3.1. Analysis of qm and KL Values of the Langmuir Isotherm
Next to the Freundlich isotherm, the Langmuir isotherm is the most commonly used to describe the results of adsorption studies. One of the advantages of this isotherm is that it is possible to determine precisely the coefficient q
m, which determines the capacity of the monolayer. This coefficient gives the maximum adsorption capacity of a given compound, assuming that a single adsorption layer is formed. The q
m value, given, e.g., in mg/g or mmol/g, is readily used to compare, e.g., different sorbents, to assess the effect of pH and temperature on adsorption effects or to evaluate the effects of sorbent modifications. The constant q
m can also be used, for example, to calculate the specific surface area of a sorbent. Many authors use the q
m value to compare the properties of sorbents with other researchers [
51,
52,
53]. The question is whether these values can be compared if they have been calculated from the non-linear form (1) or from the different linear forms (3)–(7). The linear forms of the Langmuir isotherm are not a mathematical transformation of the isotherm equation, as is the case with the linear form of the Freundlich isotherm. It is, therefore, a certain superposition of various linear models on the Langmuir isotherm model. It should also be noted that different authors use different linear forms of the Langmuir isotherm to calculate constants. In an attempt to assess the differences resulting from the way in which the Langmuir isotherm constants are calculated, the coefficients from the non-linear form and the four linear forms were determined. The Langmuir isotherms calculated in this way are included in the
supplement in Tables S1–S11. The 68 isotherm constants are included in the tables, with published values marked with “*”(constants calculated from non-linear forms).
Table S1–S11 summarises the values of the capacity quotient of the monolayer calculated from the quotient of the linear forms and the non-linear form (q
ml/q
mn). Whereby q
mn denotes the value calculated from the non-linear equations and q
mI denotes the value calculated from the linear forms.
Table 3 already shows only the range of values obtained, and the mean, median, and number of quotients of q
ml/q
mn ≥ or < from 1 (detailed data in
Tables S1–S11). For each of the linear forms, the monolayer capacity results obtained are both smaller and larger than those obtained from the non-linear form. The smallest differences were observed when the constant q
mI was calculated from linear form II (Hanes–Woolf). This is the form most commonly used by researchers to determine constants from the linear form. The ratio q
mI/q
mn ranged from 0.93 to 0.10, the arithmetic mean was 1, and the median was 0.99. The q
m values obtained from linear form II (4) differed little from those obtained from the non-linear form. Only slightly larger differences were obtained between the constants calculated from linear form III and from the non-linear form. The greatest differences in q
m values were observed when the constants were calculated from the I linear form of the Langmuir isotherm (3). For this form, the ratio q
mI/q
mn ranged from 0.83 to 1.75. For the I form of the isotherm, the highest arithmetic mean of 1.09 and median of 1.08 were also obtained.
Considering the frequency of use of linear forms for the calculation of Langmuir isotherms, it should be noted that linear form II is used most frequently. Less frequently, but also frequently, linear form I is used, for which the differences obtained between the constants calculated from the non-linear form are the highest. Forms III and IV are used very infrequently, mainly when authors compare multiple linear forms with each other.
Table 3 also shows the number of isotherms for which the obtained q
m values from the linear equations are less than, equal to, or greater than the constants determined from the non-linear forms (q
ml/q
mn < 1; q
ml/q
mn = 1; q
ml/q
mn > 1).
Figure 1 shows the q
ml/q
mn values obtained (based on the data presented in
Tables S1–S11). It was found that it was not possible to determine unambiguously that the obtained q
m values from the linear form would be greater or less than those obtained from the non-linear form. When q
m was calculated from the II linear form, in 40 cases (58.8%), q
m values higher than those calculated from the non-linear form were obtained, and in 19 cases (28.0%), the obtained q
m values were lower. When the other non-linear forms of the Langmuir isotherm were used, most of the q
m values were lower than the values obtained from the non-linear form.
