Abstract
Titanium dioxide (TiO2), a widely used semiconductor in photocatalysis owing to its adequate potential for water hydrolysis, chemical stability, low toxicity, and low cost. However, its efficiency is limited by fast charge-carrier recombination and poor visible light absorption. Coupling TiO2 with a p-type semiconductor, such as nickel oxide (NiO), forming a p-n heterojunction, decreases the recombination of charge carriers and increases photocatalytic activity. In this work, the surface of TiO2 modified with NiO nanoparticles (NPs) induced by radiolysis for photocatalytic hydrogen production was studied. The photocatalytic activity of NiO/TiO2 was evaluated using methanol as a hole scavenger under UV–visible light. All modified samples presented superior photocatalytic activity compared to bare TiO2. The dynamics of the charge carriers, a key electronic phenomenon in photocatalysis, was investigated by time-resolved microwave conductivity (TRMC). The results highlight the crucial role of Ni-based NPs modification in enhancing the separation of the charge carrier and activity under UV–visible irradiation. Furthermore, the results revealed that under visible irradiation, NiO-NPs inject electrons into the conduction band of titanium dioxide.
1. Introduction
The world urgently needs a green energy transition to combat rising greenhouse gases, which drive climate change and threaten ecosystems and human societies. The depletion of fossil fuel resources and their environmental impact underscore the need for sustainable energy alternatives. Furthermore, the shift to renewable energy sources like wind and solar is essential to mitigate climate change and ensure energy security. Thus, in this search for efficient and sustainable energy solutions, photocatalytic hydrogen production is a promising field that harnesses solar radiation, a renewable and intermittent energy source, to transform solar energy into chemical energy and store it in hydrogen bonds (H2). In this sense, semiconductor-based materials have garnered significant attention due to their potential applications in photocatalysis, photovoltaics, sensors, and ethanol oxidation technologies [1]. Among these materials, titanium dioxide (TiO2) and nickel oxide (NiO) stand out for their unique properties and versatility. TiO2 is well known for its adequate potential for water hydrolysis, low toxicity, low cost, and outstanding chemical and thermal stability [2], making it a prominent material for photocatalytic applications. However, its wide band gap (~3.2 eV for anatase and ~3.0 eV for rutile phases) [3] and fast charge-carriers’ recombination rate limit its efficiency under visible light. To overcome these limitations and improve the photocatalytic activity of TiO2, different strategies have been explored, including surface modification of titania with transition metals and plasmonic nanoparticles (NPs), transition metal oxides, graphitic carbon nitride, conjugated polymer nanostructures, etc. [4,5,6]. In particular, surface modification of TiO2 with noble metal NPs (e.g., Pt, Pd, Rh, Au, and Ag) can lead to important enhancement of the photocatalytic activity for H2 evolution [7,8,9,10,11,12]. Nevertheless, the elevated cost and limited availability of noble metals restrict their practical applications. Thus, there is a growing need to replace noble metals with abundant and cost-effective alternatives, such as transition metals. Earth-abundant metals, such as nickel (Ni)-based cocatalysts, are explored in photocatalysis because of their low cost and good efficiency [13,14]. Ni-based cocatalysts, such as metallic nickel (Ni0), nickel oxide (NiO) [12,15,16,17], and nickel hydroxide Ni(OH)2 nanoparticles [18,19], are very efficient cocatalysts when integrated with TiO2 semiconductor (SC) for hydrogen generation [20]. It has been observed that Ni0-NPs can be present at the TiO2/NiO-NPs interface and can lead to the formation of an ohmic junction with TiO2, which helps migration of photogenerated electrons to the metal [21,22]. NiO is a p-type SC with a band gap of about 3.5 eV [23,24,25,26], and forms, with TiO2, a p-n heterojunction that creates an internal electric field, reducing the charge-carriers’ recombination and facilitating interfacial charge transfer [21,23,27,28,29]. This p-n heterojunction plays a vital role in enhancing the photocatalytic activity of TiO2 under UV-vis light irradiation.
Accordingly, innovative materials such as NiO/TiO2 composites could play a crucial role in this transition to green energy by enhancing the efficiency and feasibility of renewable energy technologies. Recent studies have demonstrated that NiO/TiO2 composites exhibit superior performance in various applications, such as solar fuels’ generation and degradation of organic pollutants. Beyond these, NiO/TiO2 composites are increasingly being explored for different applications in contemporary industries, such as power generation, by enhancing the efficiency of photovoltaic cells to improve solar energy conversion rates; energy storage by developing advanced batteries and supercapacitors with higher capacity, longer lifespan, and faster charge–discharge cycles; and environmental remediation by efficiently degrading harmful pollutants in air and water. Industries ranging from automotive to consumer electronics are applying these advanced materials to drive innovation and improve product performance. For example, in the transportation sector, the development of NiO/TiO2-based materials can lead to more efficient fuel cells and batteries, directly impacting the efficiency and sustainability of electric and hybrid vehicles. In energy storage, the integration of these materials into batteries and supercapacitors promises significant advances in storage capacity and energy density, crucial for both consumer electronics and grid-scale energy solutions. Likewise, sensors and electronics will benefit from the creation of more sensitive and accurate sensors for gas detection and other environmental challenges.
In this work, the surface of TiO2 (commercial Degussa-P25) has been modified with Ni-NPs induced by radiolytic reduction of two different salt precursors, nickel (II) acetylacetonate and nickel (II) formate. Radiolytic reduction leads to a very homogeneous deposition of Ni-NPs on TiO2, obtaining a better deposition of NPs with the nickel (II) acetylacetonate precursor. The choice of the metal salt precursor used for the surface modification of TiO2 is essential to design efficient photocatalysts; therefore, through a comprehensive analysis of the structural, optical, and electronic properties of the nanomaterial, we seek to elucidate the mechanisms underlying the improved performance of NiO/TiO2 composites. When combined with TiO2, NiO can significantly improve the overall efficiency of the composite by enhancing charge separation, reducing recombination rates, and extending light absorption to the visible region. These improvements arise from the synergistic interactions between NiO and TiO2, which facilitate better utilization of a wider solar spectrum range and improved electron–hole pair dynamics, resulting in a good photocatalytic activity for H2 evolution under UV–visible light.
2. Materials and Methods
2.1. Chemical Reagents
Commercial titania (TiO2-P25, 80% anatase, and 20% rutile, Evonik, Essen, Germany) was used as photocatalytic support. Nickel (II) acetylacetonate (Ni(C5H7O2)2, Sigma-Aldrich, St. Louis, MO, USA, ≥99%) and nickel (II) formate dihydrate (Ni(HCO2)2•2H2O, Alfa Aesar, Lancashire, UK) were used as metal precursors. Ethanol (C2H5OH, VMR Chemicals, Radnor, PA, USA, 99.94%), methanol (CH3OH, Sigma Aldrich, ≥99%), deionized water (Milli-Q with 18.2 MΩ, Molsheim, France), and nitrogen gas (N2, Air Liquide, Paris, France) were used in all experiments.
2.2. Synthesis Method
The photocatalysts were prepared by radiolysis (Figure 1), using two different metal precursors: nickel (II) acetylacetonate and nickel (II) formate. TiO2 was surface-modified with different amounts of Ni: the experimental design of metal loadings was 0.1, 0.5, 1.0, 3.5, and 5.0 wt.% Ni/TiO2. The required amounts of metal precursors were dissolved in ethanol to prepare each photocatalyst. The solutions were first sonicated for 10 min, stirred for 30 min, and degassed with N2 gas. The mixture was then irradiated using a 60Co panoramic gamma source. The doses used were sufficient for the complete reduction of NiII complexes to their zerovalent state Ni0 (Table S1). After the radiolysis process, the photocatalysts were separated by centrifugation. The powders were washed with ethanol 3 times at 6000 rpm for 10 min. The photocatalysts were then dried at 60 °C for 24 h. Radiolytic reduction is a powerful method to synthesize metal NPs in solutions and on supports using simple physicochemical conditions (ambient pressure and temperature, and no additional chemical-reducing agents) [30]. High-energy radiation (γ-rays, X-rays, electrons, or ion beams) of alcohols induces excitation and ionization of the solvent [31,32]. This process leads to the formation of solvated electrons () and alcohol radicals ( in the case of ethanol) (see Equation (1)), which are strong reducing species. The reduction is very homogeneous in the medium with control of size and shape due to the high reducing power of solvated electrons and alcohol radicals [33]. Their redox potentials determined in water are and , respectively [30,33]. This approach enables the reduction of metal complexes, which are challenging to reduce using conventional chemical methods at room temperature [31]. The solvated electrons and alcohol radicals reduce the NiII complexes to their zerovalent Ni0 state. The coalescence occurs, leading to the formation of metallic nanoparticles.
