Recent Developments in Layered Double Hydroxides as Anticorrosion Coatings
Abstract
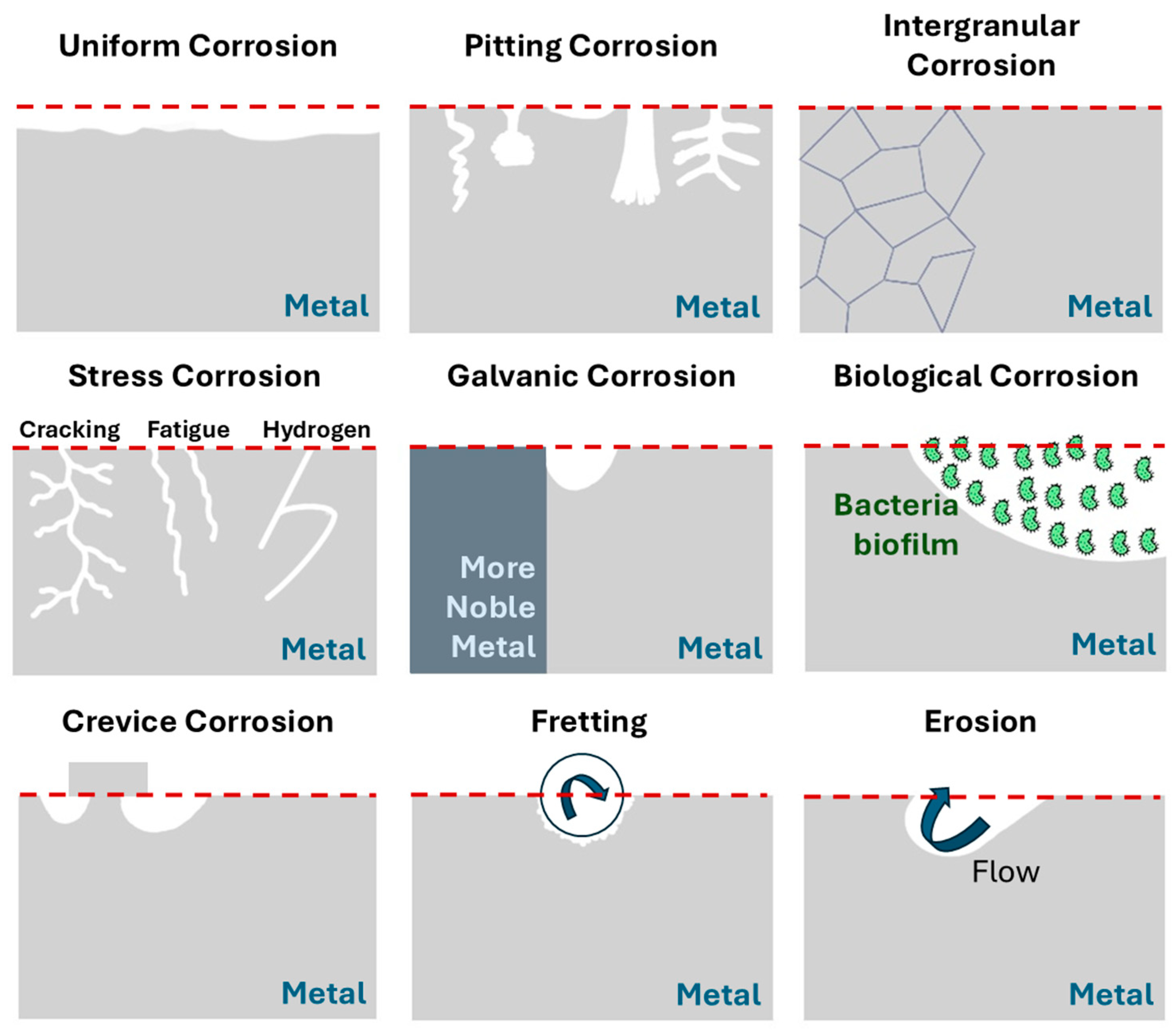
1. Introduction
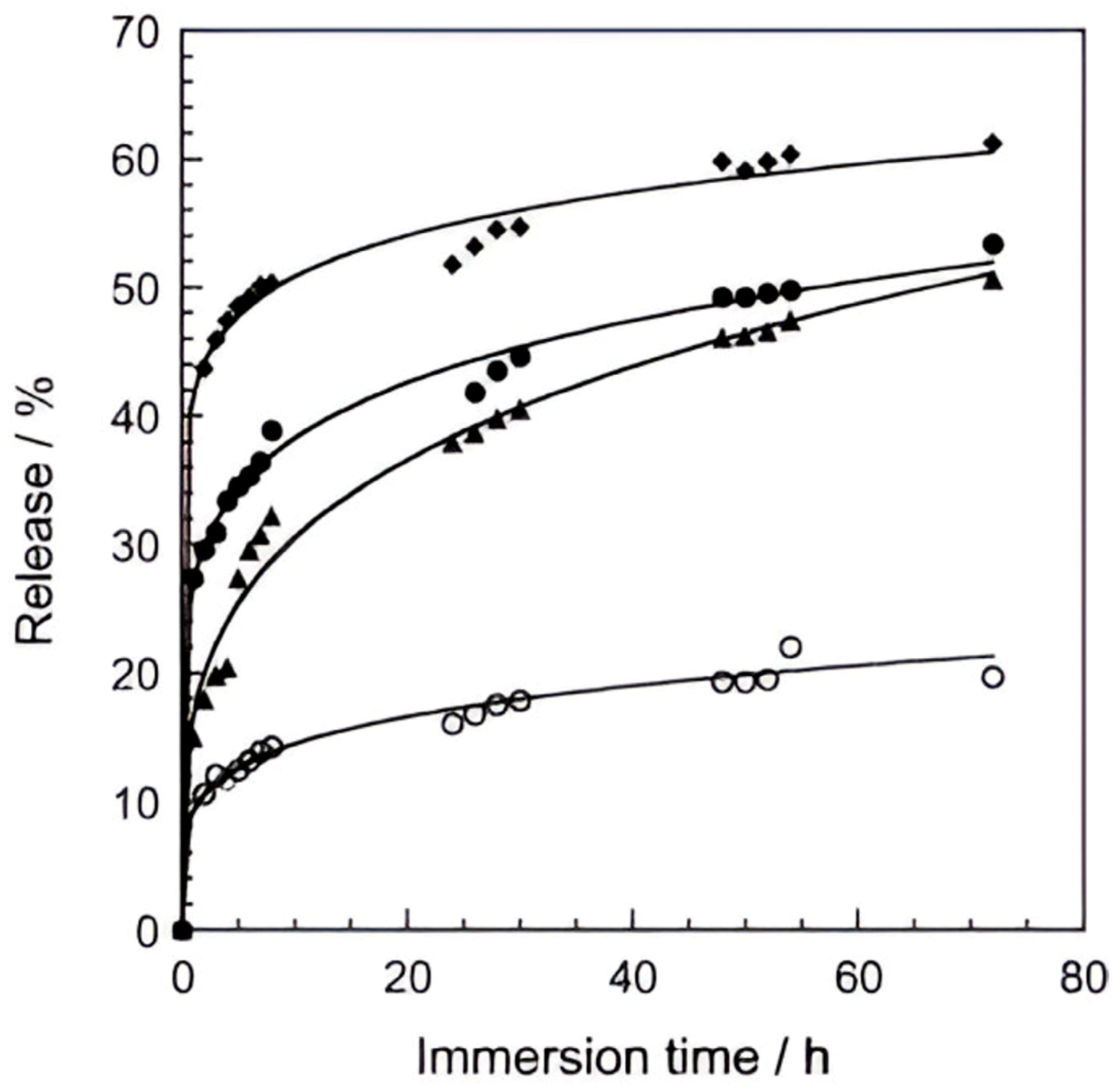
- Active protection, through the controlled release of corrosion inhibitors (e.g., molybdate, benzoate) intercalated in the LDH layers. These inhibitors are released in response to environmental triggers such as pH shifts or the presence of chloride ions, providing a self-healing function to the coating [36,38].

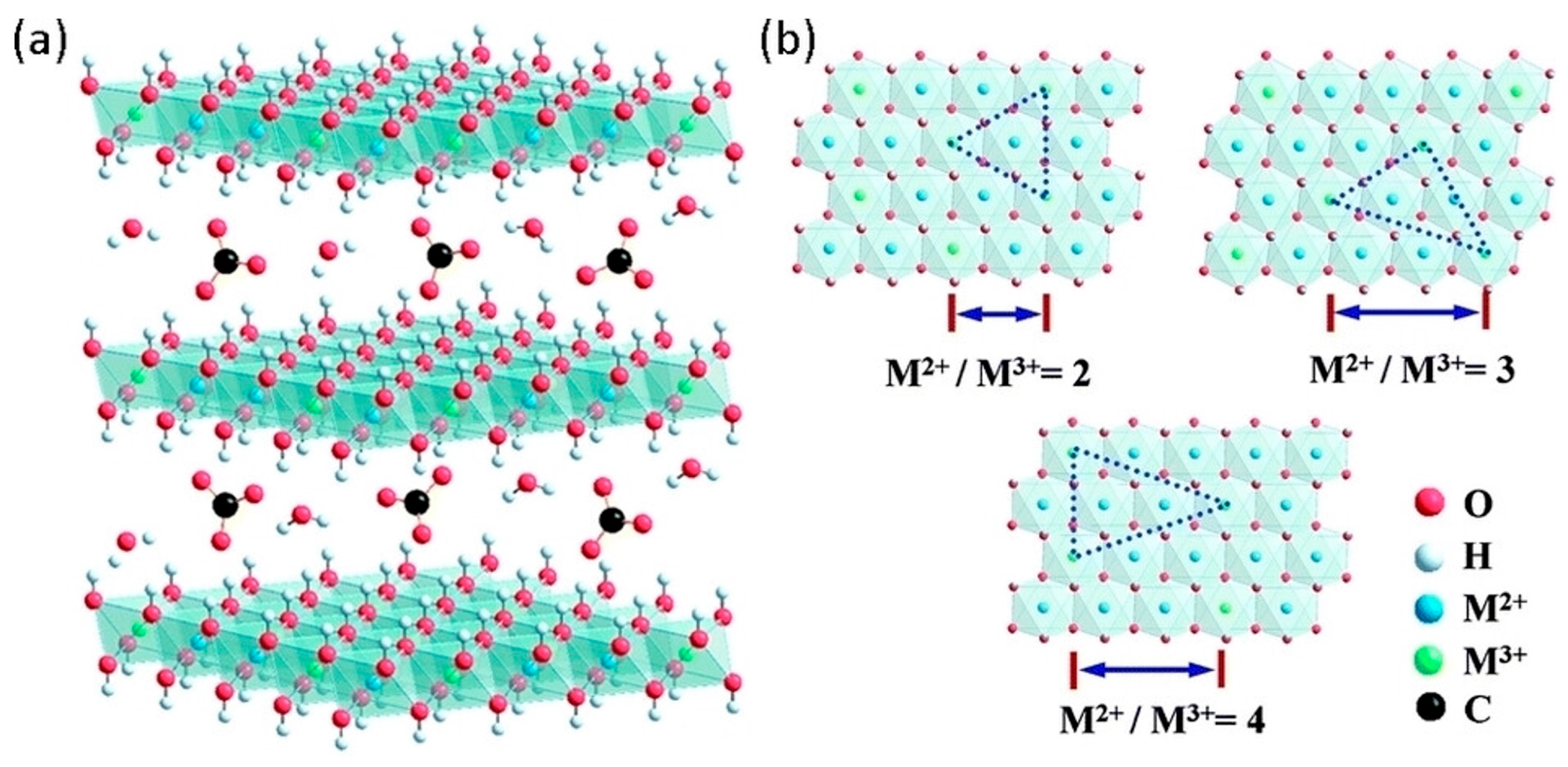
2. Structure and Properties
3. Synthesis Procedures of LDHs
- -
- Direct Methods: LDHs are directly synthesized onto the substrate, in the form of well-organized, continuous, and uniform layers. Those layers play the role of Conversional Coatings (CCs) that can be employed as synthesized or post-treated. In this last case, they can preserve their structure (as the cases of polymer–LDH hybrid coatings, superhydrophobic LDH CC, LDH CC functionalized via ion exchange, etc.), or they can be transformed (through thermal treatment harnessing the memory effect or by controllable recrystallization of LDH CC) into Metal Organic Framework Conversional Coatings (MOF CCs).
- -
- Indirect Methods: LDHs can be integrated in the form of powders (or pigments) with base material and the resultant coating is applied over the surface. Indirect methods are additional treatments and subsequent modifications of preformed LDHs not yet in the form of CCs.