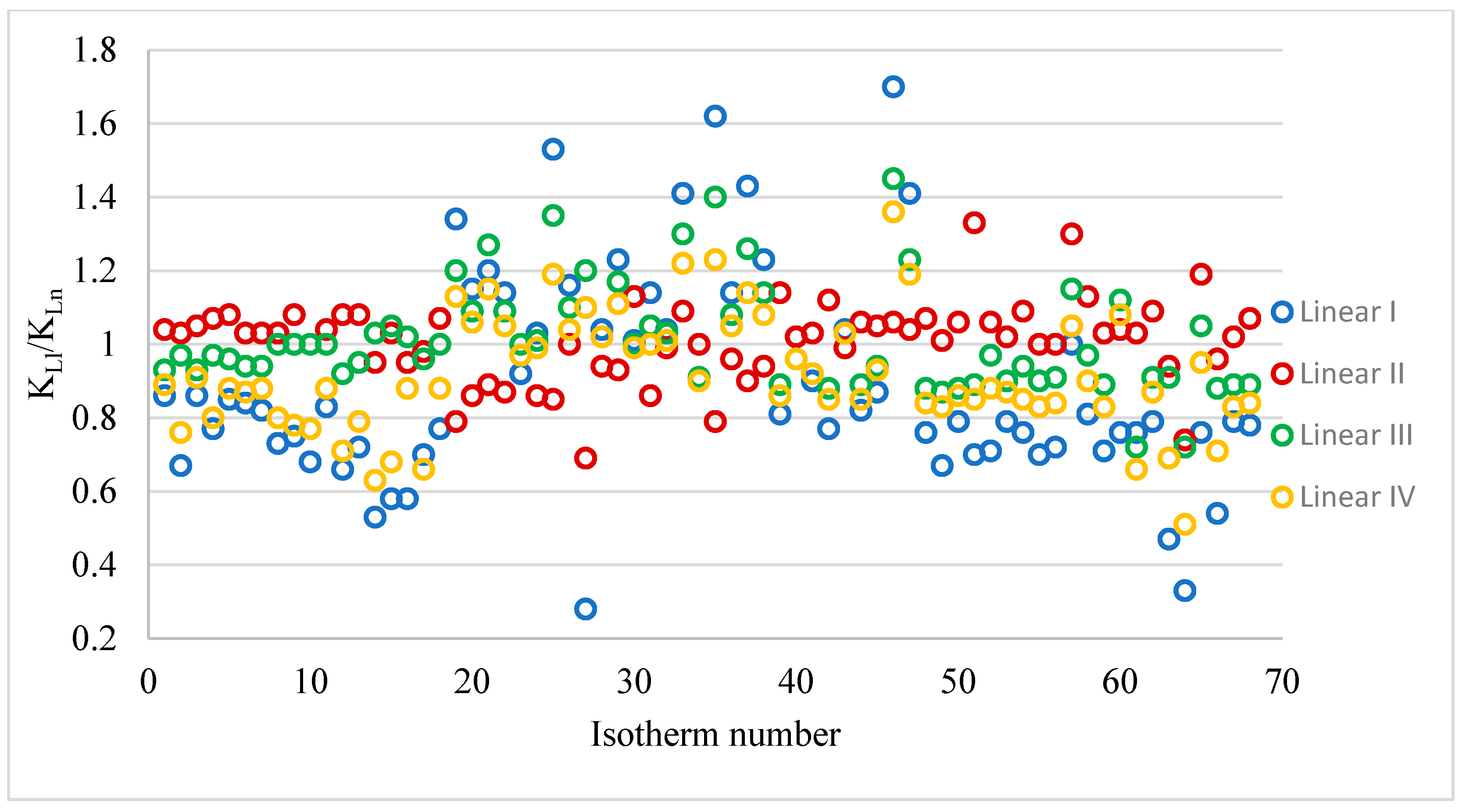
All values of the K
L constant and the K
LI/K
Ln quotients obtained from the linear and non-linear forms are provided in the
supplement in Tables S1–S11.
Table 4 summarises the K
LI/K
Ln results giving the range of quotient values, arithmetic means, medians and the number(percentage) of K
LI/K
Ln <, =, > values from 1.