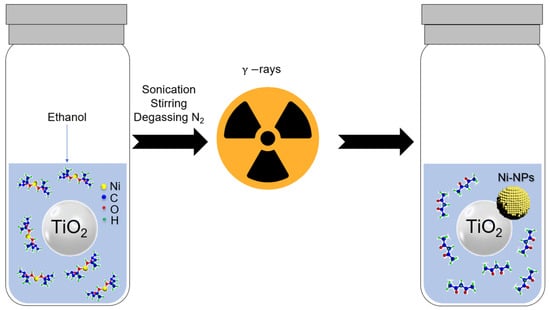
Figure 1.
Synthesis of photocatalysts, Ni-based NPs/TiO2, by radiolysis method.
2.3. Characterization of NiO/TiO2
The morphology, size, and dispersion of nickel nanoparticles on the TiO2 surface were analyzed by transmission electron microscopy (TEM).
The crystal structure was analyzed by high-resolution transmission electron microscopy (HRTEM). Electron energy loss spectroscopy (EELS) was employed to determine elemental nickel and its oxidation states on the TiO2 surface using a FEI TECNAI F30 microscope (Hillsboro, OR, USA) equipped with a tungsten field emission gun operating at 300 keV.
Inductively coupled plasma optical emission spectrometry (ICP-OES) was used for elemental analysis, which provided information on the mass content of Ni on the TiO2 surface.
UV-vis diffuse reflectance spectroscopy (DRS) with a spectrophotometer (Cary 5000 Series, Agilent Technologies, Santa Clara, CA, USA) was used to investigate the optical properties of the samples. The measurements were conducted in the wavelength range of 200–800 nm.
The crystal structure of the synthesized photocatalysts was investigated by X-ray diffraction (XRD) using a SmartLab RIGAKU diffractometer (Tokyo, Japan) with Cu Kα radiation, 40 kV, 44 mA, λ = 0.15406 nm in the 2θ range from 20° to 80°, with steps of 0.01° s−1.
Furthermore, the surface elemental composition and oxidation states of nickel nanoparticles on the TiO2 surface were analyzed by X-ray photoelectron spectroscopy (XPS). XPS analysis was conducted using a ThermoFisher K-alpha spectrometer (Waltham, MA, USA) featuring a monochromated Al Kα X-ray Source (1486.68 eV) with a 400 µm spot size, providing an irradiated area of ~1 mm2. Measurements were carried out on powdered samples under a base pressure of 3 × 10−9 mbar. The hemispherical analyzer operated in constant analyzer energy mode with a pass energy of 200 eV and a step of 1 eV for the acquisition of survey spectra, and a pass energy of 50 eV and a step of 0.1 eV for the acquisition of narrow spectra. A “dual beam” flood gun was employed to neutralize the charge buildup. The binding energies were calibrated against the neutral carbon binding energy set at 284.8 eV. The precision in binding energy is ±0.2 eV. CasaXPS software (2.3.25 version) was used to record and treat the spectra [34]. The fitting procedure was carried out by first subtracting a Shirley-type background, followed by applying symmetrical line shapes for peaking fitting. The synthetic line shapes were sums of Gauss and Lorentzian functions with 30% Lorentzian character. Line shapes extracted from well-characterized Ni0, NiO, and Ni(OH)2 references were used for the fitting of Ni2p core-level spectra.
The time-resolved microwave conductivity (TRMC) technique was used to investigate the dynamics of charge carriers under UV and visible light excitations. The incident microwaves were generated by a Gunn diode (30 GHz). A tunable laser (EKSPLA, Vilnius, Lithuania, NT342B) between 200 and 2000 nm, equipped with an optical parametric oscillator (OPO), was used as a pulsed light source. The wavelengths used were 360 nm and 420 nm, with an excitation energy of 1.1 mJ and 2.3 mJ, respectively. When a semiconductor material is excited by a laser pulse, the TRMC technique is used to calculate the relative change () in the microwave power reflected by the material. Equation (2) shows that this change () can be linked to a slight perturbation of the sample conductivity.
The primary data obtained from TRMC measurements include the maximum signal (Imax), indicating the quantity of excess charge carriers induced by laser excitation, along with the subsequent decay, which reflects the reduction in free electron concentration over time The results obtained from this analysis reveal a reduction in the number of mobile electrons (indicated by a lower Imax value) in the conduction band of the modified semiconductor, resulting in a shortened lifetime of the photogenerated electrons in the samples. Three phenomena related to metal deposition are responsible for this decrease in Imax: (a) the shielding effect created by the NPs, (b) the surface recombination centers created by the synthesis process, and (c) the quick electron transfers from TiO2 to Ni-based NPs (occurring in less than 10 ns) [10,12]. These phenomena lead to the loss of charge carriers during the laser pulse. The principles of this technique and the phenomena have already been explained in previous articles [10,12,21,33,35,36,37].
2.4. Photocatalytic Hydrogen Generation Tests
The photocatalytic reactions were carried out in a sealed quartz reactor under vigorous stirring and an inert atmosphere. A total of 20 mg of the photocatalyst was dispersed in a 20 mL solution of 25% v/v methanol in water. Methanol was used as an electron donor to inhibit the oxidation reaction caused by the holes. Before irradiation, the reactor containing the photocatalyst in suspension (methanol/water) was degassed with nitrogen (N2) to remove oxygen (O2). Subsequently, the solution was exposed to UV–visible light using a Peschl photoreactor. The amount of H2 produced was measured by gas chromatography (Agilent System Technologies 7820A GC). A gas volume of 0.2 mL was injected into the gas chromatograph every hour for a duration of 5 h. For visible light experiments, a 300 W LOT-Oriel Xenon Lamp (250 to 2000 nm) equipped with a water filter (a large quartz cell) was positioned between the lamp and the reactor to block the infrared light and prevent sample heating. An optical filter was used to limit irradiation to the visible (λ ≥ 420 nm). The amount of hydrogen was measured by gas chromatography every hour for a duration of 5 h (Micro GC Fusion, INFICON, Bad Ragaz, Switzerland). The stability of the photocatalyst with cycling was also studied. After the photocatalytic test, the reactor was covered with aluminum foil and put in the dark. The next day, the reactor was degassed again and irradiated under the same conditions until 5 cycles were completed.
3. Results and Discussion
3.1. Characterization of the Photocatalysts
The morphology, particle size, and distribution of Ni-NPs on the TiO2 surface were analyzed using TEM. Due to the similar atomic numbers of Ni and Ti, distinguishing the Ni nanoparticles by TEM was challenging. TEM images show that reduction of nickel (II) acetylacetonate precursor by radiolysis led to nanoparticles of about 8 nm for the 3.5 wt.% Niacac-based NPs/TiO2 sample, as illustrated in Figure 2a. However, with Ni formate as the precursor, the Ni-based NPs are not distinguishable by TEM for the 3.5 wt.% Niformate-based NPs/TiO2 sample (Figure S1a), which may be due to the small size of the NPs and low metal loading. The crystal structure was analyzed by HRTEM for the 3.5 wt.% Niacac-based NPs/TiO2 sample (Figure 2b). The analysis revealed interplanar distances of 0.23 nm and 0.35 nm, corresponding to the lattice planes of NiO (111) and TiO2 anatase (101), respectively [21,38]. This result confirms the formation of a p-n heterojunction between NiO-NPs and titania. NiO-NPs are formed by back oxidation of Ni0-NPs (induced by radiolysis) when exposed to air during drying. Instead, the crystal structure of Ni-NPs was not detected in the 3.5 wt.% Niformate-based NPs/TiO2 sample due to the small size of Ni-NPs (Figure S1b). Only an interplanar distance of 0.35 nm was observed, corresponding to the anatase phase of TiO2 (101).
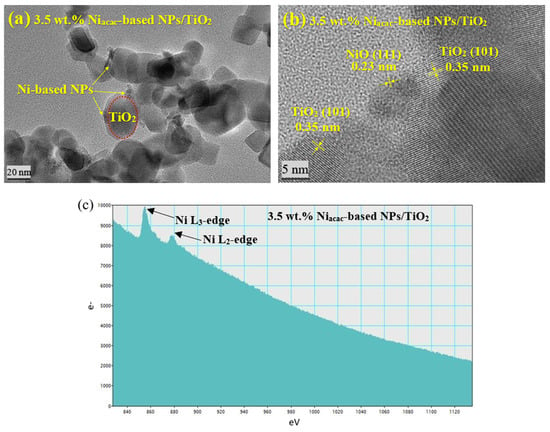
Figure 2.
