Effect of Amino Trimethylene Phosphonic Acid and Tartaric Acid on Compressive Strength and Water Resistance of Magnesium Oxysulfate Cement
Abstract
1. Introduction
2. Materials and Test Methods
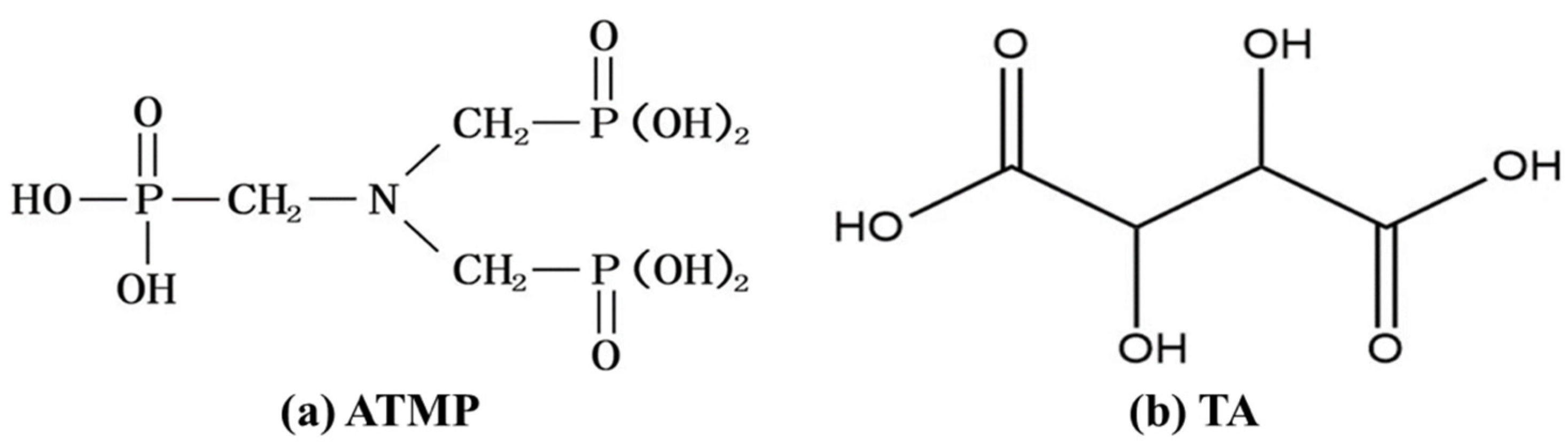
2.1. Materials
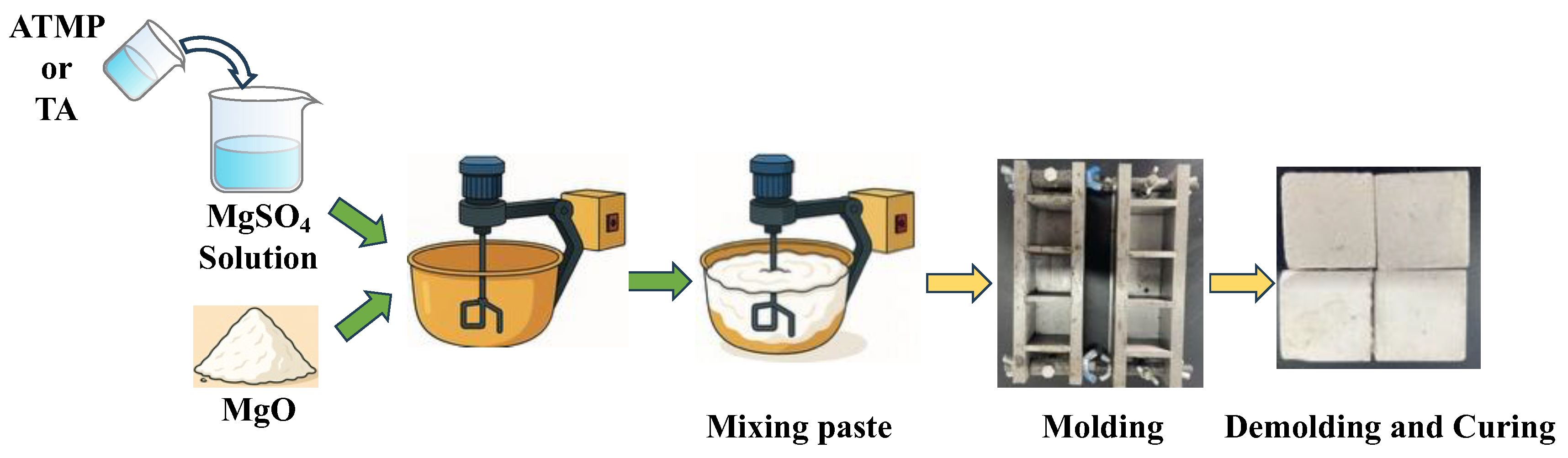
2.2. Sample Preparation
2.3. Test Methods
- (1)
- Hydration heat
- (2)
- Conductivity and pH value of MOS suspension
- (3)
- Conductivity of solution
- (4)
- Hydration analysis
3. Results and Discussion
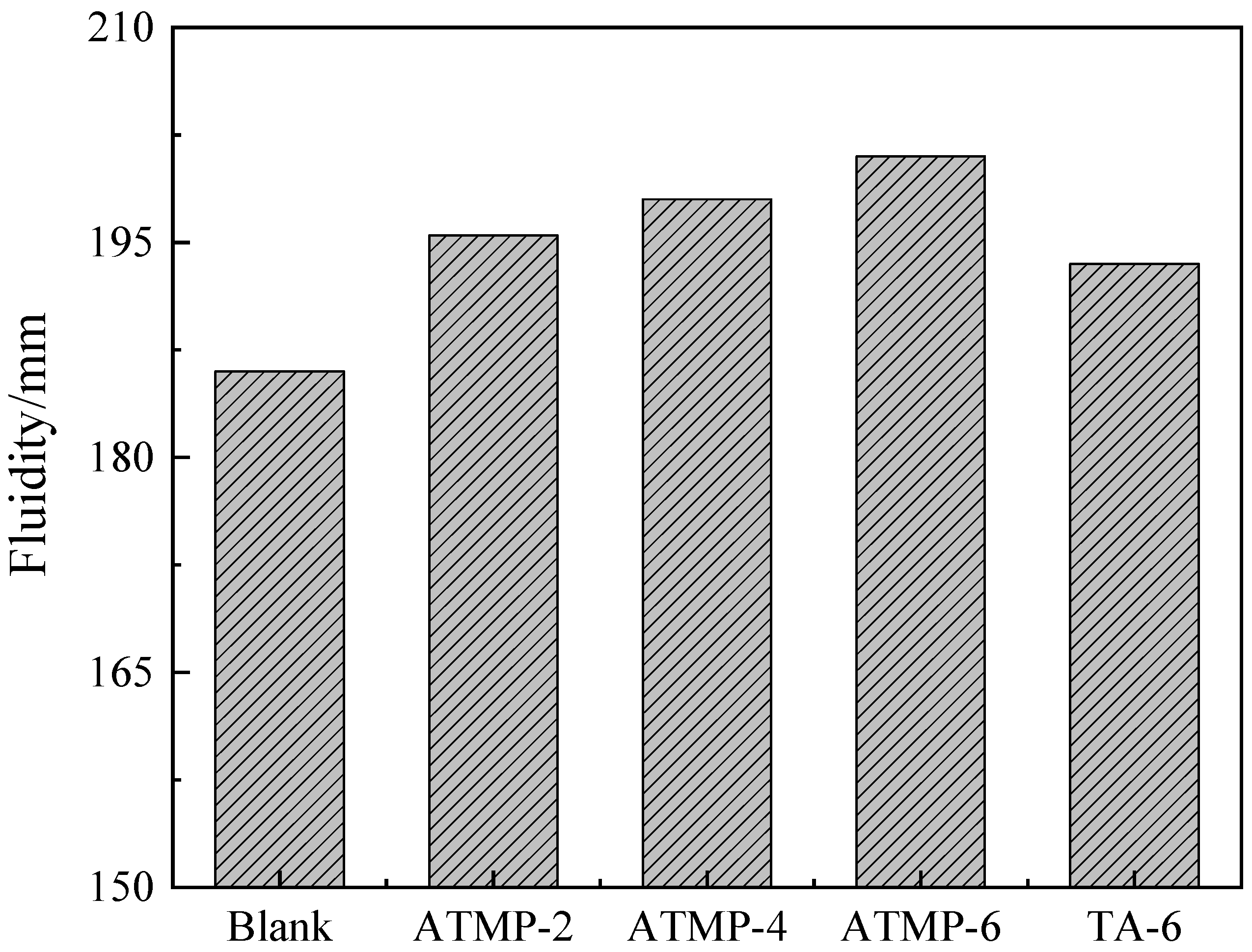
3.1. Fluidity and Setting Time
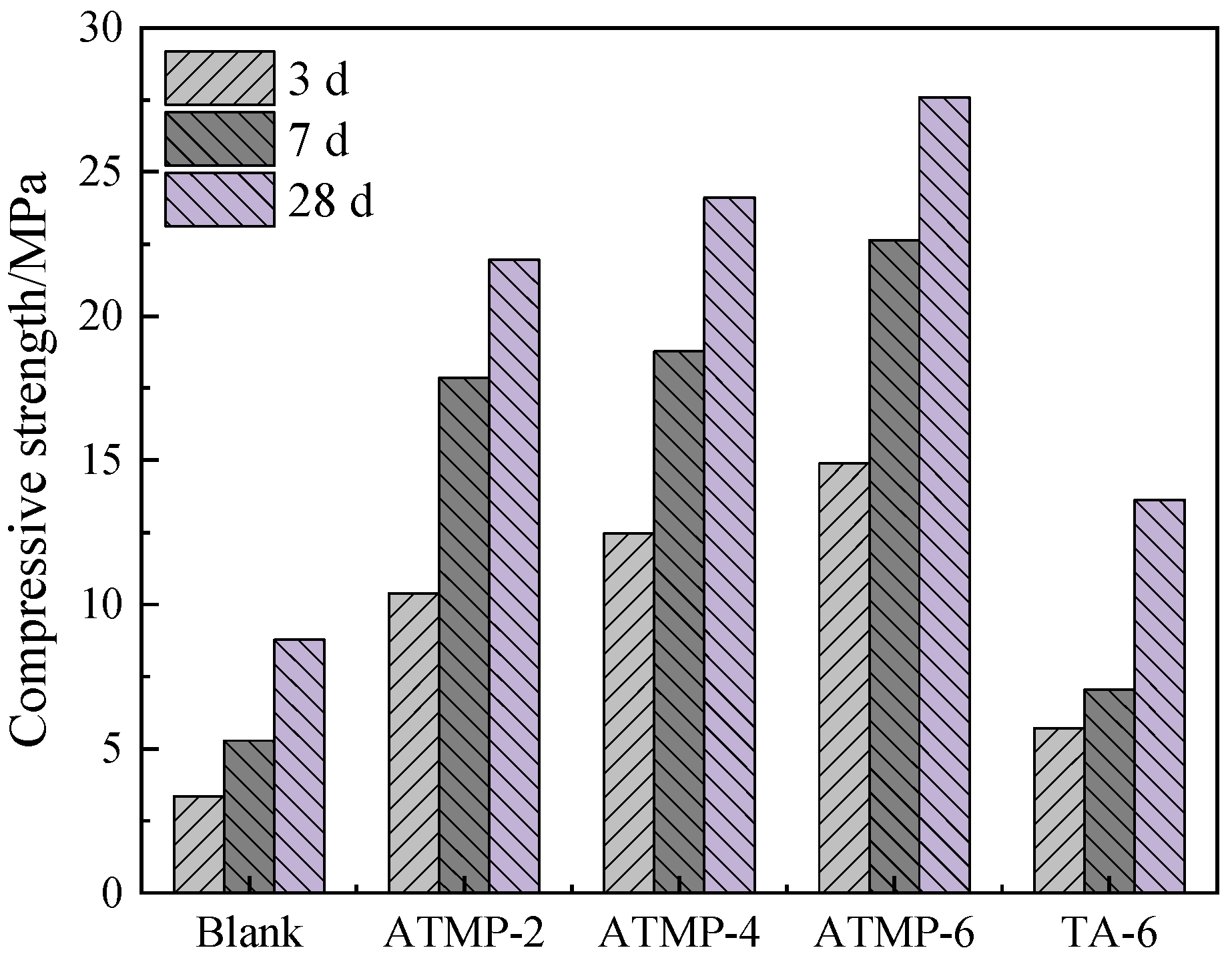
3.2. Compressive Strength
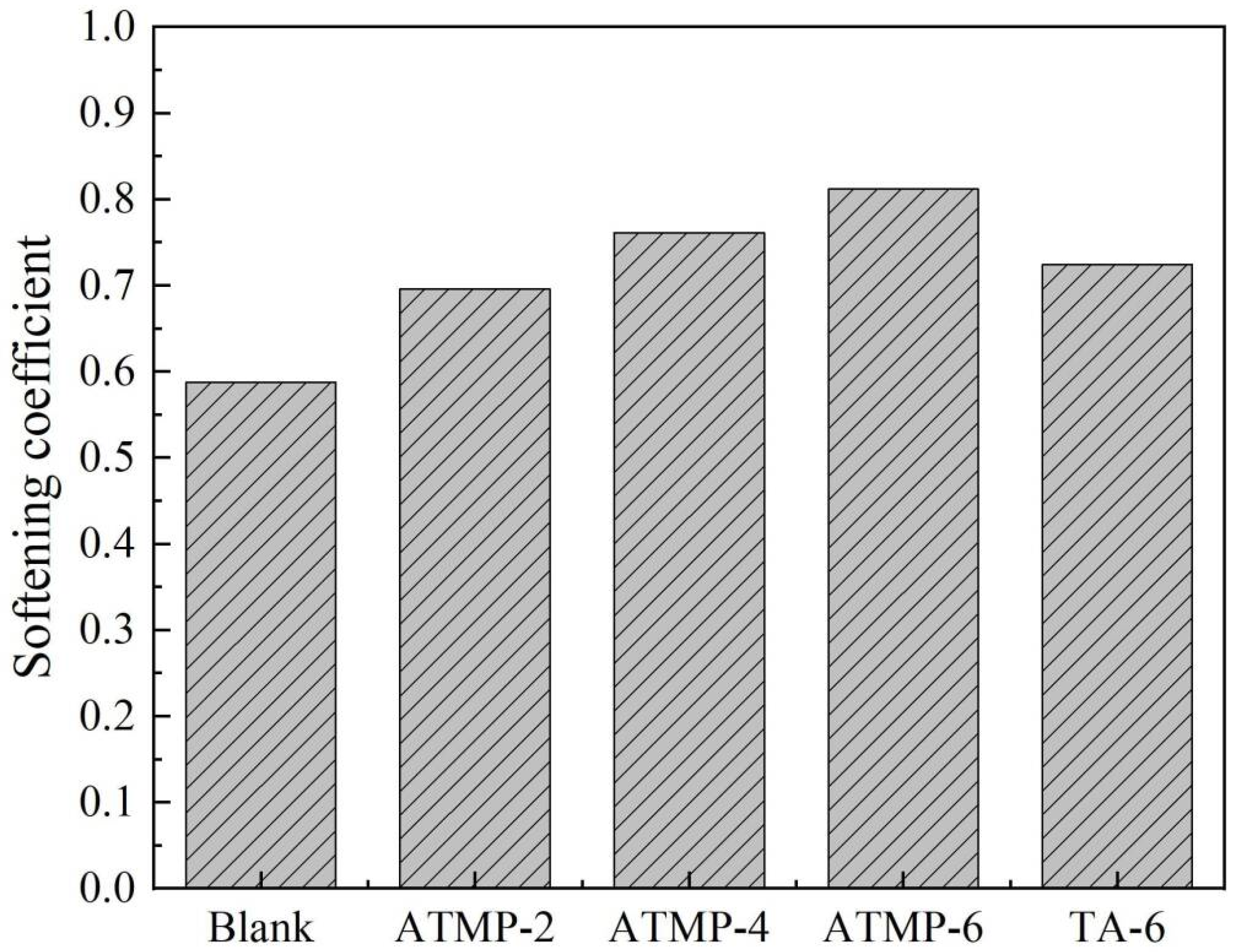
3.3. Water Resistance
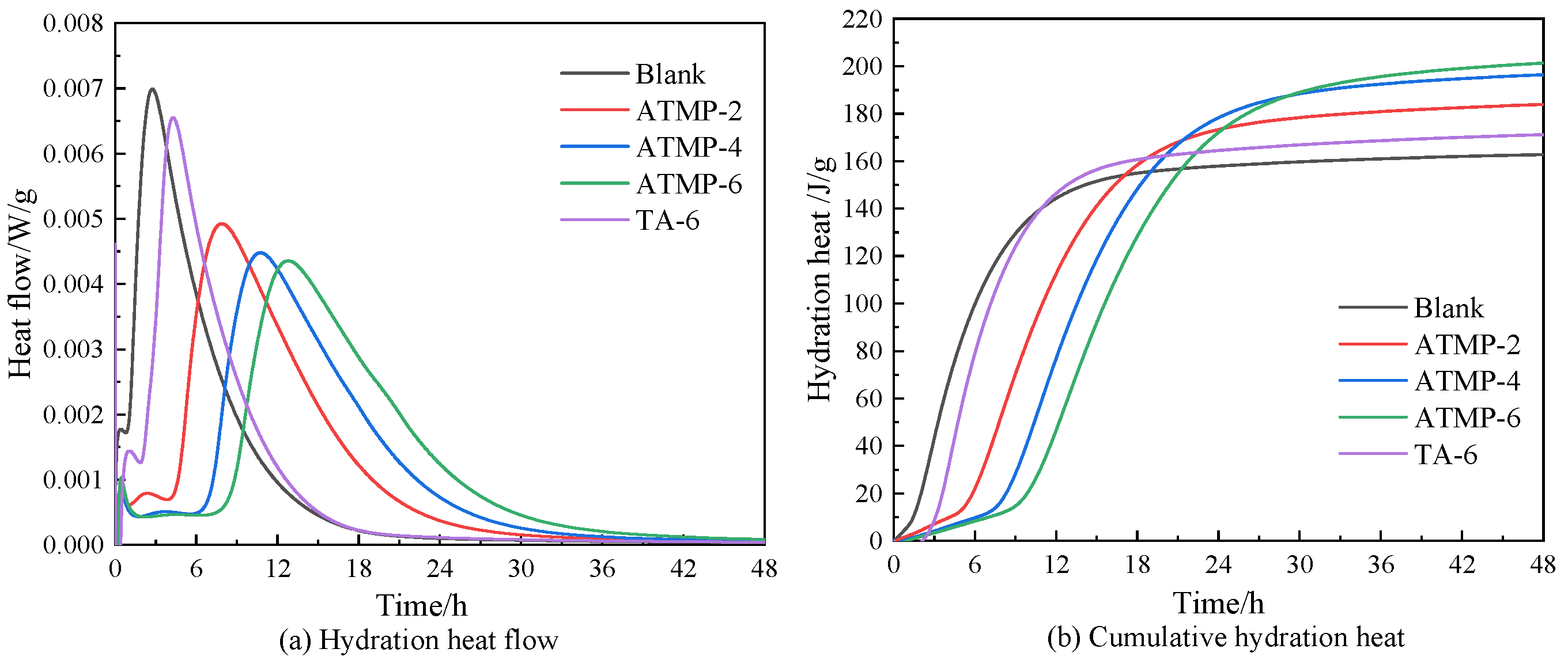
3.4. Hydration Heat
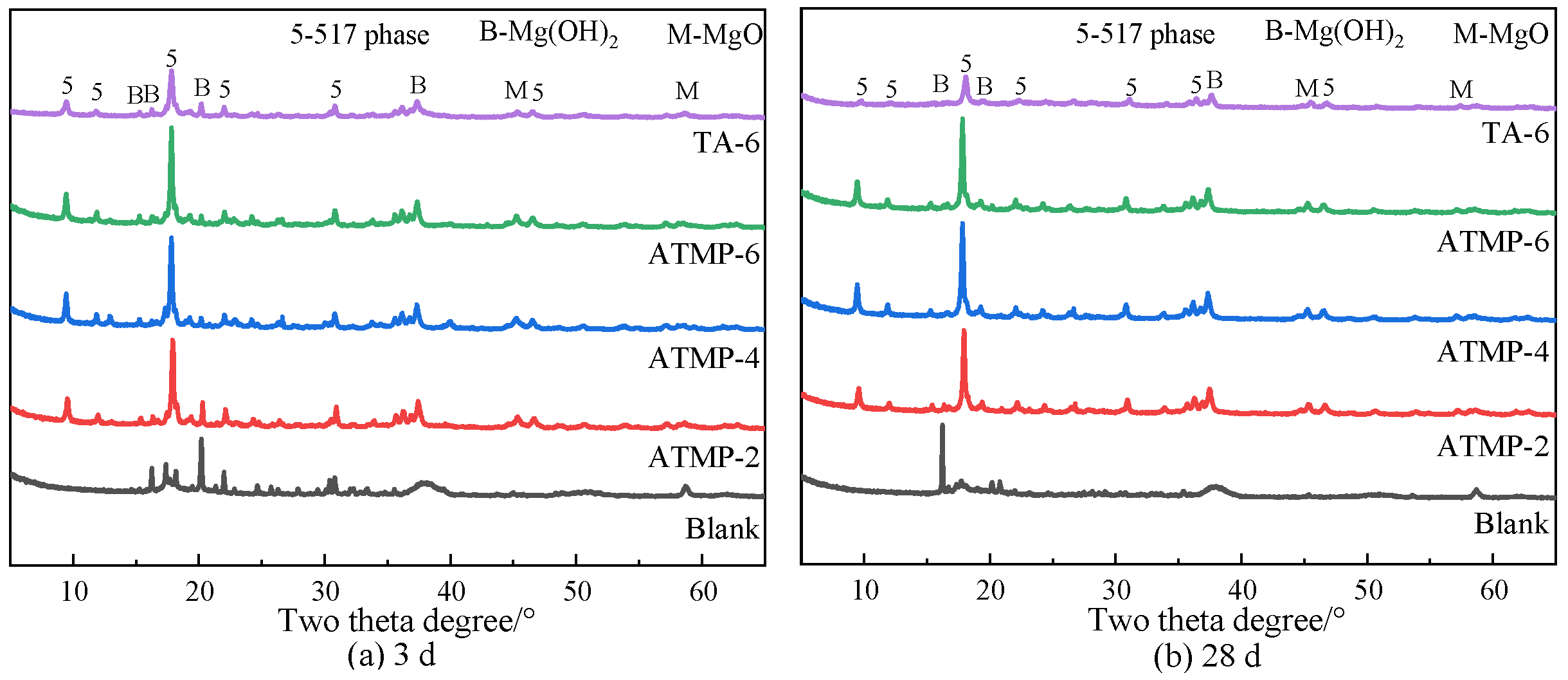
3.5. Hydrates Analysis
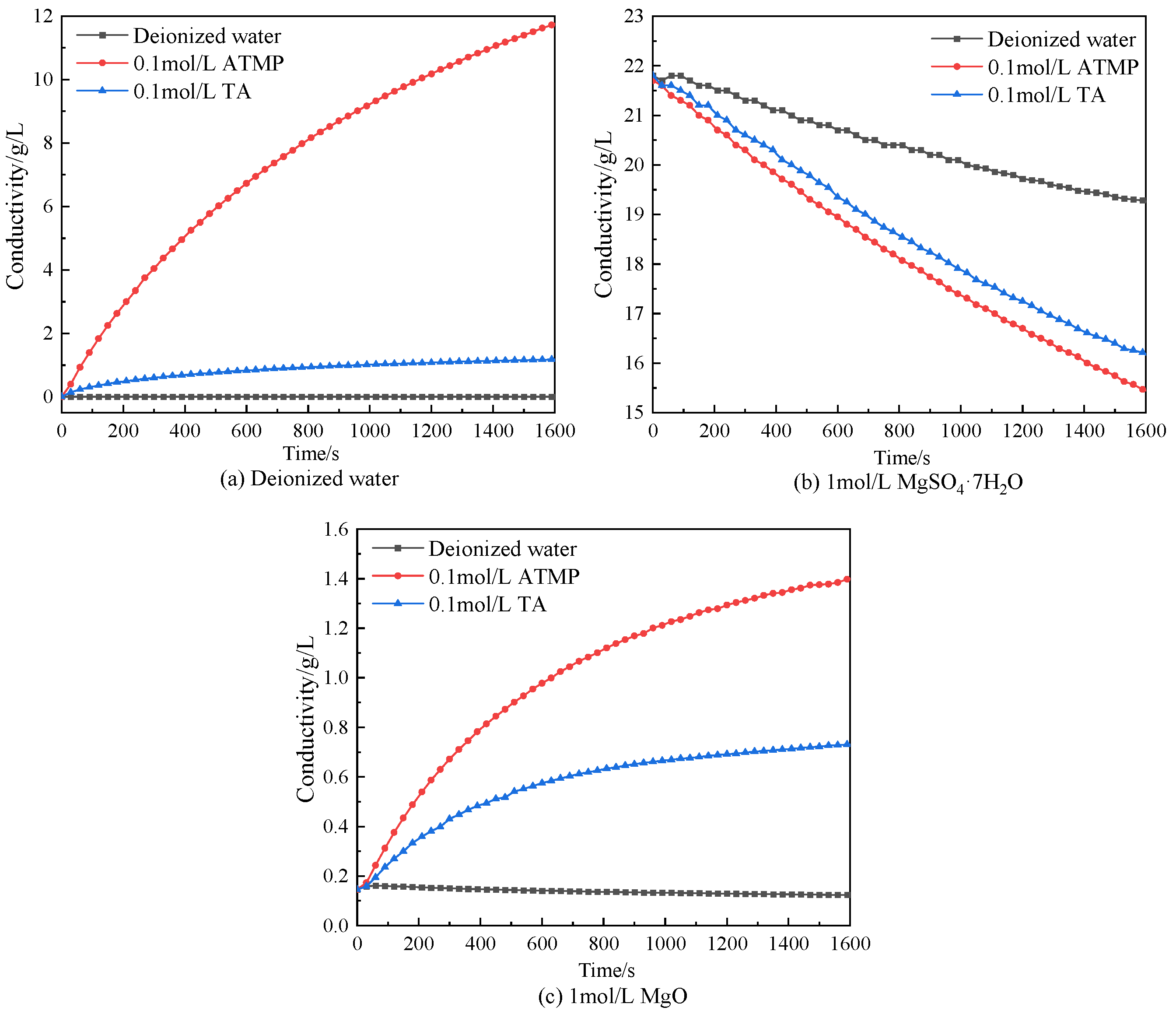
3.6. Effect of ATMP and TA on pH Value of MOS
3.7. Mechanism
4. Conclusions
- (1)
- Both ATMP and TA could improve the fluidity, delay the setting process, increase the strength, and enhance water resistance. The reason being that ATMP and TA hindered the hydration in the initial few hours while they accelerated the hydration later, which was proved by the results of analyzing the hydrates, hydration heat, and the pH value. The mechanism behind the initial hindrance and later acceleration was closely related to two aspects: H+ from ATMP and TA to hinder the generation of Mg(OH)2; chelation with Mg2+ in the liquid phase resulting in a supersaturated solution with a higher saturation, which also hindered the generation of Mg(OH)2 and then accelerated the generation of the 517 phase.