Figure 2 shows the K
LI/K
Ln quotient values for the different linear forms. The obtained K
L values from the linear equations differ to a much greater extent from those values obtained from the non-linear equations than was observed for the constant q
m. The smallest differences between the K
L values calculated from the linear and non-linear equations were obtained for the linear equation form II (range K
LI/K
Ln 0.69–1.33). For this linear form, an arithmetic mean close to unity (1.01) and a median of 1.03 were obtained. The largest differences were obtained when the K
L constants were calculated from the I linear form of the Langmuir isotherm. In this case, the range of the quotient tested was from 0.28 to 1.70. The arithmetic mean and median in this case were much smaller than 1: 0.89 and 0.81, respectively. By calculating the K
L value from the linear forms, regardless of the equation used, it is possible to obtain values both smaller and larger than those calculated from the non-linear form.
The calculation of Langmuir isotherms using non-linear and linear forms is a topic that has been considered by other authors. Hamzaoui et al. 2018 analysed four different isotherms [
54]. In two cases, the linear form IV gave the most similar qm results to those calculated from the non-linear form: in one case form II and in one case form III. Analysis of the K
L values revealed that in four cases, the most similar values to those calculated from the non-linear form were obtained when the linear IV form of the Langmuir isotherm was used and in one case when the linear II form was used. In these articles, conclusions were drawn on the basis of the analysis of few isotherms and mainly on the basis of evaluation by a single statistical measure (coefficient of determination). Similar observations to those presented in this article were made by Yadav and Singh (2017) during their study of fluoride sorption [
55]. They found that the use of linear forms resulted in lower qm values and higher K
L values compared to the results obtained from non-linear forms. The most similar values of Langmuir constants to those calculated from the non-linear form were obtained when linear form II was used. Calculations of q
m and K
L from linear form I were the furthest from those obtained from the non-linear form. Similar relationships were also obtained, for example, by Subramanyam and Das 2014 and Tonk and Rápó 2022, and they obtained both smaller and larger values of q
m and K
L from the linear equations compared to those obtained from the non-linear equation [
56,
57].
Table 5 shows selected values of q
ml/q
mn and K
LI/K
Ln calculated from literature data.
The literature reports, as well as the results presented in this article, do not indicate a single linear form whose results are most similar to those obtained from the non-linear form of the Langmuir isotherm. Nevertheless, the most commonly used linear form II (Hanes–Woolf—often referred to as form I in the literature) is also the form that most often (but not always) gives the most similar qm and KL values to those calculated from the non-linear form. It should be noted that each of the proposed linearisations has certain limitations, as does the initial Langmuir isotherm [
58].
Note that x (Ce) and y (Ce/q) are not independent. It follows that the correlation between Ce and Ce/q is overestimated. This can lead to a good fit of the results even when they are inconsistent with the Langmuir model. In contrast, the Lineweaver–Burke linearisation (I form), whose results deviate most from those obtained from the non-linear forms, is very sensitive to low values of q. The dependence of 1/q on 1/Ce leads to a clustering of points. Thus, the variability at low q values, i.e., high 1/q values, is particularly important. Any inaccuracy in the range of low equilibrium concentrations, and thus low capacities, affects the results very significantly. In the other linearisations (III Eadie-Hofstee and IV Scatchard), the x and y values are not independent and the correlation between x and y may be underestimated. For this reason, these equations may fit poorly even with data consistent with the Langmuir isotherm model.