(a) TEM micrograph, (b) HRTEM micrograph, and (c) EELS spectrum of 3.5 wt.% Niacac-based NPs/TiO2-modified sample.
EELS was used to reveal the presence of Ni and its oxidation states. Figure 2c displays the Ni-L2 and Ni-L3 edges at energy levels of 872 eV and 855 eV, respectively, for the 3.5 wt.% Niacac-based NPs/TiO2 sample.
These edges are associated with the Ni2+ oxidation state [39]. The appearance of the L2,3 ionization edges of nickel is attributed to the excited internal electron transitions (L2 edge) and (L3 edge) to the empty valence states of character s and d [40,41]. In the case of the 3.5 wt.% Niformate-based NPs/TiO2 sample (Figure S1c), no peak corresponding to the Ni-L2 and Ni-L3 edges was observed. ICP-OES was used for elemental analysis, providing insights into the mass content of Ni on the TiO2 surface. For 3.5 wt.% Niacac-based NPs/TiO2 and 3.5 wt.% Niformate-based NPs/TiO2 samples, the metal content is 1.30 wt% and 0.13 wt%, respectively. This result confirms that the precursor nickel (II) acetylacetonate is better than nickel (II) formate for the deposition of Ni-NPs on the TiO2 surface by radiolysis.
The optical properties of the modified photocatalysts and bare titanium dioxide were examined using UV-vis DRS. All samples showed strong absorption in the 200–400 nm range, which was attributed to the TiO2 support (Figure 3a and Figure S2a).
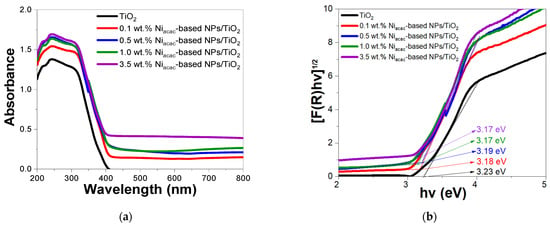
Figure 3.
(a) DRS spectra and (b) their Tauc plot of Niacac-based NPs/TiO2-modified samples and bare TiO2.
The modified samples showed a small redshift of the band edge compared to bare TiO2. The absorption in the visible range may be due to d–d transitions of Ni2+ [19,22,42,43,44] with Ni0-NPs being sensitive to oxygen. The band energy () can be calculated using the reflectance function F(R), where R is the reflectance; see Equation (3). The scattering and absorption coefficients are denoted by K and S, respectively. F(R) is proportional to the absorption coefficient (see Equation (4)), where is Planck’s constant, is the photon frequency, is the energy of the band gap, and is a factor depending on the transition probability and a constant in the optical frequency range. The factor n depends on the nature of the electron transition and takes a value of 2 or 1/2 for the direct and indirect transition band gaps, respectively [45].
The band gap energies for the modified samples and bare TiO2 were calculated with the Tauc plot method, considering the indirect transition of anatase-phase TiO2 [3]. Figure 3b shows the calculated band gap energies for the bare TiO2 (3.23 eV), 0.1 (3.18 eV), 0.5 (3.19 eV), 1.0 (3.17 eV), and 3.5 (3.17 eV) wt.% Niacac-based-NPs/TiO2 samples. Similarly, Figure S2b shows the band gap energies of the bare TiO2 (3.26 eV), 0.1 (3.26 eV), 0.5 (3.26 eV), 1.0 (3.26 eV), and 3.5 (3.18 eV) wt.% Niformate-based NPs/TiO2 samples. A slight reduction in the band gap energy is observed for the Niacac-modified samples, due to the higher content of Ni-based NPs deposited on the TiO2 surface, compared to the Niformate-modified samples. From Figure 3a, it is worth noting that NiO-NPs exhibit absorption in the visible region [22,43,44].
The crystal structure of the photocatalysts was studied by XRD (Figure S3). The diffracted peaks of the samples coincide with the reference peaks of the anatase crystalline phase (JCPDS file No. 21-1272) peaks are found at 2θ values at 24.8°, 37.3°, 47.6°, 53.5°, 55.1°, and 62.2°; correspond to (101), (004), (200), (105), (211), and (204) planes. For the rutile crystalline phase (JCPDS file No. 21-1276), peaks are at 2θ values 27.0°, 35.6°, 40.8°, and 54.0°, corresponding to (110), (101), (200), and (211) planes [46].
No diffracted peaks corresponding to the crystalline phases of Ni-NPs were observed. This observation is consistent with previous studies, where researchers also reported the absence of nickel peaks due to the low adsorbed nickel content and very small size of Ni-NPs [19,28,42]. Furthermore, no shifts were observed in the diffraction peaks of the samples modified with Ni-NPs. This suggests that the presence of Ni-NPs has no effect on the lattice structure of TiO2, and that they are solely absorbed on the TiO2 surface.
XPS was used to examine the oxidation states and surface chemical composition of Ni-NPs on the TiO2 surface. The XPS survey for the 3.5 wt.% Niacac-based NPs/TiO2 sample shows peaks corresponding to Ni, Ti, O, and C, indicating the presence of these elements on the surface of the modified sample; see Figure S4a. However, in the case of the 3.5 wt.% Niformate-based NPs/TiO2 sample, only peaks for Ti, O, and C were detected (see Figure S4b). The absence of Ni peaks in the latter sample is due to the low content of Ni-NPs on the TiO2 surface, as determined by ICP-OES. The narrow-scan XPS spectra of the 3.5 wt.% Niacac-based NPs/TiO2 sample were analyzed for Ni 2p3/2, Ti 2p, O 1s, and C 1s (Figure 4, Figure S5a, Figure S5b, and Figure S5c), respectively.
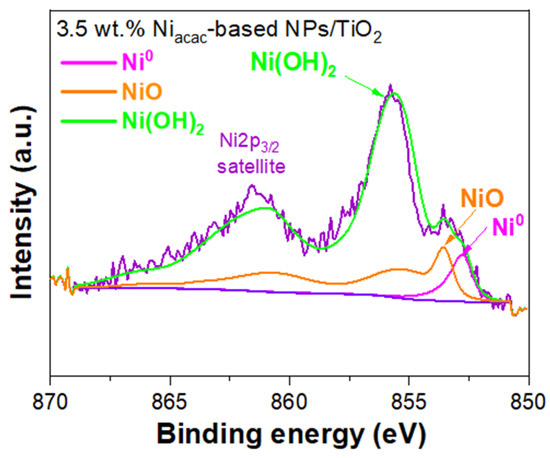
Figure 4.
Narrow-scan XPS spectra of Ni 2p3/2 of 3.5wt% Niacac-based NPs/TiO2-modified sample.
The Ni2p3/2 signal was fitted using synthetic line shapes and the fit parameters from the reference [47]. A Shirley-type background was subtracted from all spectra. The Ni2p signal shows contributions coming from Ni0 (852.7 eV), NiO (853.6 eV), and Ni(OH)2 (855.6 eV) on the TiO2 surface (Figure 4). The atomic percentages obtained for Ni0, NiO, and Ni(OH)2 are 6.63%, 33.60% and 59.77%, respectively. Ni (II) complexes have been reduced by radiolysis, leading to Ni0-NPs on TiO2. But these small NPs are sensitive to air and oxidize when exposed to air, which explains why the oxidized species are the main components of the Ni2p3/2 core-level spectrum. Ni0-NPs are expected to be located at the interface between NiO-NPs and TiO2, leading to the formation of an ohmic junction [21]. In the core level spectrum of Ti2p (Figure S5a), two symmetric peaks were observed at 458.7 eV and 464.5 eV, representing Ti 2p3/2 and Ti 2p1/2, respectively, which are the components of spin–orbit coupling [19,24]. The XPS spectrum of O 1s (Figure S5b) displayed an asymmetric peak, likely arising from adsorbed hydroxide species [24,48]. The spectrum of O 1s showed two peaks at 530.0 eV and 531.2 eV corresponding to lattice oxygen of TiO2 anatase and hydroxyl species, respectively [29,49]. The C 1s spectrum (Figure S5c) showed the peaks at 284.8 eV, 286.0 eV, and 288.8 eV, corresponding to C-C, C-O, and C = O, respectively, attributed to carbon contamination [50].