- -
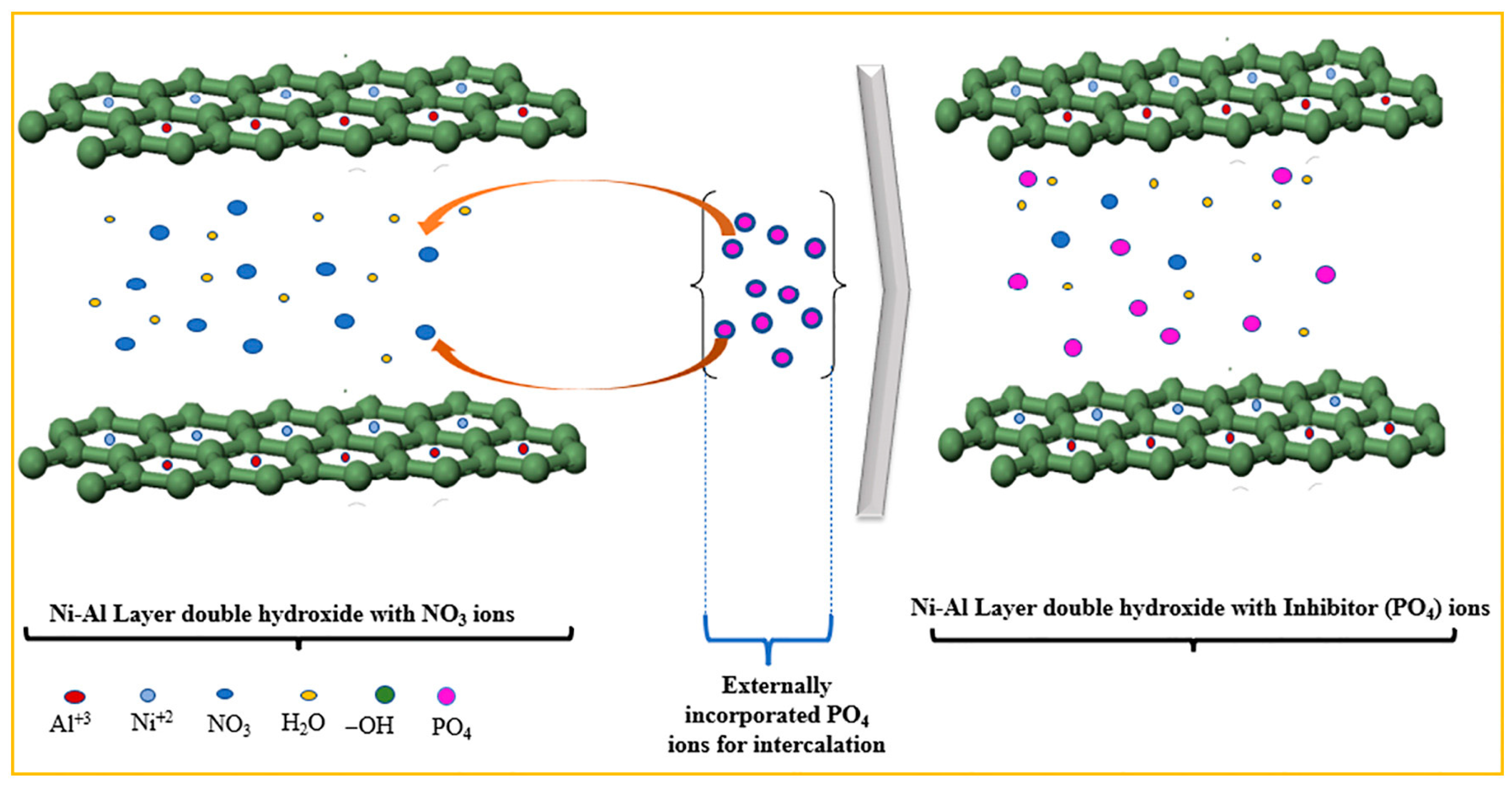
- Direct exchange—The direct mechanism involves the exchange of anions present between the lamellar layers of LDHs (e.g., NO3− o Cl−) with anions present in the reaction medium, driven by electrostatic interactions and hydrogen bonds with the OH-M2+/M3+ layer. The initial stage can lead to a partial expansion of the lattice, facilitating anion transfer. Molecular Dynamic (MD) simulations show that anion substitution is controlled by both the ionic size and the affinity for the lamellar layers [81].
- -
- Exchange via acid attack—In an acidic environment, the OH− groups of the LDH structure are protonated and the lamellar network partially dissolves, releasing the intercalated anions (e.g., CO32−). This controlled dissolution opens up space for the entry of new anions (e.g., Cl− o NO3−) once the environment returns to a less acidic pH [82]. This approach allows the exchange of “strong” anions, exploiting structural instability under acidic conditions.
- -
- Exchange by formation of surfactant salts—In this method, a surfactant anion (e.g., dodecyl sulphate, DBS) is intercalated into the interlamellar spaces, significantly changing the interlayer spacing. Subsequently, when interacting with a cationic surfactant (e.g., cetyltrimethylammonium bromide, CTAB), an organic salt is generated that “exits the lattice”, leading to a forced expulsion (“squeeze-out” mechanism) of the original anion and opening space for the intercalation of new anions [83,84]. This allows the formation of lamellar films with a porous structure and selective encapsulation capability.
- -
- Delamination-Filling Mechanism (Exfoliation-Assembly)
- -
- Reconstruction Mechanism
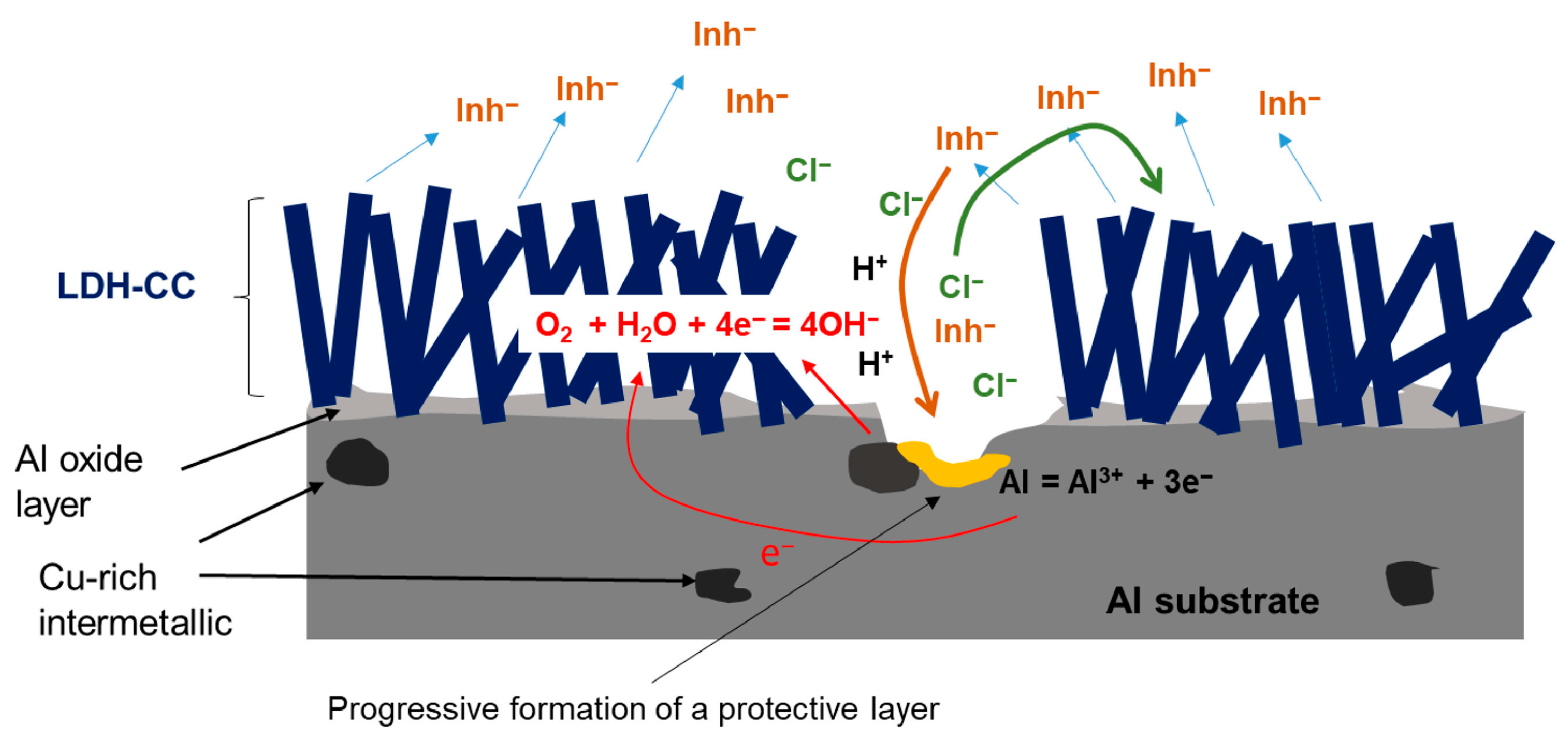
4. Corrosion Protection Mechanism
4.1. Cationic Storage
4.2. Anionic Storage
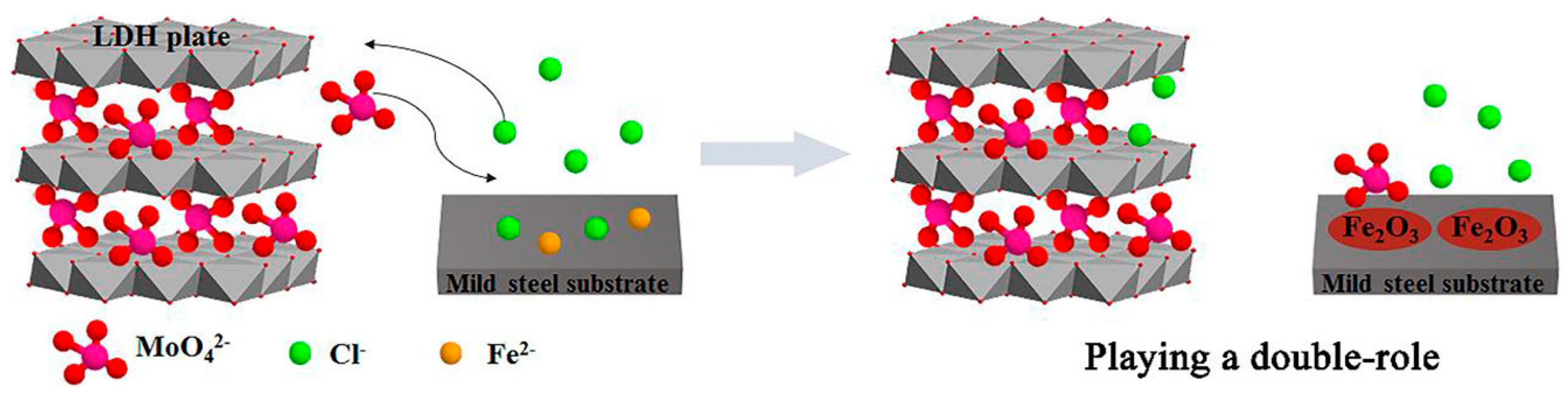
4.3. Chloride Entrapment
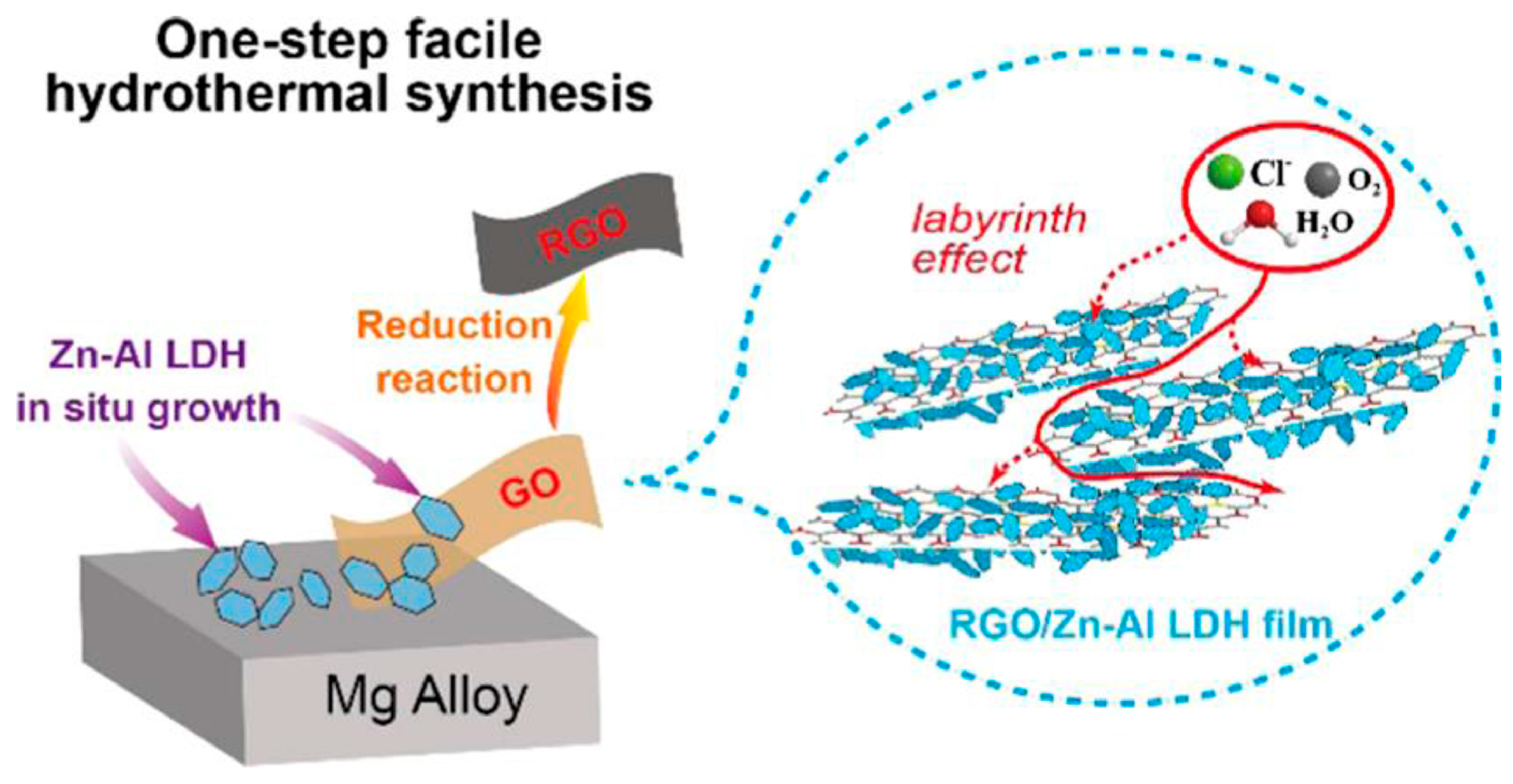
4.4. Labyrinth and Physical Barrier Mechanism
4.5. Self-Healing Mechanism
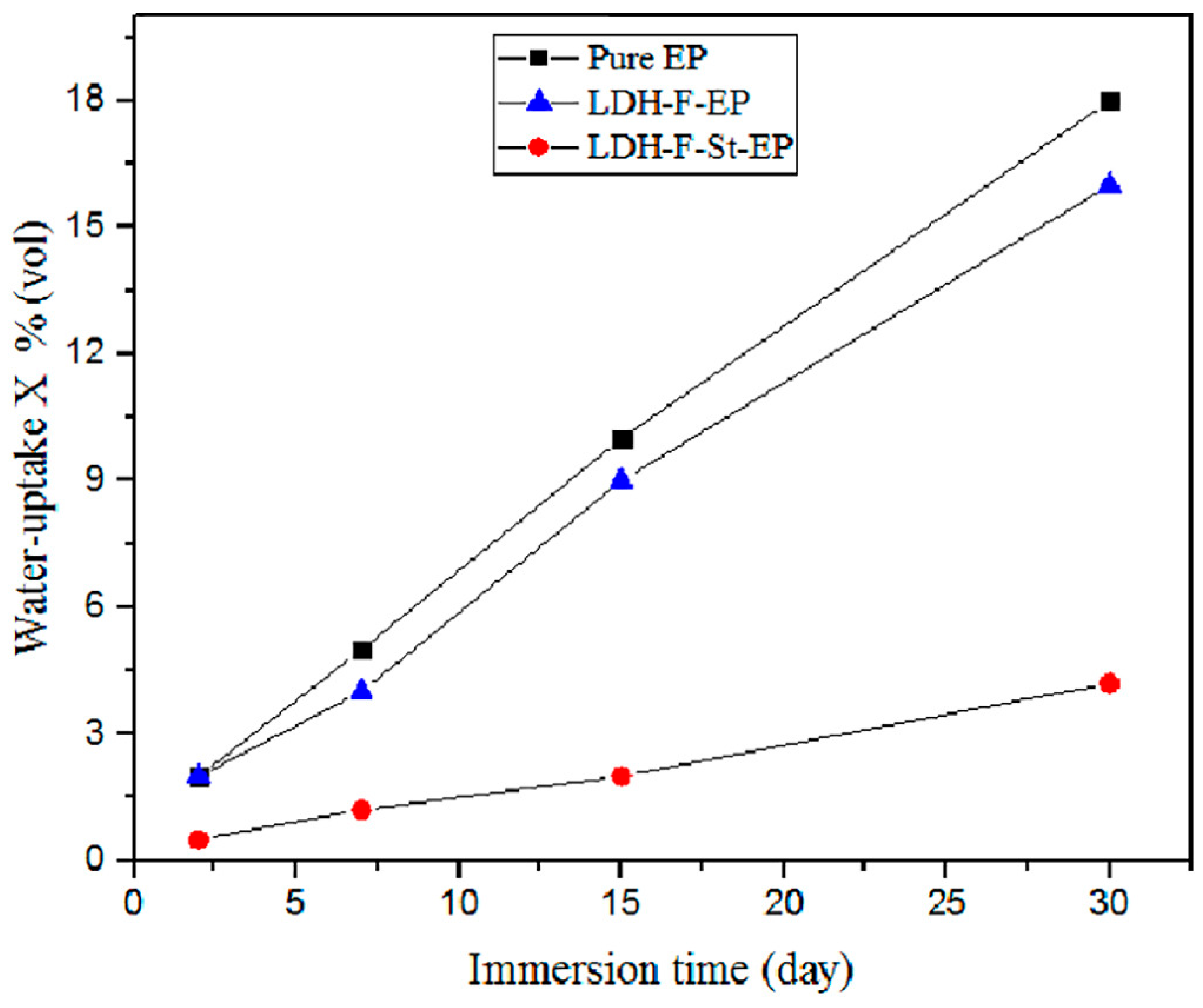
4.6. Hydrophobicity
4.7. Substrate Microstructure and Quality of LDH Coating
5. Commercialization Outlook and Limitations of LDH-Based Coatings
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- NACE Study Estimates Global Cost of Corrosion. Available online: https://www.rustbullet.com/cost-of-corrosion/?srsltid=AfmBOoor60Cy-USkAyUAObxGazC-ThrGmbPpliLgqczvovNQZCWmvT-Q (accessed on 23 June 2025).