- (2)
- By contrast, with the same mole dosage of H+, MOS with ATMP-2 displayed a higher compressive strength than TA-6, and with the same mole dosage, ATMP demonstrated greater efficacy in improving fluidity, delaying the setting process, enhancing the compressive strength, and improving water resistance. This was because the chelating ability of ATMP was stronger than that of TA in hindering the formation of Mg(OH)2 and then inducing the generation of the 517 phase, and these results also indicated that the chelating ability of the anions played a key role in both the retarding effect and performance enhancement.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, Z.; Zhang, L.; Fang, L.; Guo, Y.; Cheng, F. Effect of seed emulsion on the early hydration behavior of basic magnesium sulfate cement. Constr. Build. Mater. 2023, 383, 131316. [Google Scholar] [CrossRef]
- Barbieri, V.; Gualtieri, M.L.; Manfredini, T.; Siligardi, C. Lightweight concretes based on wheat husk and hemp hurd as bio-aggregates and modified magnesium oxysulfate binder: Microstructure and technological performances. Constr. Build. Mater. 2021, 284, 122751. [Google Scholar] [CrossRef]
- Azevedo, A.G.S.; Savastano, H. Assessment of carbonation as a complementary strategy to increase the durability of Magnesium Oxysulfate (MOS)-based fiber cement boards. Constr. Build. Mater. 2024, 438, 137086. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Sun, Y.; Yu, B.; Hu, H.; Li, D.; Hu, Z.; Wang, S.; Meng, J.; Yu, B. Investigation of the performance of basic magnesium sulfate cement mortar. Adv. Cem. Res. 2023, 35, 432–451. [Google Scholar] [CrossRef]
- Feng, T.; Wen, J.; Tan, Y. Dynamic damage mechanism of basic magnesium sulfate cement composites: Experiments and 3D mesoscopic modeling study. Mech. Mater. 2024, 194, 105011. [Google Scholar] [CrossRef]
- Gomes, C.M.; de Oliveira, A.D.S. Chemical phases and microstructural analysis of pastes based on magnesia cement. Constr. Build. Mater. 2018, 188, 615–620. [Google Scholar] [CrossRef]
- You, J.; Zhang, Y.; Yang, C.; Song, Q.; Sun, Y. Hierarchical pore structure for enhanced carbonation in basic magnesium sulfate cement: Mechanisms from modification to post-carbonation evolution. Cem. Concr. Res. 2025, 193, 107876. [Google Scholar] [CrossRef]
- Wu, C.; Huang, L. Properties and effective improvement approaches of basic magnesium sulfate cement under high-temperature pre-curing. Constr. Build. Mater. 2024, 411, 134561. [Google Scholar] [CrossRef]
- Douba, A.; Badjatya, P.; Kawashima, S. Enhancing carbonation and strength of MgO cement through 3D printing. Constr. Build. Mater. 2022, 328, 126867. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, Q.; Chen, X.; Cheeseman, C.; Wang, H.; Chang, J. Unlocking the role of silica gel in enhancing mechanical properties and water resistance of magnesium oxysulfate cement. Cem. Concr. Compos. 2025, 157, 105941. [Google Scholar] [CrossRef]