The TRMC signals highlight the significant impact of TiO2 surface modification with Ni-based NPs on the charge-carrier dynamics under UV and visible light excitation (Figure 5 and Figure S6). Just after the signal pulse, all samples reached Imax values, indicating electron transfer from the valence band (VB) to the conduction band (CB) of TiO2. Simultaneously, NiO with a bandgap of 3.5 eV is also excited, and electron transfer from the VB of NiO to its CB occurs [21,22,51]. Subsequently, faster decays and reduced Imax values were observed for the modified samples compared to bare TiO2 (Figure 5a and Figure S6a).
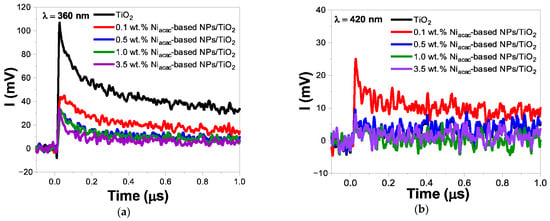
Figure 5.
TRMC signals of Niacac-based NPs/TiO2-modified samples and bare TiO2 at different wavelengths: (a) λ = 360 nm, and (b) λ = 420 nm. The laser energy of these wavelengths was 1.1 mJ and 2.3 mJ, respectively.
Electron transfer from titania to NiO-NPs is not thermodynamically allowed due to their higher position of CB level of NiO compared to the anatase phase of TiO2 [12]. However, the XPS results revealed a trace amount of Ni0 leading to the formation of an ohmic junction with TiO2. It is anticipated that these Ni0-NPs are located at the interface between NiO-NPs and TiO2, promoting the migration of photogenerated electrons to the metal [21,22]. The TRMC results provide strong evidence that Ni0-NPs effectively scavenge electrons from the conduction band of TiO2, which is beneficial for photocatalytic activity. [10,36,52] Simultaneously, the holes accumulated in the valence band of TiO2 will transfer to the NiO-VB, leading to more efficient charge-carrier separation [53]. In the case of the samples modified with nickel (II) acetylacetonate, the signal decay is faster than that of the nickel (II) formate salt precursor, and this signal decay is faster with increasing the metal loading (Figure 5a). The sample with 3.5 wt.% Niacac-based NPs/TiO2 exhibits the fastest decay rate among the different metal loadings (0.1, 0.5, and 1 wt.%). In the case of formate precursor modified samples, only a small amount of Ni is deposited on the TiO2 surface. However, surface modification of TiO2 induces a decay of the TRMC signals (Figure S6a).
Furthermore, for the surface-modified TiO2 photocatalysts, detectable TRMC signals were observed under visible light excitation at 420 nm (Figure 5b and Figure S6b). This suggests that NiO-NPs are excited by visible light due to their d–d transitions of Ni2+ [19,42,43,44]. The transfer of the photogenerated electrons from the CB of NiO-NPs to the CB of TiO2 is facilitated. For Niacac-modified samples, only 0.1 wt.% shows a detectable signal higher than that of bare TiO2 (Figure 5b). Nevertheless, for the 0.5, 1.0, and 3.5 wt.% samples, no detectable TRMC signals were obtained. This is likely due to the rapid electron transfer occurring within the system, and even if the TRMC technique allows us to obtain a nanosecond-scale signal, we cannot define the first 10 nanoseconds during the pulse. In the case of the Niformate-modified samples, all the loadings show higher signals than bare TiO2 (Figure S6b).
3.2. Photocatalytic Hydrogen Generation
The hydrogen generation is significantly enhanced when the TiO2 surface is modified with Ni-based NPs (obtained by radiolysis) under UV–visible light, as shown in Figure 6a.
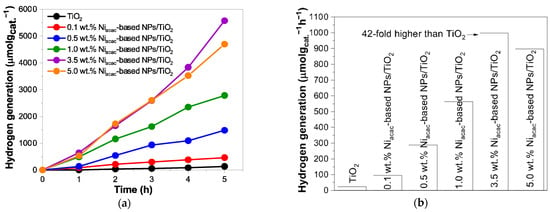
Figure 6.
(a) Photocatalytic hydrogen generation for Niacac-based NPs/TiO2 samples and bare TiO2 under UV–visible light, and (b) their hydrogen generation rates (µmol gcat.−1 h−1) under UV–visible light irradiation from 25% v/v methanol aqueous solution.
Ni-based NPs act as active sites that promote hydrogen formation [12,20,21,22]. The p-n heterojunction formed between NiO-NPs and TiO2 induces an internal electric field that reduces charge-carrier recombination and facilitates interfacial charge transfer. The hydrogen evolution rate of TiO2 was 23.3 µmol gcat.−1 h−1. The results demonstrate that the Niacac-based NPs/TiO2 samples with theoretical Ni loadings of 0.1, 0.5, 1.0, 3.5, and 5.0 wt.% on the TiO2 surface exhibit increasingly higher and notable hydrogen generation rates, corresponding to 95.9, 287.8, 563.3, 998.4, and 897.2 µmol gcat.− 1 h−1, respectively, as shown in Figure 6b. In particular, the most active sample was 3.5 wt.% Niacac-based NPs/TiO2 (998.4 µmol gcat.−1 h−1) after 5 h of irradiation, which is 42 times greater than that of bare TiO2 (23.3 µmol gcat.−1 h−1). These outcomes highlight that the achievement of the highest H2 generation rates is dependent on optimal metal loading. This highlights the importance of carefully controlling the metal loading for optimal performance. The decrease in the activity with higher loading than 5.0% may be due to higher charge-carrier recombinations. However, the hydrogen generation rates with the Niformate-based NPs/TiO2 samples were much lower compared to Niacac-TiO2 because of the very small amount of Ni deposited with the Ni formate precursor, see Figure S7b. In fact, the deposition of Ni-based NPs on TiO2 is more effective with Ni acetylacetonate as precursor, as proved by the ICP-OES results. The samples 3.5 wt.% Niacac-based NPs/TiO2 and 3.5 wt.% Niformate-based NPs/TiO2 were tested under visible light, showing a slight activity with hydrogen generation rates of 1.5 and 0.6 µmol gcat.−1 h−1, respectively. The sample 0.1 wt.% Niacac-based NPs/TiO2 shows a higher TRMC signal than bare TiO2 (Figure 5b) under visible excitation, but the hydrogen generation rate (0.4 µmol gcat.−1 h−1) was lower than that of 3.5 wt.% Niacac-based NPs/TiO2. This may be due to a higher amount of NiO or NiOH and a lower amount of metallic Ni0 NPs, which may play the role of cocatalysts for H recombination and H2 formation.
Finally, the photocatalytic stability with cycling of the best sample 3.5 wt.% Niacac-based NPs/TiO2 is studied and presented in Figure 7.
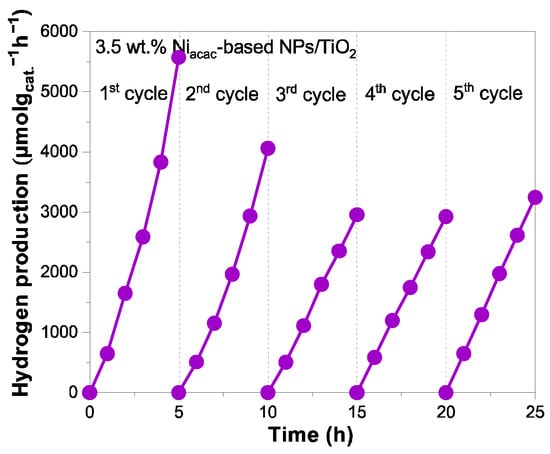
Figure 7.
Photocatalyst stability with cycling for 3.5 wt.% Niacac-based NPs/TiO2 sample under UV–visible light irradiation from 25% v/v methanol aqueous solution.
