Valorization of a Lanthanum-Modified Natural Feedstock for Phosphorus Recovery from Aqueous Solutions: Static and Dynamic Investigations
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock Preparation
2.2. Preparation of Lanthanum-Modified Material
2.3. Analytical Techniques Used for Materials Characterization
2.4. Preparation of P Synthetic Solutions and Analyses
2.5. Phosphorus Recovery in Batch Mode
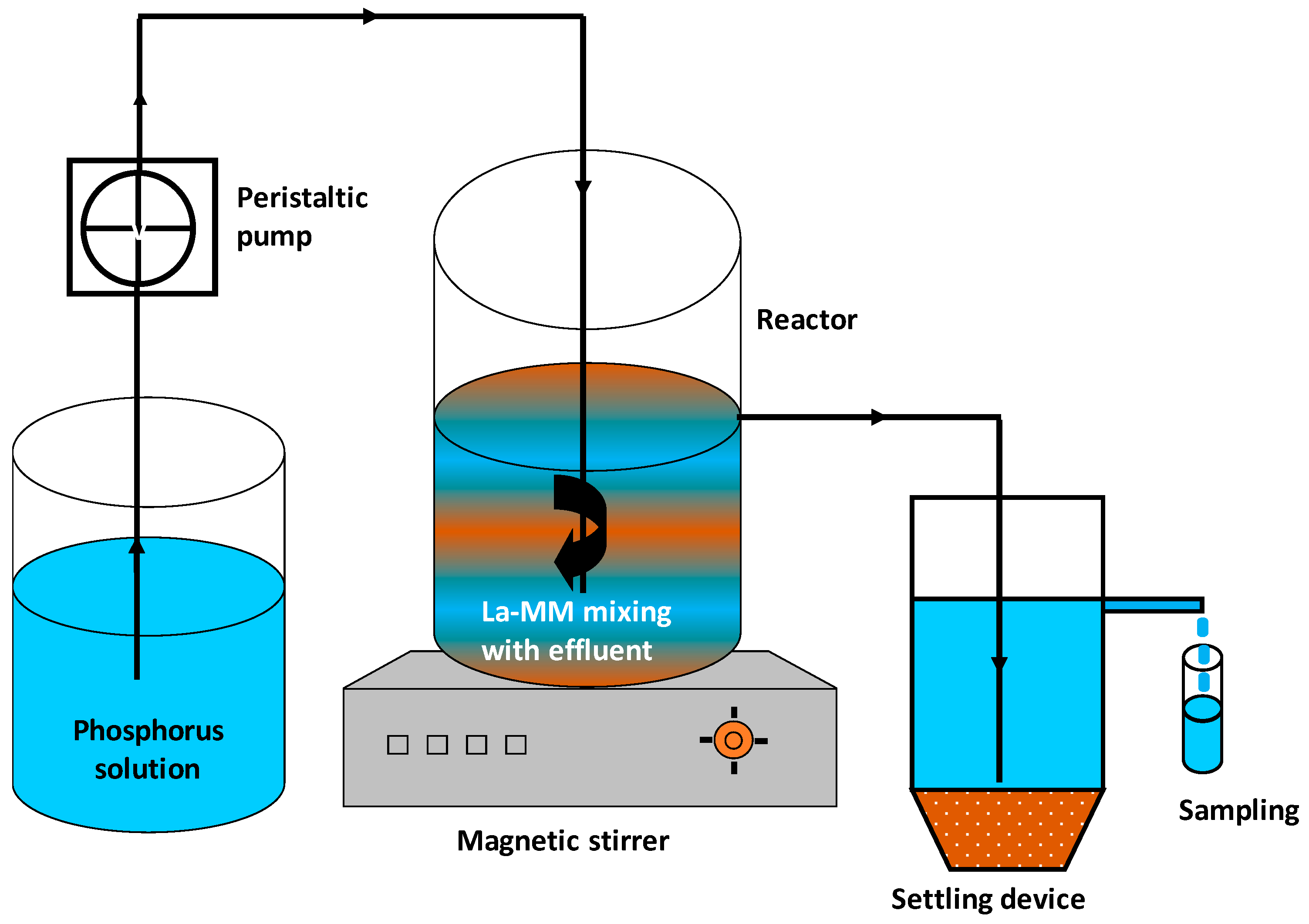
2.6. Phosphorus Recovery in CSTR Mode
2.7. Phosphorus Recovery from Real Wastewater
2.8. Heavy Metal Release from the P-Loaded Material
2.9. Statistical Analysis
3. Results and Discussion
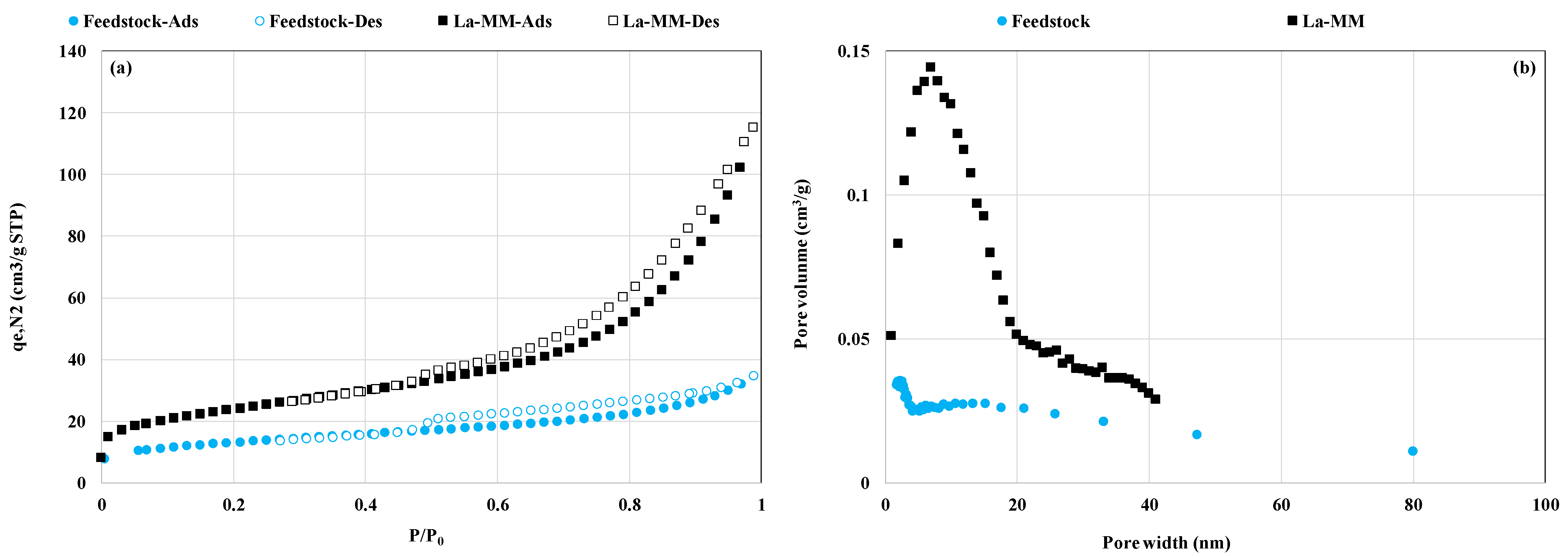
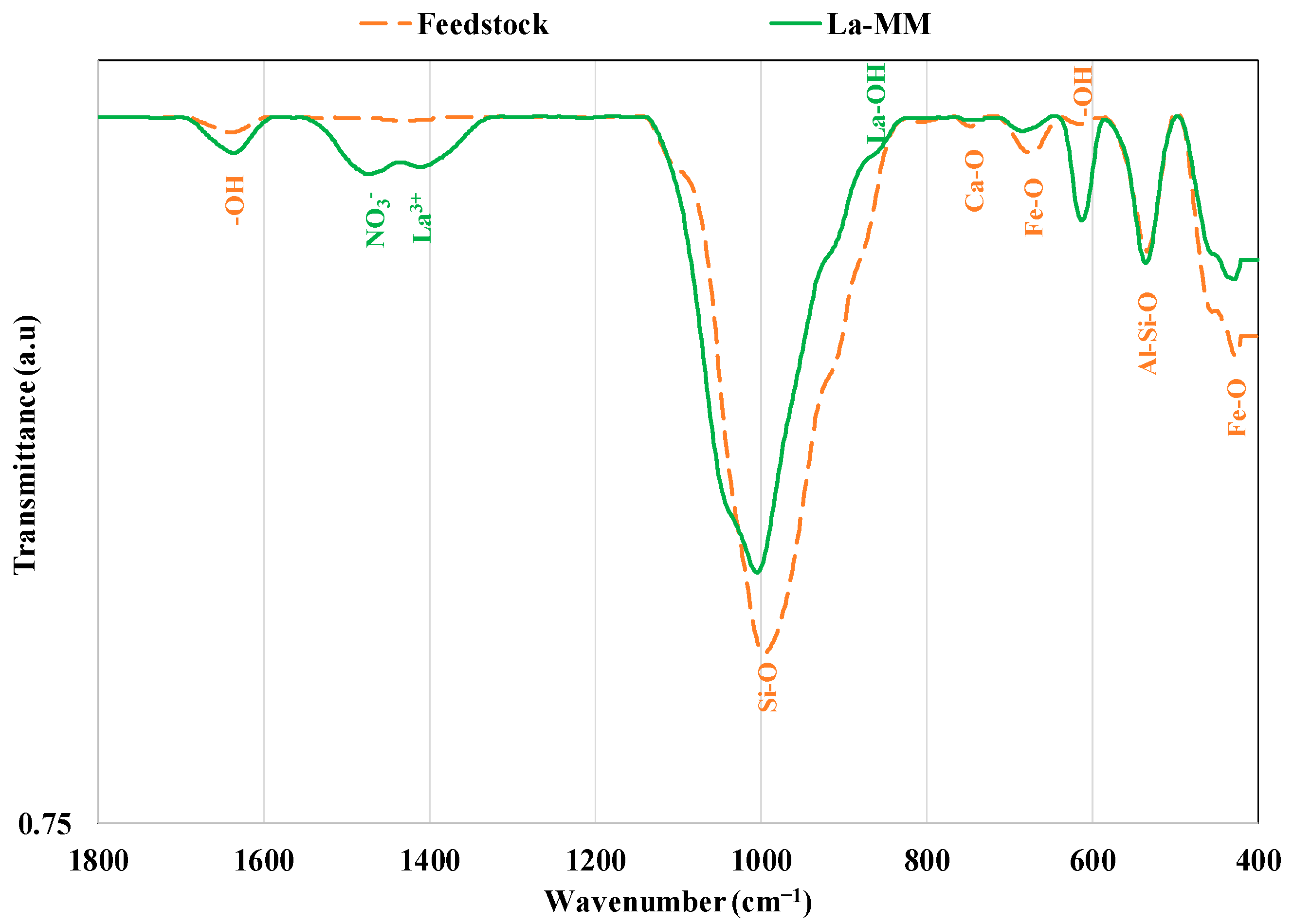
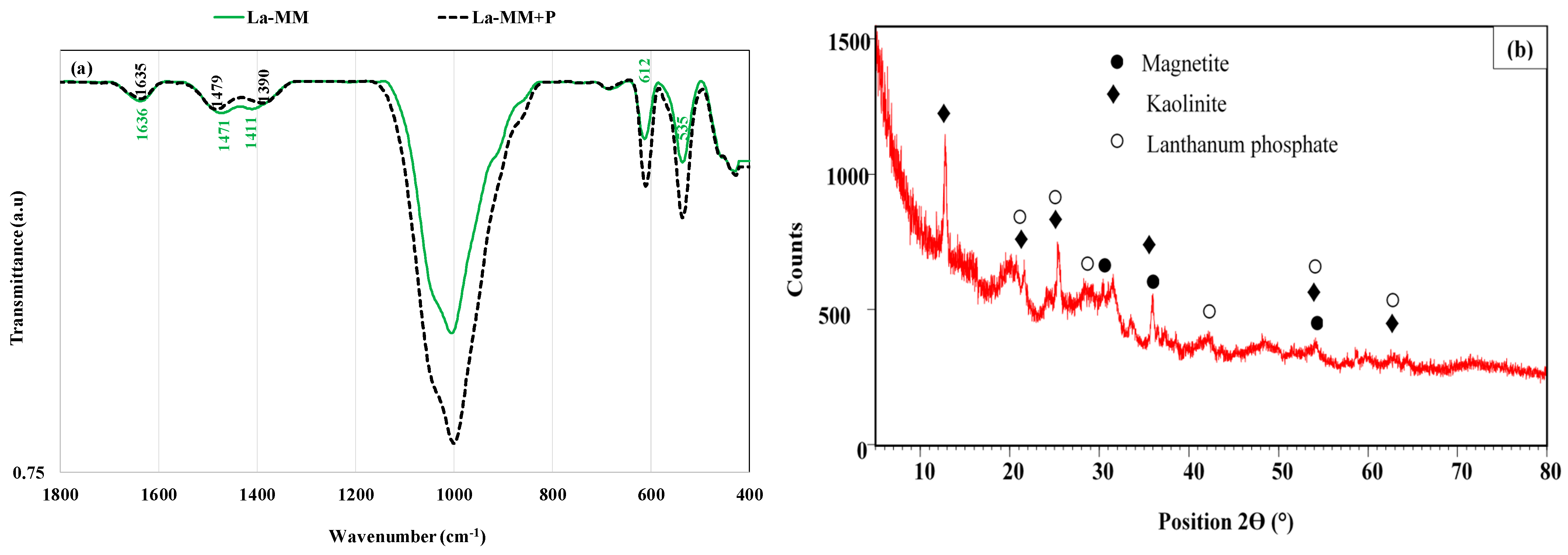
3.1. Materials Characterization
3.2. Batch Experimental Results
3.2.1. Effect of Contact Time and Initial Aqueous pH
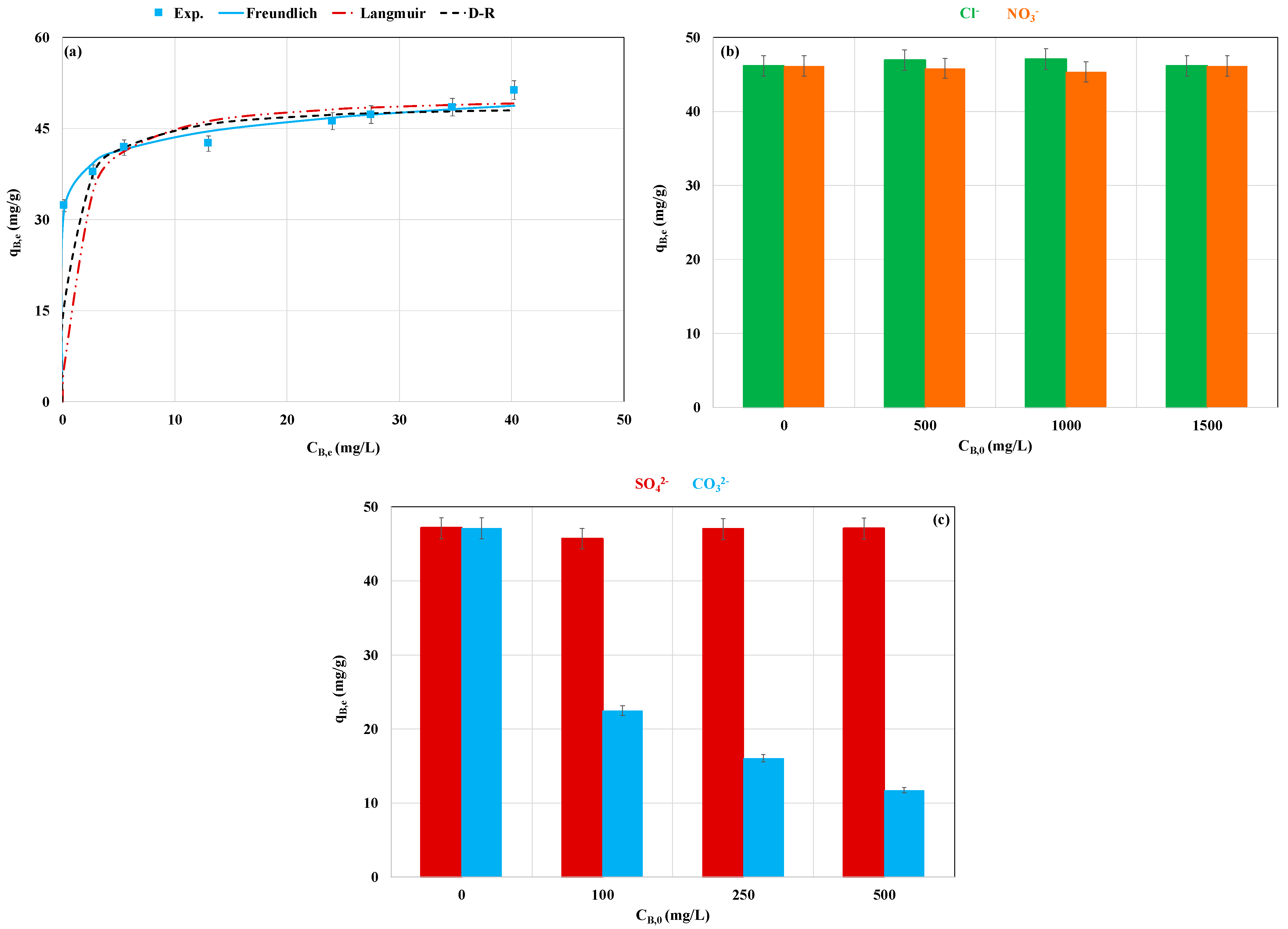
3.2.2. Effect of Initial P Concentration and Competition with Foreign Anions
3.3. CSTR Experimental Results
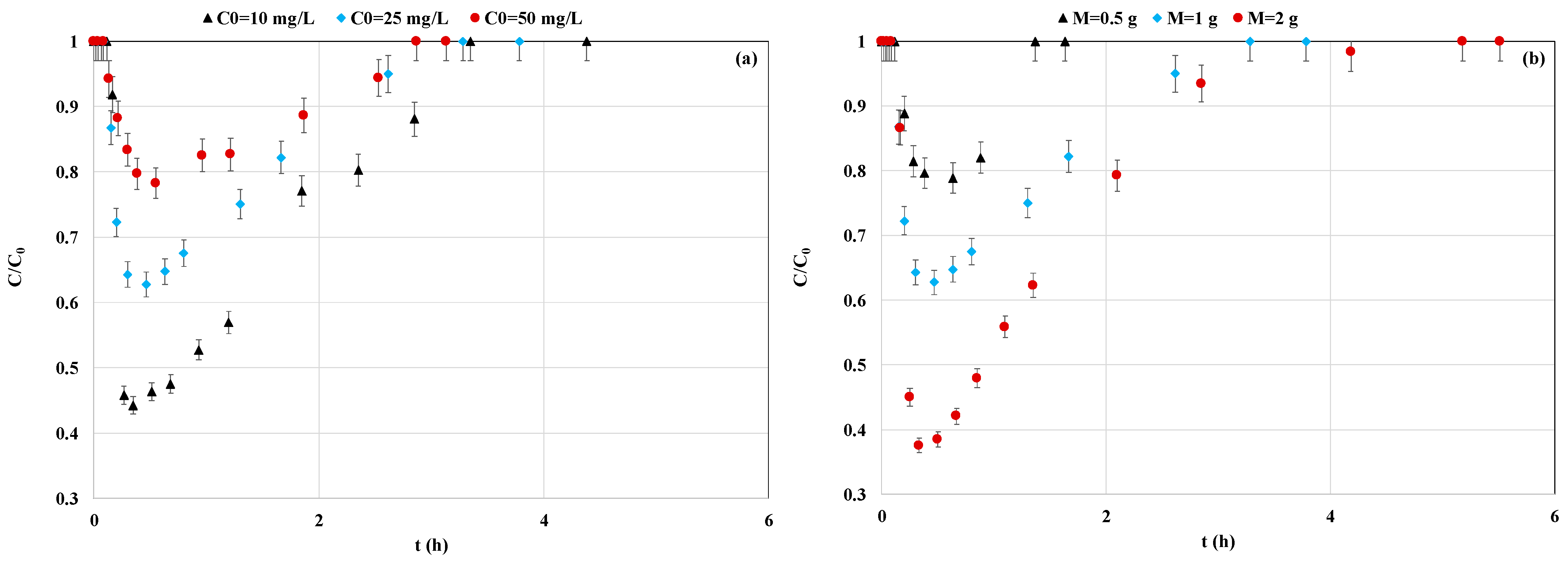
3.3.1. Impact of P Initial Concentrations
3.3.2. Impact of Adsorbent Mass
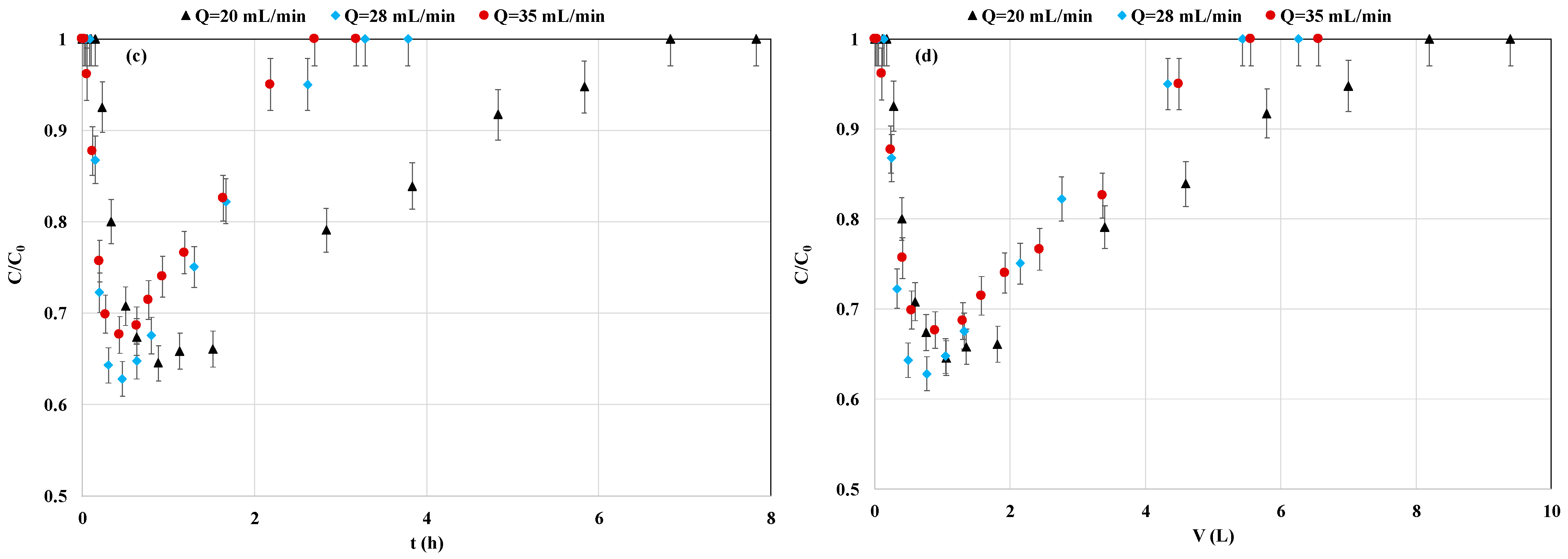
3.3.3. Impact of Flow Rate
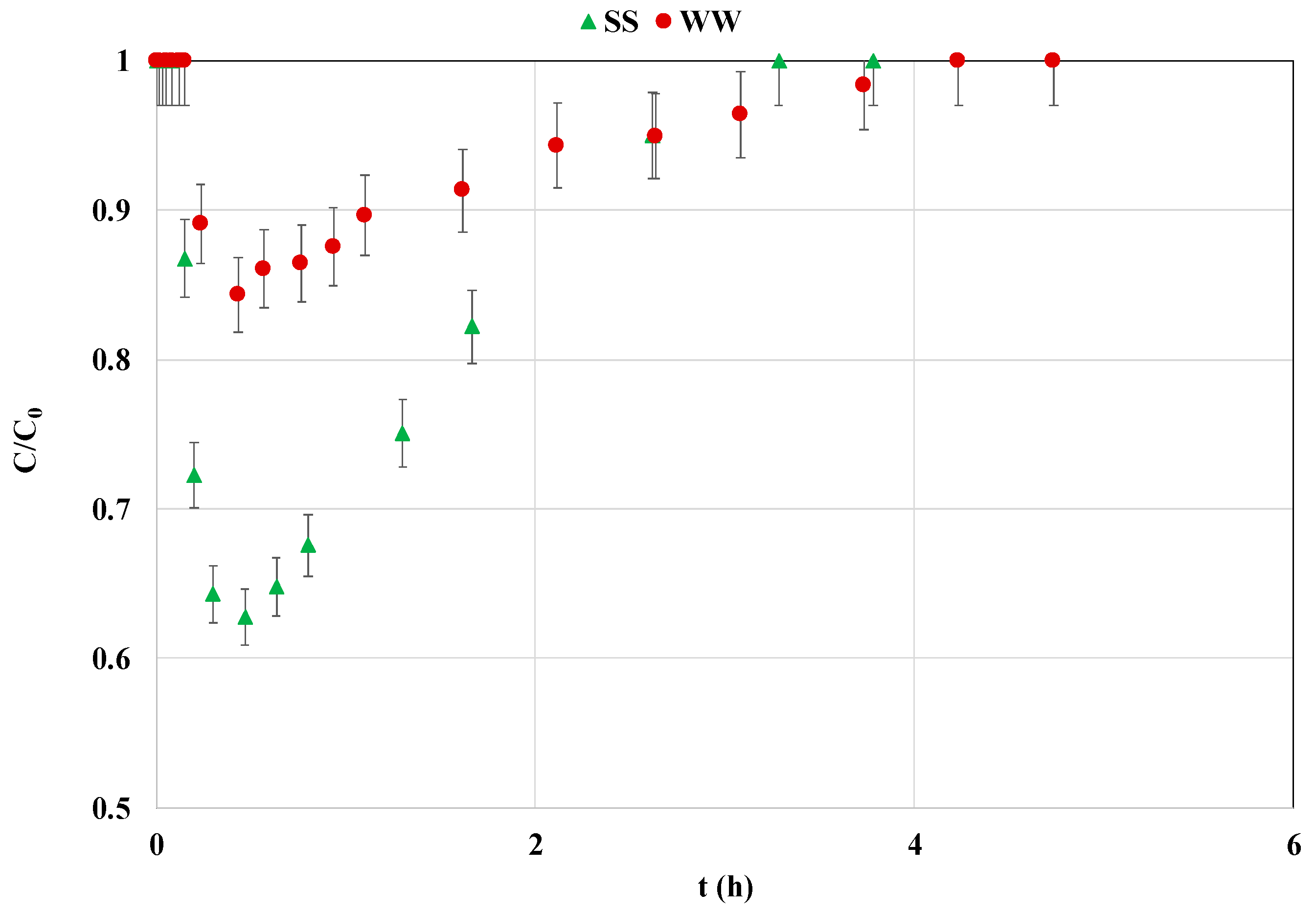
3.4. Effect of Using Actual Wastewater
3.5. Efficiency Comparison with Other Engineered Materials
3.6. Heavy Metals Release
3.7. P Recovery Mechanisms Exploration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Illakwahhi, D.T.; Maheswara, R.V.; Bajarang, B.L.S. Phosphorus Reserves Depletion, Concentration in a Single Geolocation, and the Likelihood of Weaponization for Geopolitics: A Scenario Analysis. East Afr. J. Sci. Technol. Innov. 2024, 6, 1–20. [Google Scholar]
- Qadir, M.; Drechsel, P.; Jiménez Cisneros, B.; Kim, Y.; Pramanik, A.; Mehta, P.; Olaniyan, O. Global and Regional Potential of Wastewater as a Water, Nutrient and Energy Source. Nat. Resour. Forum 2020, 44, 40–51. [Google Scholar] [CrossRef]
- Hei, P.; Yang, T.; Huang, L.; Liu, Y.; Yang, J.; Shang, Y.; Feng, L.; Huang, G. Unraveling Eutrophication Controversies: Innovative Strategies and Holistic Perspectives. Crit. Rev. Environ. Sci. Technol. 2025, 55, 147–168. [Google Scholar] [CrossRef]
- Śniatała, B.; Al-Hazmi, H.E.; Sobotka, D.; Zhai, J.; Mąkinia, J. Advancing Sustainable Wastewater Management: A Comprehensive Review of Nutrient Recovery Products and Their Applications. Sci. Total Environ. 2024, 937, 173446. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wan, Y.; Zhang, Y.; Huang, J.; Yang, Y.; Tsang, D.C.W.; Wang, H.; Chen, H.; Gao, B. Recovery of Phosphorus from Wastewater: A Review Based on Current Phosphorous Removal Technologies. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1148–1172. [Google Scholar] [CrossRef] [PubMed]
- Pratt, C.; Soares, A. New Opportunities for Biologically and Chemically New Opportunities for Biologically and Chemically Mediated Adsorption and Precipitation of Phosphorus from Wastewater. Curr. Opin. Biotechnol. 2025, 92, 103261. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Guo, J.; Hossain, M.F.; Lu, J.; Lu, Q.; Zhou, Y.; Zhou, Y. Recent Advances in the Removal and Recovery of Phosphorus from Aqueous Solution by Metal-Based Adsorbents: A Review. Resour. Conserv. Recycl. 2024, 204, 107464. [Google Scholar] [CrossRef]
