Preparation of Permeable Porous Alumina Ceramics by Gel Casting Combined with Particle Stacking and Sintering Method
Abstract
1. Introduction
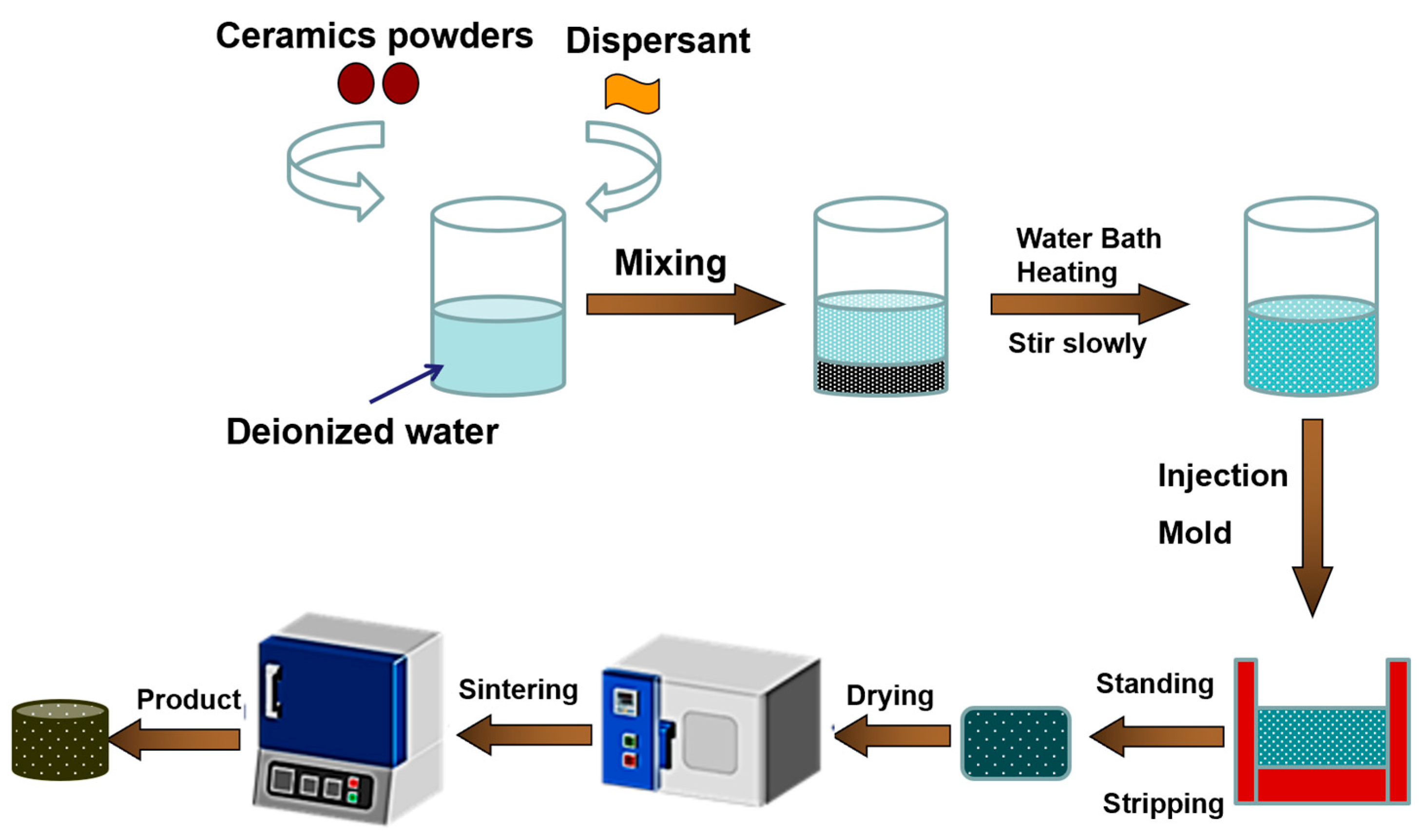
2. Materials and Methods
3. Results and Discussion
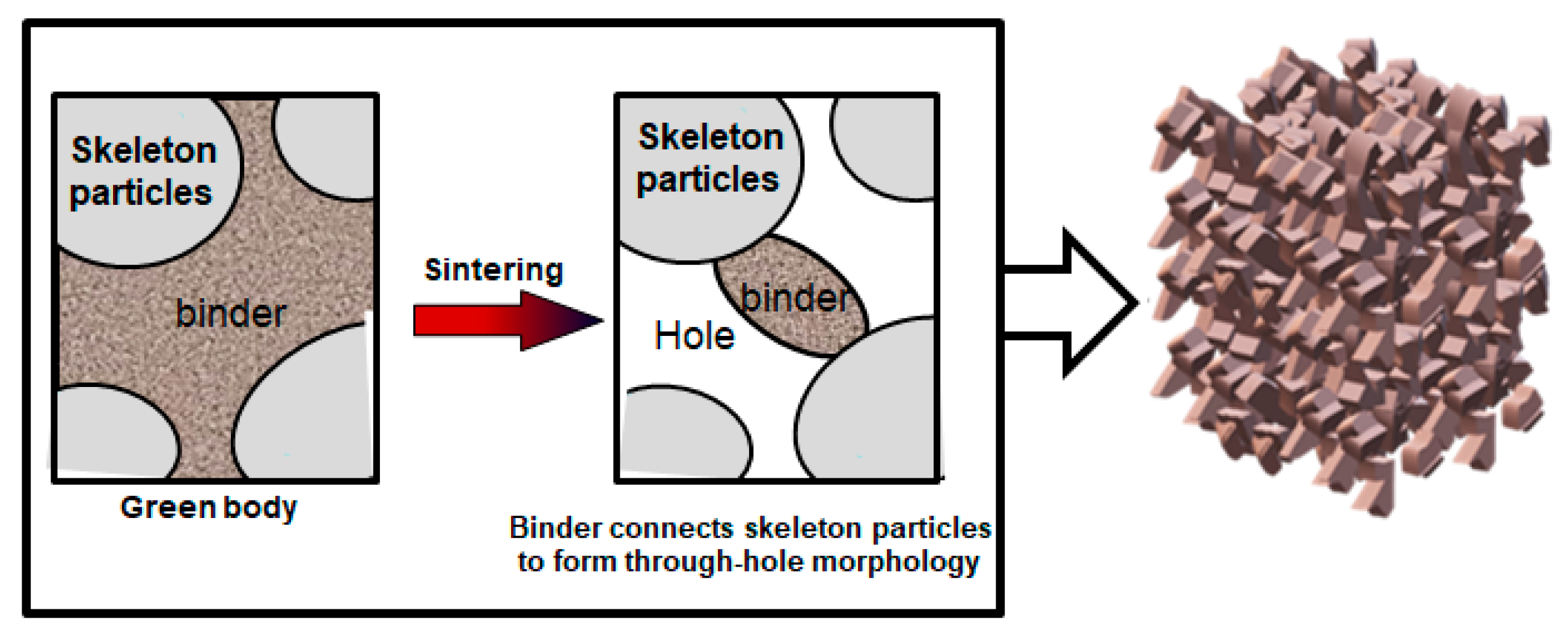
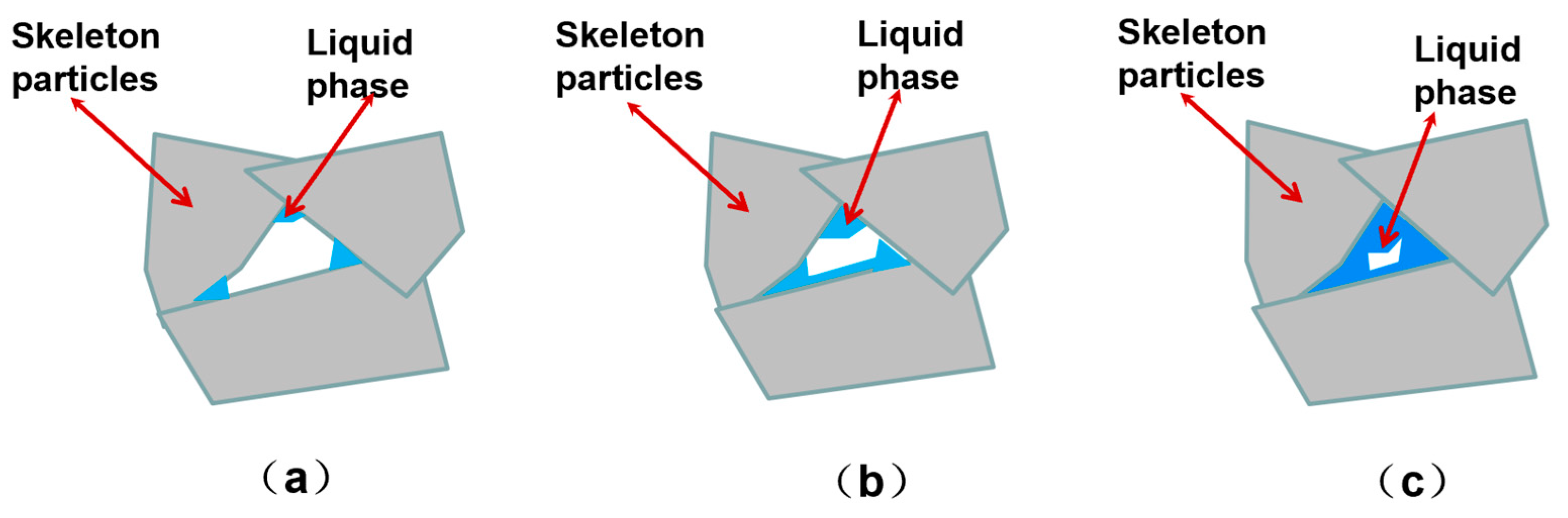
3.1. Mechanism of Forming Through-Holes by Gel Casting Combined with Particle Stacking and Sintering Method
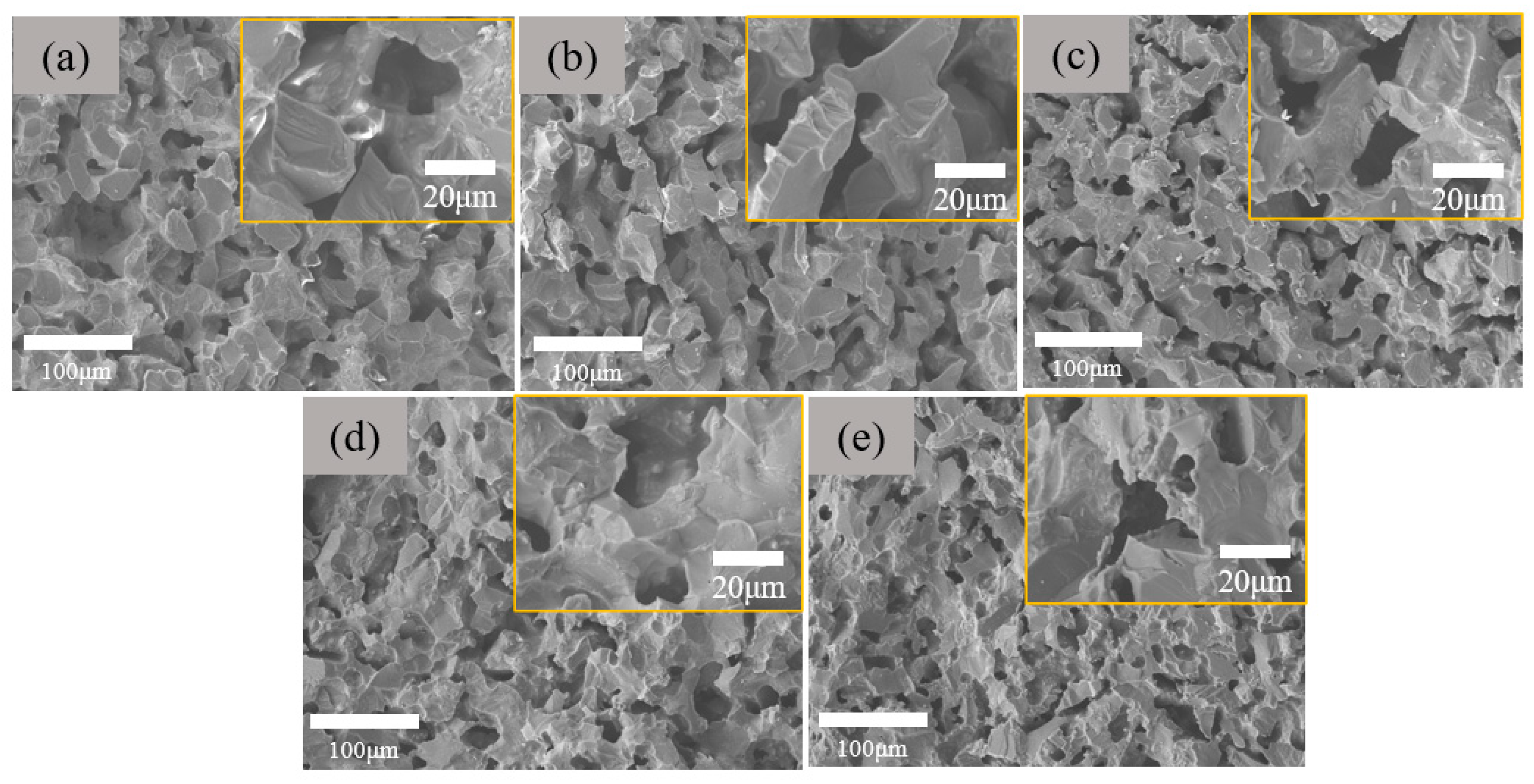
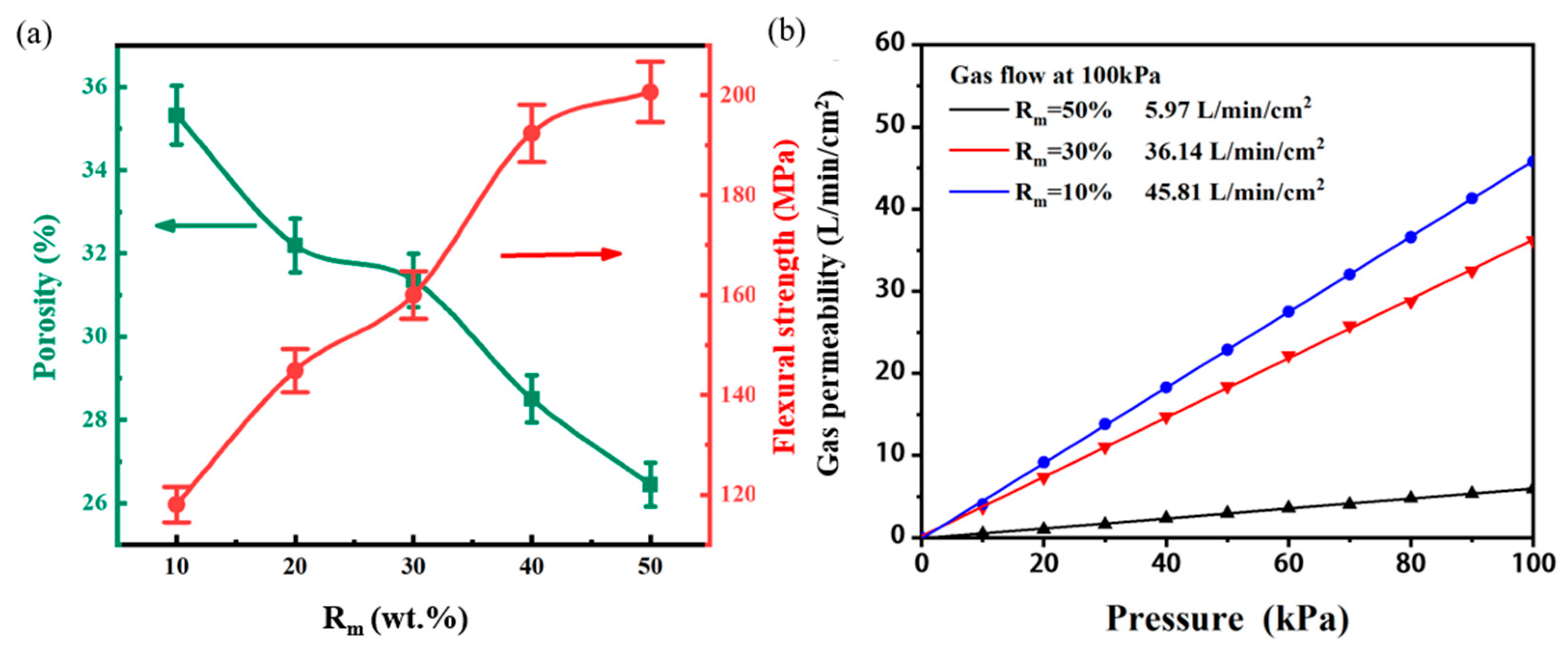
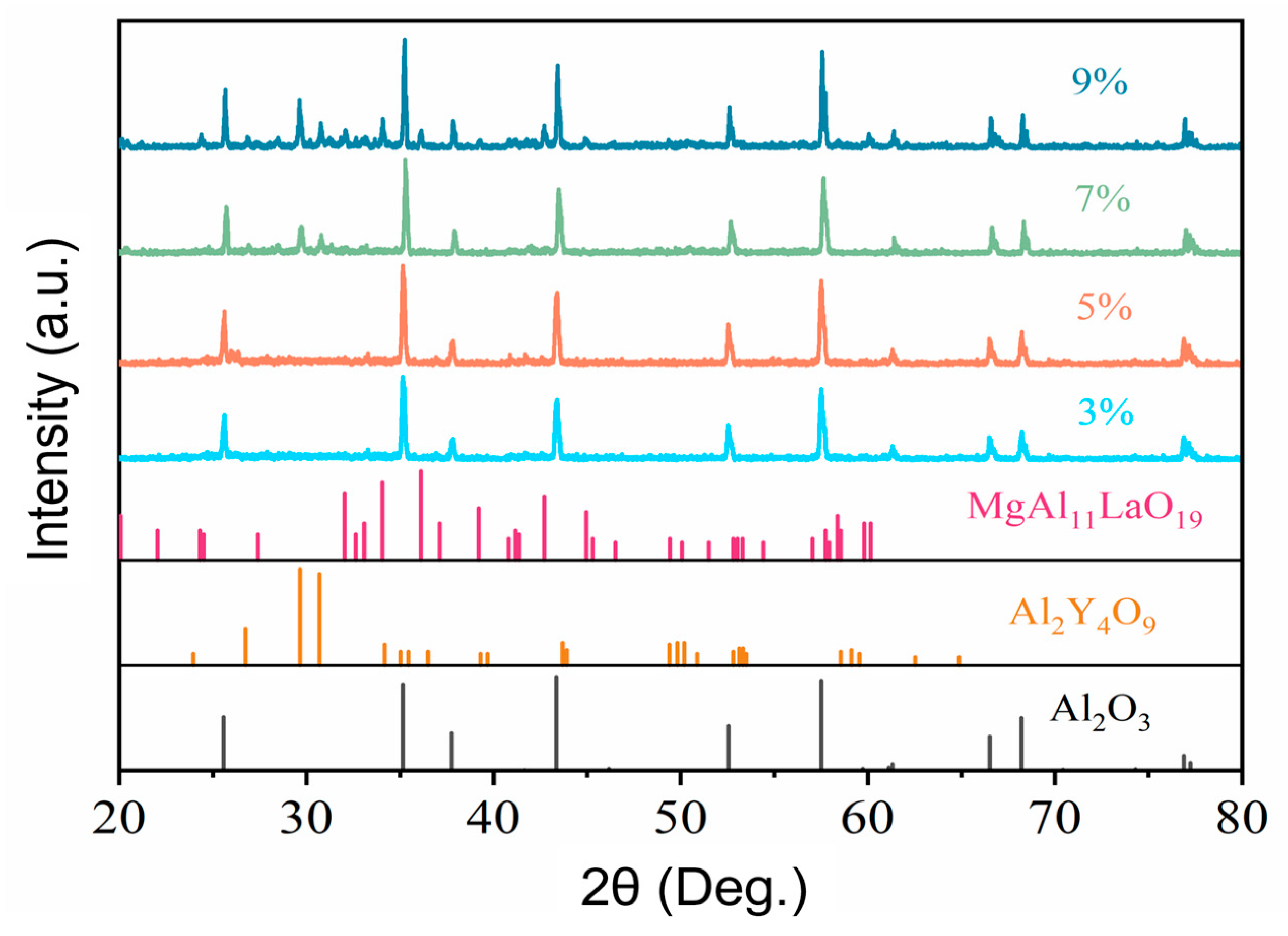
3.2. Influence of Aggregate–Matrix Ratio on Morphological Structure and Properties of Through-Porous Ceramic Materials
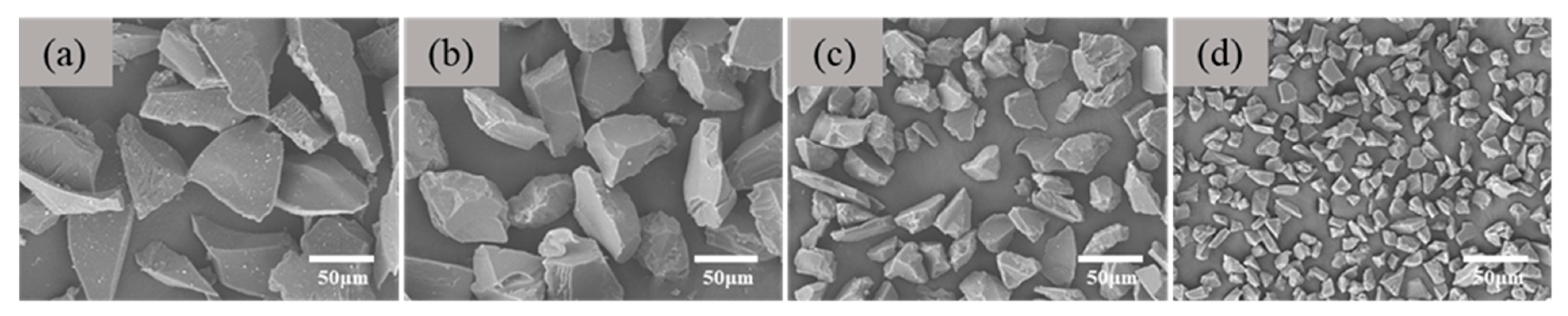
3.3. Influence of Aggregate Size on the Morphological Structure and Properties of Through-Porous Ceramic Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, Z.; Yao, D.; Xia, Y.; Zuo, K.; Yin, J.; Liang, H.; Zeng, Y.-P. Highly porous silica foams prepared via direct foaming with mixed surfactants and their sound absorption characteristics. Ceram. Int. 2020, 46, 12942–12947. [Google Scholar] [CrossRef]