The hydrogen generation decreases after the second cycle. This decrease may be due to nickel leaching or to the oxidation of the small Ni0 clusters. In fact, nickel leaching is known in the field of catalysis [38,54,55]. And one way to avoid this leaching in catalysis is to alloy Ni with noble metals [56,57]. After the third cycle, a plateau is obtained, evidencing photocatalytic stability, which is crucial for practical applications. The surface chemical composition and oxidation states of Ni-NPs on the TiO2 surface were analyzed by XPS before and after cycling. Figure S8 shows the XPS surveys of the 3.5 wt.% Niacac-based NPs/TiO2 sample before and after five cycles. After cycling, the peak corresponding to Ni 2p at 855.9 eV slightly decreases in intensity, confirming the nickel leaching. Figure 8 shows the comparison of the narrow-scan XPS spectra of Ni 2p3/2 for the 3.5 wt.% Niacac-based NPs/TiO2 sample before and after cycling. The Ni 2p3/2 signal revealed the presence of NiO (853.6 eV) and Ni(OH)2 (855.7 eV) on the TiO2 surface. Ni0-NPs were not present after cycling.
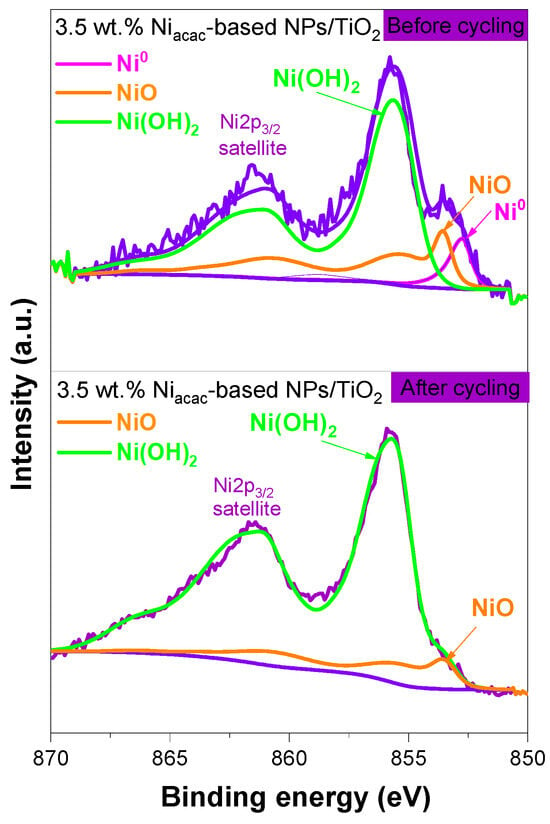
Figure 8.
Narrow-scan XPS spectra of Ni2p3/2 of 3.5 wt.% Niacac-based NPs/TiO2 sample before and after cycling.
Our results were compared with the literature on Ni-based NPs as cocatalysts on support semiconductors (Table S2). The comparison reveals notable differences in the efficiency of different catalysts under different conditions. The hydrogen generation rate of our best sample, 3.5 wt.% Niacac-based NPs/TiO2, is 998.4 µmol gcat.−1 h−1, ∼42-fold higher than that of bare TiO2 NPs, under UV–visible light irradiation. For comparison with other systems reported in the literature, Ni(OH)2/TiO2 nanotubes demonstrated approximately 12 times higher rate than TiO2 [19], and Ni/C/TiO2 showed 9 times higher rate than TiO2 [58]. NiO/CdS has been reported to be active under visible light (Table S2), with CdS being a semiconductor with a small band gap of 2.42 eV [59,60,61]. However, CdS is a toxic compound and tends to corrode under light irradiation, resulting in harmful Cd2+. For photocatalytic application, it is essential to use non-toxic and stable supports.
3.3. Proposed Photocatalytic Hydrogen Mechanism
Hereby, we propose a photocatalytic hydrogen generation mechanism for the Ni-based NPs/TiO2 system under UV–visible light irradiation, as shown in Figure 9. However, the XPS analysis after cycling shows the disappearance of the Ni0 signal, with only NiO and Ni(OH)2 remaining detectable. This suggests that Ni0 is not stable under reaction conditions over multiple cycles, and this is associated with a decrease in H2 generation in the first three cycles. The mechanism probably shifts after the first couple of cycles, relying more heavily on the NiO-TiO2 p-n junction.
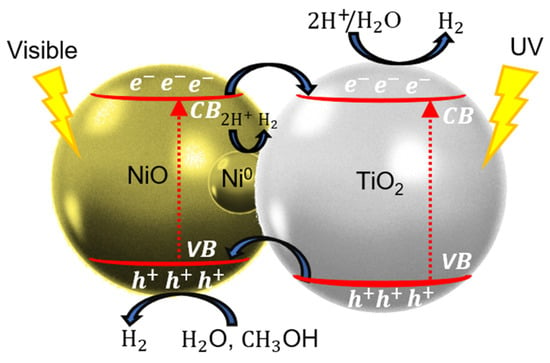
Figure 9.
Photocatalytic mechanism of Ni-based NPs/TiO2 sample under UV–visible light excitation.
Recent research papers have investigated the photocatalytic hydrogen generation of p-n NiO/TiO2 heterojunction for H2 production using simulated solar light [16,17,21,25,62,63]. When NiO/TiO2 photocatalysts are exposed to solar light, electron–hole pairs are generated in both TiO2 and NiO semiconductors (Figure 9 and Equation (5)). In the photocatalytic process, the relative locations of the semiconductors’ CB and VB are crucial because they create an internal electric field that reduces charge-carriers’ recombinations and facilitates interfacial charge transfer. Electrons photogenerated in the NiO-CB migrate to the TiO2-CB, and holes photogenerated in TiO2-VB migrate to the NiO-VB (Equation (6)). This charge transfer decreases charge-carriers’ recombinations, leading to H2 production by proton (H+) reduction (Equation (7)) or water (H2O) reduction (Equation (8)). Photogenerated electrons reaching the surfaces of TiO2 and NiO-NPs can reduce H+ to H2.
Additionally, the TRMC results provide strong evidence that Ni0 NPs effectively scavenge electrons from the CB of TiO2 under UV light, which is beneficial for photocatalytic activity [36,52]. As we mentioned before, the XPS results revealed a trace amount of Ni0 leading to the formation of an ohmic junction with TiO2. These Ni0-NPs are located at the interface between NiO-NPs and TiO2, as shown in Figure 9, facilitating the migration of photogenerated electrons to the metal [21,22]. The photogenerated electrons reaching the surface Ni0-NPs can also reduce H+ to H2.
To prevent the oxidation reaction, a sacrificial agent is used: methanol (CH3OH). It effectively scavenges the holes of the VB of NiO to generate H2 [12,24]. Methanol and water molecules react with the holes, producing hydroxyl radicals (•OH) (see Equations (9) and (10)), which subsequently oxidize CH3OH molecules into formaldehyde (CH2O), formic acid (HCOOH), and carbon dioxide (CO2); see Equations (11)–(14). These compounds are the primary intermediates in the reforming of methanol to H2. The choice of methanol as a hole scavenger is advantageous owing to its high H/C ratio and the absence of C-C bonds, which reduces the risk of carbon formation and fouling of the photocatalyst [10,64]. Furthermore, the TRMC results under visible excitation confirmed detectable signals, indicating that NiO produces photogenerated electrons and holes due to its d-d transitions [19,22,42,43,44]. Under visible-light irradiation, these photogenerated electrons migrate from the CB of NiO to the CB of TiO2.