- Jellali, S.; Hadroug, S.; Al-Wardy, M.; Al-Nadabi, H.; Nassr, N.; Jeguirim, M. Recent Developments in Metallic-Nanoparticles-Loaded Biochars Synthesis and Use for Phosphorus Recovery from Aqueous Solutions. A Critical Review. J. Environ. Manag. 2023, 342, 118307. [Google Scholar] [CrossRef] [PubMed]
- Gizaw, A.; Zewge, F.; Kumar, A.; Mekonnen, A.; Tesfaye, M. A Comprehensive Review on Nitrate and Phosphate Removal and Recovery from Aqueous Solutions by Adsorption. AQUA-Water Infrastruct. Ecosyst. Soc. 2021, 70, 921–947. [Google Scholar] [CrossRef]
- Wang, Y.; Munir, T.; Wu, X.; Huang, Y.; Li, B. Phosphorus Recovery and Reuse: Innovating with Biochar in the Circular Economy. Sci. Total Environ. 2025, 973, 179143. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yu, S.; Shi, C.; Li, C. Research Progress of Simultaneous Nitrogen and Phosphorus Removal Adsorbents in Wastewater Treatment. J. Environ. Chem. Eng. 2024, 12, 114844. [Google Scholar] [CrossRef]
- Bouanga Boudiombo, J.S.; Madden, D.G.; Cusack, B.; Cronin, P.; Ryan, A. State of the Art and Prospects of Zeolites and Metal Organic Frameworks (MOFs) for Nitrogen and Phosphorus Removal in Dairy Wastewater. Chemosphere 2023, 329, 138531. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-X.; Huang, Y.-X.; Wu, Q.-F.; Yao, W.; Lu, Y.-Y.; Huang, B.-C.; Jin, R.-C. A Review of the Application of Iron Oxides for Phosphorus Removal and Recovery from Wastewater. Crit. Rev. Environ. Sci. Technol. 2024, 54, 405–423. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, S.; Zhang, X.; Zheng, S. Phosphorus Removal and Recovery from Secondary Effluent in Sewage Treatment Plant by Magnetite Mineral Microparticles. Powder Technol. 2017, 306, 68–73. [Google Scholar] [CrossRef]
- Ahmed, S.; Lo, I.M.C. Phosphate Removal from River Water Using a Highly Efficient Magnetically Recyclable Fe3O4/La(OH)3 Nanocomposite. Chemosphere 2020, 261, 128118. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xie, Y.; Lu, H.; Xin, Y.; Altaf, R.; Zhu, S.; Liu, D. Facile Preparation of Dual La-Zr Modified Magnetite Adsorbents for Efficient and Selective Phosphorus Recovery. Chem. Eng. J. 2021, 413, 127530. [Google Scholar] [CrossRef]
- Li, Z.; Wei, Y.; Wu, H.; Yuan, P.; Bu, H.; Tan, X. Efficient and Sustainable Phosphate Removal and Recovery from Wastewater with Zinc-Substituted Magnetite. Sep. Purif. Technol. 2025, 360, 130642. [Google Scholar] [CrossRef]
- Li, Z.; Wei, Y.; Wu, H.; Yuan, P. Efficient and Regenerative Phosphate Removal from Wastewater Using Stable Magnetite/Magnesium Iron Oxide Nanocomposites. Environ. Res. 2025, 264, 120268. [Google Scholar] [CrossRef] [PubMed]
- Altaf, R.; Liu, D.; Jaafarzadeh, N.; Zou, J.; Zhou, Y.; Wu, B.; Lin, X.; Liu, D. Phosphorus Removal and Recovery from Livestock Wastewater by Using Modified Zirconium-lanthanum Magnetite. J. Water Process Eng. 2025, 71, 107385. [Google Scholar] [CrossRef]