- Voigt, C.; Jäckel, E.; Taina, F.; Zienert, T.; Salomon, A.; Wolf, G.; Aneziris, C.G.; Le Brun, P. Filtration Efficiency of Functionalized Ceramic Foam Filters for Aluminum Melt Filtration. Metall. Mater. Trans. B 2017, 48, 497–505. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, W.; Li, N.; Li, Y.; Dai, Y.; Zhang, Z.; Ma, S. Fabrication of mullite-corundum foamed ceramics for thermal insulation and effect of micro-pore-foaming agent on their properties. J. Alloys Compd. 2019, 785, 1030–1037. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, Y.; Li, B.; Pan, G. Immobilizing nitrifying bacteria with Fe2O3-CaO-SiO2 porous glass-ceramics. Int. J. Appl. Glass Sci. 2019, 10, 228–234. [Google Scholar] [CrossRef]
- Guo, W.; Hu, T.; Qin, H.; Gao, P.; Xiao, H. Preparation and in situ reduction of Ni/SiCxOy catalysts supported on porous SiC ceramic for ethanol steam reforming. Ceram. Int. 2021, 47 Pt A, 13738–13744. [Google Scholar] [CrossRef]
- Kim, J.; Ha, J.-H.; Lee, J.; Song, I.-H. Optimization for Permeability and Electrical Resistance of Porous Alumina-Based Ceramics. J. Korean Ceram. Soc. 2016, 53, 548–556. [Google Scholar] [CrossRef]
- Zhang, F.; Jia, M.; Wang, J.; Xu, M.; Wei, C.; Li, W.; Li, Z. Freeze-Casting of Arbitrary-Shaped Porous Ceramics. Adv. Eng. Mater. 2024, 26, 2301984. [Google Scholar] [CrossRef]
- Kotani, M.; Nishiyabu, K.; Matsuzaki, S.; Tanaka, S. Processing of polymer-derived porous SiC body using allylhydridopolycarbosilane (AHPCS) and PMMA microbeads. J. Ceram. Soc. Japa. 2011, 119, 563–569. [Google Scholar] [CrossRef]
- Hu, L.; Wang, C.A.; Huang, Y.; Sun, C.; Lu, S.; Hu, Z. Control of pore channel size during freeze casting of porous YSZ ceramics with unidirectionally aligned channels using different freezing temperatures. J. Eur. Ceram. Soc. 2010, 30, 3389–3396. [Google Scholar] [CrossRef]