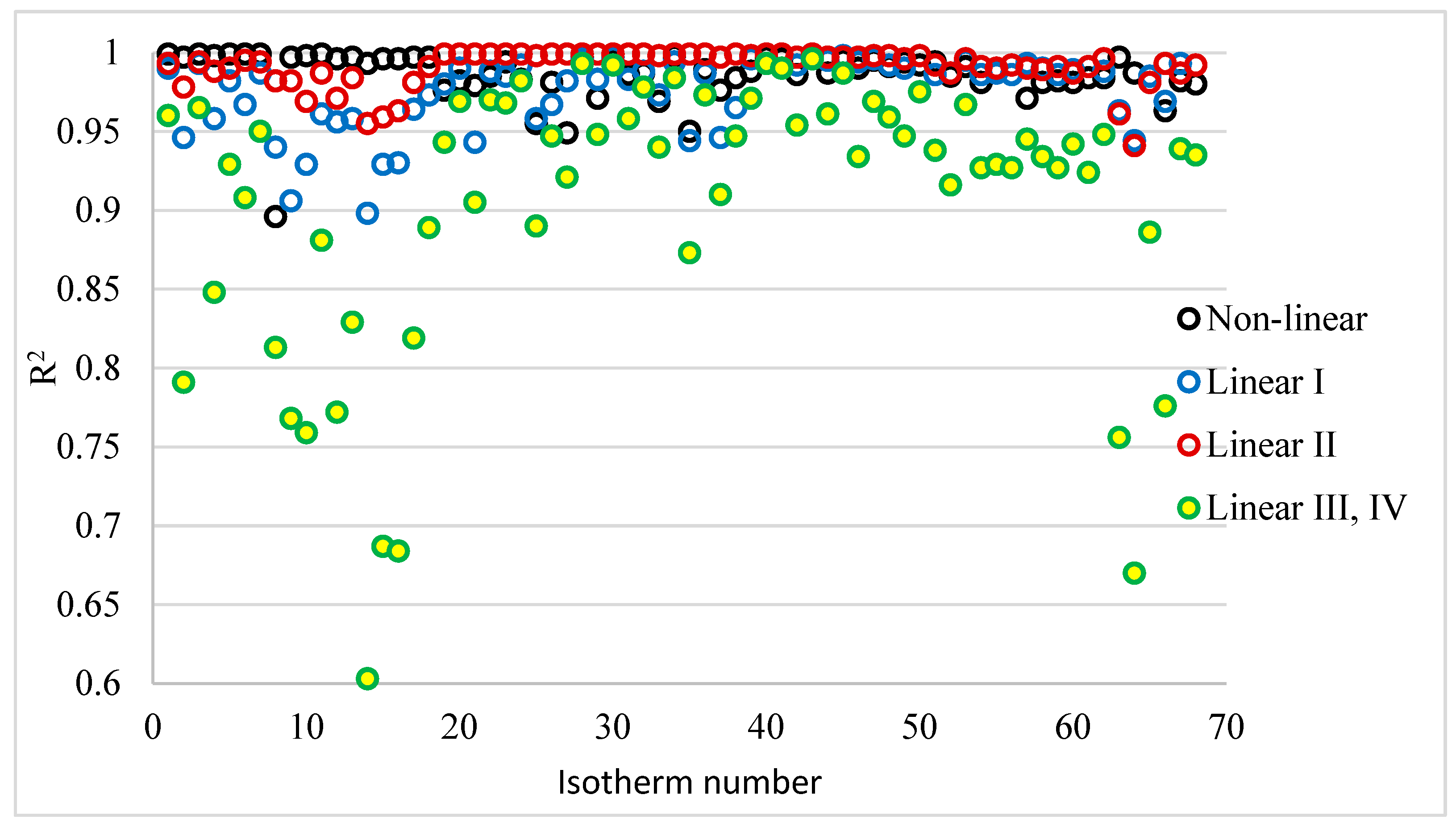
3.2. Evaluation of Linear and Non-Linear Forms of the Langmuir Isotherm Based on the Coefficient of Determination R2
The coefficient of determination R
2 is used to assess the fit of models to the test results obtained. It is usually the only statistical tool used for evaluating models of i.i.d. isotherms. In the
supplement, the calculated Langmuir isotherms (non-linear and linear forms) with R
2 coefficients of determination are shown in
Tables S1–S11. For linear forms III and IV, the coefficients of determination are the same. This is because form III is a plot of the dependence of q on q/Ce, and form IV is the dependence of q/Ce on q. Detailed values of the coefficient of determination are presented in
Figure 3.
Table 6 shows the ranges of the obtained R
2 coefficients, arithmetic means, and medians. They show that the best fit was obtained when the II linear form of the Langmuir isotherm was used. The range of values, mean, and median for this linear form were the highest, and were clearly higher not only than the other linear forms, but also than the coefficients of determination of the non-linear form.
The highest coefficients of determination for the II linear form of the Langmuir isotherm were not obtained in every case. For each isotherm study, the forms were ranked according to the magnitude of R
2. Five forms of isotherms were analysed: non-linear (N), linear I form (I), linear II form (II), linear III form (III), and linear IV form (IV). However, since the determination coefficients for form III and IV are the same, there are four places in
Table 7. The first place was assigned to the form with the highest R
2 value and the fourth place to the form for which the R
2s were lowest.