- Rajendran, S.; Sai Bharadwaj, A.V.S.L.; Barmavatu, P.; Palani, G.; Trilaksanna, H.; Kannan, K.; Meenakshisundaram, N. A Review on Lanthanum-Based Materials for Phosphate Removal. ChemEngineering 2024, 8, 23. [Google Scholar] [CrossRef]
- Al-Nadabi, H.; Jellali, S.; Hamdi, W.; Al-Tamimi, A.; Al-Raeesi, A.; Al-Sidairi, A.; Al-Busaidi, W.; Al-Hanai, A.; Al-Zeidi, K.; Al-Wardy, M.; et al. Synthesis of Lanthanum-Modified Natural Magnetite: Characterization and Valorization for Phosphorus Recovery from Aqueous Solutions. Materials 2025, 18, 2283. [Google Scholar] [CrossRef] [PubMed]
- Maity, D.; Agrawal, D.C. Synthesis of Iron Oxide Nanoparticles under Oxidizing Environment and Their Stabilization in Aqueous and Non-Aqueous Media. J. Magn. Magn. Mater. 2007, 308, 46–55. [Google Scholar] [CrossRef]
- Fu, H.; Yang, Y.; Zhu, R.; Liu, J.; Usman, M.; Chen, Q.; He, H. Superior Adsorption of Phosphate by Ferrihydrite-Coated and Lanthanum-Decorated Magnetite. J. Colloid Interface Sci. 2018, 530, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Jellali, S.; Khiari, B.; Al-balushi, M.; Al-harrasi, M.; Al-sabahi, J. Novel Calcium-Rich Biochar Synthesis and Application for Phosphorus and Amoxicillin Removal from Synthetic and Urban Wastewater: Batch, Columns, and Continuous Stirring Tank Reactors Investigations. J. Water Process Eng. 2024, 58, 104818. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, R.; Xu, T.; Xu, Y.; Ge, F.; Xi, Y.; Zhu, J.; He, H. Co-Adsorption of Phosphate and Zinc(II) on the Surface of Ferrihydrite. Chemosphere 2016, 144, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Malbenia John, M.; Benettayeb, A.; Belkacem, M.; Ruvimbo Mitchel, C.; Hadj Brahim, M.; Benettayeb, I.; Haddou, B.; Al-Farraj, S.; Alkahtane, A.A.; Ghosh, S.; et al. An Overview on the Key Advantages and Limitations of Batch and Dynamic Modes of Biosorption of Metal Ions. Chemosphere 2024, 357, 142051. [Google Scholar] [CrossRef] [PubMed]
- Jellali, S.; Khiari, B.; Al-balushi, M.; Al-sabahi, J.; Hamdi, H.; Bengharez, Z.; Al-abri, M.; Al-nadabi, H.; Jeguirim, M. Use of Waste Marble Powder for the Synthesis of Novel Calcium-Rich Biochar: Characterization and Application for Phosphorus Recovery in Continuous Stirring Tank Reactors. J. Environ. Manag. 2024, 351, 119926. [Google Scholar] [CrossRef] [PubMed]
- Azzaz, A.A.; Jellali, S.; Souissi, R.; Ergaieg, K.; Bousselmi, L. Alkaline-Treated Sawdust as an Effective Material for Cationic Dye Removal from Textile Effluents under Dynamic Conditions: Breakthrough Curve Prediction and Mechanism Exploration. Environ. Sci. Pollut. Res. 2017, 24, 18240–18256. [Google Scholar] [CrossRef] [PubMed]
- Jellali, S.; Hadroug, S.; Al-Wardy, M.; Hamdi, H.; Al-Sabahi, J.; Zorpas, A.; Hamdi, W.; Al-Raeesi, A.; Jeguirim, M. Phosphorus Recovery from Aqueous Solutions by a Mg/Al-Modified Biochar from Date Palm Wastes in Column Mode: Adsorption Characteristics and Scale-up Design Parameters Assessment. Biomass Convers. Biorefinery 2024. [Google Scholar] [CrossRef]