- Krauss Juillerat, F.; Gonzenbach, U.T.; Elser, P.; Studart, A.R.; Gauckler, L.J. Microstructural control of self-setting particle-stabilized ceramic foams. J. Am. Ceram. Soc. 2011, 94, 77–83. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, T.; Huang, G. State-of-the-art research progress and challenge of the printing techniques, potential applications for advanced ceramic materials 3D printing. Mater. Today Commun. 2024, 40, 110001. [Google Scholar] [CrossRef]
- Man, Y.; Ding, G.; Xudong, L.; Xue, K.; Qu, D.; Xie, Z. A review on porous ceramics with hierarchical pore structure by 3D printing-based combined route. J. Asian Ceram. Soc. 2021, 9, 1377–1389. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Z.; Xu, M.; Wang, S.; Li, N.; Yang, J. A review of 3D printed porous ceramics. J. Eur. Ceram. Soc. 2022, 42, 3351–3373. [Google Scholar] [CrossRef]
- Omatete, O.O. Gelcasting-a new ceramic forming process. Cerem Bull 1991, 70, 1641–1649. [Google Scholar]
- Young, A.C.; Omatete, O.O.; Janney, M.A.; Menchhofer, P.A. Gelcasting of alumina. J. Am. Ceram. Soc. 1991, 74, 612–618. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Gan, K.; Zhang, X.; Qu, Y.; Ma, N.; Yang, J. A novel gelcasting of alumina suspension using curdlan gelation. Ceram. Int. 2015, 41, 10520–10525. [Google Scholar] [CrossRef]