- Li, B.; Chen, B.; Liu, N.; Jiang, Z. Understanding the positive effects of silica fume on the high-temperature resistance and water resistance of magnesium oxysulfate cement. Constr. Build. Mater. 2024, 445, 137820. [Google Scholar] [CrossRef]
- Chen, X.; Chen, B.; Chang, J.; Xu, X.; Sun, E.; Wang, S.; Guan, Y. Improved mechanical strength of magnesium oxysulfate cement using ferric sulfate. J. Build. Eng. 2023, 67, 106007. [Google Scholar] [CrossRef]
- Wang, D.; Jin, K.; Wang, N.; Zhang, H.; Wang, J.; Zhou, X. Compressive strength and water resistance of magnesium oxysulfate (MOS) cement incorporating magnesium slag. Constr. Build. Mater. 2024, 453, 139053. [Google Scholar] [CrossRef]
- Wang, R.; Ji, X.; Zhou, R.; Jin, C.; Sun, W.; Wang, Z.; Yan, Y. Fabrication of high-strength, water-resistant homogeneous magnesium oxysulfate cement via synergistic modification with citric acid and sodium alginate. Constr. Build. Mater. 2024, 457, 139473. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, Z.; Yang, H.; Liu, X.; Qiao, Z. Properties and hydration mechanism of foamed magnesium oxysulfate cement under acid modification. Case Stud. Constr. Mater. 2024, 20, e03003. [Google Scholar] [CrossRef]