- Bian, H.; Wang, M.; Huang, J.; Liang, R.; Du, J.; Fang, C.; Shen, C.; Man, Y.B.; Wong, M.H.; Shan, S.; et al. Large Particle Size Boosting the Engineering Application Potential of Functional Biochar in Ammonia Nitrogen and Phosphorus Removal from Biogas Slurry. J. Water Process Eng. 2024, 57, 104640. [Google Scholar] [CrossRef]
- Fang, L.; Liu, R.; Li, J.; Xu, C.; Huang, L.Z.; Wang, D. Magnetite/Lanthanum Hydroxide for Phosphate Sequestration and Recovery from Lake and the Attenuation Effects of Sediment Particles. Water Res. 2018, 130, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Jellali, S.; Azzaz, A.A.; Jeguirim, M.; Hamdi, H.; Mlayah, A. Use of Lignite as a Low-Cost Material for Cadmium and Copper Removal from Aqueous Solutions: Assessment of Adsorption Characteristics and Exploration of Involved Mechanisms. Water 2021, 13, 164. [Google Scholar] [CrossRef]
- Huang, W.; Jin, Z.; Yang, H.; Qu, Y.; Che, F.; Xu, Z.; Dong, J.; Wang, K. Interception of Phosphorus Release from Sediment by Magnetite/Lanthanum Carbonate Co Modified Activated Attapulgite Composite: Performance and Mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131139. [Google Scholar] [CrossRef]
- Gong, W.; Qi, C.; Huang, L.; Tian, Z.; Huang, Z.; Tao, C.; Lin, H.; Guo, L.; Yu, Z. Adsorption of Phosphorus in Wastewater by Lanthanum-Modified Magnetic Sewage Sludge Biochar. Desalin. Water Treat. 2024, 320, 100603. [Google Scholar] [CrossRef]
- Mendez, J.C.; Hiemstra, T. Surface Area of Ferrihydrite Consistently Related to Primary Surface Charge, Ion Pair Formation, and Specific Ion Adsorption. Chem. Geol. 2020, 532, 119304. [Google Scholar] [CrossRef]
- Hao, H.; Wang, Y.; Shi, B. NaLa(CO3)2 Hybridized with Fe3O4 for Efficient Phosphate Removal: Synthesis and Adsorption Mechanistic Study. Water Res. 2019, 155, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Saikia, N.J.; Bharali, D.J.; Sengupta, P.; Bordoloi, D.; Goswamee, R.L.; Saikia, P.C.; Borthakur, P.C. Characterization, Beneficiation and Utilization of a Kaolinite Clay from Assam, India. Appl. Clay Sci. 2003, 24, 93–103. [Google Scholar] [CrossRef]
- Sengyang, P.; Rangsriwatananon, K.; Chaisena, A. Preparation of Zeolite N from Metakaolinite by Hydrothermal Method. J. Ceram. Process. Res. 2015, 16, 111–116. [Google Scholar]
- García-Sánchez, J.J.; Solache-Ríos, M.; Martínez-Gutiérrez, J.M.; Arteaga-Larios, N.V.; Ojeda-Escamilla, M.C.; Rodríguez-Torres, I. Modified Natural Magnetite with Al and La Ions for the Adsorption of Fluoride Ions from Aqueous Solutions. J. Fluor. Chem. 2016, 186, 115–124. [Google Scholar] [CrossRef]
- Hou, T.; Shanmugasundaram, A.; Nguyen, H.; Lee, D.-W. Fabrication of Surface-Functionalized PUA Composites to Achieve Superhydrophobicity. Micro Nano Syst. Lett. 2019, 7, 12. [Google Scholar] [CrossRef]