- Shahbazi, H.; Tataei, M. A novel technique of gel-casting for producing dense ceramics of spinel (MgAl2O4). Ceram. Int. 2019, 45, 8727–8733. [Google Scholar] [CrossRef]
- Mishra, M.; Bora, J.J.; Goswamee, R.L. Improvement of the mechanical strength of alumina preforms by coating with montmorillonite/LDH gels. Appl. Clay Sci. 2011, 53, 8–14. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, H.; Meng, X.; Xiao, G.; Chen, Z.; Yi, M.; Zhang, J.; Liu, W.; Xu, C. Influence of Embedding Microcapsules on Tribological Properties of Alumina Ceramics Prepared by Gel Casting. Materials 2025, 18, 2110. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.R.; Calvino, M.M.; Šiler, P.; Cába, L.; Milioto, S.; Lisuzzo, L.; Lazzara, G.; Cavallaro, G. Self-Standing Biohybrid Xerogels Incorporating Nanotubular Clays for Sustainable Removal of Pollutants. Small 2025, 21, 2405215. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Yang, M.; Wang, F.; Hao, L.; Xu, X.; Wang, G.; Agathopoulos, S. Porous Al2O3 plates prepared by combing foaming and gel-tape casting methods for efficient collection of oil from water. Chem. Eng. J. 2019, 370, 658–665. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, C.A. Porous yttria-Stabilized Zirconia Ceramics Fabricated by Nonaqueous-Based Gelcasting Process with PMMA Microsphere as Pore-Forming Agent. J. Am. Ceram. Soc. 2013, 96, 266–271. [Google Scholar] [CrossRef]
- Xia, B.; Wang, Z.; Gou, L.; Zhang, M.; Guo, M. Porous mullite ceramics with enhanced compressive strength from fly ash-based ceramic microspheres: Facile synthesis, structure, and performance. Ceram. Int. 2022, 48, 10472–10479. [Google Scholar] [CrossRef]
- Qi, F.; Xu, X.; Xu, J.; Wang, Y.; Yang, J. A Novel Way to Prepare Hollow Sphere Ceramics. J. Am. Ceram. Soc. 2014, 97, 3341–3347. [Google Scholar] [CrossRef]