- Liang, H.; Liu, J.; Kang, B.; Zhang, Y.; He, Y.; Yang, H. Optimizing 1-hydroxyethylidene-1,1-diphosphonic acid and mechanically-activated iron ore tailings to prepare low-cost and retarded magnesium oxysulfate cement. Case Stud. Constr. Mater. 2022, 17, e01631. [Google Scholar] [CrossRef]
- Tan, Y.; Yu, H.; Yang, D.; Feng, T. Basic magnesium sulfate cement: Autogenous shrinkage evolution and mechanism under various chemical admixtures. Cem. Concr. Compos. 2022, 128, 104412. [Google Scholar] [CrossRef]
- Wu, C.; Chen, W.; Zhang, H.; Yu, H.; Zhang, W.; Jiang, N.; Liu, L. The hydration mechanism and performance of Modified magnesium oxysulfate cement by tartaric acid. Constr. Build. Mater. 2017, 144, 516–524. [Google Scholar] [CrossRef]
- Chengyou, W.; Huifang, Z.; Hongfa, Y. Preparation and properties of modified magnesium oxysulfate cement derived from waste sulfuric acid. Adv. Cem. Res. 2015, 28, 178–188. [Google Scholar] [CrossRef]
- Wang, N.; Yu, H.; Bi, W.; Tan, Y.; Zhang, N.; Wu, C.; Ma, H.; Hua, S. Effects of sodium citrate and citric acid on the properties of magnesium oxysulfate cement. Constr. Build. Mater. 2018, 169, 697–704. [Google Scholar] [CrossRef]
- Runčevski, T.; Wu, C.; Yu, H.; Yang, B.; Dinnebier, R.E.; Jennings, H. Structural Characterization of a New Magnesium Oxysulfate Hydrate Cement Phase and Its Surface Reactions with Atmospheric Carbon Dioxide. J. Am. Ceram. Soc. 2013, 96, 3609–3616. [Google Scholar] [CrossRef]