- Veerasingam, S.; Venkatachalapathy, R. Estimation of Carbonate Concentration and Characterization of Marine Sediments by Fourier Transform Infrared Spectroscopy. Infrared Phys. Technol. 2014, 66, 136–140. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Z.; Song, Z.; Cao, S.; Li, X.; Wang, Y.; Zhan, Z.; Du, M.; Teng, D.; Lv, D.; et al. Preparation of La/Mg Modified Sheep Dung Activated Carbon and Its Adsorption Characteristics for Phosphorus in Wastewater. Desalin. Water Treat. 2024, 317, 100013. [Google Scholar] [CrossRef]
- Mo, J.; Li, Q.; Sun, X.; Zhang, H.; Xing, M.; Dong, B.; Zhu, H. Capacity and Mechanisms of Phosphate Adsorption on Lanthanum-Modified Dewatered Sludge-Based Biochar. Water 2024, 16, 418. [Google Scholar] [CrossRef]
- Elkhlifi, Z.; Kamran, M.; Maqbool, A.; El-Naggar, A.; Ifthikar, J.; Parveen, A.; Bashir, S.; Rizwan, M.; Mustafa, A.; Irshad, S.; et al. Phosphate-Lanthanum Coated Sewage Sludge Biochar Improved the Soil Properties and Growth of Ryegrass in an Alkaline Soil. Ecotoxicol. Environ. Saf. 2021, 216, 112173. [Google Scholar] [CrossRef] [PubMed]
- Hadroug, S.; Jellali, S.; Issaoui, M.; Kwapinska, M.; Jeguirim, M.; Leahy, J.J.; Kwapinski, W. Poultry Manure Conversion into Eco-Friendly Materials: Synthesis of Mg-/Al-Based Biochars, Characterization and Application for Phosphorus Recovery from Aqueous Solutions. Biomass Convers. Biorefinery 2023, 14, 25379–25393. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, H.; Lu, Y.Y.; Ren, Z.Q.; Gao, N.; Wang, J.J.; Huang, B.C.; Jin, R.C. In-Situ Synthesis of Lanthanum-Coated Sludge Biochar for Advanced Phosphorus Adsorption. J. Environ. Manag. 2025, 373, 123607. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Han, X.; Liu, W.; Wang, X.; Shi, Z.; Chen, C.; Mao, T.; Yin, F.; Chen, Z. Lanthanum-Doped Zinc-Iron Spinel/Ulva Lactuca Linnaeus Biochar: Enhanced Electron Transfer for Superior Phosphorus-Containing Contaminants Adsorption. Environ. Technol. Innov. 2025, 37, 104008. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Y.; He, S.; Zhan, Y.; Zhang, Z.; Wang, D.; Zhang, Z. Synthesis and Evaluation of Zirconia/Magnetite/Zeolite Composite for Controlling Phosphorus Release from Sediment: A Laboratory Study. Ecol. Eng. 2020, 151, 105874. [Google Scholar] [CrossRef]
- Wu, B.; Wan, J.; Zhang, Y.; Pan, B.; Lo, I.M.C. Selective Phosphate Removal from Water and Wastewater Using Sorption: Process Fundamentals and Removal Mechanisms. Environ. Sci. Technol. 2020, 54, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lu, Y.; Du, M.; Chen, Q.; Yan, H.; Lin, Y. Nano La(OH)3 Modified Lotus Seedpod Biochar: A Novel Solution for Effective Phosphorus Removal from Wastewater. J. Environ. Manag. 2024, 356, 120502. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Yin, T.; Li, N.; Zhang, H.; Shi, A.; Abdukayum, A.; Gao, S.; Hu, G. Reticulated Lanthanum (La) Carbonate-Carbon Composite for Efficient Phosphorus Removal from Eutrophic Wastewater. Chin. Chem. Lett. 2025, 36, 110398. [Google Scholar] [CrossRef]
- Wahab, M.A.; Hassine, R.B.; Jellali, S. Removal of Phosphorus from Aqueous Solution by Posidonia Oceanica Fibers Using Continuous Stirring Tank Reactor. J. Hazard. Mater. 2011, 189, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Song, Z.; Zou, S.; Wang, D. Effect of Carbonaceous Materials on Phosphorus Removal in Flow-through Packed Column Systems. Environ. Sci. Pollut. Res. 2024, 31, 60555–60567. [Google Scholar] [CrossRef] [PubMed]