Table 7 summarises the number of isotherm forms occupying the corresponding first, second, third, and fourth places in the series. In order to assess the fit of the isotherms to the test results, the sum of the products of place in the series × number of isotherms (l × i) is given in
Table 6. The smaller the value of the sum of the products in question, the higher the place in the series in question. From the analysis of the results in
Table 7, it can be seen that the R
2 value for the II form was 40 times the first place among the 68 isotherms analysed. For the non-linear form, R
2 was in first place 21 times and in third place as many as 31 times. For the III and IV forms, the R
2 coefficient for each isotherm was the lowest and ranked fourth in all cases analysed. Based on the sum of the products, the forms can be ranked as follows: II form > non-linear > I form > III = IV form.
From the data in
Table 6 and
Table 7, it can be seen that superimposing the linear II model on the Langmuir isotherm model allows a better fit of such a corrected isotherm to the test results obtained. This may be due to a mismatch between the Langmuir model and the test results presented in the paper. The model has many limitations, one of which is the assumption that the sorbent surface is homogeneous. In the studies presented here, commercial and modified activated carbons were considered, which had different surface chemistries. It is therefore possible that the superimposition of a linear model on the Langmuir isotherm model gives a better fit to the experimental results compared to the unmodified Langmuir model.
Higher values of the coefficients of determination for the linear form compared to the non-linear form have also been obtained by other researchers. In the case of Deb et al. 2023 [
21] and Yadav and Singh 2017 [
55], higher coefficients of determination were obtained for linear form I and II compared to the non-linear form. Hamzaoui et al. 2018 obtained higher coefficients of determination only for the II linear form compared to the non-linear form [
54]. The other forms were characterised by lower R
2 values. Different results were obtained by Tonk et al. 2022 [
57]. They obtained the highest R
2 coefficients when the non-linear form was used.
The most common isotherms used to describe sorption are the Langmuir and Freundlich isotherms. Among other things, the R
2 factor is used to assess which of these isotherms better describes the test results obtained.
Tables S1–S11 give the R
2 coefficients for the Freundlich isotherms determined from the non-linear forms.
Table 8 compares how many coefficients of determination are greater, equal, and less for the isotherms in question. Depending on the form of Langmuir isotherm determination used, however, different conclusions can be drawn. When the R
2 determined from the non-linear equation of the Langmuir isotherm was compared, as many as 64 models had higher coefficients of determination than the Freundlich isotherm. When the Langmuir isotherm was calculated from the most popular linear form II, only 49 cases resulted in higher R
2 compared to the Freundlich isotherm. For the rarely used linear forms III and IV, larger R
2 coefficients for the Frondlich isotherm were obtained in 35 cases. The use of the non-linear form or linear forms of the Langmuir isotherms may also influence the choice of the isotherm model that best describes the sorption process. Similar results have also been obtained by other researchers [
44,
55].
3.3. Evaluation of Linear and Non-Linear Forms of Langmuir Isotherms Based on Different Statistical Tools
Most researchers assess the fit of the isotherms to the test results solely on the basis of the coefficient of determination R
2. However, this coefficient has some limitations, e.g., it does not take into account the number of variables in the model. Increasing the number of variables always increases the R
2, which can lead to more complex models having higher R
2 values. The coefficient of determination tells us nothing about measurement errors or the distribution of the residuals. It is therefore important to treat the coefficient of determination R
2 as an important (but not the only) indicator for model evaluation. In addition, the coefficient of determination was originally designed for linear regressions and does not always perform well when calculating non-linear equations. The same formula was used to calculate the R
2 value regardless of whether linear or non-linear regression was analysed. In the case of linear regression, R
2 determines what portion of the research is explained by the model, and the remainder is unexplained variance. For non-linear models, there is no longer such a clear statistical interpretation of the R
2 value as in the case of linear regression. This is because the models do not have an analytical solution, so R
2 is calculated after fitting the model based on predictions. The R
2 value may be less intuitive in this case, and its interpretation is more difficult. Therefore, several statistical indices are increasingly being used in scientific articles to evaluate adsorption isotherm models [
23,
46,
59,
60,
61].