- Singh, A.; Zeng, X. Global economic impact of corrosion and advanced protection strategies. Corros. Sci. 2022, 200, 110399. [Google Scholar]
- Khalil, M.; Al-Abdulkareem, M. Materials degradation and corrosion challenges in industry: A comprehensive review. Mater. Today Commun. 2023, 34, 105981. [Google Scholar]
- Balangao, J.K. Corrosion of Metals: Factors, Types and Prevention Strategies. J. Chem. Health Risks 2024, 14, 79–87. [Google Scholar]
- Alkadir Aziz, I.A.; Annon, I.A.; Abdulkareem, M.H.; Hanoon, M.M.; Alkaabi, M.H.; Shaker, L.M.; Alamiery, A.A.; Wan Isahak, W.N.; Takriff, M.S. Insights into Corrosion Inhibition Behavior of a 5-Mercapto-1,2,4-triazole Derivative for Mild Steel in Hydrochloric Acid Solution: Experimental and DFT Studies. Lubricants 2021, 9, 122. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Yousif, E.; Isahak, W.N.R.W.; Al-Azzawi, W.K. A Review of Inorganic Corrosion Inhibitors: Types, Mechanisms, and Applications. Tribol. Ind. 2023, 45, 313–339. [Google Scholar] [CrossRef]
- Dawood, M.A.; Alasady, Z.M.K.; Abdulazeez, M.S.; Ahmed, D.S.; Sulaiman, G.M.; Kadhum, A.A.H.; Shaker, L.M.; Alamiery, A.A. The corrosion inhibition effect of a pyridine derivative for low carbon steel in 1 M HCl medium: Complemented with antibacterial studies. Int. J. Corros. Scale Inhib. 2021, 10, 1766–1782. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.; Rhee, K.Y. Electronic Effect vs. Molecular Size Effect: Experimental and Computational based Designing of Potential Corrosion Inhibitors. Chem. Eng. J. 2021, 430, 132645. [Google Scholar] [CrossRef]
- Abtan, N.; Al-Hamid, M.; Kadhim, L.; Sayyid, F.; Noori, F.; Kadum, A.; Al-Amiery, A.; Al-Azzawi, W. Unlocking the Power of 4-Acetamidoantipyrine: A Promising Corrosion Inhibitor for Preserving Mild Steel in Harsh Hydrochloric Acid Environments. Prog. Color Color. Coat. 2023, 17, 85–96. [Google Scholar]
- Nnoka, M.; Alaso Jack, T.; Szpunar, J. Effects of different microstructural parameters on the corrosion and cracking resistance of pipeline steels: A review. Eng. Fail. Anal. 2024, 159, 108065. [Google Scholar] [CrossRef]
- Tait, W.S. Chapter 27—Controlling Corrosion of Chemical Processing Equipment. In Handbook of Environmental Degradation of Materials, 3rd ed.; Kutz, M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 583–600. [Google Scholar]
- Kim, Y.-J.; Bahn, C.B.; Baek, S.H.; Choi, W.; Song, G.D. Crevice chemistry and corrosion in high temperature water: A review. Nucl. Eng. Technol. 2024, 56, 3112–3122. [Google Scholar] [CrossRef]
- Cao, X.; Huang, F.; Huang, C.; Liu, J.; Cheng, Y.F. Preparation of graphene nanoplate added zinc-rich epoxy coatings for enhanced sacrificial anode-based corrosion protection. Corros. Sci. 2019, 159, 108120. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, W.; Wang, L. State of the art and current trends on the metal corrosion and protection strategies in deep sea. J. Mater. Sci. Technol. 2025, 215, 192–213. [Google Scholar] [CrossRef]
- Niu, G.; Yuan, R.; Wang, E.; Yang, X.; Liu, Z.; Li, Z.; Zhang, Z.; Gong, N.; Li, K.; Su, B.; et al. Unravelling the influence of Mo on the corrosion mechanism of Ni-advanced weathering steel in harsh marine atmospheric environments. J. Mater. Sci. Technol. 2024, 195, 41–62. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A. Hybrid Nanomaterials as Next-Generation Corrosion Inhibitors for Metals and Alloys. In Innovations and Applications of Hybrid Nanomaterials; Khanna, V., Sharma, P., Mahajan, P., Eds.; IGI Global: Hershey, PA, USA, 2024; pp. 91–117. [Google Scholar]
- Kumar, P.; Soni, I.; Jayaprakash, G.; Kumar, S.; Rao, S.; Flores-Moreno, R.; Swamirayachar, S. Experimental and theoretical studies of hexylmeythylimidazolium tetrafluoroborate ionic liquid as cathodic corrosion inhibitor for mild steel. Inorg. Chem. Commun. 2022, 146, 110110. [Google Scholar] [CrossRef]
- Auepattana-Aumrung, K.; Crespy, D. Self-healing and anticorrosion coatings based on responsive polymers with metal coordination bonds. Chem. Eng. J. 2023, 452, 139055. [Google Scholar] [CrossRef]
- Shaw, B.A.; Kelly, R. What is Corrosion? Electrochem. Soc. Interface 2006, 15, 24–26. [Google Scholar] [CrossRef]
- Mondal, K.; Nunez, L.; Downey, C.; Van Rooyen, I. Recent Advances in the Thermal Barrier Coatings for Extreme Environments. Mater. Sci. Energy Technol. 2021, 4, 208–210. [Google Scholar] [CrossRef]
- Hussain, A.; Nuthalapati, S.; Pichumani, M.; Chakra, C. Research progress in organic zinc rich primer coatings for cathodic protection of metals—A comprehensive review. Prog. Org. Coat. 2021, 153, 106040. [Google Scholar] [CrossRef]
- Heimann, R.B. Magnesium alloys for biomedical application: Advanced corrosion control through surface coating. Surf. Coat. Tech. 2021, 405, 126521. [Google Scholar] [CrossRef]
- Okokpujie, I.; Tartibu, L.; Musa-Basheer, H.; Adeoye, A. Effect of Coatings on Mechanical, Corrosion and Tribological Properties of Industrial Materials: A Comprehensive Review. J. Bio-Tribo-Corros. 2023, 10, 2. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; You, Z.; Liu, S.; Guan, K.; Wu, R.; Wang, J.; Feng, J. Towards developing Mg alloys with simultaneously improved strength and corrosion resistance via RE alloying. J. Magnes. Alloys 2021, 9, 41–56. [Google Scholar] [CrossRef]
- Olajire, A. Recent advances on organic coating system technologies for corrosion protection of offshore metallic structures. J. Mol. Liq. 2018, 269, 572–606. [Google Scholar] [CrossRef]
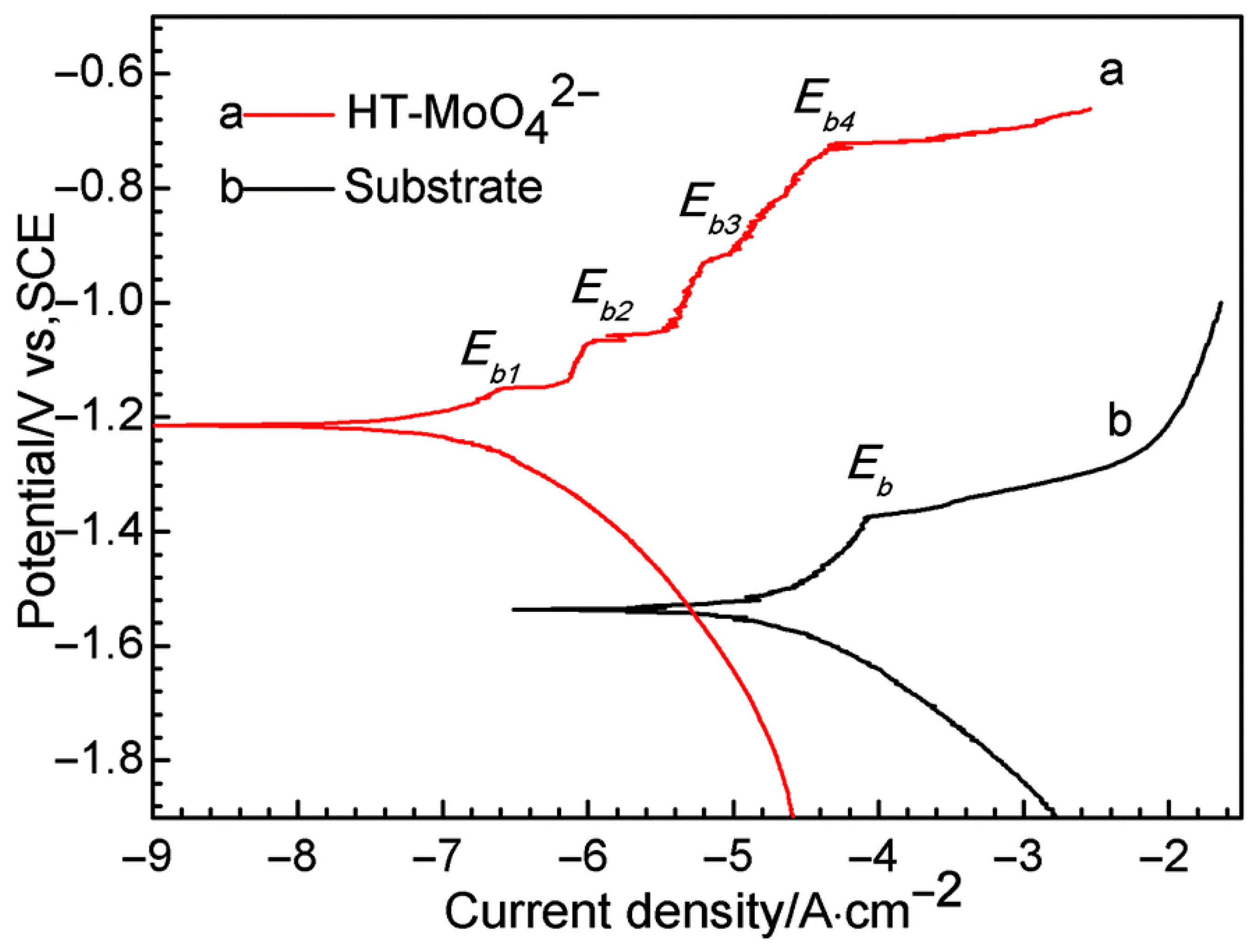
- Li, T.; Ma, Y.; Liu, J.-K.; Liu, J.-C.; Liu, Z.-X.; Ma, Y.-S.; Liu, P.-P. In-situ construction of lamellar stacked zinc molybdate-zinc/aluminium layered double hydroxides heterojunction material with synergistic corrosion protection. Prog. Org. Coat. 2024, 189, 108294. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Chen, H. Enhancement of corrosion resistance of organic coatings by incorporation of lamellar fillers: A review. Prog. Org. Coat. 2022, 163, 106541. [Google Scholar]
- Wang, Y.; Zhao, W. Role of mica and glass flakes in improving barrier properties of polymeric anticorrosive coatings. Surf. Coat. Tech. 2021, 407, 126747. [Google Scholar]
- Kumar, S.; Singh, R. Effect of filler size and loading on the mechanical and anticorrosive properties of epoxy-based coatings. J. Coat. Technol. Res. 2023, 20, 213–225. [Google Scholar]
- Zhang, J.; Li, J.; Chen, Y. Challenges in filler dispersion for polymer composites: Impact on corrosion protection. Polym. Degrad. Stab. 2022, 197, 109890. [Google Scholar]
- Zhang, X.; Wang, Y.; Li, Q. Two-dimensional nanomaterials as functional fillers for anticorrosive coatings: A review. Prog. Org. Coat. 2023, 178, 107638. [Google Scholar]
- Chen, L.; Liu, J.; Huang, Y. Barrier effects of graphene and hexagonal boron nitride nanosheets in anticorrosive coatings. Surf. Coat. Tech. 2022, 432, 128093. [Google Scholar]
- Patel, R.; Singh, S.; Kumar, P. Mechanical reinforcement and corrosion resistance of polymer composites with 2D nanomaterials. Compos. Sci. Technol. 2022, 227, 109469. [Google Scholar]
- Ahmad, S.; Nawaz, M.; Mohammad, S.; Shakoor, R.A.; Kahraman, R.; Al Tahtamouni, T.M. Modified Ni–Al Layer Double Hydroxides as Nanoparticles for Self-Healing Anti-Corrosion Composite Coating. Surf. Coat. Technol. 2024, 476, 130172. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, T.; Zhou, X. Multifunctional LDH-based nanocomposites for enhanced corrosion protection and mechanical performance. Corros. Sci. 2023, 209, 110091. [Google Scholar]
- Leal, D.A.; Kuznetsova, A.; Silva, G.M.; Tedim, J.; Wypych, F.; Marino, C.E.B. Layered Materials as Nanocontainers for Active Corrosion Protection: A Brief Review. Appl. Clay Sci. 2022, 225, 106537. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Jiao, Z.-J.; Cui, L.-Y.; Huang, Y.-D.; Li, S.-Q.; Liu, C.-B.; Zeng, R.-C. Advances in LDHs for Corrosion-Resistant Protection of Mg and Al Alloys: A Review. J. Magnes. Alloys 2025, 13, 923–947. [Google Scholar] [CrossRef]
- Majidi, R.; Danaee, I.; Vrsalović, L.; Zarei, D. Development of a Smart Anticorrosion Epoxy Coating Containing a pH-Sensitive GO/MOF Nanocarrier Loaded with 2-Mercaptobenzothiazole Corrosion Inhibitor. Mater. Chem. Phys. 2023, 308, 128291. [Google Scholar] [CrossRef]
- Altalhi, A.A.; Mohamed, E.A.; Negm, N.A. Recent advances in layered double hydroxide (LDH)-based materials: Fabrication, modification strategies, characterization, promising environmental catalytic applications, and prospective aspects. Energy Adv. 2024, 3, 2136–2151. [Google Scholar] [CrossRef]
- Chhetri, S.; Samanta, P.; Murmu, N.C.; Kuila, T. Anticorrosion Properties of Epoxy Composite Coating Reinforced by Molybdate-Intercalated Functionalized Layered Double Hydroxide. J. Compos. Sci. 2019, 3, 11. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, C.; Rad, S.; He, H.; Qin, L. A Comprehensive Review of Layered Double Hydroxide-Based Carbon Composites as an Environmental Multifunctional Material for Wastewater Treatment. Processes 2022, 10, 617. [Google Scholar] [CrossRef]
- Jiang, Y.; Shen, Z.; Tang, C.S.; Shi, B. Synthesis and application of waste-based layered double hydroxide: A review. Sci. Total Environ. 2023, 903, 166245. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, S.M.; Shehata, M.R.; El-Ela, F.I.A.; Farghali, A.; Zaher, A.; Mahmoud, R.K. Sustainable waste management and recycling of Zn-Al layered double hydroxides after adsorption of levofloxacin as a safe anti-inflammatory nano-material. RSC Adv. 2020, 10, 27633–27651, Erratum in RSC Adv. 2020, 10, 29128. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Chen, R.; Zhang, J.; Hu, L.; Qiu, X. Developed Recyclable CaFe-Layered Double Hydroxide for Efficient Cadmium Immobilization in Soil: Performance and Bioavailability. Minerals 2024, 14, 656. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Q.; Khalaf, A.H.; Lin, B.; Tang, J. Long-Term Anti-Corrosion Performance of Ultra-High Content Inhibitor Loaded Gel-Epoxy Solid Inhibitor with Temperature-Responisve Effect. Appl. Sci. 2025, 15, 3964. [Google Scholar] [CrossRef]