- Meina, L.; Qiao, M.; Zhang, Q.; Xu, S.; Wang, D. Study on the Dynamic Adsorption and Recycling of Phosphorus by Fe–Mn Oxide/Mulberry Branch Biochar Composite Adsorbent. Sci. Rep. 2024, 14, 1235. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.C.P.; Nguyen, T.P.; Nguyen, X.C.; Nguyen, X.H.; Nguyen, T.A.H.; Nguyen, T.T.N.; Vo, T.Y.B.; Nguyen, T.H.G.; Nguyen, T.T.H.; Vo, T.D.H.; et al. Adsorptive Removal of Phosphate from Aqueous Solutions Using Low-Cost Modified Biochar-Packed Column: Effect of Operational Parameters and Kinetic Study. Chemosphere 2022, 309, 136628. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Muñoz, A.; Flórez, E.; Ocampo-Perez, R.; Acelas, N. Effective Phosphorus Removal Using Transformed Water Hyacinth: Performance Evaluation in Fixed-Bed Columns and Practical Applications. PLoS ONE 2024, 19, e0312432. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.; Wilson, A.E.; Torbert, H.A.; Wang, D. Sorptive Removal of Phosphorus by Flue Gas Desulfurization Gypsum in Batch and Column Systems. Chemosphere 2023, 320, 138062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, W.; Yang, D.; Xiang, J.; Chen, Y. Removal and Recovery of Phosphorus from Secondary Effluent Using Layered Double Hydroxide-Biochar Composites. Sci. Total Environ. 2022, 844, 156802. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhao, X.; Zhou, Y.; Li, F.; Liu, W.; Huang, Y.; Zhang, H.; Ma, J.; Hu, G. Tri-Functional Lanthanum-Based Biochar for Efficient Phosphorus Recovery, Bacterial Inhibition, and Soil Fertility Enhancement. Biochar 2023, 5, 16. [Google Scholar] [CrossRef]
- Koilraj, P.; Sasaki, K. Selective Removal of Phosphate Using La-Porous Carbon Composites from Aqueous Solutions: Batch and Column Studies. Chem. Eng. J. 2017, 317, 1059–1068. [Google Scholar] [CrossRef]
- Zeng, S.; Kan, E. Sustainable Use of Ca(OH)2 Modified Biochar for Phosphorus Recovery and Tetracycline Removal from Water. Sci. Total Environ. 2022, 839, 156159. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Zhu, Z.; Chen, Z.; Wang, X.; Zhang, Y.; Chen, H. Synthesis, Characterization and Application of Dewatered Municipal Sludge-Based Creamsite and Its Phosphorus Adsorption Characteristics. J. Clean. Prod. 2023, 391, 136216. [Google Scholar] [CrossRef]
- Li, H.; Ru, J.; Yin, W.; Liu, X.; Wang, J.; Zhang, W. Removal of Phosphate from Polluted Water by Lanthanum Doped Vesuvianite. J. Hazard. Mater. 2009, 168, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhao, Y.; Zhang, Z.; Zhan, Y.; Zhang, Z.; Wang, Y.; Yu, Y.; Wu, X. Immobilization of Mobile and Bioavailable Phosphorus in Sediments Using Lanthanum Hydroxide and Magnetite/Lanthanum Hydroxide Composite as Amendments. Sci. Total Environ. 2019, 687, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.S.S.; Lam, K.H.; Lee, J.M.N.; Lau, T.C. Removal of Phosphate from Water by a Highly Selective La(III)-Chelex Resin. Chemosphere 2007, 69, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zeng, W.; Ren, Z.; Jia, Z.; Wu, G.; Peng, Y. Performance Difference of Hydrated Phosphorophilic Metal Oxides in Modifying Diatomite and Recovering Phosphorus from Wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126763. [Google Scholar] [CrossRef]