4. Conclusions
The modification of the TiO2 surface with Ni nanoparticles by radiolysis leads to small Ni-NPs dispersed on titania. The deposition of Ni-NPs with Ni acetylacetonate as a precursor is more efficient (compared to Ni formate), as confirmed by TEM, HRTEM, EELS, ICP-OES, DRS, and XPS techniques. The modified samples exhibited good photocatalytic activity for H2 evolution under UV–visible light and also slight activity under visible light. Notably, modification of TiO2 with the precursor nickel (II) acetylacetonate leads to higher hydrogen generation rates than that of nickel (II) formate. The Ni metal loading for Niacac-modified samples was optimized for photocatalytic hydrogen generation performance. Among the modified samples, the 3.5 wt.% Niacac-based NPs/TiO2 sample demonstrated the most outstanding increase in photocatalytic activity, with a rate approximately 42-fold higher than that of bare TiO2. The TRMC results provide strong evidence that Ni0-NPs effectively scavenge electrons from the CB of TiO2 under UV light, and Ni oxide NPs inject electrons into the conduction band of TiO2 under visible light. Additionally, the formation of p-n heterojunction between NiO-NPs and TiO2 played a crucial role in creating an internal electric field that reduced charge-carrier recombination and facilitated interfacial charge transfer. The modification of TiO2 with Ni-based NPs enhances charge-carrier separation and induces high H2 generation under UV–visible light. The photocatalytic activity reaches a stable rate after the second cycle. These findings advance our understanding of charge-carrier dynamics and provide valuable insights to optimize photocatalytic systems for sustainable energy applications. For further studies, we are developing photocatalysts with titania modified with bi- and tri-metallic alloys based on Ni. Alloying Ni with a noble metal such as Pt or Au is expected to enhance the photocatalytic activity and the stability of the photocatalyst.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ma18153513/s1, Figure S1: (a) TEM micrograph, (b) HRTEM micrograph, and (c) EELS spectrum of 3.5 wt.% Niformate-based NPs/TiO2; Figure S2: (a) DRS spectra and their (b) Tauc plot of Niformate-based NPs/TiO2-modified samples and bare TiO2; Figure S3: X-ray diffraction (XRD) patterns of (a) Niformate-based NPs/TiO2 and (b) Niacac-based NPs/TiO2-modified samples and bare TiO2 with the reference peaks of the anatase and rutile crystalline phases; Figure S4: Survey XPS spectra of (a) 3.5 wt% Niacac-based NPs/TiO2 and (b) 3.5 wt% Niformate-based NPs/TiO2 samples; Figure S5: Narrow-scan XPS spectra of (a) Ti 2p, (b) O 1s, and (c) C 1s of 3.5 wt% Niacac-based NPs/TiO2-modified sample; Figure S6: TRMC signals of Niformate-based NPs/TiO2-modified samples and bare TiO2 at different wavelengths: (a) λ = 360 nm and (b) λ = 420 nm. The laser energy of these wavelengths was 1.1 and 2.3 mJ, respectively; Figure S7: (a) Photocatalytic hydrogen generation for Niformate-based NPs/TiO2 samples and bare TiO2 under UV–visible light, and (b) their hydrogen generation rates (µmol gcat.−1 h−1) under UV–visible light irradiation from 25% v/v methanol aqueous solution; Figure S8: Survey XPS spectra of 3.5 wt.% Niacac-based NPs/TiO2 sample before and after cycling; Table S1: Doses for the modified samples; Table S2: Comparison of H2 evolution under UV–visible light of the different photocatalysts. References [15,19,23,24,28,42,43,58,59,60,61] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, H.R.; methodology, H.R., X.Y. and A.A.M.-M.; software, A.A.M.-M. and D.D.; validation, H.R., X.Y., D.D., C.C.-J., J.L.R.L. and A.A.M.-M.; formal analysis, A.A.M.-M.; investigation, X.Y. and A.A.M.-M.; resources, H.R., J.L.R.L., C.C.-J. and D.D.; data curation, X.Y. and A.A.M.-M.; writing—original draft preparation, A.A.M.-M.; writing—review and editing, H.R., J.L.R.L., D.D., X.Y. and A.A.M.-M.; visualization, A.A.M.-M.; supervision, H.R., X.Y. and J.L.R.L.; project administration, H.R. and J.L.R.L.; funding acquisition, H.R. and J.L.R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CONAHCYT-Mexico, grant number 995288, and CAMPUS FRANCE through a scholarship from the French Embassy in Mexico.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
A.A.M.-M. acknowledges the Labex NanoSaclay and the National Laboratory Research in Nanoscience and Nanotechnology (LINAN), IPICYT; Héctor Gabriel Silva Pereyra for EELS characterization. M.C. Beatriz Adriana Rivera-Escoto and Ignacio Guadalupe Becerril Juárez for XRD characterization. The authors also acknowledge Mireille Benoit for her help at the Institut de Chimie Physique.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| H2 | Hydrogen |
| TiO2 | Titanium Dioxide |
| Ni | Nickel |
| NiO | Nickel Oxide |
| Ni0 | Metallic Nickel |
| Ni(OH)2 | Nickel Hydroxide |
| NPs | Nanoparticles |
| Acac | Acetylacetonate |
| N2 | Nitrogen |
| O2 | Oxygen |
| UV | Ultraviolet |
| CB | Conduction Band |
| VB | Valence Band |
| NHE | Normal Hydrogen Electrode |
| TEM | Transmission Electron Microscopy |
| HRTEM | High-Resolution Transmission Electron Microscopy |
| EELS | Electron Energy Loss Spectroscopy |
| ICP-OES | Inductively Coupled Plasma Optical Emission Spectrometry |
| DRS | Diffuse Reflectance Spectroscopy |
| XRD | X-Ray Diffraction |
| XPS | X-ray Photoelectron Spectroscopy |
| TRMC | Time-Resolved Microwave Conductivity |
| GC | Gas Chromatography |
| CONAHCYT | Consejo Nacional de Humanidades, Ciencias y Tecnologías |
| CNRS | Centre National de la Recherche Scientifique |
| UMR | Unité Mixte de Recherche |
| IPICYT | Instituto Potosino de Investigación Científica y Tecnológica |
References
- Sun, H.; Li, L.; Chen, Y.; Kim, H.; Xu, X.; Guan, D.; Hu, Z.; Zhang, L.; Shao, Z.; Jung, W. Boosting ethanol oxidation by NiOOH-CuO nano-heterostructure for energy-saving hydrogen production and biomass upgrading. Appl. Catal. B Environ. 2023, 325, 122388. [Google Scholar] [CrossRef]
- Yuan, X.; Floresyona, D.; Aubert, P.H.; Bui, T.T.; Remita, S.; Ghosh, S.; Brisset, F.; Goubard, F.; Remita, H. Photocatalytic degradation of organic pollutant with polypyrrole nanostructures under UV and visible light. Appl. Catal. B 2019, 242, 284–292. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- Méndez-Medrano, M.G.; Kowalska, E.; Lehoux, A.; Herissan, A.; Ohtani, B.; Bahena, D.; Briois, V.; Colbeau-Justin, C.; Rodríguez-López, J.L.; Remita, H. Surface Modification of TiO2 with Ag Nanoparticles and CuO Nanoclusters for Application in Photocatalysis. J. Phys. Chem. C. 2016, 120, 5143–5154. [Google Scholar] [CrossRef]
- Yuan, X.; Kobylanski, M.P.; Cui, Z.; Li, J.; Beaunier, P.; Dragoe, D.; Colbeau-Justin, C.; Zaleska-Medynska, A.; Remita, H. Highly active composite TiO2-polypyrrole nanostructures for water and air depollution under visible light irradiation. J. Environ. Chem. Eng. 2020, 8, 104178. [Google Scholar] [CrossRef]
- Rafique, M.; Hajra, S.; Irshad, M.; Usman, M.; Imran, M.; Assiri, M.A.; Ashraf, W.M. Hydrogen Production Using TiO2-Based Photocatalysts: A Comprehensive Review. ACS Omega 2023, 8, 25640–25648. [Google Scholar] [CrossRef]