- Chen, Z.; Scharnagl, N.; Zheludkevich, M.L.; Ying, H.; Yang, W. Micro/nanocontainer-based intelligent coatings: Synthesis, performance and applications—A review. Chem. Eng. J. 2023, 451, 138582. [Google Scholar] [CrossRef]
- Kang, J.; Xiao, Y.; Li, L.; Qiao, L.; Liu, C.; Sun, P.; Liu, D.; Ip, W.F.; Pan, H. Ternary Synergy in Layered Double Hydroxides for Efficient and Stable Nitrate Reduction. Adv. Funct. Mater. 2025; early view. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Yao, W.; Wu, J.; Yuan, Y.; Xie, Z.; Jiang, B.; Pan, F. Synergistic effect of graphene oxide/ternary Mg-Al-La layered double hydroxide for dual self-healing corrosion protection of micro-arc oxide coating of magnesium alloy. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130339. [Google Scholar] [CrossRef]
- Jing, C.; Dong, B.; Raza, A.; Zhang, T.; Zhang, Y. Corrosion inhibition of layered double hydroxides for metal-based systems. Nano Mater. Sci. 2021, 3, 47–67. [Google Scholar] [CrossRef]
- Tabish, M.; Yasin, G.; Anjum, M.J.; Malik, M.U.; Zhao, J.; Yang, Q.; Manzoor, S.; Murtaza, H.; Khan, W.Q. Reviewing the current status of layered double hydroxide-based smart nanocontainers for corrosion inhibiting applications. J. Mater. Res. Technol. 2021, 10, 390–421. [Google Scholar] [CrossRef]
- Bouali, A.C.; Serdechnova, M.; Blawert, C.; Tedim, J.; Ferreira, M.G.S.; Zheludkevich, M.L. Layered double hydroxides (LDHs) as functional materials for the corrosion protection of aluminum alloys: A review. Appl. Mater. Today 2020, 21, 100857. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, D.; Li, X.; Lin, J.; Wang, C.; Dong, S.; Lin, C. Enhanced Corrosion Resistance of Superhydrophobic Layered Double Hydroxide Films with Long-Term Stability on Al Substrate. ACS Appl. Mater. Interfaces 2018, 10, 15150–15162. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Somvanshi, D.; Singh, R.K.; Mahanta, A.K.; Misra, N.; Paij, P. Functionalized polyvinyl chloride/layered double hydroxide nanocomposites and its thermal and mechanical properties. J. Appl. Polym. Sci. 2020, 137, 48894. [Google Scholar] [CrossRef]
- Aminifazl, A.; Karunarathne, D.J.; Golden, T.D. Synthesis of Silane Functionalized LDH-Modified Nanopowders to Improve Compatibility and Enhance Corrosion Protection for Epoxy Coatings. Molecules 2024, 29, 819. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.A.; Asghar, H.; Fedel, M. Double Doped Cerium-Based Superhydrophobic Layered Double Hydroxide Protective Films Grown on Anodic Aluminium Surface. J. Alloys Compd. 2020, 844, 156112. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Zhang, X.; Li, M.; Chen, Y.; Si-Bo, K.; Lu, W.; Wu, C.; Zhou, H.; Chen, S.; et al. MgAl Layered Double Hydroxide Nanoplate Coatings as Corrosion Inhibitors for Long-Term Protection of Q235 Steel. ACS Appl. Nano Mater. 2025, 8, 4500–4512. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Secchi, M.; Iqbal, M.A.; Montagna, M.; Zanella, C.; Fedel, M. MgAl-LDH/graphene protective film: Insight into LDH-graphene interaction. Surf. Coat. Technol. 2020, 401, 126253. [Google Scholar] [CrossRef]
- Vieira, D.E.L.; Sokol, D.; Smalenskaite, A.; Kareiva, A.; Ferreira, M.G.S.; Vieira, J.M.; Salak, A.N. Cast Iron Corrosion Protection with Chemically Modified MgAl Layered Double Hydroxides Synthesized Using a Novel Approach. Surf. Coat. Technol. 2019, 375, 158–163. [Google Scholar] [CrossRef]
- Liu, J.; Gao, X.; Chen, T. LDHs and Their Derivatives for Electrochemical Energy Storage and Conversion Systems: Design, Insights and Applications. ChemElectroChem 2024, 11, e202400156. [Google Scholar] [CrossRef]
- Zhou, Q.; Dai, X.; Li, K.; Zhang, C.; Zhang, X.; Du, Z.; Yi, S.; Yang, P.; Rao, J.; Zhang, Y. Facile Synthesis of a 2D Multilayer Core–Shell MnO2@LDH@MMT Composite with a Nanoflower Shape for Electromagnetic Wave Absorption. Cryst. Eng. Comm. 2022, 24, 6546–6557. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Li, R.; Feng, X.; Pang, X.; Rao, J.; Cong, D.; Yin, C.; Zhang, Y. Active Corrosion Protection of Mg–Al Layered Double Hydroxide for Magnesium Alloys: A Short Review. Coatings 2021, 11, 1316. [Google Scholar] [CrossRef]
- Jin, W.; Park, D.-H. Functional Layered Double Hydroxide Nanohybrids for Biomedical Imaging. Nanomaterials 2019, 9, 1404. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Dong, B.; Zhang, Y. Chemical Modifications of Layered Double Hydroxides in the Supercapacitor. Energy Environ. Mater. 2020, 3, 346–379. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Yu, X.; Jing, X.; Zhang, M.; Jiang, Z. Hydrotalcite conversion coating on Mg alloy and its corrosion resistance. J. Alloys Compd. 2010, 494, 271–274. [Google Scholar] [CrossRef]
- Li, K.; Yin, C.; Dai, X.; Zhang, J.; Yi, S.; Rao, J.; Zhang, Y. Facile Synthesis and Incomplete Sulfidation of Nickel–Cobalt–Aluminum Ternary Layered Hydroxide Binder-Free Electrode with Enhanced Supercapacitor Properties. J. Energy Storage 2022, 55, 105722. [Google Scholar] [CrossRef]
- Yang, Z.; Fischer, H.; Polder, R. Modified Hydrotalcites as a New Emerging Class of Smart Additive of Reinforced Concrete for Anticorrosion Applications: A Literature Review. Mater. Corros. 2013, 64, 1066–1074. [Google Scholar] [CrossRef]
- Zhang, C.; Li, K.; Sun, T.; Liu, X.; Dai, X.; Zhou, Q.; Wang, D.; Zhang, X.; Ding, J.; Huang, X.; et al. Biomimetic Sea Urchin-like Nano-Ferrite Structures for Microwave Absorption. ACS Appl. Nano Mater. 2024, 7, 3001–3011. [Google Scholar] [CrossRef]
- Sun, Q.; Guo, Z.; Shu, T.; Li, Y.; Li, K.; Zhang, Y.; Li, L.; Ning, J.; Yao, K.X. Lithium-Induced Oxygen Vacancies in MnO2@MXene for High-Performance Zinc-Air Batteries. ACS Appl. Mater. Interfaces 2024, 16, 12781–12792. [Google Scholar] [CrossRef] [PubMed]
- Kamal, N.A.; Pungot, N.H.; Che Soh, S.K.; Ahmad Tajuddin, N. Facile and Green Hydrothermal Synthesis of MgAl/NiAl/ZnAl Layered Double Hydroxide Nanosheets: A Physiochemical Comparison. Pure Appl. Chem. 2024, 96, 1319–1334. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Lu, Q.; Li, Y.; Jiang, R.; Liu, Y.; Zheng, X.; Zhao, N.; Li, J.; Deng, Y.; et al. High Entropy Layered Double Hydroxides with Highly Adjustable Components for Enhancing Electrocatalytic Oxygen Evolution. ACS Appl. Mater. Interfaces 2023, 15, 38423–38432. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.-Y.; Sun, C.; Zhang, H.; Xu, H.; Xu, H.; Zhang, Y.; Fu, Q.; Ma, D.-D. Fluorine Lodged High Valent High Entropy Layered Double Hydroxide for Efficient, Long Lasting Zinc–Air Batteries. Angew. Chem. Int. Ed. 2024; early view. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Y.; Dong, M. Recent Advances in Layered Double Hydroxides Membranes: Insights into Multiple Mass Transport. Adv. Funct. Mater. 2024, 34, 2312452. [Google Scholar] [CrossRef]
- Zhang, W.; Buchheit, R.G. Hydrotalcite Coating Formation on Al–Cu–Mg Alloys from Oxidizing Bath Chemistries. Corrosion 2002, 58, 591–660. [Google Scholar] [CrossRef]
- Leggat, R.B.; Zhang, W.; Buchheit, R.G. Performance of Hydrotalcite Conversion Treatment on AA2024 When Used in a Coating System. Corrosion 2002, 58, 322–328. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ren, Y.; Wang, H.; Chen, F. Double-Doped LDH Films on Aluminum Alloys for Active Protection. Mater. Lett. 2017, 192, 33–35. [Google Scholar] [CrossRef]
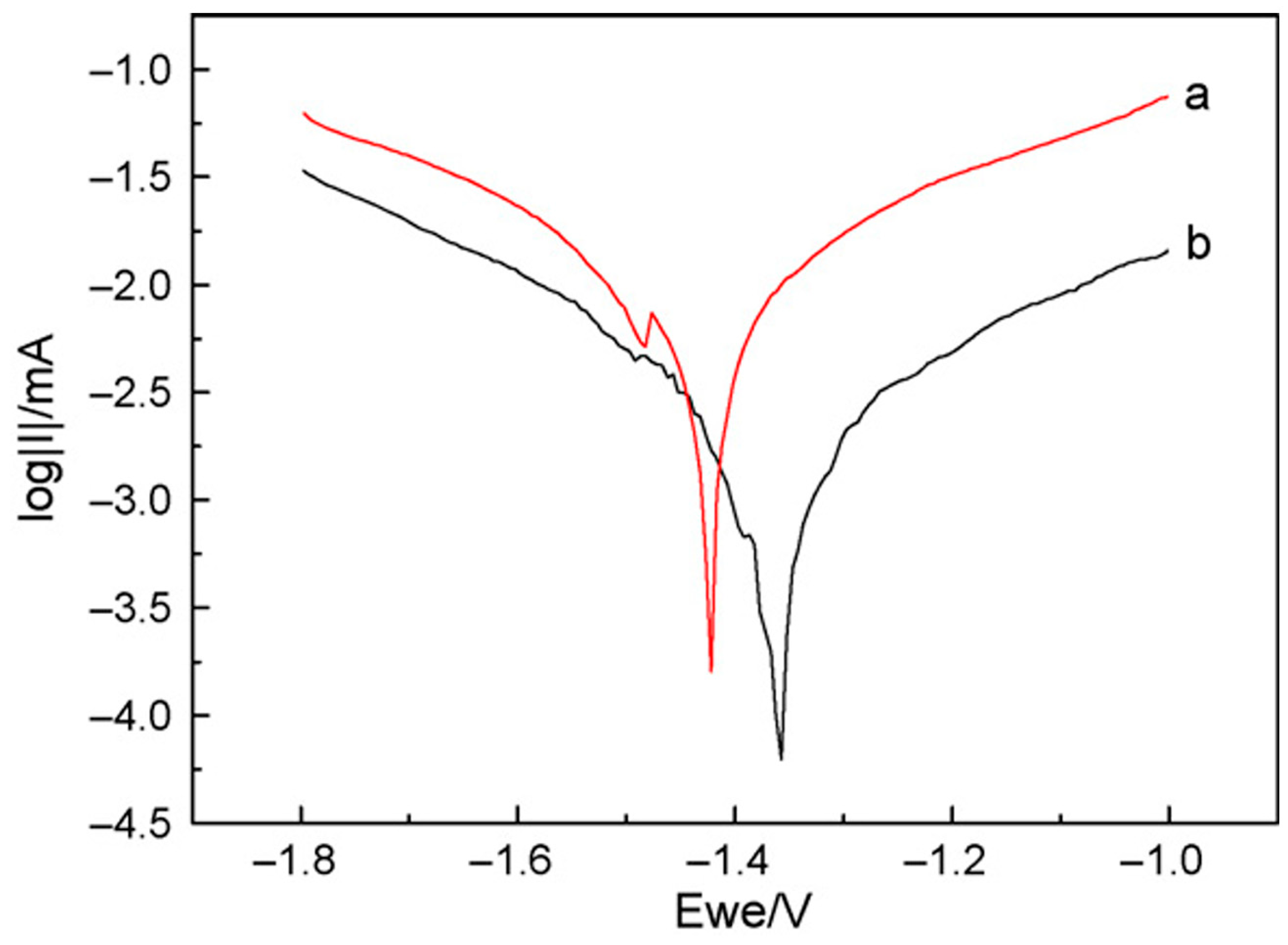
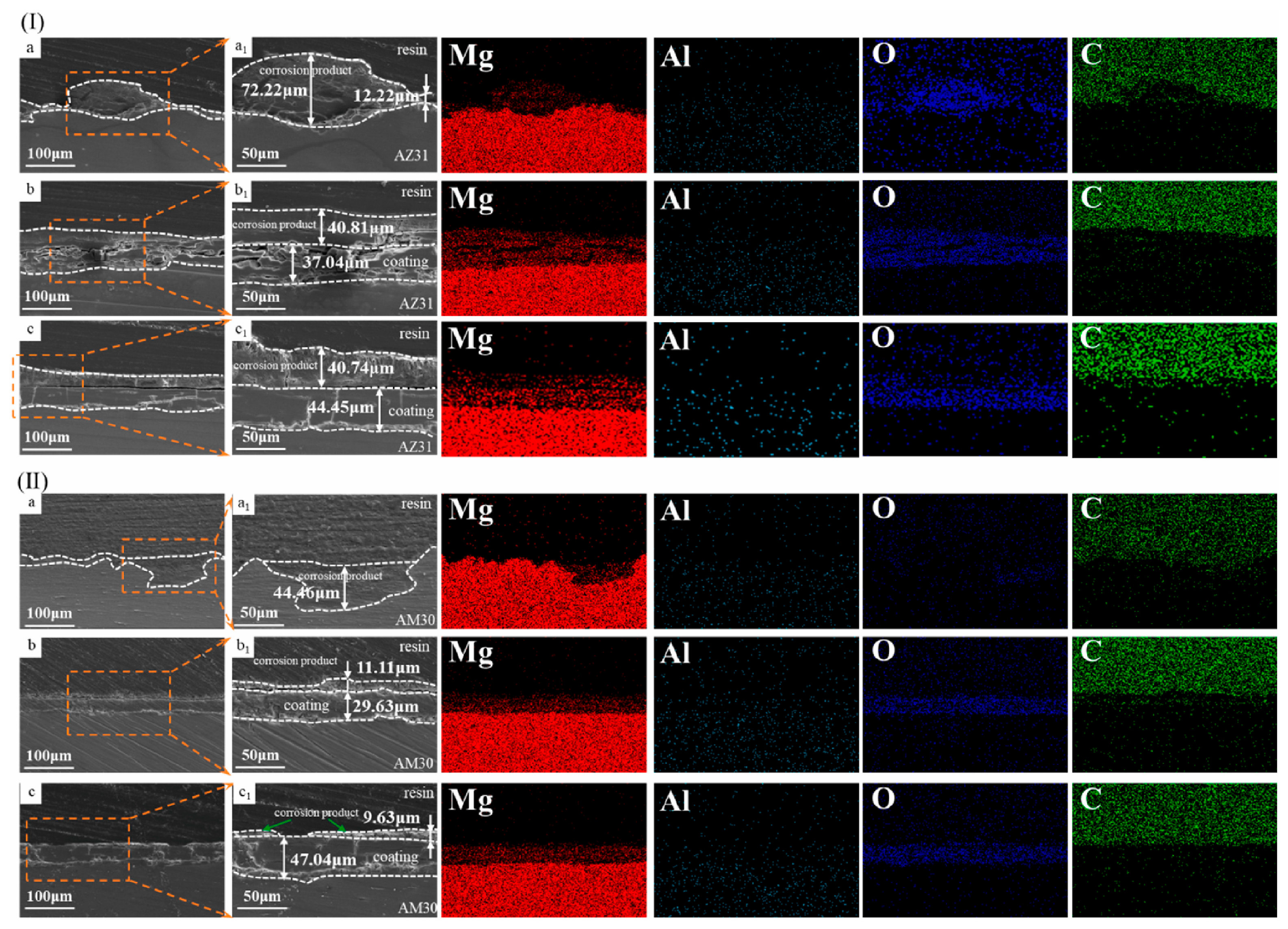
- Zhao, Y.-J.; Zhang, F.; Cui, L.-Y.; Li, S.-Q.; Liu, C.-B.; Zeng, R.-C. Corrosion Resistance of In Situ Steam LDH Coating on AZ31 and AM30 Alloys: Influence of NaOH and Al–Mn Phase. Smart Mater. Manuf. 2024, 2, 100045. [Google Scholar] [CrossRef]
- Rives, V.; Ulibarri, M.A. Layered Double Hydroxides (LDH) Intercalated with Metal Coordination Compounds and Oxometalates. Coord. Chem. Rev. 1999, 181, 61–120. [Google Scholar] [CrossRef]
- Li, W.; Liu, A.; Tian, H.; Wang, D. Controlled Release of Nitrate and Molybdate Intercalated in Zn–Al–Layered Double Hydroxide Nanocontainers towards Marine Anticorrosion Applications. Colloid Interface Sci. Commun. 2018, 24, 18–23. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Q.; Ji, F.; Jiang, L.; Liu, C.; Shen, Q.; Liu, Q. Phosphate Removal Performances of Layered Double Hydroxides (LDH) Embedded Polyvinyl Alcohol/Lanthanum Alginate Hydrogels. Chem. Eng. J. 2022, 431, 133665. [Google Scholar] [CrossRef]
- Das, J.; Patra, B.S.; Baliarsingh, N.; Parida, K.M. Adsorption of Phosphate by Layered Double Hydroxides in Aqueous Solutions. Appl. Clay Sci. 2006, 32, 252–260. [Google Scholar] [CrossRef]
- Zhao, X.J.; Zhu, Y.Q.; Xu, S.M.; Liu, H.M.; Yin, P.; Feng, Y.L.; Yan, H. Anion Exchange Behavior of M(II)Al Layered Double Hydroxides: A Molecular Dynamic and DFT Study. Phys. Chem. Chem. Phys. 2020, 22, 19758–19768. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sun, M.; Wang, X.; Lu, Y.; Tang, N.; Gao, L.; Wang, Q.; Hu, L. Preparation and Corrosive Anion-Curing Capability of Layered Double Hydroxide (LDH)/Montmorillonite Composites. Clays Clay Miner. 2023, 71, 461–477. [Google Scholar] [CrossRef]
- Brahma, D.; Saikia, H. Surfactants Assisted Synthesis of CuAl-Sodium Dodecyl Sulfate Layered Double Hydroxide and Its Adsorptive Removal of Methyl Red Dye from Aqueous Solution. Inorg. Nano-Met. Chem. 2023, 53, 605–620. [Google Scholar] [CrossRef]
- Cao, J.; Wu, Y.; Zhao, W. Review of Layered Double Hydroxide (LDH) Nanosheets in Corrosion Mitigation: Recent Developments, Challenges, and Prospects. Materials 2025, 18, 1190. [Google Scholar] [CrossRef] [PubMed]
- Cermelj, K.; Ruengkajorn, K.; Buffet, J.-C.; O’Hare, D. Layered Double Hydroxide Nanosheets via Solvothermal Delamination. J. Energy Chem. 2019, 35, 88–94. [Google Scholar] [CrossRef]