- Thomas, S.S.; Viswanathan, N.; Periyasamy, S.; Al-Asbahi, B.A. Enriched Phosphate Adsorption Using Lanthanum Organic Frameworks Decorated Papaya Biochar Supported Alginate Composite. J. Mol. Liq. 2025, 426, 127286. [Google Scholar] [CrossRef]
- He, Q.; Zhao, H.; Teng, Z.; Wang, Y.; Li, M.; Hoffmann, M.R. Phosphate Removal and Recovery by Lanthanum-Based Adsorbents: A Review for Current Advances. Chemosphere 2022, 303, 134987. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, Y.; Bud, J.; Liu, J.; Takahashi, M.; Tsubouchi, N. Adsorption of Phosphate from Aqueous Using Iron Hydroxides Prepared by Various Methods. J. Environ. Chem. Eng. 2021, 9, 104645. [Google Scholar] [CrossRef]


| Name | Equation |
|---|---|
| Kinetic model | |
| Pseudo first-order model (PFOM) | |
| Pseudo second-order (PSOM) | |
| Boundary layer diffusion | |
| Intraparticle diffusion | |
| Isotherm model | |
| Langmuir | |
| Freundlich | |
| Dubinin–Radushkevich (D-R) | |
| Material | Mineral Contents (%) | pHpzc | BET Analysis | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | Fe | Si | Al | Mg | Cr | Ca | Ni | Mn | Cl | Zn | Cd | P | Pb | Hg | BET-SA (m2 g−1) | TPV (cm3 g−1) | APS (nm) | ||
| Feedstock [21] | 69.10 | 12.40 | 7.91 | 6.50 | 1.25 | 1.09 | 0.93 | 0.30 | 0.04 | 0.02 | 0.01 | 0.001 | ND | ND | ND | - | 44.3 | 0.045 | 6.16 |
| La-MM | 81.92 | 3.79 | 3.23 | 2.77 | ND | 1.23 | 0.19 | 0.10 | 0.12 | 5.87 | 0.0029 | 0.0005 | ND | ND | ND | 6.83 | 82.7 | 0.160 | 10.54 |
| Kinetic model | qB,e,exp (mg g−1) | 47.33 |
| PFO model | qB,e,calc | 47.29 |
| k1 (min−1) | 0.0049 | |
| R2 | 0.968 | |
| MAPE (%) | 48.0 | |
| PSO model | k2 (g mg−1 min−1) | 0.00067 |
| qB,e,calc (mg g −1) | 46.31 | |
| R2 | 0.919 | |
| MAPE (%) | 30.8 | |
| Diffusion model | DBL (×10−13 m2 s−1) | 0.848 |
| R2 | 0.989 | |
| DITP (×10−13 m2 s−1) | 1.333 | |
| R2 | 0.955 |
| Isotherm | Parameter | Value |
|---|---|---|
| Freundlich | n | 12.5 |
| KF | 36.2 | |
| R2 | 0.949 | |
| MAPE (%) | 2.3 | |
| Langmuir | KL (L mg−1) | 0.798 |
| qm,L (mg g−1) | 50.7 | |
| R2 | 0.801 | |
| MAPE (%) | 13.9 | |
| D-R | qm,D-R (mg g−1) | 48.6 |
| E (kJ mol−1) | 8.4 | |
| R2 | 0.802 | |
| MAPE (%) | 8.9 |
| Adsorbent | Adsorption Experimental Conditions | qm,L for Batch and qe,d for Dynamic (mg g−1) | Reference |
|---|---|---|---|
| Batch assays | |||
| La-modified natural vesuvianite, China | C0-B: 1–5 mg L−1; pH: 7.1; DB: 0.3 g L−1; tB: 40 h; T: 20 °C | 6.7 | [63] |
| La-modified synthetic magnetite | C0, B:1–10 mg L−1; pH: 7.0; DB: 0.1 g L−1; tB = 2 h; T = 25 °C | 13.4 | [31] |
| La-modified synthetic magnetite | C0,B: 5–30 mg L−1; pH: 7.0; DB: 0.4 g L−1; tB: 24 h; T: 25 °C | 17.3 | [64] |
| La-modified synthetic porous carbon | C0,B: 3.1–62 mg L−1; pH: 7.3; DB: 0.5 g L−1; tB: 24 h; T: 25 °C | 32.4 | [60] |