Several statistical tools were used to evaluate the linear and non-linear models used, in addition to the coefficient of determination R
2: average relative error (ARE), sum of squares of errors (SSEs), chi-squared statistics (λ
2), sum of the absolute errors (SAEs), the hybrid fractional error function (HYBRID), and the root mean square error (RMSE). These are commonly used non-linear error functions [
48].
The values of these indices for all isotherms are given in
Supplementary Tables S1–S11. It should be emphasised that in the case of the fitting coefficient R
2, the better the fit of the test results for a given isotherm, the closer the R
2 value is to 1. In the case of the other indices, the fit is better when they reach lower values. The studies analysed in this paper relate to different sorbents; therefore, the concentrations and number of points used to determine the isotherms vary. It is therefore not possible to compare these indices for the entire pool of studies, but only to compare the results for individual isotherms. In The tables in the following chapters are limited to ranking isotherms, assuming that the isotherm with the lowest value of the analyzed indicator occupies the first place, and the one with the highest value of this indicator occupies the fifth place.The sum of all error values for the 68 isotherms analysed, the range of values they take, and the median are also given. This is additional (less important) information because, as mentioned earlier, the indices for isotherms performed under different conditions should not be directly compared. However, a comparison of the sets of results investigating the different ways of calculating the Langmuir isotherm constants (non-linear and four linear forms) is most appropriate.
3.3.1. Average Relative Error (ARE)
The average relative error (ARE) is used to assess the accuracy of the model and is particularly useful for a wide range of data values. It aims to minimise the fractional error over the entire concentration range. When calculating the ARE, the quotients of the differences between the experimental data and those obtained from the analysed model and the experimental data should be calculated. The resulting values should be divided by the number of measurement points (with an average over all measurement points) and multiplied by 100 to obtain the result as a percentage. Since ARE can be applied to different scales and sizes of data, it can be used to describe studies with different ranges of [
12,
61]. This is the situation in the studies analysed because, for example, we are comparing the adsorption of different compounds from solutions of different concentrations.
Tables S1–S11 show ARE results for the linear and non-linear forms of the Langmuir isotherm, and
Table 9 shows the places in the series occupied by the different isotherm types. It was assumed that first place in the series denotes the isotherm form with the lowest ARE value (the best fit of the model to the test results), and fifth place is reserved for the highest value. Most often (in 32%), the first place in the series is occupied by the third linear form of the Langmuir isotherm. The last place (in 49%) is occupied by the first linear form of the Langmuir isotherm. The sum of the products of the number of isotherms and place in the series was also calculated, with the smallest value of this product obtained indicating the best fit of the test results to the isotherm form in question. On this basis, considering all 68 isotherms, these forms can be ranked as follows: III > IV > N > I > II. This ranking takes into account the comparison of isotherm forms among themselves, but does not analyse the value of the average relative error. Therefore, the sum of the ARE for all 68 isotherms analysed, the range of values, and the median were calculated. Considering the sum of the average relative error, the isotherm forms were ranked in a different order: N > III > IV > II > I. A slightly different ranking can be obtained for the median analysis: III > N > IV > II > I. It is therefore difficult to assess unequivocally, on the basis of ARE, the suitability of the forms for describing the research results. Linear form II, on the basis of R
2, was assessed as best describing the research results. In this case, this form, depending on how ARE is interpreted, ranks either fourth or fifth in the series, i.e., next to form I, it describes the research results most poorly.
In studies by many authors analysing different isotherms, their evaluation based on R
2 and ARE varies [
13,
54,
62].
3.3.2. Sum of the Absolute Errors (SAEs)
Sum of the absolute errors is the total absolute sum of the deviations of the values obtained from the model from the values obtained from the tests. It is, therefore, one way of measuring the accuracy of the model. A major drawback is that it provides a better fit at higher adsorbate concentrations [
50,
56,
63].
SAE values for all isotherms are summarised in
Tables S1–S11.
Table 10 shows which place in the series the analysed forms of the Langmuir isotherms occupy based on SAE values. The smaller the SAE value, the better the fit of the isotherm model to the test results obtained. The best fit is the isotherm calculated from the non-linear form (40 isotherms ranked first in the series). This is also confirmed by the smallest values of the sum of errors for all isotherms, the range of values, and the median. The weakest fit on the basis of the analysed parameters was obtained when the Langmuir isotherm was calculated from the I non-linear form. In this case, the forms of the isotherms can be ranked according to the following series: N > II > III > IV > I. This ranking does not coincide with the ranks obtained from the analysis of R
2 values. A different selection of isotherms based on R
2 and based on SAE has also been observed by other researchers [
48,
64].