- Ozkul, M.H.; Baskoca, A.; Artirma, S. Influence of prolonged agitation on water movement related properties of water reducer and retarder admixtured concretes. Cem. Concr. Res. 1997, 27, 721–732. [Google Scholar] [CrossRef]
- GB/T 1346-2011; Test Methods for Water Requirement for Normal Consistency, Setting Time and Soundness of Cement. Standards Press of China: Beijing, China, 2011. (In Chinese)
- GB/T 8077-2023; Test Methods for Homogeneity of Concrete Admixtures. Standards Press of China: Beijing, China, 2023. (In Chinese)
- Yang, Z.Q.; Hou, K.P.; Guo, T.T. Research on Time-varying Behavior of Cement Grouts of Different Water-cement Ratios. In Proceedings of the International Conference on Green Building, Materials and Civil Engineering (GBMCE 2011), Shangri La, China, 22–23 August 2011; p. 4398. [Google Scholar]
- GB/T 17671-2021; Test Method for Compressive Strength of Cement Mortar. Standards Press of China: Beijing, China, 2021. (In Chinese)
- GB/T 4111-2013; Test Methods for Concrete Blocks and Bricks. Standards Press of China: Beijing, China, 2013. (In Chinese)
- Feng, G.; Huang, X.; Zheng, E.; Jiang, F.; Yang, Q.; Jin, W.; Wu, Q.; Liu, J.; Wang, D.; Huang, Y. Non-solvent displacement nonaqueous precipitation fabrication of novel foldable HAp ceramic paper without fiber and its performance. Ceram. Int. 2024, 50, 29819–29830. [Google Scholar] [CrossRef]
- Feng, G.; Xie, W.; Jiang, F.; Shao, C.; Yu, J.; Wu, Q.; Zhang, Q.; Yang, Q.; Jin, W.; Liu, J. Non-solvent displacement nonaqueous precipitation method for core-shell materials preparation: Synthesis of C@ZrSiO4 black pigment. Ceram. Int. 2023, 49, 38148–38156. [Google Scholar] [CrossRef]