| La-modified natural magnetite decorated with ferrihydrite, China | C0,B: 2–120 mg L−1; pH: 6.28; DB: 1 g L−1; tB: 24 h; T: 25 °C | 44.8 | [23] |
| La-modified synthetic magnetite and attapulgite | C0,B: 1–300 mg L−1; pH: 7.0; DB:1 g L−1; tB: 24 h; T: 25 °C | 51.7 | [33] |
| La-modified synthetic magnetite | C0,B: 0.5–250 mg L−1; pH: 7.0; DB: 0.1 g L−1; tB: 5 h; T: 23 °C | 253.8 | [15] |
| La-modified natural magnetite at a percentage of 35%, Oman | C0,B: 15–92 mg L−1; pH: natural (6.2); DB: 1 g L−1; tB:24 h; T: RT | 50.7 | This work |
| Dynamic assays (column or CSTR) | |||
| La-modified commercial resin | Column mode. C0,D: 155 mg L−1; m: - g; Q: 3 mL min−1; T: RT | 1.3 | [65] |
| Raw algal biomass | CSTR mode. C0,D: 50 mg L−1; m: 8.3 g L−1; Q: 40 mL min−1; T: RT | 3.3 | [52] |
| La-modified lotus seedpod derived biochar | Column mode. C0,D: 0.5 mg L−1; m: 1 g; Q: 3 mL min−1; T: 25 °C | 6.1 | [50] |
| La-modified synthetic porous carbon | Column mode. C0,D: 6.2 mg L−1; m: 0.5 g; Q: 0.5 mL min−1; T: 25 °C | 6.6 | [60] |
| La-modified diatomite | Column mode. C0,D: 5 mg L−1; m: 0.2 g; Q: 0.5 mL min−1; T: RT | 11.6 | [66] |
| La-carbonate carbon composite | Column mode. C0,D: 2 mg L−1; m: 1 g; Q: 15 mL min−1; T: 25 °C | 91.8 | [51] |
| Ca-modified biochar | Column mode. C0,D: 50 mg L−1; m: 10 g; Q: 5 mL min−1; T: RT | 66.4 | [24] |
| CSTR mode. C0,D: 50 mg L−1; m: 0.6 g; Q: 10 mL min−1; T: RT | 94.9 | ||
| La-modified natural magnetite at a percentage of 35%, Oman | CSTR mode. C0,D: 25 mg L−1; m: 1 g; Q:20 mL min−1; T: RT | 33.8 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Nadabi, H.; Jellali, S.; Hamdi, W.; Al-Raeesi, A.; Al-Muqaimi, F.; Al-Tamimi, A.; Al-Sidairi, A.; Al-Hanai, A.; Al-Busaidi, W.; Al-Zeidi, K.; et al. Valorization of a Lanthanum-Modified Natural Feedstock for Phosphorus Recovery from Aqueous Solutions: Static and Dynamic Investigations. Materials 2025, 18, 3383. https://doi.org/10.3390/ma18143383
Al-Nadabi H, Jellali S, Hamdi W, Al-Raeesi A, Al-Muqaimi F, Al-Tamimi A, Al-Sidairi A, Al-Hanai A, Al-Busaidi W, Al-Zeidi K, et al. Valorization of a Lanthanum-Modified Natural Feedstock for Phosphorus Recovery from Aqueous Solutions: Static and Dynamic Investigations. Materials. 2025; 18(14):3383. https://doi.org/10.3390/ma18143383
Chicago/Turabian StyleAl-Nadabi, Hamed, Salah Jellali, Wissem Hamdi, Ahmed Al-Raeesi, Fatma Al-Muqaimi, Afrah Al-Tamimi, Ahmed Al-Sidairi, Ahlam Al-Hanai, Waleed Al-Busaidi, Khalifa Al-Zeidi, and et al. 2025. "Valorization of a Lanthanum-Modified Natural Feedstock for Phosphorus Recovery from Aqueous Solutions: Static and Dynamic Investigations" Materials 18, no. 14: 3383. https://doi.org/10.3390/ma18143383
APA StyleAl-Nadabi, H., Jellali, S., Hamdi, W., Al-Raeesi, A., Al-Muqaimi, F., Al-Tamimi, A., Al-Sidairi, A., Al-Hanai, A., Al-Busaidi, W., Al-Zeidi, K., Al-Wardy, M., & Jeguirim, M. (2025). Valorization of a Lanthanum-Modified Natural Feedstock for Phosphorus Recovery from Aqueous Solutions: Static and Dynamic Investigations. Materials, 18(14), 3383. https://doi.org/10.3390/ma18143383