3.3.3. Sum of Squares of Errors (SSEs)
Sum of squares of errors (SSEs) is used, particularly in linear regression, to assess the fit of models to actual results. It is the sum of squares of the differences between the predicted values and the actual values obtained during the tests. If the SSE takes on low values then the model is a good fit to the test results. SSE is, therefore, a similar measure to SAE, but squaring the differences means that large errors will affect the results more than small errors. The magnitude of the SSE will therefore depend, among other things, on the concentrations (for low concentrations, the differences will be smaller, and for higher concentrations these differences will be larger).
The SSE values for all 68 isotherms analysed, calculated from five forms of Langmuir models, are given in
Tables S1–S11.
Table 11 shows the places in the series (the first place in the series is obtained by the form of isotherm for which the smallest SSE values were obtained, and the last place in the series when the SSE is the largest). The following series was obtained in this case: N > II > III > IV > I. This series coincides with the ranking of these forms of isotherms based on the sum of the values of all errors, and on the median. This series overlaps with the series formed on the basis of the SAE discussed in
Section 3.3.2. However, these are not the same results, because on the basis of SSE, the Langmuir isotherm calculated from the non-linear version for 61 isotherms was ranked 1, while on the basis of SAE it was ranked 1 for only 40 isotherms.
The evaluation of the forms from which the Langmuir isotherm coefficients were calculated on the basis of SAE does not coincide with the evaluation on the basis of R
2. On the basis of the R
2 value, linear form II was selected as the best descriptor of the results of the study. When assessing the fit of the isotherm form to the test results using SSE and SAE, the lowest results for these quantities were found when the non-linear form of the Langmuir isotherm was used. Other researchers also noted different results when they evaluated isotherms using R
2 and SSE. Raoof and Nahid 2024 [
59] and Qayoom et al. 2017 [
63] obtained the same R
2 values for the two isotherms, but quite different SSE values. Other researchers have also obtained a different ranking of isotherms based on R
2 and SSE [
48,
50].
3.3.4. Chi-Squared Statistics (λ2)
The non-linear chi-squared statistics measure (λ2) is one of the most commonly used measures for comparing different isotherms (when analysing articles whose authors considered other statistical measures, not just R2). It is calculated as the sum of the squares of the differences between the experimental data and the values calculated from the model and divided by the value predicted by the model. The smaller the difference between the actual value and the one obtained from the model, the smaller the λ2 value and the better the fit of the model to the experimental data.
The values of λ
2 have been placed in
Tables S1–S11 and
Table 12 shows the places in the ranks created from the values of λ
2. It was assumed, similarly to the previous statistical measures, that the first place in the row would be obtained by the form of the isotherm for which the smallest values of λ
2 were obtained, and the last place in the row was obtained when λ
2 was the largest. The following series was obtained in this case: III > N > IV > II > I. Such a series, however, does not take into account the value of this measure, but only compares these measures between different forms of calculating the coefficients of the Langmuir equation. The series looks slightly different when we add up the values of λ
2 for the 68 isotherms analysed: N > III > II > IV > I. However, regardless of whether we analyse the place in the series on the basis of the number of isotherms or the value of the sum of λ
2, linear form I is the least descriptive of the actual results.
The evaluation of the forms from which the Langmuir isotherm coefficients were calculated based on λ
2 does not coincide with the evaluation based on R
2. Similar observations were also noted by other researchers [
29,
50,
54].
3.3.5. Root Mean Square Error (RMSE)
The root mean square error (RMSE) is related to the SSE error (RMSE = √(SSE/n)). The RMSE measures the average error of the model fit, with the measure taking into account the number of measurement points. In addition, the unit of RMSE is the same as the data, which allows for easier interpretation of the results obtained compared to SSE. It is a more versatile measure because it takes into account the number of data, which makes it possible to compare different models that differ in the number of measurements. However, as errors are squared, larger errors, occurring at the higher concentrations used in the study, have the greatest impact on the magnitude of the RMSE. Several larger errors can significantly increase the RMSE even when the others have small values.