- Leroux, F.; Adachi-Pagano, M.; Intissar, M.; Chauvière, S.; Forano, C.; Besse, J.-P. Delamination and Restacking of Layered Double Hydroxides. J. Mater. Chem. 2001, 11, 105–112. [Google Scholar] [CrossRef]
- Okudaira, K.; Kameshima, Y.; Isobe, T.; Nakajima, A.; Okada, K. Preparation and Delamination Behavior of Lactate/Layered Double Hydroxide (LDH) Composites Using Reconstruction Method. Jpn. J. Colour Mater. 2011, 84, 2–6. [Google Scholar] [CrossRef][Green Version]
- Kang, G.-H.; Park, I.-K. Reconstruction and Intercalating Anion Exchange of ZnAl-Layered Double Hydroxide. Ceram. Int. 2022, 48, 3030–3036. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic Applications of Layered Double Hydroxides: Recent Advances and Perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef] [PubMed]
- Mikhailau, A.; Maltanava, H.; Poznyak, S.K.; Salak, A.N.; Zheludkevich, M.L.; Yasakau, K.A.; Ferreira, M.G.S. One-Step Synthesis and Growth Mechanism of Nitrate Intercalated ZnAl LDH Conversion Coatings on Zinc. Chem. Commun. 2019, 55, 6878–6881. [Google Scholar] [CrossRef] [PubMed]
- Salak, A.N.; Tedim, J.; Kuznetsova, A.I.; Zheludkevich, M.L.; Ferreira, M.G.S. Anion Exchange in Zn–Al Layered Double Hydroxides: In Situ X-Ray Diffraction Study. Chem. Phys. Lett. 2010, 495, 73–76. [Google Scholar] [CrossRef]
- Shkirskiy, V.; Keil, P.; Hintze-Brüning, H.; Leroux, F.; Vialat, P.; Lefèvre, G.; Ogle, K.; Volovitch, P. Factors Affecting MoO42− Inhibitor Release from Zn2Al-Based Layered Double Hydroxide and Their Implication in Protecting Hot-Dip Galvanized Steel by Means of Organic Coatings. ACS Appl. Mater. Interfaces 2015, 7, 25180–25192. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Yu, M.; Li, S.; Xue, B.; Yin, X. Influence of Embedded ZnAlCe–NO3− Layered Double Hydroxides on the Anticorrosion Properties of Sol–Gel Coatings for Aluminum Alloy. Prog. Org. Coat. 2015, 81, 93–100. [Google Scholar] [CrossRef]
- Dai, X.; Wu, L.; Xia, Y.; Chen, Y.; Zhang, Y.; Jiang, B.; Xie, Z.; Ci, W.; Zhang, G.; Pan, F. Intercalation of Y in Mg–Al Layered Double Hydroxide Films on Anodized AZ31 and Mg–Y Alloys to Influence Corrosion Protective Performance. Appl. Surf. Sci. 2021, 551, 149432. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, L.; Ling, H.; Diao, Y.; Pang, X.; Wang, Y.; Gao, K. Design and fabrication of enhanced corrosion resistance Zn–Al layered double hydroxide films based on anion exchange mechanism on magnesium alloys. Appl. Surf. Sci. 2017, 404, 246–253. [Google Scholar] [CrossRef]
- Jiang, D.; Zhou, H.; Wan, S.; Cai, G.-Y.; Dong, Z.-H. Fabrication of superhydrophobic coating on magnesium alloy with improved corrosion resistance by combining micro-arc oxidation and cyclic assembly. Surf. Coat. Technol. 2018, 339, 155–166. [Google Scholar] [CrossRef]
- Jiang, D.; Xia, X.; Hou, J.; Cai, G.; Zhang, X.; Dong, Z. A novel coating system with self-reparable slippery surface and active corrosion inhibition for reliable protection of Mg alloy. Chem. Eng. J. 2019, 373, 285–297. [Google Scholar] [CrossRef]
- Su, Y.; Qiu, S.; Yang, D.; Liu, S.; Zhao, H.; Wang, L.; Xue, Q. Active anti-corrosion of epoxy coating by nitrite ions intercalated Mg–Al LDH. J. Hazard. Mater. 2020, 391, 122215. [Google Scholar] [CrossRef] [PubMed]
- Hang, T.T.X.; Truc, T.A.; Duong, N.T.; Pébère, N.; Olivier, M.-G. Layered double hydroxides as containers of inhibitors in organic coatings for corrosion protection of carbon steel. Prog. Org. Coat. 2012, 74, 343–348. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, C.; Ma, R.; Sun, Q.; Yan, X.; Li, J.; Li, S.; Liu, Y. Insight into the Essence of Solubility and Agglomeration of Chloride-Layered Double Hydroxides. Ind. Eng. Chem. Res. 2024, 63, 17441–17449. [Google Scholar] [CrossRef]
- Poznyak, S.K.; Snihirova, D.V.; Taryba, M.G.; Zheludkevich, M.L.; Ferreira, M.G.S. Zn–Al and Mg–Al LDH intercalated with organic corrosion inhibitors: Structural characterization and release studies. Surf. Coat. Technol. 2009, 203, 1084–1091. [Google Scholar]
- Poznyak, S.K.; Tedim, J.; Rodrigues, L.M.; Salak, A.N.; Zheludkevich, M.L.; Dick, L.F.P.; Ferreira, M.G.S. Novel Inorganic Host Layered Double Hydroxides Intercalated with Guest Organic Inhibitors for Anticorrosion Applications. ACS-Appl. Mater. Interfaces 2009, 1, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Zheludkevich, M.L.; Tedim, J.; Ferreira, M.G.S. “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, D.; Zhang, F.; Pan, J.; Lin, C. Layered double hydroxide (LDH) for multifunctionalized corrosion protection of metals: A review. J. Mater. Sci. Technol. 2022, 102, 232–263. [Google Scholar] [CrossRef]
- Yang, Z.; Fischer, H.; Cerezo, J.; Mol, J.M.C.; Polder, R. Modified hydrotalcites for improved corrosion protection of reinforcing steel in concrete—Preparation, characterization, and assessment in alkaline chloride solution. Mater. Corros. 2016, 67, 721–738. [Google Scholar] [CrossRef]
- Lin, J.K.; Hsia, C.L.; Uan, J.Y. Characterization of Mg,Al–hydrotalcite conversion film on Mg alloy and Cl− and CO32− anion-exchangeability of the film in a corrosive environment. Scr. Mater. 2007, 56, 927–930. [Google Scholar] [CrossRef]
- Azhar, A.; Zaki, H.M.; Zain, Z.M.; Jalil, M.N.; Azly, M.E.K. Optimizing corrosion resistance: How pH shapes the inhibition mechanism of ZnAl–NO3− LDH on mild steel. Pure Appl. Chem. 2025, 97, 381–399. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, H.; Chang, L.; Yu, Z.; Xiao, Z.; Tang, S.; Huang, C.; Fan, J.; Yang, S. First-principles study on interlayer spacing and structure stability of NiAl-layered double hydroxides. ACS Omega 2022, 7, 39169–39180. [Google Scholar] [CrossRef] [PubMed]
- Ayemi, G.J.; Marcelin, S.; Thérias, S.; Leroux, F.; Normand, B. Synergy effect between layered double hydroxide (LDH) and EDDS for corrosion inhibition of carbon steel. Appl. Clay Sci. 2022, 222, 106497. [Google Scholar] [CrossRef]
- Li, W.; Wang, P.; Wang, S.; Duan, Z.; Liu, L.; Wang, Y.; Xu, M. In situ synthesis of graphene oxide-sealed LDHs coatings: A novel approach to enhancing corrosion resistance and tribological performance on magnesium alloys. Coatings 2023, 13, 1544. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Q.Y.; Zhang, J.T.; Hu, J.M. Electrodeposited graphene/layered double hydroxides micro/nanocontainers for both passive and active corrosion protection. npj Mater. Degrad. 2024, 8, 22. [Google Scholar] [CrossRef]
- Yu, F.; Camilli, L.; Wang, T.; Mackenzie, D.M.A.; Curioni, M.; Akid, R.; Bøggild, P. Complete long-term corrosion protection with chemical vapor deposited graphene. Carbon 2018, 132, 78–84. [Google Scholar] [CrossRef]
- Ayemi, G.J.; Blivet, C.; Marcelin, S.; Thérias, S.; Leroux, F.; Normand, B. Efficiency of layered double hydroxides in epoxy coating as corrosion inhibitor reservoirs for carbon steel. Appl. Clay Sci. 2024, 258, 107510. [Google Scholar] [CrossRef]
- Nyongombe, G.; Maaza, M.; Siaj, M.; Dhlamini, S. Improving the oxygen evolution reaction performance of ternary layered double hydroxides by tuning all three cations’ electronic structures. Nanomaterials 2025, 15, 177. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.J.; Zhao, J.; Ali, H.; Tabish, M.; Murtaza, H.; Yasin, G.; Malik, M.U.; Khan, W.Q. A review on self-healing coatings applied to Mg alloys and their electrochemical evaluation techniques. Int. J. Electrochem. Sci. 2020, 15, 3040–3053. [Google Scholar] [CrossRef]
- Olya, N.; Ghasemi, E.; Ramezanzadeh, B.; Mahdavian, M. Synthesis, characterization and protective functioning of surface decorated Zn–Al layered double hydroxide with SiO2 nanoparticles. Surf. Coat. Technol. 2020, 387, 125512. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D. Synthesis, characterization, and controlled release anticorrosion behavior of benzoate intercalated Zn–Al layered double hydroxides. Mater. Res. Bull. 2011, 46, 1963–1968. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Cui, H.; Chen, X.; Xu, R.; Zhang, Y.; Li, L. A multi-level biomimetic LDH coatings with super hydrophobicity, corrosion resistance, anti-icing and anti-fouling properties on magnesium alloy. J. Magnes. Alloys, 2025, in press. [CrossRef]
- Cao, K.; Yu, Z.; Zhu, L.; Yin, D.; Chen, L.; Jiang, Y.; Wang, J. Fabrication of superhydrophobic layered double hydroxide composites to enhance the corrosion-resistant performances of epoxy coatings on Mg alloy. Surf. Coat. Technol. 2021, 407, 126763. [Google Scholar] [CrossRef]
- Chen, K.; Dai, J.; Zhang, X. Improvement of corrosion resistance of magnesium alloys for biomedical applications. Corros. Rev. 2015, 33, 101–117. [Google Scholar] [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Ambrogi, V.; Bolli, E.; Ceccarelli, M.; Kaciulis, S.; Mezzi, A.; Montanari, R.; Pakhomova, E.; Richetta, M.; Varone, A. Surface modifications of biodegradable AZ31 alloy after immersion in physiological solution. Surf. Interface Anal. 2023, 55, 474–479. [Google Scholar] [CrossRef]
- Shuai, C.; Li, S.; Peng, S.; Feng, P.; Lai, Y.; Gao, C. Biodegradable metallic bone implants. Mater. Chem. Front. 2019, 3, 544–562. [Google Scholar] [CrossRef]
- Pompa, L.; Rahman, Z.U.; Munoz, E.; Haider, W. Surface characterization and cytotoxicity response of biodegradable magnesium alloys. Mater. Sci. Eng. C 2015, 49, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, O.; Ceccarelli, M.; Russo, M.; Torres-San-Miguel, C.R.; Urriolagoitia-Calderon, G. Experimental dynamic tests of rib implants. In Advances in Italian Mechanism Science; Springer: Cham, Switzerland, 2019; Volume 2019, pp. 353–361. [Google Scholar]
- Arrequin, J.L.; Montanari, R.; Ceccarelli, M.; Ambrogi, V.; Richetta, M.; Torres-San-Miguel, C.R.; Varone, A. Design solutions from material selection for rib fixators. Mater. Sci. Forum 2021, 1016, 303–308. [Google Scholar] [CrossRef]