- Yang, Q.; Zeng, Z.; Xu, J.; Zhang, H.; Ding, J. Effect of La2O3 on Microstructure and Transmittance of Transparent Alumina Ceramics. J. Rare Earths 2006, 24, 72–75. [Google Scholar] [CrossRef]
- Park, C.W.; Yoon, D.Y. Effects of SiO2, CaO2, and MgO Additions on the Grain Growth of Alu mina. J. Am. Ceram. Soc. 2000, 83, 2605–2609. [Google Scholar] [CrossRef]


| Samples | Rm | Al2O3 (320 mesh) | α-Al2O3 (1–3 μm) | Kaoling Powders (4000 mesh) | Sintering Aids | ||
|---|---|---|---|---|---|---|---|
| Y2O3 | La2O3 | Mg2(OH)2CO3 | |||||
| A | 10% | 114.75 g | 12.75 g | 15.00 g | 3.75 g | 2.25 g | 1.5 g |
| B | 20% | 102.00 g | 25.50 g | 15.00 g | 3.75 g | 2.25 g | 1.5 g |
| C | 30% | 89.25 g | 38.25 g | 15.00 g | 3.75 g | 2.25 g | 1.5 g |
| D | 40% | 76.50 g | 51.00 g | 15.00 g | 3.75 g | 2.25 g | 1.5 g |
| E | 50% | 63.75 g | 63.75 g | 15.00 g | 3.75 g | 2.25 g | 1.5 g |
| Longitudinal Size of Particle Size (μm) | 200 mesh | 320 mesh | 600 mesh | 1000 mesh |
|---|---|---|---|---|
| Maximum | 126 ± 10 μm | 82 ± 8 μm | 39 ± 4 μm | 20 ± 2 μm |
| Minimum | 45 ± 5 μm | 38 ± 4 μm | 21 ± 2 μm | 11 ± 1 μm |
| Average Value | 81 ± 8 μm | 52 ± 5 μm | 28 ± 3 μm | 15 ± 1 μm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Chen, Y.; Wu, Z.; Liu, Y. Preparation of Permeable Porous Alumina Ceramics by Gel Casting Combined with Particle Stacking and Sintering Method. Materials 2025, 18, 3463. https://doi.org/10.3390/ma18153463
Cheng Z, Chen Y, Wu Z, Liu Y. Preparation of Permeable Porous Alumina Ceramics by Gel Casting Combined with Particle Stacking and Sintering Method. Materials. 2025; 18(15):3463. https://doi.org/10.3390/ma18153463
Chicago/Turabian StyleCheng, Zhe, Yuanqing Chen, Zhenping Wu, and Yang Liu. 2025. "Preparation of Permeable Porous Alumina Ceramics by Gel Casting Combined with Particle Stacking and Sintering Method" Materials 18, no. 15: 3463. https://doi.org/10.3390/ma18153463
APA StyleCheng, Z., Chen, Y., Wu, Z., & Liu, Y. (2025). Preparation of Permeable Porous Alumina Ceramics by Gel Casting Combined with Particle Stacking and Sintering Method. Materials, 18(15), 3463. https://doi.org/10.3390/ma18153463