The RMSE values are shown in
Tables S1–S11, and
Table 13 shows the places in the series created based on the RMSE. Due to the definition of the RMSE and SSE error, the places in the series defined for these measures are the same. The series that can be created from the place in series is the same as the series for SSE: N > II > III > IV > I. A similar series occurs when analysing the values of the sum of all RMSEs for the 68 isotherms. The values of SSE and RMSE differ significantly, but the ranks are similar. This is mainly due to the fact that the same number of measurement points (8 points) were used to determine most of the analysed isotherms (50), and for 18 isotherms, 7 points were used. Analysing the sum of the RMSEs for the 68 isotherms resulted in the same series.
As with the errors analysed previously, the assessment of the best form of the Langmuir isotherm based on RMSE does not coincide with that based on R
2. Similar observations have been presented in other articles [
13,
65].
3.3.6. The Hybrid Fractional Error Function (HYBRID)
The previously discussed SSE/RMSE measures are inaccurate in certain cases (low concentrations). The hybrid fractional error function (HYBRID) was developed especially for low values of q
e (such values occur at low concentrations). It takes into account not only the number of degrees of freedom (n), but also the number of parameters (
p). It is, therefore, especially relevant when comparing models with different numbers of parameters [
66]. The HYBRID is expressed in %, so it is easy to compare values from different data sets. However, for very small q
e,exp, HYBRID can take on very large values.
The HYBRID values are included in
Tables S1–S11, and
Table 14 shows the places in the series created from the HYBRID values. Based on the sum of the ixl products, a series was formed, according to which the best-fitting form of the Langmuir isotherm is linear form III and the worst-fitting is linear form I (III > N > IV > II > I). However, taking the sum of all HYBRID values for the 68 isotherms analysed, a slightly different series was obtained: N > III > IV > II > I. In this case, the calculation of the Langmuir isotherm parameters from the non-linear form turned out to be closest to the actual values.
3.3.7. Comparison of Langmuir Isotherm Forms Based on Different Statistical Measures
Table 15 summarises the series describing the fit of the different forms (non-linear and four linear) from which the Langmuir isotherm coefficients were calculated. Two ranking criteria were used. The first is the ranked position in the series created for each set of measurement points for the isotherm. First place means for R
2 the value closest to 1 and for the other errors the lowest values. First place thus means the best fit of the real data to the model on the basis of the statistical measures in question. The second criterion is the sum of the errors for the 68 isotherms considered. In the case of R
2, the best fit of the model occurs when this sum is the largest, and for the other errors analysed when their sum is the smallest. Taking these two criteria into account for some errors (R
2; ARE; λ
2; HYBRID) we have slightly different series. Taking into account the sum of the errors for all the static measures analysed, the best fit of the Langmuir model to the test results was found when it was calculated from the non-linear form. However, taking the order criterion, it was found that for most of the isotherms, calculating them from the Hanes–Woolf model allows larger R
2 values to be obtained than for those calculated from the non-linear model. Analysing this criterion for the statistical measure ARE, λ
2 and HYBRID, it was found that the linear form of the Edie-Hofstee isotherm had lower errors than the model calculated from the non-linear form. In other cases, the non-linear form proved to be the best.
When comparing linear forms with each other, it is not possible to unambiguously designate one of them as best describing the actual results. It is, however, characteristic that the linear form I (Lineweaver–Burk) describes the results most poorly for almost all statistical measures. The exception is the coefficient of determination, which is most often used to evaluate adsorption isotherms. In the articles, the Hanes–Woolf linear model is used most often, but the Lineweaver–Burk model comes second, which coincides with the series created for R2. However, based on the other statistical measures, it can be concluded that using the linear Lineweaver–Burk form results in the largest errors.
Subramanyam and Das [
56], in their analysis of six different errors, also found that the linear I form (Lineweaver–Burk) has the weakest fit to real data. The non-linear form of the Langmuir isotherm best describes the results of the study. For the three error types, the II linear form (Hanes–Woolf) came first: the form (Scatchard) in two cases and the III form (Edie-Hofstee) in one case.