- Chung, Y.H.; Han, K.; Lin, C.Y.; O’Neill, D.; Mul, G.; Mei, B.; Yang, C.M. Photocatalytic hydrogen production by photo-reforming of methanol with one-pot synthesized Pt-containing TiO2 photocatalysts. Catal. Today 2020, 356, 95–100. [Google Scholar] [CrossRef]
- Chen, Y.; Soler, L.; Armengol-Profitós, M.; Xie, C.; Crespo, D.; Llorca, J. Enhanced photoproduction of hydrogen on Pd/TiO2 prepared by mechanochemistry. Appl. Catal. B 2022, 309, 121275. [Google Scholar] [CrossRef]
- Fang, S.; Liu, Y.; Sun, Z.; Lang, J.; Bao, C.; Hu, Y.H. Photocatalytic hydrogen production over Rh-loaded TiO2: What is the origin of hydrogen and how to achieve hydrogen production from water? Appl. Catal. B 2020, 278, 119316. [Google Scholar] [CrossRef]
- Méndez-Medrano, M.G.; Kowalska, E.; Lehoux, A.; Herissan, A.; Ohtani, B.; Rau, S.; Colbeau-Justin, C.; Rodríguez-López, J.L.; Remita, H. Surface Modification of TiO2 with Au Nanoclusters for Efficient Water Treatment and Hydrogen Generation under Visible Light. J. Phys. Chem. C 2016, 120, 25010–25022. [Google Scholar] [CrossRef]
- Gogoi, D.; Namdeo, A.; Golder, A.K.; Peela, N.R. Ag-doped TiO2 photocatalysts with effective charge transfer for highly efficient hydrogen production through water splitting. Int. J. Hydrogen Energy 2020, 45, 2729–2744. [Google Scholar] [CrossRef]
- Luna, A.L.; Dragoe, D.; Wang, K.; Beaunier, P.; Kowalska, E.; Ohtani, B.; Bahena Uribe, D.; Valenzuela, M.A.; Remita, H.; Colbeau-Justin, C. Photocatalytic Hydrogen Evolution Using Ni-Pd/TiO2: Correlation of Light Absorption, Charge-Carrier Dynamics, and Quantum Efficiency. J. Phys. Chem. C. 2017, 121, 14302–14311. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, R. Nickel-based cocatalysts for photocatalytic hydrogen production. Appl. Surf. Sci. 2015, 351, 779–793. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, J.; Cheng, B.; Yu, J.; Xu, J. Nickel-based cocatalysts for photocatalysis: Hydrogen evolution, overall water splitting and CO2 reduction. Mater. Today Phys. 2020, 15, 100279. [Google Scholar] [CrossRef]
- Sreethawong, T.; Suzuki, Y.; Yoshikawa, S. Photocatalytic evolution of hydrogen over mesoporous TiO2 supported NiO photocatalyst prepared by single-step sol-gel process with surfactant template. Int. J. Hydrogen Energy 2005, 30, 1053–1062. [Google Scholar] [CrossRef]
- Zheng, D.; Zhao, H.; Wang, S.; Hu, J.; Chen, Z. NiO-TiO2 p-n heterojunction for solar hydrogen generation. Catalysts 2021, 11, 1427. [Google Scholar] [CrossRef]
- Rawool, S.A.; Pai, M.R.; Banerjee, A.M.; Arya, A.; Ningthoujam, R.S.; Tewari, R.; Rao, R.; Chalke, B.; Ayyub, P.; Tripathi, A.K.; et al. pn Heterojunctions in NiO:TiO2 composites with type-II band alignment assisting sunlight driven photocatalytic H2 generation. Appl. Catal. B 2018, 221, 443–458. [Google Scholar] [CrossRef]
- Yu, J.; Hai, Y.; Cheng, B. Enhanced photocatalytic H2-production activity of TiO2 by Ni(OH)2 cluster modification. J. Phys. Chem. C 2011, 115, 4953–4958. [Google Scholar] [CrossRef]
- Lakshmana Reddy, N.; Cheralathan, K.K.; Durga Kumari, V.; Neppolian, B.; Muthukonda Venkatakrishnan, S. Photocatalytic Reforming of Biomass Derived Crude Glycerol in Water: A Sustainable Approach for Improved Hydrogen Generation Using Ni(OH)2 Decorated TiO2 Nanotubes under Solar Light Irradiation. ACS Sustain. Chem. Eng. 2018, 6, 3754–3764. [Google Scholar] [CrossRef]
- Xie, L.; Hao, J.G.; Chen, H.Q.; Li, Z.X.; Ge, S.Y.; Mi, Y.; Yang, K.; Lu, K.Q. Recent advances of nickel hydroxide-based cocatalysts in heterogeneous photocatalysis. Catal. Commun. 2022, 162, 106371. [Google Scholar] [CrossRef]
- Wang, C.; Dragoe, D.; Colbeau-Justin, C.; Haghi-Ashtiani, P.; Ghazzal, M.N.; Remita, H. Highly Dispersed Ni-Pt Bimetallic Cocatalyst: The Synergetic Effect Yields Pt-Like Activity in Photocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2023, 15, 42637–42647. [Google Scholar] [CrossRef]
- Luna, A.L.; Novoseltceva, E.; Louarn, E.; Beaunier, P.; Kowalska, E.; Ohtani, B.; Valenzuela, M.A.; Remita, H.; Colbeau-Justin, C. Synergetic effect of Ni and Au nanoparticles synthesized on titania particles for efficient photocatalytic hydrogen production. Appl. Catal. B 2016, 191, 18–28. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, J.; Zhao, Z.; Guo, W.; Qiu, J.; Mou, X.; Li, A.; Claverie, J.P.; Liu, H. NiO-TiO2 p-n heterostructured nanocables bridged by zero-bandgap rGO for highly efficient photocatalytic water splitting. Nano Energy 2015, 16, 207–217. [Google Scholar] [CrossRef]
- Uddin, M.T.; Nicolas, Y.; Olivier, C.; Jaegermann, W.; Rockstroh, N.; Junge, H.; Toupance, T. Band alignment investigations of heterostructure NiO/TiO2 nanomaterials used as efficient heterojunction earth-abundant metal oxide photocatalysts for hydrogen production. Phys. Chem. Chem. Phys. 2017, 19, 19279–19288. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Ke, J.; Wang, S.; Wang, L.; Xiao, H. Black NiO-TiO2 nanorods for solar photocatalysis: Recognition of electronic structure and reaction mechanism. Appl. Catal. B 2018, 224, 705–714. [Google Scholar] [CrossRef]
- D’Amario, L.; Föhlinger, J.; Boschloo, G.; Hammarström, L. Unveiling hole trapping and surface dynamics of NiO nanoparticles. Chem. Sci. 2018, 9, 223–230. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Abbasi, M.A.; Liu, X.; Alsalhi, M.S.; Willander, M. The synthesis of NiO/TiO2 heterostructures and their valence band offset determination. J. Nanomater. 2014, 2014, 928658. [Google Scholar] [CrossRef]
- Melián, E.P.; Suárez, M.N.; Jardiel, T.; Rodríguez, J.M.D.; Caballero, A.C.; Araña, J.; Calatayud, D.G.; Díaz, O.G. Influence of nickel in the hydrogen production activity of TiO2. Appl. Catal. B 2014, 152–153, 192–201. [Google Scholar] [CrossRef]
- Yu, C.; Li, M.; Yang, D.; Pan, K.; Yang, F.; Xu, Y.; Yuan, L.; Qu, Y.; Zhou, W. NiO nanoparticles dotted TiO2 nanosheets assembled nanotubes P-N heterojunctions for efficient interface charge separation and photocatalytic hydrogen evolution. Appl. Surf. Sci. 2021, 568, 150981. [Google Scholar] [CrossRef]
- Remita, H.; Lampre, I. Synthesis of Metallic Nanostructures Using Ionizing Radiation and Their Applications. Materials 2024, 17, 364. [Google Scholar] [CrossRef]
- Ray, P.; Clément, M.; Martini, C.; Abdellah, I.; Beaunier, P.; Rodriguez-Lopez, J.L.; Huc, V.; Remita, H.; Lampre, I. Stabilisation of small mono- and bimetallic gold-silver nanoparticles using calix [8]arene derivatives. New J. Chem. 2018, 42, 14128–14137. [Google Scholar] [CrossRef]
- Myron, J.J.J.; Freeman, G.R. The radiolysis of ethanol liquid phase. Can. J. Chem. 1965, 43, 35–43. [Google Scholar] [CrossRef]
- Tahiri Alaoui, O.; Herissan, A.; Le Quoc, C.; Zekri, M.E.M.; Sorgues, S.; Remita, H.; Colbeau-Justin, C. Elaboration, charge-carrier lifetimes and activity of Pd-TiO2 photocatalysts obtained by gamma radiolysis. J. Photochem. Photobiol. A Chem. 2012, 242, 34–43. [Google Scholar] [CrossRef]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- Luna, A.L.; Matter, F.; Schreck, M.; Wohlwend, J.; Tervoort, E.; Colbeau-Justin, C.; Niederberger, M. Monolithic metal-containing TiO2 aerogels assembled from crystalline pre-formed nanoparticles as efficient photocatalysts for H2 generation. Appl. Catal. B 2020, 267, 118660. [Google Scholar] [CrossRef]