- Das, P.; Sampath Kumar, T.S.; Sahu, K.K.; Gollapudi, S. Corrosion, stress corrosion cracking and corrosion fatigue behavior of magnesium alloy bioimplants. Corros. Rev. 2022, 40, 289–333. [Google Scholar] [CrossRef]
- Vinogradov, A.; Merson, E.; Myagkikh, P.; Linderov, M.; Brilevsky, A.; Merson, D. Attaining high functional performance in biodegradable Mg-alloys: An overview of challenges and prospects for the Mg–Zn–Ca system. Materials 2023, 16, 1324. [Google Scholar] [CrossRef] [PubMed]
- Pakhomova, E.; Varone, A.; Mezzi, A.; Fava, A.; Manis, C.; Loy, F.; Palombi, A.; Cao, G. Surface characterization of AZ31 alloy after long-term immersion in simulated body fluid. Crystals 2023, 13, 1692. [Google Scholar] [CrossRef]
- Xin, Y.; Hu, T.; Chu, P.K. In vitro studies of biomedical magnesium alloys in a simulated physiological environment: A review. Acta Biomater. 2011, 7, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, T.; Nahan, K.; Guo, X.; Zhang, Z.; Dong, Z.; Chen, S.; Chou, D.T.; Hong, D.; Kumta, P.N.; et al. In vivo characterization of magnesium alloy biodegradation using electrochemical H2 monitoring, ICP-MS, and XPS. Acta Biomater. 2017, 50, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Geng, X.; Zhang, X. Stress corrosion cracking of magnesium alloys: A review. J. Magnes. Alloys 2023, 11, 1906–1930. [Google Scholar] [CrossRef]
- Jafari, S.; Raman, R.K.S.; Davies, C.H.J. Stress corrosion cracking of an extruded magnesium alloy (ZK21) in a simulated body fluid. Eng. Fract. Mech. 2018, 201, 47–55. [Google Scholar] [CrossRef]
- Rahim, S.A.; Joseph, M.A.; Sampath Kumar, T.S.; Hanas, T. Recent progress in surface modification of Mg alloys for biodegradable orthopedic applications. Front. Mater. 2022, 9, 848980. [Google Scholar] [CrossRef]
- Kannan, B.; Dietzel, W.; Blawert, C.; Atrens, A.; Lyon, P. Stress corrosion cracking of rare-earth containing magnesium alloys ZE41, QE22 and Elektron 21 (EV31A) compared with AZ80. Mater. Sci. Eng. A 2008, 480–481, 529–539. [Google Scholar] [CrossRef]
- Choudhary, L.; Raman, R.K.S.; Hofstetter, J.; Uggowitzer, P.J. In vitro characterization of stress corrosion cracking of aluminium-free magnesium alloys for temporary bio-implant applications. Mater. Sci. Eng. C 2018, 90, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Rim, K.T.; Koo, K.H.; Park, J.S. Toxicological evaluations of rare earths and their health impacts to workers: A literature review. Saf. Health Work 2013, 4, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Istrate, B.; Munteanu, C.; Bălțatu, M.S.; Cimpoeșu, R.; Ioanid, N. Microstructural and electrochemical influence of Zn in MgCaZn biodegradable alloys. Materials 2023, 16, 2487. [Google Scholar] [CrossRef] [PubMed]
- Roche, V.; Koga, G.Y.; Matias, T.B.; Kiminami, C.S.; Bolfarini, C.; Botta, W.J.; Nogueira, R.P.; Jorge, A.M. Degradation of biodegradable implants: The influence of microstructure and composition of Mg-Zn-Ca alloys. J. Alloys Compd. 2019, 774, 168–181. [Google Scholar] [CrossRef]
- Singh, B.P.; Singh, R.; Mehta, J.S.; Prakash, C. Fabrication of biodegradable low elastic porous Mg-Zn-Mn-HA alloy by spark plasma sintering for orthopaedic applications. IOP Conf. Ser. Mater. Sci. Eng. 2017, 225, 012050. [Google Scholar] [CrossRef]
- Linyuan, H.; Xuan, L.; Feng, X.; Chenglin, C.; Jing, B. Biocorrosion behaviour of micro-arc-oxidized AZ31 magnesium alloy in different simulated dynamic physiological environments. Surf. Coat. Technol. 2019, 361, 240–248. [Google Scholar]
- Zhicheng, L.; Zhenzhen, S.; Xian, W.; Qing, Z. Corrosion resistance and cytotoxicity of AZ31 magnesium alloy with N+ ion implantation. Mater. Technol. 2019, 34, 730–736. [Google Scholar] [CrossRef]
- Merson, E.D.; Poluyanov, V.A.; Myagkikh, P.N.; Bunev, A.S.; Merson, D.L.; Vinogradov, A. Improving corrosion and stress corrosion cracking performance of machined biodegradable alloy ZX20 by HF-treatment. Metals 2023, 13, 1660. [Google Scholar] [CrossRef]
- Tong, P.; Sheng, Y.; Hou, R.; Iqbal, M.; Chen, L.; Li, J. Recent progress on coatings of biomedical magnesium alloy. Smart Mater. Med. 2022, 3, 104–116. [Google Scholar] [CrossRef]
- Rahman, M.; Li, Y.; Wen, C. HA coating on Mg alloys for biomedical applications: A review. J. Magnes. Alloys 2020, 8, 929–943. [Google Scholar] [CrossRef]
- Mohajernia, S.; Pour-Ali, S.; Hejazi, S.; Saremi, M.; Kiani-Rashid, A.R. Hydroxyapatite coating containing multi-walled carbon nanotubes on AZ31 magnesium: Mechanical-electrochemical degradation in a physiological environment. Ceram. Int. 2018, 44, 8297–8305. [Google Scholar] [CrossRef]
- Antoniac, I.; Miculescu, F.; Cotrut, C.; Ficai, A.; Rau, J.V.; Grosu, E.; Antoniac, A.; Tecu, C.; Cristescu, I. Controlling the degradation rate of biodegradable Mg–Zn–Mn alloys for orthopedic applications by electrophoretic deposition of hydroxyapatite. Materials 2020, 13, 263. [Google Scholar] [CrossRef] [PubMed]
- Peron, M.; Bin Afif, A.; Dadlani, A.L.; Berto, F.; Torgersen, J. Improving stress corrosion cracking behavior of AZ31 alloy with conformal thin titania and zirconia coatings for biomedical applications. J. Mech. Behav. Biomed. Mater. 2020, 111, 104005. [Google Scholar] [CrossRef] [PubMed]
- Khatun, H.; Rahman, M.; Mahmud, S.; Ali, O.; Akter, M. Current advancements of hybrid coating on Mg alloys for medical applications. Results Eng. 2023, 18, 101162. [Google Scholar] [CrossRef]
- Richetta, M.; de Crescenzo, C.; Narducci, R.; Montanari, R.; Varone, A. Status and challenges in biomedical applications of LDHs. Key Eng. Mater. 2023, 967, 121–130. [Google Scholar] [CrossRef]
- Tan Jesslyn, K.E.; Balan, P.; Birbilis, N. Advances in LDH coatings on Mg alloys for biomedical applications: A corrosion perspective. Appl. Clay Sci. 2021, 202, 105948. [Google Scholar] [CrossRef]
- Jiang, H.; Li, F.; Zeng, X. Microstructural characteristics and deformation of magnesium alloy AZ31 produced by continuous variable cross-section direct extrusion. J. Mater. Sci. Technol. 2017, 33, 573–579. [Google Scholar] [CrossRef]
- Alvarez-Lopez, M.; Pereda, M.D.; del Valle, J.; Fernandez-Lorenzo, M.; Garcia-Alonso, M.C.; Ruano, O.A.; Escudero, M.L. Corrosion behaviour of AZ31 magnesium alloy with different grain sizes in simulated biological fluids. Acta Biomater. 2010, 6, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Liu, S.; Du, Y.; Lei, T.; Zeng, R.; Yuan, T. Effect of the second phases on corrosion behavior of the Mg–Al–Zn alloys. J. Alloys Compd. 2017, 695, 2330–2338. [Google Scholar] [CrossRef]
- Anuradha, J.; Mitun, D.; Vamsi, K.B. Effect of heat treatment on microstructure, mechanical, corrosion and biocompatibility of Mg-Zn-Zr-Gd-Nd alloy. J. Alloys Compd. 2020, 821, 153462. [Google Scholar]
- Rodrigues, P.S.; Zenobio, I.R.; da Silva, T.I.; Fernandes, C.Q.C.; de Sousa, T.G.; de Castro, J.A.; da Fonseca, G.S.; Huguenin, J.A.O.; Ferreira, E.A. Effect of Aging on Corrosion Resistance of AZ31 Magnesium Alloy. J. Mater. Eng. Perform. 2024, 33, 3413–3425. [Google Scholar] [CrossRef]
- Ramesh, S.; Kumar, G.; Jagadeesh, C.; Anne, G.; Nayaka, H.S. Effect of Equal Channel Angular Pressing on Properties Evaluation of Biodegradable Mg-Zn-Mn Alloy. J. Bio-Tribo-Corros. 2021, 7, 69. [Google Scholar] [CrossRef]
- Wang, L.S.; Jiang, J.H.; Saleh, B.; Xie, Q.Y.; Xu, Q.; Liu, H.; Ma, A.B. Controlling Corrosion Resistance of a Biodegradable Mg–Y–Zn Alloy with LPSO Phases via Multi-pass ECAP Process. Acta Metall. Sin. Engl. Lett. 2020, 33, 1180–1190. [Google Scholar] [CrossRef]
- Kalayeha, P.M.; Mehdi Malekana, M.; Bahmanib, A.; Lotfpoura, M.; Fatemid, S.M.; Zonoozie, S.B. Combination of severe plastic deformation and heat treatment for enhancing the corrosion resistance of a new Magnesium alloy. J. Alloys Compd. 2022, 927, 166939. [Google Scholar] [CrossRef]
- Medeiros, M.P.; Lopes, D.R.; Kawasaki, M.; Langdon, T.G.; Figueiredo, R.B. An Overview on the Effect of Severe Plastic Deformation on the Performance of Magnesium for Biomedical Applications. Materials 2023, 16, 2401. [Google Scholar] [CrossRef] [PubMed]
- Prithivirajan, S.; Naik, G.M.; Narendranath, S.; Desai, V. Recent progress in equal channel angular pressing of magnesium alloys starting from Segal’s idea to advancements till date—A review. Int. J. Lightweight Mater. Manuf. 2023, 6, 82–107. [Google Scholar] [CrossRef]
- Peron, M.; Skaret, P.C.; Fabrizi, A.; Varone, A.; Montanari, R.; Roven, H.J.; Ferro, P.; Berto, F.; Torgersen, J. The effect of Equal Channel Angular Pressing on the stress corrosion cracking susceptibility of AZ31 alloy in simulated body fluid. J. Mech. Behav. Biomed. Mater. 2020, 106, 103724. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yao, M.; Ning, Y.; Yu, J.; Xing, Z.; Gao, S.; Zhang, F.; Xia, X.; Zhao, G.; Liu, P.; et al. The effects of ECAP, Mn and Ca elements on the microstructure and corrosion properties of magnesium alloys. Int. J. Lightweight Mater. Manuf. 2024, 19, 100850. [Google Scholar] [CrossRef]
- Berto, F.; Bonollo, F.; Fabrizi, A.; Fava, A.; Ferro, P.; Montanari, R.; Narducci, R.; Pakhomova, E.; Palombi, A.; Richetta, M.; et al. Layered Double Hydroxide Growth on Equal Channel Angular Pressing-Processed AZ31 Alloy. Adv. Eng. Mater. 2025, 27, 2500100. [Google Scholar] [CrossRef]
- Forticaux, A.; Dang, L.; Liang, H.; Jin, S. Controlled synthesis of double layered hydroxides nanoplates driven by screw dislocations. Nano Lett. 2015, 15, 3403–3409. [Google Scholar] [CrossRef] [PubMed]
- Richetta, M.; Ciotta, E.; Montanari, R.; Narducci, R.; Pizzoferrato, R.; Varone, A. Effect of Al substrate microstructure on layered double hydroxide morphology. J. Mater. Sci. 2019, 54, 12437–12449. [Google Scholar] [CrossRef]
- Peltier, F.; Thierry, D. Review of Cr-Free Coatings for the Corrosion Protection of Aluminum Aerospace Alloys. Coatings 2022, 12, 518. [Google Scholar] [CrossRef]
- Kisuma Chemicals. Available online: https://www.kisuma.com/ (accessed on 9 July 2025).
