enhanced Bone Regeneration. Adv. Sci. 2025, 12, e2417208. [Google Scholar] [CrossRef]
- Tang, J.; Hu, J.; Bai, X.; Wang, Y.; Cai, J.; Zhang, Z.; Geng, B.; Pan, D.; Shen, L. Near-Infrared Carbon Dots With Antibacterial and Osteogenic Activities for Sonodynamic Therapy of Infected Bone Defects. Small 2024, 20, e2404900. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhao, R.; Tang, Y.; Yi, M.; Ge, Z.; Wang, D.; Fang, Q.; Xiong, Z.; Duan, A.; Liu, W.; et al. Highly Biocompatible Polyester-Based Piezoelectric Elastomer with Antitumor and Antibacterial Activity for Ultrasound-Enhanced Piezoelectric Therapy. ACS Appl. Mater. Interfaces 2023, 15, 55308–55322. [Google Scholar] [CrossRef]
- Angulo-Pineda, C.; Srirussamee, K.; Palma, P.; Fuenzalida, V.M.; Cartmell, S.H.; Palza, H. Electroactive 3D Printed Scaffolds Based on Percolated Composites of Polycaprolactone With Thermally Reduced Graphene Oxide for Antibacterial and Tissue Engineering Applications. Nanomaterials 2020, 10, 428. [Google Scholar] [CrossRef]
- Wang, S.; Qiao, Y.; Liu, X.; Zhu, S.; Zheng, Y.; Jiang, H.; Zhang, Y.; Shen, J.; Li, Z.; Liang, Y.; et al. Reduced Graphene Oxides Modified Bi2Te3 Nanosheets for Rapid Photo-Thermoelectric Catalytic Therapy of Bacteria-Infected Wounds. Adv. Funct. Mater. 2022, 33, 2210098. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, X.; Wang, H.; Feng, X.; Lei, J.; He, Y.; Wei, J.; Zhang, Y.; Tan, L.; Yang, C. Two-dimensional Nb2C-based nanoplatform augmented sonodynamic antibacterial therapy and bone regeneration. Sci. China Mater. 2023, 66, 2913–2924. [Google Scholar] [CrossRef]
- Xu, M.; Wu, S.; Ding, L.; Lu, C.; Qian, H.; Qu, J.; Chen, Y. Engineering ultrasound-activated piezoelectric hydrogels with antibacterial activity to promote wound healing. J. Mater. Chem. B 2023, 11, 4318–4329. [Google Scholar] [CrossRef]
- Xu, J.; Ma, X.; Wang, J.; Zhang, C.; Liu, X.; Qu, Y.; Zhao, M.; Li, W.; Huang, W.; Li, Y.Q. Environmental Charge-Mediated Nanopiezocatalysis for Sonodynamic Therapy. Nano Lett. 2025, 25, 9156–9165. [Google Scholar] [CrossRef]
- Xing, X.; Zhao, S.; Xu, T.; Huang, L.; Zhang, Y.; Lan, M.; Lin, C.; Zheng, X.; Wang, P. Advances and perspectives in organic sonosensitizers for sonodynamic therapy. Coord. Chem. Rev. 2021, 445, 214087. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Z.; Liu, Z.; Zhang, J.; Zhang, Y.; Ding, Y.; Huang, T.; Xiang, D.; Wang, Z.; Dai, Y.; et al. Piezoelectric nanocomposites for sonodynamic bacterial elimination and wound healing. Nano Today 2021, 37, 101104. [Google Scholar] [CrossRef]
- Wu, J.; Li, B.; Shao, Y.; Wu, X.; Zhao, W. Piezoelectricity enhances MoSe2 nanoflowers adsorption of the antibacterial dye malachite green under sonication. Environ. Chem. Lett. 2020, 18, 2141–2148. [Google Scholar] [CrossRef]
- Guan, S.; Xu, W.; Tan, J.; Zhang, X.; Liu, X.; Liu, L.; Qian, S.; Hou, Z.; Zhu, H.; Qiu, J.; et al. Metainterface Heterostructure Enhances Sonodynamic Therapy for Disrupting Secondary Biofilms. ACS Nano 2024, 18, 15114–15129. [Google Scholar] [CrossRef]
- He, X.; Hou, J.T.; Sun, X.; Jangili, P.; An, J.; Qian, Y.; Kim, J.S.; Shen, J. NIR-II Photo-Amplified Sonodynamic Therapy Using Sodium Molybdenum Bronze Nanoplatform against Subcutaneous Staphylococcus aureus Infection. Adv. Funct. Mater. 2022, 32, 2203964. [Google Scholar] [CrossRef]
- Maleki, A.; Seyedhamzeh, M.; Yuan, M.; Agarwal, T.; Sharifi, I.; Mohammadi, A.; Kelicen-Ugur, P.; Hamidi, M.; Malaki, M.; Al Kheraif, A.A.; et al. Titanium-Based Nanoarchitectures for Sonodynamic Therapy-Involved Multimodal Treatments. Small 2023, 19, e2206253. [Google Scholar] [CrossRef]
- Ning, X.; Hao, A.; Cao, Y.; Lv, N.; Jia, D. Boosting piezocatalytic performance of Ag decorated ZnO by piezo-electrochemical synergistic coupling strategy. Appl. Surf. Sci. 2021, 566, 150730. [Google Scholar] [CrossRef]