- Remita, H.; Méndez-Medrano, M.G.; Colbeau-Justin, C. Effect of Modification of TiO2 with Metal Nanoparticles on its Photocatalytic Properties Studied by Time Resolved Microwave Conductivity. In Visible Light-Active Photocatalysis; Ghosh, S., Ed.; Wiley-VCH: Weinheim, Germany, 2018; pp. 129–164. [Google Scholar]
- Méndez-Medrano, A.A.; Bahena-Uribe, D.; Dragoe, D.; Clavaguéra, C.; Colbeau-Justin, C.; Palomares Báez, J.P.; Rodríguez-López, J.L.; Remita, H. Enhanced Photocatalytic Activity of Surface-Modified TiO2 with Bimetallic AuPd Nanoalloys for Hydrogen Generation. Sol. RRL 2024, 8, 2400106. [Google Scholar] [CrossRef]
- Yuan, X.; Dragoe, D.; Beaunier, P.; Uribe, D.B.; Ramos, L.; Méndez-Medrano, M.G.; Remita, H. Polypyrrole nanostructures modified with mono- and bimetallic nanoparticles for photocatalytic H2 generation. J. Mater. Chem. A 2020, 8, 268–276. [Google Scholar] [CrossRef]
- Langenberg, E.; Rebled, J.; Estradé, S.; Daumont, C.J.M.; Ventura, J.; Coy, L.E.; Polo, M.C.; García-Cuenca, M.V.; Ferrater, C.; Noheda, B.; et al. Long-range order of Ni2+ and Mn4+ and ferromagnetism in multiferroic (Bi0.9La0.1)2NiMnO6 thin films. J. Appl. Phys. 2010, 108, 123907. [Google Scholar] [CrossRef]
- Potapov, P.L.; Kulkova, S.E.; Schryvers, D.; Verbeeck, J. Structural and chemical effects on EELS L3,2 ionization edges in Ni-based intermetallic compounds. Phys. Rev. B 2001, 64, 184110. [Google Scholar] [CrossRef]
- De Groot, F.M.F.; Hu, Z.W.; Lopez, M.F.; Kaindl, G.; Guillot, F.; Tronc, M. Differences between L3 and L2 x-ray absorption spectra of transition metal compounds. J. Chem. Phys. 1994, 101, 6570–6576. [Google Scholar] [CrossRef]
- Wang, W.; Liu, S.; Nie, L.; Cheng, B.; Yu, J. Enhanced photocatalytic H2-production activity of TiO2 using Ni(NO3)2 as an additive. Phys. Chem. Chem. Phys. 2013, 15, 12033–12039. [Google Scholar] [CrossRef]
- Chen, W.T.; Chan, A.; Sun-Waterhouse, D.; Llorca, J.; Idriss, H.; Waterhouse, G.I.N. Performance comparison of Ni/TiO2 and Au/TiO2 photocatalysts for H2 production in different alcohol-water mixtures. J. Catal. 2018, 367, 27–42. [Google Scholar] [CrossRef]
- Chen, J.; Wang, M.; Hu, J.; Han, J.; Yu, H.; Guo, R. TiO2 nanosheet/NiO nanorod/poly(dopamine) ternary hybrids towards efficient visible light photocatalysis. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 128197. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Li, W.; Liang, R.; Hu, A.; Huang, Z.; Zhou, N. Generation of oxygen vacancies in visible light activated one-dimensional iodine TiO2 photocatalysts. RSC Adv. 2014, 4, 36959–36966. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Zou, D.; Yi, Y.; Song, Y.; Guan, D.; Xu, M.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. BaCe0.16Y0.04Fe0.8O3-δ nanocomposite: A new high-performance cobalt-free triple-conducting cathode for protonic ceramic fuelcells operating at reduced temperatures. J. Mater. Chem. A 2022, 10, 5381–5390. [Google Scholar] [CrossRef]
- Zhao, H.; Li, C.F.; Liu, L.Y.; Palma, B.; Hu, Z.Y.; Renneckar, S.; Larter, S.; Li, Y.; Kibria, M.G.; Hu, J.; et al. n-p Heterojunction of TiO2-NiO core-shell structure for efficient hydrogen generation and lignin photoreforming. J. Colloid Interface Sci. 2021, 585, 694–704. [Google Scholar] [CrossRef]
- Lai, B.; Mei, F.; Gu, Y. Bifunctional Solid Catalyst for Organic Reactions in Water: Simultaneous Anchoring of Acetylacetone Ligands and Amphiphilic Ionic Liquid “Tags” by Using a Dihydropyran Linker. Chem. Asian J. 2018, 13, 2529–2542. [Google Scholar] [CrossRef]
- Kim, S.I.; Thiyagarajan, P.; Jang, J.H. Great improvement in pseudocapacitor properties of nickel hydroxide via simple gold deposition. Nanoscale 2014, 6, 11646–11652. [Google Scholar] [CrossRef]
- Hai, Z.; El Kolli, N.; Uribe, D.B.; Beaunier, P.; José-Yacaman, M.; Vigneron, J.; Etcheberry, A.; Sorgues, S.; Colbeau-Justin, C.; Chen, J.; et al. Modification of TiO2 by bimetallic Au-Cu nanoparticles for wastewater treatment. J. Mater. Chem. A 2013, 1, 10829–10835. [Google Scholar] [CrossRef]
- Chen, J.; Wang, M.; Han, J.; Guo, R. TiO2 nanosheet/NiO nanorod hierarchical nanostructures: P–n heterojunctions towards efficient photocatalysis. J. Colloid Interface Sci. 2020, 562, 313–321. [Google Scholar] [CrossRef]
- Rosen, B.M.; Quasdorf, K.W.; Wilson, D.A.; Zhang, N.; Resmerita, A.M.; Garg, N.K.; Percec, V. Nickel-catalyzed cross-couplings involving carbon-oxygen bonds. Chem. Rev. 2011, 111, 1346–1416. [Google Scholar] [CrossRef]
- Dong, Y.; Jv, J.J.; Li, Y.; Li, W.H.; Chen, Y.Q.; Sun, Q.; Ma, J.P.; Dong, Y. Bin. Nickel-metalated porous organic polymer for Suzuki-Miyaura cross-coupling reaction. RSC Adv. 2019, 9, 20266–20272. [Google Scholar] [CrossRef]
- Kaniukov, E.Y.; Shumskaya, A.E.; Kutuzau, M.D.; Bundyukova, V.D.; Yakimchuk, D.V.; Borgekov, D.B.; Ibragimova, M.A.; Korolkov, I.V.; Giniyatova, S.G.; Kozlovskiy, A.L.; et al. Degradation mechanism and way of surface protection of nickel nanostructures. Mater. Chem. Phys. 2019, 223, 88–97. [Google Scholar] [CrossRef]
- De, S.; Zhang, J.; Luque, R.; Yan, N. Ni-based bimetallic heterogeneous catalysts for energy and environmental applications. Energy Environ. Sci. 2016, 9, 3314–3347. [Google Scholar] [CrossRef]
- Zhao, X.; Xie, W.; Shao, X.; Wang, Z.; Yang, B.; Yang, C.; Wang, J.; Su, X. An effective Ni/C co-catalyst for promoting photocatalytic hydrogen evolution over TiO2 nanospheres. Mater. Sci. Semicond. Process. 2022, 148, 106775. [Google Scholar] [CrossRef]
- Chen, X.; Chen, W.; Lin, P.; Yang, Y.; Gao, H.; Yuan, J.; Shangguan, W. In situ photodeposition of nickel oxides on CdS for highly efficient hydrogen production via visible-light-driven photocatalysis. Catal. Commun. 2013, 36, 104–108. [Google Scholar] [CrossRef]
- Quiroz-Cardoso, O.; Oros-Ruiz, S.; Solís-Gómez, A.; López, R.; Gómez, R. Enhanced photocatalytic hydrogen production by CdS nanofibers modified with graphene oxide and nickel nanoparticles under visible light. Fuel 2019, 237, 227–235. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, Z.; Liu, X.; Jia, H.; Du, P. Cadmium sulfide/graphitic carbon nitride heterostructure nanowire loading with a nickel hydroxide cocatalyst for highly efficient photocatalytic hydrogen production in water under visible light. Nanoscale 2016, 8, 4748–4756. [Google Scholar] [CrossRef]
- Sun, B.; Zhou, G.; Gao, T.; Zhang, H.; Yu, H. NiO nanosheet/TiO2 nanorod-constructed p-n heterostructures for improved photocatalytic activity. Appl. Surf. Sci. 2016, 364, 322–331. [Google Scholar] [CrossRef]
- Mannaa, M.A.; Qasim, K.F.; Alshorifi, F.T.; El-Bahy, S.M.; Salama, R.S. Role of NiO Nanoparticles in Enhancing Structure Properties of TiO2 and Its Applications in Photodegradation and Hydrogen Evolution. ACS Omega 2021, 6, 30386–30400. [Google Scholar] [CrossRef]
- Ortega Méndez, J.A.; López, C.R.; Pulido Melián, E.; González Díaz, O.; Doña Rodríguez, J.M.; Fernández Hevia, D.; Macías, M. Production of hydrogen by water photo-splitting over commercial and synthesised Au/TiO2 catalysts. Appl. Catal. B 2014, 147, 439–452. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).