- Gu, K.; Maierdan, Y.; Chen, B. Effects of ethylenediamine tetra-acetic acid (EDTA) and its disodium salt derivative (EDTA-Na) on the characteristics of magnesium oxysulfate (MOS) cement. Compos. Part B Eng. 2022, 232, 109654. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, B.; Mou, J.; Wang, D. Improved hydration resistance of magnesia by EDTA and ammonium phosphate as additives. Sci. Eng. Compos. Mater. 2017, 24, 29–33. [Google Scholar] [CrossRef]
- Barbieri, V.; Gualtieri, M.L.; Manfredini, T.; Siligardi, C. Hydration kinetics and microstructural development of a magnesium oxysulfate cement modified by macromolecules. Constr. Build. Mater. 2020, 248, 118624. [Google Scholar] [CrossRef]
- Amaral, L.F.; Oliveira, I.R.; Bonadia, P.; Salomão, R.; Pandolfelli, V.C. Chelants to inhibit magnesia (MgO) hydration. Ceram. Int. 2011, 37, 1537–1542. [Google Scholar] [CrossRef]





| Sample | Mole Ratio | ||||
|---|---|---|---|---|---|
| MgO | MgSO4·7H2O | H2O | ATMP | TA | |
| Blank | 4 | 1 | 16 | 0 | 0 |
| ATMP-2 | 4 | 1 | 16 | 0.002 | 0 |
| ATMP-4 | 4 | 1 | 16 | 0.004 | 0 |
| ATMP-6 | 4 | 1 | 16 | 0.006 | 0 |
| TA-6 | 4 | 1 | 16 | 0 | 0.006 |
| Scheme | Hydration Heat (J/g) | ||||
|---|---|---|---|---|---|
| 4 h | 10.8 h | 24 h | 30 h | 48 h | |
| Blank | 68.04 | 140.07 | 157.50 | 159.43 | 148.66 |