- SMALLMATEK—Smart Materials for Critical Applications. Available online: https://www.smallmatek.pt/ (accessed on 9 July 2025).
- BASF—We Create Chemistry. Available online: https://www.basf.com/ (accessed on 9 July 2025).
- Clariant International Ltd. Available online: https://www.clariant.com/ (accessed on 9 July 2025).
- Kyowa Chemical Industry Co., Ltd. Available online: https://www.kyowa-chem.jp/ (accessed on 9 July 2025).
- Sakai Chemical Industry Co., Ltd. Available online: https://www.sakai-chem.co.jp/ (accessed on 9 July 2025).

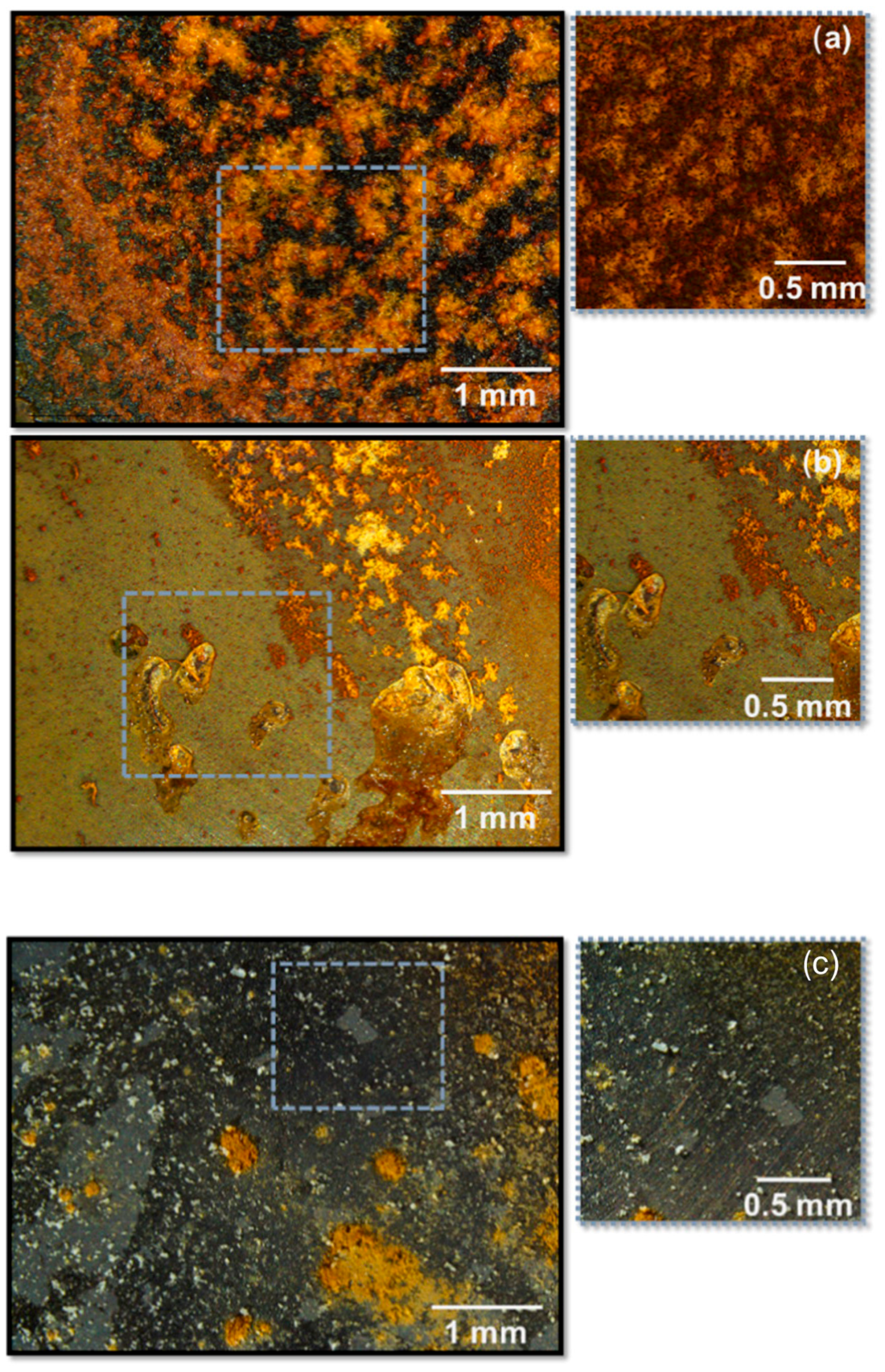




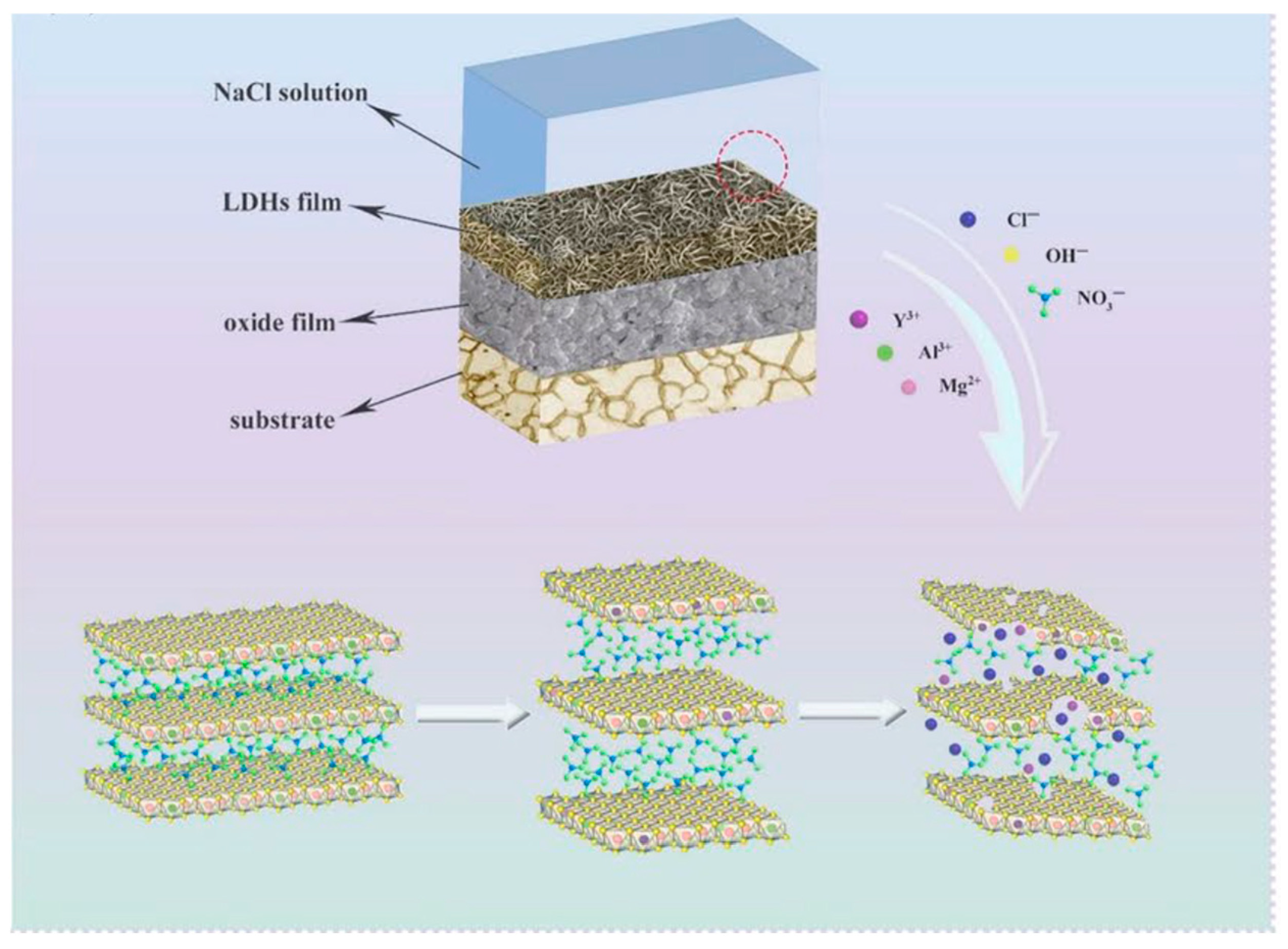
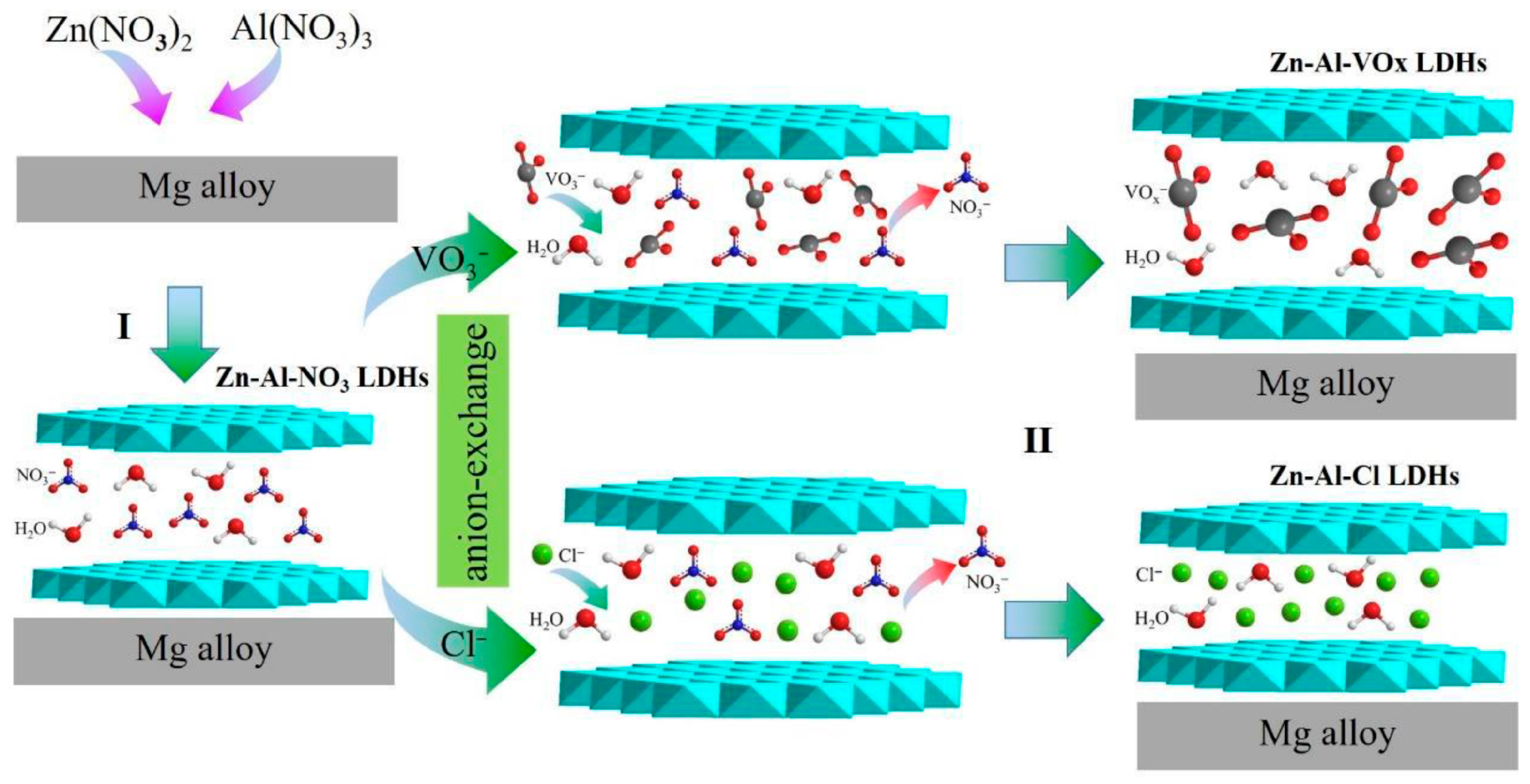

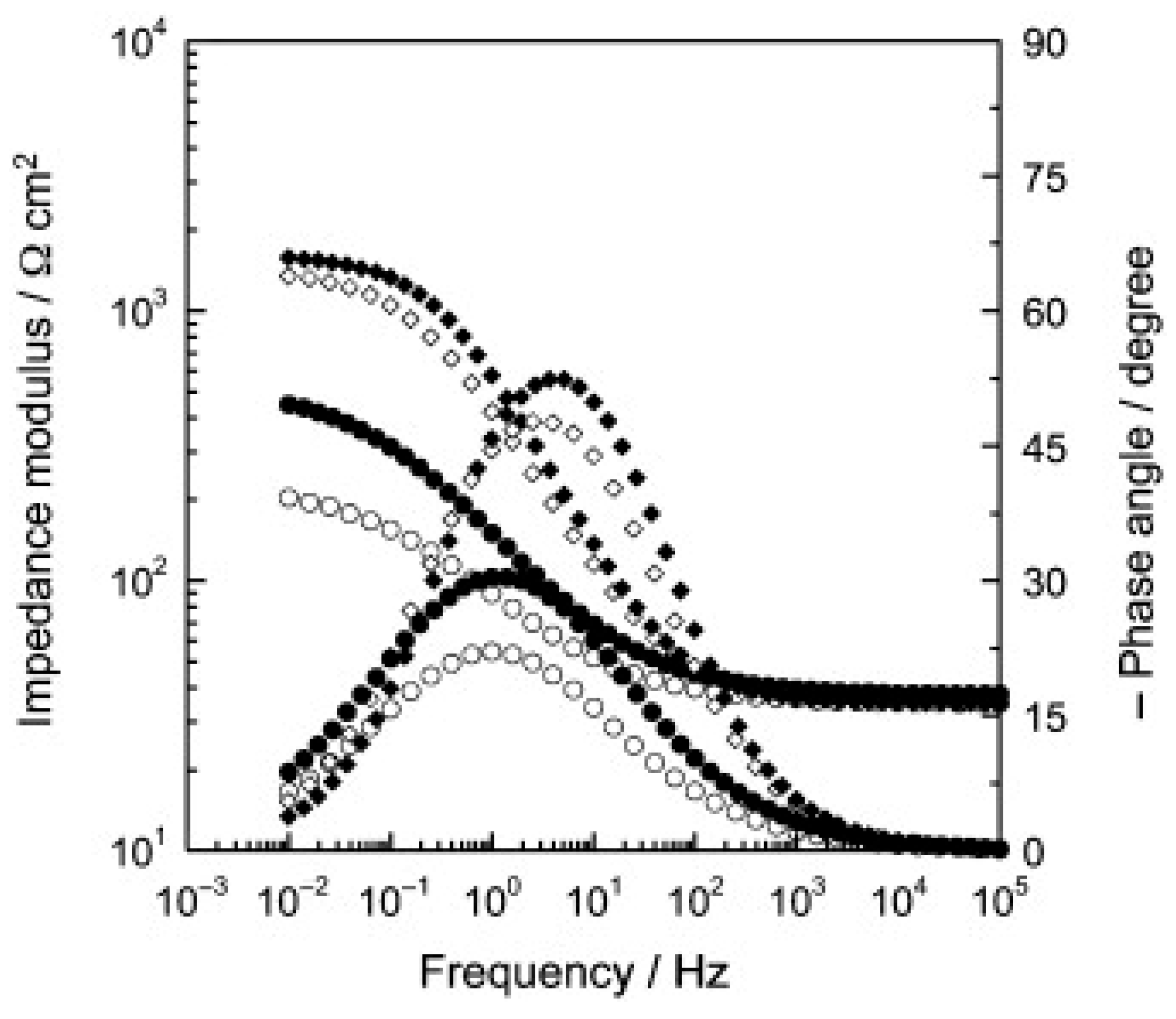
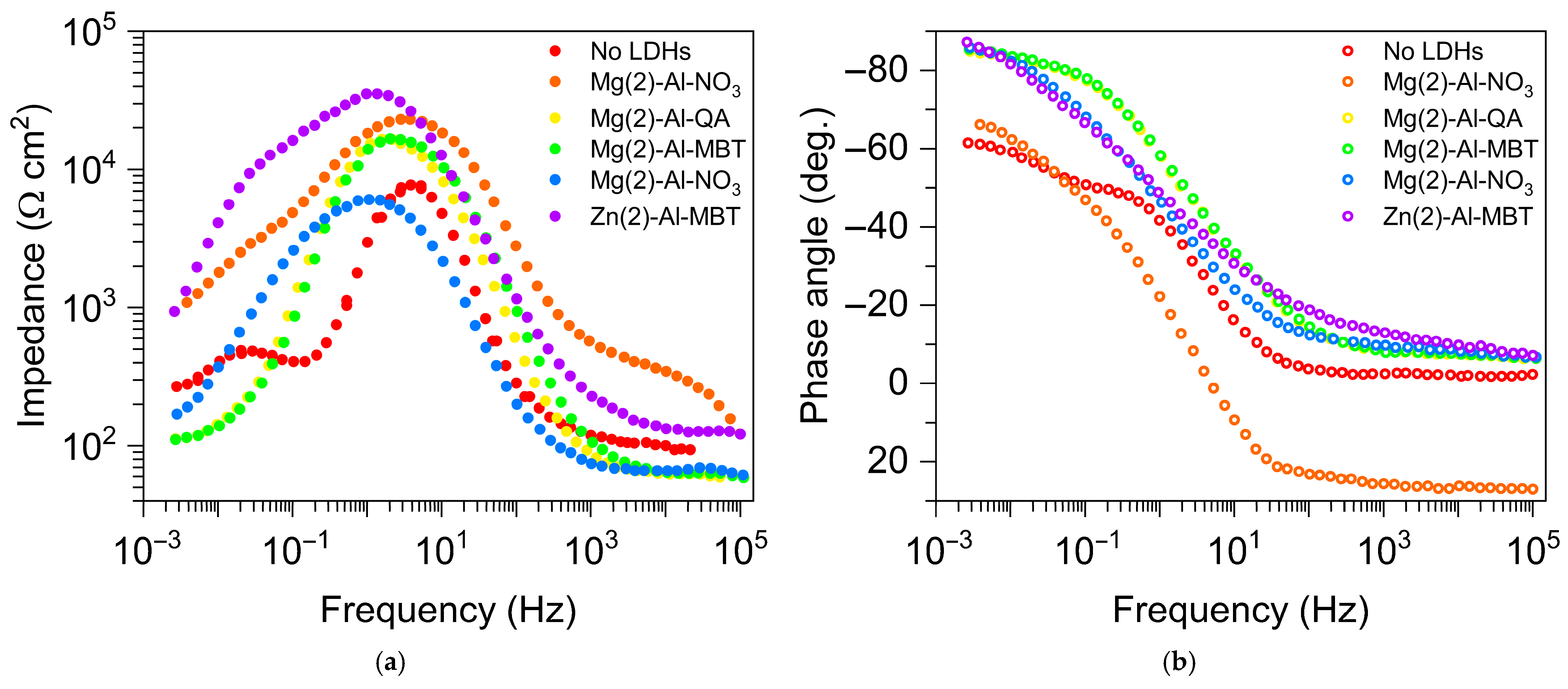


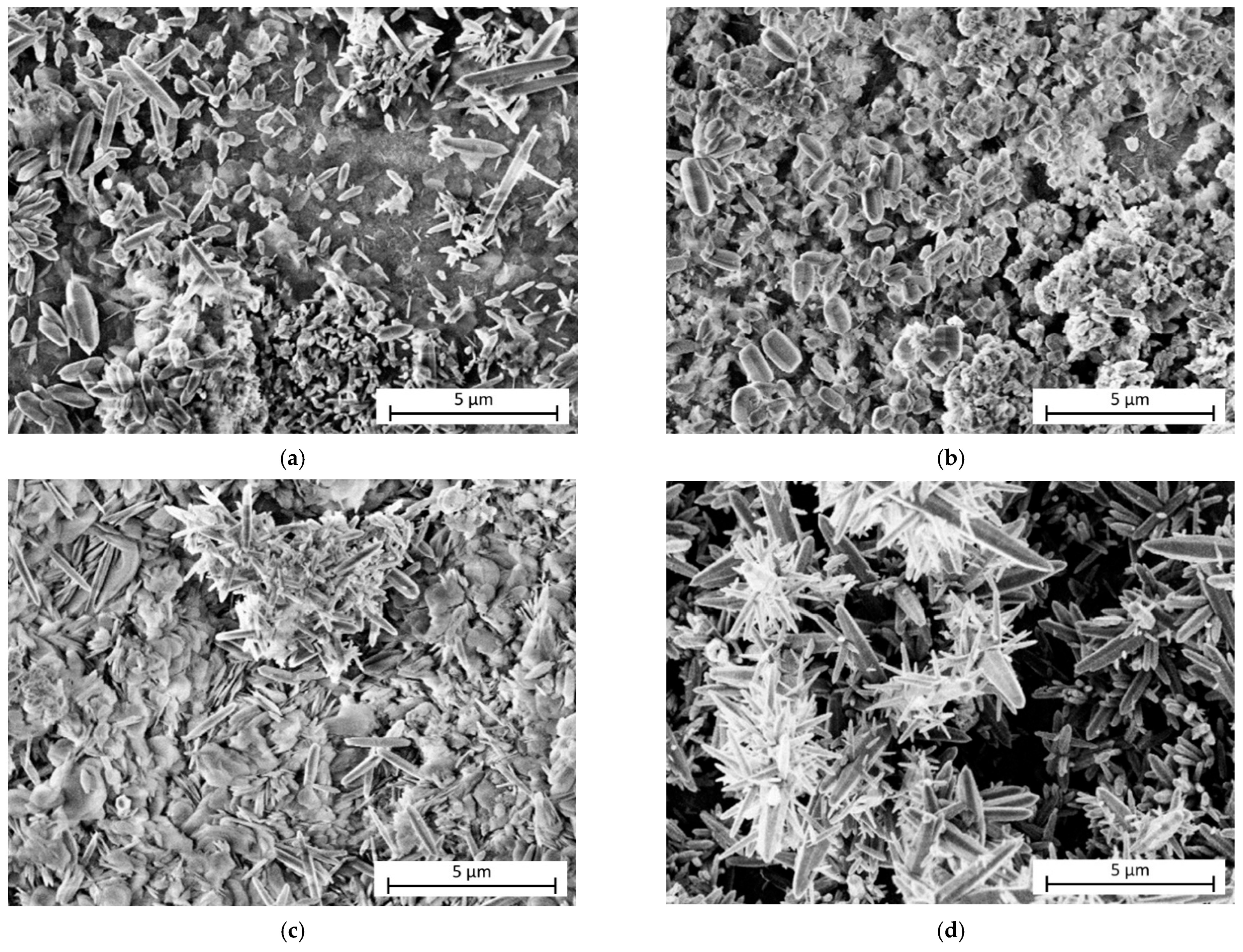

| Direct Methods | Indirect Methods |
|---|---|
|
|
| Mechanism | Benefits | Limitations |
|---|---|---|
| Direct exchange | Simple, ion selectivity-based | Slow, dependent on affinity and ion size |
| Exchange via acid attack | Enables exchanges with strongly bound anions (e.g., CO32−) | Causes structural degradation and requires acidic environment |
| Exchange by formation of surfactant salts | Allows for forced expulsion and specific encapsulation | Requires specific surfactants and organic solvents |