- Wang, H.; Mu, N.; He, Y.; Zhang, X.; Lei, J.; Yang, C.; Ma, L.; Gao, Y. Ultrasound-controlled MXene-based Schottky heterojunction improves anti-infection and osteogenesis properties. Theranostics 2023, 13, 1669–1683. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Wang, J.; Geng, X.; Jia, B.; Wang, L.; Li, Y.Q.; Geng, B.; Huang, W. Graphene Quantum Dots Nanoantibiotic-Sensitized TiO2-x Heterojunctions for Sonodynamic-Nanocatalytic Therapy of Multidrug-Resistant Bacterial Infections. Adv. Healthc. Mater. 2024, 13, e2400659. [Google Scholar] [CrossRef] [PubMed]
- de Bitencourt Rodrigues, H.; Oliveira de Brito Lira, J.; Padoin, N.; Soares, C.; Qurashi, A.; Ahmed, N. Sonoelectrochemistry: Ultrasound-assisted Organic Electrosynthesis. ACS Sustain. Chem. Eng. 2021, 9, 9590–9603. [Google Scholar] [CrossRef]
- He, Y.; Hua Liu, S.; Yin, J.; Yoon, J. Sonodynamic and chemodynamic therapy based on organic/organometallic sensitizers. Coord. Chem. Rev. 2021, 429, 213610. [Google Scholar] [CrossRef]
- Wu, J.; Huang, J.; Yu, J.; Xu, M.; Liu, J.; Pu, K. Exosome-Inhibiting Polymeric Sonosensitizer for Tumor-Specific Sonodynamic Immunotherapy. Adv. Mater. 2024, 36, e2400762. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, D.; Huang, H.; Yang, D.; Song, X.; Feng, W.; Jing, X.; Chen, Y. Nanosonosensitizer Optimization for Enhanced Sonodynamic Disease Treatment. Adv. Mater. 2024, 36, e2409663. [Google Scholar] [CrossRef]
- He, F.; Li, W.; Liu, B.; Zhong, Y.; Jin, Q.; Qin, X. Progress of Piezoelectric Semiconductor Nanomaterials in Sonodynamic Cancer Therapy. ACS Biomater. Sci. Eng. 2024, 10, 298–312. [Google Scholar] [CrossRef]
- Li, D.; Yang, Y.; Li, D.; Pan, J.; Chu, C.; Liu, G. Organic Sonosensitizers for Sonodynamic Therapy: From Small Molecules and Nanoparticles toward Clinical Development. Small 2021, 17, e2101976. [Google Scholar] [CrossRef]
- Lin, G.; Nash, G.T.; Luo, T.; Ghosh, I.; Sohoni, S.; Christofferson, A.J.; Liu, G.; Engel, G.S.; Lin, W. 2D Nano-Sonosensitizers Facilitate Energy Transfer to Enhance Sonodynamic Therapy. Adv. Mater. 2023, 35, e2212069. [Google Scholar] [CrossRef]
- Mao, C.; Jin, W.; Xiang, Y.; Zhu, Y.; Wu, J.; Liu, X.; Wu, S.; Zheng, Y.; Cheung, K.M.C.; Yeung, K.W.K. Realizing Highly Efficient Sonodynamic Bactericidal Capability through the Phonon-Electron Coupling Effect Using Two-Dimensional Catalytic Planar Defects. Adv. Mater. 2023, 35, e2208681. [Google Scholar] [CrossRef]
- Deng, C.; Zheng, M.; Han, S.; Wang, Y.; Xin, J.; Aras, O.; Cheng, L.; An, F. GSH-activated Porphyrin Sonosensitizer Prodrug for Fluorescence Imaging-guided Cancer Sonodynamic Therapy. Adv. Funct. Mater. 2023, 33, 2300348. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Zhang, Z.; Yu, Z.; Sun, B.; Yang, Y.; Wang, G.; Shuai, C. Ultrasound-Activated BiOI/Ti3C2 Heterojunctions in 3D-Printed Piezocatalytic Antibacterial Scaffolds for Infected Bone Defects. Materials 2025, 18, 3533. https://doi.org/10.3390/ma18153533
Xie J, Zhang Z, Yu Z, Sun B, Yang Y, Wang G, Shuai C. Ultrasound-Activated BiOI/Ti3C2 Heterojunctions in 3D-Printed Piezocatalytic Antibacterial Scaffolds for Infected Bone Defects. Materials. 2025; 18(15):3533. https://doi.org/10.3390/ma18153533
Chicago/Turabian StyleXie, Juntao, Zihao Zhang, Zhiheng Yu, Bingxin Sun, Yingxin Yang, Guoyong Wang, and Cijun Shuai. 2025. "Ultrasound-Activated BiOI/Ti3C2 Heterojunctions in 3D-Printed Piezocatalytic Antibacterial Scaffolds for Infected Bone Defects" Materials 18, no. 15: 3533. https://doi.org/10.3390/ma18153533
APA StyleXie, J., Zhang, Z., Yu, Z., Sun, B., Yang, Y., Wang, G., & Shuai, C. (2025). Ultrasound-Activated BiOI/Ti3C2 Heterojunctions in 3D-Printed Piezocatalytic Antibacterial Scaffolds for Infected Bone Defects. Materials, 18(15), 3533. https://doi.org/10.3390/ma18153533