| ATMP-2 | 9.77 | 98.79 | 173.68 | 178.54 | 186.88 |
| ATMP-4 | 6.54 | 59.14 | 178.73 | 188.06 | 192.31 |
| ATMP-6 | 4.92 | 30.81 | 172.25 | 189.68 | 201.26 |
| TA-6 | 33.24 | 140.07 | 163.97 | 166.71 | 165.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhou, Z.; Qiu, L.; Lu, K.; Xu, D.; Zhang, S.; Zhang, S.; Jian, S.; Tan, H. Effect of Amino Trimethylene Phosphonic Acid and Tartaric Acid on Compressive Strength and Water Resistance of Magnesium Oxysulfate Cement. Materials 2025, 18, 3473. https://doi.org/10.3390/ma18153473
Zhou Y, Zhou Z, Qiu L, Lu K, Xu D, Zhang S, Zhang S, Jian S, Tan H. Effect of Amino Trimethylene Phosphonic Acid and Tartaric Acid on Compressive Strength and Water Resistance of Magnesium Oxysulfate Cement. Materials. 2025; 18(15):3473. https://doi.org/10.3390/ma18153473
Chicago/Turabian StyleZhou, Yutong, Zheng Zhou, Lvchao Qiu, Kuangda Lu, Dongmei Xu, Shiyuan Zhang, Shixuan Zhang, Shouwei Jian, and Hongbo Tan. 2025. "Effect of Amino Trimethylene Phosphonic Acid and Tartaric Acid on Compressive Strength and Water Resistance of Magnesium Oxysulfate Cement" Materials 18, no. 15: 3473. https://doi.org/10.3390/ma18153473
APA StyleZhou, Y., Zhou, Z., Qiu, L., Lu, K., Xu, D., Zhang, S., Zhang, S., Jian, S., & Tan, H. (2025). Effect of Amino Trimethylene Phosphonic Acid and Tartaric Acid on Compressive Strength and Water Resistance of Magnesium Oxysulfate Cement. Materials, 18(15), 3473. https://doi.org/10.3390/ma18153473