| Mechanism | Benefits | Limitations/Technical Challenges |
|---|---|---|
| Delamination-Filling | High area nanosheet production; flexible reassembly | High cost, use of solvents, difficulty in scaling |
| Reconstruction | Rapid structure reformation; simple | May not fully restore crystalline order |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varone, A.; Narducci, R.; Palombi, A.; Rasulzade, S.; Montanari, R.; Richetta, M. Recent Developments in Layered Double Hydroxides as Anticorrosion Coatings. Materials 2025, 18, 3488. https://doi.org/10.3390/ma18153488
Varone A, Narducci R, Palombi A, Rasulzade S, Montanari R, Richetta M. Recent Developments in Layered Double Hydroxides as Anticorrosion Coatings. Materials. 2025; 18(15):3488. https://doi.org/10.3390/ma18153488
Chicago/Turabian StyleVarone, Alessandra, Riccardo Narducci, Alessandra Palombi, Subhan Rasulzade, Roberto Montanari, and Maria Richetta. 2025. "Recent Developments in Layered Double Hydroxides as Anticorrosion Coatings" Materials 18, no. 15: 3488. https://doi.org/10.3390/ma18153488
APA StyleVarone, A., Narducci, R., Palombi, A., Rasulzade, S., Montanari, R., & Richetta, M. (2025). Recent Developments in Layered Double Hydroxides as Anticorrosion Coatings. Materials, 18(15), 3488. https://doi.org/10.3390/ma18153488










